Abstract
Acanthamoeba keratitis (AK) is a rare but severe ocular infection with a significant risk of vision loss. Contact lens use is the main risk factor for AK. The orthokeratology (OK) lens, a specially designed contact lens, has been used worldwide as an effective method of myopia control. However, the OK lens is associated with an increased risk of Acanthamoeba infection. Many primary practitioners are concerned about this infection because of its relative rarity, the lack of promising therapeutic medications, and the need for referral. We herein report two cases of AK associated with OK lenses, present a systematic review of such cases, and discuss the possible reasons for the higher incidence rate of this infection in patients who wear OK lenses. We combined the clinical knowledge and skills of corneal specialists and lens experts with the sole objective of addressing these OK lens-related AK cases. We found that the most common risk factors were rinsing the lenses or lens cases with tap water. Prompt and accurate diagnosis along with adequate amoebicidal treatment are essential to ensure desirable outcomes for OK lens wearers who develop AK. Appropriate OK lens parameters and regular checkups are also important.
Keywords: Acanthamoeba keratitis, orthokeratology, ocular infection, biguanides, diamidines, myopia control, rigid contact lens
Introduction
Acanthamoeba keratitis (AK), a rare and severe vision-threatening corneal infection, is a potentially disastrous complication of contact lens use and is being increasingly recognized with the popularity of contact lens use worldwide.1,2 The orthokeratology (OK) lens, a specially designed rigid contact lens, has been widely used in the past two decades as one of the most effective modalities for myopia control. Parents and clinicians are more open to the use of this novel lens in children in an attempt to retard myopia progression and thus decrease the risk of sight-threatening complications related to high myopia, such as chorioretinal abnormalities,3 glaucoma,3 myopic maculopathy,3 and cataract.4 However, along with the popularity of OK lens use, there has been a dramatic rise in the number of ocular infections,5 among which AK is one of the most serious.
Because of the relative rarity of AK and the need to transfer patients to a corneal specialist when AK is highly suspected, many primary practitioners are not familiar with the clinical profile or therapeutic plan of AK, even they are concerned about this potentially disastrous complication during their practice. In this article, we report two cases of AK related to OK lens use and analyze clinical cases of OK lens-associated AK published in the PubMed, Embase, and Cochrane Library databases. The reference lists of these studies were also searched. By combining the clinical knowledge and skills of corneal specialists and lens experts, we discuss the potential causative agents that increase the risk of infection and examine the reasons for the increased likelihood of Acanthamoeba infection in OK lens wearers. We hope that sharing our experience with AK case management and publication of this systematic review will help clinicians interested in this subject to rationally assess the risks that patients may face.
Materials and methods
We performed a retrospective review of the medical records of all consecutive patients seen in the Eye Clinic at the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology during the 12-month period from January 2019 to December 2019. In this review, we identified two cases of AK related to OK lens use. We followed the CARE guidelines of the EQUATOR Network in our reporting of these cases. Data regarding the patients’ demographic details, causes and duration of symptoms, presenting and final visual acuity, and interventions were collected. Both patients had undergone a complete eye examination under slit-lamp biomicroscopy (Topcon Corporation, Tokyo, Japan) and confocal microscopy (HRT3; Heidelberg Engineering, Heidelberg, Germany). We obtained all patients’ verbal consent before the treatment began. To protect the patients’ privacy, all patient details were de-identified in this study. The requirement for approval by an ethics committee or institutional review board was waived because of the nature of this study (case report and literature review).
Literature search and screening
We screened the main medical databases of PubMed, Embase, and the Cochrane Library to identify relevant articles published from January 2000 to March 2020 using the following search strategy:
(1) rigid contact lens OR orthokeratology lens OR orthokeratology OR reverse geometry lens or corneal reshaping OR rigid contact lens; (2) Acanthamoeba keratitis OR Acanthamoeba OR eye infection OR keratitis OR cornea ulcer OR microbial keratitis OR bacterial keratitis; (3) (1) AND (2)
To include all cases that met the criteria for inclusion in our analysis, we also reviewed the reference lists and citations of all retrieved studies. According to predefined inclusion and exclusion criteria, the titles, abstracts, and full copies of all included articles were sequentially assessed. Articles were included if they met the following criteria:
(1) The study sample comprised patients with AK who had a history of OK lens use. (2) The study focused on the complications of OK lens use. (3) The study focused on the diagnosis or management of AK. (4) The following information and raw data were available: age, sex, duration of OK lens use, risk factors, treatment before diagnosis, symptom duration before diagnosis, visual acuity at presentation, clinical symptoms and signs, diagnostic method, duration and mode of treatment, surgery needed, and outcomes. (5) The article was written in English.
Articles were excluded if they met the following criteria:
(1) The article contained duplicate data. (2) The article was an abstract, comment, review, or editorial review. (3) The article had inadequate data or had relevant raw data that could not be extracted.
Research quality assessments
The inherent nature of retrospective studies renders most of them susceptible to a certain level of bias, such as selection bias, recall bias, and/or detection bias. As a result, none of the relevant published cases were excluded from this review based on risk of bias.
Results
Case 1
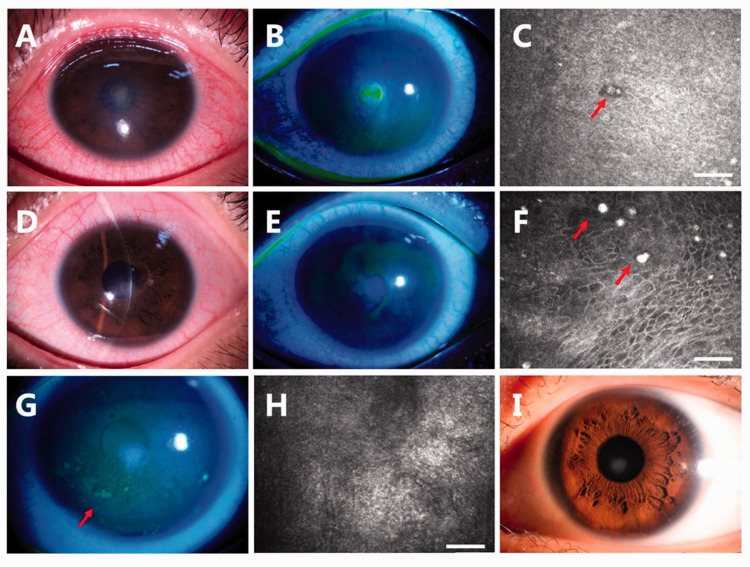
A 21-year-old female college student presented with a 2-day history of pain, tearing, and vision loss in her left eye. She had used OK lenses for myopia correction for 3 years. She often rinsed the lenses with tap water. Her visual acuity was counting fingers at 15 cm on the left side. Slit-lamp examination revealed an anterior to mid-stromal corneal infiltrate (Figure 1(a)) measuring 2 × 2 mm with fluorescent staining (Figure 1(b)). The corneal stroma was severely edematous, and Descemet’s membrane was wrinkled. Confocal laser microscopy revealed structures highly resembling amoebic cysts (Figure 1(c)). She was treated with 0.02% chlorhexidine eye drops hourly. Rapid relief of the symptoms and healing of the ulcer were noted during the next 4 days (Figure 1(d) and (e)). However, amoebic cysts were still found by confocal microscopy (Figure 1(f)). The 0.02% chlorhexidine eye drops were used every 2 hours for the following 4 weeks. The patient reported eye dryness and a foreign body sensation at the 1-month visit. Conjunctival injection and a punctate corneal epithelial defect were noted (Figure 1(g)). The previous ulcer had healed, and no amoebic cysts were found (Figure 1(h)). The patient was treated with 0.3% sodium hyaluronate eye drops to promote epithelial cell recovery, and 0.02% chlorhexidine was applied four times a day. When the patient presented for a follow-up examination at 70 days after onset, her corrected vision was 20/20 with a small patch of corneal opacity (Figure 1(i)). The eye drops were discontinued after amoebic cysts were confirmed to be absent by confocal microscopy. She developed no recurrence during the 6-month follow-up.
