Abstract
Background
Leukoreduction is a routine procedure in human transfusion medicine but is uncommon in veterinary.
Objectives
To evaluate the effect of leukoreduction on the quality of canine whole blood (WB) and blood products during storage.
Animals
Ten canine blood donors.
Methods
This is a case series study. An amount of 450 mL of blood was collected from each dog. Five WB and 5 packed red blood cells (pRBC) bags were divided into 2 units each: leukoreduced (LR) and non‐leukoreduced (nLR). RBC count, erythrocytes' mean osmotic fragility (MOF), 2,3‐diphosphoglycerate (2,3‐DPG), adenosine triphosphate (ATP), percentage of hemolysis, potassium (K), lactate, glucose, and cytokines were measured weekly from day of donation (T0) to day 35 (T35); pH, coagulation times, and clotting factors were evaluated at T0 and T35 from WB and in fresh frozen plasma after 1 year of storage.
Results
Leukoreduction showed positive effects on lactate (T35: LR WB 14.42 mmol/L SD 2.71, nLR WB 22.42 mmol/L SD 1.86, LR pRBC 20.88 mmol/L SD 2.65, nLR pRBC 36.81 mmol/L SD 2.34; P < .0001), pH (T35: LR WB 6.88 SD 0.16, nLR WB 6.69 SD 0.20, P = .02; LR pRBC 6.57 SD 0.23, nLR pRBC 6.22 SD 0.11; P < .001), and K (LR pRBC 4.08 mmol/L SD 0.88, nLR pRBC 5.48 mmol/L SD 0.90; P < .001). Increasing values of IL8 were observed in nLR units during storage (T0: 4167 ± 11 888 pg/mL; T35: 6367 ± 11 612 pg/mL).
Conclusion and Clinical Importance
LR blood units are recommended to critically ill dogs with marked inflammatory conditions.
Keywords: blood, dog, leukoreduction, storage lesions, transfusion
Abbreviations
- 2,3‐DPG
2,3‐diphosphoglycerate
- aPTT
activated partial thromboplastin time
- ATP
adenosine triphosphate
- CPDA‐1
citrate‐phosphate‐dextrose‐adenine
- FFP
fresh frozen plasma
- H index
hemolysis index
- Hb
hemoglobin
- HCT
hematocrit
- IL‐6
interleukin‐6
- K
potassium
- LR pRBC
leukoreduced packed red blood cells
- LR WB
leukoreduced whole blood
- LR
leukoreduction
- MCV
mean corpuscular volume
- MOF
mean osmotic fragility
- nLR pRBC
non‐leukoreduced packed red blood cells
- nLR WB
non‐leukoreduced whole blood
- pRBC
packed red blood cells
- PT
prothrombin time
- RBC
red blood cells
- SAGM
saline‐adenine‐glucose‐mannitol
- WB
whole blood
- WBC
white blood cells
1. INTRODUCTION
Transfusion of whole blood (WB) and blood components, such as packed red blood cells (pRBC) and plasma, has gained importance in veterinary medicine. Whole blood contains red blood cells (RBC), leukocytes, platelets, and plasma proteins including clotting factors. To optimize blood use, WB can be separated into pRBC by centrifugation removing the supernatant plasma, and it is indicated for managing hemolysis and nonregenerative anemia. 1
Despite of additive solutions, morphological and biochemical changes occur in stored blood, reducing the function and the survival of RBCs and potentially causing adverse effects in recipients. 2 , 3 The entity of these changes, named “storage lesions,” differs among species 4 , 5 and additive solutions. 6 , 7 , 8
In human transfusion medicine, the growing literature on the adverse effects of blood storage and the possible clinical implications for transfusion recipients has recently identified the quality of blood stored as 1 of the most critical issues. 9 , 10 Consequently, methods of preserving blood to optimize its characteristics and limit degradation during storage are required. In particular, the metabolites of leukocytes such as cytokines, histamine, elastase, and acid phosphatase seem fundamental to the development of storage lesions and post‐transfusion reactions. 11 The leukoreduction of the human blood units reduces number of white blood cells (about 1‐5 x 106 per blood unit). 12 The prestorage filtration, soon after the blood donation, reduces the lesions of RBCs during the storage period by removing the leukocytes before their fragmentation and avoiding the accumulation of cytokines of leukocyte origin in stored blood and blood components. 13 , 14
In veterinary transfusion medicine, the biochemical changes of canine pRBC during storage and the effect of leukoreduction on the in vitro quality of RBCs have been scarcely investigated. 4 , 7 , 8 , 15
The aims of this study were as follows: (a) to investigate the effect of prestorage leukoreduction on the in vitro biochemical changes, which occur in canine WB stored in CPDA‐1 and in pRBC stored in saline‐adenine‐glucose‐mannitol (SAGM) medium, during 35 days of storage; (b) to investigate the coagulative profile and the concentration of the clotting factors in WB soon after donation and at the end of the storage period (35 days), as well as in fresh frozen plasma (FFP) after 1 year of storage.
2. MATERIALS AND METHODS
2.1. Animal selection and sampling protocol
The study, including blood collection and samples analysis, was performed in 2 years. Ten dogs have been selected from the list of blood donors of the canine blood bank of the Istituto Zooprofilattico Sperimentale delle Venezie (IZSVe), Legnaro (Italy). According to the Italian Ministry of Health guidelines, the donors were clinically healthy, over 25 kg, between 2 and 8 years, regularly vaccinated, and protected against endo‐ and ectoparasites. 16
Five double and 5 triple bag collection systems, with an integrated leukoreduction filter, were used (AB bags, Futurlab srl, Italy). All the bag systems were equipped with a self‐cleaning valve that allowed blood samples collection ensuring sterility during the study period. Approximately 450 mL of blood has been collected, from the jugular vein of each donor, in a primary bag and gently mixed with the anticoagulant preservative solution (63 mL of CPDA‐1 for double systems and CPD for the triple systems).
Blood collected in the double system was cooled for 2 hours at 4°C, and half of the WB was passed through the leukoreduction filter into the secondary bag by gravity, obtaining leukoreduced whole blood (LR WB); the remaining WB was left unfiltered (non‐leukoreduced [nLR] WB) in the primary bag. Both units were stored at 4°C in a blood bank refrigerator for 35 days.
Blood collected in triple systems was centrifuged at 4050g for 12 minutes at 4°C in a refrigerated centrifuge (Multifuge 4 KR, Thermo Scientific), within 2 hours after the donation. The plasma was driven to the empty satellite bag by a manual plasma extractor and stored at −30°C as FFP. After being suspended in 100 mL of SAGM medium, half of the pRBC were filtered obtaining leukoreduced pRBC (LR pRBC) units, and the remaining half was left unfiltered (nLR pRBC) in the primary bag. Both units were stored at 4°C in a blood bank refrigerator for 35 days.
