Abstract
This review article aims to address mysteries existing between Interstitial Lung Abnormality (ILA) and Nonspecific Interstitial Pneumonia (NSIP). The concept and definition of ILA are based upon CT scans from multiple large-scale cohort studies, whereas the concept and definition of NSIP originally derived from pathology with evolution to multi-disciplinary diagnosis. NSIP is the diagnosis as Interstitial Lung Disease (ILD) with clinical significance, whereas only a part of subjects with ILA have clinically significant ILD. Eventually, both ILA and NSIP must be understood in the context of chronic fibrosing ILD and progressive ILD, which remains to be further investigated.
Abbreviations: AIP, acute interstitial pneumonia; ATS/ERS, American Thoracic Society/European Respiratory Society; RB-ILD, respiratory bronchiolitis-associated interstitial lung disease; BIP, bronchiolitis obliterans with interstitial pneumonia; BOOP, bronchiolitis obliterans organizing pneumonia; CTD, connective tissue disease; DIP, desquamative interstitial pneumonia; fNSIP, fibrosing nonspecific interstitial pneumonia; GGO, ground-glass opacities; GIP, giant cell interstitial pneumonia; HRCT, high-resolution CT; IIP, idiopathic interstitial pneumonia; ILA, interstitial lung abnormality; ILD, interstitial lung disease; LIP, lymphoid interstitial pneumonia; NSIP, nonspecific interstitial pneumonia; UIP, usual interstitial pneumonia
Keywords: Interstitial lung abnormality (ILA), Nonspecific interstitial pneumonia (NSIP), Connective tissue disease (CTD), Interstitial lung disease (ILD), CT, HRCT, Pulmonary fibrosis
1. Introduction
Interstitial lung abnormality (ILA) is defined as “incidental identification of non-dependent abnormalities, including ground-glass or reticular abnormalities, lung distortion, traction bronchiectasis/bronchiolectasis, honeycombing, and non-emphysematous cysts involving at least 5% of a lung zone in individuals in whom interstitial lung disease is not suspected” [1,2]. (Table 1) In the background, there was a long history of evolution of the concept from usual interstitial pneumonia (UIP) to nonspecific interstitial pneumonia (NSIP) during the development of the guidelines for UIP with multidisciplinary efforts of pathologists, radiologists, and pulmonologists [3,4]. In the clinical practice, we notice an overlap of CT findings between ILA and NSIP. ILA is defined solely by CT findings, and NSIP is originally defined by pathological findings, which is currently based upon multi-disciplinary conference consensus decision. Therefore, it is important to review CT findings of NSIP and pathology of ILA to understand this phenomenon of an overlap in CT findings between the two entities in the context of the spectrum of pulmonary fibrosis [5]. Traction bronchiectasis/bronchiolectasis has been recognized as an important prognostic biomarker for fibrotic lung disease. This review describes the above contents with CT and pathology illustrations and Tables in addition to the discussion on the current options for management of fibrotic lung diseases (Table 2).
Table 1.
Definition and Subcategories of Interstitial Lung Abnormality (ILA).
| Definition |
|---|
| Incidental identification of non-dependent abnormalities, including ground-glass or reticular abnormalities, lung distortion, traction bronchiectasis, honeycombing, and non-emphysematous cysts involving at least 5% of a lung zone (upper, middle, and lower lung zones are demarcated by the levels of the inferior aortic arch and right inferior pulmonary vein) in individuals in whom interstitial lung disease is not suspected. |
| Subcategories of ILA |
|---|
| Non-subpleural: ILAs without predominant subpleural localization |
| Subpleural non-fibrotic: ILAs with a predominant subpleural localization and without evidence of fibrosisa |
| Subpleural fibrotic: ILAs with a predominant subpleural localization and with evidence of pulmonary fibrosisa |
Modified from the Position Paper by the Fleischner Society (Lancet Respir Med 2020, Reference #2).
Fibrosis is characterized by the presence of architectural distortion with traction bronchiectasis or honeycombing (or both).
Table 2.
Histologic Diagnosis and HRCT Features of Idiopathic Non-specific Interstitial Pneumonia.
| Histologic Features |
|---|
| Cellular Pattern |
| Mild to moderate interstitial chronic inflammation |
| Type II pneuocyte hyperplasia in areas of inflammation |
| Fibrosing Pattern |
| Dense or loose interstitial fibrosis with uniform appearance |
| Frequent lung architectural preservation |
| Mild or moderate chronic interstitial inflammation |
| HRCT Findings |
|---|
| Bilateral symmetric and predominantly lower lung zone involvement |
| Reticular opacity with traction bronchiectasis and lower lobe volume loss |
| Diffuse or subpleural in axial planes; sometimes spare subpleural lungs |
| Based on American Thoracic Society Project for idiopathic nonspecific interstitial pneumonia (Travis WD et al; reference #40) |
2. Emerging concept of ILA with subcategories
The diagnosis and classification of idiopathic interstitial pneumonia (IIP) had been based on pathology [3]. In 2002, new classification of IIP was proposed by ATS/ERS [4]. This guideline crucially placed greater importance to clinical and radiologic assessment, mentioning that clinical and radiologic information showed high positive predictive value [6]. Along with more attention to high-resolution CT (HRCT) in the diagnosis of IIP, asymptomatic interstitial lung disease (ILD) had been reported in HRCT in older patients, smokers and rheumatoid arthritis patients [[7], [8], [9], [10]]. The high attenuation areas incidentally found on HRCT were reported to be associated with the decline of pulmonary function [[10], [11], [12], [13]]. In 2011, the concept of ILA was advocated as subclinical CT findings suggesting the presence of interstitial changes in the lungs [11]. The ILA was observed in from 2 to 10 % of several smokers’ cohorts and sometimes showed interval progression [10,[12], [13], [14], [15], [16], [17]]. It has the various features similar to those of UIP in terms of genetic factor, reduced pulmonary function, exercise intolerance, and worsened all-cause mortality [1,2,[13], [14], [15], [16], [17], [18], [19], [20], [21], [22]]. These results suggested the possibility of spectrum from ILA to UIP [1,2,5,18]. According to the diagnostic criteria of idiopathic pulmonary fibrosis proposed by Lynch et al. in 2018 [23], ILA was described as the area of uncertainty.
ILA was subcategorized by distribution and presence of its abnormalities. Washko et al. [13] have reported the distribution patterns: centrilobular, subpleural and mixed patterns. Centrilobular pattern was excluded from the definition of ILA in the criteria on Fleischner Society Position paper because of little relevancy to pulmonary interstitial fibrosis [2,19]. Previous studies implied that subpleural reticulation, traction bronchiectasis/ bronchiolectasis and nonemphysematous cyst in subjects with ILA were associated with poorer prognosis [[24], [25], [26], [27], [28]]. In the long-term observation, subpleural non-fibrotic ILA sometimes progressed to subpleural fibrotic ILA, namely ILA with subpleural reticulation, traction bronchiolectasis or honeycombing [1,2,15,17,25]. These results emphasized the importance of detecting subpleural fibrotic ILA [1,2]. Subpleural fibrotic ILA might be considered one of potential precursor of UIP [1,2,23,25]. Following these results, ILA is now classified with three subcategories: non-subpleural non-fibrotic, subpleural non-fibrotic and subpleural fibrotic ILAs [2].
3. Definition of NSIP originally derived from pathology
The idiopathic interstitial pneumonias (IIPs) were first classified as a set of histopathologic patterns in the 1960s by Liebow and Carrington [29] into usual interstitial pneumonia (UIP), desquamative interstitial pneumonia (DIP), bronchiolitis obliterans with interstitial pneumonia (BIP), giant cell interstitial pneumonia (GIP) and lymphoid interstitial pneumonia (LIP). The BIP evolved into bronchiolitis obliterans organizing pneumonia (BOOP) in pathologic term, then into cryptogenic organizing pneumonia [[30], [31], [32]].
Much discussion has been made on the controversy regarding what patterns should be included in the classification system and what these patterns represent for clinical disease [33]. Consequently, GIP has been classified into a pneumoconiosis (hard-metal pneumoconiosis) [34] and LIP finally as interstitial pneumonias after once being considered preneoplastic disorder such as a kind of lymphoproliferative disease (with more clear understanding of proteomics and genomics as to lung lymphomas) [33].
Nonspecific interstitial pneumonia (NSIP) was first defined as one of a different group of pulmonary fibrosis by Katzenstein and Fiorelli in 1994 [35]. Katzenstein and Fiorelli [35] in 1994 reported pathologic features of new interstitial pneumonia named nonspecific interstitial pneumonia/fibrosis. The histologic whole mark is varying proportions of interstitial inflammation and fibrosis that appeared to be occurring over a single time span (i.e., the process, temporally uniform). And the authors suggested that because it may have varying etiologies including underlying connective tissue disease (CTD), organic dust or other exposures, and prior acute lung injury, less often, it may reflect a nonrepresentative biopsy of another process. In addition to NSIP, new histopathologic patterns have been described such as respiratory bronchiolitis-associated interstitial lung disease (RB-ILD) and acute interstitial pneumonia (AIP) [[36], [37], [38]].
