Abstract
Objectives
To identify key long non-coding (lnc)RNAs responsible for the epithelial–mesenchymal transition (EMT) of CNE1 nasopharyngeal carcinoma cells and to investigate possible regulatory mechanisms in EMT.
Methods
CNE1 cells were divided into transforming growth factor (TGF)-β1-induced EMT and control groups. The mRNA and protein expression of EMT markers was determined by real-time quantitative PCR and western blotting. Differentially expressed genes (DEGs) between the two groups were identified by RNA sequencing analysis, and DEG functions were analyzed by gene ontology and Kyoto Encyclopedia of Genes and Genomes analyses. EMT marker expression was re-evaluated by western blotting after knockdown of a selected lncRNA.
Results
TGF-β1-induced EMT was characterized by decreased E-cadherin and increased vimentin, N-cadherin, and Twist expression at both mRNA and protein levels. Sixty lncRNA genes were clustered in a heatmap, and mRNA expression of 14 dysregulated lncRNAs was consistent with RNA sequencing. Knockdown of lnc-PNRC2-1 increased expression of its antisense gene MYOM3 and reduced expression of EMT markers, resembling treatment with the TGF-β1 receptor inhibitor LY2109761.
Conclusion
Various lncRNAs participated indirectly in the TGF-β1-induced EMT of CNE1 cells. Lnc-PNRC2-1 may be a key regulator of this and is a potential target to alleviate CNE1 cell EMT.
Keywords: Nasopharyngeal carcinoma, epithelial–mesenchymal transition, long non-coding RNA, transforming growth factor-β1, lnc-PNRC2-1, MYOM3
Introduction
Nasopharyngeal carcinoma (NPC) is a common cancer of the nasopharynx or upper larynx. The distribution of patients with NPC is mainly concentrated in East Asia and Africa, with higher incidences occurring in Guangdong Province, China.1 Radiation therapy is adopted by most patients with NPC; however, this treatment often leads to local recurrence and distant metastasis which can be fatal.2,3 Therefore, exploring the pathogenesis of NPC is of vital importance for determining the optimal treatment.
Epithelial–mesenchymal transition (EMT) is closely related to cancer metastasis and the development of cancer. It results in tumor cells losing adhesion and gaining invasiveness, causing tumor spread and metastasis.4 The occurrence of EMT involves the down-regulation of E-cadherin and the up-regulation of N-cadherin,5 so these proteins are considered to be important markers of EMT. Liu et al.6 found that the inhibition of movement and colony formation in NPC was associated with the decreased expression of F-actin, phosphorylated focal adhesion kinase and paxillin, and a Slug protein, and the increased expression of E-cadherin. Based on this observation, investigating the correlation between EMT and NPC and exploring the specific mechanism by which EMT influences NPC are expected to be promising for the prospective treatment of NPC.
A growing number of studies have revealed that long non-coding (lnc)RNAs play an important role in the EMT of many cancers. In head and neck cancers in particular, many dysfunctional lncRNAs regulate transcripts associated with EMT invasion/metastasis.7 For example, the expression of lncRNA TUG1 was higher in NPC tissues and cell lines than in healthy tissues, while the inhibition of TUG1 significantly reduced the proliferation, migration, and invasion of NPC cells.8 Zhou et al.9 found that the expression of lncRNA YBX1 was increased in CNE1 cells with EMT, and that its expression negatively correlated with E-cadherin expression, and positively correlated with that of vimentin. The down-regulation of YBX1 partially inhibited the transforming growth factor (TGF)-β1-induced migration of CNE1 cells, suggesting that targeting lncRNA is a promising strategy for NPC treatment. However, the specific lncRNAs responsible for EMT in NPC remain unclear.
In this study, EMT-like cells were induced by TGF-β1, after which an EMT-related lncRNA expression profile was obtained and analyzed using gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. The key regulator lnc-PNRC2-1 was selected for further analysis and its function in TGF-β1-induced EMT was verified using small interfering (si)RNA in CNE1 cells.
Materials and methods
Cell culture and treatment
The human well-differentiated nasopharyngeal carcinoma cell line CNE1 was provided by the Stem Cell Bank (Chinese Academy of Sciences, Shanghai, China). Cells were incubated in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin (all from Life Technologies, Waltham, MA, USA). Cells were grown at 37°C in a humidified incubator with 5% CO2. After incubation for 2 or 3 days, cells in good condition were used for subsequent experiments.
CNE1 cells were seeded at a density of 1 × 106 per well of 6-well plates. EMT was induced by the treatment of 5 ng/mL TGF-β1 (Abcam, Cambridge, MA, USA). The TGF-β1 signaling inhibitor LY2109761 was used to investigate the role of TGF-β1 in inducing EMT. Cells without TGF-β1 treatment were used as controls. CNE1 cells with or without TGF-β1 treatment were incubated for 24 hours, then their morphology was observed using phase contrast microscopy (Olympus, Tokyo, Japan).