From each bag, 10 mL of WB, LR WB, pRBC, and LR pRBC was collected weekly (T0, T7, T14, T21, T28, and T35), starting from the day of donation (T0), by using a sterile vacutainer system for hematological and biochemical analyses.
2.2. Hematological analysis
Residual leukocytes in LR units were investigated immediately after filtration using a commercial kit BDLeucocount with FACSCalibur flow cytometer (BD Biosciences, San Jose, California), following the manufacturer's instructions.
Hematological analysis was performed on a Cell‐Dyn 3700 analyzer (Abbott Laboratories, Abbott Park, Illinois); RBC morphology was assessed by light microscopy on Wright‐Giemsa stained slides (Aerospray, Delcon, Italy) and the average number of abnormal cells (echinocytes, spherocytes, schistocytes, and other shapes) was recorded per 1000x microscopic monolayer field and of at least 5 fields observed. 17
Erythrocytes' mean osmotic fragility (MOF) was measured as previously described. 5 Briefly, WB was incubated for 30 minutes at room temperature in isotonic‐to‐hypotonic sodium chloride solutions (0.90, 0.75, 0.65, 0.60, 0.55, 0.50, 0.45, 0.40, 0.30, 0.20, 0.10, and 0.00% NaCl). After centrifugation at 250 g for 10 minutes, the hemolysis index (H index) was determined using the supernatant on a clinical chemistry analyzer (Cobas C501, Roche Diagnostics GmbH, Mannheim, Germany). The H index is a semiquantitative spectrophotometric measurement of the free hemoglobin (Hb) concentration in mg/dL. The analytical sensitivity of the H index method is of 5 mg/dL. The mean osmotic fragility values are equivalent to NaCl concentration at which 50% of RBC lyzed.
The pH was measured at T0 and T35 (GLP21 pH meter, Crison Instruments, Lainate, Italy). Intraerythrocyte 2,3‐diphosphoglycerate (2,3‐DPG) and ATP concentrations were evaluated as previously described. 5 Briefly, 500 μL of RBC pellet was treated with 1.5 mL of refrigerated 8% trichloroacetic acid and then centrifuged at 3076g for 10 minutes at +4°C: the supernatant was neutralized with sodium carbonate (Na2CO3) 1 M and frozen at −80°C until the day of analysis. The 2,3‐DPG concentration was evaluated by UV spectrophotometry with an enzymatic assay (2‐3‐DPG, Roche Diagnostics GmbH, Mannheim, Germany), while the ATP concentration was determined by a luminescence assay system (ATPLite, Perking Elmer Inc., Waltham, Massachusetts). 18 , 19 The results of both analyses have been normalized with the Hb concentration obtained from the RBC pellet before acid extraction.
The remaining part of the blood sample was centrifuged at 1200g for 15 minutes at 4°C. The plasma was then frozen at −20°C in order to be analyzed as described later.
2.3. Plasma analysis
The plasma H index was used to determine the free Hb concentration in mg/dL. The percentage of hemolysis was calculated with the following formula 20 :
The concentration values of potassium (K), lactate, and glucose were analyzed in plasma samples using a clinical chemistry analyzer (Cobas C501, Roche Diagnostics GmbH, Mannheim, Germany). In particular, K and glucose concentrations were determined using commercial kits from Roche Diagnostics, while lactate concentration was determined using a Randox colorimetric kit (Randox Laboratories Ltd., Crumlin, County Antrim, United Kingdom).
The concentration values of canine interleukin‐6 (IL‐6), IL‐8, and tumor necrosis factor (TNFα) were assessed in all plasma samples with the kit Invitrogen ProcartaPlex Multiplex Immunoassay (Thermo Fisher Scientific, Waltham, Massachusetts). Assays were performed in duplicate according to the manufacturer's guidelines. The lower limits of detection for IL‐6, IL‐8, and TNFα were 8.2 pg/mL, 8.2 pg/mL, and 2.7 pg/mL, respectively.
2.4. Coagulation analysis
The coagulation profile, prothrombin time (PT), activated partial thromboplastin time (aPTT), and fibrinogen, as well as coagulation factors (VII, VIII, IX, XI, and XII), were determined in WB at T0 and T35, as well as in FFP at 1 year of storage. The ACL7000 instrumentation and dedicated kits (Instrumentation Laboratory, Bedford, Massachusetts) were used for PT, aPTT, and fibrinogen, while the STA Compact instrumentation and related commercial kits (Stago Italia SRL, Milan, Italy) were used for coagulation factors.
2.5. Statistical analysis
Descriptive analyses were performed to evaluate the distributions of the collected parameters and potential outliers. The effects of the type of bag (WB vs pRBC), type of unit (LR vs nLR), sampling time, and their interactions on parameters distribution were assessed using linear mixed models. Accounting for the study design, the dog was included in the models as random effect while the type of unit and sampling time within each dog were considered repeated effects. Regarding 2,3‐DPG, ATP, osmotic fragility, percentage of hemolysis, glucose, K and lactate, the Kronecker product, built with the unstructured and first‐order autoregressive covariance structures, was used for modeling the repeated effects type of unit and sampling time, respectively. For RBC parameters, coagulation profile data, and pH, the sampling points considered in the statistical analyses were T0 and T35, modeled in the repeated effect using a compound symmetry covariance structure. The Akaike Information Criterion (AIC) and the residual diagnostics were used to evaluate the goodness of fit of the models and to support the choice of the covariance structures. The comparison of the coagulation data in FFP and all combinations between type of bags and sampling time was performed using nonparametric K‐sample test on the equality of medians. Statistical significance was set to P < .05. All the analyses were performed using SAS v.9.4.
3. RESULTS
In all the LR WB and LR pRBC units, residual leukocytes' count resulted fewer than 1 × 106 WBC/units (0.002 ± 0.003 × 106 WBC/unit). When significant differences were not observed between LR and nLR units, they were considered together as WB and pRBC.
The mean values with SD of the RBC‐related indices are summarized in Table 1. During storage, hematocrit (HCT) and mean corpuscular volume (MCV) increased significantly in nLR pRBC; at T35, significant differences were observed between LR and nLR pRBC for HCT and MCV, being higher in nLR units.
TABLE 1.