In 2002, the American Thoracic Society/European Respiratory Society (ATS/ERS) defined seven disease categories of IIPs and proposed standardized terminology and diagnostic criteria [4]. In 2013, the ATS/ERS updated the 2002 ATS/ERS classification and claimed that the update is a supplement to the previous 2002 IIP classification document [39].
Nonspecific interstitial pneumonia was accepted by the 2002 American Thoracic Society/European Respiratory Society classification of idiopathic interstitial pneumonias, but it was regarded as provisional, pending further study [4]. However, the idiopathic NSIP is a distinct form of IIP. The diagnosis requires a dynamic integrated multidisciplinary approach because the histologic pattern can be seen in other disorders such as hypersensitivity pneumonitis [40]. Whereas UIP is the most common histopathologic pattern (47–64 %) seen in cases of IIPs, the exact incidence and prevalence of the NSIP need to be studied. Idiopathic NSIP is regarded to be much less than UIP in its incidence and to represent 14–36 % of the whole IIPs [41]. Moreover, the NSIP foci can be seen in the same patients and even in the same lobe showing UIP [42]. It remains unclear, however, whether these cases of NSIP represent early or inactive phase of idiopathic pulmonary fibrosis (pathologic UIP). The histopathologic features of NSIP are seen in connective tissue disease-related interstitial lung disease and hypersensitivity pneumonitis [40].
4. CT findings of NSIP
The CT findings of NSIP was reported first by Park et al. [43]. In their report, while reporting seven cases of NSIP, the predominant radiographic findings were areas of patchy parenchymal opacification present bilaterally in the middle and lower lung zones. The most common CT findings identified on thin-section CT were patchy areas of GGO present alone or with areas of consolidation. At follow-up CT, the initial parenchymal abnormalities resolved completely in three patients, improved in another three, and persisted in one. According to a report from the American Thoracic Society project [40], characteristic features of NSIP include reticular opacities with lower lung zone predominance, associated with traction bronchiectasis and lobar volume loss. By high-resolution computed tomography, the lower lung zones were predominantly involved in 92 % (62 of 67 cases); 46 % had a peripheral distribution; 47 %were diffuse. Most showed a reticular pattern (87 %) with traction bronchiectasis (82 %) and volume loss (77 %) [40]. Kim et al. [44] compared CT and pathologic findings and concluded that the patchy subpleural areas of GGO mixed with irregular reticular lesions or bronchial dilatation were caused by varying degrees of interstitial inflammation, fibrosis, or both. In 13 patients with nonspecific interstitial pneumonia, areas of GGO decrease significantly on follow-up CT whereas the areas of reticular lesions decreased slightly, and the extent of decrease in GGO correlated significantly with that of functional improvement [45]. The fNSIP demonstrated disease progression on serial CT and clinical studies and most important CT prognostic factor was fibrotic score (the extent of reticulation plus honeycombing) [46,47].
Thin-section CT is useful for identifying NSIP: 78 % of diagnostic accuracy was reported by Silva et al. [48] In this study, typical features of fNSIP on CT are subpleural GGO and reticular lesions with sometimes mild traction bronchiectasis. The lesions may show distribution of both along the bronchovascular bundles and subpleural lungs (Fig. 1). Particularly in fNSIP related to the connective tissue disease, the lesions demonstrate lower lung zone predominance (Fig. 2). On CT, UIP features (probable UIP on CT) can be overlapped with fNSIP with subpleural fibrosis accompanying traction bronchiectasis/bronchiolectasis [23]. Similarly, the UIP can be overlapped with fNSIP with patchy involvement, and subpleural or paraseptal distribution of fibrosis except the features of temporal heterogeneity and the features of the presence of fibroblastic foci on pathology [42]. The distinction between fNSIP and chronic hypersensitivity pneumonitis is often difficult on HRCT, even though lobular areas with decreased attenuation and vascularity and centrilobular small nodules are seen with upper and middle lung zone distribution in chronic hypersensitivity pneumonia [48]. Regardless of these issues, radiologic assessment for fNSIP is important owing to the difference in patient management and patient survival (effective corticosteroid and cytotoxic drug use and longer overall survival in fNSIP, compared with UIP) from UIP [49].
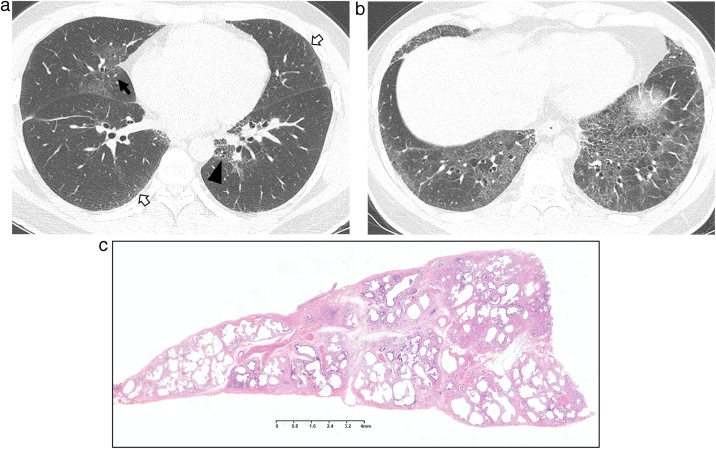
Fig. 1.
Idiopathic fibrosing nonspecific interstitial pneumonia in a 40-year-old man.
(a, b) Lung window images of CT scans obtained at levels of right interior pulmonary vein (a) and liver dome (b), respectively, show reticular lesions and traction bronchiectasis and bronchiolectasis (arrowheads) along bronchovascular bundles (arrows) and along subpleural lungs (open arrows).
(c) Low-power magnification of lung obtained from left lower lobe by video-assisted transthoracic surgery (VATS) demonstrates temporally uniform interstitial fibrosis with minimal architectural distortion.
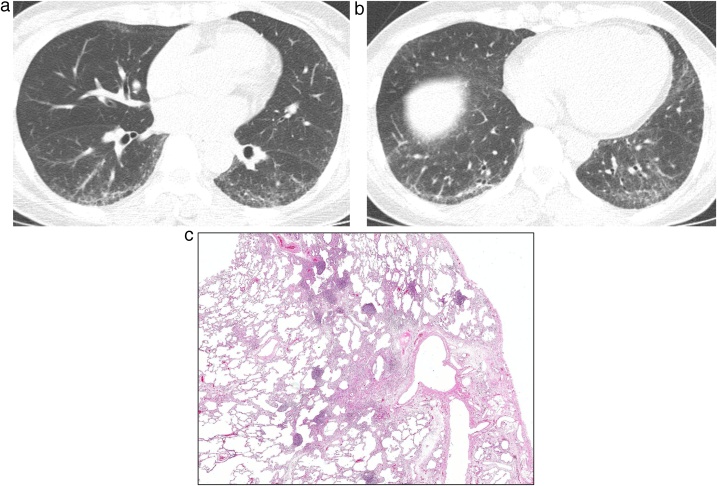
Fig. 2.
Fibrosing nonspecific interstitial pneumonia in a 43-year-old woman with interstitial pneumonia with autoimmune features (IPAF; fluorescent antinuclear antibody [FANA], 1:640).
(a, b) Lung window images of CT scans obtained at levels of right interior pulmonary vein (a) and liver dome (b), respectively, show reticular lesions and traction bronchiolectasis (arrowhead in b) in bilateral subpleural lungs.
(c) Low-power magnification of lung obtained from right lower lobe by VATS demonstrates mild pleural fibrosis, and airway centered fibrosis containing lymphoid aggregates including some with germinal centers. Although not specific, lymphoid follicles with germinal centers are often associated with connective tissue disease.
The NSIP is associated more commonly with CTD than UIP. This relationship between CTD and NSIP can influence on patients’ overall survival depending on the sort of underlying connective tissue disease [50]. With treatment, serial CT scans of polymyositis/dermatomyositis patients with NSIP show significant improvement in abnormal GGO and consolidation, and radiologic progression of lung fibrosis is limited [51]. And the CT scan features and clinical course of NSIP in polymyositis/dermatomyositis patients are relatively uniform [51,52].
Previous studies investigated the relationship between pathology and radiology of the NSIP. They showed that in CT interpretation, observers frequently have difficulty in distinguishing NSIP from UIP or other IIPs because of overlapped features among them [[53], [54], [55]]. Sumikawa et al. [56] have reported that about 90 % of pathological NSIP showed NSIP pattern at HRCT, while 28 % of pathological UIP showed NSIP pattern at HRCT. They also reported that less reticulation and more GGO on HRCT play an important role with favoring NSIP than UIP in distinction. These results might have reflected on some degree of cellular component of pathological NSIP. The retrospective review of each figure from several studies showed that CT findings of fNSIP or other IIP cases are similar to those of fibrotic ILA [40,49,[53], [54], [55],[57], [58], [59]]. In the latest clinical practice guideline by Raghu et al. [60], CT features of both fibrotic ILA and fNSIP are similar to those of either CT probable UIP or indeterminate UIP pattern, depending on the degree and distribution of fibrosis. Fibrosing NSIP shows worse overall survival than cellular NSIP, and subpleural fibrotic ILA eventually progresses to pulmonary fibrosis and resultant increased mortality [25,61].
