Abstract
Objective
To summarize the clinical and pathological features of patients with cardiac lymphoma.
Methods
The general conditions, clinical features, pathological types, and prognostic indices of 37 patients with cardiac lymphoma treated in our hospital were analyzed.
Results
Among the 37 patients, only one had primary cardiac lymphoma, and the other 36 patients had secondary cardiac lymphoma. The cardiac manifestations were mainly chest tightness, shortness of breath, increased heart rates, and electrocardiographic abnormality caused by pericardial effusion, but myocardial enzyme levels were normal in all patients. Only three patients displayed solid heart-occupying manifestations. These lesions were mainly located in the right atrium, and the masses were all larger than 5 cm. The pathological type was diffuse large B cell lymphoma that did not arise from the germinal center in all three patients.
Conclusions
Cardiac lymphoma was mostly secondary, and pericardial effusion was the primary objective sign. Moreover, cardiac lymphoma was characterized by a high international prognostic index, late stage, and high rates of T and NK cell lymphoma. Most cases were accompanied by serous cavity effusion, extranodal involvement of important organs, elevated lactate dehydrogenase levels, hypoalbuminemia, and normal myocardial enzyme levels.
Keywords: Lymphoma, heart, pericardial effusion, international prognostic index, T cell, B cell, extranodal involvement
Introduction
There is no lymphoid tissue present in the heart; thus, it is rarely involved in lymphomas originating from the lymphatic hematopoietic system.1 Lymphomas originating from the heart (or only affecting the heart) are called primary cardiac lymphomas.2 This type of lymphoma is extremely rare, accounting for less than 1% of all extranodal lymphomas and an even lower percentage of all lymphomas.2 Previous studies reported that the tumor can spread to the pericardium and myocardium during the course of the disease, which is called secondary cardiac lymphoma, in 9% to 24% of patients with lymphoma. In cardiac lymphoma (primary and secondary) involving different parts of the heart, the clinical manifestations can include chest tightness, suffocation, cardiac insufficiency, pericardial effusion, and arrhythmia.3 However, the occurrence of solid heart tumors is rare. Because most lymphomas involving the heart are secondary, the cardiac presentations are easily hidden by the symptoms of other organs, and thus, the condition is easily missed or misdiagnosed.4 This study retrospectively analyzed the clinical characteristics and pathological conditions of patients with cardiac lymphoma.
Materials and methods
Patients
Patients with heart-involved lymphoma diagnosed in the Department of Hematology, First Medical Center, PLA General Hospital from January 1, 2009 to December 31, 2019 were enrolled in our clinical trial. The inclusion criteria were mainly based on the surgical pathology, pericardial effusion puncture or its morphological detection, and immunotyping-based diagnosis. Imaging methods such as ultrasound, CT, nuclear magnetic resonance, or PET-CT were used for patients who could not undergo biopsy. Moreover, patients with inflammatory diseases such as tuberculosis and pericardial effusion caused by hypoproteinemia or heart disease were excluded. The decline or disappearance of pericardial effusion or cardiac mass after chemotherapy served as the evidence for judging cardiac involvement.
This research was approved by the Ethics Committee of Chinese PLA General Hospital (approval number 0009-0047) on the basis of the Declaration of Helsinki. We clearly confirmed that written informed consent was obtained from all patients, and all relevant documents were stored at our hospital.
LDH measurement
An LDH assay kit (fluorometric, ab197000, Abcam, Cambridge, UK) was used to assess LDH concentrations in samples. Briefly, reagents were mixed for the assays (samples, standards, and positive control) to be performed. The reaction mix was created using the following formula: X µL component × (number of samples + number of standards + number of positive controls + 1). Then, 50 µL of the reaction mix were added to each standard, sample, and positive control well. The fluorescence intensity was measured in each well using excitation and emission wavelengths of 535 and 587 nm, respectively, in the kinetic mode every 2 to 3 minutes at 37°C.
β2-MG measurement
A kit for measuring β2-MG concentrations was obtained from Shanghai Yanjin Biotechnology Co., Ltd. (Shanghai, China). In the assay, purified human β2-MG antibody was used to coat the microtiter plate to create a solid-phase antibody. Human β2-MG was added to the antibody-coated wells and combined with HRP-labeled β2-MG antibody to form an antibody–antigen–enzyme-labeled antibody complex. After thorough washing, TMB was added for color development. TMB is converted into a blue color following catalysis by HRP and then to yellow in the presence of acid. The intensity of the color is positively correlated with β2-MG levels in the sample. The absorbance (optical density) was measured using a microplate reader at a wavelength of 450 nm, and the human β2-MG content in the sample was measured using a standard curve.
