Abstract
INTRODUCTION:
Plants and animals respond to pathogen invasion through intracellular nucleotide-binding leucine-rich repeat receptors (NLRs) that directly interact with pathogen proteins or indirectly detect pathogen-derived alterations in the host proteome. Upon recognition of pathogen invasion, NLRs trigger an immune response that resolves in a variety of ways depending on the type of NLR being activated. The overall architecture of NLRs is highly conserved, consisting of a C-terminal leucine-rich repeat (LRR) platform that determines substrate specificity and a central nucleotide-binding oligomerization domain. The N-terminal domain varies between NLRs and determines the mechanism used by the host to activate the immune response. Thus, NLRs in plants have been classified according to their N-terminal domain into Toll/interleukin 1 receptor (TIR) NLRs (TNLs), coiled-coil NLRs (CNLs), and RPW8- like coiled-coil NLRs (RNLs). Pathogen detection and oligomerization of the NLR activates these N-terminal domains by bringing them in close contact. In all three cases, association of the N-terminal domain leads to localized cell death and expression of disease resistance. The TIR domains of TNLs have been shown to have oligomerization-dependent NADase activity that is required for promoting cell death, but it is not understood how the interactions between TIR domains renders them catalytically active.
RATIONALE:
The structure of the ROQ1 (recognition of XopQ 1)–XopQ (Xanthomonas outer protein Q) complex, an immune receptor bound to its pathogen substrate, was used as a model to study the mechanism of direct binding, oligomerization, and TIR domain activation of TNLs. ROQ1 has been shown to physically interact with the Xanthomonas effector XopQ, causing it to oligomerize and trigger a TIR-dependent hypersensitive cell death response. We coexpressed, extracted, and purified the assembled ROQ1-XopQ complex from ROQ1’s native host, Nicotiana benthamiana, and solved its structure by cryo–electron microscopy to 3.8-Å resolution. The interactions described in our structure were further confirmed by in vivo mutational analysis.
RESULTS:
Our structure reveals that ROQ1 forms a tetrameric resistosome upon recognizing XopQ. The LRR and a post-LRR domain named the C-terminal jelly-roll/Ig-like domain (C-JID), form a horseshoe-shaped scaffold that curls around the pathogen effector, thereby recognizing multiple regions of the substrate. Binding of the ROQ1 LRR to XopQ occurs through surface-exposed residues that make up the scaffold of the domain, as well as an elongated loop between two LRRs that forms a small amphipathic a-helix at the site of interaction. The mode of substrate recognition by the C-JID is reminiscent of that used by immunoglobulins to bind their antigen. Similar to the complementary-determining regions of antibodies, interconnecting loops emerging from the C-JID b-sandwich structure make substrate-specific contacts with XopQ. In particular, an extended loop of the C-JID dives into the active-site cleft of XopQ and interacts with conserved residues required for nucleoside binding, suggesting that ROQ1 not only recognizes its substrate but also inhibits its ligand-binding function.
The nucleotide-binding domain (NBD), helical domain 1 (HD1) and the winged-helix domain (WHD), termed NB-ARC because of their presence in Apaf-1, R proteins, and CED-4 (ARC), are responsible for ROQ1 oligomerization in an ATP-bound state. Individual protomers intercalate in a similar fashion as found in other NLR structures, promoting association between the N-terminal TIR domains. The TIR domains bind to each other through two distinct interfaces (called AE and BE), causing them to form a dimer of dimers. BE-interface contacts cause a conformational rearrangement in a loop, called the BB-loop, at the periphery of the TIR domain active site that exposes the putative catalytic glutamate that is suggested to cleave NAD+. These results provide a rationale for the previously determined oligomerization dependence of TIR domain NADase activity.
CONCLUSION:
We propose a step-by-step mechanism for ROQ1 immune signaling based on our structure of the activated complex and on previous biochemical studies. The LRR and C-JID of ROQ1 recognize the pathogen effector through direct contacts with its surface and active-site residues. Detection of the substrate releases autoinhibitory contacts between the NB-ARC domain and the LRR, allowing the NB-ARC domain to transition to an ATP-bound, oligomerization-prone state. Complex assembly brings the TIR domains in close contact, leading to opening of the NADase active site in an interface-dependent manner. Cleavage of NAD+ by the TIR domain results in the release of adenosine diphosphate ribose, a signaling molecule that triggers cytosolic Ca2+ influx, a widely used chemical cue in response to various biotic and abiotic stresses, leading to downstream activation of localized cell death and disease resistance.▪
Graphical Abstract
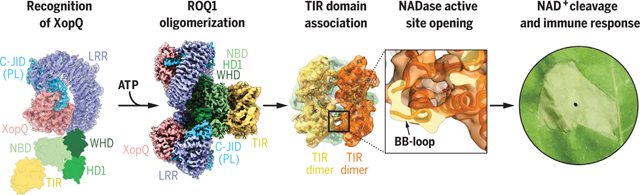
Proposed mechanism of ROQ1 activation. The LRR and C-JID of ROQ1 recognize the pathogen effector XopQ. ROQ1 oligomerizes through the NB-ARC domain (NBD, HD1, WHD) in an ATP-bound state. TIR domain association causes a conformational rearrangement of the BB-loop and opens the NADase active site. Catalytic activity of the TIR domains further signals the immune response, resulting in cell death.
Plants and animals detect pathogen infection using intracellular nucleotide-binding leucine-rich repeat receptors (NLRs) that directly or indirectly recognize pathogen effectors and activate an immune response. How effector sensing triggers NLR activation remains poorly understood. Here we describe the 3.8-angstrom-resolution cryo–electron microscopy structure of the activated ROQ1 (recognition of XopQ 1), an NLR native to Nicotiana benthamiana with a Toll-like interleukin-1 receptor (TIR) domain bound to the Xanthomonas euvesicatoria effector XopQ (Xanthomonas outer protein Q). ROQ1 directly binds to both the predicted active site and surface residues of XopQ while forming a tetrameric resistosome that brings together the TIR domains for downstream immune signaling. Our results suggest a mechanism for the direct recognition of effectors by NLRs leading to the oligomerization-dependent activation of a plant resistosome and signaling by the TIR domain.
Plants have a sophisticated and finely tuned innate immune system that recognizes invading phytopathogens to protect from infection and disease. Pathogen recognition is facilitated by both membrane-anchored pattern recognition receptors and intracellular innate immune receptors (1). The latter include the nucleotide-binding leucine-rich repeat receptors (NLRs) (2). Although some NLR immune receptors directly bind pathogen effector proteins, others, such as ZAR1, monitor effector-mediated alterations of host targets to activate effector-triggered immunity (ETI) (3–5). ETI activation is often accompanied by localized cell death referred to as the hypersensitive response (HR). Animals also use NLR proteins as intracellular immune receptors to recognize potential pathogens, and the NLR domain architecture is highly conserved, with each region playing a specific role in its mechanism of action (6). Plant NLRs generally consist of three domains: an N-terminal region that is either a coiled-coil (CC) domain or a Toll-like interleukin-1 receptor (TIR) domain, a central nucleotide-binding (NB) domain conserved in APAF-1, other R-proteins, and CED-4 (NB-ARC), and the C-terminal leucine-rich repeat (LRR) domain (2). Plant NLRs are divided into TIR-NLRs (TNLs), CC-NLRs (CNLs), and RPW8-like CC (CCR)-NLRs (RNLs) based on their N-terminal domains, with experimental evidence consistently suggesting that oligomerization of the N-terminal domains is required for signal transduction and expression of disease resistance (3).
Although the activation mechanism of a plant CNL resistosome has been elucidated (7, 8), the mechanism of TNL activation remains elusive. There is still no structural evidence for TNL resistosome formation. TIR domains of both plant and animal NLRs were reported to have a nicotinamide adenine dinucleotide (NAD+) nucleosidase activity that requires TIR domain oligomerization to trigger hypersensitive cell death (9, 10). Whether the NADase activity of the TIR domain is fully responsible for ETI activation and why NAD+ cleaving only happens in the presence of TIR self-association require further investigation.