5. An overlap of CT findings between ILA and NSIP
ILA and NSIP have common radiologic features such as ground-glass opacity (GGO), reticular abnormality and traction bronchiectasis [1,2,4]. Both subpleural fibrotic ILA and fNSIP frequently manifest reticulation or traction bronchiectasis coexistent with GGO on HRCT [2,4,15,40]. They often showed interval progression [2,15,17,25,49,57]. However, ILA is derived from subclinical radiologic findings on CT, whereas NSIP is being diagnosed with MDD approach because of the possibility of secondary NSIP related to connective tissue disease, hypersensitivity pneumonitis, infection, and drug-related pneumonitis [2,3,18,40,62,63].
6. Pathological considerations of ILA
ILA is a radiologic term. The primary source of histopathologic findings reported in ILA is background lung in specimens resected for benign and malignant nodules and masses; studies by Miller et al. [64] and Hung et al. [65] are the most relevant in this setting.
In 2018, Miller et al. [64] examined 424 patients with paired chest CT scans and histopathologic samples obtained from lung nodule resections. Of 424 patients, 26 (6%) had ILA, 257 (61 %) did not have ILA, and 141 (31 %) were indeterminate for ILA. The histopathologic findings most strongly associated with ILA were subpleural fibrosis in 12 (46 %), fibroblast foci in 7 (28 %), honeycombing in 2 (8%), UIP in 2 (8%), and atypical adenomatous hyperplasia in 9 (35 %). In 2019, Hung et al. [65] evaluated 406 resections from 397 patients and found parenchymal lung abnormalities in 101 of 397 (25 %) patients: 10 % of these were fibrotic interstitial changes including smoking-related interstitial fibrosis (7%), UIP (1%), NSIP (0.7 %), and undefined fibrosis (1%); followed by granulomatous processes (8%), and other findings (6%) including thrombotic angiopathy (2%), Langerhans cell histiocytosis (1.5 %), constrictive bronchiolitis (1%), atypical lymphoid infiltrates (1%), and amyloidosis (0.5 %); and aspiration (4%).
Both studies included histopathologic correlation with ILA, but the design of the two studies was different. Miller et al. [64] hypothesized that ILA could represent early/mild idiopathic pulmonary fibrosis (IPF) and that histopathology from ILA might be supportive. Their cohort was comprised of patients who had undergone lung nodule resection, had chest CT scans within 3 months before surgery, and who had no clinical history of ILD. In contrast, Hung et al. [65] set out to determine the prevalence of incidental non-neoplastic lung disease in patients undergoing resection of benign and malignant mass lesions who also had chest CT scans within 2 months before surgery. While both studies preceded the Fleischner Position Paper on ILA [2], the approach taken by Miller et al. [64] most closely approximates the Fleischner criteria for identifying patients with ILA and is the first blinded study that compares chest CT imaging and histopathology specifically for the purpose of determining the histopathology associated with ILA.
Miller et al. [64] and Hung et al. [65] are well done studies that suggest smoking-related fibrosis [66,67] occurs in a much greater portion of ILAs than UIP and fibrotic NSIP. Interestingly, Miller et al. [64] and Hung et al. [65] found fibrotic changes in 52 % and 51 % of specimens respectively with no radiologic ILA, demonstrating that lung fibrosis can be below the resolution of chest CT. The method for sample section could not be controlled and is a limitation in both of these retrospective studies; non-neoplastic lung sections were randomly taken by the prosector handling the gross specimen, rather than selecting tissue based on the radiologic location of ILA.
Future studies have an opportunity to build on our current knowledge and to address several issues in ILA. First, radiologic-pathologic correlation is most reliable when tissue for histopathologic examination is selected from radiologicly identified areas of abnormality. Contact radiography of specimens as described by Itoh et al. [68] could guide tissue selection for histopathologic examination and greatly improve radiologic-pathologic correlation leading to a deeper understanding of ILA.
Second, distinguishing normal from minimal/early change is not well established, and findings are often ignored both in radiology and pathology for fear of erroneously suggesting a diagnosis of diffuse fibrotic lung disease [69,70]. On the opposite end of the spectrum, in their study of inter-observer variation between pathologists in diffuse parenchymal lung disease, Nicholson et al. [71] reported an overall kappa coefficient of agreement of 0.38 for first choice diagnosis, while Watadani et al. [72] reported a kappa coefficient of agreement of 0.40−0.58 in their chest CT assessment of honeycombing in lungs. Inter-observer variability for radiology and pathology in the assessment of ILA is unknown.
Third, the background lung in specimens resected for neoplasms should not be ignored, in particular, the presence of fibrosis including smoking-related fibrosis needs to be reported [67], and a pattern given if possible (Figs. 3,4). Lung cancer protocols should be reviewed and modified to expand sampling of background lung to better support radiologic-pathologic correlation in ILA.
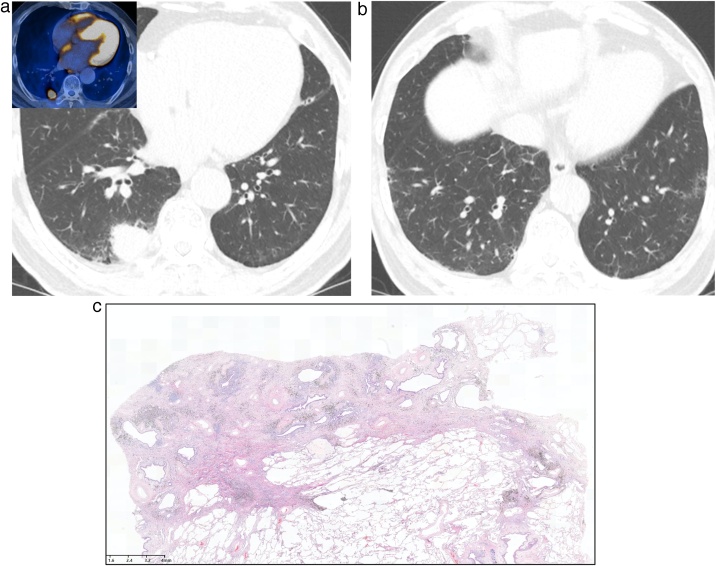
Fig. 3.
Interstitial lung abnormality combined with lung adenocarcinoma in a 71-year-old man.
(a, b) Lung window images of CT scans obtained at levels of right interior pulmonary vein (a) and liver dome (b), respectively, show subpleural reticular lesions mixed with some ground-glass opacity in both lungs. Also note a 26-mm-sized nodule in right lower lobe. Inset in a: high fluorodeoxyglucose uptake within nodule at positron emission tomography indicating malignant nature of nodule.
(c) Low-power magnification of lung obtained from a right lower lobectomy demonstrates focal subpleural fibrosis with cystic spaces and anthracosis. Histologically similar images shown by Miller et al64 and Hung et al65 were classified as UIP.
Finally, terminology for lung fibrosis is limited, pathologists have only two terms - UIP and fibrosing NSIP. These are histologic patterns of lung injury with considerable histologic variation from case to case, and differing etiologies that are overlapping [4,39]. Histologic UIP and NSIP are defined as diffuse chronic patterns of lung fibrosis, so the use of these terms in cases with limited findings on imaging, namely ILA, is questionable as illustrated in Fig. 3, Fig. 4. Pathologists cannot determine extent of fibrosis on limited histologic sampling; chest CTs serve as pathologists “gross lung examination” for assessing the extent of fibrosis in both UIP and NSIP. The best terminology for pathologists to use when findings are limited on chest CT should be carefully considered, given the implications of rendering a diagnosis of UIP or fibrosing NSIP.
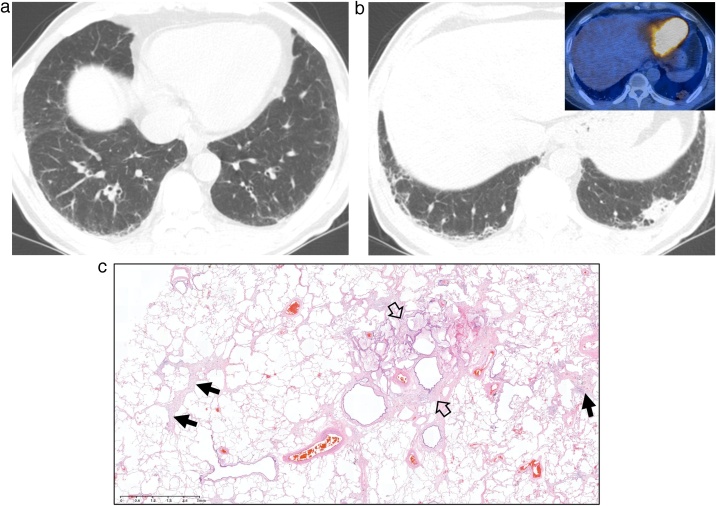
Fig. 4.