Immunoglobulin measurement
In total, 20 mL of serum, 20 mL of physiological saline, and 10 mL of (NH4)2SO4 saturated solution (added dropwise) were mixed to create a 20% (NH4)2SO4 solution. After thorough mixing, the solution was allowed to stand for 30 minutes. The solution was centrifuged at 1000 × g for 20 minutes, and the precipitate was discarded to remove fibrin. Then, 30 mL of (NH4)2SO4 saturated solution were added to the supernatant to create a 50% (NH4)2SO4 solution, which was mixed thoroughly and allowed to stand for 30 minutes. The solution was centrifuged at 1000 × g for 20 minutes, and the supernatant was discarded. The precipitate was dissolved in 20 mL of normal saline, and then 10 mL of (NH4)2SO4 saturated solution were added to create a 33% (NH4)2SO4 solution, which was mixed thoroughly and allowed to stand for 30 minutes. The solution was centrifuged at 1000 × g for 20 minutes, and the supernatant was discarded to remove albumin. The aforementioned steps were repeated two or three times. The precipitate was dissolved in 10 mL of normal saline and placed in a dialysis bag. The mixture was subjected to desalination, dialysis in normal water overnight, and then dialysis in normal saline at 4°C for 24 hours, with the fluid changed several times during the process. SO42− levels in the dialysate were measured using 1% BaCl2, and NH4 content was measured using Nessler’s reagent (1–2 drops of the reagent were added to 3–4 mL of the dialysate, and a brick red color indicated the presence of NH4). The process was repeated until no SO42− was present or until NH4 was detected. Each sample was centrifuged to remove precipitation (contaminants), and the supernatant represented the crude IgG content. The samples were then separated using a DEAE-cellulose chromatography column, eluted with 0.01 mol/L PBS (0.03 mol/L NaCl, pH 7.4), and the eluate was collected.
The purity of IgG was assessed using immunoelectrophoresis. Specifically, the sample to be tested was added to the well, and after electrophoresis, anti-IgG serum was added to the tank. The agar was allowed to diffuse for 24 hours, and the result was observed. If the extracted IgG was pure, only one arc-shaped precipitation line would appear, and the precipitation line was located in the γ-globulin region. For this identification, immunoelectrophoresis of whole serum and antiserum antibodies was performed simultaneously for comparison.
Immunohistochemistry
Lymphoma tissues were collected from the control and experimental groups and washed with PBS, and tissue blocks smaller than 0.5 cm × 0.5 cm × 0.1 cm were taken. For fixation and embedding, samples were fix with 4% paraformaldehyde, followed by successive incubations with 70% ethanol for 30 minutes, 80% ethanol for 30 minutes, 90% ethanol twice for 30 minutes each, 95% ethanol twice for 30 minutes each, and 100% ethanol for 30 minutes The samples were cleared twice with xylene for 15 minutes each, incubated with 55°C paraffin wax three times for 1 hour each, and embedded using a stainless steel mold. For sectioning, tissue sections with a thickness of 5 µm were attached to a polylysine-coated glass slide overnight at 60°C.
For deparaffinization, sections were immersed in xylene twice for 5 minutes each, 100% ethanol twice for 5 minutes each, 95% ethanol twice for 5 minutes each, 90% ethanol twice for 5 minutes each, 85% ethanol for 5 minutes, and 70% ethanol twice for 5 minutes each. Samples were then rinsed with tap water, followed by PBS twice. For antigen retrieval, heat retrieval was performed via incubation with citrate buffer solution for 20 minutes, followed by three washes in PBS for 5 minutes each. Samples were then incubated with 3% hydrogen peroxide for 10 minutes at room temperature and then washed three times with PBS for 5 minutes each. Normal goat serum blocking solution was added dropwise to the slices for 20 minutes at room temperature. Excess liquid was shaken off without washing, and the primary antibody was added dropwise to the slices, followed by an overnight incubation at 4°C. Slides were washed three times with PBS for 5 minutes each. Biotinylated secondary antibody (IgG) was added dropwise to the slices, which were incubated at 37°C for 20 minutes. Slides were washed three times with PBS for 5 minutes each. HRP-labeled streptavidin working solution (SA/HRP) was added dropwise to the slices, followed by incubation at 37°C for 20 minutes. Slides were washed three times for 5 minutes each.
For DAB color development, 1 mL of distilled water in the DAB color development kit and one drop each of color developers A, B, and C were mixed thoroughly and added to each specimen, and color was allowed to develop for 6 minutes. Samples were washed thoroughly, restained with hematoxylin for 1 minute, and fully washed. Samples were then subjected to 1% hydrochloric acid alcohol differentiation, incubated with 1% amine water inverted blue, and fully washed.
For mounting, samples were incubated with 70% ethanol for 5 minutes, 80% ethanol for 5 minutes, 90% ethanol twice for 5 minutes each, 95% ethanol twice for 5 minutes each, and 100% ethanol twice for 5 minutes each, followed by cleared in xylene twice for 5 minutes each. Samples were subjected to neutral resin mounting and microscopic observation. For this purpose, the positive tissue phases of the experimental and control groups were selected, and samples were examined under a microscope at ×400 magnification. For image analysis, a meaningful organization phase was selected, and the data were collected, analyzed, and saved.
