Abstract
Objective
This was a prospective study to investigate whether progesterone affects sperm activity by regulating the cyclic AMP-protein kinase A (cAMP-PKA) signalling pathway via α/β hydrolase domain-containing protein 2 (ABHD2).
Methods
Spermatozoa were collected from healthy and infertile men (with oligoasthenospermia or abnormal acrosome; n = 30/group). The expression of and mutations in ABHD2 were detected by quantitative PCR, western blot, and gene sequencing. The expression of ABHD2 in the presence of progesterone was detected in all groups, and cAMP and PKA levels were detected by ELISA in fertile men after treatment with ABHD2 antibody and PKA inhibitor H-89, respectively.
Results
Expression of ABHD2 mRNA and protein were reduced in spermatozoa from infertile compared with fertile men. Four gene mutation sites were detected in spermatozoa from the infertile groups. Progesterone increased mRNA and protein levels of ABHD2 in healthy spermatozoa but not in spermatozoa from infertile men. The levels of cAMP and PKA were increased by progesterone in healthy spermatozoa, and the progesterone-increased cAMP and PKA were decreased by ABHD2 antibody and H-89, respectively.
Conclusion
Progesterone regulates the ABHD2-mediated cAMP-PKA signalling pathway in healthy spermatozoa, which provides a new target for clinical diagnosis and treatment of infertility.
Keywords: Sperm, progesterone, abhydrolase domain containing 2, acylglycerol lipase, cyclic AMP-protein kinase A signalling pathway, gene mutation, oligoasthenospermia
Introduction
Infertility usually refers to the failure of a couple to conceive within 1 year of normal sexual activity without contraceptive measures. The proportion of infertile couples in the world is about 15%, of which male factors account for 30% to 40%.1 An increasing number of infertile couples have received or will receive assisted reproductive technology (ART) treatment.2 Sperm selection is an important step in ART treatment because abnormal spermatozoa increase the risk of genetic defects in offspring.3 At present, a variety of sperm selection or preparation methods for ART have been developed, including the upstream method, density gradient centrifugation, electrophoresis technology, magnetic cell sorting with annexin V-coated beads, hyaluronic acid binding, microfluidic technology, and morphological examination of motile sperm organelles, among others.2,4 However, these sperm processing and preparation techniques only use the physical and chemical properties of sperm for selection, bypassing the natural selection process through biological properties during in vivo fertilisation.5,6
Progesterone regulates various functions of human spermatozoa, including capacitation, motility, hyperactivation, acrosomal reaction, and chemotaxis.7–9 Because progesterone can stimulate capacitation in some spermatozoa, exposure to progesterone has been used as a chemokine to prepare the best spermatozoa.7,8,10 Progesterone regulates sperm function through a variety of signal pathways, including phosphorylation of heat shock protein 9011 and Ca2+ cation channels (CatSper).12,13 The CatSper-Ca2+ signalling pathway is very important for the regulation of human sperm function. Studies have shown that the hormones progesterone and prostaglandin in the reproductive system regulate the physiological function of human spermatozoa mainly through changes in Ca2+ concentration mediated by CatSper channels.14 In addition, many reports indicate that some synthetic fragrances and a variety of environmental endocrine disruptors can affect the physiological function of human sperm by activating CatSper channels.12,14
Progesterone directly activates the spermatozoa-specific calcium channel CatSper; in addition, it induces extracellular calcium influx and ultimately regulates spermatozoa-related physiological functions. The CatSper inhibitor mibefradil cannot completely block the effect of progesterone on mature human spermatozoa.15 Recently, it was reported that progesterone could hydrolyse endogenous cannabinoid, which inhibits the CatSper channel, by binding and activating the highly expressed type II domain dehydrogenase ABHD2 (α/β hydrolase domain-containing protein 2) receptor on human spermatozoa.16–18 The intracellular signalling mechanism underlying the regulation of sperm function by progesterone remains to be elucidated.19
ABHD2 (a member of the metabolic serine hydrolases superfamily) is considered a membrane receptor of progesterone16 and the core functional protein of the nongenomic effects of progesterone in spermatozoa.20 Activation of the cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA) pathway is an early event during capacitation.21 Recently, it was demonstrated that the Ca2+ channel CatSper is not activated by cAMP-PKA signalling: CatSper was not activated directly by intracellular cAMP or indirectly by the cAMP-PKA pathway.22 However, there is no further direct evidence that ABHD2 is a human sperm progesterone receptor. Because progesterone and ABHD2 are both involved in the regulation of sperm function, in this study, we aimed to test whether progesterone differentially regulates ABHD2 expression in normal and abnormal spermatozoa, and to assess the involvement of cAMP-PKA signalling pathway. Male infertility as it relates to sperm motility is a complicated disease, with mutations and polymorphisms in multiple genes. We also detected mutations in the ABHD2 gene, which could be useful in determining the failure of sperm–egg binding and explaining some unknown aspects of infertility.