Mean levels and SDs at different sampling times (T0, T35) for erythrocyte indices and pH in whole blood (WB) and packed red blood cells (pRBC)
| Mean (SD) | |||||
|---|---|---|---|---|---|
| Parameter (laboratory reference range) | Time | nLR WB | LR WB | nLR pRBC | LR pRBC |
| RBC (5.37‐8.40 M/μL) | T0 | 5.37 (0.50) | 5.20 (0.57) | 8.40 (0.68) | 8.24 (0.56) |
| T35 | 5.39 (0.69) | 5.28 (0.62) | 8.42 (0.71) | 7.70 (1.18) | |
| HGB (13.0‐19.5 g/dL) | T0 | 13.10 (1.20) | 12.48 (1.38) | 20.66 (1.43) | 19.96 (0.85) |
| T35 | 13.06 (1.56) | 12.67 (1.70) | 20.62 (1.56) | 18.62 (2.73) | |
| HCT (39‐56%) | T0 | 38.48 (4.60) | 37.28 (5.26) | 59.72 (3.67) | 58.64 (2.67) |
| T35 | 38.96 (5.51) | 37.76 (5.55) | 68.50 (4.54) | 57.18 (8.31) | |
| MCV (64‐75 fL) | T0 | 71.52 (3.33) | 71.48 (3.22) | 71.20 (1.79) | 71.24 (1.82) |
| T35 | 72.26 (2.82) | 71.30 (3.09) | 81.48 (3.38) | 74.34 (3.47) | |
| MCH (21.3‐26.7 pg) | T0 | 24.38 (0.58) | 24.00 (0.65) | 24.64 (0.66) | 24.26 (0.70) |
| T35 | 24.28 (0.90) | 24.00 (0.90) | 24.50 (0.41) | 24.22 (0.59) | |
| MCHC (32.1‐36.3 g/dL) | T0 | 34.16 (1.20) | 33.64 (1.29) | 34.60 (0.44) | 34.04 (0.38) |
| T35 | 33.62 (1.29) | 33.72 (1.44) | 30.10 (0.95) | 32.58 (0.75) | |
| RDW (11.5‐16.7%) | T0 | 15.78 (0.43) | 15.32 (0.78) | 18.52 (1.57) | 17.62 (1.05) |
| T35 | 16.44 (1.04) | 16.42 (1.13) | 19.88 (2.58) | 17.94 (1.75) | |
| pH | T0 | 7.13 (0.18) | 7.19 (0.17) | 7.09 (0.06) | 7.10 (0.03) |
| T35 | 6.70 (0.20) | 6.88 (0.16) | 6.22 (0.11) | 6.57 (0.23) | |
Note: HCT, MCV, and MCHC: significant difference (P < .01) between T0 and T35 for nLR pRBC and between nLR and LR pRBC at T35; pH, significant difference (P < .01) between T0 and T35 for all bags and between LR and nLR at T35.
Abbreviations: Hb, hemoglobin; HCT, hematocrit; MCH, mean hemoglobin concentration; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; LR, leukoreduced; nLR, not leukoreduced; pRBCs, packed red blood cells; RBC, red blood cells; RDW, red blood cell distribution width.
An almost complete PLT depletion was observed after leukoreduction, with platelet count at T0 of 142 ± 72 K/μL and 396 ± 271 K/μL in nLR WB and nLR pRBC units, and of 0.36 ± .68 K/μL and 0.26 ± .37 K/μL in LR WB and LR pRBC, respectively.
On microscopic examination, RBC morphology showed an increasing presence of echinocytes during storage, starting from a total absence or weak presence (1+, corresponding to an average of 5‐10 echinocytes per 1000x field) at T0, to a moderate presence (2+, corresponding to an average of 11‐100 echinocytes per 1000x field) at T35. 17 During storage, we did not observe other abnormal shapes on blood films.
At T0, no differences in MOF were observed between bags. In WB bags, the MOF values showed a slight but significant decrease (P = .003) from T0 (0.44% NaCl) to T35 (0.42% NaCl). Moreover, at T35, pRBC bags showed significantly higher mean values than WB (P = .03), particularly due to an increasing trend over time of nLR pRBC (P < .001). In fact, a significant difference between LR and nLR pRBC units was observed from T14 (Figure 1A).
FIGURE 1.
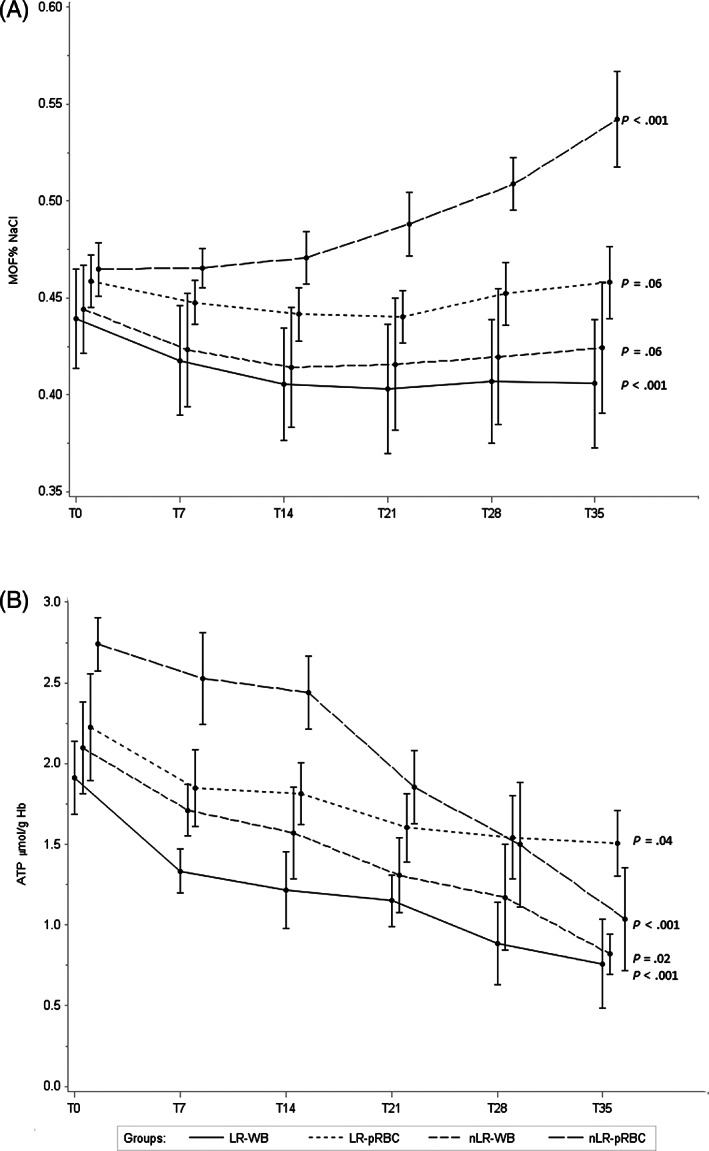
Mean levels at each observation time (T0, T7, T21, T28, and T35) of, A, mean osmotic fragility (MOF) and B, ATP for each type of blood units; error bars show SEs for each observation. Time variable was shifted for allowing the display of the groups of blood units
At T0, the pH mean values were not statistically different between bags. At T35, significant lower values were detected in all units compared with T0, with significantly lower values in nLR than in LR units for both types of bags (P < .001) (Table 1).