Statistical analysis
The comparative analysis of median age was performed using the rank sum test. Differences in the rates of various clinical manifestations between B and T lymphomas were tested using Fisher’s exact test. P < 0.05 indicated statistical significance.
Results
General situation
This study included 37 patients with lymphoma involving the heart, including 26 men and 11 women with a median age of 46 years (range, 17–81). The median age of patients with B cell lymphoma was 45 years (range, 17–77), and that of patients with T or NK cell lymphoma was 53.5 years (range, 36–81). Patient age did not differ between these groups. The baseline characteristics, treatment types, and outcomes of patients are presented in Table 1.
Table 1.
Baseline characteristics, treatment type, and outcomes of the patients.
| B cell lymphoma (n = 23) | T and NK cell lymphoma (n = 14) | |
|---|---|---|
| Age (years) | ||
| <30 | 5 | 0 |
| 31–40 | 5 | 3 |
| 41–50 | 6 | 3 |
| 51–60 | 1 | 4 |
| 61–70 | 4 | 2 |
| 71–80 | 2 | 1 |
| >80 | 0 | 1 |
| Sex | ||
| Male | 17 | 10 |
| Female | 6 | 4 |
| Dropsy of serous cavity | ||
| Hydrothorax | 20 | 10 |
| Ascites | 13 | 10 |
| Mediastinal lymph node enlargement | 19 | 10 |
| Other involvement | 1 (0–3) | 1 (0–3) |
| ECOG | 1 (0–4) | 1 (0–4) |
| B symptoms | 18 | 11 |
| First-line therapy | ||
| R-CHOP | 22 | 7 |
| R-EPOCH | 1 | 6 |
| Response (CR + PR) to first-line treatment after 3–4 cycles of chemotherapy | ||
| R-CHOP | 17/22 | 4/7 |
| R-EPOCH | 0/1 | 3/7 |
| Response to second- and third-line therapy (include radiation or stem cell transplant) | 3/6 | 2/7 |
| 1-year survival | 22 | 10 |
CR, complete response; ECOG, Eastern Cooperative Oncology Group; PR, partial response; R-CHOP, rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone therapy; R-EPOCH, rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride therapy.
Patient history
One female patient had a 5-year history of rheumatoid arthritis, for which she took immunosuppressive agents. Moreover, one female patient had a 3-year history of Sjogren’s syndrome but no history of immunosuppressive therapy. Meanwhile, one female patient had breast cancer 4 years previously, and she received chemotherapy. In addition, two patients with thyroid cancer underwent surgical resection. The other patients had no histories of immunodeficiency disease and malignancy, nor had they used immunosuppressive agents.
Pathological types
Among the pathological types, 14 patients had lymphomas derived from T and NK cells (two cases of NKT lymphoma; two cases of angioimmunoblastic T cell lymphoma, T2 type; nine cases of unspecified peripheral T cell lymphoma; one case of ALK positive anaplastic large cell lymphoma), and 23 patients had lymphomas derived from B cells (one case of follicular grade 2 lymphoma; three cases of follicular-transformed large B lymphoma; two cases of Burkitt lymphoma; nine cases of diffuse large B cell lymphoma non-specific type; six cases of primary mediastinal diffuse large B cell lymphoma; two cases of high-grade B cell lymphoma).
Clinical stage and range of lesion involvement
One patient had only cardiac involvement (stage I), and this lesion was classified as primary cardiac lymphoma. The other 36 patients had secondary cardiac lymphomas, all of which were clinical stage IV, but there were no patients with stage IV lymphoma only based on the presence of pericardial effusion. In addition to cardiac involvement, 23 patients had serous peritoneal and/or pleural effusions, and 29 patients had both pleural effusion and enlarged mediastinal lymph nodes. Among 23 patients with B cell lymphoma, pleural effusion, enlarged mediastinal lymph nodes, and ascites were identified in 20, 19, and 13 patients, respectively. In 14 patients with T (NK) cell lymphoma and pleural effusion, pleural effusion, enlarged mediastinal lymph nodes, and ascites were each found in 10 patients cases. According to Fisher’s exact test, the rates of pleural effusion, enlarged mediastinal lymph nodes, and ascites did not differ between patients with T (NK) cell and B cell lymphoma. Eighteen patients displayed extranodal involvement at more than two sites excluding the heart, included the lungs, liver, pancreas, gastrointestinal tract, spleen, kidneys, adrenal gland, bladder, breast, testes, bone marrow, and central nervous system. Moreover, seven patients displayed involvement in more than three extranodal organs. However, the rate of extranodal involvement, excluding serous effusion, did not differ between patients with T (NK) cell and B cell lymphoma. Twenty-nine patients displayed B symptoms, including 11 patients with T (NK) cell lymphoma and 18 patients with B cell lymphoma.
