Abstract
Background
Hypercalcemia is commonly observed in cats with azotemic chronic kidney disease (CKD). Dietary phosphate restriction is considered standard of care but may contribute to the development of hypercalcemia. The optimal dietary management strategy for these cats is unclear.
Objectives
To describe the effect of feeding a moderately phosphate‐restricted diet (MP; 1.5 g/Mcal phosphorus; Ca : P ratio, 1.3) to cats with concurrent azotemic CKD and ionized hypercalcemia.
Animals
Client‐owned cats with ionized hypercalcemia (ionized calcium [iCa] concentration >1.4 mmol/L) at diagnosis of CKD (n = 11; baseline hypercalcemics) or after CKD diagnosis while eating a phosphate‐restricted clinical renal diet (0.8 g/Mcal phosphorus; Ca : P ratio, 1.9; n = 10; RD hypercalcemics).
Methods
Changes in variables over time, after starting MP at visit 1, were assessed using linear mixed model analysis within each group of cats. Data are reporte as median [25th, 75th percentiles].
Results
At visit 1, iCa was 1.47 [1.42, 1.55] mmol/L for baseline hypercalcemics and 1.53 [1.5, 1.67] mmol/L for RD hypercalcemics. Blood iCa decreased (P < .001) when RD hypercalcemics were fed MP, with iCa <1.4 mmol/L in 8/10 cats after 2.2 [1.8, 3.7] months. Plasma phosphate concentrations did not change. In contrast, the baseline hypercalcemic group overall showed no change in iCa but a decrease in plasma phosphate concentration during 8.8 [5.5, 10.6] months on the MP diet, although 4/11 individual cats achieved iCa <1.4 mmol/L by 3.4 [1.0, 6.2] months.
Conclusions and Clinical Importance
Attenuation of dietary phosphate restriction could result in normalization of iCa in cats that develop hypercalcemia while eating a clinical renal diet.
Keywords: CKD, diet, feline, renal
Abbreviations
- β
beta; slope of the line
- BCS
body condition score
- BHCa
baseline hypercalcemic group
- Ca : P
calcium‐to‐phosphorus ratio
- Ca × P
total calcium‐phosphate product
- CKD
chronic kidney disease
- FGF23
fibroblast growth factor 23
- HCO3−
venous bicarbonate concentration
- IRIS
International Renal Interest Society
- PTH
parathyroid hormone
- RDHCa
renal diet hypercalcemic group
- USG
urine specific gravity
1. INTRODUCTION
Azotemic chronic kidney disease (CKD) is a common condition in older cats, 1 , 2 and is associated with changes in calcium homeostasis in this species. Plasma total hypercalcemia was observed in 60 of 191 cats (31%) diagnosed with azotemic CKD, 3 and an increase in the trend of plasma total calcium concentrations was observed in 40 of 71 (56%) of cats transitioned to a phosphate‐restricted clinical renal diet over 200 days. 4 Ionized calcium concentration is poorly predicted from extrapolation of total calcium concentrations in cats 3 , 5 but because it is the biologically active form, its direct measurement provides a better indication of disrupted calcium homeostasis than does sole measurement of total calcium concentration. Ionized hypercalcemia is seen concurrently with CKD in cats, but it is not always apparent whether the hypercalcemia has resulted in development of CKD, or the CKD has led to the development of hypercalcemia. In some cases, ionized hypercalcemia has been reported to develop after prescription of a phosphate‐restricted clinical renal diet, 6 although this outcome was only observed in 2/15 cats.
Dietary phosphate restriction may contribute to the development of hypercalcemia because lower dietary phosphate and a higher dietary calcium‐to‐phosphorus (Ca : P) ratio possibly could lead to enhanced intestinal calcium absorption. 7 , 8 This hypothesis is supported by the observation that withdrawal of the phosphate‐restricted clinical renal diet restored normocalcemia in the 2 aforementioned cats. 6 However, hyperphosphatemia is associated with increased risk of progression of azotemia and death in cats with CKD, 9 , 10 , 11 and dietary phosphate restriction is recognized as an effective management strategy in cats with CKD, decreasing plasma phosphate and parathyroid hormone (PTH) concentrations and improving survival. 6 , 12 , 13 , 14 , 15 As a result, the feeding of a phosphate‐restricted clinical renal diet has become standard of care for cats with azotemic CKD. Management strategies for ionized hypercalcemia in cats with azotemic CKD, whether present at CKD diagnosis, or documented after introduction of a clinical renal diet have not been explored previously.
Our aim was to describe the effect of feeding a moderately phosphate‐restricted diet on plasma markers of calcium‐phosphate homeostasis in cats with azotemic CKD and concurrent ionized hypercalcemia.
2. METHODS
The clinical records of 2 companion animal practices in central London (Beaumont Sainsbury Animal Hospital, Camden and People's Dispensary for Sick Animals, Bow) were retrospectively searched for cats with a prior or new diagnosis of azotemic CKD, and that had a new diagnosis of ionized hypercalcemia made between 1 January 2014 and 31 December 2018. A diagnosis of azotemic CKD had been made based on plasma creatinine concentration >2 mg/dL in conjunction with urine specific gravity (USG) <1.035, or plasma creatinine concentration >2 mg/dL on 2 consecutive visits 2 to 4 weeks apart. Ionized hypercalcemia was based on a blood ionized calcium concentration >1.40 mmol/L, to exclude small transient increases above the previously calculated reference interval for cats ≥9 years of age of 1.19 to 1.37 mmol/L. 16 All clinic appointments and blood samples had been performed between 0900 and 1300, and all clients were telephoned the day before and asked to fast their cats overnight before their appointment.
Owners of cats that had been normocalcemic at diagnosis of CKD had been advised to feed a protein and phosphate restricted renal diet (RD, Feline Veterinary Diet Renal, Royal Canin SAS, Aimargues, France; 0.8 g/Mcal phosphorus; Ca : P ratio 1.9) according to a standardized clinical protocol. Cats that subsequently developed hypercalcemia with ionized calcium concentration >1.4 mmol/L on 2 consecutive visits, or >1.5 mmol/L at any time, were included in the study if they had subsequently been transitioned to a moderately protein and phosphate‐restricted diet (MP, Feline Veterinary Care Nutrition Senior Consult Stage 2, Royal Canin SAS; 1.5 g/Mcal phosphorus; Ca : P ratio 1.3) on the visit ionized hypercalcemia was diagnosed. Full diet compositions are presented in Table 1. These cats were assigned to the renal diet hypercalcemic (RDHCa) group. Cats that were hypercalcemic (ionized calcium concentration >1.4 mmol/L) at the time of azotemic CKD diagnosis were included in the study if they had been transitioned immediately onto MP without receiving RD first; confirmation of increased ionized calcium concentration at a second visit was not a requirement. These cats were assigned to the baseline hypercalcemic (BHCa) group.