At T0, no differences in ATP values were observed between bags. An overall significant decreasing trend was observed for both types of bags (P < .001) during storage. The pRBC units showed slightly higher mean values than WB at T7 (P = .05) and T14 (P = .03). Comparing LR vs nLR units, in WB units no differences were observed during storage, while LR pRBC showed a less pronounced decreasing trend than nLR (P = .04) (Figure 1B).
At T0, no differences in 2,3‐DPG concentrations were observed between bags. Both WB and pRBC units showed a significant decrease during storage; particularly, from T21 to the end of the storage period, the pRBC units had significantly lower mean 2,3‐DPG values than WB units (T35: WB 11.66 μmol/g Hb, pRBC 5.10 μmol/g Hb, P = .02). No differences were observed between LR and nLR units (Figure 2A).
FIGURE 2.
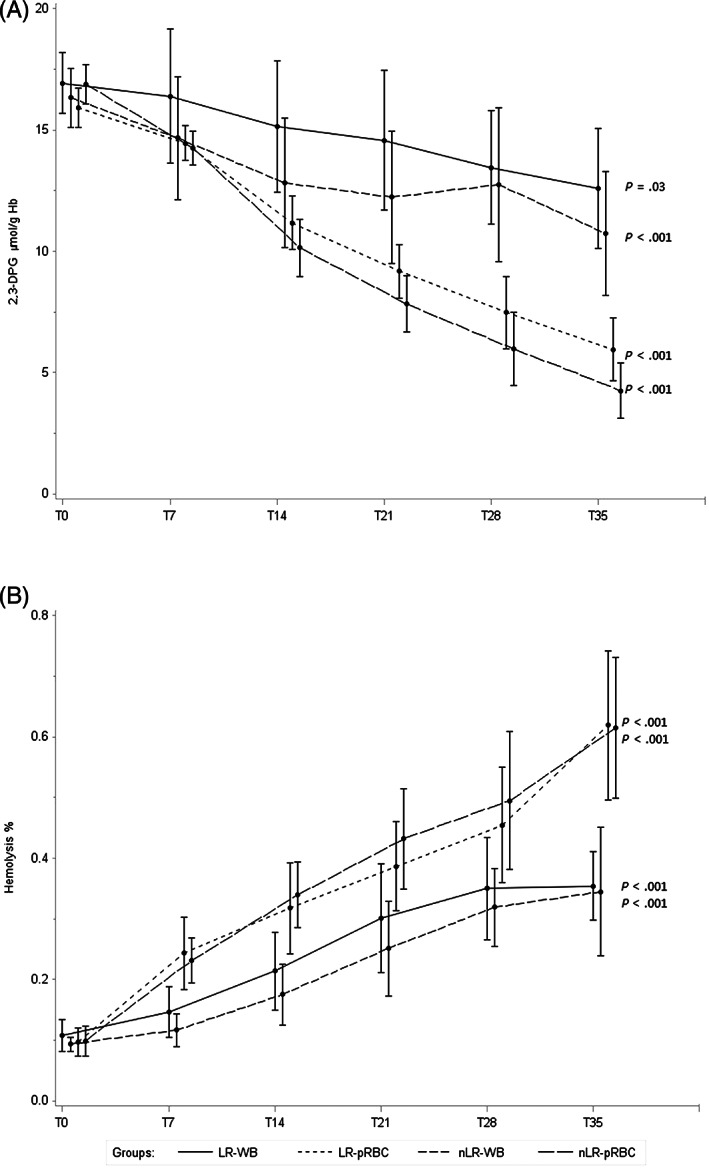
Mean levels at each observation time (T0, T7, T21, T28, and T35) of, A, 2,3‐DPG and, B, hemolysis for each type of blood units; error bars show SEs for each observation. Time variable was shifted for allowing the display of the groups of blood units
The hemolysis (%) measured at T0 showed no significant differences between bags. An overall significant increasing trend was observed for both type of bags (P < .001), showing higher mean values in pRBC than in WB at T35 (0.66% vs 0.41%, P = .03). No differences were observed between LR and nLR units during storage (Figure 2B).
The K mean concentration at T0 was significantly higher in WB (3.52 mmol/L) than in pRBC bags (1.95 mmol/L) (P < .001). A significant increasing trend was observed in both types of bags (P < .001). Higher mean values of K were observed in nLR pRBC with respect to LR starting form T21, while no difference was found comparing LR and nLR WB units (Figure 3A).
FIGURE 3.
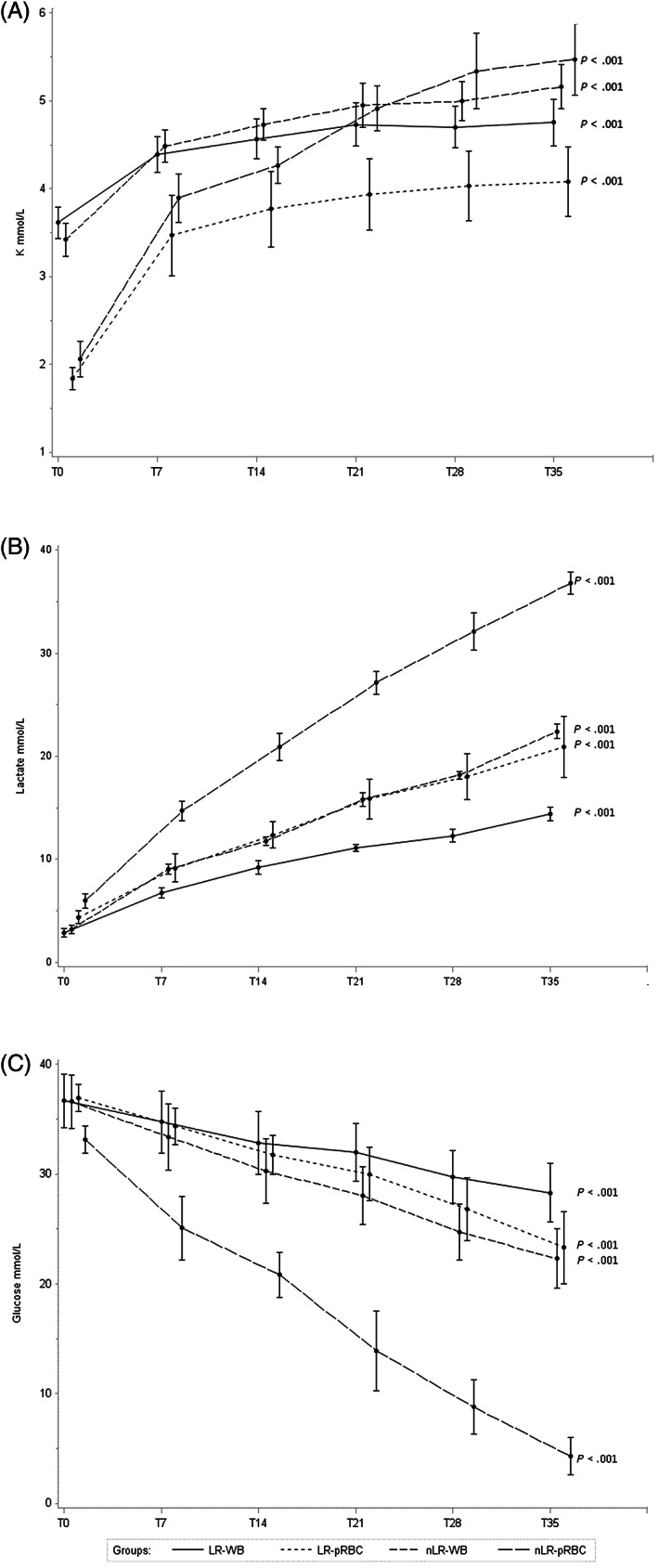
Mean levels at each observation time (T0, T7, T21, T28, and T35) of, A, lactate, B, potassium, and C, glucose for each type of blood units; error bars show SEs for each observation. Time variable was shifted for allowing the display of the groups of blood units
At T0, the mean concentration of plasma lactate showed no significant differences between bags, but it significantly increased during storage in all types of bags (P < .001); pRBC showed higher values than WB bags starting from T7 (P = .004); LR units showed significantly lower values than nLR ones, starting from T7 for pRBC and from T21 for WB (Figure 3B).