Material and methods
Ethics statement
This work was approved by the reproductive medicine ethics committee of Shanghai Ji’Ai Genetic and Infertility Diagnosis and Treatment Center (JIAIE2017-08). All patients provided written informed consent.
Specimens
Semen samples were taken from infertile patients (duration of infertility longer than 1 year) and classified by the Shanghai Ji’ai Genetic and Infertility Center into two groups: (1) oligoasthenospermia: spermatozoa concentration of 5 to 15 × 106/mL and progressive motility rate (PR) <32% or PR+ normal morphology (NR) <40%; and (2) abnormal sperm acrosome reaction: normal morphology, abnormal sperm function (spontaneous) detected based on fluorescein isothiocyanate and Pisum sativum agglutinin (FITC-PSA) staining, the World Health Organization standard. The control group (volunteers) were fertile (normal morphology ≥4%) men who met the spermatozoa donation standard of the National Health Commission Human Sperm Bank. Thirty participants (n = 30) were included in each group, and all participants were younger than 35 years of age. This trial followed the CONSORT statement (http://www.consort-statement.org/). The patients and volunteers were abstinent for 2 to 7 days, and spermatozoa were obtained through masturbation.
Purification, separation, and treatment of spermatozoa
Spermatozoa were purified by Percoll density gradient centrifugation.23 Spermatozoa samples from the control, oligoasthenospermia, and abnormal acrosome groups were collected. Then, 100% and 50% equal volumes of Percoll separation solution and spermatozoa sample (a 1:1 mix of Percoll plus spermatozoa and a 1:2 mix of Percoll plus spermatozoa, respectively) were added into the tube from bottom to top and centrifuged at 500 × g for 20 minutes at room temperature. The white sperm precipitations were isolated, washed twice with phosphate-buffered saline (PBS), resuspended in HEPES buffer (#15630106, Thermo Fisher Scientific, Waltham, MA, USA), and placed in a 37°C incubator for subsequent experimental studies.
Sensitivity of spermatozoa to progesterone
Spermatozoa from oligoasthenospermia and control samples were incubated in a 5% CO2 incubator at 37°C for 3.5 hours to enable sperm capacitation. Then, 3 μM progesterone was added and samples were incubated for 2 hours. Finally, expression levels of ABHD2 were assessed.
Effects of ABHD2 and PKA on progesterone-regulated sperm function
To detect the role of ABHD2 in sperm capacitation, spermatozoa from fertile (control) participants were incubated in a 5% CO2 incubator at 37°C for 3.5 hours to enable sperm capacitation. Then, the spermatozoa were incubated with 3 μM progesterone for 2 hours with or without pretreatment with 100 μmol/L ABHD2 antibody (ab230417, Abcam, Cambridge, UK) or the PKA inhibitor H89 (MCE, Shanghai, China) for 15 minutes. Then, the effects of progesterone on cAMP, PKA, and acrosome reaction in spermatozoa were studied.
Western blot analysis
The spermatozoa samples were precipitated and lysed using cooled radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitor to lyse the cells and then centrifuged at 10,000 × g and 4°C for 12 minutes to collect the protein. The protein was quantified by the bicinchoninic acid (BCA) method (Beyotime Biotechnology, Shanghai, China), and the loading buffer was added. After mixing, the cells were soaked in 100°C water for 5 minutes and cooled on ice. After sodium dodecyl sulfate-PAGE, the protein was transferred onto a polyvinylidene difluoride membrane, blocked in 5% nonfat milk for 1 hour, incubated with ABHD2 antibody (1:1000, ab230417, Abcam) overnight at 4°C, washed in PBS 3 times (10 minutes each), and incubated with horseradish peroxidase (HRP)-labelled goat anti-rabbit IgG secondary antibody for 1 hour at room temperature. Then, enhanced chemiluminescence (ECL) with a substrate chromogenic solution (Thermo Fisher Scientific) was used to detect the signal by using the Bio-Rad Gel Doc XR+ (BioRad Laboratories, Hercules, CA, USA), and intensity was quantified using ImageJ (National Institutes for Health, Bethesda, MD, USA).