TABLE 1.
Ingredients and mean dietary composition for the low protein and phosphate “renal” diet and the moderately protein and phosphate restricted diet MP fed to cats before and during this study respectively. Nutrients expressed per Mcal of Metabolizable Energy with Energy calculated by the manufacturer using the National Research Council 2006 equation
| Nutrient | Low protein and phosphate restricted renal diet | Moderately protein and phosphate restricted MP diet |
|---|---|---|
| Energy (Kcal/kg) | 3925 | 3789 |
| Moisture (g/Mcal) | 20 | 21 |
| Protein (g/Mcal) | 59 | 74 |
| Fat (g/Mcal) | 43 | 37 |
| EPA‐DHA (g/Mcal) | 1.1 | 2.1 |
| NFE (g/Mcal) | 112 | 109 |
| Starch (g/Mcal) | 97 | 89 |
| Crude fiber (g/Mcal) | 12 | 14 |
| Total dietary fiber (g/Mcal) | 27 | 34 |
| Minerals (g/Mcal) | 15 | 15 |
| Calcium (g/Mcal) | 1.5 | 2.0 |
| Phosphate (g/Mcal) | 0.8 | 1.5 |
| Calcium : phosphate ratio | 1.9 | 1.3 |
| Sodium (g/Mcal) a | 1.0 | 1.1 |
| Potassium (g/Mcal) | 2.3 | 1.8 |
| Chloride (g/Mcal) | 2.7 | 2.0 |
| Vitamin D3 (IU/Mcal) | 204 | 185 |
| Vitamin A (IU/Mcal) | 5990 | 6070 |
| Ingredients |
Renal diet: maize flour, rice, animal fats, wheat gluten, vegetable fibers, maize gluten, soya protein isolate, maize, hydrolyzed animal proteins, minerals, chicory pulp, dehydrated poultry protein, fish oil, soya oil, mono ‐ and diglycerides of palmitic and stearic acids esterified with citric acid, psyllium husks and seeds, fructo‐oligo‐saccharides, marigold extract (source of lutein). MP: Maize, wheat gluten, maize flour, dehydrated poultry protein, wheat, maize gluten, animal fats, rice, vegetable fibers, hydrolyzed animal proteins, chicory pulp, fish oil, soya oil, minerals, tomato (source of lycopene), psyllium husks and seeds, FOS, GLM 0.3%, hydrolyzed yeast (source of mannan‐oligo‐saccharides), hydrolyzed crustaceans (source of glucosamine), borage oil, marigold extract (source of lutein), hydrolyzed cartilage (source of chondroitin). |
|
Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FOS, fructo‐oligo‐saccharides; GLM, New Zealand green‐lipped mussel extract; NFE, nitrogen‐free extract.
Prior to 2014, renal diet contained 0.8 g/Mcal sodium. All other nutrient concentrations were consistent during the study period.
Owners had been requested to present their cats in for reexamination at 4 to 6 weeks after diet change and subsequently every 2 months. Cats receiving amlodipine besylate for treatment of systemic hypertension were eligible for inclusion. Cats with a diagnosis of diabetes mellitus, treated with corticosteroids, or that were diagnosed with hyperthyroidism at any time were excluded. Cats recruited to the study formed part of a large observational cohort for which owner consent was obtained.
Clinical data for up to 12 months after transition onto MP were retrieved from the electronic clinical records including signalment data, weight, body condition score (BCS, 1‐9), muscle condition score (MCS 0‐3 representing severe [0], moderate [1], mild [2], and no [3] muscle wastage) biochemical variables, venous pH and bicarbonate (HCO3 −) concentration, PCV, systolic blood pressure, and USG. After venipuncture blood ionized calcium concentration, venous pH and HCO3 − concentration had been measured on whole blood using a hand‐held analyzer (iSTAT 1 point‐of‐care analyzer, Abbott Point of Care Inc, Princeton, New Jersey). Plasma biochemistry had been performed on heparinized plasma by a commercial laboratory (Idexx laboratories, Wetherby, UK). Plasma intact fibroblast growth factor 23 (FGF23) and PTH concentrations had been measured using an FGF23 ELISA (FGF‐23 ELISA Kit, Kainos Laboratories, Tokyo, Japan) and PTH immunoradiometric assay (total intact PTH immunoradiometric assay‐coated bead version, 3KG600, Scantibodies, Santee, California), previously validated for use with feline samples. 17 , 18 Parathyroid hormone measurements below the lower limit of detection of the assay (<5.2 pg/mL) were assigned a value of half of this limit (2.6 pg/mL) as previously described. 17
Normality of variables was assessed by visual inspection of histograms and, because not all variables had a Gaussian distribution, results are reported as median [25th, 75th percentiles]. Variables with highly right‐skewed distributions subsequently were logarithmically transformed (ln). For each group, a linear mixed effects model, using time (measured in months as equivalent to 30.4 days) as a fixed effect and cat as random effect, was used to assess if the rate of change in variables over time was significantly different from zero within the group. Normality of the residuals was checked by visually inspecting the histograms. Differences between groups were not assessed because they were not deemed directly comparable. The clinic visit at which MP was started was designated as the baseline visit (time 0). Quadratic terms for time (time2) were included in initial models to account for potential nonlinear changes of variables over time, and subsequently removed if not significant. Because of a very small number of data points occurring after the 6 month time point in the RDHCa group, only data up until 6 months were included in the model for this group. Analyses were performed using R version 3.6.0 (https://www.R-project.org/). The <lme4> and <lmerTest> packages were used for the linear mixed effects model analyses; all graphs were generated using <lattice> and <latticeExtra> packages.