Interstitial lung abnormality and mucin-producing lung adenocarcinoma in a 73-year-old man.
(a, b) Lung window images of CT scans obtained at levels of suprahepatic inferior vena cava (a) and liver dome (b), respectively, show reticular lesions and traction bronchiolectasis (arrowheads) in both lungs. Also note a 23-mm-sized lung nodule in left lung base. Inset: rather mild fluorodeoxyglucose uptake within nodule at positron emission tomography indicating mucin-producing nature of lung cancer.
(c) Low-power magnification of lung obtained from a left lower lobe lobectomy demonstrates focal subpleural fibrosis (arrows) with cystic spaces (open arrows). These histologic findings do not meet pathologic criteria for the diagnosis of UIP or fibrosing NSIP.
7. Traction bronchiectasis/bronchiolectasis as a prognostic marker for fibrotic lung disease
In radiologic-pathologic correlation studies, radiologic traction bronchiectasis is associated with fibroblast foci in pathologically indicating the diagnosis of definite UIP or probable UIP [60,73]. Radiologic honeycombing also corresponded to histological traction bronchiolectasis [74]. It is considered that abnormal remodeling as dysplastic proliferation of bronchiole is also the cause of traction bronchiectasis [74,75]. Traction bronchiectasis seems to represent the temporary state of its continuous and irreversible spectrum from early ILD to honeycombing [75,76]. Previous studies have reported the association between radiologic traction bronchiectasis and mortality. In ILA and hypersensitivity pneumonitis, the presence of traction bronchiectasis was shown as one of the most influential factors for predicting worse overall survival [25,77]. The severity of traction bronchiectasis also had the correlation with overall survival [26,59,78]. Accordingly, traction bronchiectasis/bronchiolectasis can be serving as a potentially prognostic marker. It is considered important to detect traction bronchiectasis/bronchiolectasis on HRCT especially in subpleural fibrotic ILA cases, with frequently little or no honeycombing [28].
8. Current management strategies
The first step in management of ILD is the identification of underlying diseases or conditions such as CTD or environmental/occupational exposures, since treatment and prognosis of ILD varies greatly depending on the associated etiologies [62,63,79,80]. The NSIP is the most frequently associated ILD in CTD, although there are certain predilections of specific ILD patterns for different CTDs [[81], [82], [83]]. In addition, interstitial pneumonitis in hypersensitivity pneumonitis, human immunodeficiency virus, drug-related pneumonitis, or lymphoproliferative diseases may present with NSIP pattern [50,84].
The treatment regimen for NSIP with CTD depends on the type of underlying CTD. For systemic sclerosis-associated ILD for which the most common pattern is NSIP, cyclophosphamide with or without subsequent azathioprine has shown to decrease declining in lung function [85,86]. A recent randomized trial comparing mycophenolate mofetil and cyclophosphamide demonstrated similar efficacy in reducing the decrease of lung function between two drugs with mycophenolate mofetil showing better tolerance and less toxicity [87]. Other CTDs that may manifest ILD features include Sjögren’s syndrome, rheumatoid arthritis, systemic lupus erythematosus, mixed connective tissue disease, and inflammatory myositis. There are no guidelines or consensus on the treatment of ILD associated with those CTDs due to lack of controlled trials. However, corticosteroid is often the first-line medication, while immunosuppressants such as azathioprine, cyclophosphamide, mycophenolate mofetil, or calcineurin inhibitors are considered as conjunction, in steroid refractory cases, or in cases where corticosteroid is intolerable due to complications [83,88,89].
With regard to idiopathic NSIP, asymptomatic patients with mild disease may be followed without treatment [84]. However, for those with progressive disease or moderate to severe disease, corticosteroid treatment seems to be beneficial in stabilizing pulmonary function [90,91]. Other pharmacological options are azathioprine, cyclophosphamide, mycophenolate mofetil, or rituximab [84]. Nonetheless, there are no controlled trials available to confirm the dose and duration of medication as well as validate the efficacy of drugs.
For subset of patients with either idiopathic or secondary NSIP who suffer from progression regardless of aforementioned treatment, antifibrotic agents may be an option. Nintedanib and pirfenidone are the two antifibrotic agents available in the market which have successfully delayed the progression and suppressed acute exacerbation of idiopathic pulmonary fibrosis [92,93]. In recent multicenter prospective phase III placebo-controlled trials, nintedanib helped reduce the annual rate of declining in pulmonary function for systemic sclerosis-ILD and progressive fibrosing interstitial lung diseases including CTD-ILD and chronic hypersensitivity pneumonitis [94,95]. The RELIEF study, a phase II multicenter prospective randomized controlled trial on efficacy and safety of pirfenidone for progressive ILD which is comprised of CTD-ILD, fibrotic NSIP, asbestosis-related fibrosis, and chronic hypersensitivity pneumonitis has been recently completed [96].
Given its prevalence, substantial rate of progression, and impact on survival [15,17,22,25], need for monitoring and treatment strategies of ILA is increasingly recognized. However, the optimal management protocol for ILA has not yet been established. A recent position paper from Fleischner Society proposed a management algorithm for ILA [2]. The proposed schema suggests examination of evidence of a clinically significant ILD such as respiratory symptoms or decreased pulmonary function as well as the extent of disease on chest CT as the first step. If such evidence does not exist, management strategies of active monitoring versus expectant management are decided based on the presence of risk(s) for progression (e.g., smoking, exposure to inhalation agents, usual interstitial pneumonia pattern). Active monitoring recommends clinical and pulmonary function reassessment in 3–12 months and follow-up CT at 12–24 months for progression. Nonetheless, the appropriateness of this protocol including intervals of follow-up studies requires future validation. Furthermore, the benefit of treatment with immunosuppressive drugs or antifibrotic agents for progressive ILA which constitutes about 50 % of patients is not known. Further studies are warranted to establish strategies for evaluating and monitoring of ILA and to identify the significance of pharmacological treatment for disease progression and survival.
9. Conclusion
The presence of ILA is defined by CT findings, and NSIP is a clinicopathologic entity of ILD originally defined by pathology. There is an overlap between ILA and NSIP in terms of both CT findings and even pathological features. ILA, a radiologic entity, shall further evolve among different (non-subpleural, subpleural non-fibrotic, and subpleural fibrotic ILAs) subgroups in the aspects of 1) clinically significant ILD; 2) active monitoring method; and 3) expectant management. NSIP, as a clinicopathologic ILD, requires further evaluation including the search for the underlying conditions such as CTD. The underlying pathology of fibrotic ILA includes UIP, fNSIP, and airway enlargement and fibrosis (smoking-related lung disease). Furthermore, HRCT features of subpleural fibrotic ILA, fNSIP pattern and CT probable or indeterminate UIP pattern may overlap among the three patterns. The communication between radiologists and multi-disciplinary team of pulmonologists and pathologists may be performed with common and pertinent terms and mutual understanding of the nature of radiologically defined ILA and pathologically defined NSIP.
Ethical statement
This review paper includes no personal information of subjects.
This study does not include animal experiment. We use only demographic and image data, which is de-identified to all the co-authors.
Thus, we state that this study is based on the declaration of Helsinki.
Disclaimer
The views expressed in this article are those of the author (TJF) and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or the U.S. Government.
Funding
Grant Support (Hatabu):NIHR01CA203636, 5U01CA209414-03, NIH/NHLBI2R01HL111024-06, NIH R01HL135142, NIH/NHLBI 1R01HL130974.
Declaration of Competing Interest
Dr. Hatabu reports grants from Canon Medical Systems Inc, grants from Konica Minolta Inc, personal fees from Mitsubishi Chemical Co, personal fees from Canon Medical Systems Inc, outside the submitted work. Other authors have no conflicts of interest to disclose.