Lactate dehydrogenase (LDH), β2-microglobulin, immunoglobulin, and albumin
Thirty-one and 26 patients exhibited LDH and β2-microglobulin elevation, respectively. Moreover, 31 patients had hypoalbuminemia. Immunoglobulin levels were normal in all patients.
International prognostic index (IPI)
Because of the large age span and multiple disease types of the patients, this study re-assessed the prognosis of all patients using the IPI to facilitate statistical analysis. The IPI was 0, 1, 2, 3, 4, and 5 in 0, 0, 5, 5, 16, and 11 patients, respectively.
Heart-related manifestations
Eleven patients exhibited mild chest tightness and shortness of breath, 10 patients had an increased heart rate, and four patients had difficulty breathing or an inability to lie down. Moreover, electrocardiogram revealed arrhythmia and T wave abnormalities in 4 and 13 patients, respectively. All patients presented with normal myocardial enzyme levels. Imaging revealed three cases of solid occupying masses in the heart, and all 37 patients had pericardial effusion. The patients with solid occupying masses in the heart underwent transthoracic transesophageal echocardiography.
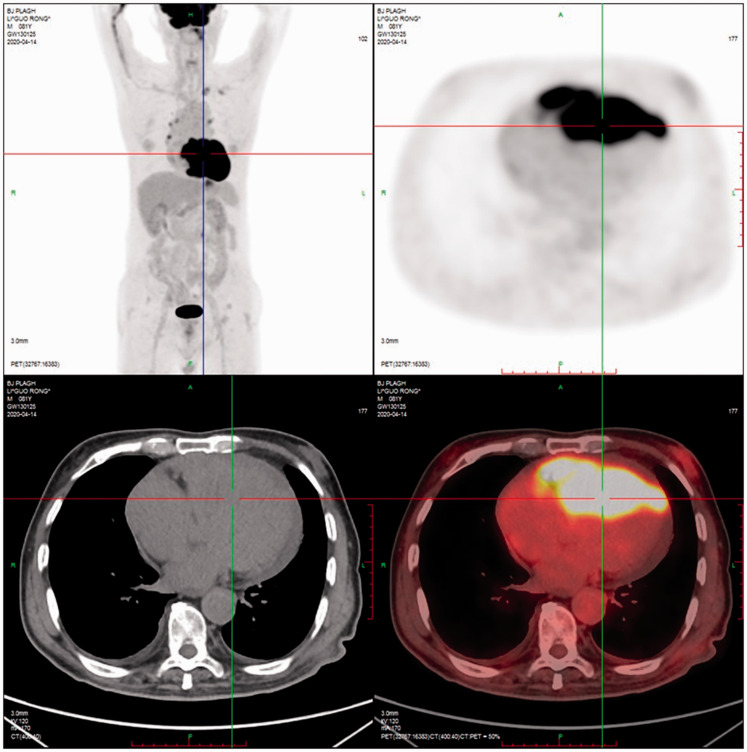
Patient 1 had a low-echo mass (6.0 × 4.0 cm2) in the anterior wall of the right atrium, which was enlarged, and the mass protruded into the superior vena cava (4.2 × 2.0 cm2) and displayed poor mass mobility and a small level of pericardial effusion (Figure 1). Thoracotomy was performed, revealing that the tumor extensively involved the heart, and a portion of the tumor (3.5 × 0.8 × 0.8 cm3) was removed for examination.
Figure 1.
Cardiac tumor of patient 1. The patient had a low-echo mass (6.0 × 4.0 cm2) in the anterior wall of the right atrium, which was enlarged, and the mass protruded into the superior vena cava (4.2 × 2.0 cm2) and displayed poor mass mobility and a small amount of pericardial effusion.