Gene expression by reverse transcription quantitative PCR
RNA was extracted from spermatozoa using the Trizol reagent (Sigma-Aldrich Co., St. Louis, MO, USA) and centrifuged at 12,000 × g and 4°C for 5 minutes. Reverse transcription (in a total reaction of 20 μL) was performed according to the instructions of the Takara reverse transcription kit (RR047A; Takara, Dalian, China), with the following protocol: 42°C for 5 minutes, 37°C for 20 minutes, and 80°C for 1 minute. Quantitative (q)PCR was performed on a Roche 480 fluorescence quantitative PCR instrument (Roche, Mannheim, Germany) in a 25-μL reaction system containing 2.5 μL of 200 μM dNTPs, 4 μL of 6 mM Mg2+, 1 μL of 600 nM primers, 0.5 μL of 200 nM SYBR Green, 0.3 μL of 1.5 U Taq, and 5 μL (100 ng) of template. The primer sequences were as follows: ACTB (β-actin) forward: 5′-ATCTCCTTCTGCATCCTGTCGG-3′, reverse: 5′-CATGGAGTCCTGGCATCCACGA-3′; ABHD2 forward: 5′-CCTCATGTTTGCCTTCTTTGC-3′, reverse: 5′-GGCGGGTACATTTCTCCATC-3′. The protocol was 95°C for 15 minutes; 95°C for 20 s, 60°C for 45 s, with 40 cycles.
Detection of cAMP content in spermatozoa
A human cAMP ELISA Kit (Suzhou Calvin Biotechnology Co. Ltd., Suzhou, China) was used. The kit was removed from a 4°C refrigerator and equilibrated at room temperature for 20 minutes. Then, 50 μL of 0, 1.5, 3, 6, 12, and 24 nmo1/L concentrations of the standard sample, 10 μL of sperm sample (2 × 105 spermatozoa), and 40 μL of distilled, deionised H2O (ddH2O) were added into the wells of a six-well plate. Then, 100 μL of HRP was added into each well, and the plates were incubated in a 37°C water bath for 60 minutes before being washed five times in ddH2O. Finally, 50 μL of substrates A and B were added to each well and incubated at 37°C for 15 minutes in the dark. The reactions were stopped by adding 50 μL of termination solution. The absorbance was immediately detected at 450 nm using a microplate reader, and the concentration of cAMP was calculated according to a standard curve.
Detection of PKA activity
A GENMED kit was used for the quantitative detection of cell PKA activity (GMS5059.1, Shanghai Jiemei Gene Pharmaceutical Technology Co. Ltd. Shanghai, China). After centrifugation of the original sperm samples from all three groups at 300 × g and 4°C for 5 minutes, the supernatant (2 × 105 spermatozoa) was carefully aspirated. First, 3 mL of GENMED lysis solution (reagent B) was added to each pellet, vortexed vigorously for 15 s, incubated in an ice tank for 30 minutes, and centrifuged at 16,000 × g and 4°C for 5 minutes. Then, 23 μL of GENMED buffer (reagent C) and 2 μL of GENMED substrate solution (reagent D) were added into each centrifuge tube with 50 μL of standard solution or sample and 25 μL of GENMED reaction solution (reagent E), and incubated for 30 minutes at 4°C. Finally, 25 μL of GENMED enzymatic solution (reagent F) was added and incubated for 30 minutes at room temperature. The reaction was terminated by adding 25 μL of GENMED termination solution (reagent G) and fluorescence was immediately detected by fluorescence microplate reader. The relative fluorescence reading (in relative fluorescence units, RFU) was obtained at 25°C with excitation and emission wavelengths of 485 and 530 nm. The PKA concentration and activity of samples were calculated according to a standard curve.
Assessment of acrosome integrity by FITC-PSA staining
A sperm acrosome staining kit (Anhui Anke Bioengineering, Beijing, China) was used. First, 0.2 to 0.4 mL of Biggers–Whitten–Whittingham (BWW) culture medium was added to the spermatozoa and placed at 37°C for 1 hour. Then, the top 1 mL was pipetted into a conical test tube, 5 mL of saline was added, and the mixture was centrifuged at 1000 × g for 10 minutes. After centrifugation, slides were prepared with 5 μL of spermatozoa suspension, dried naturally, and fixed in 95% alcohol for 30 minutes. The FITC-PSA staining solution was added to the slides and placed at 4°C for 1 hour. The slides were then washed in ddH2O, sealed, and observed under a confocal laser microscope with excitation at 450 to 490 nm. The proportion of spermatozoa with intact acrosomes was scored through fluorescence. There were three to four spermatozoa in each field of view, and for each group of samples, we examined five fields of view, and calculated the acrosome reaction rate by counting the fluorescence intensity of each sperm acrosome.
