Abstract
The aim of this study was to improve the quality of semen samples by using a novel double-tube (DT) method. The DT method was developed to select sperm and compared with traditional swim-up (SU) technique for 31 semen samples. Sperm DNA integrity were tested with TUNEL and SCSA. Content of antisperm antibodies (ASA) in the semen was measured by ELISA and MAR. Levels of the caspase-3 in the sperm were assessed by western blotting. After SU and DT, 15 couples and 16 couples were underwent IVF-ET. The number of RCDs, the percentage of SDF and DFI, ASA and the level of caspase-3 were significantly decreased after DT and SU (p = .001 and p < .001). When the DT and SU compared, there were significant changes in the number of RCD, the percentage of SDF and DFI, ASA and the level of caspase-3 (p < 0.05-0.001). There was a higher cleavage rate (p = .017) and a lower abortion rate (p < .05) in DT-IVF group than in SU-IVF group. DT selection yielded spermatozoa with low RCDs, DFI, ASA, and caspase-3 which would be benefit for ART.
Keywords: sperm selection, swim-up, double tube method, DNA fragmentation, Caspase-3
Introduction
The selection of ideal spermatozoa before intrauterine insemination (IUI), in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) is important. The swim-up (SU) method and density gradient centrifugation (DGC) are commonly used to sperm separation or isolation during the assisted reproductive technologies (ARTs) process (Beck-Fruchter et al., 2016; Li et al., 2018; Muratori et al., 2019). The DGC method can select spermatozoa with higher motility and better morphology than original semen, but it may increase the oxidative DNA damage in spermatozoa preparation (Aitken et al., 2014; Muratori et al., 2016). Sperm DNA fragmentation (SDF) after DGC procedure is higher than the SU method (Muratori et al., 2019). The method of selecting sperm should be improved.
The purpose of sperm selection is to obtain better sperm quality, including sperm with normal morphology, and without DNA fragmentation, round cells (germ cells and leukocytes), epithelial cells, debris such as isolated sperm heads and tails, and bacteria (RCD). The presence of large numbers of nonviable spermatozoa in the prepared sample can inhibit capacitation (Miller et al., 2019). Although several sperm separation techniques have been developed, there is no sturdy method, reliable enough to provide a good-quality fraction of functional spermatozoa from the whole semen to be used in assisted reproductive technologies (ART) which include IVF and IVF-related procedures such as intra-cytoplasmic sperm injection (ICSI) and, according to some definitions, intra-uterine insemination (IUI), also known as artificial insemination.
Antisperm antibodies (ASAs) may reduce sperm motility and some antibodies that bind to sperm antigens result in functional impairment of sperm and thus its inability to fertilize the oocyte (Chamley and Clarke, 2007). In semen, ASAs may be separated by filtration or DGC method, and improved the recovery of ASA-free spermatozoa (Grundy et al., 1991; Ryan et al., 1994). High rates of DNA damage, activate caspase-3 and DNA fragmentation are frequently associated with poor semen parameters and male infertility (Miller et al., 2019; Paasch et al., 2003). In selecting appropriate spermatozoa for ICSI processes, the active caspase-3 expression, DNA fragmentation, and apoptosis index may be decreased by the hyaluronic acid binding method and zeta preparation procedure (Rezaei-Agdam et al., 2019). There is no report for improvement of the quality of semen samples with a novel double-tube (DT) method. The devise of DT has been patented (2011; ZL200910014401.1; China).
Based on these observations, we presented a novel DT selection method and hypothesized that sperm motility, morphology, DNA integrity, RCD, ASAs, and caspase-3 activity may be improved by DT selection sperm device.
Materials and Methods
Subjects
Between February 2018 and February 2019, semen sample was collected from 31 men (ranged 27–45-year-old, means of 33.5 ± 4.5) who attended the andrology/in vitro fertilization (IVF) unit at the Maternal and Child Health Care Hospital of Shandong Province. Prior to this study, subjects were informed of the investigations and provided consent. The inclusion criteria included: (1) infertility for more than 12 months; (2) infertile couples who agree to IVF treatment; and (3) oligozoospermia or asthenozoospermia or teratozoospermia or oligoasthenoteratozoospermia, or ASAs positive in semen. The exclusion criteria were: (1) current or past diseases such as hepatic, renal, adrenal, or thyroid disorders; (2) vasectomy or anastomosis; and (3) taking drugs for male infertility in the past 3 months, especially sex hormone drugs and immunosuppressants. The study protocol was reviewed and approved by Ethics Committee/Institutional Review Board (approval number: ART2018-1-03), and the study was conducted according to guidelines described in the Declaration of Helsinki. Written informed consent was provided by all subjects prior to study inclusion. Compensations for all subjects were free routine semen analysis twice, one anti-sperm antibody test and one sperm DNA fragmentation assay.
Collection of Semen Samples and Analysis
Semen samples were collected by masturbation after 3 to 5 days of sexual abstinence and were analyzed, according to the 2010 guidelines of the World Health Organization (World Health Organization, 2010). Samples were collected in sterile, non-toxic polypropylene containers following a 25-min duration of liquefaction. Microscopic evaluations were performed using a phase-contrast microscope (IX51; Olympus, Tokyo, Japan) at 400× magnification. The computer-assisted semen analysis (CASA) of Sperm Class Analyzer® (SCA, Spain) was used. The selected separation swim-up technique was carried out according to the standard specifications. Each sample was divided into two equal aliquots, and each was processed in parallel using the SU and the DT of different sperm selection procedures.