References
- 1.Hatabu H., Hunninghake G.M., Lynch D.A. Interstitial lung abnormality: recognition and perspectives. Radiology. 2019;291:1–3. doi: 10.1148/radiol.2018181684. [DOI] [PubMed] [Google Scholar]
- 2.Hatabu H., Hunninghake G.M., Richeldi L., Brown K.K., Wells A.U., Remy-Jardin M., Verschakelen J., Nicholson A.G., Beasley M.B., Christiani D.C., San José Estépar R., Seo J.B., Johkoh T., Sverzellati N., Ryerson C.J., Graham Barr R., Goo J.M., Austin J.H.M., Powell C.A., Lee K.S., Inoue Y., Lynch D.A. Interstitial lung abnormalities detected incidentally on CT: a position paper from the Fleischner Society. Lancet Respir. Med. 2020;8:726–737. doi: 10.1016/S2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katzenstein A.L., Myers J.L. Idiopathic pulmonary fibrosis clinical relevance of pathologic classification clinical features of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 1998;157:1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society, European Respiratory Society American thoracic Society/European respiratory society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 5.Hino T., Lee K.S., Han J., Hata A., Ishigami K., Hatabu H. Spectrum of pulmonary fibrosis from interstitial lung abnormality to usual interstitial pneumonia: importance of identification and quantification of traction bronchiectasis in patient management. Korean J. Radiol. 2020;21 doi: 10.3348/kjr.2020.1132. (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunninghake G.W., Zimmerman M.B., Schwartz D.A., King T.E., Jr, Lynch J., Hegele R., Waldron J., Colby T., Müller N., Lynch D., Galvin J., Gross B., Hogg J., Toews G., Helmers R., Cooper J.A., Jr, Baughman R., Strange C., Millard M. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2001;164:193–196. doi: 10.1164/ajrccm.164.2.2101090. [DOI] [PubMed] [Google Scholar]
- 7.Rosas I.O., Ren P., Avila N.A., Chow C.K., Franks T.J., Travis W.D., McCoy J.P., Jr, May R.M., Wu H.P., Nguyen D.M., Arcos-Burgos M., MacDonald S.D., Gochuico B.R. Early interstitial lung disease in familial pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2007;176:698–705. doi: 10.1164/rccm.200702-254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copley S.J., Wells A.U., Hawtin K.E., Gibson D.J., Hodson J.M., Jacques A.E.T., Hansell D.M. Lung morphology in the elderly: comparative CT study of subjects over 75 years old versus those under 55 years old. Radiology. 2009;251:566–573. doi: 10.1148/radiol.2512081242. [DOI] [PubMed] [Google Scholar]
- 9.Gochuico B.R., Avila N.A., Chow C.K., Novero L.J., Wu H.P., Ren P., MacDonald S.D., Travis W.D., Stylianou M.P., Rosas I.O. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch. Intern. Med. 2008;168:159–166. doi: 10.1001/archinternmed.2007.59. [DOI] [PubMed] [Google Scholar]
- 10.Lederer D.J., Enright P.L., Kawut S.M., Hoffman E.A., Hunninghake G., Van Beek E.J.R., Austin J.H.M., Jiang R., Lovasi G.S., Barr R.G. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am. J. Respir. Crit. Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Washko G.R., Lynch D.A., Matsuoka S., Ross J.C., Umeoka S., Diaz A. Identification of early interstitial lung disease in smokers from the COPDGene study. Acad. Radiol. 2010;17:48–53. doi: 10.1016/j.acra.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsushima K., Sone S., Yoshikawa S., Yokoyama T., Suzuki T., Kubo K. The radiological patterns of interstitial change at an early phase: over a 4-year follow-up. Respir. Med. 2010;104:1712–1721. doi: 10.1016/j.rmed.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Washko G.R., Hunninghake G.M., Fernandez I.E., Nishino M., Okajima Y., Yamashiro T., Ross J.C., Estépar R.S.J., Lynch D.A., Brehm J.M., Andriole K.P., Diaz A.A., Khorasani R., D’Aco K., Sciurba F.C., Silverman E.K., Hatabu H., Rosas I.O., COPDGene Investigators Lung volumes and emphysema in smokers with interstitial lung abnormalities. N. Engl. J. Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sverzellati N., Guerci L., Randi G., Calabrò E., La Vecchia C., Marchianò A., Pesci A., Zompatori M., Pastorino U. Interstitial lung diseases in a lung cancer screening trial. Eur. Respir. J. 2011;38:392–400. doi: 10.1183/09031936.00201809. [DOI] [PubMed] [Google Scholar]
- 15.Jin G.Y., Lynch D., Chawla A., Garg K., Tammemagi M.C., Sahin H., Misumi S., Kwon K.S. Interstitial lung abnormalities in a CT lung cancer screening population : Prevalence and progression rate. Radiology. 2013;268:563–571. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunninghake G.M., Hatabu H., Okajima Y., Gao W., Dupuis J., Latourelle J.C., Nishino M., Araki T., Zazueta O.E., Kurugol S., Ross J.C., San José Estépar R., Murphy E., Steele M.P., Loyd J.E., Schwarz M.I., Fingerlin T.E., Rosas I.O., Washko G.R., O’Connor G.T., Schwartz D.A. MUC5B promoter polymorphism and interstitial lung abnormalities. N. Engl. J. Med. 2013;368:2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Araki T., Putman R.K., Hatabu H., Gao W., Dupuis J., Latourelle J.C., Nishino M., Zazueta O.E., Kurugol S., Ross J.C., Estépar R.S.J., Schwartz D.A., Rosas I.O., Washko G.R., O’Connor G.T., Hunninghake G.M. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am. J. Respir. Crit. Care Med. 2016;194:1517–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putman R.K., Rosas I.O., Hunninghake G.M. Genetics and early detection in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2014;189:770–778. doi: 10.1164/rccm.201312-2219PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Putman R.K., Gudmundsson G., Araki T., Nishino M., Sigurdsson S., Gudmundsson E.F., Eiríksdottír G., Aspelund T., Ross J.C., Estépar R.S.J., Miller E.R., Yamada Y., Yanagawa M., Tomiyama N., Launer L.J., Harris T.B., El-Chemaly S., Raby B.A., Cho M.H., Rosas I.O., Washko G.R., Schwartz D.A., Silverman E.K., Gudnason V., Hatabu H., Hunninghake G.M. The MUC5B promoter polymorphism is associated with specific interstitial lung abnormality subtypes. Eur. Respir. J. 2017;50 doi: 10.1183/13993003.00537-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doyle T.J., Washko G.R., Fernandez I.E., Nishino M., Okajima Y., Yamashiro T., Divo M.J., Celli B.R., Sciurba F.C., Silverman E.K., Hatabu H., Rosas I.O., Hunninghake G.M., COPDGene Investigators Interstitial lung abnormalities and reduced exercise capacity. Am. J. Respir. Crit. Care Med. 2012;185:756–762. doi: 10.1164/rccm.201109-201618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podolanczuk A.J., Oelsner E.C., Barr R.G., Hoffman E.A., Armstrong H.F., Austin J.H.M., Basner R.C., Bartels M.N., Christie J.D., Enright P.L., Gochuico B.R., Stukovsky K.H., Kaufman J.D., Nath P.H., Newell J.D., Palmer S.M., Rabinowitz D., Raghu G., Sell J.L., Sieren J., Sonavane S.K., Tracy R.P., Watts J.R., Williams K., Kawut S.M., Lederer D.J. High attenuation areas on chest computed tomography in communitydwelling adults: the MESA study. Eur. Respir. J. 2016;48:1442–1452. doi: 10.1183/13993003.00129-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Putman R.K., Hatabu H., Araki T., Gudmundsson G., Gao W., Nishino M., Okajima Y., Dupuis J., Latourelle J.C., Cho M.H., El-Chemaly S., Coxson H.O., Celli B.R., Fernandez I.E., Zazueta O.E., Ross J.C., Harmouche R., Estépar R.S.J., Diaz A.A., Sigurdsson S., Gudmundsson E.F., Eiríksdottír G., Aspelund T., Budoff M.J., Kinney G.L., Hokanson J.E., Williams M.C., Murchison J.T., MacNee W., Hoffmann U., O’Donnell C.J., Launer L.J., Harrris T.B., Gudnason V., Silverman E.K., O’Connor G.T., Washko G.R., Rosas I.O., Hunninghake G.M. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315:672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynch D.A., Sverzellati N., Travis W.D., Brown K.K., Colby T.V., Galvin J.R., Goldin J.G., Hansell D.M., Inoue Y., Johkoh T., Nicholson A.G., Knight S.L., Raoof S., Richeldi L., Ryerson C.J., Ryu J.H., Wells A.U. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir. Med. 2018;6:138–153. doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- 24.Edey A.J., Devaraj A.A., Barker R.P., Nicholson A.G., Wells A.U., Hansell D.M. Fibrotic idiopathic interstitial pneumonias: HRCT findings that predict mortality. Eur. Radiol. 2011;21:1586–1593. doi: 10.1007/s00330-011-2098-2. [DOI] [PubMed] [Google Scholar]