As expected, glucose levels showed a significant decrease in all blood units, particularly evident in LR pRBC units (4.35 mmol/L at T35) (Figure 3C).
During storage, the IL‐8 mean concentration remained at a stable low level in LR units (T0: 137 ± 221 pg/mL; T35: 150 ± 245 pg/mL at T35), while increasing values were observed in nLR units during storage (T0: 4167 ± 11 888 pg/mL; T35: 6367 ± 11 612 pg/mL at T35). Due to the high variability among units, no statistical evaluation was performed. Interleukin‐6 and TNFα concentrations remained under the detection limit in all bags at all storage times.
Regarding the coagulation profile, the PT showed no significant differences between LR and nLR WB units at T0 and T35; notwithstanding significant higher mean values were observed at T35 for both units (P < .001). The mean values of PT measured in WB at T0 and T35 and in FFP after 1 year showed no significant differences. The aPTT mean values were higher in LR than in nLR units (P = .04), and they increased significantly at T35 (P = .03); differently, no significant differences were observed between T0 and the FFP, as well as between T35 and the FFP. The fibrinogen mean concentration was significantly lower in LR WB than in nLR units (P < .001); moreover, a significant decrease was observed at T35 in both units, compared with T0 (P < .001). Notwithstanding, no significant differences were observed between T0 and the FFP as well as between T35 and the FFP (Table 2).
TABLE 2.
Mean levels and SDs of coagulation variables in whole blood at different sampling times (T0, T35) and in fresh frozen plasma at 1 year of storage
| LR WB | nLR WB | P a | FFP | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter (laboratory reference range) | T0 | T35 | T0 | T35 | LR vs nLR | T0 vs T35 | T0 |
P for the comparisons with all combinations between type of bags and sampling time |
| PT (5.4‐7.8 seconds) | 9.38 (1.45) | 10.64 (1.82) | 9.28 (1.51) | 11.66 (2.87) | ns | <.001 | 9.57 (1.45) | ns |
| aPTT (9.4‐15.6 seconds) | 19.22 (4.42) | 20.26 (2.13) | 13.74 (0.91) | 15.52 (2.13) | .036 | .031 | 17.01 (2.62) | P = .02 with nLR at T0 |
| Fibrinogen (108‐416 mg/dL) | 141.60 (17.4) | 125.00 (19.4) | 145.80 (16.27) | 134.40 (19.78) | <.001 | <.001 | 135.44 (46.24) | ns |
| FVII (62%‐150%) | 62.40 (15.63) | 53.20 (15.74) | 62.40 (17.01) | 54.80 (13.26) | ns | <.001 | 55.33 (13.3) | ns |
| FVIII (60%‐135%) | 72.20 (14.97) | 42.00 (11.11) | 84.00 (14.28) | 52.40 (17.83) | ns | <.001 | 59.23 (24.38) | ns |
| FIX (55%‐110%) | 60.00 (11.51) | 46.80 (6.14) | 66.20 (11.37) | 53.60 (7.73) | .024 | <.001 | 52.33 (9.48) | ns |
| FXI (50%‐110%) | 61.50 (34.20) | 52.20 (40.22) | 93.25 (17.63) | 83.40 (10.85) | na | na | 63.59 (8.18) | na |
| FXII (60%‐125%) | 89.00 (26.87) | 78.40 (15.29) | 102.25 (16.88) | 89.40 (14.94) | na | na | 76.52 (10.68) | na |
Note: na indicates statistical analyses not performed due to the reduced number of observations and high observed variability.
Abbreviations: aPTT, activated partial thromboplastin time; FFP, fresh frozen plasma; LR, leukoreduced; nLR, non‐leukoreduced; PT, prothrombin time; WB, whole blood.
For all parameters, no significant interaction between type of bags and sampling time. Mean values between nLR WB and LR WB.
The mean concentration of factors VII, VIII, and IX showed no significant differences between nLR WB and LR WB. During storage, a significant decrease of the mean concentration was observed for all the clotting factors (P < .05). However, no significant differences were observed between T0 and FFP and between T35 and FFP. Factors XI and XII showed high variability among bags; thus, no statistical evaluation has been performed (Table 2).