Sperm Obtained Through Routine Swim-up Method
Swim-up technique was carried out by the method of using the sperm preparation medium (World Health Organization, 2010) (FertiMedium, BRED-021, Bred Life Science, Shenzhen, China). One ml of semen in a 15-mL conical centrifuge tube was gently overlayered with a 1.2 mL volume of sperm preparation medium. The tube was inclined at an angle of about 45 °C, to increase the surface area of the semen-culture medium interface, and then incubated for 1 h at 37 °C. After gently returning the tube to the upright position, 1.0 mL of the uppermost medium was pipetted to another test tube. Then sperm parameters of the uppermost medium were analyzed.
Double Tube (DT) Device Design and Sperm Selection
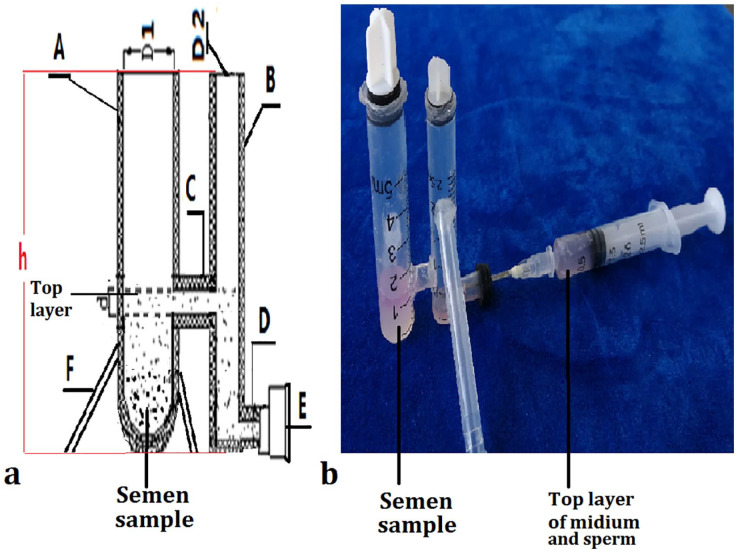
The structure and principle of the DT selection sperm device is presented in Figure 1. The DT was designed to have two straight tubes [(A) and (B)] in which motile spermatozoa are migrated along the horizontal tube (C) to tube (B), and other components in semen such as immotile sperm, “round cells” (germ cells and leukocytes), epithelial cells, and debris (isolated sperm heads and tails) and bacteria (RCD) will be left in a tube (A). The tube (A), tube (B), and the connected tube (C) constituted the H-tube, and tube (B) and tube (D) constituted the L-tube. Two types of the DT device (large and small) were designed. The inside diameter and the height of the tube (A) (D1 and h) were 0.8 cm or 0.6 cm, and 3.5 cm or 3.0 cm, and the tube (B) (D2 and h) were 0.6 cm or 0.4 cm and 3.0 cm or 2.5 cm; and the inside diameter and the length (d and L) of the tube (C) and tube (D) were 0. 4 or 0.3 cm and 0.6 cm or 0.3 cm, respectively.
Figure 1.
Double tube (DT) selection sperm device. (a), designed diagram, the tube (A) and tube (B), first straight tube and second straight tube. Tube (C), the connected tube. Tube (D), the output pipe. E, rubber plugs. F, the stent for fixing the DT. (b), physical map of DT with semen and sperm preparation medium. (c), the top layer of sperm medium sucked into an empty needle.
For the double tube from fresh semen procedure, 1.5 ml of the sperm preparation medium was firstly placed into the tube (A), and flowed into the tube (B). Semen sample (1.0 mL for large and 0.5 mL for small double tube) was then gently placed underneath tube (A) using a Pasteur pipette. The DT was placed in an incubator at 37 °C for 1 hr. Subsequently, 1.0–1.5 mL from large type and 0.5–0.8 mL from small type of the top layer with high quality sperm were collected from the tube (D) using an empty needle sucked (see Figure 1). Sperm parameters of the collected mixture were tested.
After the SU and the DT of sperm selection procedures, sperm numbers and motility were evaluated automatically using CASA system. In order to obtain reliable results, each measure consisted an average value of three different captured fields. Motility grades were classified into progressive (progressive rate, PR), non-progressive (NP) motile spermatozoa and immotile spermatozoa.
Vitality Test Using Eosin–Nigrosin (EN) and Hypo-Osmotic Swelling (HOS)
The test for sperm vitality was carried out by vital staining of spermatozoa with EN and HOS according to the method described by World Health Organization (2010). The score of HOS/EN was according to the method described by Zhang et al. (2015).
Diff-Quik Staining for Sperm Morphology Analysis
Sperm morphology assessment was tested by using Diff-Quik staining as the World Health Organization (2010) criteria. The Diff-Quik rapid staining kit (D030, Jiancheng, Nanjing, China) was used. Approximately 200 spermatozoa were assessed per slide for the percentage of normal forms or of normal and abnormal forms.
Calculation of Round Cells and Debris (RCD) Other than Spermatozoa
Other components in semen and the collected mixture such as RCD were counted. For the swim-up and the double tube, 1.0–2.0 mL of medium was collected and centrifuged for 10 min at 400 g, respectively. After discarding the supernatant, the pellet was mixed with 0.1 mL of sperm preparation medium. The concentration of RCD was calculated relative to that of spermatozoa by assessing fixed and stained semen smears made from undiluted semen and from the collected and concentrated mixture of the swim-up and the double tube (World Health Organization, 2010).