To further our understanding of the molecular events that control the direct recognition of pathogen effectors and activation of TNL immune receptors, we transiently coexpressed Xanthomonas euvesicatoria type III effector XopQ (Xanthomonas outer protein Q) and its TNL receptor ROQ1 (recognition of XopQ1) in Nicotiana benthamiana eds1–1 mutant leaves, copurified them by sequential affinity chromatography, and solved a 3.8-Å cryo–electron microscopy (cryo-EM) structure of the assembled protein complex. XopQ is highly conserved across various Xanthomonas species and has been shown to have nucleoside hydrolase activity, to physically interact with 14-3-3 proteins of the plant host, and to suppress ETI (11). Nevertheless, the precise mechanism of XopQ that promotes pathogen virulence remains unclear. Recognition of XopQ by ROQ1 has been shown to trigger downstream ETI signal transduction, leading to a hypersensitive cell death response and resistance to pathogen invasion (11, 12). Our structural data reveal that ROQ1 directly binds XopQ to activate a tetrameric resistosome. We identified a series of necessary contacts for XopQ recognition by ROQ1 and describe the structure of a post-LRR (PL) domain that is essential in effector binding. We also describe the overall oligomeric state of ROQ1 and the interfaces formed by the NB-ARC domain in an open conformation. Finally, we provide an explanation for the requirements of oligomerization in TIR activation, which involves opening of the NADase active site in an interface-dependent manner. Together, our results provide the structural basis for direct effector recognition, oligomerization, and activation of TNLs that reveals how these immune receptors detect pathogens and signal an immune response.
Overall structure of the ROQ1 resistosome
XopQ recognition by ROQ1 triggers a rapid cell death response in wild-type N. benthamiana leaves, making it difficult to obtain sufficient protein for expression and purification (11). All plant TNLs require the downstream EDS1 protein to achieve cell death and express disease resistance (13). To obtain live tissue for protein purification, we transiently coexpressed ROQ1 and XopQ by Agrobacterium-mediated transformation in CRISPR-induced eds1–1 mutants of N. benthamiana known to prevent ROQ1-induced cell death (12). Cryo-EM imaging and analysis of the affinity-purified complex yielded a reconstruction at 3.8-Å overall resolution with C4 symmetry imposed (figs. S1 and S2) and showed that the ROQ1 protomers assemble into a tetrameric, four-leaf clover structure with XopQ present in a 1:1 ratio (Fig. 1). Further image processing was required to allow building of atomic models (fig. S3). We found that the nucleotide-binding domain (NBD), helical domain 1 (HD1), and winged helix domain (WHD) provide the necessary contacts for ROQ1 oligomerization and bring together the four TIR domains. The LRR features the characteristic horseshoe shape and wraps around the XopQ effector protein, recognizing its surface residues. The cryo-EM map also reveals a PL domain at the C-terminal end of the LRR connected by a short 10-residue linker (Figs. 1A and 2). To improve the density of ROQ1 bound to XopQ, we applied symmetry expansion and focused refinement around the LRR-PL-XopQ region (figs. S1 and S3). The improved reconstruction showed that the XopQ effector is in its open conformation, exposing the cleft of the predicted nucleoside hydrolase active site (Fig. 2E). XopQ’s specific substrate remains unidentified, but previous studies have shown that XopQ binds adenosine diphosphate ribose (ADPR), an important immune signaling molecule in plants, consistent with its immunosuppressive function (14).
Fig. 1. Overall structure of the ROQ1-XopQ complex.
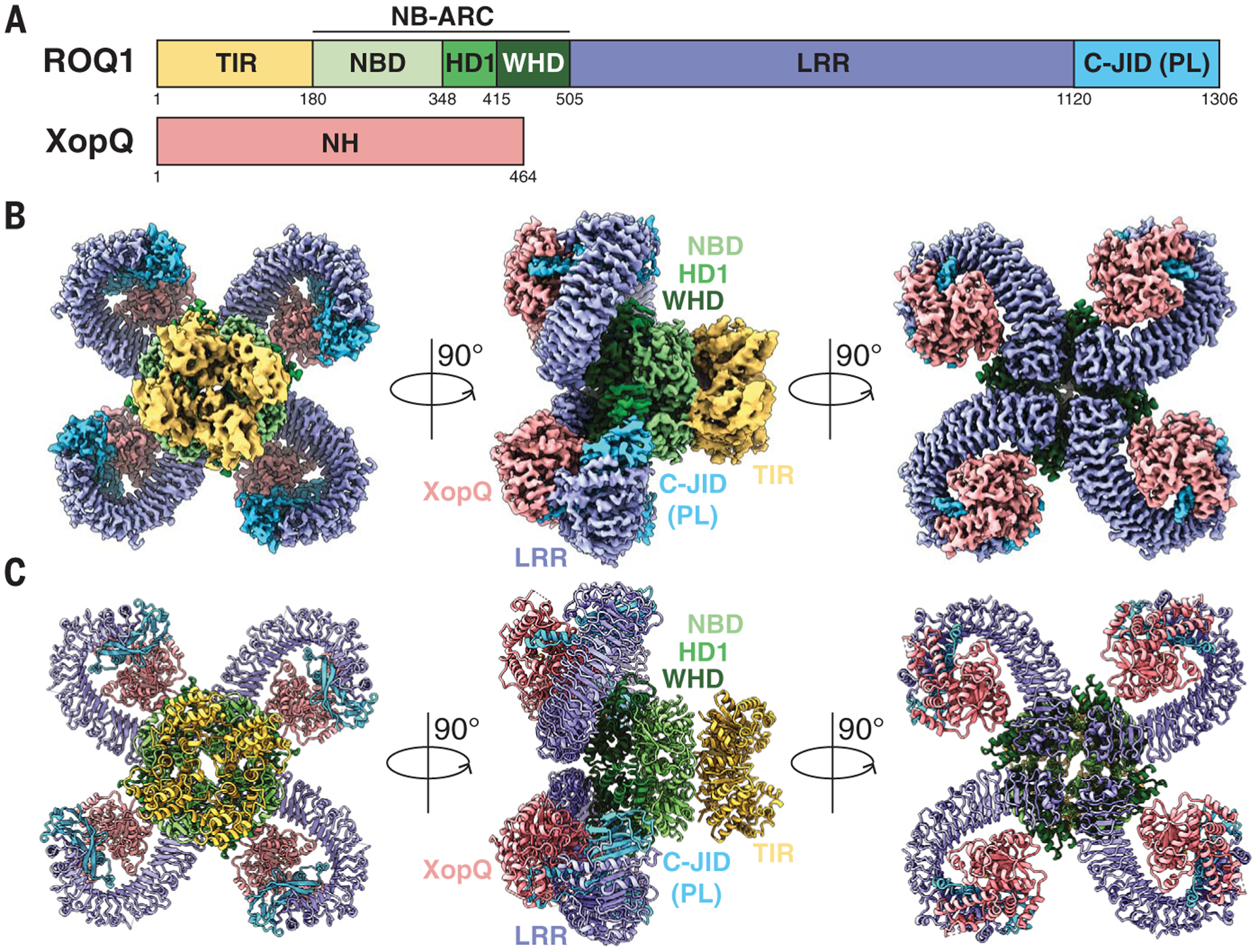
(A) Schematic representations of ROQ1 and XopQ with color-coded domain architecture: TIR, yellow; NB-ARC NDB, HD1, and WHD, light green, green, and dark green, respectively; LRR, violet; C-JID (or PL domain), light blue; and XopQ, salmon. (B and C) Composite density map of the ROQ1-XopQ complex from three cryo-EM reconstructions (B) and corresponding atomic model (C) shown in three orthogonal views. Colors are according to the nomenclature in (A).
Fig. 2. Structure of the ROQ1 LRR and C-JID (PL domain) binding to XopQ.