Real-time reverse transcription quantitative PCR (RT-qPCR)
RNA was extracted from CNE1 cells using TRIzol reagent (Life Technologies) in accordance with the manufacturer’s instructions. rRNA was eliminated completely using an rRNA removal kit (Takara Bio, Beijing, China) to ensure accurate lncRNA analysis. Then, 1 µg of extracted total RNA was added to the reverse transcription system for cDNA synthesis at 42°C for 2 minutes. Subsequently, the relative RNA level was detected using SYBR qPCR Master Mix (Takara Bio). A total of 20 µL of StepOne Real-Time PCR system was subjected to the following thermal conditions: pre-denaturation at 95°C for 5 minutes, followed by 30 cycles of 95°C for 10 s, 60°C for 30 s, and an extension at 72°C for 30 s. RT-qPCR primers are listed in Table 1. The GAPDH gene was used as an internal reference and the relative mRNA level of target genes was calculated using the 2−ΔΔCt method.
Table 1.
Primer sequences for RT-qPCR.
| Gene name or lnc-RNA | Sequence (5′–3′) |
|---|---|
| AL392086.2 | F: GAAAAGGACAAGTGTCACCC |
| R: GGTAATGTTCCAGTCCAGTCTC | |
| AC097480.1 | F: GCTCCTCCTGAAAAGAGACAG |
| R: CTGAAGTCCTGGTTAGCATG | |
| LINC02257 | F: TCTCGCACTGTCATCCTGG |
| R: CGTAGTCAGCATACCTGTGTG | |
| AC008695.1 | F: CAGGCCATTGCTAACGAAC |
| R: GTAACTGTGGTCCCAAGGC | |
| HSD11B1-AS1 | F: GAGATGATGGAGAGTATGCAG |
| R: GTTCCATACCATCACTTAGGC | |
| AL591178.1 | F: CATCCCCTTGTAAACACTCC |
| R: GGTATCCATCTCTCCTTCTGG | |
| AC093001.1 | F: GTGAACACATCCGAACATCAG |
| R: GTCGCCAAAATGTGTCTGG | |
| AC087762.1 | F: GAAAGAGGAAGATGTGGGCTC |
| R: AACTGGCTTACAACATTCCC | |
| NALCN-AS1 | F: GTGGAGGATGGAGCTGATAAC |
| R: CTAATGAGGTTGGGGAAAGTG | |
| AP001341.1 | F: GTCACAGGATGGACCAGATG |
| R: GATTCAACGAGAGAGCAGGAG | |
| AC0022400.2 | F: CCCTTCTGTTGATTTGTTGG |
| R: GGATTGGTTCTGTTCTTGGATG | |
| AL360169.2 | F: GACTGAGGCAAGAGAATCGC |
| R: GTTTGGTAGACAAGCCTGCC | |
| AC092343.1 | F: GGAGTACAACCACCTGGGAAC |
| R: GCCAAATCTGGGTCATCAATC | |
| MAGEC2 | F: GGAGGGAGCACTTCGTCTATG |
| R: GGACTCTCTCTTCCACATCTTTC | |
| MYOM3 | F: GATGGAGCTGGGAACACAAAG |
| R: GCCTGGATGAAGGTGTTTTC | |
| E-cadherin | F: CTCCAGGACTTAGAATAGTGCC |
| R: GACTCCTCCATTCCTTCCAG | |
| N-cadherin | F: GGGTGAACTTGGTTTTTGGAC |
| R: CATAAAATCCCAGTGCTTCTG | |
| Vimentin | F: GAAGGAGGAAATGGCTCGTC |
| R: GGTATCAACCAGAGGGAGTG | |
| Twist | F: CATCGACTTCCTCTACCAGG |
| R: CTCCTTCTCTGGAAACAATGAC |
RT-qPCR, real-time quantitative reverse transcription PCR; lnc-RNA, long non-coding RNA; F, forward; R, reverse.