Antisperm Antibody (ASA) assay
The ASA test was used to identify a special protein (antibody) that attacks sperm. The kit for ASA detection was purchased from Immunoglobulin A (H108) and Immunoglobulin G (H106) Assay Kit (Nanjing Jiancheng, China). Content of the ASA production in the semen was measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions. Briefly, 100 mL of semen sample or the mixed sperm medium by swim-up and double tube method were added into the ELISA plate coated with a primary antibody (1: 100) for human spermatozoa protein and then incubated for 30 min at 37 °C. After washing, horseradish peroxidase-conjugated secondary antibody (1: 200) was added into each well of the plate and incubated for 30 min. After washing, 50 micro liter of a substrate TMB (3,3′,5,5′-tetramethylbenzidine) was added to yield a color reaction. Absorbance values were determined at a wavelength of 450 nm using a microplate reader. The experimental operation and analysis of results were implemented strictly in accordance with the kit instructions. An ASA level of <75 IU was considered negative, whereas an ASA level of >75 IU was considered positive.
The IgG-ASA-coated motile sperm counts were evaluated with mixed agglutination reaction test (Sperm MAR test). The direct MAR test (Hinting et al., 1988) was performed on a microscope slide by mixing one drop (approximately 10 μL in volume) of fresh semen or the mixed sperm medium by swim-up and double tube method, one drop of latex particles coated with IgG and one drop of antiserum from rabbit against human IgG (SpermMar Kit, FertiPro, Belgium). In this assay, the motile sperm covered by latex particles of <50% was considered as ASA negative, whereas the motile sperm covered by latex particles of >50% was considered as ASA positive.
TUNEL Assay
The nicked DNA in pre-treatment semen, swim-up and double tube sorted spermatozoa was assessed by the TUNEL assay according to the manufacturer’s instruction In-Situ Cell Death Detection Kit (Roche Diagnostics GmbH, Mannheim, Germany) and as published (Qiu et al., 2012). The score of TUNEL was according to the method described by Qiu et al. (2012).
Sperm Chromatin Structure Assay (SCSA) Using Flow Cytometry
The SCSA is a flow cytometric technique which identifies spermatozoa with abnormal chromatin packaging defined as susceptibility to acid-induced DNA denaturation in situ. The DNA fragmentation index (DFI) were detected. The flow cytometric method was performed based on the manufacturer’s instruction and the SCSA procedure was described by Zhang et al. (2018). When the DFI was more than 30%, the sperm function was abnormal.
Protein Extraction and Western Blotting
Spermatozoa collected from semen, double tube and Swim-up mixture were centrifuged and washed twice with PBS. After washing, the sperm cells were sonicated for 5 s at 4 °C in 100 μl of lysis buffer consisting of 50 mM Tris/HCl, 150 mM NaCl, 1% Triton X-100, 1% deoxycholate, 1 mM EGTA, 0.4 mM EDTA, a protease inhibitor cocktail. The protein concentrations were determined using a BCA Protein Assay Kit (Pierce, 23227, Shanghai, China). The homogenates were clarified by centrifugation at 10,000 g (15 min 4 °C), and the supernatants were used for protein analysis after dilution with 4× SDS sample buffer. The total proteins from sperm were separated by 10%–15% w/v sodium dodecyl sulfate-polyacrylamide gel electrophoresis transferred to a polyvinylidene difluoridemembrane. Five per cent skim milk powder blocked for 2 hr at room temperature. Caspase-3 was detected using a rabbit polyclonal antibody to caspase-3 (1:300; Proteintech, Chicago, IL, USA), followed by a mouse anti-rabbit secondary antibody (1:2000; Multi Sciences Biotech, Chicago, IL, USA). For the internal control β-actin (Beyotime, China), the ratio is 1:5000. After blocking, the membranes were incubated overnight at 4 °C overnight. On the following day, the membranes were washed twice, 10 minutes each. The antigen–antibody complexes were visualized in the presence of electro-chemiluminescence. The intensities of protein bands on the blots were evaluated by using Amersham Imager 600 UV (AI 600, GE, USA). The intensity and molecular weight of the bands were quantified and normalized against the values for β-actin.
In vitro Fertilization and Embryo Transfer (IVF-ET)-Assisted Reproduction Scheme
Thirty-one subjects were randomized to either the swim up (SU) group or double tube (DT) group (SU-IVF group: 15 cases, DT-IVF group: 16 cases). Routine long protocol of gonadotrophin releasing hormone agonists in controlled ovarian hyperstimulation was applied. Controlled ovarian stimulation was performed using gonadotropin-releasing hormone (GnRH) (Merck KGaA, Darmstadt, Germany) agonist, recombinant follicular-stimulating hormone (FSH) (Merck KGaA, Darmstadt, Germany) and human chorionic gonadotropin (HCG) (Livzon, Zhuhai, China). Standard techniques were used in IVF treatment, including fertilization, embryo culture, and embryo transfer at the Reproductive Medical Centre of Maternal and Child Health Care Hospital of Shandong Province. ET was performed on the third day of in vitro culture, β-HCG detection 2 weeks after transplant and ultrasonic examination 4 weeks after transplant. A fertilized egg and fetal heart beat were determined as clinical pregnancy which was followed by the follow-up to delivery.
Statistical Analysis
The statistical analysis of the study was performed using an IBM SPSS (Version 22.0. Armonk, NY: IBM Corp.). Data are shown as the mean ± SD. Parametric data were analyzed statistically using Student’s t-tests. Paired tests were used for comparing data before and after sperm separation. The exact Pearson Chi-Square test was used for the number of seminal ASAs and MAR positivity, and clinical pregnancy rate and the rate of abortion by using IVF-ET between pre-treatment, SU or DT method. The difference was considered statistically significant for p values <.05.