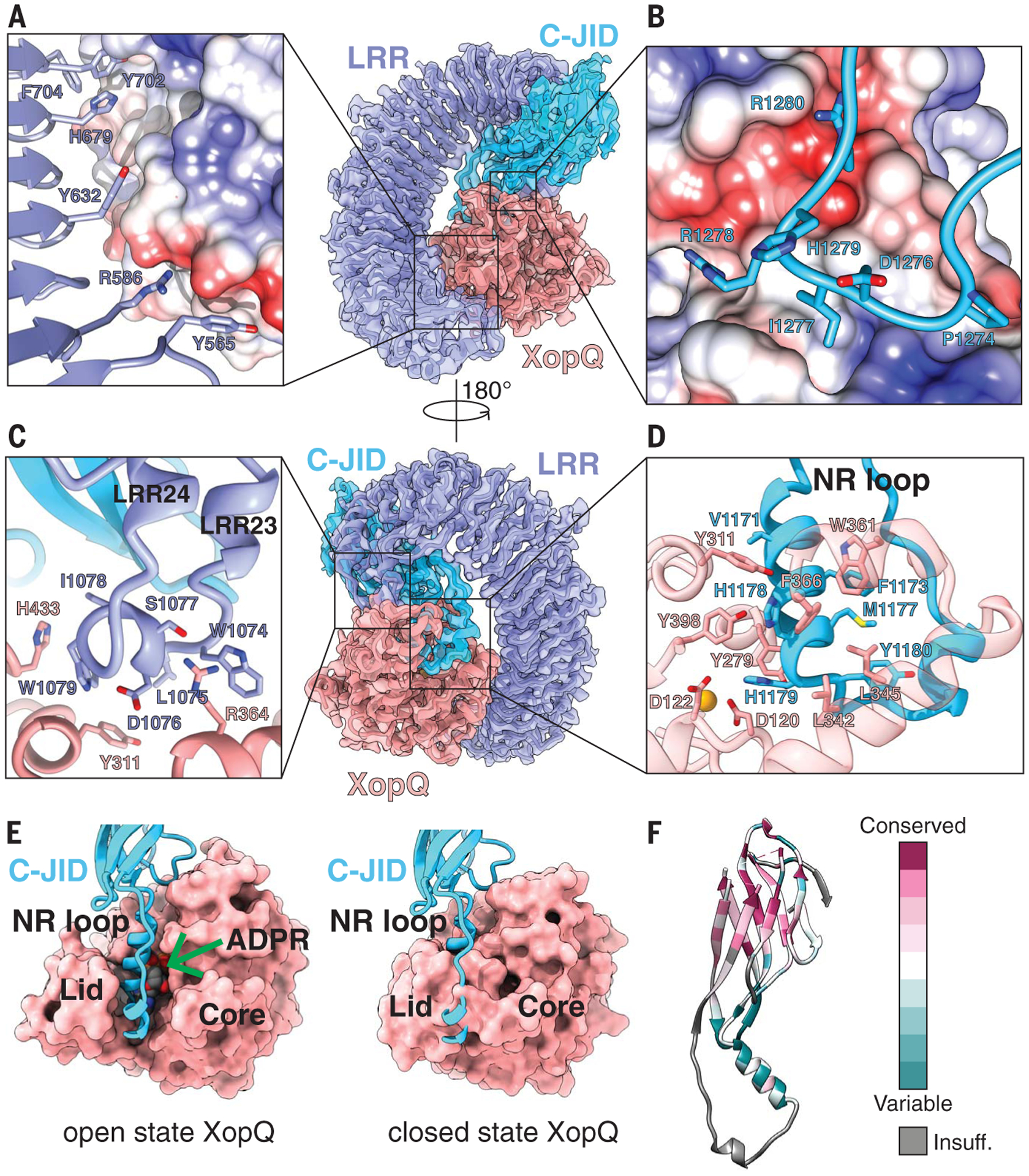
(A) Surface contacts between the N-terminal region of the LRR, shown with a violet ribbon, and XopQ, represented by its Coulombic surface potential. (B) Surface contacts made by the loop between β-strands 7 and 8 of the C-JID domain (light blue) and XopQ. (C) The elongated LRR between repeats 23 and 24 (violet) interacting with XopQ (salmon). (D) Interactions between the NR loop (light blue) and active-site residues of XopQ required for ADPR binding. Catalytic Ca2+ is shown in gold. (E) Left: Structure of XopQ in the open conformation built from our cryo-EM density, with the NR loop inserted into the active-site cleft. The position of ADPR (green arrow) from the close state of XopQ (PDB: 4P5F) is modeled to show its overlapping position with the NR loop. Right: ADPR-bound, closed state of XopQ. The NR loop is modeled to demonstrate the clashes that would occur upon XopQ closure. (F) Residue conservation of the C-JID. Regions where too few sequences aligned to calculate a reliable conservation score are colored in gray (labeled “Insuff.”).
Recognition of XopQ by ROQ1
The 24 LRRs of ROQ1 form a 150-Å-long scaffold that bends around XopQ, displaying key contact residues along its surface (Fig. 2). We found that the LRR of ROQ1 interacts with the effector in two different ways. First, in the region where XopQ is in close contact with the LRR scaffold, several side chains exposed on the surface of the LRR directly interact with the substrate (Fig. 2A). A similar mechanism is used by the LRRs of the CNL ZAR1 to recognize RKS1 and TLR3 to recognize double-stranded RNA (8, 15). Most of these residues have large aromatic side chains that recognize hydrophobic patches and grooves on the surface of XopQ. Second, in regions where the LRR scaffold is too far away to interact with the effector directly, we found an elongated linker between two LRRs (LRRs 23 and 24) that reaches over to bind XopQ (Fig. 2C). A small amphipathic a-helix is formed at the site of contact, with hydrophobic side chains recognizing conserved residues at the outer edge of XopQ’s active site cleft (Y311XopQ, H433XopQ) (Fig. 2C). The extended linker then loops back toward the scaffold and forms the next repeat in the LRR. Mutating the residues that form the hydrophobic face of the α-helix (L1075ROQ1, W1079ROQ1, I1078ROQ1) to alanines resulted in loss of the HR phenotype, suggesting that these interactions are critical for XopQ recognition (fig. S4).
A 10-residue linker (amino acids 1120 to 1129) connects the C-terminal end of the LRR to the PL domain, which also interacts with XopQ (Fig. 2, B and D). This domain folds into a β-sandwich, with nine antiparallel β-strands arranged into two β-sheets (fig. S5). The last LRR forms hydrogen bonds with one of the β-strands, thereby rigidifying the conformation between the two domains. Because PLs serving in pathogen detection have been found in other TNLs but remain poorly characterized, we sought to further investigate the possible structural homology of the ROQ1 PL domain with published structures (16). Analysis using the CATH database (17) revealed proteins with immunoglobulin-like and jelly-roll folds as the closest structural homologs (fig. S5A). Furthermore, the PL domain of RPP1, another TNL, shares a similar structure to the PL domain in ROQ1 (18) despite having low sequence identity (14.29%) (fig. S5). The core of this domain in RPP1 also folds into a β-sandwich structure that forms hydrogen bonds with the last β-strand of the LRR (18). Both share the same β-strand topology, with the exception of a ninth C-terminal β-strand present in Roq1 (fig. S5B). The major structural differences in the ROQ1 and RPP1 PL domain are found in the loops that interconnect the β-strands and serve to recognize their respective substrates. Domains fused to the C terminus of LRRs are found in many other NLRs (3, 16) and are likely to differ in structure and function. Therefore, to address the specific type of PL domain used by ROQ1 and RPP1, we refer to it as the C-terminal jelly-roll/Ig-like domain (C-JID).
The mode of recognition used by the C-JID to detect the foreign protein is reminiscent of the way immunoglobulins bind to their antigen (fig. S6). Loops emerging from the β-sandwich structure target sites in XopQ to form substrate-specific contacts with the pathogen protein in a manner that resembles the complementarity-determining regions found in antibodies. The loop between β-strands 7 and 8 of the ROQ1 C-JID simultaneously recognizes a hydrophobic pocket through the insertion of an isoleucine (I1277ROQ1) and an area of negative potential targeted by R1280ROQ1 (Fig. 2B). Disrupting these interactions with a ROQ1 double mutant (R1280D and I1277A) prevented HR in tobacco leaves, suggesting that these interactions are essential for XopQ detection by ROQ1 (fig. S4).