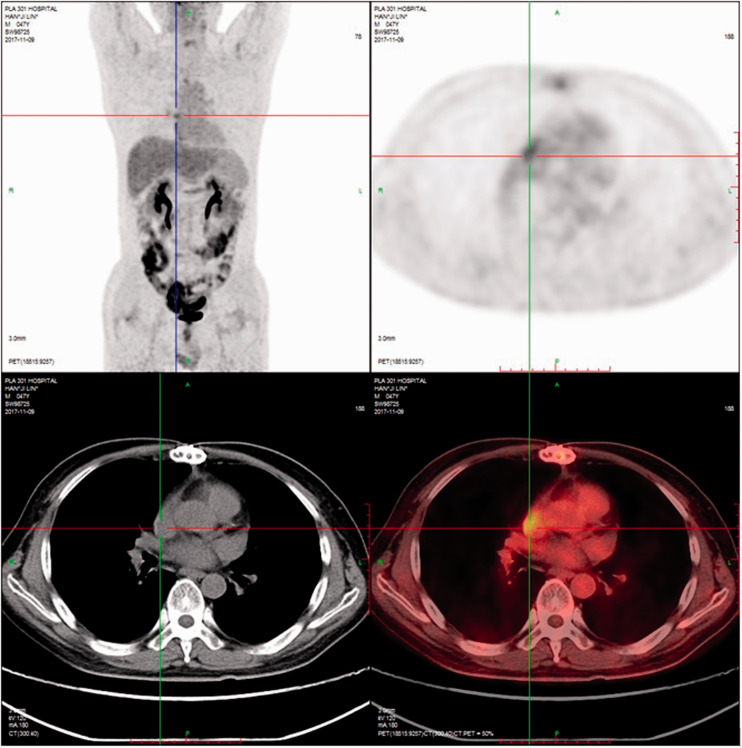
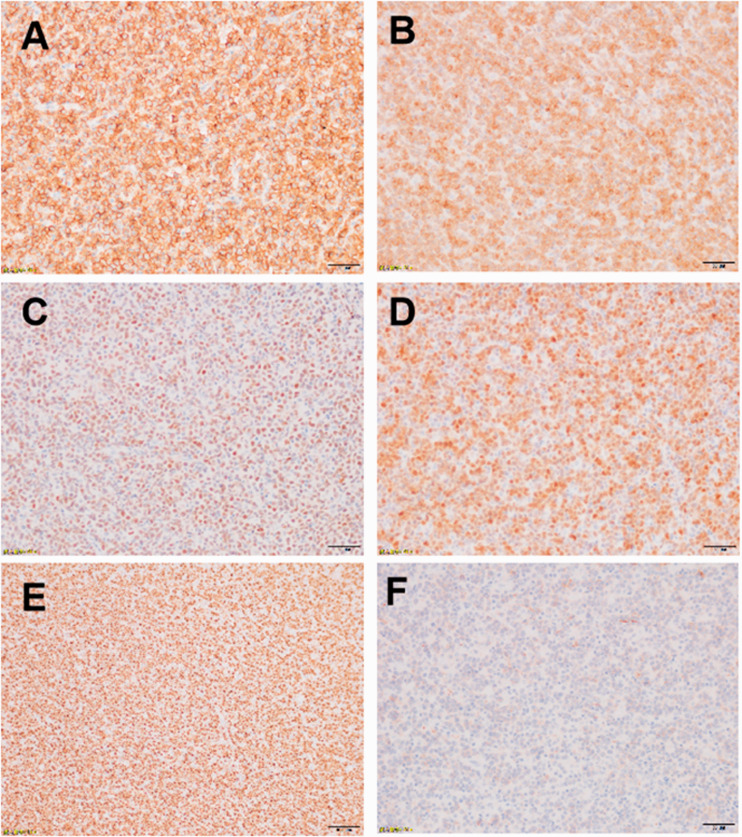
Patient 2 had a solid hypoechoic mass with an area of 5.0 × 3.5 cm2 adjacent to the anterior lobe of the tricuspid valve in the right atrium. There was moderate to large regurgitation of the tricuspid valve, and the right atrium was enlarged with a small amount of pericardial effusion. A solid mass with an area of 3.2 × 0.9 cm2 was present in the pericardial cavity of the left ventricular posterior wall (Figure 2). Surgical exploration found that the tumor was massive, and the tumor tissue in the right atrium, anterior tricuspid valve, septum, and coronary sinus was removed. However, the adhesion of the pedicle of the tumor tissue to the anterior septal angle of the tricuspid valve and Koch’s triangle prevented its complete removal. A permanent pacemaker was implanted in the epicardium. Excisional lymph node biopsy with immunohistochemistry was positive for CD20 (+++; Figure 3A), Bcl-2 (+, >80%; Figure 3B), Bcl-6 (+; Figure 3C), Mum-1 (+; Figure 3D), Ki-67 (+, >80%; Figure 3E), and CD10 (slightly +; Figure 3F). After treatment with rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone (R-CHOP) therapy or rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride therapy and transplantation, clinical symptom remission was obtained, and the cardiac tumor was removed.
Figure 2.
Cardiac tumor of patient 2. Patient 2 had a solid hypoechoic mass (5.0 × 3.5 cm2) adjacent to the anterior lobe of the tricuspid valve in the right atrium. There was moderate to large regurgitation of the tricuspid valve, and the right atrium was large and contained a small amount of pericardial effusion. A solid mass with an area of 3.2 × 0.9 cm2 was present in the pericardial cavity of the left ventricular posterior wall.
Figure 3.
Excisional lymph node biopsy with immunohistochemistry in a patient with B cell lymphoma. A. CD20 (+++). B. Bcl-2 (+, >80%). C. Bcl-6 (+). D. Mum-1 (+). E. Ki-67 (+, >80%). F. CD10 (slightly +).