4. DISCUSSION
The primary aim of our study was to evaluate the in vitro quality of canine WB and pRBC during 35 days storage and to evaluate the possible effect of leukoreduction on hematological and biochemical parameters. In our study, leukoreduction successfully met the US FDA threshold of 5 × 106 WBC/unit 21 and the less than 1 × 106 WBC/unit standard of the Council of Europe. 22 Moreover, leukoreduction caused an almost complete platelets' depletion, confirming the reduction greater than 99% in LR blood canine units. 23 In cold‐stored WB, the platelet count declines by 1% to 2% per day, with limited impact on clotting time or on clot strength as measured by thromboelastography. Animal studies have confirmed that platelets from stored WB participate in clot formation. 24 Leukoreducing WB using a platelet‐saving filter did not compromise hemostatic properties. 25
In our study, the evaluated erythrocytes indices remained stable during storage, except for MCV that slightly increased particularly in nLR pRBC. The increase in MCV is described for stored human, 14 feline, 5 and canine RBCs. 26 The increase of MCV might be due to the progressive impairment of the ATP‐dependent Na‐K pumps of the RBC membrane, which cannot function properly during storage, 14 and to the prolonged contact with the anticoagulant solution. 27 The higher MCV value observed in nLR units might have also resulted from the presence of leukocyte products, which can affect erythrocyte membrane properties. 14
Membranous changes that affect RBC during storage can decrease their elasticity, causing a gradual echinocytic shape transformation. 14 In our study, echinocytosis showed a mild increase in all bags during the storage period. Echinocytosis is considered a reversible morphological alteration and is likely attributable to RBC anticoagulant contact and to the duration of storage time, as well as to the constant depletion of ATP and 2,3‐DPG. 9 , 27
The osmotic fragility test is conventionally used to assess erythrocyte resistance to hemolysis, using a series of hypotonic solutions of NaCl. 28 In healthy animals, the MOF (corresponding at the NaCl concentration at which 50% of RBCs lyzed) occurs around 0.5% NaCl. 29 In our study, pRBC showed higher MOF values than WB starting from T28, particularly due to the increasing trend in nLR pRBC units from T14. However, the mean value at the end of the storage was still to be considered as normal in all type of bags. The blood sample storage conditions could have a strong influence on erythrocyte shape, volume, and integrity because of the continuous anaerobic metabolism of these blood elements, leading to increased vulnerability at a relatively high osmotic strength. 28 In a recent study, the MOF of RBCs increased constitutively during storage, but it was significantly lower in WB than in pRBC units, throughout the storage period. 30
Loss of RBC integrity during storage results in hemolysis, causing the release of free Hb in the red cell suspending media. The rate of in‐bag hemolysis depends on the individual donor, the preservative solution, and the duration of storage. 31 Many other factors are believed to cause hemolysis in blood units, such as preparative procedures, mechanical stress, bacterial contamination, temperature, osmotic and pH changes, presence of leukocytes, and intrinsic RBC membrane defects. 32 In our study, the percentage of hemolysis was negligible at T0 in all the bags, suggesting that the preparative procedures, including separation and filtration, did not adversely affected RBCs. During storage, an increasing trend has been observed, being significantly higher in the pRBC bags than in WB at T35, but remaining below the most stringent regulatory guidelines, fixed at <0.8% for LR WB by the Council of Europe Guide for blood components. 22 Differently from pRBC, WB units retain all the components of the donation, including plasma, that might exert pleiotropic cytoprotective effects on stored RBCs by providing survival factors, nutrients, amino acids, erythropoietin, and ROS scavengers, such as albumin and uric acid. 33 , 34 in vitro studies reported that, while the supernatant of long‐stored pRBCs induces hemolysis in fresh RBCs, incubation of stored RBCs with fresh plasma at 37°C results in reduced hemolysis. 35 Recently, Tzounakas et al 30 reported that warming of cold blood supplemented by CPDA‐1 up to 17°C in less than 8 hours before component processing led to increased incidence of in‐bag hemolysis in pRBC but not in WB units, supporting the protective effect of plasma on the biopreservation of RBCs.
In our study, the leukoreduction did not have any evident effect on the percentage of hemolysis during storage. It has been reported that RBCs may be damaged if they are forced through leukoreduction filters and that hemolysis is more likely to occur in undiluted RBC concentrates with high hematocrits than in WB. 32 On the other hand, many studies reported that the presence of leukocytes in unfiltered RBC units might contribute to the increase in hemolysis during storage. 32 None of these 2 conditions seems to have occurred in our investigation.
In our study, glucose concentration significantly decreased in all types of bags during storage, with the lowest values in nLR pRBC. Lactate concentrations significantly increased in all bags, being significantly higher in pRBC than in WB starting from T14. Leukoreduction had significant effect on lactate production in both types of bags, with lower lactate concentrations in LR units from T7 in pRBC and from T21 in WB.
There was a significant negative correlation between glucose and lactate, as well as between glucose and MOF, in all types of bags (data not shown). Moreover, a significant positive correlation was found in nLR pRBC between lactate and MOF.
In addition to lactate accumulation, the glycolytic process leads to protons' accumulation, causing a progressive acidosis of stored blood. 31 The average pH values decreased significantly during the storage period in all types of bags, with lower values in the pRBC than in WB bags. Leukoreduced units showed significantly higher values than nLR ones. LR units have significantly higher pH than other groups during 28 days of storage in other studies. 36
Due to the progressive inactivation of the ATP‐dependent Na‐K pumps of the RBC membrane, human‐stored RBC solutions develop increased potassium concentrations and decreased sodium concentrations during storage. 9 In our investigation, K levels increased significantly in all types of bags, with the lowest concentrations observed in LR pRBC at the end of the storage period. Similar results have been reported in canine LR pRBC, stored in different additive solutions, 8 as well as in feline WB stored in CPDA‐1 for 35 days. 5 In contrast, a study of nLR feline pRBC reported a decrease in K mean levels. 37 The impact of the increased concentrations of K in blood products on the canine recipients is currently unknown.
In our study, mean ATP concentration decreased from T0 to T35 in all types of bags, and leukoreduction had no statistical significant effect, even though a less pronounced decreasing trend in LR pRBC units was observed. All type of bags maintained mean ATP values at T35 over the indicated threshold, as previously published data for dogs. 7 , 8 , 38
The erythrocyte 2,3‐DPG is a glycolytic intermediate that can modify of Hb‐oxygen affinity in many species, including human beings, dogs, and rats. The decrease in 2,3‐DPG mean concentration is well described in human and canine stored blood, resulting in a significantly lower delivery of oxygen to the tissues. 9 In our study, the pRBC bags had significantly lower values than WB from T21 until the end of the storage period. In contrast to published data, 15 we observed that the leukoreduction did not improve significantly the 2,3‐DPG erythrocyte concentration; the decrease of 2,3‐DPG seems to be independent of leukocyte presence, but ascribable to alteration of the glycolytic pathway and to the fall of pH values. In fact, it is known that a major factor causing the degradation of 2,3‐DPG is the decrease in pH during storage. 6
In our survey, the IL‐8 median concentration remained at low levels in LR WB and LR pRBC units, during all the storage period, while an increasing trend was observed in nLR units. Similarly, previous studies found that leukoreduction prevented IL‐8 accumulation in canine pRBC, while it significantly increased in nLR units during storage. 36 , 39 , 40 Even though IL‐8 has no direct pyrogenic effects, it has been speculated that the passive inoculation of cytokines might have a synergistic effect on the cytokine network of critically ill patients, contributing to the systemic inflammatory response. 41 Notwithstanding, the inflammatory effects of IL‐8 passive infusion on canine blood recipients remain unknown.