The greatest number of contacts between the C-JID and XopQ are made by a 33-residue loop (amino acids 1163 to 1196) that dives into the active-site cleft of the effector and positions side chains in close contact with conserved sites required for ADPR binding (Fig. 2, D and E) (14). We refer to this loop as the NR loop for its ability to bind residues in XopQ responsible for nucleoside recognition. Two α-helical segments of the loop bring together large hydrophobic side chains that interface with the interior lid region of XopQ (Fig. 2, D and E). The conserved XopQ residues targeted in this region (W361XopQ, F366XopQ, L345XopQ) serve to recognize the base moiety of ADPR (14). Active-site residues that would otherwise stabilize the α-phosphate of the ligand (Y311XopQ and Y398XopQ) are recognized by ROQ1 V1171ROQ1, H1178 ROQ1, and H1179ROQ1 (14). Additionally, H1179ROQ1 interacts with D120XopQ, which is involved in the recognition of one of the sugar moieties in ADPR (14). In summary, the conserved residues in XopQ involved in recognizing the base, α-phosphate, and ribose moieties in ADPR are targeted by the ROQ1’s NR loop. Mutating the NR loop to a short flexible linker (-SGGGSGGS-) resulted in the loss of HR, suggesting that the ROQ1 mutant could no longer recognize XopQ (fig. S4). Comparison of our structure of XopQ, which is in an open state, with the closed, ADPR-bound state (PDB: 4P5F) shows that the NR loop overlaps with ADPR and thus would prevent the ligand from entering the active-site cleft or interacting with XopQ (Fig. 2E). The presence of the NR loop may also block XopQ from transitioning to the closed state because the NR loop would clash with the lid region capping the active site. These observations led us to hypothesize that ROQ1 not only recognizes the pathogen effector but may also inhibit its mechanism of ligand binding (19).
The C-JID of ROQ1 has a conserved β-sandwich core that may be found in the NLRs of other members of the nightshade family (Fig. 2F). We ran a BLAST search using the sequence of the C-JID (amino acids 1129 to 1306) and found multiple hits corresponding to resistance genes in other species of tobacco, as well as in various species of potatoes, peppers, and morning glories. The more conserved residues are within the strands of the β-sandwich, whereas the loop residues pointing toward XopQ are more variable (Fig. 2F). The NR loop is only found in three other tobacco species, with minor sequence differences (V1171→I, Y1195→F). This pattern of conservation suggests that the variable loops emerging from the C-JID core of related NLRs could serve to recognize different pathogen effectors using a mechanism similar to that used by ROQ1. Such a strategy would be akin to that of sequence variations in the complementarity-determining regions of antibodies that enable them to recognize a diversity of epitopes.
Previous studies suggested that it was difficult to identify mutations in XopQ that could evade ROQ1 recognition (20). This is consistent with our results demonstrating that the LRR and C-JID of ROQ1 make multiple contacts with XopQ, suggesting that this gene may be durable in the field and difficult for the pathogen to evade. In the future, these contacts could be modified to build synthetic receptors targeting various pathogen effectors, resulting in new recognitional specificities.
Oligomerization of ROQ1
NLRs are generally thought to exist in an inhibited state mediated by either intra- or intermolecular contacts that prevent oligomerization between protomers and activation of the immune response (6, 21). Structural studies of inactive NLRs suggested that these inhibitory contacts hold the nucleotide-binding region (NBD, HD1, WHD) in a closed state (7, 22, 23). Upon activation, these interactions must be disrupted to transition to the oligomerization-prone state, where the WHD is moved away from the nucleotide-binding site, thereby displacing the ADP-specific MHD motif on the WHD and allowing adenosine triphosphate (ATP) or deoxyadenosine triphosphate (dATP) binding (NLRs have been shown to bind both ATP and dATP in their active state; in this study, we used ATP) (8, 24–27). We expect ROQ1 to be similarly regulated by autoinhibitory contacts with the LRR based on evidence demonstrating that a truncated version of ROQ1 missing the LRR and C-JID regions spontaneously triggers an immune response in the absence of effectors (12). We sought to determine whether the C-JID could play a role in autoinhibition. Removing the C-JID of ROQ1 (DPL) resulted in loss of HR in planta, suggesting that the LRR, not the C-JID, is involved in making the intramolecular contacts that obstruct a conformational switch to the active state (fig. S4).
Four ROQ1 protomers oligomerize through the NB-ARC domains upon substrate recognition (12). Our density map reveals the molecular contacts between the three subdomains of the NB-ARC (NBD, HD1, and WHD) in the context of the resistosome, as well as the presence of ATP at the nucleotide-binding pocket, consistent with an activated state of ROQ1 (Fig. 3). The ATP molecule is stabilized at the interface between the NBD and HD1, with the NBD recognizing the β-phosphate through the canonical P loop (K224 and T225). The two aspartates (D300 and D301) in the Walker B motif are in close proximity to a Mg2+ ion that is further coordinated by the β- and γ-phosphate of ATP as well as T225 of the P-loop (Fig. 3A) (28). Further recognition of the ligand is provided by R329 of the TTR motif (amino acids 327 to 329) that interacts with the ATP γ-phosphate and by residues forming a pocket around the base moiety (L190, I193, L356). The role of R329 in sensing the ATP molecule is highlighted by a loss of HR phenotype when it is mutated to an alanine (fig. S4).
Fig. 3. Oligomerization interfaces between NB-ARC domains.
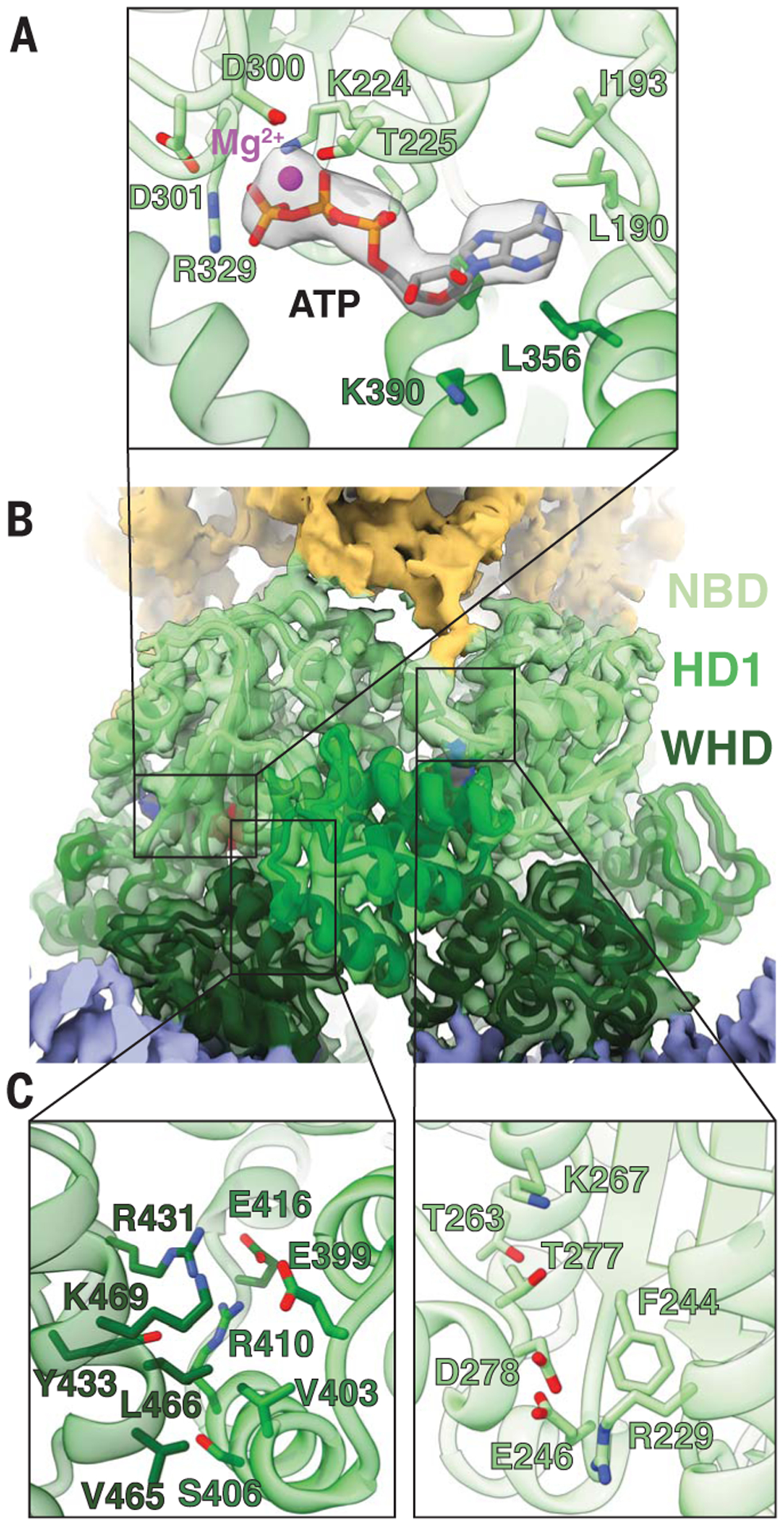
(A) ATP modeled in the cryo-EM density (4.8σ) near an oligomerization interface, showing the side chains of residues involved in ATP and Mg2+ (magenta) binding. (B) Interface between two NB-ARC domains of neighboring protomers. (C) Left: Contacts between the WHD and HD1. Right: Contacts between neighboring NBDs. Colors are according to the nomenclature in Fig. 1.