Figure 1.
Case 1. (a, b) Slit-lamp examination revealed a corneal infiltrate measuring 2 × 2 mm with fluorescent staining. (c) Confocal laser microscopy revealed structures highly resembling the amoebic cyst (red arrow). (d, e) Resolution of the ulcer was noted after 4 days of treatment. (f) Amoebic cysts were still found by confocal microscopy (red arrows). (g) A punctate corneal epithelial defect was noted at the 1-month visit. (red arrow), but (h) no amoebic cysts were found. (i) A mild corneal opacity was present at the 70-day visit. Bar = 50 microns.
Case 2
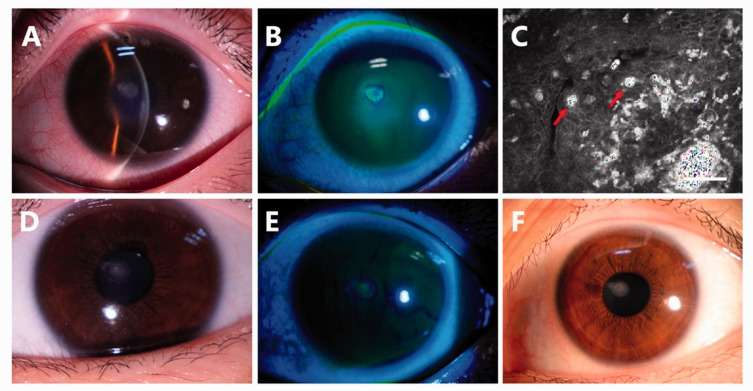
A 17-year-old female senior high school student was referred to our cornea clinic with a 4-day history of pain, tearing, and vision loss in the left eye. She had used OK lenses for vision correction for 1 year. She often touched the lenses directly with her wet hands after washing her hands with tap water. She had received an antiviral treatment for 3 days at the previous clinic, but the symptoms worsened before referral to our cornea clinic. Upon presentation, her visual acuity was counting fingers at 15 cm. Slit-lamp examination revealed stromal edema (Figure 2(a)) and a corneal ulcer measuring 2 × 2 mm with fluorescent staining (Figure 2(b)). Confocal laser microscopy revealed structures highly resembling amoebic cysts within the ulcer (Figure 2(c)). She was treated with 0.02% chlorhexidine eye drops hourly for 7 days. The corneal edema subsided (Figure 2(d)) and the fluorescein staining was weakly positive (Figure 2(e)). The 0.02% chlorhexidine eye drops were used every 2 hours for the following 4 weeks and then reduced to four times a day for another 4 weeks. At the 2-month visit, her corrected vision was 20/20 with slight corneal opacity (Figure 2(f)). The eye drops were discontinued when the absence of amoebic cysts was confirmed by confocal laser microscopy. She developed no recurrence during the 7-month follow-up.
Figure 2.
Case 2. Slit-lamp examination revealed (a) stromal edema and (b) a corneal ulcer measuring 2 × 2 mm with fluorescent staining. (c) Confocal laser microscopy revealed structures highly resembling amoebic cysts in the ulcer. After 7 days of treatment, (d) the corneal edema had subsided and (e) fluorescein staining was weakly positive. (f) The ulcer had healed with a small corneal opacity at the 2-month visit. Bar = 50 microns.
In total, 289 articles were identified using the index words from the three databases (PubMed, n = 175; Embase, n = 110; Cochrane Library, n = 4). After removing 127 duplicate articles and 3 articles with no authors by Endnote software or manually, 159 articles were screened by reading their titles and abstracts. When summarizing these individual cases, we collected the complete patient information, OK lens wearing status, diagnostic examination findings, and treatment plan. Based on our strict inclusion and exclusion criteria, we identified 13 relevant case reports for data analysis after removal of review articles, general serial reports, and irrelevant reports. These 13 case reports involved 24 eyes of 20 patients using OK lenses (Table 1). Among all reported cases, the patients’ ages ranged from 9 to 41 years and the highest incidence of AK occurred in the 10- to 19-year age range. The mean age at presentation was 19.4 ± 8.2 years, and female preponderance was identified (male:female ratio of 1.0:2.3). The most common risk factor for AK in OK lens wearers was rinsing the lenses or cases with tap water. Practices such as inappropriate lens care procedures, patient noncompliance with the practitioner’s instructions, and persistent lens use despite discomfort emerged as potential risk factors for AK in OK lens wearers. The performance of microbiological culture as the diagnostic method was reported in 83% of the cases. Among them, one patient had bilateral symptoms but was culture-positive on only one side. Confocal microscopy was used for confirmation in three eyes. The median duration from onset of symptoms to diagnosis was 20 days. Among all 20 patients, 42% had poor outcomes as defined by best-corrected visual acuity of less than 20/40 or requirement of surgery.
Table 1.
Summary of reported cases of Acanthamoeba keratitis in OK lens wearers.