DNA extraction and ABHD2 sequencing analysis
The gene extraction kit (4202050, Simgen, Hangzhou, China) and DNA purification kit were used with second-generation sequencing (ABI3730; Applied Biosystems, Foster City, CA, USA). The sequencing results were compared with GenBank to evaluate mutations.
Statistical analysis
Spermatozoa samples from infertile patients (n = 30 for each of two groups) and fertile controls (n = 30) were included. We did not perform a sample size calculation; thus, the limited number of samples may affect the statistical significance of the results. Statistical analyses were performed using ANOVA with pairwise t-tests. Post hoc analysis was conducted using SPSS software version 19.0 (IBM Corp., Armonk, NY, USA). Data are expressed as mean ± standard deviations from four independent experiments (n = 4). The significance threshold (p-value) was set at 0.05.
Results
ABHD2 was significantly decreased in infertile men
The expression of ABHD2 mRNA in spermatozoa in the control, oligoasthenospermia, and abnormal acrosome groups was detected by quantitative reverse transcription (qRT)-PCR (Figure 1a). Compared with that of the control group (1.06 ± 0.25), the relative expression of ABHD2 mRNA in the oligoasthenospermia (0.73 ± 0.25) and abnormal acrosome (0.31 ± 0.12) groups was significantly decreased (p < 0.01).
Figure 1.
Expression of ABHD2 in spermatozoa from men in the fertile control, oligoasthenospermia, and abnormal acrosome groups. (a) Quantitative reverse transcription-PCR detection of ABHD2 mRNA; (b) western blot detection and quantitation of ABHD2. n = 30 per group; **p < 0.01; error bars represent standard deviations.
ABHD2, abhydrolase domain containing 2, acylglycerol lipase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Western blot was used to detect the expression of ABHD2 protein in spermatozoa from the control, oligoasthenospermia, and abnormal acrosome groups (Figure 1b). Compared with the control group, the level of ABHD2 in the oligoasthenospermia and abnormal acrosome groups was significantly decreased (p < 0.01) (Figure 1b).
Four gene mutation sites were detected in spermatozoa from infertile men
The sequencing results of ABHD2 in DNA from infertile men and controls in our study were compared with the sequence of ABHD2 in Genbank (accession number: AC013565.10). No mutations were found in spermatozoa from controls. In spermatozoa from men with oligoasthenospermia, a C to G mutation at position 480 was found, with the codon mutated from CTC to CTG, but this change did not alter the encoded amino acid (Leu). Another mutation, deletion of a T at position 803, resulted in a change in the amino acid sequence. Three mutations in ABHD2 were found in spermatozoa with abnormal acrosomes, including a deletion of G at position 477, and deletions at positions 804 and 805, which caused a frameshift mutation in the ABHD2 coding region and a change in the amino acid sequence (Figure 2).
Figure 2.
Mutation sites of ABHD2 gene in spermatozoa from men in the fertile control, oligoasthenospermia, and abnormal acrosome groups, including a C to G at position 480 in the oligoasthenospermia group, and a G deletion at 477 and a CC deletion at bases 804 and 805 in the abnormal acrosome group. No mutations in ABHD2 were detected in the healthy control group.
ABHD2, abhydrolase domain containing 2, acylglycerol lipase.
The progesterone-induced increase of sperm ABHD2 was decreased in men with oligoasthenospermia
Spermatozoa from men in the oligoasthenospermia and control groups were treated with progesterone, and expression of ABHD2 was detected by western blot (Figure 3). Results showed that expression of ABHD2 protein in the oligoasthenospermia group was significantly decreased (p < 0.01) compared with that of the control group (Figure 3a and b). Progesterone significantly enhanced (p < 0.01) expression of ABHD2 protein in the control group, but not in the oligoasthenospermia group (Figure 3A and B), suggesting that the sensitivity of spermatozoa to progesterone was decreased in infertile men with oligoasthenospermia.
Figure 3.
Effect of progesterone on expression of ABHD2 in spermatozoa from men in the fertile control and oligoasthenospermia groups. Spermatozoa were treated with progesterone for 2 hours. (A) Western blot; (b) quantitative data. n = 30 per group; **p < 0.01; error bars represent standard deviations.