Patient 3 had a solid hypoechoic mass with an area of 7.6 × 4.7 cm2 in the right atrium that blocked part of the tricuspid valve orifice. The right atrium of the patient was large, and the lesion exhibited extensive pericardial effusion. Thoracotomy revealed that the tumor tissue was completely fused with the right atrium and right ventricular myocardium. The tumor tissue was completely removed from the right atrium, tricuspid valve leaflet, and coronary sinus. The pathological diagnosis of all three patients was diffuse large B cell lymphoma that did not arise from the germinal center.
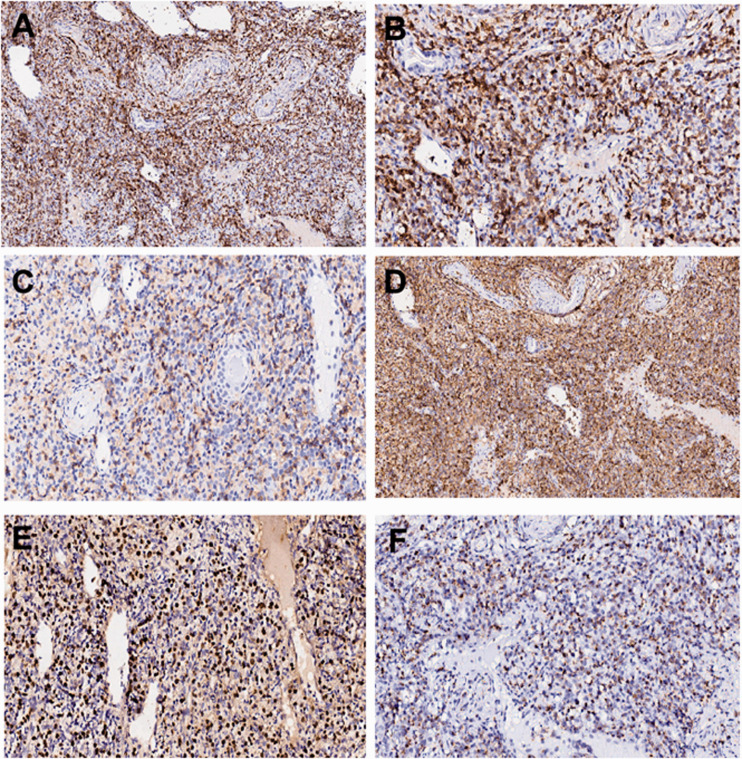
Moreover, the results of excisional lymph node biopsy with immunohistochemistry in a patient with T and NK cell lymphoma are presented in Figure 4 (CD20 [+++; Figure 4A], CD3 [+++; Figure 4B], CD4 [+; Figure 4C], CD45RO [+++; Figure 4D], EBER [+++; Figure 4E], and TIA1 [+; Figure 4F).
Figure 4.
Excisional lymph node biopsy with immunohistochemistry in a patient with T and NK cell lymphoma. A. CD20 (+++). B. CD3 (+++). C. CD4 (+). D. CD45RO (+++). E. EBER (+++). F. TIA1 (+).
Discussion
Primary cardiac lymphoma is extremely rare, accounting for less than 2% of heart tumors. It also accounts for less than 1% of extranodal lymphomas and a lower percentage of all lymphomas.5–8 Because of the difficulty in obtaining samples and lack of specificity in clinical symptoms, primary cardiac lymphoma is difficult to diagnose. Most cases with cardiac lymphoma were diagnosed by autopsy.9,10 The most common pathological type is diffuse large B cell lymphoma, followed by Burkitt lymphoma, T cell lymphoma, small lymphocyte lymphoma, and plasmablastic lymphoma.5,11,12 The most common pathological types of secondary cardiac lymphoma are diffuse large B cell lymphoma, T-lymphoblastic lymphoma, and Hodgkin lymphoma.14 Because most cardiac lymphomas are secondary, tumors outside the heart appear before the heart tumors. Through invasion of the mediastinum or surrounding mass, lymphatic circulation, or blood dissemination, the heart is involved, and heart-related symptoms appear.15–20 Therefore, most of the relevant cardiac symptoms are late or mild,21,22 meaning they can be easily ignored.