Results
Sperm Count, Motility, Vitality, Normal Morphology and RCDs After Separation by Swim-up and Double tube
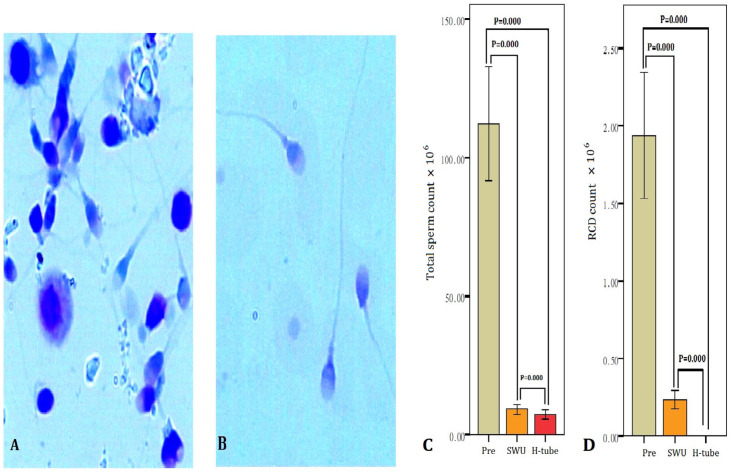
The initial sperm concentration, total sperm number, the percentage of motility, vitality (normal HOS/EN), normal morphology and RCD of 31 subjects (pre-selection) were 9.46–162.26 × 106/ml, 23.9–486.5 × 106, 5%–39%, 12%–74%, 2%–16%, and 0.30–10.60 × 106/ml, respectively. Twelve of 31 subjects were oligospermatozoa (sperm counts <15 × 106/ml and total sperm number <39× 106) (38.7%). ASAs positive in seminal plasma (more than 75 IU) were found in 8 infertile men (25.8%), and spermatozoa MAR positive (more than 50%) were in 6 cases (6/31, 19.4%). All of them were with sperm motility of less than 40% (asthenozoospermia), and eight were teratozoospermia (normal morphology of less than 4%) (25.8%). Sixteen of 31 subjects were with RCDs of more than 1.0 × 106/ml in semen (51.6%). As presented in Table 1, the percentage of motility, vitality and normal morphology were evaluated from the semen samples of 31 subjects after subjecting each sample to each of the two separation techniques. The data revealed a significant increase (p < .001) in the percentage of motility, vitality and normal morphology using swim-up technique and double tube method as compared with pre-selection. The percentage of sperm with normal morphology, motility and vitality after double tube selection was significantly higher (independent samples t test, p < .001) than swim-up treatment. The total sperm number and RCD were found to be significantly decreased in both methods compared with pre-selection (p < .001) (Figure 2). Sperm morphology and RCDs, as assessed by Diff-Quik staining, are displayed in Figure 2.
Table 1.
Sperm Parameters After Separation by Swim-up and Double Tube (Means ± SD).
| Group | n | Pre-selection | Swim-up | Double tube |
|---|---|---|---|---|
| Motility (PR + NP) (%) | 31 | 17.4 ± 9.2 | 74.9 ± 15.1a | 94.2 ± 4.8a,b |
| Vitality (HOS/EN) (%) | 31 | 30.3 ± 16.6 | 78.7 ± 19.5a | 96.4 ± 3.4a,c |
| Normal morphology (%) | 31 | 7.0 ± 4.0 | 35.2 ± 22.1a | 44.8 ± 23.6a,d |
RCDs: round cells and debris.
p < .001, paired-samples t test, pre-selection vs swim-up and double tube. bp = .001, paired-samples t test, double tube vs swim-up. c=0.002, paired-samples t test, double tube vs swim-up. d<0.001, paired-samples t test, double tube vs swim-up.
Figure 2.
The total sperm number and RCD before treatment and after double tube (DT) and swim-up. (A) RCD in original semen. (B) After DT. (A) and (B): Diff-Quik staining, bright field optics at ×1000 magnification with oil. (C) Total sperm number pre-treatment and after swim-up and DT methods. (D) RCD decreased by using swim-up and DT methods (p = .004 and p = .009). SWU, swim-up; Pre, pre-treatment.
The Effect of Selection with Swim-up and Double Tube Methods on Sperm DNA Fragmentation (SDF)
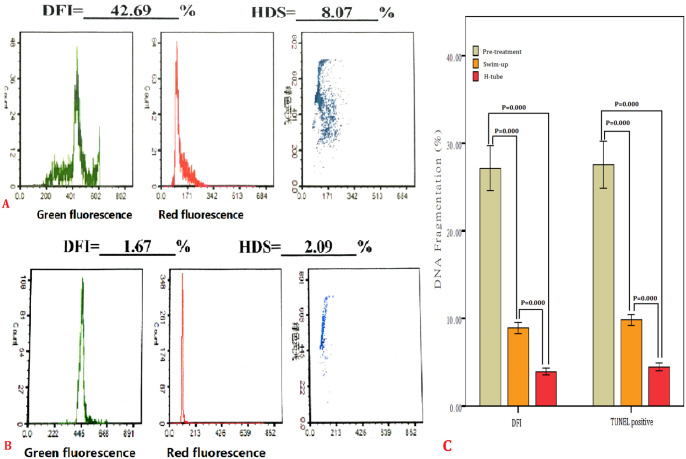
The percentage of SDF and DFI in the initial semen samples was 26.0% and 24.8%, respectively. Table 2 shows the significant decrease (p < .001) in percentage of SDF and DFI using swim-up and double tube as compared to the initial semen samples. The percentages of SDF and DFI by the double tube were lower than that of swim-up (p < .001). Sperm with DNA fragments or integrity are presented in Figure 3.
Table 2.