ABHD2, abhydrolase domain containing 2, acylglycerol lipase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
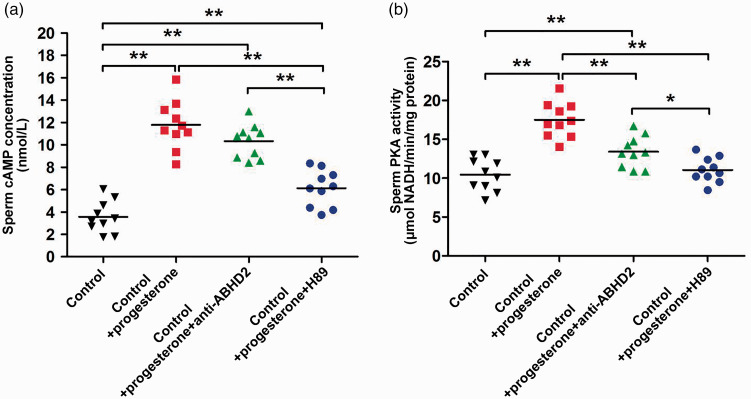
Progesterone regulates the ABHD2-mediated cAMP-PKA signalling pathway
The levels of PKA and cAMP in spermatozoa from fertile controls in the presence of progesterone were detected using ABHD2 antibody (Figure 4A) and PKA inhibitor H89 (Figure 4B). Compared with that in the control group without progesterone, PKA activity was significantly increased after progesterone treatment (17.51 ± 2.25 vs. 10.44 ± 2.08, with vs. without progesterone; p < 0.01). Anti-ABHD2 antibody (13.41 ± 2.00) and H89 inhibitor (11.05 ± 1.59) significantly decreased the PKA levels in spermatozoa treated with progesterone (p < 0.01).
Figure 4.
Levels of PKA and cAMP in spermatozoa in the presence of progesterone. Spermatozoa were treated with progesterone with or without ABHD2 antibody or PKA inhibitor H89 for 2 hours. (A) ELISA detection of cAMP, (B) cell PKA activity. n = 10 per group, *p < 0.05; **p < 0.01; error bars represent standard deviations.
PKA, protein kinase A; cAMP, cyclic adenosine monophosphate; ABHD2, abhydrolase domain containing 2, acylglycerol lipase; NADH, nicotinamide adenine dinucleotide.
Compared with that in the control group without progesterone, cAMP activity was significantly increased after treatment with progesterone (11.78 ± 2.16 vs. 3.57 ± 1.41, with vs. without progesterone; p < 0.01). Anti-ABHD2 antibody (10.33 ± 1.49) and H89 inhibitor (6.13 ± 1.62) significantly decreased cAMP levels in normal spermatozoa treated with progesterone (p < 0.01).
Progesterone may regulate sperm fertilisation via ABHD2 and cAMP-PKA signalling pathway
The acrosome reaction of spermatozoa was detected by FITC-PSA staining (Figure 5). Results showed that the acrosome was intact in spermatozoa from the control group with or without progesterone. The fluorescence of the sperm head was bright and uniform, and the equatorial zone also showed fluorescent bands (Figure 5A). Anti-ABHD2 antibody and H89 inhibitor inhibited fluorescence of the sperm head and fluorescent bands of equatorial zone, respectively (Figure 5B).
Figure 5.
Sperm acrosome reaction was detected by FITC-PSA staining. Spermatozoa from fertile controls were treated with progesterone with or without ABHD2 antibody or the PKA inhibitor H89 for 2 hours. (A) Confocal images (arrows indicate the differences between spermatozoa); and (B) quantification of intensity. **p < 0.01; error bars represent standard deviations.
FITC, fluorescein isothiocyanate; PSA, Pisum sativum agglutinin; ABHD2, abhydrolase domain containing 2, acylglycerol lipase; PKA, protein kinase A.
Discussion
In the present study, we demonstrated higher expression of ABHD2 in normal sperm and when adding P4 compared with spermatozoa from infertile men with oligoasthenospermia or abnormal acrosome reaction. Moreover, addition of progesterone increased levels of cAMP and PKA in normal spermatozoa, but not in spermatozoa from men with oligoasthenospermia or abnormal acrosome reaction. We found that expression of ABHD2 in spermatozoa from the oligoasthenospermia and abnormal acrosome reaction groups was decreased compared with that of the control group, in accordance with the low fertilisation rate associated with these groups in clinic-assisted reproduction. ABHD2 may be a useful new indicator for evaluating sperm fertility and sperm–egg binding function in assisted reproduction.