LncRNA sequencing and bioinformatics
Total RNA was extracted from both groups of CNE1 cells, followed by purity detection using the Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). After the instrument was set to zero, the absorbance value of 2.5 µL of the RNA solution being tested was measured. Then, transcriptome analysis for differential lncRNA expression was performed by Novogene Co., Ltd. (Beijing, China). LncRNA sequencing findings were validated by RT-qPCR, and lncRNA functional analysis was conducted using bioinformatics including GO and KEGG pathway analyses (https://www.kegg.jp/).10
Western blotting
Western blotting analysis of EMT markers was based on a previously published method with slight modifications.11 CNE1 cells were digested using radioimmunoprecipitation assay cracking liquid (Beyotime Biotechnology, Shanghai, China) and collected proteins were preliminarily separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. The membrane was washed twice with phosphate-buffered saline (PBS) and incubated in blocking liquid for 2 hours. After discarding the remaining liquid, the membrane was incubated overnight at 4°C with primary antibodies including anti-E cadherin (dilution 1:1000), anti-N cadherin (dilution 1:1000), anti-vimentin (dilution 1:1000), and anti-Twist (dilution 1:1000). The anti-β-actin antibody (dilution 1:500) was used as a control. All antibodies were purchased from Abcam. A corresponding secondary antibody (horseradish peroxidase-conjugated goat anti-rat IgG [H+L], Servicebio, Wuhan, China) was incubated at room temperature for 2 hours and detected using ECL chromogenic solution (Thermo Fisher Scientific). The density of protein bands was determined using a Tanon-4600 quantitative image analyzer (Tanon Science and Technology, Shanghai, China).
Cell transfection
CNE1 cells were seeded into 24-well plates with RPMI 1640 medium 24 hours before transfection. LncRNA siRNA and the negative control (si-NC) were constructed by GenePharma Co., Ltd (Shanghai, China). A total of 50 µL of Lipofectamine® 3000 transfection reagent (Invitrogen, Carlsbad, CA, USA) and 50 µL of siRNA were respectively diluted in 450 µL of Opti-MEM, then mixed to form the transfection complex. This mixture was immediately added to cells then incubated for 6 hours at 37°C. Cells were washed twice with PBS, then cultured in fresh complete medium containing 10% FBS for 24 hours.
Statistical analysis
All tests were performed in triplicate and data are shown as means ± standard deviation (SD). Data analysis was performed using GraphPad Prism 7.0 software (San Diego, CA, USA). Comparisons among multiple groups were made using one-way analysis of variance followed by Tukey’s test. Comparisons between two groups were determined using the Student’s t-test. Differences of P < 0.05, P < 0.01, or P < 0.001 were regarded as statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001).
Results
TGF-β1 induced EMT in CNE1 cells
CNE1 cells induced by TGF-β1 were shown to undergo EMT-like morphological changes including a spindle-like appearance, decreased intercellular adhesion, and increased extensive intercellular space (Figure 1a). RT-qPCR revealed significantly reduced mRNA expression of E-cadherin (P < 0.01), and significantly increased mRNA expression of N-cadherin, vimentin, and Twist (P < 0.05, Figure 1b). Additionally, western blotting showed that the protein expression of four EMT marker proteins was consistent with their corresponding mRNA expression (P < 0.001, Figure 1c). These results indicate that EMT was successfully induced by TGF-β1 in CNE1 cells.
Figure 1.
TGF-β1 induced EMT in CNE1 NPC cells. (a) Morphology of indicated cells was shown under Optical microscope. Magnification ×100. The EMT-like morphological changes identified by relatively losing cell-to-cell adhesions and acquiring spindle-like phenotype. Black arrow shows the extensive intercellular space. (b) mRNA expression of E-cadherin, vimentin, N-cadherin, and Twist in TGF-β1-induced and control cells as assessed by RT-qPCR. (c) Protein expression of E-cadherin, vimentin, N-cadherin, and Twist in TGF-β1-induced and control cells as assessed by western blotting. *P < 0.05, **P < 0.01 vs. TGF-β1(–).
TGF, transforming growth factor; NPC, nasopharyngeal carcinoma; RT-qPCR, real-time quantitative reverse transcription PCR; EMT, epithelial–mesenchymal transition.
Differentially expressed lncRNA profiles and their functions in EMT
LncRNA sequencing identified 7569 DEGs, including 4180 that were up-regulated and 3389 that were down-regulated in TGF-β1-induced CNE1 cells compared with controls. A heatmap of the top 60 DEGs was produced following bioinformatics analysis (Figure 2a), revealing the clustering of lncRNAs whose expression was significantly altered in CNE1 cells undergoing TGF-β1-induced EMT. Twenty DEGs (10 up-regulated and 10 down-regulated genes) were selected for verification by RT-qPCR. Six of these showed negative amplification (although this could reflect sequencing errors), but the expression of the remaining 14 agreed with the bioinformatics analysis (Figure 2b). Because very few predicted target mRNAs of lnc-PNRC2-1 were identified, we analyzed all predicted target mRNAs of all differentially expressed lncRNAs. Among the up-regulated lncRNAs, novel transcript lncRNA AL591178.1 (lnc-PNRC2-1) up-regulation was the most significant (P < 0.01). GO analysis suggested that DEGs were mainly enriched in functional regions related to ribosome function, protein translation and transportation, signal recognition particle-dependent translational protein targeting to the membrane, protein targeting to the endoplasmic reticulum (ER), and protein localization to the ER (Figure 3a). Consistently, KEGG analysis showed that these DEGs were mainly enriched in corresponding pathways associated with myocardial function, neurodegenerative diseases, and substrate metabolism and development, such as dilated cardiomyopathy, autoimmune thyroid disease, retinol metabolism, and gonadotropin-releasing hormone signaling pathways (Figure 3b). Because more protein translation and energy are required in EMT, this suggests that these genes play important roles in TGF-β1-induced EMT.