3. RESULTS
Seventy cats with a diagnosis of azotemic CKD were identified that had an ionized calcium concentration of >1.4 mmol/L between 1 January 2014 and 31 December 2018. Twenty‐two cats were excluded because of a concurrent diagnosis of hyperthyroidism and 3 cats were excluded because of treatment with corticosteroids. Therefore, 45 cats were reviewed for their suitability for inclusion in the study based on their dietary history. Of these 45 cases, 11 cats were diagnosed with ionized hypercalcemia while receiving a renal diet, but were not transitioned onto MP because of only having transient ionized hypercalcemia, with ionized calcium concentration >1.4 mmol/L but <1.5 mmol/L on 1 visit only. The median ionized calcium concentration for these 11 cats was 1.42 mmol/L (range, 1.4‐1.44 mmol/L). Another 4 cats met the criteria for transition onto MP but had continued on RD. Of these, only 1 cat stayed on RD exclusively and continued to have ionized hypercalcemia at last known follow‐up (392 days later). The remaining 3 cats had a decrease or normalization of ionized calcium concentrations when their diet subsequently was changed to half RD and half another food, or RD was stopped altogether.
Five cats had no ionized calcium measurement available from the date of CKD diagnosis (no baseline measurement) and therefore could not be categorized into the BHCa or RDHCa group. Four cats were lost to follow‐up or died after transition onto MP with no follow‐up data available. Therefore, 21 cats were available for analysis, of which 11 were designated to the BHCa group and 10 were designated to the RDHCa group. Baseline information for both groups is presented in Table 2.
TABLE 2.
Baseline clinicopathological variables of cats with azotemic CKD and ionized hypercalcemia at diagnosis of CKD (BHCa group) or that developed ionized hypercalcemia after introduction of a renal diet (RDHCa group)
| Variables | BHCa group (n = 11) Median [25th, 75th percentile] | n | RDHCa group (n = 10) Median [25th, 75th percentile] | n |
|---|---|---|---|---|
| Ionized calcium (mmol/L) | 1.47 [1.42, 1.55] | 11 | 1.53 [1.51, 1.67] | 10 |
| Total calcium (mg/dL) | 10.8 [10.4, 11.4] | 11 | 11.7 [11.2, 12.5] | 10 |
| Phosphate (mg/dL) | 3.9 [3.7, 4.3] | 11 | 4.3 [4.2, 4.7] | 10 |
| Ca × P (mg2/dL2) | 44.7 [38.7, 50.8] | 11 | 52.8 [49.0, 56.1] | 10 |
| PTH (pg/mL) | 10.2 [5.4, 47.0] | 8 | 2.6 [2.6, 8.4] | 7 |
| FGF23 (pg/mL) | 2295 [1227, 2527] | 7 | 1489 [634, 6408] | 7 |
| Creatinine (mg/dL) | 2.4 [2.2, 2.7] | 11 | 2.4 [2.0, 2.8] | 10 |
| Potassium (mEq/L) | 3.8 [3.5, 4.2] | 11 | 3.5 [3.2, 3.8] | 10 |
| Sodium (mEq/L) | 152 [150, 153] | 11 | 152 [150, 152] | 10 |
| HCO3 − (mEq/L) | 19.1 [17.4, 22.4] | 11 | 21.2 [17.9, 23.0] | 10 |
| Venous pH | 7.34 [7.30, 7.38] | 11 | 7.36 [7.31, 7.40] | 10 |
| PCV (%) | 32 [30, 36] | 11 | 33 [29, 34] | 10 |
| Total protein (g/dL) | 7.1 [7.0, 7.9] | 11 | 7.2 [7.1, 8.0] | 10 |
| Albumin (g/dL) | 3.1 [2.8, 3.2] | 11 | 2.9 [2.6, 3.2] | 10 |
| USG | 1.017 [1.014, 1.020] | 4 | 1.016 [1.014‐1.021] | 3 |
| SBP (mm Hg) | 138 [126, 141] | 9 | 136 5[117, 152] | 10 |
| Sex (n [%] male) | 7 (64) | 11 | 2 (20) | 10 |
| Age (years) | 16.9 [15.1, 17.3] | 11 | 14.6 [12.8, 18.1] | 10 |
| BCS (1‐9) | 3 [3, 3] | 10 | 3.5 [2, 6] | 10 |
| Body weight (kg) | 3.55 [2.96, 4.08] | 9 | 3.25 [2.63, 4.25] | 10 |
The BHCa group consisted of 11 cats, of which 10 were domestic shorthair and 1 was a domestic longhair, 7 were male (1 intact) and 4 were female (all neutered). Nine cats were in International Renal Interest Society (IRIS) stage 2 CKD, 1 in IRIS stage 3, and 1 in stage 4 CKD (www.iris-kidney.com). One cat was receiving amlodipine for hypertension and 2 cats were receiving meloxicam for osteoarthritis. At baseline, 2 cats (including the stage 4 cat) had plasma total calcium concentrations above reference interval (>2.95 mmol/L). One of the IRIS stage 2 cats had a plasma phosphate concentration exceeding the IRIS stage‐specific phosphate target range (www.iris-kidney.com) at baseline. The median number of follow‐up visits, including the baseline visit, was 4 [3, 5] visits, with a median follow‐up time of 8.8 [5.5, 10.6] months. During follow‐up, after starting the MP diet, plasma creatinine concentration, HCO3 − concentration, and body weight significantly decreased over time (Figure 1; Table 3A). Plasma phosphate and blood potassium concentrations showed significant nonlinear changes over time (Figures 1 and 2; Table 3A). No significant changes were observed for blood ionized calcium, plasma total calcium, PTH, or FGF‐23 concentrations (Figure 2; Table 3A). No cat had an increase in plasma creatinine concentration of >25% during follow‐up. Plasma phosphate concentration decreased to below the IRIS stage‐specific target in the 1 cat where it had been increased at baseline, but increased to above the IRIS stage‐specific target range in 1 CKD stage 2 and the stage 4 cat. Ionized calcium concentration decreased to <1.4 mmol/L in 4/11 cats after a median of 3.4 [1.0, 6.2] months, but remained >1.4 mmol/L in the other 7 cats. Total calcium × phosphate product (Ca × P) exceeded 70 mg2/dL2 during follow‐up in the 2 cats with plasma phosphate concentrations above the targets for their IRIS stages. No significant changes in BCS or MCS were observed during the follow‐up period (P ≥ .25, data not shown). Two cats had constipation and 2 cats had decreased activity levels during follow‐up while having ionized hypercalcemia. Only 1 of these cats subsequently became normocalcemic and the activity level of this cat was reported to improve.