Genetic methods have been used for some time to study the impact of male infertility, but the specific mechanisms underlying gene regulation and diagnosis and treatment methods remain unclear.24 For example, mutations in the CatSper1 or CatSper2 genes cause male genetic infertility.25 At present, it is believed that the regulatory effect of progesterone on sperm function is closely related to the sperm-specific Ca2+ channel CatSper.14,15 Moreover, progesterone activates the CatSper channels of human spermatozoa through ABHD2, which increases the influx of Ca2+ ions and activates downstream signals.26 We performed Sanger sequencing of the ABHD2 gene on semen DNA samples and found no mutations in the control group, one mutation in each of two patients with oligoasthenospermia, and three mutations in two patients with abnormal acrosome reactions. The pathogenicity of these mutations needs to be further studied by functional experiments. ABHD2 is the core protein that mediates the nongenomic effects of progesterone in spermatozoa.18 However, it is not known how ABHD2 activates intracellular signalling molecules. Expression of ABHD2 protein in normal spermatozoa was increased after the addition of progesterone. Although spermatozoa are transcriptionally and translationally silent, our results are consistent with progesterone upregulating the membrane progesterone receptor within a short time (2 hours) without nuclear gene expression.20 However, the expression of ABHD2 was not significantly increased by addition of progesterone in abnormal spermatozoa compared with that without progesterone. The same incubation duration has been used in other published studies.27
The cAMP-PKA pathway is an intracellular signalling pathway involved in nongenomic effects of steroid hormone regulation and mammalian sperm capacitation.28 Its regulation is mainly through activation of adenylate cyclase to increase the intracellular content of cAMP, activating cAMP-dependent PKA, and leading to phosphorylation of serine, threonine, and tyrosine in spermatozoa, which in turn regulates sperm capacitation, hyperactivity, and acrosome reaction.18 At present, the specific mechanism of the progesterone membrane receptor in spermatozoa and the effects of progesterone on spermatozoa are not clear. It has been confirmed that ABHD2 is a membrane receptor of progesterone, and the core functional protein of the nongenomic effects of progesterone in spermatozoa.20 In the present study, we found that progesterone can activate the sperm acrosome reaction, allowing sperm to pass through the zona pellucida and oocyte membrane and finally complete fertilisation. Progesterone activates the CatSper channel of human spermatozoa through ABHD2 and then causes hyperactivity, chemotaxis, and acrosome reaction.17,29,30 It was recently found, using mass spectrometry, that ABHD2 inhibitor could reduce the occurrence of acrosome reaction.18 Thus, reduced levels of ABHD2 might be responsible for abnormal acrosome reaction. Progesterone increases cAMP content in a Ca2+-dependent manner through the ABHD2 receptor.17,18 In the current study, progesterone increased PKA activity and cAMP levels in spermatozoa from controls. After addition of ABHD2 antibody and PKA inhibitor H89, both PKA activity and cAMP induced by progesterone were decreased. Anti-ABHD2 antibody reduces the level of Ca2+ in spermatozoa,31,32 which might decrease the cAMP content and thus inhibit sperm hyperactivity. In addition, FITC-PSA staining was used to detect the acrosome reaction of spermatozoa. ABHD2 antibody and PKA inhibitor H89 inhibited the increase in PSA fluorescent staining of sperm heads after addition of progesterone, suggesting that the binding progesterone and ABHD2 protein contributes to the regulation of cAMP-PKA signalling pathway and activation of sperm Ca2+-dependent physiological functions such as acrosome reaction. However, the PKA inhibitor could target CatSper,22 and the role of PKA or CatSper should be assessed in future studies by using other tools.
Early findings have shown that progesterone can regulate CatSper channels through competitive analogues such as testosterone and hydrocortisone.24 The results for ABHD2 presented in the current study help explain some cases of unexplained infertility. Progesterone regulates spermatozoa via the ABHD2-mediated intracellular cAMP-PKA pathway. The ABHD2 signalling pathway is an important component in the evaluation of fertilisation and male infertility. Future studies should include a larger number of cases. CatSper has also been associated with motility and hyperactivation7,33–35 and, considering the localisation of human ABHD2, it seems that ABHD2 is involved in capacitation, motility, and hyperactivity, although further studies are needed to clarify these roles.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research was supported by scientific research project of Shanghai Municipal Commission of health and family planning (grant no. 201740158) and Shanghai Municipal Science and Technology Major Project (grant no. 2017SHZDZX01).