- 25.Putman R.K., Gudmundsson G., Axelsson G.T., Hida T., Honda O., Araki T., Yanagawa M., Nishino M., Miller E.R., Eiriksdottir G., Gudmundsson E.F., Tomiyama N., Honda H., Rosas I.O., Washko G.R., Cho M.H., Schwartz D.A., Gudnason V., Hatabu H., Hunninghake G.M. Imaging patterns are associated with interstitial lung abnormality progression and mortality. Am. J. Respir. Crit. Care Med. 2019;200:175–183. doi: 10.1164/rccm.201809-1652OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hida T., Nishino M., Hino T., Lu J., Putman R.K., Gudmundsson E.F., Araki T., Valtchinov V.I., Honda O., Yanagawa M., Yamada Y., Hata A., Jinzaki M., Tomiyama N., Honda H., Estepar R.S.J., Washko G.R., Johkoh T., Christiani D.C., Lynch D.A., Gudnason V., Gudmundsson G., Hunninghake G.M., Hatabu H. Traction Bronchiectasis/Bronchiolectasis is associated with interstitial lung abnormality mortality. Eur. J. Radiol. 2020;129 doi: 10.1016/j.ejrad.2020.109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoyer N., Wille M.M.W., Thomsen L.H., Wilcke T., Dirksen A., Pedersen J.H., Saghir Z., Ashraf H., Shaker S.B. Interstitial lung abnormalities are associated with increased mortality in smokers. Respir. Med. 2018;136:77–82. doi: 10.1016/j.rmed.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Hino T., Hida T., Nishino M., Lu J., Putman R.K., Gudmundsson E.F., Hata A., Araki T., Valtchinov V.I., Honda O., Yanagawa M., Yamada Y., Kamitani T., Jinzaki M., Tomiyama N., Ishigami K., Honda H., Estepar R.S.J., Washiko G.R., Johkoh T., Christiani D.C., Lynch D.A., Gudnason V., Gudmundsson G., Hunninghake G.M., Hatabu H. Progression of traction Bronchiectasis/bronchiolectasis in interstitial lung abnormalities is associated with increased all-cause mortality: age Gene/Environment susceptibility-reykjavik study. Eur. J. Radiol. Open. 2020 doi: 10.1016/j.ejro.2021.100334. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liebow A.A., Carrington C.B. The interstitial pneumonias. In: Simon M., Potchen E.J., Lemay E., editors. Frontiers in Pulmonary Radiology. Grune and Stratton; New York: 1969. pp. 102–141. [Google Scholar]
- 30.Epler G.R., Colby T.V., McLoud T.C., Carrington C.B., Gaensler E.A. Bronchiolitis obliterans organizing pneumonia. N. Engl. J. Med. 1985;312:152–158. doi: 10.1056/NEJM198501173120304. https://doi.org/NEJM198501173120304. [DOI] [PubMed] [Google Scholar]
- 31.Davison A.G., Heard B.E., McAllister W.A., Turner-Warwick M.E. Cryptogenic organizing pneumonitis. Q. J. Med. 1983;52:382–394. [PubMed] [Google Scholar]
- 32.Lee K.S., Kullnig P., Hartman T.E., Müller N.L. Cryptogenic organizing pneumonia: CT findings in 43 patients. Am. J. Roentgenol. 1994;162:543–546. doi: 10.2214/ajr.162.3.8109493. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson A.G. Interstitial pneumonias. In: Tomashefski J.F., Cagle P.T., Farver C.F., Fraire A.E., editors. Dail and Hammar’s Pulmonary Pathology. 2008. pp. 695–721. [Google Scholar]
- 34.Choi J.W., Lee K.S., Chung M.P., Han J., Chung M.J., Park J.S. Giant cell interstitial pneumonia: high-resolution CT and pathologic findings in four adult patients. Am. J. Roentgenol. 2005;184:268–272. doi: 10.2214/ajr.184.1.01840268. [DOI] [PubMed] [Google Scholar]
- 35.Katzenstein A.L., Fiorelli R.F. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am. J. Surg. Pathol. 1994;18:136–147. [PubMed] [Google Scholar]
- 36.Myers J.L., Veal C.F., Jr, Shin M.S., Katzenstein A.L. Respiratory bronchiolitis causing interstitial lung disease. A clinicopathologic study of six cases. Am. Rev. Respir. Dis. 1987;135:880–884. doi: 10.1164/arrd.1987.135.4.880. [DOI] [PubMed] [Google Scholar]
- 37.Yousem S.A., Colby T.V., Gaensler E.A. Respiratory bronchiolitis-associated interstitial lung disease and its relationship to desquamative interstitial pneumonia. Mayo Clin. Proc. 1989;64:1373–1380. doi: 10.1016/s0025-6196(12)65379-8. [DOI] [PubMed] [Google Scholar]
- 38.Katzenstein A.L., Myers J.L., Mazur M.T. Acute interstitial pneumonia: a clinicopathologic, ultrastructural, and cell kinetic study. Am. J. Surg. Pathol. 1986;10:256–267. [PubMed] [Google Scholar]
- 39.Travis W.D., Costabel U., Hansell D.M., King T.E., Jr, Lynch D.A., Nicholson A.G., Ryerson C.J., Ryu J.H., Selman M., Wells A.U., Behr J., Bouros D., Brown K.K., Colby T.V., Collard H.R., Cordeiro C.R., Cottin V., Crestani B., Drent M., Dudden R.F., Egan J., Flaherty K., Hogaboam C., Inoue Y., Johkoh T., Kim D.S., Kitaichi M., Loyd J., Martinez F.J., Myers J., Protzko S., Raghu G., Richeldi L., Sverzellati N., Swigris J., Valeyre D., ATS/ERS Committee on Idiopathic Interstitial Pneumonias An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Travis W.D., Hunninghake G., King T.E., Jr, Lynch D.A., Colby T.V., Galvin J.R., Brown K.K., Chung M.P., Cordier J.-F., du Bois R.M., Flaherty K.R., Franks T.J., Hansell D.M., Hartman T.E., Kazerooni E.A., Kim D.S., Kitaichi M., Koyama T., Martinez F.J., Nagai S., Midthun D.E., Müller N.L., Nicholson A.G., Raghu G., Selman M., Wells A. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am. J. Respir. Crit. Care Med. 2008;177:1338–1347. doi: 10.1164/rccm.200611-1685OC. [DOI] [PubMed] [Google Scholar]
- 41.Kim D.S., Collard H.R., King T.E., Jr Classification and natural history of the idiopathic interstitial pneumonias. Proc. Am. Thorac. Soc. 2006;3:285–292. doi: 10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flaherty K.R., Travis W.D., Colby T.V., Toews G.B., Kazerooni E.A., Gross B.H., Jain A., Strawderman R.L., Flint A., Lynch J.P., Martinez F.J. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2001;164:1722–1727. doi: 10.1164/ajrccm.164.9.2103074. [DOI] [PubMed] [Google Scholar]
- 43.Park J.S., Lee K.S., Kim J.S., Park C.S., Suh Y.L., Choi D.L., Kim K.J. Nonspecific interstitial pneumonia with fibrosis: radiographic and CT findings in seven patients. Radiology. 1995;195:645–648. doi: 10.1148/radiology.195.3.7753988. [DOI] [PubMed] [Google Scholar]
- 44.Kim T.S., Lee K.S., Chung M.P., Han J., Park J.S., Hwang J.H., Kwon O.J., Rhee C.H. Nonspecific interstitial pneumonia with fibrosis: high-resolution CT and pathologic findings. Am. J. Roentgenol. 1998;171:1645–1650. doi: 10.2214/ajr.171.6.9843306. [DOI] [PubMed] [Google Scholar]
- 45.Kim E.Y., Lee K.S., Chung M.P., Kwon O.J., Kim T.S., Hwang J.H. Nonspecific interstitial pneumonia with fibrosis: serial high-resolution CT findings with functional correlation. Am. J. Roentgenol. 1999;173:949–953. doi: 10.2214/ajr.173.4.10511155. [DOI] [PubMed] [Google Scholar]
- 46.Shin K.M., Lee K.S., Chung M.P., Han J., Bae Y.A., Kim T.S., Chung M.J. Prognostic determinants among clinical, thin-section CT, and histopathologic findings for fibrotic idiopathic interstitial pneumonias: tertiary hospital study. Radiology. 2008;249:328–337. doi: 10.1148/radiol.2483071378. [DOI] [PubMed] [Google Scholar]
- 47.Jeong Y.J., Lee K.S., Müller N.L., Chung M.P., Chung M.J., Han J., Colby T.V., Kim S. Usual interstitial pneumonia and non-specific interstitial pneumonia: serial thin-section CT findings correlated with pulmonary function. Korean J. Radiol. 2005;6:143–152. doi: 10.3348/kjr.2005.6.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silva C.I.S., Müller N.L., Lynch D.A., Curran-Everett D., Brown K.K., Lee K.S., Chung M.P., Churg A. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology. 2008;246:288–297. doi: 10.1148/radiol.2453061881. [DOI] [PubMed] [Google Scholar]
- 49.Lee H.Y., Lee K.S., Jeong Y.J., Hwang J.H., Kim H.J., Chung M.P., Han J. High-resolution CT findings in fibrotic idiopathic interstitial pneumonias with little honeycombing: serial changes and prognostic implications. AJR Am. J. Roentgenol. 2012;199:982–989. doi: 10.2214/AJR.11.8192. [DOI] [PubMed] [Google Scholar]