FIGURE 1.
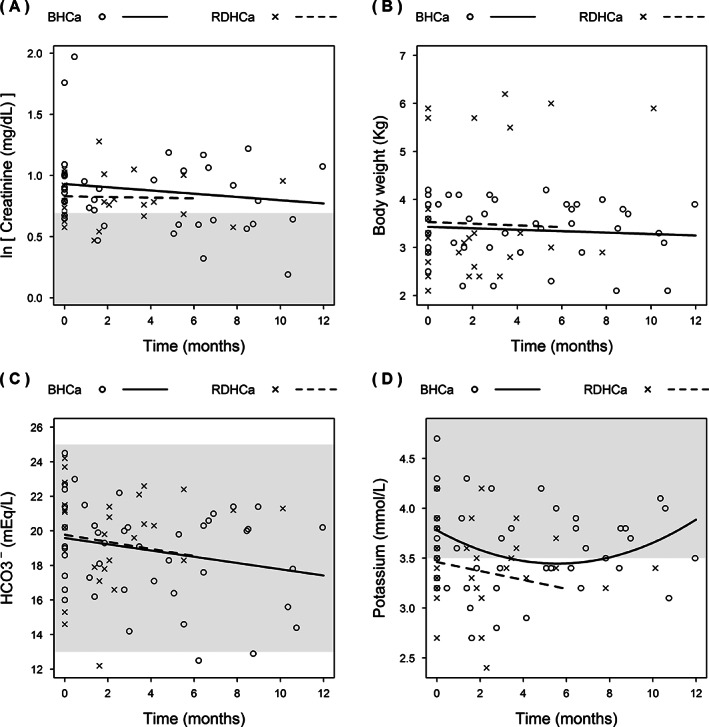
Change in plasma creatinine concentrations (A), body weight (B), blood bicarbonate (HCO3 −) concentration (C), and potassium concentrations (D) over time in cats with ionized hypercalcemia at diagnosis of azotemic CKD (BHCa, n = 11) and cats that developed ionized hypercalcemia during the course of CKD (RDHCa, n = 10) that were transferred to a moderately protein and phosphate restricted diet. Reference intervals are shown with a shaded area where applicable. Because of a very small number of data points occurring after the 6 month time point in the RDHCa group, only data up until 6 months were included in the model for this group
TABLE 3.
Linear mixed model analysis of changes in variables after introduction of Royal Canin Senior Consult Stage 2 diet showing (A) cats with ionized hypercalcemia at diagnosis of azotemic CKD (BHCa group, n = 11) and (B) cats that developed ionized hypercalcemia after being diagnosed with azotemic CKD (RDHCa group, n = 10). Quadratic terms for time (time2) were included in initial models to account for potential nonlinear changes of variables over time, and subsequently removed if not significant
| (A) | β (month) | SE | 95% CI | P‐value | β (month2) | SE | 95% CI | P‐value |
|---|---|---|---|---|---|---|---|---|
| Ionized calcium (mmol/L) | 0.006 | 0.005 | −0.003 to 0.015 | .18 | ||||
| Total calcium (mg/dL) | 0.014 | 0.044 | −0.080 to 0.099 | .75 | ||||
| ln PTH (pg/mL) | 0.002 | 0.010 | −0.018 to 0.022 | .81 | ||||
| ln Phosphate (mg/dL) | −0.064 | 0.026 | −0.116 to (−0.014) | .02 | 0.006 | 0.003 | 0.001‐0.011 | .04 |
| Ca × P (mg2/dL2) | −0.186 | 0.382 | −0.988 to 0.560 | .63 | ||||
| ln Creatinine (mg/dL) | −0.013 | 0.006 | −0.026 to (−0.001) | .04 | ||||
| ln FGF23 (pg/mL) | −0.035 | 0.022 | −0.081 to 0.009 | .13 | ||||
| Potassium (mmol/L) | −0.120 | 0.035 | −0.187 to (−0.050) | <.01 | 0.011 | 0.003 | 0.004‐0.017 | <.01 |
| Sodium (mmol/L) | −0.039 | 0.066 | −0.168 to 0.094 | .56 | ||||
| Albumin (g/dL) | 0.007 | 0.004 | −0.001 to 0.014 | .11 | ||||
| Total protein (g/dL) | 0.011 | 0.012 | −0.013 to 0.034 | .37 | ||||
| HCO3‐ (mEq/L) | −0.181 | 0.072 | −0.325 to (−0.039) | .02 | ||||
| pH | −0.002 | 0.001 | −0.004 to 0.000 | .1 | ||||
| Body weight (kg) | −0.015 | 0.005 | −0.025 to (−0.005) | <.01 |
| (B) | β (month) | SE | 95% CI | P‐value | β (month2) | SE | 95% CI | P‐value |
|---|---|---|---|---|---|---|---|---|
| Ionized calcium (mmol/L) | −0.056 | 0.013 | −0.081 to (−0.030) | <.01 | ||||
| Total calcium (mg/dL) | −1.133 | 0.204 | −1.524 to (−0.719) | <.01 | 0.158 | 0.042 | 0.073‐0.239 | <.01 |
| ln PTH (pg/mL) | 0.322 | 0.078 | 0.147 to 0.476 | <.01 | ||||
| ln phosphate (mg/dL) | −0.026 | 0.023 | −0.072 to 0.022 | .29 | ||||
| Ca × P (mg2/dL2) | −2.83 | 1.18 | −5.21 to (−0.398) 0.019 | .03 | ||||
| ln Creatinine (mg/dL) | −0.003 | 0.016 | −0.035 to 0.029 | .87 | ||||
| ln FGF23 (pg/mL) | −0.167 | 0.118 | −0.407 to 0.072 | .19 | ||||
| Potassium (mmol/L) | −0.045 | 0.023 | −0.089 to 0.003 | .07 | ||||
| Sodium (mmol/L) | 0.040 | 0.186 | −0.345 to 0.042 | .83 | ||||
| Albumin (g/dL) | 0.008 | 0.016 | −0.025 to 0.042 | .65 | ||||
| Total protein (g/dL) | 0.001 | 0.036 | −0.076 to 0.071 | .98 | ||||
| HCO3 − (mEq/L) | −0.205 | 0.189 | −0.576 to 0.197 | .31 | ||||
| pH | −0.005 | 0.005 | −0.013 to 0.004 | .31 | ||||
| Body weight (kg) | −0.018 | 0.025 | −0.068 to 0.034 | .5 |
FIGURE 2.