| Author/Year | No. of patients | Age (years)/Sex | Duration of OK lens use | Risk factors | Eye infected | Duration before Dx | Treatment before Dx | Symptoms at presentation | Signs at presentation | Diagnostic method | Treatment | Final outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hirabayashi et al.63 2019 | 1 | 17 F | Not mentioned | Not mentioned | OS | 5 months | Antivirals and topical corticosteroids for presumed herpetic disease for several months | UCVA: hand motions | Moderate ptosis, severe conjunctival injection, a large ring infiltrate with diffuse opacity involving 75% of the cornea with moderate stromal edema | 1. Confocal microscopy 2. Corneal scraping and culture |
1. PHMB + chlorhexidine + moxifloxacin 2. Cyclopentolate 3. Oral valacyclovir 4. Oral voriconazole 5. Oral miltefosine |
After 1 year 1. Significant scarring of the cornea with severe opacification and diffuse vascularization 2. VA: hand motions |
| Greenwell et al.64 2013 | 2 | 29 F | Not mentioned | Often washed lenses with tap water | OS | 2 weeks | Antifungal eye drops | 1. Severe left eye pain, photophobia, and foreign body sensation 2. BCVA: OD 6/5, OS 6/9 |
1. Epithelial disturbance on the temporal cornea, comprising epithelial thickening 2. Punctate erosion with ring‐shaped subepithelial opacity | Corneal culture | 1. PHMB 2. Propamidine changed to gentamicin |
After 6 months 1. BCVA: 6/6 2. Back to OK lens use |
| 36 F | Not mentioned | Cleaned lens case under tap water | OS | 9 days | Topical antibiotic treatment + antifungal eye drops | 1. Left eye pain, photophobia and watering 2. VA: OD 6/5, OS 6/7.5 |
Persistent dendriform epitheliopathy | Corneal scraping and culture | 1. PHMB + propamidine | After 4 months UCVA: 6/7.5 |
||
| Kent et al.65 2012 | 1 | 10 F | Not mentioned | 1. Used bottled spring water to clean lenses 2. Swam in pools wearing lenses |
OD | Not mentioned | Topical antibiotic treatment | 1. A red eye without ocular pain 2. VA: hand motions |
A stromal ring infiltrate | Corneal culture | Antiprotozoan therapy without details | At last follow-up UCVA: 20/200 |
| Kim and Kim66 2010 | 1 | 22 F | 5 days | Used tap water for contact lens management | OU | 1 month | Ganciclovir + fluconazole | Ocular pain, photophobia, and decreased vision | 1. Ring-shaped stromal infiltrates, radial infiltration resembling perineuritis, and disciform keratitis in both corneas 2. OD worse |
1. Corneal scrapings stained with Gram stain 2. Bacterial culture 3. Culture of corneal scrapings, lens case, and lenses 4. Inverted phase contrast microscopy + light microscopy |
1. PHMB 2. Oral itraconazole for 2 weeks 3. Levofloxacin + tobramycin 4. Atropine |
After 1 month 1. BCVA: OD 20/100, OS 20/25 2. Corneal epithelium had healed but stromal opacity remained |
| Xuan et al.67 2008 | 1 | 16 F | 2 years | Not mentioned | OU | 3 weeks | Treated for contact lens-induced infectious keratitis for 3 weeks | VA: OD 20/25, OS: counting fingers/50 cm | 1. Epithelial surface irregularity, stromal infiltrates and peripheral polyneuritis in right eye 2. A 2-mm central corneal ulcer with stromal infiltrates in left eye |
Corneal culture | 1. PHMB 2. Oral itraconazole |
After 2 months 1. BCVA: OD 20/20, OS 20/25 2. Right cornea was clear except paracentral dot opacity, left healed with faint diffuse central stromal opacity |
| Lee et al.68 2007 | 4 | 15 F | 1 year | 1. Used lens cleaner and MPS irregularly 2. Rinsed lens with tap water |
OU | 1 week | Topical antibiotic treatment | Severe bilateral ocular pain, photophobia | Radial keratoneuritis | Corneal scraping and culture | 1. PHMB + chlorhexidine tapered 3 months 2. Oral itraconazole 10 days |
After 6 months 1. Cornea clear 2. BCVA: OU 20/20 |
| 14 M | 3 years | Rinsed lens with tap water | OS | 3 weeks | Antibacterial and corticosteroid eye drops | Redness and severe pain | A central corneal ulcer and keratoneuritis | Corneal scraping and culture | 1. PHMB + chlorhexidine tapered 3 months 2. Oral itraconazole 10 days |
After 3 months 1. Ulcer healed with scarring 2. BCVA: 20/100 |
||
| 16 F | 15 months | Rinsed lens with tap water | OD | 18 days | Not mentioned | Photophobia and pain | A paracentral corneal ulcer | Corneal scraping and culture | 1. PHMB + chlorhexidine tapered 3 months 2. Oral itraconazole 10 days |
After 5 months 1. Subepithelial corneal scarring 2. BCVA: 20/25 |
||
| 15 F | 3 years | Rinsed lens with tap water | OS | 15 days | Topical antibiotic treatment | Pain, tearing, and redness | A large central corneal ulcer | Corneal scraping and culture | 1. PHMB + chlorhexidine tapered 3 months 2. Oral itraconazole 10 days |
After 5 months 1. Central corneal scarring 2. BCVA: 20/40 |
||
| Robertson et al.24 2007 | 1 | 19 M | 3 years | Rinsed with tap water and stored in tap water in case | OS | 4 months | 1. Antibacterial eye drops 2. Phototherapeutic keratectomy 3. Oral acyclovir |
Not described | Large abscess extending deeply into the stroma | Confocal microscopy | Propamidine + PHMB + chlorhexidine 13 months | After 13 months 1. VA: light perception 2. Secondary angle-closure glaucoma |
| Wong et al.25 2007 | 1 | 9 M | 5 months | Visited swimming pool 3 days previously | OD | 1 week | Topical antibiotic treatment | 1. Pain, photophobia, and redness 2. BCVA: 20/40 |
1. Conjunctival injection and superficial punctate keratitis with multiple central subepithelial infiltrates 2. Perineural infiltrates |
Corneal scraping and culture | Propamidine + PHMB 4 months | After 4 months 1. BCVA: 20/25 2. Faint residual central subepithelial scars |
| Lee et al.69 2006 | 1 | 15 F | 10 months | MPS on basis, denied tap water | OU | 1 week | Topical antibiotic treatment | 1. Severe ocular pain and photophobia in both eyes 2. VA: OD 15/100, OS 10/100 |
1. Severe conjunctival injection 2. Radial keratoneuritis |
Corneal scraping and culture | 1. PHMB + chlorhexidine 2. Oral itraconazole 10 days |
After 6 months 1. BCVA: OU 20/20 2. Cornea clear without impairment |
| Wilhelmus70 2005 | 1 | 16 F | Not mentioned | Not mentioned | OD | 1 month | Antibacterial and corticosteroid eye drops | UCVA: hand motions | A ring-shaped stromal infiltrate | 1. Corneal biopsy 2. Culture |
1. Hexamidine + chlorhexidine 2. Oral itraconazole 10 days 3. Topical prednisolone 1% and oral prednisone later added for stromal keratitis and anterior scleritis |
After 6 months 1. BCVA: 20/100 after 1 year 1. Penetrating keratoplasty 2. Postkeratoplasty VA: 20/20 |
| Yepes et al.71 2005 | 1 | 41 F | 18 months | Denied | OD | >2 weeks | Antibacterial and corticosteroid eye drops | BCVA: OD 20/200, 20/70 with a pinhole; OS 20/25 | 1. Right upper eyelid edema, diffuse conjunctival injection 2. Incomplete superficial circinate irregular infiltrate, diffuse superficial punctate subepithelial infiltrates |
Corneal culture | 1. Polymyxin B, neomycin, gramicidin 2. Propamidine |
After 15 Mos 1. BCVA: hand motion 2. Densely scarred and vascularized cornea 3. A mature cataract 4. Scheduled for a combined corneal transplant, cataract extraction and intraocular lens implantation |
| Sun et al.72 2003 | 4 | 18 M | 6 months | Not mentioned | OD | Not mentioned | Not mentioned | 1. Photophobia and pain 2. VA: counting fingers |
A central ulcer | Corneal scraping | Chlorhexidine +topical neomycin sulfate + metronidazole | After 2 months 1. BCVA: 20/50 2. Subepithelial corneal nebula remained |
| 15 F | 1 year | Not mentioned | OD | 20 days | Not mentioned | 1. Severe pain, photophobia 2. VA: counting fingers at 1 foot |
1. A central ulcer 2. Radial keratoneuritis |
Corneal scraping | Chlorhexidine + topical neomycin sulfate + metronidazole | After 5 months 1. No impairment of visual acuity 2. Corneal ulcer had healed |
||
| 19 M | 6 months | Not mentioned | OD | 3 weeks | Not mentioned | 1. Redness and severe pain 2. VA: hand motions |
1. A large, paracentral corneal ulcer 2. Radial keratoneuritis 3. Flares and cells in the anterior chamber |
Corneal scraping | Chlorhexidine + topical neomycin sulfate + metronidazole | After 2 months 1. Corneal scarring 2. BCVA: 20/75 |