In our study, IL‐6 and TNFα concentrations remained always under the detection limit in all bags and at all storage times. In a previous study, IL‐6 concentration was higher in canine WB than in LR or irradiated WB at every storage time, but it did not increase in a time‐dependent manner, while TNFα was under the detection limit in all the groups. 36
A significant increase in aPTT values and a simultaneous decrease in fibrinogen, factors VII, VIII, and IX concentration were observed in WB between T0 and T35. No significant differences were observed between WB at T0 and FFP, as well as between WB at T35 and FFP, demonstrating that, contrary to a common belief, the WB maintains good levels of clotting factors during all the storage period, including those considered more labile factors. Evaluating the effect of leukoreduction on concentration of clotting factors, a slight but significant difference between LR and nLR WB units was observed for aPTT, fibrinogen, and factor IX. In humans, it is known that LR FFP has lower concentrations of factors VII, VIII, and XI, as well as it has longer aPTT. 42 Differently, a recent study on canine FFP showed no significant differences between LR and nLR units. 43 Further studies in both human and veterinary medicine showed that leukoreduction causes a significant decline in FVIII activity and fibrinogen concentration. 44 , 45 In our study, the mean concentration of factor VIII was lower in LR WB than in nLR ones at T0 and T35, even though the difference was not statistically significant. Currently, it is unknown how leukoreduction can affect activity and concentration of clotting factors, even though it is supposed that the type of filter and the length and temperature of storage before leukoreduction may be involved in this mechanism. 42 The different results obtained by different studies in veterinary medicine may be because of the low number of samples considered, the differences in the LR procedure, and the high variability of these parameters. Since no specific standard exist, and studies indicate a high variability among donors, further clinical and therapeutic trials are needed to define the minimal clotting factors levels in blood products to support surgical hemostasis.
The main limitation of this study is the small number of subjects enrolled because of ethical and practical reasons, as well as the high variability of some variables. Nevertheless, the results obtained are coherent with the literature already available on canine pRBC, and add new insight into canine WB biochemical characteristics during storage time and into canine FFP after 1 year of storage.
In conclusion, our results demonstrate the good biochemical quality of both canine WB and pRBC during 35 days of storage; moreover, better biochemical conditions in WB than in pRBC, particularly for the percentage of hemolysis, MOF, pH, lactate, and 2,3‐DPG, probably due to the protective effects of survival and nutritive factors contained in plasma, have been recorded. WB can be used in all cases of anemia to restore the correct RBC volume, but it is primarily recommended for managing acute, severe hemorrhage. In both human and veterinary transfusion medicine, pRBC separation from WB is a common practice, with the advantages to economize blood use, to prolong storage time, and to better address the patient component deficiency. pRBC is more indicated than WB in case of normovolemic anemia, while FFP in case of clotting factors deficiency.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Informed client consent was obtained by dog owners participating in the study. The study was approved by the Ethical Committee of the Istituto Zooprofilattico Sperimentale delle Venezie.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Funding provided by Italian Ministry of Health (Projects IZS VE 04/15 RC). The authors thank the technical staff at the Veterinary Medical Center “della Riviera” for their assistance in conducting this study. The authors also thank the owners and their dogs for their willingness to participate in this study.
Stefani A, Capello K, Carminato A, et al. Effects of leukoreduction on storage lesions in whole blood and blood components of dogs. J Vet Intern Med. 2021;35:936–945. 10.1111/jvim.16039
Funding information Italian Ministry of Health, Grant/Award Number: IZS VE 04/15 VE
REFERENCES
- 1. Kisielewicz C, Self IA. Canine and feline blood transfusions: controversies and recent advances in administration practices. Vet Anaesth Anal. 2014;41:233‐242. [DOI] [PubMed] [Google Scholar]
- 2. Hess JR. Measures of stored red blood cell quality. Vox Sang. 2014;107:1‐9. [DOI] [PubMed] [Google Scholar]
- 3. Vandromme MJ, McGwin G, Weinberg JA. Blood transfusion in the critically ill: does storage age matter? Scand J Trauma Resusc Emerg Med. 2009;17:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson CR, Pashmakova MB, Heinz JA, et al. Biochemical evaluation of storage lesion in canine packed erythrocytes. J Small Anim Pract. 2017;58:678‐684. [DOI] [PubMed] [Google Scholar]
- 5. Crestani C, Stefani A, Carminato A, et al. In vitro assessment of quality of citrate‐phosphate‐dextrose‐adenine‐1 preserved feline blood collected by a commercial closed system. J Vet Intern Med. 2018;32(3):1051‐1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kurup PA, Arun P, Gayathri NS, et al. Modified formulation of CPDA for storage of whole blood, and of SAGM for storage of red blood cells, to maintain the concentration of 2,3‐diphosphoglycerate. Vox Sang. 2003;85(4):253‐261. [DOI] [PubMed] [Google Scholar]
- 7. Wardrop KJ, Tucker RL, Mugnai K. Evaluation of canine red blood cells stored in a saline, adenine, and glucose solution for 35 days. J Vet Intern Med. 1997;11:5‐8. [DOI] [PubMed] [Google Scholar]
- 8. Lacerda LA, Hlavac NRC, Terra SR, Back FP, Jane Wardrop K, González FHD. Effects of four additive solutions on canine leukoreduced red cell concentrate quality during storage. Vet Clin Pathol. 2014;43:362‐370. [DOI] [PubMed] [Google Scholar]
- 9. Obrador R, Musulin S, Hansen B. Red blood cell storage lesion. J Vet Emerg Crit Care. 2015;25:187‐199. [DOI] [PubMed] [Google Scholar]
- 10. Gilliss BM, Looney MR, Gropper MA. Reducing noninfectious risks of blood transfusion. Anesthesiology. 2011;115:635‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rajesh K, Harsh S, Amarjit K. Effects of prestorage leukoreduction on the rate of febrile nonhemolytic transfusion reactions to red blood cells in a tertiary care hospital. Ann Med Health Sci Res. 2015;5:183‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brecher ME. Collection, preparation, storage, and distribution of components from whole blood donations. In: Brecher ME, ed. Technical Manual. 15th ed. Bethesda: AABB; 2005:175‐202. [Google Scholar]
- 13. Walter R, Brand B, Mark M, Schnyder L, Stifanic M, Reinhart WH. Effects of leucocyte depletion on rheologic properties of human CPDA‐1 blood. Vox Sang. 2000;79:151‐155. [DOI] [PubMed] [Google Scholar]
- 14. Sollberger T, Walter R, Brand B, Contesse J, Meredith DO, Reinhart WH. Influence of prestorage leucocyte depletion and storage time on rheologic properties of erythrocyte concentrates. Vox Sang. 2002;82:191‐197. [DOI] [PubMed] [Google Scholar]
- 15. Ergül Ekiz E, Arslan M, Akyazi Í, et al. The effects of prestorage leukoreduction and storage duration on the in vitro quality of canine packed red blood cells. Turkish J Vet Anim Sci. 2012;36:711‐717. [Google Scholar]
- 16. Ministero Della Salute Dipartimento Della Sanità Pubblica e Dell'Innovazione . Linea guida relativa all'esercizio delle attività riguardanti la medicina trasfusionale in campo veterinario 2016;25:5–18. [Google Scholar]
- 17. Harvey J. Hematology procedures. Veterinary Hematology: A Diagnostic Guide and Color Atlas. St Louis, MO: Saunders/Elsevier; 2012:11‐32. [Google Scholar]
- 18. Gorman MW, Marble DR, Ogimoto K, Feigl EO. Measurement of adenine nucleotides in plasma. Luminescence. 2003;18:173‐181. [DOI] [PubMed] [Google Scholar]
- 19. Gorman MW, Feigl EO, Buffington CW. Human plasma ATP concentration. Clin Chem. 2007;53:318‐325. [DOI] [PubMed] [Google Scholar]
- 20. Sawant R, Jathar S, Rajadhyaksha S, Kadam P. Red cell hemolysis during processing and storage. Asian J Transfus Sci. 2007;1:47‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. FDA . Guidance for Industry: Pre‐Storage Leukocyte Reduction of Whole Blood and Blood Components Intended for Transfusion. 2012.