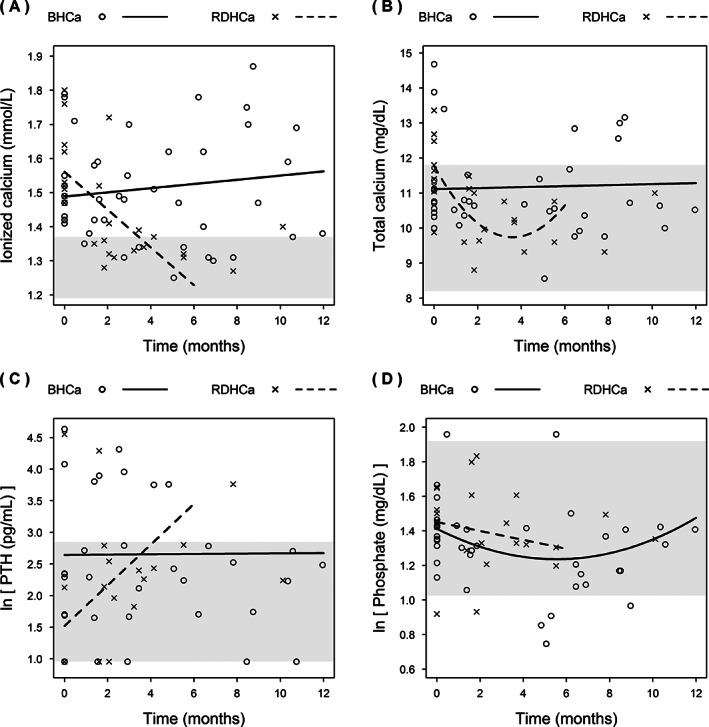
Change in blood ionized calcium concentrations (A), plasma total calcium (B), PTH (C), and phosphate concentrations (D) over time in cats with ionized hypercalcemia at diagnosis of azotemic CKD (BHCa, n = 11) and cats that developed ionized hypercalcemia during the course of CKD (RDHCa, n = 10) that were transferred to a moderately protein and phosphate restricted diet. Reference intervals are shown with a shaded area where applicable. Because of a very small number of data points occurring after the 6 month time point in the RDHCa group, only data up until 6 months were included in the model for this group
The RDHCa group consisted of 9 domestic shorthair cats and 1 Birman, 2 males and 8 females (all neutered). Nine cats were in IRIS stage 2 CKD and 1 was in IRIS stage 3. Three cats were receiving amlodipine, with 1 also receiving benazepril for hypertension, and 1 cat was receiving meloxicam for osteoarthritis. At the time of CKD diagnosis, median blood ionized calcium concentration had been 1.32 [1.25, 1.33] mmol/L. The median time since CKD diagnosis to documentation of hypercalcemia was 4.7 [3.9, 10.0] months, and all cats had started eating RD within 1 month of their CKD diagnosis. At baseline, 8/10 cats were reportedly fed 100% RD and the remaining 2 cats were fed 50% to 70% RD. At baseline, plasma total calcium concentration exceeded the reference interval in 4 cats. Plasma phosphate concentration exceeded the upper limit of the IRIS stage‐specific target ranges in 3 cats at baseline. The median number of follow‐up visits, including the baseline visit was 3 [2, 3] visits, with a median follow‐up of 3.7 [2.3, 6.1] months. Diet transition occurred after 1 incident of ionized calcium concentration >1.5 mmol/L in 7 cats and after 2 incidents of ionized calcium concentration >1.4 mmol/L in 3 cats. During follow‐up, after transition onto MP diet, blood ionized calcium concentration, plasma total calcium concentrations, and calcium‐phosphate product decreased significantly over time and plasma PTH increased significantly over time with a nonlinear change (Figure 2; Table 3B), although for 8/10 cats PTH concentrations never exceeded the upper limit of the reference interval. One cat had an increase in plasma creatinine concentration of >25% during follow‐up. Plasma FGF‐23 concentration did not change and plasma phosphate concentration did not significantly increase in the group as a whole. An increase to above the upper limit of the IRIS stage‐specific target range was observed in 1 cat and plasma phosphate concentration remained increased in another cat that was hyperphosphatemic at baseline but decreased in the other 2 previously hyperphosphatemic cats. Ionized calcium concentration decreased to below 1.4 mmol/L in 8/10 cats after a median of 2.2 [1.8, 3.7] months. The remaining 2 cats only had short follow‐up times of 1.6 and 2.1 months, with decreases in ionized calcium concentration from 1.76 to 1.52 mmol/L and 1.47 to 1.41 mmol/L, respectively. Ca × P never exceeded 70 mg2/dL2 during follow‐up for any cat in this group. No significant changes in BCS or MCS were observed during the follow‐up period (P ≥ .98, data not shown). Two cats had decreased activity levels while they had ionized hypercalcemia. Only 1 of these cats subsequently became normocalcemic, and the activity level of this cat was noted to improve.
4. DISCUSSION
We found that attenuation of dietary phosphate restriction and a lower dietary Ca : P ratio led to a significant reduction in blood ionized and total calcium concentrations in cats that developed ionized hypercalcemia when fed a clinical RD. However, no significant change in calcium concentrations was observed overall in cats that were already hypercalcemic at diagnosis of azotemic CKD when started on the same moderately phosphate‐restricted diet.
For cats that became hypercalcemic while eating RD, a significant decrease in ionized and total calcium concentrations was observed after transfer onto MP diet. Plasma phosphate and FGF23 concentrations did not change significantly. This observation might indicate firstly that feeding RD (at least in part) underlies the development of hypercalcemia in these cats, and secondly that transfer onto a less phosphate‐restricted diet normalizes calcium status without causing overt hyperphosphatemia or continuing increases in FGF23 concentrations over time in these cats; although occasional individual cats did show increases of plasma phosphate to above the upper limit of the IRIS stage‐specific target range. The significant increase in plasma PTH seems likely to be secondary to normalization of blood ionized calcium concentration. Plasma PTH concentration was suppressed to be below the lower limit of detection in 5 of the 6 cats where this information was available at the baseline visit and never exceeded the reference interval for 8/10 cats for the duration of the study. These changes are compatible with our understanding of the physiological relationship between PTH and ionized calcium concentration.
