Abstract
Self-medication is the most common practice worldwide and it may lead to irrational use of drugs. Therefore, this study aimed to assess the prevalence of self-medication practice and its associated factors among health science students. A cross-sectional study was conducted on 600 health science students in Gondar town. The data regarding self-medication practice and its associated factors were collected using a face-to-face interview on a structured questionnaire. SPSS −24 was used for data analysis and explained with univariate, and multivariate logistic regression analysis to determine the factors associated with self-medication practice (sex, age, religion, marital status, residence, department, year of study, monthly income, access to pharmacy, and peer/family pressure). A total of 554 students responded to the questionnaire with a response rate of 92.3%. Out of 554 respondents, 78.2% were practiced self-medication. Headache/fever 37.88% (n = 164) was reported as the most common complaint to practice self-medication. Among the reasons for self-medication practice, similarity of symptoms with past illness 33.49% (n = 145) was the most frequently reported. In current study, Females (AOR = 3.11, 95% CI = 1.55, 6.25), Muslim followers (AOR = 2.78, 95% CI = 1.30, 5.91), Protestant followers (AOR = 4.25, 95% CI = 1.38, 13.07), pharmacy students (AOR = 3.72, 95% CI = 1.97, 9.30), clinical nursing students (AOR = 2.88, 95% CI = 1.87, 14.48), monthly income (>500ETB) (AOR = 2.49, 95% CI = 1.12, 5.56), distance of health institution (<30 min) (AOR = 2.79, 95% CI = 1.39, 5.61), and accessibility of pharmacy (AOR = 4.85, 95% CI = 2.08, 11.29) were the independent predictors of self-medication practice. Self-medication is common in health science students in Gondar town. Health professionals should educate students on the risks and benefits of self-medication to encourage responsible self-medication. National guidelines on medicine access should be developed and strong measures should be implemented to halt the selling of medications without a proper prescription.
Keywords: self-medication, practice, health sciences
What do we already know about this topic?
Self-medication with over the counter medications is a wide-reaching public health problem.
How does your research contribute to the field?
The findings of the study will improve the students’ awareness concerning the dangerous effects of self-medication practice. The findings will also recommend the government to develop national guidelines on medicine access and strong measures should be implemented to halt the selling of medications without a proper prescription.
What are your research’s implications towards theory, practice, or policy?
The findings of the study will guide the government or policymaker to implement strategies to increase the level of awareness among the students and to facilitate responsible use of self-medications.
Introduction
Self-medication practice (SMP) is the use of medications to treat self-recognized disease; it can also be defined as recurrent or continuous use of prescribed medications for the treatment of chronic or recurrent disease; besides, it also comprises the use of the medication of family members.1 Self-medication with over the counter medications is a wide-reaching and badly-behaved practice and is more common in economically poor countries.2,3
Self-medication practice has shown a discrepancy among different residents and it is affected by numerous factors such as gender, self-care orientation, age, educational level, expenditure, medical knowledge, income, and illnesses.4,5 Self-medication practice is a worldwide health problem with serious public health implications.6 Barriers to healthcare access, lack of time, difficulties in securing a medical consultation due to administrative delays, and economic factors were the reasons to practice self-medication.5 Thus, patients having mild symptoms don’t need to get a health professional (doctor), instead, they preferred to meet the pharmacist for advice and medication for their diseases.7 Several studies conducted on self-medication practice revealed that SMP is a common practice, particularly in economically poor populations.8
Globally, the prevalence of self-medication practice is varied from 32.5% to 81.5%.9-11 A study conducted in Ethiopia showed that the prevalence of SMP ranges from 12.8% to 77.1%.12 The prevalence of self-medication practice among college students is high and previous studies have reported the prevalence of SMP as 94% in Hong Kong,13 76% in Pakistan,14 87% in India,15 86.4% in Brazil,16 98% in Palestine,17 55% in Egypt,18 and 43.2% in Ethiopia.19 Based on such findings, participant’s educational level, low income, job, absence of health insurance, and SMP experience,20,21 sex,22 residence,23 age,24 and men,25,26 were found to be the factors associated with self-medication practice.
To the best of our knowledge and the literature reviewed, there is little empirical evidence in Ethiopia, particularly in the study setting where there is no study conducted on private health science students. Previous studies didn’t assess the prevalence and associated factors of SMP, which is the area to be addressed in the present study. Previous studies didn’t also concurrently address reasons of SMP, the source of the drugs, factors affecting SMP, sources of information, and perceived disease conditions that lead to SMP. Hence, within its global burden and impact, knowing its magnitude and associated factors at private health science college is crucial and has an essential role in developing appropriate regulatory, administrative, and educational measures. Progressive increment and high prevalence of self-medication among health professionals is also a major problem for Ethiopia.27 The findings of the study will recommend the government or policymaker to implement strategies to increase the level of awareness among the students and to facilitate responsible use of self-medications in Ethiopia. Despite the bad concerns of SMP and its high spread, studies on its prevalence and associated factors in this study area are limited. Thus, this study aimed to assess the prevalence of self-medication practice and associated factors among medical students in Gondar town, North West Ethiopia.
Methods
Study Design and Period
A cross-sectional study design was used to conduct this study. The study period was from February 3 to April 5, 2020, in Gondar town, Northwest Ethiopia.