In agreement with published NLR structures in the active state (8, 24–27), the WHD of activated ROQ1 is rotated away from the nucleotide-binding site, thereby displacing the MHD motif and exposing the oligomerization interface (Fig. 3, B and C). This arrangement allows the NBD-HD1 surface of a protomer to intercalate with the NBD-WHD surface of its neighbor. The major interactions involve an HD1-WHD interface and an NBD-NBD interface (Fig. 3C). HD1 binds to the neighboring WHD using a mixture of polar and hydrophobic contacts. Residues in the fourth α-helix of HD1 (amino acids 401 to 413) play an important role in forming the ROQ1 tetramer (Fig. 3C, left). Single point mutations changing the character of these residues (E399R, V403D, and R410A) resulted in loss of HR, suggesting that ROQ1 oligomerization was disrupted (fig. S4). Similar results were observed when mutating a charged residue that brings together NBD domains (R229D) (fig. S4). In other structures of multimeric NLRs (8, 24–27), the contacts between the NBDs also involve their N-terminal linker. The equivalent linker in ROQ1 is poorly resolved in our cryo-EM map compared with the surrounding NBD, for which we observed well-defined side-chain densities, indicating that the linker region in ROQ1 is flexible in the tetrameric state. Furthermore, the same linker in other NLRs provides contacts that are in part responsible for properly positioning the NBDs relative to each other. In fact, in NLRs that form larger-order oligomers, the linker forms an α-helix, whereas in smaller complexes such as the pentameric ZAR1, the N-terminal linker forms a slim structured loop without any secondary structure, allowing for the NBD to pack more tightly (fig. S7). Similarly, the poorly defined structure in the ROQ1 linker could explain the tight packing between NBDs that results in tetramerization instead of higher oligomeric states.
Mechanisms have been proposed for the oligomerization of NLRs with differing reliance on nucleotide binding (21). In the case of ZAR1, indirect substrate recognition mediated by the guard protein RKS1 causes a conformational change in the NBD and triggers ADP release, but the individual ZAR1 protomers are still unable to oligomerize independently of ATP (7, 8). By contrast, the direct recognition of flagellin by the NLR NAIP5 induces a large conformational transition to the active state (26, 29) and has been shown to activate even when the ATP-binding P-loop motif was mutated (30). The structure of the ROQ1 NB-ARC domain closely resembles that of ZAR1 (fig. S8) and shares a 22.2% sequence similarity. Previous studies have also shown that mutation in the P-loop of ROQ1 prevented oligomerization (12), suggesting that ATP binding is required for assembly. On the basis of these observations, we expect ROQ1 to follow a similar oligomerization mechanism to ZAR1, in which substrate recognition by the LRR and C-JID of ROQ1 induces a conformational change in the NBD that releases ADP. ATP binding would then be required to transition to the oligomerization-prone state.
TIR domain oligomerization and activation
Tetramerization of the NB-ARC domains brings the TIR domains into close proximity (Fig. 4). The individual TIR domains interact with each other upon resistosome assembly, allowing them to become active NADases and trigger HR (31). The mechanism for how this association renders TIRs catalytically active remains poorly understood. Many structural studies on TNL have relied on truncated TIR-containing proteins that are missing the subunits driving oligomerization (10, 32–36). Here, we describe a mechanism for TIR association and activation in context of the fully assembled ROQ1 TNL.
Fig. 4. TIR domain interfaces and conformational rearrangement of the BB-loop.
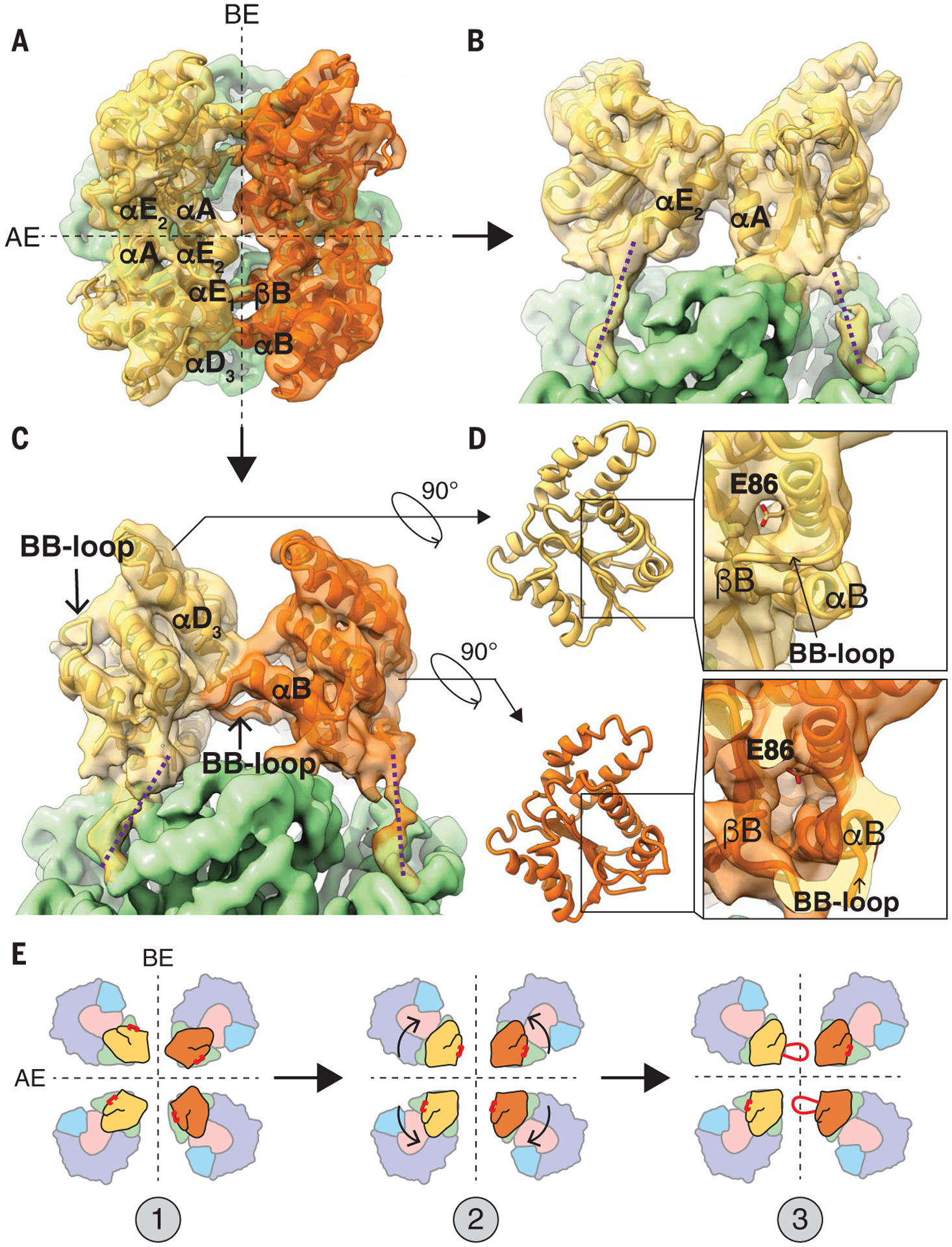
(A) Top view of ROQ1 displaying the four TIR domains organized as a dimer of dimers (each symmetric dimer shown in distinct yellow and orange). The two interfaces are marked with black dotted lines; the AE interface is formed between TIR domains shown in the same color. (B) Orthogonal view from (A) of the AE interface. (C) Orthogonal view from (A) of the BE interface marking the BB-loop positioned under the αD3 to αE1 helices. The proposed paths of the protein chain linking the TIR domain to the NBD are shown with purple dotted lines. (D) Top: NADase active site of a TIR domain for which the BB-loop is not interfacing with the DE surface. Bottom: Conformational rearrangement in the BB-loop bound to the DE surface. The side chain of the putative catalytic glutamate (E86) is shown in stick representation. (E) Hypothetical mechanism of TIR oligomerization with the position of the BB-loop in red. (1) Individual TIR domains are brought in close proximity. (2) TIR domains recognize each other at the AE and BE interface. (3) Assembly causes the conformational rearrangement in the BB-loop that opens the NADase active site. A Gaussian filter was applied to the map in (A) to (D) (width 1.5 Å) to reduce noise.