The reason for this apparent diet effect is unclear and it may be multifactorial. Notable differences between the prior RD and MP include their phosphate concentration, their calcium concentration, Ca : P ratio, and fiber content. Previous studies have shown that both the form of phosphate (organic versus inorganic) and the Ca : P ratio in diets for cats have significant influences on serum and urinary phosphate concentrations, and on renal function. 19 , 20 However, these studies have focused on relative dietary phosphate excess, because of either higher concentrations of bioavailable inorganic phosphate, or because of a low Ca : P ratio resulting in more unbound phosphate available for absorption. The RD has a lower phosphate concentration and a higher Ca : P ratio than the MP. Our study suggests that it is possible that in some cats, hypercalcemia develops secondary to excessive intestinal calcium absorption associated with an increased dietary Ca : P ratio. This may then be ameliorated by switching to a diet with a higher dietary phosphate concentration and lower Ca : P ratio, resulting in increased calcium binding in the intestinal lumen and decreased calcium absorption. A high dietary Ca : P ratio may therefore be an important risk factor for a subset of cats with CKD that are prone to ionized hypercalcemia. However, alternative causes of ionized hypercalcemia because of bone resorption or renal reabsorption cannot be completely excluded.
Another possible dietary factor associated with ionized hypercalcemia could be fiber content. Increased fiber to slow transit time and bind luminal calcium has been proposed as a strategy in the management of idiopathic hypercalcemia (IHC), 21 although not all affected cats respond to this intervention. 22 The RD has a lower crude and total fiber content than the MP; therefore, fiber content may have contributed to the changes seen in our study. Both diets had similar reported concentrations of vitamin D, but serum concentrations of 25‐hydroxyvitamin D and 1,25‐dihydroxyvitamin D were not evaluated directly and therefore it is not possible to rule out induced differences in vitamin D metabolism between the diets. Future studies should consider direct measurement of vitamin D metabolites to elucidate all of the CKD‐MBD variables involved in dietary effects on ionized hypercalcemia. Vitamin A has been shown to influence bone resorption in rodent models 23 and to decrease serum calcium concentrations in humans. 24 However, the concentrations of this vitamin were similar between RD and MP, and therefore it seems unlikely that vitamin A concentrations were driving the changes seen in ionized calcium concentrations in the RDHCa group in our study. The MP diet did not significantly change calcium concentrations in the cats that were already hypercalcemic at diagnosis of CKD. Additionally, no change in PTH concentrations was observed in this group, although plasma phosphate concentrations did significantly decrease. No attempt was made to clarify the mixture of diets these cats had been receiving before transition onto MP except confirming that none were receiving a clinical RD. Therefore, this decrease in plasma phosphate concentration likely reflects a decrease in dietary phosphate intake on the MP compared to previous diets. Two cats had plasma phosphate concentrations above the IRIS stage‐specific targets and these cats had Ca × P > 70 mg2/dL2, which is considered a risk factor for soft tissue mineralization. 25 However, the majority of this group maintained Ca × P < 70 mg2/dL2 and the moderate dietary phosphate restriction was not associated with a worsening of their hypercalcemia.
The disparity in the response to feeding the MP diet between the BHCa and RDHCa groups in our study suggests that there may be physiological differences between cats that have ionized hypercalcemia before the onset of azotemic CKD and those that appear sensitive to developing hypercalcemia after introduction of a phosphate‐restricted renal diet. This possibility highlights the importance of measuring ionized calcium concentration at diagnosis and at subsequent visits in cats with CKD. No attempt was made retrospectively to diagnose the underlying causes of the hypercalcemia seen in the BHCa group cats in our study. The causes are likely to have varied, given the range of PTH concentrations observed in this group and the variation in response of ionized calcium concentrations among individual cats over time. Concepts associated with the diets and ionized calcium concentrations seen in our study may be applicable to cats with IHC, which appears to be on the increase as a common cause of hypercalcemia in cats. 22 , 26 Additional studies would be required to clarify the response of these cats to different dietary calcium and phosphate concentrations, but it may be prudent to monitor ionized calcium concentrations carefully in IHC cats started on phosphate‐restricted or high Ca : P ratio diets in case of exacerbation of their hypercalcemia.
Neither group of cats experienced an increase in plasma creatinine concentration during the study, and 20/21 cats had <25% change in their plasma creatinine concentration, suggesting that they did not have clinically relevant progression of their CKD during this period. 10 The cats that were hypercalcemic at baseline were noted to have a significant decrease in bicarbonate and potassium concentrations and a decrease in body weight over time. The significant change in body weight was not accompanied by significant decreases in either BCS or MCS, but this may be because these more subjective measures may be underpowered to detect changes in this small group of cats. Weight loss has been noted to occur both before and after a diagnosis of azotemic CKD 27 and is of concern in older cats. Weight loss was not seen in the RDHCa group, but this may reflect the shorter follow‐up period on MP for this group or it may be that the etiologies of the ionized hypercalcemia seen in the BHCa group could have directly contributed to the weight loss seen (eg, because of decreased appetite). Our retrospective study did not include any measurement of food ingested by the cats, but this should be considered in future studies alongside careful body weight monitoring. The significant change in bicarbonate concentrations might be because of the transition onto MP, which is a moderately acidifying diet, but dietary information for the prior foods eaten by this group was not available to confirm this possibility. Cats with late stage CKD are at risk of metabolic acidosis, but 9 of 11 cats in the BHCa group were in IRIS stage 2 CKD during the study period, making this a less likely cause of these changes. Interestingly, plasma potassium concentrations changed significantly in this group over time, but these changes were nonlinear, showing an initial decrease and subsequent increase. Clinically, these changes were small and largely not considered of concern for any individual cat and the later increase in plasma potassium concentration may be a result of the death of 1 particularly hypokalemic cat just after the 4 month time point. Our study was limited by the small number of cats involved and these changes were not observed in the RDHCa group, but statistical comparison of the groups was considered invalid and not performed given that these were 2 individual cohorts of cats that were not deemed directly comparable.