Study Area
Gondar town is located 750 kilometers away from Addis Ababa, the capital city of Ethiopia. It is one of the ancient and densely populated towns in Ethiopia. Now a day, the town has 1 specialized referral hospital, 2 private general hospitals, 5 health centers, twenty-four pharmacies, 4 drug stores, 3 private health colleges, and 1 university.
Study Population
A cross-sectional study was conducted in Abyssinia, Blue Nile, and GT Health Science College, Ethiopia, and included first, second, and third-year Health Science Students (Laboratory, Clinical Nursing, and Pharmacy) through the academic year 2019/2020.
Sample Size Determination
Single population proportion formula was used with the assumption of 95% CI, 5% margin of error, the prevalence (p) of self-medication practice in Ethiopia (50%),12 and 5% for possible non-response to determine a final adjusted sample size of 372. The number of students to be included in each Health Science College was calculated based on proportion with the total number of students found in each Health Science College. Then, a systematic random sampling method was used to recruit the final interviewed students. However, to increase the power of the study, the sample size was extended to 600.
Data Collection Tool and Procedure
To assess the prevalence of SMP and its associated factors, The first part of the questionnaire consisted of the socio-demographic characteristics of participants such as gender, parent’s education, year of study, department, and average monthly allowance. The second part consisted of questions related to the frequency, duration, and practices of SMP and the source of medication. The third section included the reasons for self-medication practices and an indication of drugs for SMP. Three trained health professionals were recruited and supervised by three MSc graduate health professionals. The Completeness and fulfillment of all questions were checked by the principal investigator and data collectors. The data were collected using a face-to-face interview on a structured questionnaire. The questionnaire was developed after a detailed review of the literature.6,24,28-30 Initially, the questionnaire is prepared in English and translated into the local language (Amharic) then back to the English language to ensure consistency. A pre-test was done 2 weeks before the actual data collection on 28 participants who were not included in the final analysis. Finally, completed questionnaires were collected.
Operational Definition
In the current study, self-medication practice was defined as any use of medications without the prescriptions of health care professionals (health officers, physicians, nurses, and others who have a government license to prescribe medications) for the management of self-recognized diseases.24,31
Data Processing and Analysis
Data were checked for completeness and consistency before entry. Coded data were entered into Statistical Package for the Social Sciences (SPSS) software version 24 for analysis. Descriptive statistics like, frequency, percentage, mean, standard deviation, and median were used for data presentation. Univariate and multivariate logistic regression was applied to identify associated factors with a 95% confidence interval using a P-value <.05 as a cutoff point.
Results
Socio-Demographic Characteristics of Respondents
In the current study, a total of 600 questionnaires were disseminated to evaluate the SMP in which 554 were finalized and returned making a response rate of 92.3%. The mean age of the participants was 21.5 years (standard deviation ±3.7). More than half 57.2% (n = 317) of the respondents were female of whom 19% (n = 105) were married. The majority of the respondents 69.7% (386), were Orthodox Christian followers and the majority of the participants were permanent residents of urban areas 61.9% (n = 343). In terms of their study year distribution, the most reported department was Clinical nursing 38.1% (n = 211) followed by Pharmacy 34.7% (n = 192 and Laboratory 27.3% (n = 151). Among respondents, 42.2% (n = 234) was in the first year of study followed by second year of study 34.1% (n = 189), and third year of study 23.6%(n = 131) (Table 1).
Table 1.
Socio-Demographic Characteristics of Respondents (n = 554).
| Variables | Category | Frequency | Percent |
|---|---|---|---|
| Sex | Male | 237 | 42.8 |
| Female | 317 | 57.2 | |
| Age in year | 18-21 | 186 | 33.6 |
| 21-25 | 303 | 54.7 | |
| ≥26 | 65 | 11.7 | |
| Religion | Orthodox | 386 | 69.7 |
| Muslim | 111 | 20.0 | |
| Protestant | 57 | 10.3 | |
| Marital status | Unmarried | 431 | 77.8 |
| Married | 105 | 19.0 | |
| Divorced | 14 | 2.5 | |
| Widowed | 4 | 0.7 | |
| Residence | Rural | 211 | 38.1 |
| Urban | 343 | 61.9 | |
| Department | Pharmacy | 192 | 34.7 |
| Clinical nursing | 211 | 38.1 | |
| Laboratory | 151 | 27.3 | |
| Year of study | 1st year | 234 | 42.2 |
| 2nd year | 189 | 34.1 | |
| 3rd year | 131 | 23.6 | |
| Monthly income of participants’ | <200 ETB | 124 | 22.4 |
| 200-500 ETB | 191 | 34.5 | |
| >500 ETB | 239 | 43.1 | |
| Parents’ educational level | Illiterate | 186 | 33.6 |
| Non-formal education | 96 | 17.3 | |
| Grade 1-8 | 63 | 11.4 | |
| Grade 9-12 | 58 | 10.5 | |
| Diploma and above | 151 | 27.3 |
Prevalence, Source of Drug, and Indication of Self-Medication Practice
In this study, the prevalence of SMP was 78.2% (n = 433). Among the respondents who practice self-medication, 40.6% (n = 176) were practice more than 4 times, 26.6% (n = 115) were practice 3 times, 20.6% (n = 89) were practice once, and 12.2% (n = 53) were practice twice. Among the respondents who practice self-medication (n = 433), 28.2% (n = 122) of the participants were faced adverse drug reactions. Among self-medication users, 69.7% (n = 302) were got medication from the pharmacy, 17.8% (n = 77) were got from family, 8.8% (n = 38) were got from friends, and 3.7% (n = 16) were got the medications from other sources. About 58.4% (n = 253) of the participants were reported that the cost of the drug was cheap. However, 15.0% (n = 65) of the respondents were reported that the cost of the drug was expensive (Table 2).