Figure 2.
LncRNA sequencing and bioinformatic analysis. (a) Hierarchical clustering heatmap of differentially expressed genes. (b) RT-qPCR verification of 20 differentially expressed target genes in TGF-β1-induced and control cells. *P < 0.05, **P < 0.01 vs. TGF-β1(–).
ns, non-significant; lnc-RNA, long non-coding RNA.
Figure 3.
GO and KEGG pathway analyses of differentially expressed mRNAs. (a) GO analysis. (b) KEGG analysis.
GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
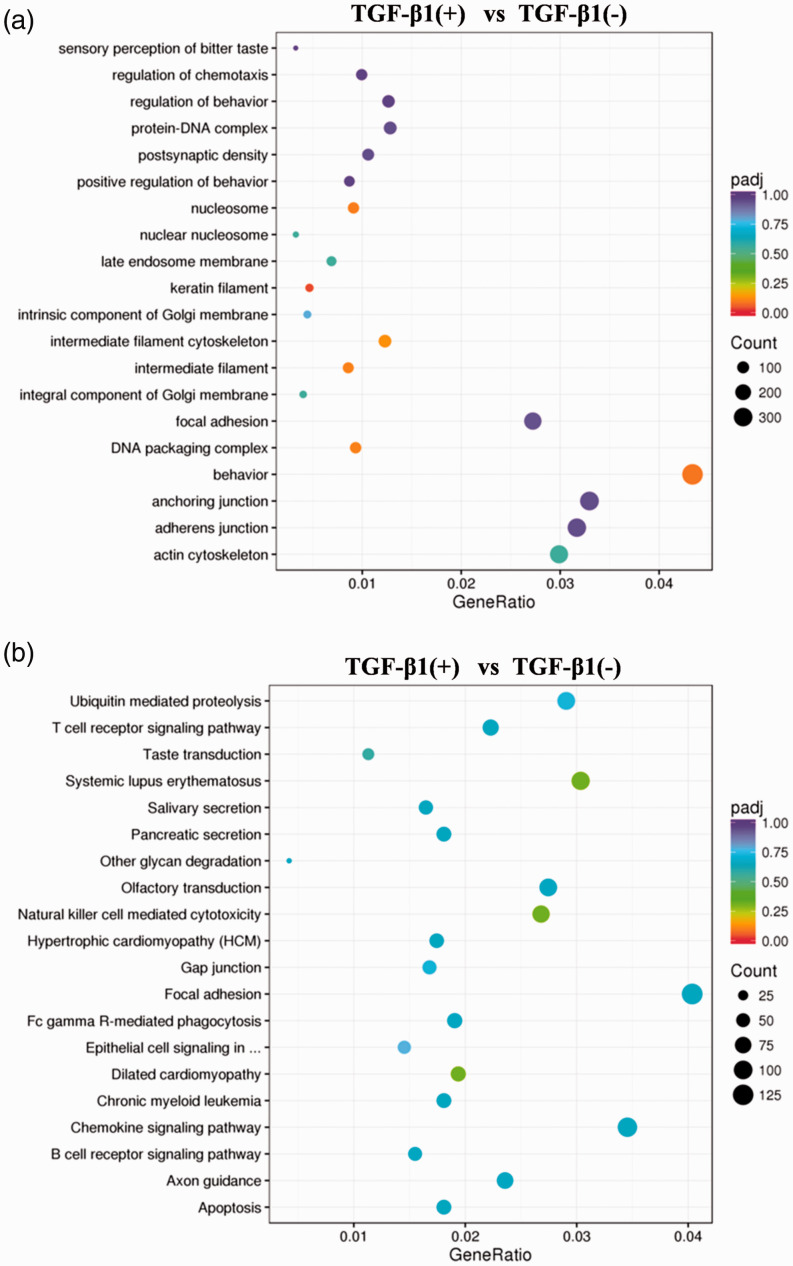
Additionally, GO (Figure 4a) and KEGG (Figure 4b) pathway analyses of the predicted target mRNAs of all differentially expressed lncRNAs indicated that the lncRNAs are mainly associated with biologic processes including behavior, regulation of chemotaxis, and regulation of behavior, and pathways regulating natural killer cell-mediated cytotoxicity, dilated cardiomyopathy, and systemic lupus erythematosus. We also observed that EMT-related genes did not overlap with any co-expressed or co-localized target genes of lnc-PNRC2-1 (data not shown), suggesting that lncRNAs may not regulate EMT directly.