||
| 17 F | 2 years | Not mentioned | OD | Not mentioned | Not mentioned | VA: 20/60 | Epithelial irregularity with a diffuse punctate defect | 1. Confocal microscopy 2. Culture |
Chlorhexidine + topical neomycin sulfate + metronidazole | After 1 month 1. Neovascularization developed in the anterior corneal stroma 2. Treatment was continued, no definitive results |
||
| Hutchinson and Apel73 2002 | 1 | 29 M | 5 years | Not mentioned | OD | 1 month | Antibacterial and corticosteroid eye drops | 1. Painful, red eye 2. VA: 6/24 |
1. Conjunctival injection 2. Epithelial and superficial corneal stromal edema 3. A superficial ring infiltrate + circular area of small linear corneal epithelial defects 4. 2+ cells in right anterior chamber |
Corneal scraping and culture | 1. PHMB + propamidine 2. Atropine 3. Oral ketoconazole 4. Prednisolone after 10 days of admission |
After 1 month 1. BCVA: 6/36 2. A non‐staining opacity remained |
OK, orthokeratology; F, female; M, male; OS, left eye; MPS, multipurpose solution; OD, right eye; OU, both eyes; Dx, diagnosis; VA, visual acuity; BCVA, best-corrected visual acuity; UCVA, uncorrected visual acuity; PHMB, polyhexamethylene biguanide
Discussion
AK is caused by a free-living protozoan. When describing this causal agent, Acanthamoeba spp., the most frequent word used is “ubiquitous” because this protozoan is commonly found in air, soil, dust, and all kinds of water including fresh and chlorinated water, showers, and swimming pools.6,7 Although Acanthamoeba species are found almost everywhere, they are considered largely a waterborne pathogen in humans because AK has only been consistently linked to alterations in the quality of domestic water supplies6–8 or contact with contaminated water.9 The Centers for Disease Control and Prevention and previous studies6,7,10–12 have indicated that the practice of rinsing lenses or lens cases with tap water is a very common risk factor for Acanthamoeba infection in OK lens wearers; this is consistent with the findings in our reviewed cases and our two patients.
There are two stages in the life cycle of Acanthamoeba species: an active trophozoite stage that exhibits vegetative growth, and an intrinsically resistant cyst stage with minimal metabolic activity.13 Mutual transformation can be observed between the trophozoite and cyst by encystment or excystment. Commonly, the trophozoite becomes a resistant cyst with food deprivation, desiccation, extreme temperature, and extreme pH or with the use of topical medications.20 While in a suitable environment or with adequate food resources, the trophozoite excysts and reproduces by binary fission.17 More than 20 genotypes have been identified to date based on the analysis of sequence differences in the diagnostic fragment 3 region of the 18S ribosomal RNA gene.14 Among all these genotypes, T4 is the main genotype in China15 and has also been found to be the most common genotype related to eye infections in most countries.16–19
More than 40 years have passed since the first case of AK was documented.21 Cases of AK have increased largely in parallel to the growing use of contact lenses, and indeed, contact lens use is recognized as the leading risk factor for AK.1,2,22 Among all kinds of contact lenses, previous studies have shown that the incidence of AK is lowest in regular rigid gas permeable (RGP) lens wearers; one study showed that the incidence rate of AK was 9.5 times lower in RGP lens wearers than in soft contact lens wearers.23 Interestingly, however, patients using RGP lenses for OK purposes have a higher incidence rate of Acanthamoeba infection.24,25 Among causes of microbial keratitis related to OK lens use, Acanthamoeba has become the second most common culprit.26
The worldwide prevalence of myopia is dramatically increasing, especially in East Asia, where the prevalence may reach 90% in university student populations.27,28 OK lenses, with their outstanding myopia control effect, have become a very popular choice among patients with myopia.29 According to incomplete statistics, more than 1.2 million OK lenses were sold in China in 2019. Although numerous studies have focused on the mechanism and clinic effects of OK lenses in myopia control,30–32 equal attention must be paid to the risk of complications. Among all complications, AK is relatively rare but vision-threatening.17 With the potential risks for young patients with this severe ocular disease, ophthalmologists and lens prescribers must become more aware of the pathological processes and management of AK because children and adolescents are the main target populations of OK lens use. Despite many studies having rapidly advanced the knowledge of Acanthamoeba infection, few studies have focused on AK in OK lens wearers. Most previous studies have drawn summative conclusions that contact lens use is the leading cause of AK without distinguishing the lens type, lens material, or lens design. However, how does OK lens use increase the incidence of AK? Below, we present a detailed analysis of the possible pathogenesis of AK and discuss its clinical manifestations, diagnostic methods, treatments, and prognosis among OK lens users.
Compared with regular rigid contact lenses, the OK lens uses the same lens material but has a different design and wear modality, adopting reverse geometry to reshape the anterior surface of the cornea. This design has two purposes: first, to provide unaided clear visual acuity during the daytime by the wearing of the OK lens at night while asleep to flatten the central corneal curvature; second, to reduce the elongation of the ocular axial length, sequentially controlling myopia progression by increasing peripheral myopic defocus, which is a putative mechanism to slow myopia development.33,34 Different from the regular alignment fit of the RGP lens, the shape of the OK lens and the inherent tight bending between the lens and cornea can induce central corneal epithelial thinning and surface cell damage, which predisposes the cornea to an increased risk of infection.35,36 On the basis of different designs of the same material, Wei et al.37 suggested that the OK lens produces a higher risk of microbial keratitis than the alignment fit RGP lens with overnight wearing because the OK lens resulted in greater cornea binding for pathogens in their study. Choo et al.38 stated that tear pooling under the reverse curve of the OK lens created higher colonization rates than the alignment fit lens, under which the tear fluid was evenly distributed. Although these two studies37,38 focused on corneal adhesion of Pseudomonas aeruginosa, the higher infection rate of Acanthamoeba in OK lens users may share the same mechanism. In addition to the lens design, the nocturnal wearing strategy of the OK lens is another main reason for the increased infection risk.39 The cornea usually goes into a relatively hypoxic status with eyelid closure during sleep, and diffuse cornea edema or microcystic epithelial changes can be seen during this time.39 When an OK lens is worn during nighttime, the state of oxygen deprivation is advanced, leading to cell death and desquamation of the stressed epithelial cells and increasing the risk of abrasion.40 Another study showed that high carbon dioxide tension served as a strong stimulus for Acanthamoeba excystation and growth.17 When the epithelium is no longer intact, pathogen adhesion and infection readily occur. In practice, many lens prescribers intend to prevent myopia progression by inducing more peripheral myopic defocus, which is hypothesized to provide effective myopia control by changing the corneal shape and aberrations. This may result in higher pressure or tighter fitting by changing the parameters of the OK lens even beyond the patient’s needs. Greater hydraulic pressure underneath the OK lens can produce larger mechanical corneal abrasions, giving this opportunistic pathogen greater exposure via breaks in the epithelium and resulting in corneal infiltration. These possible causes contribute to the increased risk of infection.