Table 2.
Prevalence and Source of the Drug for Self-Medication Practice among Students (n = 433).
| Statement | Answer | Frequency | Percent |
|---|---|---|---|
| Do you have ever practice self-medication in your lifetime? (n = 554) | Yes | 433 | 78.2 |
| No | 121 | 21.8 | |
| If your answer is yes to the above question, how many times practiced in your lifetime? (n = 433) | Once | 89 | 20.6 |
| Twice | 53 | 12.2 | |
| Three times | 115 | 26.6 | |
| ≥4 times | 176 | 40.6 | |
| Duration of self-medication? (n = 433) | For 1 day | 113 | 26.1 |
| For 2 days | 86 | 19.9 | |
| For 3 days | 68 | 15.7 | |
| Four days | 129 | 29.8 | |
| ≥ 5 days | 37 | 8.5 | |
| Do you remember the name of the drug? (n = 433) | Yes | 340 | 78.5 |
| No | 93 | 21.5 | |
| Do you always read the instruction? (n = 433) | Yes | 321 | 74.1 |
| No | 112 | 25.9 | |
| Do you have ever faced any adverse drug reactions following self-medication? (n = 443) | Yes | 122 | 28.2 |
| No | 311 | 71.8 | |
| Where did you get that medication? (n = 443) | Pharmacy | 302 | 69.7 |
| Friends | 38 | 8.8 | |
| Family | 77 | 17.8 | |
| Others (specify) | 16 | 3.7 | |
| Cost of the drug? (n = 433) | Cheap | 253 | 58.4 |
| Expensive | 65 | 15.0 | |
| Not known | 115 | 26.6 |
Symptoms’ of Illnesses for Self-Medication Practice
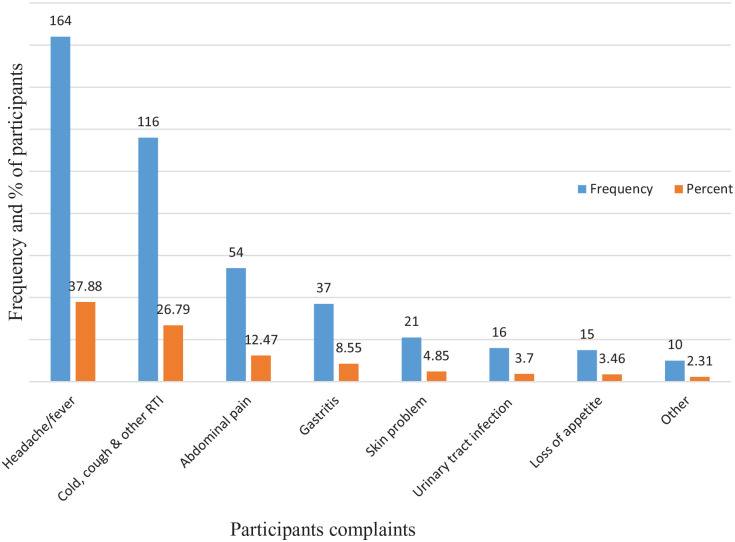
From the ailments reported by respondents, Headache/fever 37.88% (164), Common cold, cough, and other respiratory systems 26.79% (116), Abdominal pain 12.47% (54), Gastritis 8.55% (37), Skin problem 4.85% (n = 21), Urinary tract infection 3.7% (n = 16), and Loss of appetite 3.46% (n = 15) were the most reported illness for practicing self-medication (Figure 1).
Figure 1.
Indication of symptoms’ of illnesses for self-medication practice (n = 433).
Reasons of Participants for Self-Medication Practice
Perceptions of similarity of symptoms with past illness 33.49% (n = 145), illness as mild 19.17% (n = 83), self-medication is cheaper 18.24% (79), need quick relive 11.55% (n = 50), friends’ suggestion 7.16% (n = 31), and long waiting time in health service 5.77% (n = 25) were the main motives for SMP (Table 3).
Table 3.
Reasons for Self-Medication Practice Among Students (n = 433).
| Reasons | Frequency | Percent |
|---|---|---|
| The similarity of symptoms with past illness | 145 | 33.49 |
| Perceive illness as mild | 83 | 19.17 |
| Self-medication is cheaper | 79 | 18.24 |
| Need quick relive | 50 | 11.55 |
| Friends’ suggestion | 31 | 7.16 |
| Long waiting time in health service | 25 | 5.77 |
| Others | 20 | 4.62 |
Factors Associated with Self-Medication Practice
According to the multivariate logistic regression analysis, gender, religion, marital status, department, monthly income, parents’ educational level, the distance of health institution, and accessibility of pharmacy were factors associated with SMP. The current finding showed that females were 3.1 times (AOR = 3.11, 95% CI = 1.55, 6.25) more likely to practice SM than males. Respondents who are Muslim followers were nearly 3 times (AOR = 2.78, 95% CI = 1.30, 5.91) more likely to practice SM than Orthodox Christian. Likewise, Protestant followers were 4× (AOR = 4.25, 95% CI = 1.38, 13.07) more likely to practice self-medication than Orthodox Christian. Based on the department in which they enroll, Pharmacy students were nearly 4× (AOR = 3.72, 95% CI = 1.97, 9.30) more likely to practice SM as compared to laboratory students. Similarly, Clinical nursing students were nearly 3× (AOR = 2.88, 95% CI = 1.87, 14.48) more likely to practice SM as compared to Laboratory students. Based on a monthly allowance, the participants were grouped into 3, and those monthly income (>500ETB) were 2 times (AOR = 2.49, 95% CI = 1.12, 5.56) more likely to practice SM than those monthly incomes were less than 200. Accessibility of pharmacy was nearly 5 times (AOR = 4.85, 95% CI = 2.08, 11.29) more likely to experience self-medication practice compared to that inaccessibility (Table 4).