- 50.Nunes H., Schubel K., Piver D., Magois E., Feuillet S., Uzunhan Y., Carton Z., Tazi A., Levy P., Brillet P.-Y., Nicholson A.G., Kambouchner M., Valeyre D. Nonspecific interstitial pneumonia: survival is influenced by the underlying cause. Eur. Respir. J. 2015;45:746–755. doi: 10.1183/09031936.00148613. [DOI] [PubMed] [Google Scholar]
- 51.Yoo H., Hino T., Han J., Franks T.J., Im Y., Hatabu H., Chung M.P., Lee K.S. Connective tissue disease-related interstitial lung disease (CTD-ILD) and interstitial lung abnormality (ILA): evolving concept of CT findings, pathology and management. Eur. J. Radiol. Open. 2021;8 doi: 10.1016/j.ejro.2020.100311. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Arakawa H., Yamada H., Kurihara Y., Nakajima Y., Takeda A., Fukushima Y., Fujioka M. Nonspecific interstitial pneumonia associated with polymyositis and dermatomyositis: serial high-resolution CT findings and functional correlation. Chest. 2003;123:1096–1103. doi: 10.1378/chest.123.4.1096. [DOI] [PubMed] [Google Scholar]
- 53.MacDonald S.L.S., Rubens M.B., Hansell D.M., Copley S.J., Desai S.R., du Bois R.M., Nicholson A.G., Colby T.V., Wells A.U. Nonspecific interstitial pneumonia and usual interstitial pneumonia: comparative appearances at and diagnostic accuracy of thin-section CT. Radiology. 2001;221:600–605. doi: 10.1148/radiol.2213010158. [DOI] [PubMed] [Google Scholar]
- 54.Johkoh T., Müller N.L., Cartier Y., Kavanagh P.V., Hartman T.E., Akira M., Ichikado K., Ando M., Nakamura H. Idiopathic interstitial pneumonias: diagnostic accuracy of thin-section CT in 129 patients. Radiology. 1999;211:555–560. doi: 10.1148/radiology.211.2.r99ma01555. [DOI] [PubMed] [Google Scholar]
- 55.Sumikawa H., Johkoh T., Ichikado K., Taniguchi H., Kondoh Y., Fujimoto K., Tateishi U., Hiramatsu T., Inoue A., Natsag J., Ikemoto M., Mihara N., Honda O., Tomiyama N., Hamada S., Nakamura H., Müller N.L. Usual interstitial pneumonia and chronic idiopathic interstitial pneumonia: analysis of CT appearance in 92 patients. Radiology. 2006;241:258–266. doi: 10.1148/radiol.2411050928. [DOI] [PubMed] [Google Scholar]
- 56.Sumikawa H., Johkoh T., Fujimoto K., Arakawa H., Colby T.V., Fukuoka J., Taniguchi H., Kondoh Y., Kataoka K., Ogura T., Baba T., Ichikado K., Gyobu T., Yanagawa M., Honda O., Tomiyama N. Pathologically proved nonspecific interstitial pneumonia: CT pattern analysis as compared with usual interstitial pneumonia CT pattern. Radiology. 2014;272:549–556. doi: 10.1148/radiol.14130853. [DOI] [PubMed] [Google Scholar]
- 57.Silva C.I.S., Müller N.L., Hansell D.M., Lee K.S., Nicholson A.G., Wells A.U. Nonspecific interstitial pneumonia and idiopathic pulmonary fibrosis: changes in pattern and distribution of disease over time. Radiology. 2008;247:251–259. doi: 10.1148/radiol.2471070369. [DOI] [PubMed] [Google Scholar]
- 58.Gruden J.F., Panse P.M., Leslie K.O., Tazelaar H.D., Colby T.V. UIP diagnosed at surgical lung biopsy, 2000-2009: HRCT patterns and proposed classification system. AJR Am. J. Roentgenol. 2013;200:458–467. doi: 10.2214/AJR.12.9437. [DOI] [PubMed] [Google Scholar]
- 59.Sumikawa H., Johkoh T., Colby T.V., Ichikado K., Suga M., Taniguchi H., Kondoh Y., Ogura T., Arakawa H., Fujimoto K., Inoue A., Mihara N., Honda O., Tomiyama N., Nakamura H., Müller N.L. Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am. J. Respir. Crit. Care Med. 2008;177:433–439. doi: 10.1164/rccm.200611-1696OC. [DOI] [PubMed] [Google Scholar]
- 60.Raghu G., Remy-Jardin M., Myers J.L., Richeldi L., Ryerson C.J., Lederer D.J., Behr J., Cottin V., Danoff S.K., Morell F., Flaherty K.R., Wells A., Martinez F.J., Azuma A., Bice T.J., Bouros D., Brown K.K., Collard H.R., Duggal A., Galvin L., Inoue Y., Gisli Jenkins R., Johkoh T., Kazerooni E.A., Kitaichi M., Knight S.L., Mansour G., Nicholson A.G., Pipavath S.N.J., Buendía-Roldán I., Selman M., Travis W.D., Walsh S., Wilson K.C. Diagnosis of idiopathic pulmonary fibrosis an official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 61.Travis W.D., Matsui K., Moss J., Ferrans V.J. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns. Am. J. Surg. Pathol. 2000;24:19–33. doi: 10.1097/00000478-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Johkoh T., Lee K.S., Nishino M., Travis W.D., Ryu J.H., Lee H.Y., Ryerson C.J., Franquet T., Bankier A.A., Brown K.K., Goo J.M., Kauczor H.U., Lynch D.A., Nicholson A.G., Richeldi L., Schaefer-Prokop C.M., Verschakelen J., Raoof S., Rubin G.D., Powell C., Inoue Y., Hatabu H. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: a position paper from the Fleischner society. Radiology. 2021;298:550–566. doi: 10.1148/radiol.2021203427. [DOI] [PubMed] [Google Scholar]
- 63.Johkoh T., Lee K.S., Nishino M., Travis W.D., Ryu J.H., Lee H.Y., Ryerson C.J., Franquet T., Bankier A.A., Brown K.K., Goo J.M., Kauczor H.U., Lynch D.A., Nicholson A.G., Richeldi L., Schaefer-Prokop C.M., Verschakelen J., Raoof S., Rubin G.D., Powell C., Inoue Y., Hatabu H. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: a position paper from the Fleischner society. Chest. 2021 doi: 10.1016/j.chest.2020.11.027. (online ahead of print) [DOI] [PubMed] [Google Scholar]
- 64.Miller E.R., Putman R.K., Vivero M., Hung Y., Araki T., Nishino M., Washko G.R., Rosas I.O., Hatabu H., Sholl L.M., Hunninghake G.M. Histopathology of interstitial lung abnormalities in the context of lung nodule resections. Am. J. Respir. Crit. Care Med. 2018;197:955–958. doi: 10.1164/rccm.201708-1679LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hung Y.P., Hunninghake G.M., Miller E.R., Putman R., Nishino M., Araki T., Hatabu H., Sholl L.M., Vivero M. Incidental nonneoplastic parenchymal findings in patients undergoing lung resection for mass lesions. Hum. Pathol. 2019;86:93–101. doi: 10.1016/j.humpath.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawabata Y., Hoshi E., Murai K., Ikeya T., Takahashi N., Saitou Y., Kurashima K., Ubukata M., Takayanagi N., Suita H., Kanauchi S., Colby T.V. Smoking-related changes in the background lung of specimens resected for lung cancer: a semiquantitative study with correlation to postoperative course. Histopathology. 2008;53:707–714. doi: 10.1111/j.1365-2559.2008.03183.x. [DOI] [PubMed] [Google Scholar]
- 67.Yamada T., Nakanishi Y., Homma T., Uehara K., Mizutani T., Hoshi E., Shimizu Y., Kawabata Y., Colby T.V. Airspace enlargement with fibrosis shows characteristic histology and immunohistology different from usual interstitial pneumonia, nonspecific interstitial pneumonia and centrilobular emphysema. Pathol. Int. 2013;63:206–213. doi: 10.1111/pin.12054. [DOI] [PubMed] [Google Scholar]
- 68.Itoh H., Murata K., Konishi J., Nishimura K., Kitaichi M., Izumi T. Diffuse lung disease: pathologic basis for the high-resolution computed tomography findings. J. Thorac. Imaging. 1993;8:176–188. [PubMed] [Google Scholar]
- 69.Oldham J.M., Adegunsoye A., Khera S., Lafond E., Noth I., Strek M.E., Kadoch M., Chung J.H. Underreporting of interstitial lung abnormalities on lung Cancer screening computed tomography. Ann. Am. Thorac. Soc. 2018;15:764–766. doi: 10.1513/AnnalsATS.201801-053RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wright J.L., Tazelaar H.D., Churg A. Fibrosis with emphysema. Histopathology. 2011;58:517–524. doi: 10.1111/j.1365-2559.2010.03648.x. [DOI] [PubMed] [Google Scholar]
- 71.Nicholson A.G., Addis B.J., Bharucha H., Clelland C.A., Corrin B., Gibbs A.R., Hasleton P.S., Kerr K.M., Ibrahim N.B., Stewart S., Wallace W.A., Wells A.U. Inter-observer variation between pathologists in diffuse parenchymal lung disease. Thorax. 2004;59:500–505. doi: 10.1136/thx.2003.011734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watadani T., Sakai F., Johkoh T., Noma S., Akira M., Fujimoto K., Bankier A.A., Lee K.S., Müller N.L., Song J.-W., Park J.-S., Lynch D.A., Hansell D.M., Remy-Jardin M., Franquet T., Sugiyama Y. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology. 2013;266:936–944. doi: 10.1148/radiol.12112516. [DOI] [PubMed] [Google Scholar]