Through this retrospective analysis of 37 patients with cardiac lymphoma, this study found that the sex and age distributions of patients with cardiac lymphoma were similar to the overall situation of the lymphoma population, and a male predominance was identified (26:11). The median age of patients with B cell lymphoma was lower than that of patients with T cell lymphoma without significance. The vast majority of these patients had no history of immunodeficiency or immunosuppressant use. Compared with the general lymphoma population, this group of patients had a higher proportion of T and NK cell lymphoma (14 cases, 37.8%) than the general population of lymphoma. Although possible deviation associated with the single-center nature and small sample size of the study cannot be excluded, the results also indicated that patients with T and NK cell lymphoma were more prone to extranodal involvement and more extensive lesions than those with B cell lymphoma. In addition, patients had a wide range of lesions, serous cavity effusion, and a poor physical status. In addition to pericardial effusion, 23 patients had peritoneal or pleural effusion, which was much higher than the rate of total serous effusion in the general lymphoma population. Therefore, the physical status of some patients in this group was relatively poor. Meanwhile, several patients had hypoproteinemia, increased LDH and β2-microglobulin levels, and B symptoms. In particular, 31 and 26 patients had elevated LDH and β2-microglobulin levels, respectively, and 31 patients had low albumin levels. These abnormal laboratory indicators, which may be related to the prognosis of lymphoma, suggest that the prognosis of this group of patients was not optimistic. However, it cannot be excluded that these abnormalities are related to tumor stimulation and protein loss caused by serous cavity fluid accumulation, and thus, their significance must be comprehensively evaluated. These patients also exhibited a higher rate of vital organ involvement. Specifically, the rate of involvement of more than two organs excluding the heart was higher than reported previously. This indicated that in addition to direct invasion of the surrounding tissues such as the mediastinum, cardiac involvement demonstrated that lymphoma had existed for a prolonged period. The proportion of patients with mediastinal tumors and/or pleural effusion appeared to be higher in the B cell lymphoma group than in the T cell lymphoma group, whereas the proportion of patients with ascites was higher in patients with T cell lymphoma. This confirmed that T cell-derived lymphoma is more likely to spread widely, and pericardial effusion was more closely related to the wide spread of disease. Meanwhile, pericardial effusion from B cell-derived lymphoma may have more relevance to local factors. The proportion of patients with moderate to high risk according to the IPI was high. In particular, 32 patients had IPI scores exceeding 3 points. The clinical manifestations of the heart were not specific, and myocardial enzyme levels were normal. The most common manifestations of the heart included chest tightness, shortness of breath, palpitation, and increased heart rate. Meanwhile, nearly half of the patients had electrocardiographic arrhythmia and T wave abnormalities. Pericardial effusion was found in all patients via echocardiography, suggesting the importance of this test in patients with lymphoma. Primary cardiac lymphoma was rare. Among the 37 patients reported in this article, only one 68-year-old patient had primary cardiac lymphoma. The initial symptoms in this patient were cough and chest tightness. PET-CT of the lesion was limited to the heart, and ultrasound revealed a solid hypoechoic mass (7.6 × 4.7 cm2) in the right atrium. The tumor blocked part of the tricuspid valve orifice accompanied by right atrium enlargement and extensive pericardium effusion. Thoracotomy revealed that the tumor tissue was completely fused with the right atrium and right ventricular myocardium. The pathological diagnosis was diffuse large B cell lymphoma, non-germinal origin, and the immunophenotype was Bcl-6 (−), CD3 (+), CD10 (−), CD20 (+++), Kappa (++), Ki-67 (+, >75%), lambda (+, locally), CD79a (++), and Mum-1 (+).
In addition, three patients had cardiac lymphoma accompanied by solid cardiac masses, including the aforementioned patient with primary cardiac lymphoma. The pathological type of the three lesions was diffuse large B cell lymphoma that did not have a germinal center origin. The two patients with secondary cardiac lymphoma were middle-aged men (45–46 years old) with normal immune function, both of whom initially presented with symptoms of chest tightness. Their heart lesions were larger than 5 cm and located in the right atrium. Their tumors grew into the heart cavity and further infiltrated both the myocardium and superior vena cava, and they were accompanied by moderate to large levels of pericardial effusion. These two patients had electrocardiographic changes, including ST-T changes in one patient and a atrial flutter and third degree atrioventricular block in the other patient. Their lesions had a wide range and exhibited kidney and adrenal involvement. The Eastern Cooperative Oncology Group (ECOG) performance status was 2–3, and the IPI score was 2–3 points, which indicated middle-high risk. R-CHOP chemotherapy cannot completely ablate tumor masses in the heart. The curative effect was poor in patients with pericardial effusion as the manifestation of cardiac involvement compared with the findings in the patients with diffuse large B lymphoma. The three patients with solid heart spaces were all middle-aged males. The lesions were mainly located in the right atrium, the masses were all larger than 5 cm, and the pathological type was non-germinal center-derived diffuse large B cell lymphoma.
Cardiac lymphoma is easily missed or misdiagnosed, and medical personnel must increase their awareness of this malignancy.23 Echocardiography cannot be excluded in the diagnostic examination. In addition, the extensive application of PET-CT, which combines imaging and functional metabolism, effectively reveals the proliferation and metabolism of tumors throughout the body.24–26 It represented a more accurate method for the diagnosis and treatment evaluation of lymphomas with cardiac involvement.27–30 Because of the low incidence and lack of relevant large-scale clinical research on cardiac lymphoma, it is extremely meaningful to study cases or small samples. At present, it is unclear whether another treatment plan is superior to R-CHOP. In patients with late-stage disease and high prognostic indices attributable only to pericardial involvement, the need for combined hematopoietic stem cell transplantation requires further investigation.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: Financial support was received from the Chinese PLA General Hospital (MJ 2014) and Hainan Natural Science Foundation (No. 817351).