Table 4.
Univariate and Multivariate Logistic Regression Analysis of Self-Medication Practice among Students (n = 554).
| Variables | Category | Self-medication practice | COR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Sex | Male | 176 | 61 | 1 | 1 |
| Female | 243 | 74 | 1.14 (1.03-2.68)* | 3.11 (1.55-6.25)** | |
| Age in year | 18-21 | 144 | 42 | 1 | 1 |
| 21-25 | 235 | 68 | 1.05 (0.69-1.60) | 1.13 (0.58-2.20) | |
| ≥26 | 54 | 11 | 1.23 (0.63-2.43) | 1.30 (0.54-3.12) | |
| Religion | Orthodox | 292 | 94 | 1 | 1 |
| Muslim | 89 | 22 | 1.34 (0.81-2.22) | 2.78 (1.30-5.91)** | |
| Protestant | 52 | 5 | 2.63 (1.16-5.98)* | 4.25 (1.38-13.07)** | |
| Marital status | Unmarried | 338 | 93 | 1 | 1 |
| Married | 68 | 37 | 0.51 (0.32-0.80)* | 0.10 (0.05-0.23)** | |
| Divorced | 10 | 4 | 0.69 (0.21-2.24) | 0.18 (0.04-0.79)** | |
| Widowed | 3 | 1 | 0.83 (0.09-8.03) | 0.22 (0.01-3.49) | |
| Residence | Urban | 279 | 64 | 1.74 (1.17-2.57)* | 1.25 (0.72-2.15) |
| Rural | 154 | 57 | 1 | 1 | |
| Department | Pharmacy | 157 | 35 | 1.72 (1.06-2.79)* | 3.72 (1.97-9.30)** |
| Clinical nursing | 171 | 40 | 1.67 (1.04-2.68)* | 2.88 (1.87-14.48)** | |
| Laboratory | 105 | 46 | 1 | 1 | |
| Year of study | 1st year | 189 | 45 | 1.58 (1.04-2.82)* | 1.02 (0.33-3.15) |
| 2nd year | 151 | 38 | 1.45 (0.88-2.39) | 0.30 (0.04-2.52) | |
| 3rd year | 93 | 38 | 1 | 1 | |
| Monthly income of participants’ | <200 ETB | 75 | 49 | 1 | 1 |
| 200-500 ETB | 164 | 45 | 2.41 (1.49-3.87)* | 2.12 (0.98-4.55) | |
| >500 ETB | 194 | 27 | 2.91 (1.74-4.86)* | 2.49 (1.12-5.56)** | |
| Parents’ educational level | Illiterate | 164 | 22 | 2.01 (1.11-3.62)* | 1.14 (0.54-2.37) |
| Non formal education | 42 | 54 | 0.20 (0.11-0.35) | 0.04 (0.02-0.11)** | |
| Grade 1-8 | 48 | 15 | 0.86 (0.43-1.73) | 0.37 (0.14-1.02) | |
| Grade 9-12 | 49 | 9 | 1.15 (0.54-2.46) | 0.41 (0.15-1.09) | |
| Diploma and above | 121 | 30 | 1 | 1 | |
| Distance of health institution | <30 min | 179 | 22 | 2.54 (1.58-4.06) ** | 2.79 (1.39-5.61)** |
| 30 min-1 h | 88 | 28 | 1.39 (0.84-2.29) | 2.05 (1.03-4.10)** | |
| >1 h | 166 | 71 | 1 | 1 | |
| Access to pharmacy | Yes | 142 | 30 | 1.79 (1.14-2.82)* | 4.85 (2.08-11.29)** |
| No | 277 | 105 | 1 | 1 | |
| Health professional in their family | Yes | 242 | 74 | 0.85 (0.57-1.26) | 0.73 (0.40-1.32) |
| No | 191 | 47 | 1 | 1 | |
| Peer/family pressure | Yes | 245 | 70 | 0.95 (0.64-1.41) | 1.00 (0.58-1.72) |
| No | 188 | 51 | 1 | 1 | |
Variables those were significant during univariate logistic analysis at P-value ≤.05.
Variables that were found to have significant association during multivariate analysis at P-value ≤.05.