Figure 4.
GO and KEGG pathways of the predicted target mRNAs of differentially expressed lncRNAs. (a) GO pathways. (b) KEGG pathways.
GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
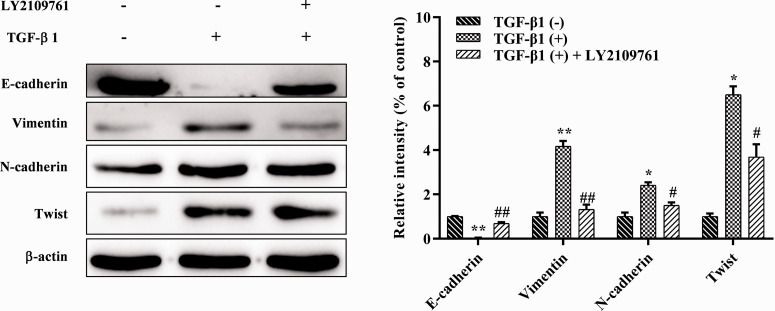
Use of a TGF-β1 inhibitor reversed TGF-β1-induced EMT
Western blotting showed that the progression of EMT in CNE1 cells was effectively inhibited by treatment with the TGF-β1 inhibitor LY2109761 (Figure 5). Compared with the EMT group, the protein expression of E-cadherin was significantly increased (P < 0.01), while that of N-cadherin, vimentin, and Twist was significantly reduced in cells treated with LY2109761 (P < 0.05). This showed that EMT can be inhibited, but not completely eliminated, in CNE1 cells, and indicated that TGF-β1 plays a key role in the induction of EMT in CNE1 cells.
Figure 5.
Verification of the role of TGF-β1 inhibitor on TGF-β1-induced EMT using western blotting. *P < 0.05, **P < 0.01 vs. TGF-β1(–); #P < 0.05, ##P < 0.01 vs. TGF-β1(+).
TGF, transforming growth factor; EMT, epithelial–mesenchymal transition.
Knockdown of lnc-PNRC2-1 inhibited EMT in CNE1 cells
Lnc-PNRC2-1 was silenced by siRNA in CNE1 cells with or without TGF-β1 to further verify its function in EMT. As shown in Figure 6a and 6b, the mRNA and protein expression of E-cadherin was significantly increased in the si-AL (siRNA targeting lnc-PNRC2-1) group (P < 0.01), while the mRNA and protein expression of N-cadherin, vimentin, and Twist was all significantly reduced compared with the si-NC group (P < 0.05), suggesting that lnc-PNRC2-1 functions in EMT in CNE1 cells. Additionally, lnc-PNRC2-1 knockdown significantly increased the expression of its antisense gene MYOM3 (https://www.genecards.org/) in CNE1 cells (P < 0.01).
Figure 6.
Lnc-PNRC2-1 knockdown inhibited EMT in CNE1 cells. (a) RT-qPCR analysis of the expression of E-cadherin, vimentin, N-cadherin, Twist, and MYOM3 in si-NC and si-AL groups. (b) Western blotting analysis of the expression of E-cadherin, vimentin, N-cadherin, Twist, and MYOM3 proteins in si-NC and si-AL groups (*P < 0.05, **P < 0.01, ***P < 0.001 vs. si-NC). (c) Lnc-PNRC2-1 knockdown inhibited TGF-β1-induced EMT of CNE1 cells. Each value represents the mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001 vs. blank; #P < 0.05, ##P < 0.01 vs. TGF-β1 group).
EMT, epithelial–mesenchymal transition; RT-qPCR, real-time quantitative reverse transcription PCR; TGF, transforming growth factor.
As shown in Figure 6c, the protein expression of E-cadherin and MYOM3 was significantly decreased in the TGF-β1 group compared with the control (P < 0.01), while that of N-cadherin, vimentin, and Twist was significantly increased (P < 0.01). However, TGF-β1-induced EMT, as represented by marker expression, was almost completely reversed in cells pre-incubated with si-AL, together with a significant increase in MYOM3 expression (P < 0.01). These results indicate that TGF-β1-induced EMT can be alleviated by inhibiting lnc-PNRC2-1 expression.
Discussion
Most NPC occurs as the subtype poorly differentiated squamous cell carcinoma. Because of its hidden lesions and the difficulty of early detection, about 60% of patients have cervical lymph node metastasis, and 20% have cranial nerve involvement.12 Increasing attention has been paid to the role of EMT in NPC, so exploring the molecular mechanism underlying the occurrence and development of NPC and identifying early diagnostic indicators are extremely important for improving its prognosis.