Sperm DNA Fragmentation and DFI Using Swim-up and Double Tube.
| Group | n | Pre-treatment | Double tube | Swim-up |
|---|---|---|---|---|
| TUNEL (positive) (%) | 31 | 27.5 ± 14.9 | 4.5 ± 2.4a | 9.8 ± 3.4a,b |
| DFI (%) | 31 | 27.2 ± 14.5 | 3.9 ± 2.3a | 8.9 ± 3.6a,b |
p = .000, paired-samples t test, pre-treatment vs swim-up and double tube. bp = .000, paired-samples t test, double tube vs swim-up.
Figure 3.
SDF pre-treatment and after double tube (DT) and swim-up. (A) SDF in original semen. (B) After DT. (C) TUNEL positive sperm and DFI significantly decreased by using swim-up and DT methods (p < .001), SDF after DT was lower than swim-up. SWU, swim-up; PRE, pre-treatment.
Changes in Antisperm Antibodies (ASAs) in Seminal Plasma and Spermatozoa
Pre-treatment seminal ASAs more than 75 IU were found in 8 infertile men (25.8%), and spermatozoa covered by latex particles of MAR more than 50% were in 6 cases (6/31, 19.4%). After SU and DT, ASAs in seminal plasma and spermatozoa were 1 case and 0, respectively (Table 3).
Table 3.
ASAs in Semninal Plasma and Sperm After Swim-up and Double Tube.
| Group | n | Pre-treatment | Double tube | x 2 | p | Swim-up | x 2 | p |
|---|---|---|---|---|---|---|---|---|
| Semen (>75 IU) | 31 | 8 (25.8%) | 0 (0) | 9.185 | .005a | 1 (3.2%) | 6.369 | .026a |
| 1.016 | .000b | |||||||
| Sperm MAR (>50%) | 31 | 6 (19.4%) | 0 (0) | 6.643 | .024a | 1 (3.2%) | 4.026 | .104a |
| 1.016 | .000b |
Swim-up and double tube vs. pre-treatment; bSwim-up vs. double tube.
The Effect of Selection with SU and DT Methods on the Expression of Caspase-3
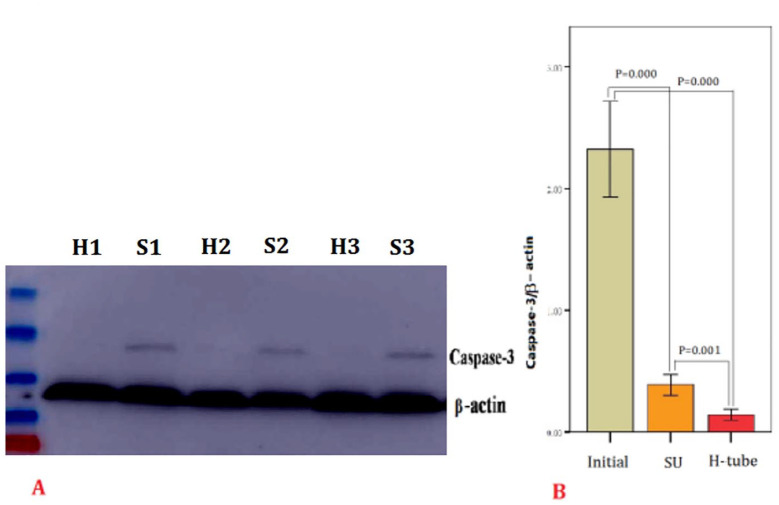
The expression of caspase-3 is presented in Figure 4. A western blot analysis of protein expression showed that caspase-3 levels were decreased by using SU and DT method compared with the initial semen samples (p < .001). There was a significant difference of caspase-3 levels of selected sperm between SU technique and DT method (p = .001).
Figure 4.
Expression of caspase-3. (A) S1, S2, and S3: the initial semen samples. H1, H2, and H3: selected sperm by double tube (DT) method. (B) Levels of caspase-3 significantly decreased by using swim-up and double tube methods (p < .001), Levels of caspase-3 after DT was lower than swim-up (p = .001). Initial: before treatment; SU: swim-up.
Comparison of IVF-ET Index Between Two Groups
In 16 males of DT-IVF group and in 15 males of SU-IVF grooup, asthenozoospermia, oligospermatozoa and teratozoospermia were 16, 7, and 4 cases, and 15, 5, and 4 cases, respectively. There was no statistical difference in maternal age, basal FSH value, Basal E2 value, antral follicle count, retrieved oocyte number and fertility rate between two groups (p > .05), presented in Table 4. There was a statistical difference in cleavage rate between two groups (p = .017), presented in Table 4.
Table 4.
Comparison of IVF-ET Index in Two Groups.
| SU-IVF (n = 15) | DT-IVF (n = 16) | t value | p value | |
|---|---|---|---|---|
| Age (years) | 31.1 ± 3.5 | 31.5 ± 3.1 | 0.691 | 0.501 |
| Basal FSH value (U/L) | 6.9 ± 1.9 | 7.3 ± 1.4 | 0.953 | 0.357 |
| Basal E2 value (pmol/L) | 182.9 ± 20.6 | 183.1 ± 23.6 | 0.031 | 0.976 |
| Antral follicle count | 11.0 ± 1.9 | 11.3 ± 2.3 | 0.503 | 0.623 |
| Retrieved oocyte number | 11.5 ± 1.8 | 11.8 ± 2.3 | 0.354 | 0.728 |
| Fertility rate (%) | 74.7 ± 5.3 | 75.5 ± 6.4 | 0.581 | 0.571 |
| Cleavage rate (%) | 92.9 ± 36 | 95.6 ± 3.2 | 2.697 | 0.017a |
p < .05, DT-IVF group vs. SU-IVF group.