Our study had some limitations. As a retrospective study, albeit in a population of cats managed in a consistent manner, there were factors such as the previous diets of the BHCa cats and the time between visits that were not controlled for. Additionally, the BHCa cats only had to have ionized hypercalcemia documented on 1 occasion to be included in that group and the RDHCa cats only had to have ionized hypercalcemia documented once if >1.5 mmol/L to be transitioned onto RD. As such, some cats may have been included in both groups that would only have had transient hypercalcemia. Given the documentation of transient mild hypercalcemia observed in 11 cats on RD (that were not included in the study population), future studies should aim to ensure that cats are persistently hypercalcemic before making interventions.
Our study did not include a control group. One cat with ionized hypercalcemia was documented to have remained on RD exclusively in the long term and this cat remained hypercalcemic, but based on this 1 cat alone, it is not possible to conclude this would be the case generally. This prevents us from conclusively determining that the changes observed in variables over time were truly the result of feeding the MP diet, and precludes assessment as to whether this diet change affects survival time. However, there would be concerns about continuing to feed RD in the longer term once cats have been shown to have developed persistent ionized hypercalcemia, although the same diet has been shown to improve survival time for the majority of cats with azotemic CKD. 11 Previous survival studies did not identify calcium as a risk factor for death in cats with CKD. 9 , 11 , 28 Nonetheless, hypercalcemia could further decrease glomerular filtration rate, 29 , 30 has been associated with calcium oxalate nephrolithiasis in cats, 21 and calcium is associated with increased mortality 31 , 32 and vascular calcification 33 in human CKD patients. Clinical signs of hypercalcemia were minimal in our study, and it was difficult to conclusively prove they were attributable to the increased plasma calcium concentrations. At the present time, it remains to be determined if hypercalcemia causes a clinical problem in cats with CKD.
In conclusion, cats that appear to have ionized hypercalcemia induced by feeding a phosphate‐restricted and high Ca : P ratio clinical RD experience resolution of their hypercalcemia after transition onto MP, a less phosphate‐restricted diet with a lower Ca : P ratio, and this outcome was not associated with significant changes in kidney function during the study period. Moderate dietary phosphate restriction with MP did not result in significant changes in plasma calcium concentrations in cats that had ionized hypercalcemia at the time of azotemic CKD diagnosis, but plasma phosphate concentrations did decrease in this group. Future studies should consider the heterogeneity that appears to be present in cats with ionized hypercalcemia and azotemic CKD, attempt to investigate these differences further, and assess whether long‐term hypercalcemia has a negative health effect in cats with CKD.
CONFLICT OF INTEREST DECLARATION
Rebecca Geddes received a PhD studentship funded by Royal Canin SAS; receives funding from Petplan Charitable Trust and an RVC Internal Grant; has a consultancy agreement with Boehringer Ingelheim; has received speaking honoraria from Boehringer Ingelheim. Henk van den Broek received a PhD studentship funded by Royal Canin SAS. Rosanne Jepson receives funding from Petplan Charitable Trust, Feline Foundation for Renal Research, RVC Internal Grant and PetSavers; has consultancy agreements with Boehringer Ingelheim and CEVA; has received speaking honoraria from Boehringer Ingelheim, Hills Pet Nutrition and CEVA. Jonathan Elliott has received funding from consultancies with Elanco Ltd, CEVA Animal Health Ltd, Boehringer Ingelheim Ltd, Orion Incorp, Idexx Ltd, Nextvet Ltd, Waltham Centre for Pet Nutrition, Kindred Biosciences Inc, Invetx Inc; has received grant funding from Elanco Ltd, Waltham Centre for Pet Nutrition, Royal Canin SAS, Idexx Ltd, Zoetis Ltd and CEVA Animal Health; is a member of the International Renal Interest Society which receives a grant from Elanco Ltd. Vincent Biourge is an employee of Royal Canin SAS.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Ethics and Welfare Committee of the Royal Veterinary College (URN 2013 1258E) and the Royal Canin Ethics Review Committee.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed.
ACKNOWLEDGMENT
Funding provided by Royal Canin SAS, Aimargues, France. The Renal Research Clinic at the Royal Veterinary College acknowledges financial support from Royal Canin for its research on chronic kidney disease‐mineral and bone disorder in cats. The authors acknowledge Ms Nicola Lötter for her help with the Renal Research Clinic.