Our initial fourfold symmetric reconstruction of ROQ1-XopQ could not clearly resolve the density corresponding to the TIR domains. Further analysis (see the materials and methods) revealed that the four TIR domains do not assemble in a fourfold symmetric fashion, but rather form a twofold symmetric dimer of dimers. The change in symmetry at the TIR domains highlights the importance for flexibility in the linker that connects them to the NBDs, as discussed previously. After adjusting the symmetry for this region and performing focused refinement, the TIR domains reached an overall resolution of 4.6 Å, allowing us to visualize secondary structure elements and trace the polypeptide backbone (figs. S1 and S3). The TIR domains are arranged forming two types of interfaces. First, TIR domains engage in a head-to-head, symmetric interaction involving alpha helices the αA and αE2 of each protomer [nomenclature of TIR structural motifs as in (37), shown as interaction between the same color protomers; Fig, 4, A and B]. This interface, previously called the AE interface, is also found in many crystal structures of isolated plant TIR domains, including RPP1, RUN1, and SNC1 (fig. S9) (10, 32). Consistent with published studies on these plant TNLs, mutating residues in the αA helix of ROQ1 (H30A) disrupts HR and highlights the functional importance of these contacts (fig. S4).
In our structure of ROQ1-XopQ, TIR domains engaged in an AE-AE interaction then further dimerize head-to-tail, forming what is described as the BE interface (38) (shown as interactions between the different colored protomers; Fig. 4, A and C). In the BE interaction, the so-called BB-loop (residues between βB and αB) of one TIR domain plugs beneath the loop between αD3 and αE1 of the adjacent one (Fig. 4C). Previous mutational analyses already demonstrated a functionally important role for the BB-loop in TIR domains (10, 39). We further mutated residues in the αD3-αE1 loop (I151A and G153A) that are in close contact with the BB-loop and found that they independently resulted in loss of the HR phenotype (fig. S4).
Association between plant TIR domains at the DE surface (formed in part by the αD3-αE1 loop) has previously been observed in crystallographic studies, but their conformations are different from the ones defined by our cryo-EM analysis. For example, the TIR domains of RPP1, SNC1, and L6 face each other head-to-head at their DE surfaces with different rotational angles relative to each other instead of interacting in a head-to-tail fashion (32), perhaps because the TIR domains were visualized in isolation and the NB-ARC domain responsible for driving oligomerization was truncated. These studies highlighted the importance of the DE surface in plant TIR domain oligomerization, but the proper interactions remained unclear because of the variability in conformation between structures. The BE interaction is found in more distant phyla. The crystal structures of TIR domains from the human SARM1 (10) and MAL (40) proteins, as well as TRR-2 (unpublished, PDB: 4W8G and 4W8H) from Hydra magnipapillata share a similar BB-loop conformation to that found in the activated ROQ1 tetramer, in which it fits under the αD3-αE1 loop (fig. S10). This structural relationship suggests shared mechanistic features for TIR domain assembly and activation between animals and plants. In fact, the human SARM1 TIR domains simultaneously form AE and BE interfaces in the crystal lattice (figs. S9 and S10) (10).
Our structure now demonstrates that the BB-loop takes on two different conformations within the activated ROQ1 tetramer and undergoes a conformational switch as TIR domains interact at a BE interface. In the protomer in which the BB-loop is unbound (the one that is contributing to the BE contacts through its DE surface), it is seen in an upward position along the rim of the NADase active site (Fig. 4C). This conformation is the same as that found in the TIR crystal structures lacking BE contacts (32). In the other protomer, the BB-loop interfacing with the adjacent DE surface has been repositioned by a downward motion of ~12 Å (Fig. 4D, bottom). A highly conserved glycine residue (G52ROQ1) in the BB-loop likely provides the flexibility required to undergo this conformational switch (fig. S11). Mutating this glycine to a proline resulted in loss of HR (fig. S4). Similarly, mutating the equivalent glycine in SARM1 (G601P) was shown to hinder flexibility and prevent a transition to the engaged state, resulting in a defective BE interface with the loop stuck in the upward position and a severe decrease in NADase activity (fig. S11) (10).
Repositioning of the BB-loop induced by the BE interface opens the NADase active site (Fig. 4D). Large, positively charged side chains (of K50ROQ1, R51ROQ1, and K53ROQ1) that would otherwise crowd the entrance of the active site are moved down with the BB-loop. The structure of a SARM1 mutant (G601P), in which the BB-loop is trapped in the unengaged state, reveals a lysine inserted inside the active-site cleft; this indicates that these side chains may act to prevent substrate binding (10). Furthermore, NADase activity increased when equivalent BB-loop arginines were mutated to alanines in the plant RUN1 TIR domain (10). Together, these studies suggest that these large, positively charged side chains serve to inhibit NADase function and must be displaced for TIR activation.
Freeing the active site exposes conserved residues that have been proposed to recognize NAD+ based on biochemical and structural studies using chemical analogs (9, 10). The nicotinamide moiety of the substrate is supposed to fit in the active-site cleft of the TIR domain, bringing the covalently linked ribose in close proximity to the catalytic glutamate. Mutating the putative catalytic glutamate in ROQ1 (E86ROQ1) to an alanine abrogates HR (fig. S4), suggesting loss of NADase activity. No NAD+ was observed in our structure, which could have been cleaved by the activated TIR domain, yet we still observed a small density positioned above the TIR domain active site at the BE interface (fig. S12). An ATP molecule was modeled at this position in Ma et al. (18). The corresponding density in our map is weak compared with that of neighboring residues (W82), with only part of an ATP molecule fitting in the density even at low contour levels (fig. S12). Therefore, it was left unmodeled. Further investigation will be required to determine the position of NAD+ within the ROQ1 TIR domain active site. Mechanistic details of NAD+ cleavage and product formation remain unresolved and have been found to vary among TIR domains (31). The steps in this enzymatic reaction involve breaking the glycosidic bond that connects nicotinamide to ADPR and, in some cases, a structural rearrangement in ADPR that leads to the formation of cyclic-ADPR or variant-cyclic-ADPR (9, 41). ADPR and cyclic-ADPR have been shown to modulate the Ca2+ level in plant cells, which is a widely used chemical signal for responding to various biotic and abiotic stresses (42).
In the case of the fully assembled ROQ1 TNL, it is clear that the AE and BE interfaces are essential in TIR signaling. Both interfaces align the TIR domains in a conformation inducive to NADase active-site opening (Fig. 4E). Whether this mechanism of TIR association can be applied to other TNL remains to be determined. Similar to ROQ1, the TIR domains of the activated RPP1 tetramer form a dimer of dimers through AE- and BE-interface contacts, causing a rearrangement in the BB-loop (18). Nevertheless, there are likely to be alternative ways for TIR domains to assemble based on the number of protomers required to build the active complex, heterocomplex formation with other NLRs, and interface requirements for activation.