Early diagnosis of AK is invaluable and is associated with relatively favorable outcomes. However, the diagnosis of AK can be challenging because the corneal infections caused by Acanthamoeba sometimes have no particular characteristics or they may mimic other corneal infections, such as herpes simplex keratitis or fungal keratitis.41 Additionally, Acanthamoeba co-infections with other bacteria or viruses are common.41–43 The patients’ presentations can vary and may involve common signs of ocular inflammation, such as blurred vision, photophobia, pain, and tearing, usually involving one eye but occasionally both.44 Many articles have emphasized the degree of pain associated with AK and have even regarded it as the hallmark symptom; the pain has been described as “severe or excruciating” or “characteristically disproportionate to relatively mild clinical findings.”9 However, the diagnosis of AK cannot be ruled out if pain is absent. Some patients with AK may be pain-free or have mild pain clinically, as did our two patients. Patients with a long history of OK lens use but poor hygiene habits regarding lens care often have chronic ocular microtrauma. They may not complain of pain as a significant symptom because they may have reduced corneal sensation after a long period of OK lens wearing.
AK usually progresses slowly. Early infections generally signify changes in the epithelium, such as punctate keratopathy-induced irregularities, multifocal epithelial or subepithelial infiltration, pseudo-dendrites, and/or elevated epithelial ridges.15 AK can involve the corneal nerve and cause radial keratoneuritis, which is relatively specific, although it does not appear in all cases.9,45 Our patients had an early presentation with central corneal haze and a ground-glass appearance. In the late stages, the amoebae can deeply penetrate the corneal stroma, and the infection becomes exceedingly difficult to treat and eradicate.43,45,46 A corneal ring infiltrate is recognized as a characteristic sign and strong predictor of a poor outcome, although it appears in less than half of affected patients.2,47 Salt-like dense infiltrates and/or groove-shaped corneal melting have been reported as other helpful characteristics that are more frequently observed than ring infiltrates (61.1% vs. 41.1%, respectively).48 Hypopyon, anterior scleritis, or perforation can be seen in certain late-stage cases. The formation of cataract or elevated intraocular pressure can be caused by severe anterior chamber inflammation, which can also be attributed to long-term use of topical antiamoebic eyedrops.10,41
Numerous studies have suggested that a long symptom duration before diagnosis or a late stage of AK at presentation is associated with a longer disease course and worse visual outcome.43,45,46 One main reason for the favorable prognosis in our patients was a timely diagnosis within the first few days of symptom occurrence. In their analysis of all patients with AK from 1991 to 2013 at the Tongren Eye Center, Jiang et al.48 found that 77.8% of 260 cases were identified as advanced-stage AK at the initial presentation and that 53.3% of their patients were cured after undergoing topical antiamoebic therapy for a mean duration of 5 months.48 In our case summary, however, patients had a better therapeutic outcome and shorter length of treatment if they received an accurate diagnosis within 3 weeks (Table 1). This requires the primary practitioner to become more sensitive to possible cases and to have lower thresholds for referral or diagnostic procedures. Once the diagnosis is suspected, confirmation must begin immediately.
Conventional diagnostic methods such as smear and culture are still commonly used in the clinical setting, and culture remains the gold standard for diagnosis of Acanthamoeba. However, the sensitivity of these techniques can be unfavorable, ranging from 70% to 84% for smear and 33% to 50% for culture.41,49–51 In recent years, in vivo confocal microscopy (IVCM) has become more widely used in the clinical setting. IVCM is a very helpful method with higher sensitivity (close to 90%) and specificity (91.1%–100%) for the diagnosis of AK51,52 using the reference standard of a positive culture result. Noninvasive and rapid IVCM checks, especially for patients with an uncertain diagnosis, a poor initial treatment response, or a history of prolonged therapy for corneal infection, can allow detailed examination and permit monitoring of the disease course. In our cases, we first checked the patients’ corneas by IVCM with consideration of the whole picture of each patient’s history, invasive processing of corneal scraping, and longer return period of culture. Cysts of Acanthamoeba under IVCM appeared as high-contrast, ovoid, double-walled structures or round bodies. Polymerase chain reaction assay is another rapid novel diagnostic method that can identify Acanthamoeba 18S ribosomal RNA with 71% to 77% sensitivity.50,53 Clinically, multiple microbial workups will enhance the identification of a particular pathogen. Further studies are needed to validate the interpretation and utilization of these diagnostic tools for prompt diagnosis and treatment.
No medications have been licensed and no specific therapeutic plans have been established for Acanthamoeba infection.49,54 All treatment protocols are empirically based on clinical observations or previous publications. Despite the numerous reports and studies on the treatment of AK, different countries may have different therapeutic protocols involving considerations such as easy accessibility, medical potency, adverse effects, and costs, especially if long treatment periods are required. Although consensus is lacking, the first-line therapeutic medication in most countries is a biguanide, including polyhexamethylene biguanide 0.02% or chlorhexidine 0.02%; these are identified in the literature as the most effective cysticidal antiamoebics with low levels of corneal epithelial toxicity.49,54,55 They can be used either as monotherapy or in combination with a diamidine, such as propamidine 0.1% or hexamidine 0.1%. There is limited evidence for a better prognosis with dual therapy as opposed to monotherapy. However, many clinicians prefer a dual therapeutic plan by combining a biguanide with a diamidine for potentially synergistic or additive effects in consideration of the difficulty of eradicating resistant cysts.10,56 Various researchers have compared the respective efficacy of these medications.55,57,58 However, no significant difference has been found in the success rate (78% and 86% for chlorhexidine and polyhexamethylene biguanide, respectively).57 We used chlorhexidine alone in our patients and achieved a satisfactory result because both cases were diagnosed at an early stage.
Inclusion of steroids in AK management is controversial. Many studies have shown the need for longer therapeutic periods or poorer visual outcomes if steroids are used before diagnosis or under misdiagnosis of viral infections. Steroids can suppress patients’ immunological responses, especially the amoebic killing function of macrophages. They can also increase the number of trophozoites by facilitating excystment, leading to greater corneal destruction. Many authorities suggest avoiding steroid use during the whole course of AK treatment, especially in cases of early diagnosis.9,49,59 Some researchers recommend that patients receive steroids when severe or persistent infections are present, such as anterior scleritis or indolent ulcers.46,54 Our patients were not treated with steroids, and they responded rapidly to antiamoebic drugs.