Geddes RF, van den Broek DHN, Chang Y‐M, Biourge V, Elliott J, Jepson RE. The effect of attenuating dietary phosphate restriction on blood ionized calcium concentrations in cats with chronic kidney disease and ionized hypercalcemia. J Vet Intern Med. 2021;35:997–1007. 10.1111/jvim.16050
Funding information Royal Canin SAS, Aimargues, France
REFERENCES
- 1. Lulich JP. Feline renal failure: questions, answers, questions. Compend Contin Educ Pract Vet. 1992;14(2):127‐152. [Google Scholar]
- 2. Marino CL, Lascelles BD, Vaden SL, Gruen ME, Marks SL. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg. 2014;16(6):465‐472. 10.1177/1098612X13511446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van den Broek DH, Chang YM, Elliott J, Jepson RE. Chronic kidney disease in cats and the risk of total hypercalcemia. J Vet Intern Med. 2017;31(2):465‐475. 10.1111/jvim.14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang PK, Geddes RF, Chang YM, Jepson RE, Bijsmans E, Elliott J. Risk factors associated with disturbances of calcium homeostasis after initiation of a phosphate‐restricted diet in cats with chronic kidney disease. J Vet Intern Med. 2020. 10.1111/jvim.15996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schenck PA. Prediction of serum ionized calcium concentration by serum total calcium measurement in cats. Can J Vet Res. 2010;74(3):209‐213. [PMC free article] [PubMed] [Google Scholar]
- 6. Barber PJ. Effect of dietary phosphate restriction on renal secondary hyperparathyroidism in the cat. J Small Anim Pract. 1999;40(2):62‐70. [DOI] [PubMed] [Google Scholar]
- 7. Lee KJ, Kim KS, Kim HN, Seo JA, Song SW. Association between dietary calcium and phosphorus intakes, dietary calcium/phosphorus ratio and bone mass in the Korean population. Nutr J. 2014;13(1):114. 10.1186/1475-2891-13-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Block GA, Wheeler DC, Persky MS, et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012;23(8):1407‐1415. 10.1681/ASN.2012030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boyd LM, Langston C, Thompson K, Zivin K, Imanishi M. Survival in cats with naturally occurring chronic kidney disease (2000‐2002). J Vet Intern Med. 2008;22(5):1111‐1117. [DOI] [PubMed] [Google Scholar]
- 10. Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med. 2012;26(2):275‐281. 10.1111/j.1939-1676.2011.00874.x. [DOI] [PubMed] [Google Scholar]
- 11. King JN, Tasker S, Gunn‐Moore DA, Strehlau G. Prognostic factors in cats with chronic kidney disease. J Vet Intern Med. 2007;21(5):906‐916. [PubMed] [Google Scholar]
- 12. Elliott J, Rawlings JM, Markwell PJ, Barber PJ. Survival of cats with naturally occurring chronic renal failure: effect of dietary management. J Small Anim Pract. 2000;41(6):235‐242. [DOI] [PubMed] [Google Scholar]
- 13. Geddes RF, Elliott J, Syme HM. The effect of feeding a renal diet on plasma fibroblast growth factor 23 concentrations in cats with stable azotemic chronic kidney disease. J Vet Intern Med. 2013;27(6):1354‐1361. 10.1111/jvim.12187. [DOI] [PubMed] [Google Scholar]
- 14. Ross SJ, Osborne CA, Kirk CA, Lowry SR, Koehler LA, Polzin DJ. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J Am Vet Med Assoc. 2006;229(6):949‐957. [DOI] [PubMed] [Google Scholar]
- 15. Plantinga EA, Everts H, Kastelein AM, Beynen AC. Retrospective study of the survival of cats with acquired chronic renal insufficiency offered different commercial diets. Vet Rec. 2005;157(7):185‐187. [DOI] [PubMed] [Google Scholar]
- 16. Geddes RF, Jepson RE, Forcada Y, Elliott J, Syme HM. Associations between single nucleotide polymorphisms in the calcium sensing receptor and chronic kidney disease‐mineral and bone disorder in cats. Vet J. 2018;235:34‐41. 10.1016/j.tvjl.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 17. Geddes RF, Finch NC, Elliott J, Syme HM. Fibroblast growth factor 23 in feline chronic kidney disease. J Vet Intern Med. 2013;27(2):234‐241. 10.1111/jvim.12044. [DOI] [PubMed] [Google Scholar]
- 18. Williams TL, Elliott J, Syme HM. Calcium and phosphate homeostasis in hyperthyroid cats: associations with development of azotaemia and survival time. J Small Anim Pract. 2012;53(10):561‐571. 10.1111/j.1748-5827.2012.01253.x. [DOI] [PubMed] [Google Scholar]
- 19. Alexander J, Stockman J, Atwal J, et al. Effects of the long‐term feeding of diets enriched with inorganic phosphorus on the adult feline kidney and phosphorus metabolism. Br J Nutr. 2018;121:1‐21. 10.1017/S0007114518002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coltherd JC, Staunton R, Colyer A, et al. Not all forms of dietary phosphorus are equal: an evaluation of postprandial phosphorus concentrations in the plasma of the cat. Br J Nutr. 2019;121(3):270‐284. 10.1017/S0007114518003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McClain HM, Barsanti JA, Bartges JW. Hypercalcemia and calcium oxalate urolithiasis in cats: a report of five cases. J Am Anim Hosp Assoc. 1999;35(4):297‐301. [DOI] [PubMed] [Google Scholar]
- 22. Midkiff AM. Idiopathic hypercalcemia in cats. J Vet Intern Med. 2000;14(6):619‐626. [DOI] [PubMed] [Google Scholar]
- 23. Navia JM, Harris SS. Vitamin A influence on calcium metabolism and calcification. Ann N Y Acad Sci. 1980;355:45‐57. 10.1111/j.1749-6632.1980.tb21326.x. [DOI] [PubMed] [Google Scholar]
- 24. Johansson S, Melhus H. Vitamin A antagonizes calcium response to vitamin D in man. J Bone Miner Res. 2001;16(10):1899‐1905. 10.1359/jbmr.2001.16.10.1899. [DOI] [PubMed] [Google Scholar]
- 25. Bertazzolo W, Toscani L, Calcaterra S, Crippa L, Caniatti M, Bonfanti U. Clinicopathological findings in five cats with paw calcification. J Feline Med Surg. 2003;5(1):11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coady M, Fletcher DJ, Goggs R. Severity of ionized hypercalcemia and hypocalcemia is associated with etiology in dogs and cats. Front Vet Sci. 2019;6:276. 10.3389/fvets.2019.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freeman LM, Lachaud MP, Matthews S, Rhodes L, Zollers B. Evaluation of weight loss over time in cats with chronic kidney disease. J Vet Intern Med. 2016;30(5):1661‐1666. 10.1111/jvim.14561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Geddes RF, Elliott J, Syme HM. Relationship between plasma fibroblast growth factor‐23 concentration and survival time in cats with chronic kidney disease. J Vet Intern Med. 2015;29(6):1494‐1501. 10.1111/jvim.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levi M, Ellis MA, Berl T. Control of renal hemodynamics and glomerular filtration rate in chronic hypercalcemia. Role of prostaglandins, renin‐angiotensin system, and calcium. J Clin Invest. 1983;71(6):1624‐1632. 10.1172/jci110918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levi M, Peterson L, Berl T. Mechanism of concentrating defect in hypercalcemia. Role of polydipsia and prostaglandins. Kidney Int. 1983;23(3):489‐497. 10.1038/ki.1983.46. [DOI] [PubMed] [Google Scholar]
- 31. Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2008;52(3):519‐530. 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 32. Young EW, Albert JM, Satayathum S, et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005;67(3):1179‐1187. 10.1111/j.1523-1755.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 33. Lomashvili K, Garg P, O'Neill WC. Chemical and hormonal determinants of vascular calcification in vitro. Kidney Int. 2006;69(8):1464‐1470. 10.1038/sj.ki.5000297. [DOI] [PubMed] [Google Scholar]