The loss of E-cadherin expression is considered to be important in the process of cancer development and is also a key step of EMT.13 E-cadherin maintains the phenotype of epithelial cells, and its decrease during EMT reduces cell adhesion and causes tumor cells to shed and migrate easily, thus promoting tumor metastasis.14 Current research indicates that TGF-β is a crucial cytokine in EMT and it is often used to induce EMT in vitro.15 Recent studies have shown that cells undergoing EMT obtain characteristics similar to those of stem cells. These undifferentiated stromal cells stop expressing E-cadherin and instead express N-cadherin, Snail, a specific protease, and some totipotent transcription factors such as Oct-4 and Nanog.14 The EMT of epithelial cells is related to morphological changes and variations in molecular marker expression levels. EMT-induced genes such as Twist indirectly inhibit the activity of E-cadherin, further leading to the loss of cell polarity.16 Tumor cells deriving from epithelial cells through EMT acquire movement and invasion abilities, then transfer to healthy organs and tissues via the blood and lymph circulation.17 Our results indicate that TGF-β1 induced EMT in CNE1 cells, which manifested through reduced intercellular adhesion and spindle-like changes. Additionally, after EMT, the mRNA and protein expression of E-cadherin was significantly reduced, while that of N-cadherin, Twist, and vimentin was significantly increased. Moreover, characteristics similar to those of stem cells were obtained, which were indicative of EMT.
LncRNAs localize in the nucleus and cytoplasm where they participate in gene regulation at multiple levels such as epigenetics, transcription, and post-transcription. Recent studies have found that lncRNAs play important roles in regulating tumor proliferation, apoptosis, and EMT.18 For example, Richards et al.19 found that lncRNA-HIT promoted the invasion and migration of breast cancer cells, and that its inhibition up-regulated the expression of E-cadherin and down-regulated that of vimentin. Furthermore, high expression of lncRNA-MALAT-1 was found in bladder cancer tissues, and MALAT-1 knockdown decreased the expression of EMT regulatory factors ZEB1, ZEB2, and Slug, and significantly increased that of E-cadherin.20 Additionally, lncRNA-H19 was highly expressed in stromal-like tumor cells and tumor tissues, competitively antagonized the function of microRNA(miR)-138 and miR-200A, and increased the expression of vimentin, ZEB1, and ZEB2.21 In the present study, we detected the altered expression of multiple lncRNAs in CNE1 cells after EMT. Using differential gene clustering analysis, we identified 7569 lncRNA DEGs, from which 60 lncRNAs were selected and considered as potential key regulators in the TGF-β1-induced EMT of CNE1 cells.
Because it was difficult to list all GO terms of 7569 DEGs, we clustered the top 20 GO terms. Most of these terms were associated with ribosome function, protein translation and transportation, all of which strongly correlate with the progression of cancer. Several terms related to ribosomes have been shown to be involved in tumorigenesis,22 while ribosome-associated lncRNAs play important cellular functions and regulate both lifespan and sugar metabolism which are critical to tumor cell survival.23,24 Protein translation and transportation may be related to specific metabolism and promote the proliferation of cancer cells. For example, enzymes are key to biological processes and their regulation occurs at almost every step of cancer formation.25 Furthermore, lncRNAs involved in protein ubiquitination may participate in tumorigenesis.26
We also listed the top 20 enrichment pathways identified by KEGG analysis. Identified DEGs were mainly involved in myocardial function, disease, substrate metabolism and development, and cancer development. Myocardial abnormalities were previously shown to be essential in the development of cancer,27 while some disease-related functional pathways may contribute to tumor-related or unrelated lncRNA cleavage. Recent studies reported the association of lncRNAs with Parkinson’s disease and abnormal neurodevelopmental processes, which may also be crucial for initiating the malignant proliferation of tumor cells.28,29 The metabolism of specific substrates may also be involved in tumor development. For instance, glycosaminoglycans and other nuclear regulatory factors regulate tumorigenesis,30 while pathways associated with development that recognize functional lncRNAs are related to tumor formation.31,32 In the present study, important GO terms and pathways were identified as crucial for EMT in CNE1 cells. The expression of 14 of the 20 most dysregulated lncRNAs identified by RNA sequencing was verified using RT-qPCR. Among them, HSD11B1-AS1 (lnc-LAMB3-1), AC008695.1, and MAGEC2 have been reported to be key for tumor cell EMT.33–35 We found that the extent of EMT was significantly reduced after treatment with a TGF-β1 inhibitor, as shown by significant changes in the expression of EMT marker proteins. Taken together, these findings revealed that expression changes of lncRNAs are closely related to the occurrence and development of EMT in CNE1 cells. Because the predicted target mRNAs of lnc-PNRC2-1 were too few to perform GO or KEGG enrichment analysis, we analyzed all predicted target mRNAs of all differentially expressed lncRNAs. Our findings show that lncRNAs may not directly regulate EMT, because co-expressed or co-localized genes were not enriched in EMT-related GO terms or KEGG pathways.