Summary
Our structure of the ROQ1-XopQ complex, together with previous biochemical studies, led us to propose a mechanism for TNL immune signaling: (i) both the LRR and C-JID recognize the pathogen effector, at which point the NR loop inserts itself into the active-site cleft of XopQ and targets conserved residues required for nucleoside-binding; (ii) the NB-ARC domain is released by the LRR and undergoes a conformational switch to the ATP-bound oligomerization state; (iii) ROQ1 protomers associate into a four-leaf-clover structure and the TIR domains are brought in close contact; (iv) the TIR domains bind to each other, forming distinct AE and BE interfaces and causing a conformational rearrangement in the BB-loop of two of the subunits; and (v) the NADase active site is exposed, allowing for the cleavage of NAD+. Future work will be required to identify the exact molecular species produced by the TIR domains and how they are used by the immune system of the host to trigger a response to pathogen invasion.
Methods summary
ROQ1–3Flag and StrepII-XopQ were transformed into Agrobacterium GV3101 and coexpressed in N. benthamiana eds1–1 mutant leaves through transient agroinfiltration. Leave tissue was harvested 30 hours after infiltration and ground with a mortar and pestle. The powdered leaf material was then resuspended in a buffer supplemented with protease inhibitors and further lysed by sonication. Two rounds of centrifugation were required to separate cell debris from the soluble fraction of the lysate. Purification of the assembled Roq1-XopQ complex was performed by sequential affinity chromatography. First, we selected for ROQ1–3Flag by Flag-immunoprecipitation. Strep-tactin resin was then used to capture the StrepII-XopQ containing complex. Individual steps can be visualized on a SDS–polyacrylamide gel electrophoresis gel in fig. S13.
The purified Roq1-XopQ complex was deposited on a freshly plasma cleaned holey carbon grid (QUANTIFOIL R2/2) coated with a thin layer of carbon. The original buffer used in the purification of the sample was removed and replaced with a similar buffer containing 3% trehalose instead of NP-40 and glycerol. The grid was mounted onto a Thermo Fisher Scientific Mk. IV Vitrobot, blotted, and plunged-frozen in liquid ethane.
Data were collected using a Titan Krios cryo–electron microscope operating at 300 kV and equipped with a K3 direct electron detector camera mounted behind a BioQuantum energy filter. A total of 11,134 dose-fractionated movies were acquired in superresolution counting mode, with an electron exposure of 50 e−/Å2 and defocus values ranging from −0.9 to −2.5 μm.
All processing steps were done in Relion 3.1 (43). CTF fits were calculated in Gctf (44). Particles were selected from the motion corrected micrographs (45) using an unbiased Laplacian-of-Gaussian autopicker and were subjected to an initial round of 3D classification. One more round of refinement followed by alignment-free 3D classification resulted in a class of 15,263 particles with a broad distribution of projection directions that converged to 3.8-Å resolution [Fourier shell correlation (FSC) = 0.143]. Symmetry expansion and focused refinement were required to resolve the LRR-PL-XopQ and TIR domain regions at a resolution of 3.8 and 4.6 Å, respectively. A detailed procedure is described in figs. S2 and S3.
Individual models were built for each of the three cryo-EM reconstructions. An initial model for the NB-ARC domain, TIR domain, and XopQ were generated using structures of homologous proteins in the PDB (6J5T, 5KU7, and 4KL0, respectively). The LRR and PL domain of ROQ1 were built manually in COOT (46). Models were refined and validated in Phenix (47). A final model versus map FSC was calculated using MTRIAGE (48). Model validation statistics can be found in table S1.
For HR phenotype observation, ROQ1–3Flag mutants and StrepII-XopQ were transiently coexpressed in N. tabacum roq1–1 mutant leaves through Agrobacterium-mediated transformation. HR phenotypes were observed and imaged 2 days after infiltration. To test for protein expression, ROQ1–3Flag mutants and StrepII-XopQ were coexpressed and extracted from N. benthamiana eds1–1 mutant leaves and detected by Western blotting.
Conservation scores for residues in the ROQ1 C-JID were calculated in Consurf (49) from a multiple sequence alignment provided in the supplementary materials (sequence alignment file S1).
Supplementary Material
ACKNOWLEDGMENTS
We dedicate this work to the memory of André Martin (1929-2020). We thank P. Grob and D. Toso for electron microscopy support; A. Chintangal and P. Tobias for computational support; N. Haloupek and P. Grob for advice with cryo-EM grid preparation of NLR samples; B. Greber for advice with data processing and with model building and refinement; K. Krasileva, D. Prigozhin, and K. Verster for useful discussion on PL domain conservation and LRR-effector interactions; and B. Kobe for useful discussion on TIR domains. Data were collected at the BACEM facility, Berkeley QB3. Molecular graphics and analyses were performed with UCSF Chimera and UCSF ChimeraX.
Funding:
This work was supported by the Founders Fund from the Innovative Genomics Institute of the University of California-Berkeley (to B.J.S.) and by funds from the Tsinghua-Peking Center for Life Sciences (to T.Q.). T.Q. was supported by a Tang Distinguished Scholarship from the University of California-Berkeley. E.N. is a Howard Hughes Medical Institute Investigator.
Footnotes
Competing interests: B.J.S. is the scientific cofounder and serves on the board of directors of Mendel Biotechnology and is on the scientific advisory boards of Verinomics and the Sainsbury Laboratory. R.M. and B.J.S. are inventors on a patent application held by the University of California that covers the ability to engineer new plant immune receptors.
Data and materials availability: Materials are available from B.J.S. CryoEM density maps and fitted models have been deposited in the Electron Microscopy Data Bank (EMDB) and the Protein Data Bank (PDB). The maps for initial reconstruction of the ROQ1-XopQ complex, focused refinement around the LRR-C-JID-XopQ region, and TIR domains have been deposited with the EMDB accession codes 22381, 22380, and 22383, respectively. The refined coordinate models have been deposited with PDB accession codes 7JLV, 7JLU, and 7JLX, respectively. All other data are available either in the main paper or the supplementary materials.