Numerous alternative strategies exist in addition to these commonly used medications. For example, some Chinese clinicians have reported use of a cocktail therapy of chlorhexidine (0.02%), neomycin (0.5%), and metronidazole (0.4%).9 However, recent studies are less supportive of the use of aminoglycosides such as neomycin and paromomycin in AK therapy because of their non-cysticidal function and significant corneal toxicity. Certain antifungal medications, such as oral fluconazole, are also used less frequently in contemporary treatment plans.
For refractory late-stage cases of AK, therapeutic keratoplasty can be indicated to save the patient’s visual outcomes if the condition stops responding to medical therapy. Most specialists suggest that surgery should not be performed until the acute infection has been alleviated.46,60 The need for corneal grafting has varied during the past several decades and could be quite high in some studies. Jiang et al.48 stated that 46.7% of their patients required therapeutic grafts. In a retrospective case series of 62 patients with AK in a tertiary hospital in Taiwan during a 20-year period, 35.5% of patients underwent surgical treatment.61
Conclusion
The mechanism of orthokeratology lens-related AK is likely to involve a combination of altered corneal defenses, including mechanical trauma, oxygen deprivation, nutrient starvation, metabolic injury, eye dryness related to lens use, hypersensitivity or chemical toxicity from the lens material and lens solution, and microorganism adherence to the non-intact corneal epithelium. All of these potential pathological processes may contribute to the development of AK. When managing patients with ocular infection related to OK lens use, ophthalmologists must rely on their accrued experience and clinical acumen to determine the next steps for slowing the progression of infection, improving the patient’s prognosis, and saving the patients from potential vision loss and high medical expenses. The cornea of OK lens users must be carefully evaluated under a slit lamp at each follow-up visit. Clinicians must pay more attention to the corneal health of patients with poor compliance because these patients may be accustomed to minor irritations in their eyes and thus overlook corneal discomfort. Primary doctors also need to advance their medical knowledge and improve their ability to diagnose rare diseases that may cause severe consequences. Finally, it is very important to emphasize clinical communication and establish a system enabling rapid referral of patents. Surveillance efforts must be combined with clinical efforts to understand the deeper mechanisms of AK caused by OK lens use.
Future direction
Further studies need to collect more comprehensive data that include all parameters of lens and lens fitting conditions, which are very important because the mechanical effects of the lens vary based on these features. Besides the OK lens itself, the lens disinfection system needs to be improved because it was attributed to the sudden rise in the incidence rate of AK in the United Kingdom and the United States from 2002 to 2007.8,62 However, increasing the antiseptic ingredients in lens solutions will aggravate the known toxic and apoptotic effects on the ocular surface, especially with long-term use. Further research is needed to identify a more effective and safer lens care system.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Research Foundation of Union Hospital (2018xhyn106).
ORCID iDs: Jinfang Wu https://orcid.org/0000-0001-6041-7797
Huatao Xie https://orcid.org/0000-0003-4027-691X
References
- 1.Cope JR, Collier SA, Schein OD, et al. Acanthamoeba keratitis among rigid gas permeable contact lens wearers in the United States, 2005 through 2011. Ophthalmology 2016; 123: 1435–1441. DOI: 10.1016/j.ophtha.2016.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tu EY, Joslin CE, Sugar J, et al. Prognostic factors affecting visual outcome in Acanthamoeba keratitis. Ophthalmology 2008; 115: 1998–2003. DOI: 10.1016/j.ophtha.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho BJ, Shin JY, Yu HG. Complications of pathologic myopia. Eye Contact Lens 2016; 42: 9–15. DOI: 10.1097/ICL.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 4.Pan CW, Cheng CY, Saw SM, et al. Myopia and age-related cataract: a systematic review and meta-analysis. Am J Ophthalmol 2013; 156: 1021–1033.e1. DOI: 10.1016/j.ajo.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Chan TC, Li EY, Wong VW, et al. Orthokeratology-associated infectious keratitis in a tertiary care eye hospital in Hong Kong. Am J Ophthalmol 2014; 158: 1130–1135.e2. DOI: 10.1016/j.ajo.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Ustunturk-Onan M, Walochnik J. Identification of free-living amoebae isolated from tap water in Istanbul, Turkey. Exp Parasitol 2018; 195: 34–37. DOI: 10.1016/j.exppara.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Carnt N, Subedi D, Lim AW, et al. The prevalence and seasonal variation of Acanthamoeba in domestic tap-water in greater Sydney region, Australia. Clin Exp Optom 2020; 103: 782–786. DOI: 10.1111/cxo.13065. [DOI] [PubMed] [Google Scholar]
- 8.Radford CF, Minassian DC, Dart JK. Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br J Ophthalmol 2002; 86: 536–542. DOI: 10.1136/bjo.86.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X, Zhang Y, Li R, et al. Acanthamoeba keratitis: clinical characteristics and management. Ophthalmology 2006; 113: 412–416. DOI: 10.1016/j.ophtha.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 10.Hassan F, Bhatti A, Desai R, et al. Analysis from a year of increased cases of Acanthamoeba Keratitis in a large teaching hospital in the UK. Cont Lens Anterior Eye 2019; 42: 506–511. DOI: 10.1016/j.clae.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Taher EE, Meabed EMH, Abdallah I, et al. Acanthamoeba keratitis in noncompliant soft contact lenses users: genotyping and risk factors, a study from Cairo, Egypt. J Infect Public Health 2018; 11: 377–383. DOI: 10.1016/j.jiph.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman AB, Richdale K, Mitchell GL, et al. Water exposure is a common risk behavior among soft and gas-permeable contact lens wearers. Cornea 2017; 36: 995–1001. DOI: 10.1097/Ico.0000000000001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke DW, Niederkorn JY. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol 2006; 22: 175–180. DOI: 10.1016/j.pt.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Sun X, Wang Z, et al. Identification of 18S ribosomal DNA genotype of Acanthamoeba from patients with keratitis in North China. Invest Ophthalmol Vis Sci 2004; 45: 1904–1907. DOI: 10.1167/iovs.03-1073. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Wang Z, Qu J, et al. Acanthamoeba keratitis related to contact lens use in a tertiary hospital in China. BMC Ophthalmol 2019; 19: 202. DOI: 10.1186/s12886-019-1210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caumo K, Rott MB. Acanthamoeba T3, T4 and T5 in swimming-pool waters from Southern Brazil. Acta Tropica 2011; 117: 233–235. DOI: 10.1016/j.actatropica.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Lakhundi S, Khan NA, Siddiqui R. The effect of environmental and physiological conditions on excystation of Acanthamoeba castellanii belonging to the T4 genotype. Parasitol Res 2014; 113: 2809–2816. DOI: 10.1007/s00436-014-3941-6. [DOI] [PubMed] [Google Scholar]