Lnc-PNRC2-1 is a subcategory (RNA class) of the ENSG00000225315 gene whose antisense gene is MYOM3. Because dysregulated MYOM3 expression may affect the survival of patients with renal cancer or lung cancer,36,37 we hypothesized that MYOM3 is also associated with the EMT of NPC. As expected, we found that lnc-PNRC2-1 knockdown downregulated EMT and significantly altered the expression of its marker proteins, resembling the effects of treatment with the TGF-β receptor inhibitor LY2109761. Consistently, we also found that lnc-PNRC2-1 knockdown significantly increased MYOM3 protein expression. It has been reported that specific lncRNA antagonism can silence lncRNA expression in vivo,38 so we propose that lnc-PNRC2-1 could be used as a target to inhibit the EMT of CNE1 cells.
Conclusion
Many lncRNAs are involved in the TGF-β-induced EMT of CNE1 NPC cells. Lnc-PNRC2-1 showed the most significant change in expression in CNE1 cells after EMT, and likely mediates TGF-β-induced EMT, at least in part, indicating a novel potential target for NPC therapy. However, future studies should also investigate the other differentially expressed lncRNAs identified in this study for their role in NPC EMT.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Applied Basic Research Project Fund of the Yunnan Province Science and Technology Department and Kunming Medical University [Grant No.: 2018FE001(-244)].
ORCID iD: Xiaojiang Li https://orcid.org/0000-0002-7412-6795
References
- 1.Huang ML, Zou Y, Yang R, et al. Placenta specific 8 gene induces epithelial-mesenchymal transition of nasopharyngeal carcinoma cells via the TGF-β/Smad pathway. Exp Cell Res 2019; 374: 172–180. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Wang W, Chen S, et al. FOXA1 reprograms the TGF-β-stimulated transcriptional program from a metastasis promoter to a tumor suppressor in nasopharyngeal carcinoma. Cancer Lett 2019; 442: 1–14. [DOI] [PubMed] [Google Scholar]
- 3.He Z, Dong W, Li Q, et al. Sauchinone prevents TGF-β-induced EMT and metastasis in gastric cancer cells. Biomed Pharmacother 2018; 101: 355–361. [DOI] [PubMed] [Google Scholar]
- 4.Lee AW, Ng WT, Chan YH, et al. The battle against nasopharyngeal cancer. Radiother Oncol 2012; 104: 272–278. [DOI] [PubMed] [Google Scholar]
- 5.Huang G, Du MY, Zhu H, et al. MiRNA-34a reversed TGF-β-induced epithelial-mesenchymal transition via suppression of SMAD4 in NPC cells. Biomed Pharmacother 2018; 106: 217–224. [DOI] [PubMed] [Google Scholar]
- 6.Liu SC, Huang CM, Bamodu OA, et al. Ovatodiolide suppresses nasopharyngeal cancer by targeting stem cell-like population, inducing apoptosis, inhibiting EMT and dysregulating JAK/STAT signaling pathway. Phytomedicine 2019; 56: 269–278. [DOI] [PubMed] [Google Scholar]
- 7.Kolenda T, Guglas K, Ryś M, et al. Biological role of long non-coding RNA in head and neck cancers. Rep Pract Oncol Radiother 2017; 22: 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian W, Ren Z, Lu X. Knockdown of long non-coding RNA TUG1 suppresses nasopharyngeal carcinoma progression by inhibiting epithelial-mesenchymal transition (EMT) via the promotion of miR-384. Biochem Biophys Res Commun 2019; 509: 56–63. [DOI] [PubMed] [Google Scholar]
- 9.Zhou LL, Ni J, Feng WT, et al. High YBX1 expression indicates poor prognosis and promotes cell migration and invasion in nasopharyngeal carcinoma. Exp Cell Res 2017; 361: 126–134. [DOI] [PubMed] [Google Scholar]
- 10.Geng L, Xu X, Zhang H, et al. Comprehensive expression profile of long non-coding RNAs in Peripheral blood mononuclear cells from patients with neuropsychiatric systemic lupus erythematosus. Ann Transl Med 2020; 8: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng PH, Chieh-Yu Lai J, Hsu KW, et al. Hypoxia-induced lncRNA RP11-390F4.3 promotes epithelial-mesenchymal transition (EMT) and metastasis through upregulating EMT regulators. Cancer Lett 2020; 483: 35–45. [DOI] [PubMed] [Google Scholar]
- 12.Tang LL, Chen WQ, Xue WQ, et al. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett 2016; 374: 22–30. [DOI] [PubMed] [Google Scholar]
- 13.Tepass U, Truong K, Godt D, et al. Cadherins in embryonic and neural morphogenesis. Nat Rev Mol Cell Biol 2000; 1: 91–100. [DOI] [PubMed] [Google Scholar]
- 14.Shargh SA, Sakizli M, Khalaj V, et al. Downregulation of E-cadherin expression in breast cancer by promoter hypermethylation and its relation with progression and prognosis of tumor. Med Oncol 2014; 31: 250. [DOI] [PubMed] [Google Scholar]