- 73.Walsh S.L.F., Wells A.U., Sverzellati N., Devaraj A., von der Thüsen J., Yousem S.A., Colby T.V., Nicholson A.G., Hansell D.M. Relationship between fibroblastic foci profusion and high resolution CT morphology in fibrotic lung disease. BMC Med. 2015;13:241. doi: 10.1186/s12916-015-0479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Staats P., Kligerman S., Todd N., Tavora F., Xu L., Burke A. A comparative study of honeycombing on high resolution computed tomography with histologic lung remodeling in explants with usual interstitial pneumonia. Pathol. Res. Pract. 2015;211:55–61. doi: 10.1016/j.prp.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 75.Chilosi M., Poletti V., Murer B., Lestani M., Cancellieri A., Montagna L., Piccoli P., Cangi G., Semenzato G., Doglioni C. Abnormal re-epithelialization and lung remodeling in idiopathic pulmonary fibrosis: the role of deltaN-p63. Lab. Invest. 2002;82:1335–1345. doi: 10.1097/01.LAB.0000032380.82232.67. [DOI] [PubMed] [Google Scholar]
- 76.Piciucchi S., Tomassetti S., Ravaglia C., Gurioli C., Gurioli C., Dubini A., Carloni A., Chilosi M., Colby T.V., Poletti V. From “traction bronchiectasis” to honeycombing in idiopathic pulmonary fibrosis: a spectrum of bronchiolar remodeling also in radiology? BMC Pulm. Med. 2016;16:87. doi: 10.1186/s12890-016-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salisbury M.L., Gu T., Murray S., Gross B.H., Chughtai A., Sayyouh M., Kazerooni E.A., Myers J.L., Lagstein A., Konopka K.E., Belloli E.A., Sheth J.S., White E.S., Holtze C., Martinez F.J., Flaherty K.R. Hypersensitivity pneumonitis: radiologic phenotypes are associated with distinct survival time and pulmonary function trajectory. Chest. 2019;155:699–711. doi: 10.1016/j.chest.2018.08.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walsh S.L.F., Sverzellati N., Devaraj A., Wells A.U., Hansell D.M. Chronic hypersensitivity pneumonitis: high resolution computed tomography patterns and pulmonary function indices as prognostic determinants. Eur. Radiol. 2012;22:1672–1679. doi: 10.1007/s00330-012-2427-0. [DOI] [PubMed] [Google Scholar]
- 79.Park J.H., Kim D.S., Park I.N., Jang S.J., Kitaichi M., Nicholson A.G., Colby T.V. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am. J. Respir. Crit. Care Med. 2007;175:705–711. doi: 10.1164/rccm.200607-912OC. [DOI] [PubMed] [Google Scholar]
- 80.Navaratnam V., Ali N., Smith C.J., McKeever T., Fogarty A., Hubbard R.B. Does the presence of connective tissue disease modify survival in patients with pulmonary fibrosis? Respir. Med. 2011;105:1925–1930. doi: 10.1016/j.rmed.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 81.Kim E.A., Lee K.S., Johkoh T., Kim T.S., Suh G.Y., Kwon O.J., Han J. Interstitial lung diseases associated with collagen vascular diseases: radiologic and histopathologic findings. Radiographics. 2002;22:S151–S165. doi: 10.1148/radiographics.22.suppl_1.g02oc04s151. [DOI] [PubMed] [Google Scholar]
- 82.Oliveira R.P., Ribeiro R., Melo L., Grima B., Oliveira S., Alves J.D. Connective tissue disease-associated interstitial lung disease. Pulmonology. 2020 doi: 10.1016/j.pulmoe.2020.01.004. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 83.Kim E.J., Collard H.R., King T.E., Jr Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest. 2009;136:1397–1405. doi: 10.1378/chest.09-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tomassetti S., Ryu J.H., Piciucchi S., Chilosi M., Poletti V. Nonspecific interstitial pneumonia: what is the optimal approach to management? Semin. Respir. Crit. Care Med. 2016;37:378–394. doi: 10.1055/s-0036-1583176. [DOI] [PubMed] [Google Scholar]
- 85.Tashkin D.P., Elashoff R., Clements P.J., Goldin J., Roth M.D., Furst D.E., Arriola E., Silver R., Strange C., Bolster M., Seibold J.R., Riley D.J., Hsu V.M., Varga J., Schraufnagel D.E., Theodore A., Simms R., Wise R., Wigley F., White B., Steen V., Read C., Mayes M., Parsley E., Mubarak K., Connolly M.K., Golden J., Olman M., Fessler B., Rothfield N., Metersky M., Scleroderma Lung Study Research Group Cyclophosphamide versus placebo in scleroderma lung disease. N. Engl. J. Med. 2006;354:2655–2666. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 86.Hoyles R.K., Ellis R.W., Wellsbury J., Lees B., Newlands P., Goh N.S., Roberts C., Desai S., Herrick A.L., McHugh N.J., Foley N.M., Pearson S.B., Emery P., Veale D.J., Denton C.P., Wells A.U., Black C.M., du Bois R.M. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54:3962–3970. doi: 10.1002/art.22204. [DOI] [PubMed] [Google Scholar]
- 87.Tashkin D.P., Roth M.D., Clements P.J., Furst D.E., Khanna D., Kleerup E.C., Goldin J., Arriola E., Volkmann E.R., Kafaja S., Silver R., Steen V., Strange C., Wise R., Wigley F., Mayes M., Riley D.J., Hussain S., Assassi S., Hsu V.M., Patel B., Phillips K., Martinez F., Golden J., Connolly M.K., Varga J., Dematte J., Hinchcliff M.E., Fischer A., Swigris J., Meehan R., Theodore A., Simms R., Volkov S., Schraufnagel D.E., Scholand M.B., Frech T., Molitor J.A., Highland K., Read C.A., Fritzler M.J., Kim G.H.J., Tseng C.H., Elashoff R.M., Sclerodema Lung Study II Investigators Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir. Med. 2016;4:708–719. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gutsche M., Rosen G.D., Swigris J.J. Connective tissue disease-associated interstitial lung disease: a review. Curr. Respir. Care Rep. 2012;1:224–232. doi: 10.1007/s13665-012-0028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Solomon J.J., Fischer A. Connective tissue disease-associated interstitial lung disease: a focused review. J. Intensive Care Med. 2015;30:392–400. doi: 10.1177/0885066613516579. [DOI] [PubMed] [Google Scholar]
- 90.Park I.N., Jegal Y., Kim D.S., Do K.H., Yoo B., Shim T.S., Lim C.M., Lee S.D., Koh Y., Kim W.S., Kim W.D., Jang S.J., Kitaichi M., Nicholson A.G., Colby T.V. Clinical course and lung function change of idiopathic nonspecific interstitial pneumonia. Eur. Respir. J. 2009;33:68–76. doi: 10.1183/09031936.00158507. [DOI] [PubMed] [Google Scholar]
- 91.Lee J.Y., Jin S.M., Lee B.J., Chung D.H., Jang B.G., Park H.S., Lee S.M., Yim J.J., Yang S.C., Yoo C.G., Han S.K., Shim Y.S., Kim Y.W. Treatment response and long term follow-up results of nonspecific interstitial pneumonia. J. Korean Med. Sci. 2012;27:661–667. doi: 10.3346/jkms.2012.27.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Richeldi L., du Bois R.M., Raghu G., Azuma A., Brown K.K., Costabel U., Cottin V., Flaherty K.R., Hansell D.M., Inoue Y., Kim D.S., Kolb M., Nicholson A.G., Noble P.W., Selman M., Taniguchi H., Brun M., Le Maulf F., Girard M., Stowasser S., Schlenker-Herceg R., Disse B., Collard H.R., INPULSIS Trial Investigators Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 93.King T.E., Jr, Bradford W.Z., Castro-Bernardini S., Fagan E.A., Glaspole I., Glassberg M.K., Gorina E., Hopkins P.M., Kardatzke D., Lancaster L., Lederer D.J., Nathan S.D., Pereira C.A., Sahn S.A., Sussman R., Swigris J.J., Noble P.W., ASCEND Study Group A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 94.Distler O., Highland K.B., Gahlemann M., Azuma A., Fischer A., Mayes M.D., Raghu G., Sauter W., Girard M., Alves M., Clerisme-Beaty E., Stowasser S., Tetzlaff K., Kuwana M., Maher T.M., SENSCIS Trial Investigators Nintedanib for systemic sclerosis-associated interstitial lung disease. N. Engl. J. Med. 2019;380:2518–2528. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- 95.Flaherty K.R., Wells A.U., Cottin V., Devaraj A., Walsh S.L.F., Inoue Y., Richeldi L., Kolb M., Tetzlaff K., Stowasser S., Coeck C., Clerisme-Beaty E., Rosenstock B., Quaresma M., Haeufel T., Goeldner R.G., Schlenker-Herceg R., Brown K.K., INBUILD Trial Investigators Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 2019;381:1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 96.Behr J., Neuser P., Prasse A., Kreuter M., Rabe K., Schade-Brittinger C., Wagner J., ünther A.G. Exploring efficacy and safety of oral Pirfenidone for progressive, non-IPF lung fibrosis (RELIEF) - a randomized, double-blind, placebo-controlled, parallel group, multi-center, phase II trial. BMC Pulm. Med. 2017;17:122. doi: 10.1186/s12890-017-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]