Discussion
Several studies have shown that SMP is commonly practiced worldwide.4,32,33 The prevalence of SMP has persisted in both developing and developed countries,34,35 and the SMP is high among adolescences,36 and more common among college students.37 Economic status, informal accessibility of medications, long clinical process, widespread advertisement, medical cost, lifestyle, inaccessibility of healthcare, and past experiences are the common motives for the general public for seeking SMP.17,38,39
In the present study, the prevalence of SMP was 79.2%. Comparable findings were reported in different countries, 88.4% in India,40 81.9% in Nepal,41 62.9% in Egypt,29 64.98% in Wollo,42 72% in Iran,43 79.9% in Serbia,44 91.4% in South-western Nigeria,45 64.5% in Ethiopia,46 and 81.8% in Sudan.47 However, lower prevalence was reported in previous studies, 33.7% in Iran,48 36.7% in Nekemte,49 35.9% in South India,50 23.3% in Bahir Dar,51 27.16% in Sire town,52 24.40% in Silte zone,53 and 16% in Brazil.22 A study conducted in Ethiopia,4 was among health science students. However, in Egypt,54 and Jordan,55 the prevalence and associated factors of SMP were conducted among both health science and para health science students. The prevalence of SMP was high among health science students, this may be because of having better knowledge and education in health sciences students and it is also linked with exposure to clinical attachment. Furthermore, the difference in SMP among various studies may be due to differences in socioeconomic gaps, sample size, setting, sampling method, and law enforcement.
In the current study, the predominant diseases that led to SM were Headache/fever (37.88%), followed by Common cold and other respiratory systems (26.79%), and Abdominal pain (12.47%). These findings are consistent with previous studies conducted in Jordan,56 Nigeria,56 India,47 Ethiopia,6,46 Egypt,57 and Saudi Arabia.58 In contrast, it isn’t in line with previous studies in India and Ethiopia, that they practice SM for Diabetes mellitus, Malaria, and Epilepsy.59,60 The predominant reasons to practice self-medication were similarity of symptoms with past illness (33.49%), followed by illness as mild (19.17%), and self-medication is cheaper (18.24%). This finding is similar to studies conducted in Sire town,52 Jimma town,25 Iran,61 Meket district,6 Jigjiga,60 Eritrea,32 Mekelle University.36
In this study, 28.2% of the participants were reported adverse drug reactions following SMP which is in agreement with previous studies conducted in India (29.77%).62 Though the current result was higher than the previous studies that quantified 5% in India,63 and 9.2% in Eritrea,32 of the respondents were experienced adverse drug reactions.
Among self-medication user, 69.7% of them got medication from the pharmacy, 17.8% were got from family, 8.8% were got from friends, and 3.7% were got the medications from other sources. Several previous studies revealed that pharmacies and drug stores are common sources of medication. Besides, friends, relatives, and previous prescriptions are represented for commonly stated sources.4,29,45,48 Informal accessibility of all medications from drug stores and pharmacies might be linked to week legislation concerning access to drugs in Ethiopia. The absence of strong legislation in the country might be contributing to the increase in the number of respondents who could practice SMP. Therefore, such practices may increase the patterns of drug resistance, irrational drug use, and harm to human life.
In the present study, females were 3 times more likely to practice self-medication than males. A study conducted in Nigeria,46 Uganda,64 and Iran,65 revealed that SMP was higher in females than in males. Similarly, in a study done in Serbia, female participants practiced SM 1.4 times more likely than male participants.66 However, findings revealed an insignificant difference in SMP between females and males.4,33,46 This gender difference in SMP could be explained by the different health conditions for instance menstruation in the case of women might be the common reason for practicing self-medication more than males.
Based on their monthly income, the participants were grouped into 3, and those monthly income (>500ETB) were 2 times more likely to practice SM than those monthly incomes were less than 200. This finding is consistent with a study conducted in Eritrea and Northwest Ethiopia.31,32,54 Students with low monthly income have more chances of obtaining drugs from pharmacy/drug store without getting legal prescriptions from health professionals.
A study conducted at Mekele University reported that there was a statically significant association among study departments, pharmacy students are more likely to practice SM than other health science and para-health students.36 Based on the department in which they enroll, pharmacy students were almost 4 times more likely to practice SM when compared with laboratory students. Similarly, clinical nursing students were nearly 3 times more likely to practice SM when compared with laboratory students. This may due to the nature of the curriculum in the department, as the students of the Laboratory spend most of their practical sessions in more of a laboratory setting whereas the others were directly involved with patient care which could have increased both the accessibility and knowledge of medications.
The odds ratio of self-medication practice among respondents whose distance of health institution (<30 min) were nearly 3 times and distance of health institution (30 min-1 h) were 2 times more likely to practice self-medication as compared to the distance of health institution were greater than 1 h. Accessibility of pharmacy was nearly 5 times more likely experienced self-medication practice compared to that inaccessibility. This may be due to respondents who are near to health institutions (pharmacy) easily access the medications. However, the current finding isn’t in agreement with the previous study done at Meket district.6
Respondents who had access to pharmacies were nearly 5 times more likely to utilize SM when compared with the respondents who had not. This finding is in agreement with previous studies conducted in India,50 Nigeria,56 and China.67 This could be because of a lack of time to consult health professionals in the health facilities and lack of money. Thus, the current finding indicates that the unaffordability of the health care fees by the respondents is noted as the motive for SMP.
Conclusion
A high prevalence of self-medication practice was shown among Health Science College students. In the current study, factors like gender, religion, department, monthly income, the distance of health institution, and accessibility of pharmacy were the predictors of SMP. Thus, college students must be trained on the undesirable effects of SMP. Furthermore, a strong national guideline on medication access must be established and strong measures should be applied to stop the access of medications without a legal prescription from licensed professionals. Strengthening the students’ awareness concerning the dangerous impacts of SMP and ensure a regular regulation of drug stores/pharmacies have a key role in decreasing the practice of SM. Thus, joined efforts of individuals, communities, health facilities, and the regulatory bodies are extremely crucial.