Comparison of Pregnancy Outcome Index in Two Groups
There was no significant statistics in the rate of clinical pregnancy between DT-IVF group and SU-IVF group (p > .05). The rate of early spontaneous abortion in SU-IVF group was significant statistical higher than the DT-IVF group (p < .05) (Table 5). No ectopic pregnancy was found in the both groups.
Table 5.
Comparison of Pregnancy Outcome Index in Two Groups.
| SU group (n = 15) | DT group (n = 16) | x2 value | p value | |
|---|---|---|---|---|
| Clinical pregnancy rate | 8/15 (53.3%) | 10/16 (62.5%) | 0.267 | 0.722 |
| Rate of abortion | 5/8 (62.5%) | 1/10 (10.0%) | 5.513 | 0.043 |
Discussion
In present study, the double tube method for selection high quality spermatozoa was used and compared with swim-up technique. According to the obtained data, motion parameters (sperm motility and vitality), the percentage of recovered normal morphology and round cells and debris (RCDs) were enhanced significantly (p ≤ .001) after the double tube method (DT) and swim-up (SU). Statistically significant difference was found between swim-up and double tube method in these parameters (p < .001). The vitality, motility, and morphology of the spermatozoa are important for sperm function. The assessment of sperm vitality is one of the basic elements of semen analysis, and especially important in samples where many sperm are immotile, to distinguish between immotile dead sperm and immotile live sperm. Sperm selection techniques are the routinely utilized in the clinic for assisted reproductive technologies (ART) such as intrauterine insemination (IUI) and in vitro fertilization (IVF). With the SU technique, washing media is placed over a semen sample inside one tube, which is incubated for approximately 1 hr. During that time, sperm that are highly motile swim into the top layer, then a sterile Pasteur pipette was used to remove the supernatant containing actively motile sperms. Because the pipette was directly inserted into the top layer in swim-up tube, the immotile sperm and RCDs may be pipetted from the bottom of semen. In the present study, with the double tube method, the top layer of containing high quality sperm was flowed through the connected tube into the collected tube and did not use the pipette. The higher motility, vitality and normal morphology sperm were collected by using the double tube method than that of swim-up technique (p < .001). The RCDs were decreased to 0 (while 0.3 × 106 in swim-up, p = .009). The simple sperm wash systems with any standard medium do not effectively separate anomalous spermatozoa. The SU method has been reported to result in a higher level of non-sperm components than DGC (e.g., debris, bacteria) (Björndahl et al., 2005; Chen and Bongso, 1999). In RCDs, leukocytes are the most important for sperm function and fertility. A high number of leukocytes in semen may be associated with male subfertility (Henkel et al., 2005; World Health Organization, 2010). The presence of ROS in the seminal fluid can originate from several sources, both endogenous and exogenous (World Health Organization, 2010).
From this study, the results showed more sperm with normal morphology and more sperm with DNA integrity were acquired on the DT device. The incidence of SDF (TUNEL-positive) was lower when sperm sorting was performed by the DT device than by a conventional SU technique (P<0.001). Selection of the best spermatozoa and elimination of damaged spermatozoa are critical for successful IUI, IVF, and ICSI in infertility clinics. The advantages of the DT device are that it can select highly motile, morphologically normal, and less SDF spermatozoa from initial semen samples. In the present study, results of SDF and DFI tested by TUNEL and SCSA were similar. SCSA is considered to be a precise and repeatable test to detect DFI (Hinting et al., 1988). Our results revealed that in the initial semen, DFI and SDF were 24.8% and 26.0%, respectively, and through DT and SU, the rate of DFI and SDF was 3.9% and 4.4%, and 9.3% and 9.9%, respectively. Levels of ASAs in mixed sperm medium by SU and DT were significantly reduced which compared with pre-treatment semen (p < .05 and p < .01). Sperm MAR positive more than 50% was 0 by using DT, and significantly decreased which compared with pre-treatment spermatozoa (p < .05), but there were no significant deference between SU and pre-treatment (p = .104). Antisperm antibodies (ASAs) play important roles in male and female immunological infertility. The incidence of ASA positive maybe low in infertile males, so in the present study, inclusion criteria included ASAs positive in semen. About 6% of men referred for infertility treatment are diagnosed with membrane-bound ASA (Chamley and Clarke, 2007; Tüttelmann and Nieschlag, 2010). ASAs bounded to the sperm surface, may be tested with non-ASA-spermatozoa by separated sperm methods such as SU and DGC (Grundy et al., 1991; Ryan et al., 1994). ASA may have an adverse effect on fertilization and early embryonic development. The autoimmune antisperm reaction may be accompanied by a more significant decrease in the semen quality and an increase in the proportion of spermatozoa with DNA fragmentation (Ryan et al., 1994).
Caspase-3 is the major effector enzyme causing cell disruption during apoptosis. Grunewald et al. (2010) reported that in 20 infertile semen samples, spermatozoa with active caspase-3 are 51.8%, and after SU, spermatozoa with active caspase-3 are 25.7%. In ram sperm, the percentage of active caspase-3 is decreased and DFI is increased after SU (Pichardo et al., 2010). In human spermatozoa, after SU isolation, the percentage of vital spermatozoa with active caspase-3 is decreased (Kotwicka et al., 2008). In the present study, the level of caspase-3 selected sperm by the DT method was lower than that of SU (p < .001).