- 15.Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139: 871–890. [DOI] [PubMed] [Google Scholar]
- 16.Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol 1982; 95: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartis D, Mise N, Mahida RY, et al. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax 2014; 69: 760–765. [DOI] [PubMed] [Google Scholar]
- 18.Hauptman N, Glavač D. Long non-coding RNA in cancer. Int J Mol Sci 2013; 14: 4655–4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards EJ, Zhang G, Li ZP, et al. Long non-coding RNAs (LncRNA) regulated by transforming growth factor (TGF) β: LncRNA-hit-mediated TGFβ-induced epithelial to mesenchymal transition in mammary epithelia. J Biol Chem 2015; 290: 6857–6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ying L, Chen Q, Wang Y, et al. Upregulated MALAT-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol Biosyst 2012; 8: 2289–2294. [DOI] [PubMed] [Google Scholar]
- 21.Liang WC, Fu WM, Wong CW, et al. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget 2015; 6: 22513–22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Zhang YH, Lu G, et al. Analysis of cancer-related lncRNAs using gene ontology and KEGG pathways. Artif Intell Med 2017; 76: 27–36. [DOI] [PubMed] [Google Scholar]
- 23.Xu XD, Shao SX, Jiang HP, et al. Warburg effect or reverse Warburg effect? A review of cancer metabolism. Oncol Res Treat 2015; 38: 117–122. [DOI] [PubMed] [Google Scholar]
- 24.Marcel V, Catez F, Mertani HC, et al. [The ribosome: a new player in tumorigenesis?]. Med Sci (Paris) 2014; 30: 21–24. [DOI] [PubMed] [Google Scholar]
- 25.He X, Tan X, Wang X, et al. C-Myc-activated long noncoding RNA CCAT1 promotes colon cancer cell proliferation and invasion. Tumour Biol 2014; 35: 12181–12188. [DOI] [PubMed] [Google Scholar]
- 26.Qiu JJ, Lin YY, Ye LC, et al. Overexpression of long non-coding RNA HOTAIR predicts poor patient prognosis and promotes tumor metastasis in epithelial ovarian cancer. Gynecol Oncol 2014; 134: 121–128. [DOI] [PubMed] [Google Scholar]
- 27.Wang R, Zhao L, Ji L, et al. Myocardial infarction associated transcript (MIAT) promotes papillary thyroid cancer progression via sponging miR-212. Biomed Pharmacother 2019; 118: 109298. [DOI] [PubMed] [Google Scholar]
- 28.Soreq L, Guffanti A, Salomonis N, et al. Long non-coding RNA and alternative splicing modulations in Parkinson's leukocytes identified by RNA sequencing. PLoS Comput Biol 2014; 10: e1003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Northcott PA, Shih DJ, Peacock J, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature 2012; 488: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vigetti D, Deleonibus S, Moretto P, et al. Natural antisense transcript for hyaluronan synthase 2 (HAS2-AS1) induces transcription of HAS2 via protein O-GlcNAcylation. J Biol Chem 2014; 289: 28816–28826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drevon C, Jaffredo T. Cell interactions and cell signaling during hematopoietic development. Exp Cell Res 2014; 329: 200–206. [DOI] [PubMed] [Google Scholar]
- 32.Gloss B, Moran-Jones K, Lin V, et al. ZNF300P1 encodes a lincRNA that regulates cell polarity and is epigenetically silenced in type II epithelial ovarian cancer. Mol Cancer 2014; 13: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vishnubalaji R, Shaath H, Elkord E, et al. Long non-coding RNA (lncRNA) transcriptional landscape in breast cancer identifies LINC01614 as non-favorable prognostic biomarker regulated by TGFβ and focal adhesion kinase (FAK) signaling. Cell Death Discov 2019; 5: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kast RE, Skuli N, Cos S, et al. The ABC7 regimen: a new approach to metastatic breast cancer using seven common drugs to inhibit epithelial-to-mesenchymal transition and augment capecitabine efficacy. Breast Cancer (Dove Med Press) 2017; 9: 495–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Wang L, Liu J, et al. Expression and prognostic relevance of MAGE-A3 and MAGE-C2 in non-small cell lung cancer. Oncol Lett 2017; 13: 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. The Human Protein Atlas. MYOM3. https://www.proteinatlas.org/ENSG00000142661-MYOM3/pathology/renal+cancer (accessed 10 Febrary 2021)
- 37.http://bioinfo.henu.edu.cn/LUCAGSE26939
- 38.Krützfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with ‘antagomirs'. Nature 2005; 438: 685–689. [DOI] [PubMed] [Google Scholar]