Limitation of the Study
The current study was aimed to assess modern self-medication practice, which doesn’t take account of the traditional medication practice. As the study is cross-sectional and depends on self-reported assessment, under-reporting is more likely to occur. This study was not included the attitude, awareness of the participants towards self-medication practice, and types of medication. This enforced the generalization of the findings to all types of medications. Besides, the study was done among health science students and a comparison group from different streams (Para health) is missing. Thus, the prevalence of self-medication practice might be underestimated.
Acknowledgments
The authors would like to acknowledge the Deans of Health science college who permitted the data collection and we want to acknowledge the students of the health science college for their voluntary participation.
Footnotes
Author Contribution: ZDK contributed to design the study, write up of the final research, data interpretation, data analysis, final manuscript preparation, and supervision of the study. DAA and ABM participated in data analysis and data interpretation. EFE contributed to writing up the final research, data interpretation, and manuscript preparation. All authors read and approved the final manuscript.
Availability of Data and Materials: The data sets supporting the finding of this study can be obtained from the corresponding author upon a formal request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval and Consent to Participate: This study was approved by the ethical committee of the school of pharmacy, the University of Gondar. Informed verbal, as well as written consent, was obtained from study participants before data collection, and the purpose of the study was explained to the respondents in advance. The information collected from respondents was kept confidential.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Zemene Demelash Kifle  https://orcid.org/0000-0001-7030-2782
https://orcid.org/0000-0001-7030-2782
References
- 1. World Health Organization. Guidelines for the Regulatory Assessment of Medicinal Products for Use in Self-medication. World Health Organization; 2000. [Google Scholar]
- 2. Sarahroodi S, Maleki-Jamshid A, Sawalha AF, Mikaili P, Safaeian L. Pattern of self-medication with analgesics among Iranian University students in central Iran. J Family Commun Med. 2012;19(2):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ehigiator O, Azodo CC, Ehizele AO, Ezeja EB, Ehigiator L, Madukwe IU. Self-medication practices among dental, midwifery and nursing students. Eur J Gen Dentist. 2013;2(1):54. [Google Scholar]
- 4. Abay S, Amelo W. Assessment of Self-medication practices among medical, pharmacy, health science students in Gondar University, Ethiopia. J Young Pharm. 2010;2(3):306-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klemenc-Ketiš Z, Hladnik Ž, Kersnik J. A cross sectional study of sex differences in self-medication practices among university students in Slovenia. Collegium Antropologicum. 2011;35(2):329-334. [PubMed] [Google Scholar]
- 6. Kassie AD, Bifftu BB, Mekonnen HS. Self-medication practice and associated factors among adult household members in Meket district, Northeast Ethiopia, 2017. BMC Pharmacol Toxicol. 2018;19(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hankó B, Kázmér M, Kumli P, et al. Self-reported medication and lifestyle adherence in Hungarian patients with type 2 diabetes. Pharm World Sci. 2007;29(2):58-66. [DOI] [PubMed] [Google Scholar]
- 8. Geissler PW, Nokes K, Prince RJ, Achieng’Odhiambo R, Aagaard-Hansen J, Ouma JH. Children and medicines: self-treatment of common illnesses among Luo schoolchildren in western Kenya. Social Sci Med. 2000;50(12):1771-1783. [DOI] [PubMed] [Google Scholar]
- 9. Lam CL, Catarivas MG, Munro C, Lauder IJ. Self-medication among Hong Kong Chinese. Social Sci Med. 1994;39(12):1641-1647. [DOI] [PubMed] [Google Scholar]
- 10. Sanghani S, Zaveri H, Patel V. Self medication: prevalence and pattern in urban community. J Pharmacovigil Drug Safety. 2008;5:95-98. [Google Scholar]
- 11. Phalke V, Phalke D, Durgawale P. Self-medication practices in rural Maharashtra. Indian J Commun Med. 2006;31(1):34. [Google Scholar]
- 12. Ayalew MB. Self-medication practice in Ethiopia: a systematic review. Patient Pref Adherence. 2017;11:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Idris T, Khanum S, Uddin MS, et al. Study on self-medication practices among university students of Bangladesh. J Adv Med Med Res. 2016;14(6):1-8. [Google Scholar]
- 14. Zafar SN, Syed R, Waqar S, et al. Self-medication amongst university students of Karachi: prevalence, knowledge and attitudes. J Pak Med Assoc. 2008;58(4):214. [PubMed] [Google Scholar]
- 15. Verma RK, Mohan L, Pandey M. Evaluation of self medication among professional students in North India: proper statutory drug control must be implemented. Evaluation. 2010;3(1):60-64. [Google Scholar]
- 16. Da Silva MGC, Soares MCF, Muccillo-Baisch AL. Self-medication in university students from the city of Rio Grande, Brazil. BMC Public Health. 2012;12(1):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sawalha AF. A descriptive study of self-medication practices among Palestinian medical and nonmedical university students. Res Social Admin Pharm. 2008;4(2):164-172. [DOI] [PubMed] [Google Scholar]