- 18.Walochnik J, Scheikl U, Haller-Schober EM. Twenty years of acanthamoeba diagnostics in Austria. J Eukaryot Microbiol 2015; 62: 3–11. DOI: 10.1111/jeu.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez-Martinez D, Reyes-Batlle M, Castelan-Ramirez I, et al. Evaluation of the sensitivity to chlorhexidine, voriconazole and itraconazole of T4 genotype Acanthamoeba isolated from Mexico. Exp Parasitol 2019; 197: 29–35. DOI: 10.1016/j.exppara.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Aqeel Y, Rodriguez R, Chatterjee A, et al. Killing of diverse eye pathogens (Acanthamoeba spp., Fusarium solani, and Chlamydia trachomatis) with alcohols. PLoS Negl Trop Dis 2017; 11: e0005382. DOI: 10.1371/journal.pntd.0005382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naginton J, Watson PG, Playfair TJ, et al. Amoebic infection of the eye. Lancet 1974; 2: 1537–1540. [DOI] [PubMed] [Google Scholar]
- 22.Verani JR, Lorick SA, Yoder JS, et al. National outbreak of Acanthamoeba keratitis associated with use of a contact lens solution, United States. Emerg Infect Dis 2009; 15: 1236–1242. DOI: 10.3201/eid1508.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seal DV. Acanthamoeba keratitis update-incidence, molecular epidemiology and new drugs for treatment. Eye (Lond) 2003; 17: 893–905. DOI: 10.1038/sj.eye.6700563. [DOI] [PubMed] [Google Scholar]
- 24.Robertson DM, McCulley JP, Cavanagh HD. Severe acanthamoeba keratitis after overnight orthokeratology. Eye Contact Lens 2007; 33: 121–123. DOI: 10.1097/01.icl.0000244110.70378.8c. [DOI] [PubMed] [Google Scholar]
- 25.Wong VW, Chi SC, Lam DS. Good visual outcome after prompt treatment of acanthamoeba keratitis associated with overnight orthokeratology lens wear. Eye Contact Lens 2007; 33: 329–331. DOI: 10.1097/ICL.0b013e318030d5cf. [DOI] [PubMed] [Google Scholar]
- 26.Watt K, Swarbrick HA. Microbial keratitis in overnight orthokeratology: review of the first 50 cases. Eye Contact Lens 2005; 31: 201–208. DOI: 10.1097/01.icl.0000179705.23313.7e. [DOI] [PubMed] [Google Scholar]
- 27.Jung SK, Lee JH, Kakizaki H, et al. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in Seoul, South Korea. Invest Ophthalmol Vis Sci 2012; 53: 5579–5583. DOI: 10.1167/iovs.12-10106. [DOI] [PubMed] [Google Scholar]
- 28.Sun J, Zhou J, Zhao P, et al. High prevalence of myopia and high myopia in 5060 Chinese university students in Shanghai. Invest Ophthalmol Vis Sci 2012; 53: 7504–7509. DOI: 10.1167/iovs.11-8343. [DOI] [PubMed] [Google Scholar]
- 29.Liu YM, Xie P. The safety of orthokeratology–a systematic review. Eye Contact Lens 2016; 42: 35–42. DOI: 10.1097/ICL.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barr JT, Rah MJ, Jackson JM, et al. Orthokeratology and corneal refractive therapy: a review and recent findings. Eye Contact Lens 2003; 29: S49–S53; discussion S57-49, S192-194. DOI: 10.1097/00140068-200301001-00014. [DOI] [PubMed] [Google Scholar]
- 31.Chan B, Cho P, Cheung SW. Orthokeratology practice in children in a university clinic in Hong Kong. Clin Exp Optom 2008; 91: 453–460. DOI: 10.1111/j.1444-0938.2008.00259.x. [DOI] [PubMed] [Google Scholar]
- 32.Chang LC, Liao LL. Vision improvement and compliance with the use of orthokeratology lenses in school children: a sample from five primary schools in Northern Taiwan. Eye Contact Lens 2018; 44: 299–303. DOI: 10.1097/ICL.0000000000000518. [DOI] [PubMed] [Google Scholar]
- 33.Cooper J, Tkatchenko AV. A review of current concepts of the etiology and treatment of myopia. Eye Contact Lens 2018; 44: 231–247. DOI: 10.1097/ICL.0000000000000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vagge A, Ferro Desideri L, Nucci P, et al. Prevention of progression in myopia: a systematic review. Diseases 2018; 6: 92. DOI: 10.3390/diseases6040092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Li J, Li X, et al. Redistribution of the corneal epithelium after overnight wear of orthokeratology contact lenses for myopia reduction. Cont Lens Anterior Eye 2020; 43: 232–237. DOI: 10.1016/j.clae.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Lipson MJ, Brooks MM, Koffler BH. The role of orthokeratology in myopia control: a review. Eye Contact Lens 2018; 44: 224–230. DOI: 10.1097/ICL.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 37.Wei C, Zhu M, Petroll WM, et al. Pseudomonas aeruginosa infectious keratitis in a high oxygen transmissible rigid contact lens rabbit model. Invest Ophthalmol Vis Sci 2014; 55: 5890–5899. DOI: 10.1167/iovs.14-14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choo JD, Holden BA, Papas EB, et al. Adhesion of Pseudomonas aeruginosa to orthokeratology and alignment lenses. Optom Vis Sci 2009; 86: 93–97. DOI: 10.1097/OPX.0b013e318194e973. [DOI] [PubMed] [Google Scholar]
- 39.Fleiszig SM, Evans DJ. Pathogenesis of contact lens-associated microbial keratitis. Optom Vis Sci 2010; 87: 225–232. DOI: 10.1097/OPX.0b013e3181d408ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steele KR, Szczotka-Flynn L. Epidemiology of contact lens-induced infiltrates: an updated review. Clin Exp Optom 2017; 100: 473–481. DOI: 10.1111/cxo.12598. [DOI] [PubMed] [Google Scholar]
- 41.Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol 2009; 148: 487–499.e2. DOI: 10.1016/j.ajo.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Randag AC, Van Rooij J, Van Goor AT, et al. The rising incidence of Acanthamoeba keratitis: a 7-year nationwide survey and clinical assessment of risk factors and functional outcomes. PLoS One 2019; 14: e0222092. DOI: 10.1371/journal.pone.0222092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robaei D, Carnt N, Minassian DC, et al. Therapeutic and optical keratoplasty in the management of Acanthamoeba keratitis: risk factors, outcomes, and summary of the literature. Ophthalmology 2015; 122: 17–24. DOI: 10.1016/j.ophtha.2014.07.052. [DOI] [PubMed] [Google Scholar]