- 22. EDQM . Guide to the Preparation, Use and Quality Assurance of Blood Components. 2017.
- 23. McQuinn ER, Smith SA, Viall AK, et al. Neutrophil extracellular traps in stored canine red blood cell units. J Vet Intern Med. 2020;34:1894‐1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van der Meer PF, Klei TR, de Korte D. Quality of platelets in stored whole blood. Transfus Med Rev. 2020;34:234‐241. 10.1016/j.tmrv.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 25. Sivertsen J, Braathen H, Lunde THF, et al. Cold‐stored leukoreduced CPDA‐1 whole blood: in vitro quality and hemostatic properties. Transfusion. 2020;60:1042‐1049. [DOI] [PubMed] [Google Scholar]
- 26. Price GS, Armstrong PJ, McLeod DA, et al. Evaluation of citrate‐phosphate‐dextrose‐adenine as a storage medium for packed canine erythrocytes. J Vet Intern Med. 1988;2:126‐132. [DOI] [PubMed] [Google Scholar]
- 27. Chen D, Serrano K, Devine D. Introducing the red cell storage lesion. ISBT Sci Ser. 2016;11:26‐33. [Google Scholar]
- 28. Salvagno GL, Demonte D, Dima F, Bovo C, Lippi G. Stability of refrigerated whole blood samples for osmotic fragility test. Hematol Transfus Cell Ther. 2020;42:134‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tritschler C, Mizukami K, Raj K, Giger U. Increased erythrocytic osmotic fragility in anemic domestic shorthair and purebred cats. J Feline Med Surg. 2016;18:462‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tzounakas VL, Anastasiadi AT, Karadimas DG, et al. Temperature‐dependent haemolytic propensity of CPDA‐1 stored red blood cells vs whole blood—red cell fragility as donor signature on blood units. Blood Transfus. 2017;15:447‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hess JR, Sparrow RL, van der Meer PF, Acker JP, Cardigan RA, Devine DV. Red blood cell hemolysis during blood bank storage: using national quality management data to answer basic scientific questions. Transfusion. 2009;49:2599‐2603. [DOI] [PubMed] [Google Scholar]
- 32. Sowemimo‐Coker SO. Red blood cell hemolysis during processing. Transfus Med Rev. 2002;16:46‐60. [DOI] [PubMed] [Google Scholar]
- 33. Ames BN, Cathcart R, Schwiers EHP. Uric acid provides an antioxidant defense in humans against oxidantand radical‐caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858‐6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vota DM, Maltaneri RE, Wenker SD, Nesse AB, Vittori DC. Differential erythropoietin action upon cells induced to eryptosis by different agents. Cell Biochem Biophys. 2013;65:145‐157. [DOI] [PubMed] [Google Scholar]
- 35. Tzounakas VL, Kriebardis AG, Georgatzakou HT, et al. Glucose 6‐phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic Biol Med. 2016;96:152‐165. [DOI] [PubMed] [Google Scholar]
- 36. Yang H, Kim W, Bae J, et al. Effects of irradiation and leukoreduction on down‐regulation of CXCL‐8 and storage lesion in stored canine whole blood. J Vet Sci. 2019;20:72‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heinz JA, Pashmakova MB, Wilson CR, et al. Biochemical evaluation of the effects of storage on feline erythrocytes. J Small Anim Pract. 2016;57:637‐643. [DOI] [PubMed] [Google Scholar]
- 38. Brownlee L, Wardrop KJ, Sellon RK, Meyers KM. Use of a Prestorage Leukoreduction filter effectively removes leukocytes from canine whole blood while preserving red blood cell viability. J Vet Intern Med. 2000;14:412‐417. [DOI] [PubMed] [Google Scholar]
- 39. Corsi R, McMichael MA, Smith SA, et al. Cytokine concentration in stored canine erythrocyte concentrates. J Vet Emerg Crit Care. 2014;24:259‐263. [DOI] [PubMed] [Google Scholar]
- 40. Purcell SL, Claus M, Hosgood G, Smart L. Effect of leukoreduction on concentrations of interleukin‐8, interleukin‐1β, and tumor necrosis factor‐α in canine packed red blood cells during storage. Am J Vet Res. 2015;76:969‐974. [DOI] [PubMed] [Google Scholar]
- 41. Stack G, Baril L, Napychank P, Snyder EL. Cytokine generation in stored, white cell‐reduced, and bacterially contaminated units of red cells. Transfusion. 1995;35:199‐203. [DOI] [PubMed] [Google Scholar]
- 42. Alhumaidan HS, Cheves Mt TA, Holme S, Sweeney JD. The effect of filtration on residual levels of coagulation factors in plasma. Am J Clin Pathol. 2013;139:110‐116. [DOI] [PubMed] [Google Scholar]
- 43. Foote ML, Brooks MB, Archer TM, Wills RW, Mackin AJ, Thomason JM. Coagulation factor activity in units of leukoreduced and nonleukoreduced canine fresh‐frozen plasma. Am J Vet Res. 2019;80:846‐851. [DOI] [PubMed] [Google Scholar]
- 44. Lehmann H, Hindricks E, Hassdenteufel EM, Moritz A, Bauer N. Prospective comparative quality control study of a novel gravity‐driven hollow‐fiber whole blood separation system for the production of canine blood products. Front Vet Sci. 2019;6:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chan KS‐K, Sparrow RL. Microparticle profile and procoagulant activity of fresh‐frozen plasma is affected by whole blood leukoreduction rather than 24‐hour room temperature hold. Transfusion. 2014;54:1935‐1944. [DOI] [PMC free article] [PubMed] [Google Scholar]