ORCID iD: Xiaoxi Sun https://orcid.org/0000-0001-5011-2594
References
- 1.Leslie SW Siref LE, andKhan MAB.. Male infertility. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC., 2020. [Google Scholar]
- 2.Choe J Archer JS, andShanks AL.. In vitro fertilization. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC., 2020. [Google Scholar]
- 3.Eisenbach M, Giojalas LC. Sperm guidance in mammals - an unpaved road to the egg. Nat Rev Mol Cell Biol 2006; 7: 276–285. DOI: 10.1038/nrm1893. [DOI] [PubMed] [Google Scholar]
- 4.Liu W, Wang Z, Leng J, et al. 20(R)-ginsenoside Rg3, a product of high-efficiency thermal deglycosylation of ginsenoside Rd, exerts protective effects against scrotal heat-induced spermatogenic damage in mice. Biocell 2020; 44: 655–669. [Google Scholar]
- 5.Sun T, Cheng L, Ma J, et al. Effect of peroxiredoxin 6 on total and progressive motility of human spermatozoa after cryopreservation. Biocell 2020; 44: 323–327. DOI: 10.32604/biocell.2020.011890. [Google Scholar]
- 6.Maksoud HA, Mahfouz M, Soliman M, et al. Harmful effects of pyrethroid ester insecticide on the male reproductive system mainly through affecting testicular function and inflammatory markers. Biocell 2020; 44: 111–115. DOI: 10.32604/biocell.2020.08399. [Google Scholar]
- 7.Tamburrino L, Marchiani S, Muratori M, et al. Progesterone, spermatozoa and reproduction: an updated review. Mol Cell Endocrinol 2020; 516: 110952. DOI: 10.1016/j.mce.2020.110952. [DOI] [PubMed] [Google Scholar]
- 8.Saint-Dizier M, Mahé C, Reynaud K, et al. Sperm interactions with the female reproductive tract: a key for successful fertilization in mammals. Mol Cell Endocrinol 2020; 516: 110956. DOI: 10.1016/j.mce.2020.110956. [DOI] [PubMed] [Google Scholar]
- 9.Gerhardt K. Progesterone and endocannabinoid interaction alters sperm activation. Biol Reprod 2016; 95: 9. DOI: 10.1095/biolreprod.116.142554. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez EM, Moreno-Irusta A, Rodriguez MB, et al. Chemotactic selection of frozen-thawed stallion sperm improves sperm quality and heterologous binding to oocytes. Anim Reprod Sci 2020; 221: 106582. DOI: 10.1016/j.anireprosci.2020.106582. [DOI] [PubMed] [Google Scholar]
- 11.Sagare-Patil V, Bhilawadikar R, Galvankar M, et al. Progesterone requires heat shock protein 90 (HSP90) in human sperm to regulate motility and acrosome reaction. J Assist Reprod Genet 2017; 34: 495–503. DOI: 10.1007/s10815-017-0879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehfeld A. Revisiting the action of steroids and triterpenoids on the human sperm Ca2+ channel CatSper. Mol Hum Reprod 2020; 26: 816–824. DOI: 10.1093/molehr/gaaa062. [DOI] [PubMed] [Google Scholar]
- 13.De Amicis F, Santoro M, Guido C, et al. Progesterone through progesterone receptors affects survival and metabolism of pig sperm. Anim Reprod Sci 2012; 135: 75–84. DOI: 10.1016/j.anireprosci.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Rahban R, Nef S. CatSper: The complex main gate of calcium entry in mammalian spermatozoa. Mol Cell Endocrinol 2020; 518: 110951. DOI: 10.1016/j.mce.2020.110951. [DOI] [PubMed] [Google Scholar]
- 15.Vicente-Carrillo A, Álvarez-Rodríguez M, Rodríguez-Martínez H. The CatSper channel modulates boar sperm motility during capacitation. Reprod Biol 2017; 17: 69–78. DOI: 10.1016/j.repbio.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Miller MR, Mannowetz N, Iavarone AT, et al. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science 2016; 352: 555–559. DOI: 10.1126/science.aad6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mannowetz N, Miller MR, Lishko PV. Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids. Proc Natl Acad Sci U S A 2017; 114: 5743–5748. DOI: 10.1073/pnas.1700367114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baggelaar MP, Den Dulk H, Florea BI, et al. ABHD2 inhibitor identified by activity-based protein profiling reduces acrosome reaction. ACS Chem Biol 2019; 14: 2295–2304. DOI: 10.1021/acschembio.9b00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purnell BA. Progesterone signaling in sperm. Science 2016; 352: 546–548. [Google Scholar]