We observed that higher motile and normal morphological sperm cells with lower levels of ASAs, DNA fragmentation, caspase-3 and RCDs were selected by SU and DT methods. Through IVF-ET, the rate of fertility and clinical pregnancy were no statistical difference between DT-IVF group and SU-IVF group, while the cleavage rate was higher and the rate of abortion was lower in DT-IVF group than SU-IVF group. These suggested that the result of IVF may be impacted by levels of ASAs, DNA fragmentation, caspase-3 and RCDs in semen and sperm. The reason of no statistical difference of the rate of fertility and clinical pregnancy may be due to too small sample size (only 15 cases in SU-IVF group and 16 cases in DT-IVF group). In addition, IVF provides may have an equal change of conception in couples with ASAs in comparison with couples with no antibodies (Janssen et al., 1992). Some of ASAs may inhibit sperm capacitation and thus prevent all processes of fertilization that follow and some other antibodies may not affect capacitation and not associate with IVF outcome (Vujisić et al., 2005; Yeh et al., 1995). It is known that sperm DNA damage is as a potential cause of infertility. An inverse correlation between SDF and sperm motility has been reported (Palermo et al., 2014). Unexplained infertility may be attributed to a subtle male factor linked to compromised sperm DNA integrity. Double-strand breaks may actually affect the chromosomal integrity of the male genome with consequent contribution to embryo aneuploidy. Swim-up (SU) selection based on sperm motility excludes many spermatozoa with ultrastructural evidence of apoptosis, as confirmed by TUNEL analysis; also there is a correlation between sperm motility and sperm chromatin structure assay (SCSA) parameters; and that sperm DNA fragmentation affects sperm motility and fertilization rates (Giwercman et al., 2003; Piomboni et al., 2006). The normality of sperm nuclear DNA plays a critical role in mammalian fertilization and subsequent embryonic development. It has been demonstrated that sperm cells with damaged or fragmented-DNA can fertilize oocytes in vitro (Hourcade et al., 2010). In the present study, the sample size is small, it may have some influence on the result in the statistical processing, therefore, the sample size should be increased for further research.
Limitations, reasons for caution. In the present study, the sample size is small, it may have some influence on the result in the statistical processing; therefore, the sample size should be increased for further research. Additionally, the inclusion criteria for selection of infertile couples who must agree to do IVF treatment and ASAs positive in semen into the cohort limits the sample size.
In summary, we developed the double tube (DT) for selection sperm. The DT method was yielding a good quality fraction of normal and viable spermatozoa. However, further experiments should be performed in sperm function. Improvement of sperm selection methods is very necessary and important for male factor infertility.
Footnotes
Authors’ Note: The study was approved by the Medical and Health Technology Development Plan of Shandong Province (2015ws0267), and the Key Research and Development Program in Maternal and Child Health Care Hospital of Shandong Province (No: 2019YJ001).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yi Qiu  https://orcid.org/0000-0003-2430-7421
https://orcid.org/0000-0003-2430-7421
References
- Aitken R. J., Finnie J. M., Muscio L., Whiting S., Connaughton H. S., Kuczera L., Rothkirch T. B., De Iuliis G. N. (2014). Potential importance of transition metals in the induction of DNA damage by sperm preparation media. Human Reproduction, 29, 2136–2217. [DOI] [PubMed] [Google Scholar]
- Beck-Fruchter R., Shalev E., Weiss A. (2016). Clinical benefit using sperm hyaluronic acid binding technique in ICSI cycles: A systematic review and meta-analysis. Reproductive BioMedicine Online, 32, 286–298. [DOI] [PubMed] [Google Scholar]
- Björndahl L., Mohammadieh M., Pourian M., Söderlund I., Kvist U. (2005). Contamination by seminal plasma factors during sperm selection. Reproductive BioMedicine Online, 26, 170–173. [DOI] [PubMed] [Google Scholar]
- Chamley L. W., Clarke G. N. (2007). Antisperm antibodies and conception. Seminal Immunopathology, 29, 169–184. [DOI] [PubMed] [Google Scholar]
- Chen M. J., Bongso A. (1999). Comparative evaluation of two density gradient preparations for sperm separation for medically assisted conception. Human Reproduction, 14, 759–764. [DOI] [PubMed] [Google Scholar]
- Giwercman A., Richthoff J., Hjollund H., Bonde J. P., Jepson K., Frohm B., Spano M. (2003). Correlation between sperm motility and sperm chromatin structure assay parameters. Fertility Sterility, 80(6), 1404–1412. [DOI] [PubMed] [Google Scholar]
- Grundy C. E., Robinson J., Gordon A. G., Hay D. M. (1991). Selection of an antibody-free population of spermatozoa from semen samples of men suffering from immunological infertility. Human Reproduction, 6, 593–596. [DOI] [PubMed] [Google Scholar]
- Grunewald S., Reinhardt M., Blumenauer V., Hmeidan A. F., Glander H. J., Paasch U. (2010). Effects of post-density gradient swim-up on apoptosis signalling in human spermatozoa. Andrologia, 42, 127–131. [DOI] [PubMed] [Google Scholar]
- Henkel R., Kierspel E., Stalf T., Mehnert C., Menkveld R., Tinneberg H. R., Schill W. B., Kruger T. F. (2005). Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertility Sterility, 83, 635–642. [DOI] [PubMed] [Google Scholar]