- 18. El Ezz N, Ez-Elarab H. Knowledge, attitude and practice of medical students towards self medication at Ain Shams University, Egypt. J Prev Med Hyg. 2011;52(4):196-200. [PubMed] [Google Scholar]
- 19. Gupta V, Bansal P, Manhas R, Singh Z, Ghaiye P. Preferred system of medicine and reasons of self medication among college students in Malwa region of Punjab. J Drug Deliv Ther. 2011;1(2):27-29. [Google Scholar]
- 20. Afolabi A. Self medication, drug dependency and self-managed healthcare – a review. Pub Health Social Behav Health. 2012;16:234-253. [Google Scholar]
- 21. Yadav S, Rawal G. Self-medication practice in low income countries. Int J Pharmaceut Chem Anal. 2015;2(3):139-142. [Google Scholar]
- 22. Arrais PSD, Fernandes MEP, Pizzol TdSD, et al. Prevalence of self-medication in Brazil and associated factors. Revista de saude publica. 2016;50:13s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balamurugan E, Ganesh K. Prevalence and pattern of self medication use in coastal regions of South India. Br J Med Pract. 2011;4(3):a428. [Google Scholar]
- 24. Garofalo L, Di Giuseppe G, Angelillo IF. Self-medication practices among parents in Italy. Biomed Res Int. 2015;2015:580650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bekele A, Ararsa A. Assessment of self-medication practice and drug storage on private pharmacy clients in Jimma town, Oromia, south West Ethiopia. Trends Drug Deliv. 2019;1(3):17-29. [Google Scholar]
- 26. Marak A, Borah M, Bhattacharyya H, Talukdar K. A cross-sectional study on self-medication practices among the rural population of Meghalaya. Int J Med Sci Public Health. 2016;5(6):1134-1138. [Google Scholar]
- 27. Fekadu G, Dugassa D, Negera GZ, et al. Self-medication practices and associated factors among health-care professionals in selected hospitals of western Ethiopia. Patient Pref Adherence. 2020;14:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bekele SA, Argaw MD, Yalew AW. Magnitude and factors associated with self-medication practices among university students: the case of Arsi University, College of Health Science, Asella, Ethiopia: cross-sectional survey based study. Open Access Library J. 2016;3(6):1-15. [Google Scholar]
- 29. Helal R, Abou-ElWafa H. Self-medication in university students from the city of Mansoura, Egypt. J Environ Public Health. 2017;2017:9145193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson D, Sekhar HS, Alex T, Kumaraswamy M, Chopra RS. Self medication practice among medical, pharmacy and nursing students. Int J Pharm Pharm Sci. 2016;8(7):443-447. [Google Scholar]
- 31. Zeru N, Fetene D, Geberu DM, Melesse AW, Atnafu A. Self-medication practice and associated factors among University of Gondar College of Medicine and Health Sciences Students: a cross-sectional study. Patient Pref Adherence. 2020;14:1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Araia ZZ, Gebregziabher NK, Mesfun AB. Self medication practice and associated factors among students of Asmara College of Health Sciences, Eritrea: a cross sectional study. J Pharm Policy Pract. 2019;12(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abdi A, Faraji A, Dehghan F, Khatony A. Prevalence of self-medication practice among health sciences students in Kermanshah, Iran. BMC Pharmacol Toxicol. 2018;19(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mitsi G, Jelastopulu E, Basiaris H, Skoutelis A, Gogos C. Patterns of antibiotic use among adults and parents in the community: a questionnaire-based survey in a Greek urban population. Int J Antimicrob Agents. 2005;25(5):439-443. [DOI] [PubMed] [Google Scholar]
- 35. Al-Azzam SI, Al-Husein BA, Alzoubi F, Masadeh MM. Self-medication with antibiotics in Jordanian population. Int J Occupational Med Environ Health. 2007;20(4):373. [DOI] [PubMed] [Google Scholar]
- 36. Gutema GB, Gadisa DA, Kidanemariam ZA, et al. Self-medication practices among health sciences students: the case of Mekelle University. J Appl Pharm Sci. 2011;1(10):183. [Google Scholar]
- 37. James H, Handu SS, Al Khaja KA, Otoom S, Sequeira RP. Evaluation of the knowledge, attitude and practice of self-medication among first-year medical students. Med Principles Pract. 2006;15(4):270-275. [DOI] [PubMed] [Google Scholar]
- 38. Hussain A, Khanum A. Self medication among university students of Islamabad, Pakistan-a preliminary study. Southern Med Rev. 2008;1(1):14-16. [Google Scholar]
- 39. Galato D, Galafassi LDM, Alano GM, Trauthman SC. Responsible self-medication: review of the process of pharmaceutical attendance. Brazil J Pharm Sci. 2009;45(4):625-633. [Google Scholar]
- 40. Kaur N, Bisht B, Kaur M. Self medication practices among youngsters: a global health concern. Medico Legal Update. 2021;21(1):658-663. [Google Scholar]
- 41. Gyawali S, Shankar PR, Poudel PP, Saha A. Knowledge, attitude and practice of self-medication among basic science undergraduate medical students in a medical school in western Nepal. J Clin Diagn Res. 2015;9(12):FC17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zewdie S, Andargie A, Kassahun H. Self-medication practices among undergraduate University Students in Northeast Ethiopia. Risk Manage Healthc Policy. 2020;13:1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Niroomand N, Bayati M, Seif M, Delavari S, Delavari S. Self-medication pattern and prevalence among Iranian medical sciences students. Curr Drug Safety. 2020;15(1):45-52. [DOI] [PubMed] [Google Scholar]