- 20.Baldi E, Luconi M, Muratori M, et al. Nongenomic activation of spermatozoa by steroid hormones: facts and fictions. Mol Cell Endocrinol 2009; 308: 39–46. DOI: 10.1016/j.mce.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Puga Molina LC, Luque GM, Balestrini PA, et al. Molecular basis of human sperm capacitation. Front Cell Dev Biol 2018; 6: 72. DOI: 10.3389/fcell.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T, Young S, Krenz H, et al. The Ca(2+) channel CatSper is not activated by cAMP/PKA signaling but directly affected by chemicals used to probe the action of cAMP and PKA. J Biol Chem 2020; 295: 13181–13193. DOI: 10.1074/jbc.RA120.013218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boonsimma K, Ngeamvijawat J, Sukcharoen N, et al. Supplementing post-wash asthenozoospermic human spermatozoa with coenzyme Q10 for 1 hr in vitro improves sperm motility, but not oxidative stress. Andrologia 2020; 52: e13818. DOI: 10.1111/and.13818. [DOI] [PubMed] [Google Scholar]
- 24.Barratt CLR, Björndahl L, De Jonge CJ, et al. The diagnosis of male infertility: an analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum Reprod Update 2017; 23: 660–680. DOI: 10.1093/humupd/dmx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown SG, Publicover SJ, Barratt CLR, et al. Human sperm ion channel (dys)function: implications for fertilization. Hum Reprod Update 2019; 25: 758–776. DOI: 10.1093/humupd/dmz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naresh Kumar M, Thunuguntla VBSC, Veeramachaneni GK, et al. Molecular characterization of human ABHD2 as TAG lipase and ester hydrolase. Biosci Rep 2016; 36: e00358. DOI: 10.1042/bsr20160033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sagare-Patil V, Galvankar M, Satiya M, et al. Differential concentration and time dependent effects of progesterone on kinase activity, hyperactivation and acrosome reaction in human spermatozoa. Int J Androl 2012; 35: 633–644. DOI: 10.1111/j.1365-2605.2012.01291.x. [DOI] [PubMed] [Google Scholar]
- 28.Baro Graf C, Ritagliati C, Stival C, et al. Everything you ever wanted to know about PKA regulation and its involvement in mammalian sperm capacitation. Mol Cell Endocrinol 2020; 518: 110992. DOI: 10.1016/j.mce.2020.110992. [DOI] [PubMed] [Google Scholar]
- 29.Correia JN, Conner SJ, Kirkman-Brown JC. Non-genomic steroid actions in human spermatozoa. “Persistent tickling from a laden environment.” Semin Reprod Med 2007; 25: 208–219. DOI: 10.1055/s-2007-973433. [DOI] [PubMed] [Google Scholar]
- 30.Jiménez-González MC, Gu Y, Kirkman-Brown J, et al. Patch-clamp ‘mapping' of ion channel activity in human sperm reveals regionalisation and co-localisation into mixed clusters. J Cell Physiol 2007; 213: 801–808. DOI: 10.1002/jcp.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yun B, Lee H, Powell R, et al. Regulation of calcium release from the endoplasmic reticulum by the serine hydrolase ABHD2. Biochem Biophys Res Commun 2017; 490: 1226–1231. DOI: 10.1016/j.bbrc.2017.06.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardela J, Jauregi-Miguel A, Martinez CA, et al. Semen modulates the expression of NGF, ABHD2, VCAN, and CTEN in the reproductive tract of female rabbits. Genes (Basel) 2020; 11: 758. DOI: 10.3390/genes11070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Kang H, Peng L, et al. Pentachlorophenol inhibits CatSper function to compromise progesterone's action on human sperm. Chemosphere 2020; 259: 127493. DOI: 10.1016/j.chemosphere.2020.127493. [DOI] [PubMed] [Google Scholar]
- 34.Machado SA, Sharif M, Wang H, et al. Release of porcine sperm from oviduct cells is stimulated by progesterone and requires CatSper. Sci Rep 2019; 9: 19546. DOI: 10.1038/s41598-019-55834-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finkelstein M, Etkovitz N, Breitbart H. Ca(2+) signaling in mammalian spermatozoa. Mol Cell Endocrinol 2020; 516: 110953. DOI: 10.1016/j.mce.2020.110953. [DOI] [PubMed] [Google Scholar]