- 44.Awwad ST, Petroll WM, McCulley JP, et al. Updates in Acanthamoeba keratitis. Eye Contact Lens 2007; 33: 1–8. DOI: 10.1097/ICL.0b013e31802b64c1. [DOI] [PubMed] [Google Scholar]
- 45.Claerhout I, Goegebuer A, Van Den Broecke C, et al. Delay in diagnosis and outcome of Acanthamoeba keratitis. Graefes Arch Clin Exp Ophthalmol 2004; 242: 648–653. DOI: 10.1007/s00417-003-0805-7. [DOI] [PubMed] [Google Scholar]
- 46.Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite 2015; 22: 10. DOI: 10.1051/parasite/2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamazoe K, Yamamoto Y, Shimazaki-Den S, et al. Visual outcome in Japanese patients with Acanthamoeba keratitis. Eye (Lond) 2012; 26: 517–522. DOI: 10.1038/eye.2011.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang C, Sun X, Wang Z, et al. Acanthamoeba keratitis: clinical characteristics and management. Ocul Surf 2015; 13: 164–168. DOI: 10.1016/j.jtos.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology 2017; 124: 1678–1689. DOI: 10.1016/j.ophtha.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goh JWY, Harrison R, Hau S, et al . Comparison of in vivo confocal microscopy, PCR and culture of corneal scrapes in the diagnosis of Acanthamoeba keratitis. Cornea 2018; 37: 480–485. [DOI] [PubMed] [Google Scholar]
- 51.Chidambaram JD, Prajna NV, Palepu S, et al. In vivo confocal microscopy cellular features of host and organism in bacterial, fungal, and Acanthamoeba keratitis. Am J Ophthalmol 2018; 190: 24–33. DOI: 10.1016/j.ajo.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang YE, Tepelus TC, Vickers LA, et al. Role of in vivo confocal microscopy in the diagnosis of infectious keratitis. Int Ophthalmol 2019; 39: 2865–2874. DOI: 10.1007/s10792-019-01134-4. [DOI] [PubMed] [Google Scholar]
- 53.Pasricha G, Sharma S, Garg P, et al. Use of 18S rRNA gene-based PCR assay for diagnosis of Acanthamoeba keratitis in non-contact lens wearers in India. J Clin Microbiol 2003; 41: 3206–3211. DOI: 10.1128/jcm.41.7.3206-3211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carnt N, Robaei D, Minassian DC, et al. Acanthamoeba keratitis in 194 patients: risk factors for bad outcomes and severe inflammatory complications. Br J Ophthalmol 2018; 102: 1431–1435. DOI: 10.1136/bjophthalmol-2017-310806. [DOI] [PubMed] [Google Scholar]
- 55.Carrijo-Carvalho LC, Sant'ana VP, Foronda AS, et al. Therapeutic agents and biocides for ocular infections by free-living amoebae of Acanthamoeba genus. Surv Ophthalmol 2017; 62: 203–218. DOI: 10.1016/j.survophthal.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 56.Cheung N, Nagra P, Hammersmith K. Emerging trends in contact lens-related infections. Curr Opin Ophthalmol 2016; 27: 327–332. DOI: 10.1097/ICU.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 57.Lim N, Goh D, Bunce C, et al. Comparison of polyhexamethylene biguanide and chlorhexidine as monotherapy agents in the treatment of Acanthamoeba keratitis. Am J Ophthalmol 2008; 145: 130–135. DOI: 10.1016/j.ajo.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 58.Sharma S, Garg P, Rao GN. Patient characteristics, diagnosis, and treatment of non-contact lens related Acanthamoeba keratitis. Br J Ophthalmol 2000; 84: 1103–1108. DOI: 10.1136/bjo.84.10.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakagawa H, Koike N, Ehara T, et al. Corticosteroid eye drop instillation aggravates the development of Acanthamoeba keratitis in rabbit corneas inoculated with Acanthamoeba and bacteria. Sci Rep 2019; 9: 12821. DOI: 10.1038/s41598-019-49128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bagga B, Garg P, Joseph J, et al. Outcome of therapeutic deep anterior lamellar keratoplasty in advanced Acanthamoeba keratitis. Indian J Ophthalmol 2020; 68: 442–446. DOI: 10.4103/ijo.IJO_307_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu HY, Chu HS, Wang IJ, et al. Clinical features and outcomes of Acanthamoeba keratitis in a tertiary hospital over 20- year period. J Formos Med Assoc 2020; 119: 211–217. DOI: 10.1016/j.jfma.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 62.Carnt N, Hoffman JM, Verma S, et al. Acanthamoeba keratitis: confirmation of the UK outbreak and a prospective case-control study identifying contributing risk factors. Br J Ophthalmol 2018; 102: 1621–1628. DOI: 10.1136/bjophthalmol-2018-312544. [DOI] [PubMed] [Google Scholar]
- 63.Hirabayashi KE, Lin CC, Ta CN. Oral miltefosine for refractory Acanthamoeba keratitis. Am J Ophthalmol Case Rep 2019; 16: 100555. DOI: 10.1016/j.ajoc.2019.100555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greenwell TH, Loh RS, Chehade M, et al. Misdiagnosis of orthokeratology-related Acanthamoeba keratitis as herpes simplex virus keratitis. Clin Exp Ophthalmol 2013; 41: 418–420. DOI: 10.1111/ceo.12047. [DOI] [PubMed] [Google Scholar]
- 65.Kent SS, Robert MC, Tokarewicz AC, et al. Painless Acanthamoeba keratitis. Can J Ophthalmol 2012; 47: 383–384. DOI: 10.1016/j.jcjo.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 66.Kim EC, Kim MS. Bilateral acanthamoeba keratitis after orthokeratology. Cornea 2010; 29: 680–682. DOI: 10.1097/ICO.0b013e3181861bf9. [DOI] [PubMed] [Google Scholar]
- 67.Xuan YH, Chung BS, Hong YC, et al. Keratitis by Acanthamoeba triangularis: report of cases and characterization of isolates. Korean J Parasitol 2008; 46: 157–164. DOI: 10.3347/kjp.2008.46.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JE, Hahn TW, Oum BS, et al. Acanthamoeba keratitis related to orthokeratology. Int Ophthalmol 2007; 27: 45–49. DOI: 10.1007/s10792-007-9055-8. [DOI] [PubMed] [Google Scholar]
- 69.Lee SJ, Jeong HJ, Lee JE, et al. Molecular characterization of Acanthamoeba isolated from amebic keratitis related to orthokeratology lens overnight wear. Korean J Parasitol 2006; 44: 313–320. DOI: 10.3347/kjp.2006.44.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilhelmus KR. Acanthamoeba keratitis during orthokeratology. Cornea 2005; 24: 864–866. DOI: 10.1097/01.ico.0000175410.28859.bd. [DOI] [PubMed] [Google Scholar]
- 71.Yepes N, Lee SB, Hill V, et al. Infectious keratitis after overnight orthokeratology in Canada. Cornea 2005; 24: 857–860. DOI: 10.1097/01.ico.0000154388.43501.f8. [DOI] [PubMed] [Google Scholar]
- 72.Sun X, Chen L, Zhang Y, et al. Acanthamoeba keratitis as a complication of orthokeratology. Am J Ophthalmol 2003; 136: 1159–1161. DOI: 10.1016/s0002-9394(03)00635-4. [DOI] [PubMed] [Google Scholar]
- 73.Hutchinson K, Apel A. Infectious keratitis in orthokeratology. Clin Exp Ophthalmol 2002; 30: 49–51. DOI: 10.1046/j.1442-9071.2002.00483.x. [DOI] [PubMed] [Google Scholar]