- 44. Karthik L, Kumar G, Keswani T, Bhattacharyya A, Chandar SS, Rao KB. Protease inhibitors from marine actinobacteria as a potential source for antimalarial compound. PLoS One. 2014;9(3):e90972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Osemene K, Lamikanra A. A study of the prevalence of self-medication practice among university students in Southwestern Nigeria. Trop J Pharm Res. 2012;11(4):683-689. [Google Scholar]
- 46. Tesfaye ZT, Ergena AE, Yimer BT. Self-medication among medical and nonmedical students at the University of Gondar, Northwest Ethiopia: a cross-sectional study. Scientifica. 2020;2020:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Borah H, Kakati R, Borah M, Deka C. Self-medication practices and its determinants among urban dwellers of Dibrugarh town, Assam. Scholars J Appl Med Sci. 2016;4(8E):3100-3106. [Google Scholar]
- 48. Ahmadi SM, Jamshidi K, Sadeghi K, Abdi A, Vahid MP. The prevalence and affecting factors on self-medication among students of Kermanshah University of Medical Science in 2014. J Clin Diagn Res. 2016;10(5):IC01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sado E, Gedif T. Drug utilization at household level in Nekemte Town and surrounding rural areas, western Ethiopia: a cross-sectional study. Open Access Library J. 2014;1(3):1. [Google Scholar]
- 50. Divya M, Bharatesh S, Vasudeva G, Varalakshmi C. Self-medication among adults in Urban Udupi Taluk, Southern India. Int J Med Public Health. 2016;6(3):126-129. [Google Scholar]
- 51. Mihretie TM. Self-medication practices with antibiotics among urban dwellers of Bahir Dar town, North West Ethiopia. Addis Ababa Univ. 2014. [Google Scholar]
- 52. Jaleta A, Tesema S, Yimam B. Self-medication practice in sire town, West Ethiopia: a cross-sectional study. Cukurova Med J. 2016;41(3):447-452. [Google Scholar]
- 53. Mossa DA, Wabe NT, Angamo MT. Self-medication with antibiotics and antimalarials in the community of Silte zone, South Ethiopia. TAF Prevent Med Bull. 2012;11(5):529-536. [Google Scholar]
- 54. Gelayee DA. Self-medication pattern among social Science University students in Northwest Ethiopia. J Pharmaceutics. 2017;2017:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Alshogran OY, Alzoubi KH, Khabour OF, Farah S. Patterns of self-medication among medical and nonmedical University students in Jordan. Risk Manage Healthc Policy. 2018;11:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abdulraheem I, Adegboye A, Fatiregun A. Self-medication with antibiotics: empirical evidence from a Nigerian rural population. J Pharmaceutical Res Int. 2016;11(5):1-13. [Google Scholar]
- 57. Zeid W, Hamed M, Mansour N, Diab R. Prevalence and associated risk factors of self-medication among patients attending El-Mahsama family practice center, Ismailia, Egypt. Bull Natl Res Centre. 2020;44(1):1-5. [Google Scholar]
- 58. Ansari M, Alanazi A, Moin A. Consumers’ awareness, attitude and associated factors towards self-medication in Hail, Saudi Arabia. PLoS One. 2020;15(4):e0232322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Loharkar N, Keche Y, Yegnanarayan R, Dharma M, Bhosale A, Makan A. Self medication use in urban population of Pune, Maharashtra, India. Scholars J Appl Med Sci. 2013;1(6):732-738. [Google Scholar]
- 60. Amaha MH, Alemu BM, Atomsa GE. Self-medication practice and associated factors among adult community members of Jigjiga town, Eastern Ethiopia. PLoS One. 2019;14(6):e0218772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jafari F, Khatony A, Rahmani E. Prevalence of self-medication among the elderly in Kermanshah-Iran. Global J Health Sci. 2015;7(2):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Parakh R, Sharma N, Kothari K, Parakh R, Parakh P. Selfmedication practice among engineering students in an engineering college in North India. J Phytopharm. 2013;2:30-36. [Google Scholar]
- 63. Subramanian P, Shanmugam N, Sivaraman U, Kumar S, Selvaraj S. Antiobiotic resistance pattern of biofilm-forming uropathogens isolated from catheterised patients in Pondicherry, India. Aust Med J. 2012;5(7):344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Niwandinda F, Lukyamuzi EJ, Ainebyona C, Ssebunya VN, Murungi G, Atukunda EC. Patterns and practices of self-medication among students enrolled at Mbarara University of Science and Technology in Uganda. Integr Pharm Res Pract. 2020;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Varpaei HA, Onsori P, Esmaeili F, et al. Self-medication practice, its causes and risk factors among people in Tehran, Iran: a descriptive-analytic study. J Commun Med. 2020;3(1):1025. [Google Scholar]
- 66. Ediriweera DS, Kasturiratne A, Pathmeswaran A, et al. Mapping the risk of snakebite in Sri Lanka-a national survey with geospatial analysis. PLoS Neglect Trop Dis. 2016;10(7):e0004813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wen Y, Lieber E, Wan D, Hong Y, Group NCHSPT. A qualitative study about self-medication in the community among market vendors in Fuzhou, China. Health Social Care Commun. 2011;19(5):504-513. [DOI] [PMC free article] [PubMed] [Google Scholar]