- Hinting A., Vermeulen L., Comhaire F. (1988). The indirect mixed antiglobulin reaction test using a commercially available kit for the detection of antisperm antibodies in serum. Fertility Sterility, 49, 1039–1044. [DOI] [PubMed] [Google Scholar]
- Hourcade J. D., Pérez-Crespo M., Fernández-González R., Pintado B., Gutiérrez-Adán A. (2010). Selection against spermatozoa with fragmented DNA after postovulatory mating depends on the type of damage. Reproductive Biology and Endocrinology, 8, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen H. J., Bastiaans B. A., Goverde H. J., Hollanders H. M., Wetzels A. A., Schellekens L. A. (1992). Antisperm antibodies and in vitro fertilization. Journal of Assisted Reproductive Genetics, 9(4), 345–349. [DOI] [PubMed] [Google Scholar]
- Kotwicka M., Filipiak K., Jedrzejczak P., Warchol J. B. (2008). Caspase-3 activation and phosphatidylserine membrane translocation in human spermatozoa: Is there a relationship? Reproductive BioMedicine Online, 16, 657–663. [DOI] [PubMed] [Google Scholar]
- Li K., Li R., Ni Y., Sun P., Liu Y., Zhang D., Huang H. (2018). Novel distance-progesterone-combined selection approach improves human sperm quality. Journal of Translational Medicine, 16, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D., Pavitt S., Sharma V., Forbes G., Hooper R., Bhattacharya S., Kirkman-Brown J., Coomarasamy A., Lewis S., Cutting R., Brison D., Pacey A., West R., Brian K., Griffin D., Khalaf Y. (2019). Physiological, hyaluronan-selected intracytoplasmic sperm injection for infertility treatment (HABSelect): A parallel, two-group, randomised trial. Lancet, 393, 416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratori M., Tarozzi N., Cambi M., Boni L., Iorio A. L., Passaro C., Luppino B., Nadalini M., Marchiani S., Tamburrino L., Forti G., Maggi M., Baldi E., Borini A. (2016). Variation of DNA fragmentation levels during density gradient sperm selection for assisted reproduction techniques: A possible new male predictive parameter of pregnancy? Medicine (Baltimore), 95, e3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratori M., Tarozzi N., Carpentiero F., Danti S., Perrone F. M., Cambi M., Casini A., Azzari C., Boni L., Maggi M., Borini A., Baldi E. (2019). Sperm selection with density gradient centrifugation and swim up: Effect on DNA fragmentation in viable spermatozoa. Scientific Reports, 9, 7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paasch U., Grunewald S., Fitzl G., Glander H. J. (2003). Deterioration of plasma membrane is associated with activation of caspases in human spermatozoa. Journal of Andrology, 24, 246–252. [DOI] [PubMed] [Google Scholar]
- Palermo G. D., Neri Q. V., Cozzubbo T., Rosenwaks Z. (2014). Perspectives on the assessment of human sperm chromatin integrity. Fertility Sterility, 102(6), 1508–1517. [DOI] [PubMed] [Google Scholar]
- Pichardo A. I., Aragón-Martínez A., Ayala-Escobar M. E., Domínguez-Vara I. A. (2010). Viability tests, active caspase-3 and -7, and chromatin structure in ram sperm selected using the swim-up procedure. Journal of Andrology, 31, 169–176. [DOI] [PubMed] [Google Scholar]
- Piomboni P., Bruni E., Capitani S., Gambera L., Moretti E., La Marca A., De Leo V., Baccetti B. (2006). Ultrastructural and DNA fragmentation analyses in swim-up selected human sperm. Archives of Andrology, 52(1), 51–59. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Wang L. G., Zhang L. H., Zhang A. D. (2012). Quality of sperm obtained by penile vibratorybratory stimulation and percutaneous vasal sperm aspiration in men with spinal cord injury. Journal of Andrology, 33, 1036–1046. [DOI] [PubMed] [Google Scholar]
- Rezaei-Agdam H., Moshari S., Nahari E., Minas A., Daliri Z., Hallaj M., Razi M. (2019). Zeta and hyaluronic acid assessments, novel sperm selection procedures, in animal model for male infertility. Andrologia, 51, e13447. [DOI] [PubMed] [Google Scholar]
- Ryan M., Drudy L., Cottell E., Harrison R. F. (1994). Preparation of antibody free spermatozoa by in vitro immunodepletion using immunobeads. Andrologia, 26, 247–250. [DOI] [PubMed] [Google Scholar]
- Tüttelmann F., Nieschlag E. (2010). Classification of andrological disorders. In Nieschlag E., Behre H. M., Nieschlag S. (eds) Andrology male reproductive health and dysfunction (pp. 87–92). Springer. [Google Scholar]
- Vujisić S., Lepej S. Z., Jerković L., Emedi I., Sokolić B. (2005). Antisperm antibodies in semen, sera and follicular fluids of infertile patients: Relation to reproductive outcome after in vitro fertilization. American Journal of Reproductive Immunology, 54(1), 13–20. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2010). WHO laboratory manual for the examination and processing of human semen (5th ed.). WHO Press, WHO. [Google Scholar]
- Yeh W. R., Acosta A. A., Seltman H. J., Doncel G. (1995). Impact of immunoglobulin isotype and sperm surface location of antisperm antibodies on fertilization in vitro in the human. Fertility and Sterility, 63(6), 1287–1292. [DOI] [PubMed] [Google Scholar]
- Zhang M. H., Zhai L. P., Fang Z. Y., Li A. N., Xiao W., Qiu Y. (2018). Effect of scrotal heating on sperm quality, seminal biochemical substances, and reproductive hormones in human fertile men. Journal of Cellular Biochemistry, 119, 10228–10238. [DOI] [PubMed] [Google Scholar]
- Zhang M. H., Zhang A. D., Shi Z. D., Wang L. G., Qiu Y. (2015). Changes in levels of seminal nitric oxide synthase, macrophage migration inhibitory factor, sperm DNA integrity and caspase-3 in fertile men after scrotal heat stress. PLoS One, October 29. [DOI] [PMC free article] [PubMed] [Google Scholar]