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Dangl JL, Horvath DM, Staskawicz BJ, Pivoting the plant immune system from dissection to deployment. Science 341, 746–751 (2013). doi: 10.1126/science.1236011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J, Chai J, Structural insights into the plant immune receptors PRRs and NLRs. Plant Physiol. 182, 1566–1581 (2020). doi: 10.1104/pp.19.01252; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamborski J, Krasileva KV, Evolution of plant NLRs: From natural history to precise modifications. Annu. Rev. Plant Biol 71, 355–378 (2020). doi: 10.1146/annurev-arplant-081519-035901; [DOI] [PubMed] [Google Scholar]
- 4.Feehan JM, Castel B, Bentham AR, Jones JDG, Plant NLRs get by with a little help from their friends. Curr. Opin. Plant Biol 56, 99–108 (2020). doi: 10.1016/j.pbi.2020.04.006; [DOI] [PubMed] [Google Scholar]
- 5.Jubic LM, Saile S, Furzer OJ, El Kasmi F, Dangl JL, Help wanted: Helper NLRs and plant immune responses. Curr. Opin. Plant Biol 50, 82–94 (2019). doi: 10.1016/j.pbi.2019.03.013; [DOI] [PubMed] [Google Scholar]
- 6.Jones JDG, Vance RE, Dangl JL, Intracellular innate immune surveillance devices in plants and animals. Science 354, aaf6395 (2016). doi: 10.1126/science.aaf6395; [DOI] [PubMed] [Google Scholar]
- 7.Wang J et al. , Ligand-triggered allosteric ADP release primes a plant NLR complex. Science 364, eaav5868 (2019). doi: 10.1126/science.aav5868; [DOI] [PubMed] [Google Scholar]
- 8.Wang J et al. , Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 364, eaav5870 (2019). doi: 10.1126/science.aav5870; [DOI] [PubMed] [Google Scholar]
- 9.Wan L et al. , TIR domains of plant immune receptors are NAD+-cleaving enzymes that promote cell death. Science 365, 799–803 (2019). doi: 10.1126/science.aax1771; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horsefield S et al. , NAD+ cleavage activity by animal and plant TIR domains in cell death pathways. Science 365, 793–799 (2019). doi: 10.1126/science.aax1911; [DOI] [PubMed] [Google Scholar]
- 11.Schultink A, Qi T, Lee A, Steinbrenner AD, Staskawicz B, Roq1 mediates recognition of the Xanthomonas and Pseudomonas effector proteins XopQ and HopQ1. Plant J 92, 787–795 (2017). doi: 10.1111/tpj.13715; [DOI] [PubMed] [Google Scholar]
- 12.Qi T et al. , NRG1 functions downstream of EDS1 to regulate TIR-NLR-mediated plant immunity in Nicotiana benthamiana. Proc. Natl. Acad. Sci. U.S.A 115, E10979–E10987 (2018). doi: 10.1073/pnas.1814856115; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lapin D, Bhandari DD, Parker JE, Origins and immunity networking functions of EDS1 family proteins. Annu. Rev. Phytopathol 58, 253–276 (2020). doi: 10.1146/annurev-phyto-010820-012840; [DOI] [PubMed] [Google Scholar]
- 14.Yu S, Hwang I, Rhee S, The crystal structure of type III effector protein XopQ from Xanthomonas oryzae complexed with adenosine diphosphate ribose. Proteins 82, 2910–2914 (2014). doi: 10.1002/prot.24656; [DOI] [PubMed] [Google Scholar]
- 15.Liu L et al. , Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science 320, 379–381 (2008). doi: 10.1126/science.1155406; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Ghelder C, Esmenjaud D, TNL genes in peach: Insights into the post-LRR domain. BMC Genomics 17, 317 (2016). doi: 10.1186/s12864-016-2635-0; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sillitoe I et al. , CATH: Expanding the horizons of structure-based functional annotations for genome sequences. Nucleic Acids Res. 47 (D1), D280–D284 (2019). doi: 10.1093/nar/gky1097; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma S et al. , Direct pathogen-induced assembly of an NLR immune receptor complex to form a holoenzyme. Science 10.1126/science.abe3069 (2020). doi: 10.1126/science.abe3069 [DOI] [PubMed] [Google Scholar]
- 19.Li W, Chiang Y-H, Coaker G, The HopQ1 effector’s nucleoside hydrolase-like domain is required for bacterial virulence in arabidopsis and tomato, but not host recognition in tobacco. PLOS ONE 8, e59684 (2013). doi: 10.1371/journal.pone.0059684; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adlung N, Bonas U, Dissecting virulence function from recognition: Cell death suppression in Nicotiana benthamiana by XopQ/HopQ1-family effectors relies on EDS1-dependent immunity. Plant J. 91, 430–442 (2017). doi: 10.1111/tpj.13578; [DOI] [PubMed] [Google Scholar]
- 21.Xiong Y, Han Z, Chai J, Resistosome and inflammasome: Platforms mediating innate immunity. Curr. Opin. Plant Biol 56, 47–55 (2020). doi: 10.1016/j.pbi.2020.03.010; [DOI] [PubMed] [Google Scholar]
- 22.Steele JFC, Hughes RK, Banfield MJ, Structural and biochemical studies of an NB-ARC domain from a plant NLR immune receptor. PLOS ONE 14, e0221226 (2019). doi: 10.1371/journal.pone.0221226; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Z et al. , Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science 341, 172–175 (2013). doi: 10.1126/science.1236381; [DOI] [PubMed] [Google Scholar]
- 24.Pang Y et al. , Structure of the apoptosome: Mechanistic insights into activation of an initiator caspase from Drosophila. Genes Dev. 29, 277–287 (2015). doi: 10.1101/gad.255877.114; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi S et al. , Crystal structure of the Caenorhabditis elegans apoptosome reveals an octameric assembly of CED-4. Cell 141, 446–457 (2010). doi: 10.1016/j.cell.2010.03.017; [DOI] [PubMed] [Google Scholar]
- 26.Tenthorey JL et al. , The structural basis of flagellin detection by NAIP5: A strategy to limit pathogen immune evasion. Science 358, 888–893 (2017). doi: 10.1126/science.aao1140; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L et al. , Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science 350, 404–409 (2015). doi: 10.1126/science.aac5789; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonardi V, Cherkis K, Nishimura MT, Dangl JL, A new eye on NLR proteins: Focused on clarity or diffused by complexity? Curr. Opin. Immunol 24, 41–50 (2012). doi: 10.1016/j.coi.2011.12.006; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X et al. , Structural basis for specific flagellin recognition by the NLR protein NAIP5. Cell Res. 28, 35–47 (2018). doi: 10.1038/cr.2017.148; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halff EF et al. , Formation and structure of a NAIP5-NLRC4 inflammasome induced by direct interactions with conserved N- and C-terminal regions of flagellin. J. Biol. Chem 287, 38460–38472 (2012). doi: 10.1074/jbc.M112.393512; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayless AM, Nishimura MT, Enzymatic functions for Toll/interleukin-1 receptor domain proteins in the plant immune system. Front. Genet 11, 539 (2020). doi: 10.3389/fgene.2020.00539; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X et al. , Multiple functional self-association interfaces in plant TIR domains. Proc. Natl. Acad. Sci. U.S.A 114, E2046–E2052 (2017). doi: 10.1073/pnas.1621248114; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams SJ et al. , Structural basis for assembly and function of a heterodimeric plant immune receptor. Science 344, 299–303 (2014). doi: 10.1126/science.1247357; [DOI] [PubMed] [Google Scholar]
- 34.Hyun K-G, Lee Y, Yoon J, Yi H, Song J-J, Crystal structure of Arabidopsis thaliana SNC1 TIR domain. Biochem. Biophys. Res. Commun 481, 146–152 (2016). doi: 10.1016/j.bbrc.2016.11.004; [DOI] [PubMed] [Google Scholar]
- 35.Bernoux M et al. , Structural and functional analysis of a plant resistance protein TIR domain reveals interfaces for self-association, signaling, and autoregulation. Cell Host Microbe 9, 200–211 (2011). doi: 10.1016/j.chom.2011.02.009; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams SJ et al. , Structure and function of the TIR domain from the grape NLR protein RPV1. Front. Plant Sci 7, 1850 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y et al. , Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature 408, 111–115 (2000). doi: 10.1038/35040600; [DOI] [PubMed] [Google Scholar]
- 38.Nimma S, Ve T, Williams SJ, Kobe B, Towards the structure of the TIR-domain signalosome. Curr. Opin. Struct. Biol 43, 122–130 (2017). doi: 10.1016/j.sbi.2016.12.014; [DOI] [PubMed] [Google Scholar]
- 39.Vyncke L et al. , Reconstructing the TIR side of the myddosome: A paradigm for TIR-TIR interactions. Structure 24, 437–447 (2016). doi: 10.1016/j.str.2015.12.018; [DOI] [PubMed] [Google Scholar]
- 40.Ve T et al. , Structural basis of TIR-domain-assembly formation in MAL- and MyD88-dependent TLR4 signaling. Nat. Struct. Mol. Biol 24, 743–751 (2017). doi: 10.1038/nsmb.3444; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Essuman K et al. , TIR domain proteins are an ancient family of NAD+-consuming enzymes. Curr. Biol 28, 421–430.e4 (2018). doi: 10.1016/j.cub.2017.12.024; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Y et al. , Abscisic acid signaling through cyclic ADP-ribose in plants. Science 278, 2126–2130 (1997). doi: 10.1126/science.278.5346.2126; [DOI] [PubMed] [Google Scholar]
- 43.Scheres SHW, RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol 180, 519–530 (2012). doi: 10.1016/j.jsb.2012.09.006; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang K, Gctf: Real-time CTF determination and correction. J. Struct. Biol 193, 1–12 (2016). doi: 10.1016/j.jsb.2015.11.003; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng SQ et al. , MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017). doi: 10.1038/nmeth.4193; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emsley P, Cowtan K, Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr 60, 2126–2132 (2004). doi: 10.1107/S0907444904019158; [DOI] [PubMed] [Google Scholar]
- 47.Adams PD et al. , PHENIX: Building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr 58, 1948–1954 (2002). doi: 10.1107/S0907444902016657; [DOI] [PubMed] [Google Scholar]
- 48.Afonine PV et al. , New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Crystallogr. D Struct. Biol 74, 814–840 (2018). doi: 10.1107/S2059798318009324; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashkenazy H et al. , ConSurf 2016: An improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44 (W1), W344–W350 (2016). doi: 10.1093/nar/gkw408; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