ORCID iD: Jian Bo https://orcid.org/0000-0003-0285-8775
References
- 1.He XL, Yu F, Guo T, et al . T-cell lymphoblastic lymphoma presenting with pleural effusion: A case report. Respir Med Case Rep 2014; 12: 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muthusamy P, Ebrom S, Cohle SD, et al . Pericardial involvement as an initial presentation of anaplastic large cell lymphoma. Can Fam Physician 2014; 60: 638–641. [PMC free article] [PubMed] [Google Scholar]
- 3.El Sayed MJ, Gharzuddin W, Kharfan-Dabaja MA. T-cell acute lymphoblastic leukemia presenting as a cardiac tamponade. Austin J Emergency & Crit Care Med 2014; 1: 4. [Google Scholar]
- 4.McDonnell PJ, Mann RB, Bulkley BH. Involvement of the heart by malignant lymphoma: a clinicopathologic study. Cancer 1982; 49: 944–951. [DOI] [PubMed] [Google Scholar]
- 5.Petersen CD, Robinson WA, Kurnick JE. Involvement of the heart and pericardium in the malignant lymphomas. Am J Med Sci 1976; 272: 161–165. [DOI] [PubMed] [Google Scholar]
- 6.Chaves FP, Quillen K, Xu D. Pericardial effusion: a rare presentation of adult t-cell leukemia/lymphoma. Am J Hematol 2004; 77: 381–383. [DOI] [PubMed] [Google Scholar]
- 7.Ako J, Eto M, Kim S, et al. Pericardial constriction due to malignant lymphoma. Jpn Heart J 2000; 41: 673–679. [DOI] [PubMed] [Google Scholar]
- 8.Deline JM, Cable DG. Clustering of recurrent pericarditis with effusion and constriction in a family. Mayo Clin Proc 2002; 77(1): 39–43. [DOI] [PubMed] [Google Scholar]
- 9.Grebenc ML, Rosado De Christenson ML, Burke AP, et al. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics 2000; 20: 1073–1103. [DOI] [PubMed] [Google Scholar]
- 10.Abraham KP, Reddy V, Gattuso P. Neoplasms metastatic to the heart: review of 3314 consecutive autopsies. Am J Cardiovasc Pathol 1990; 3: 195–198. [PubMed] [Google Scholar]
- 11.Klatt EC, Heitz DR. Cardiac metastases. Cancer 1990; 65:1456–1459. [DOI] [PubMed] [Google Scholar]
- 12.Majano-Lainez RA. Cardiac tumors: a current clinical and pathological perspective. Crit Rev Oncog 1997; 8: 293–303. [DOI] [PubMed] [Google Scholar]
- 13.Al-Mehisen R, Al-Mohaissen M, Yousef H. Cardiac involvement in disseminated diffuse large B-cell lymphoma, successful management with chemotherapy dose reduction guided by cardiac imaging: A case report and review of literature. World J Clin Cases 2019; 7: 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel J, Melly L, Aheppard MN. Primary cardiac lymphoma: B- and T-cell cases at a specialist UK centre. Ann Oncol 2010; 21: 1041–1045. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen JD, Carrasquillo JA, Little RF, et al . Fluorodeoxyglucose positron emission tomography in the presence of cardiac metastases. Clin Nucl Med 2003; 28: 979–980. [DOI] [PubMed] [Google Scholar]
- 16.Abramowitz Y, Hiller N, Perlman G, et al. The diagnosis of primary cardiac lymphoma by right heart catheterization and biopsy using fluoroscopic and transthoracic echocardiographic guidance. Int J Cardiol 2007; 118: e39–e40. [DOI] [PubMed] [Google Scholar]
- 17.Kang SM, Rim SJ, Chang HJ, et al. Primary cardiac lymphoma diagnosed by transvenous biopsy under transesophageal echocardiographic guidance and treated with systemic chemotherapy. Echocardiography 2003; 20: 101–103. [DOI] [PubMed] [Google Scholar]
- 18.Jurkovich D, De Marchena E, Bilsker M, et al. Primary cardiac lymphoma diagnosed by percutaneous intracardiac biopsy with combined fluoroscopic and transesophageal echocardiographic imaging. Catheter Cardiovasc Interv 2000; 50: 226–233. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda H, Nakamura S, Nishimaki H, et al. Primary lymphoma of the heart: case report and literature review. Pathol Int 2004; 54: 187–195. [DOI] [PubMed] [Google Scholar]
- 20.Koehler F, Borges AC, Fotuhi PC. Large B cell lymphoma with cardiac infiltration. Heart 2003; 89: 1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alzeerah MA, Singh R, Jarrous A. Large B-cell lymphoma of the atria. Tex Heart Inst J 2003; 30: 74–75. [PMC free article] [PubMed] [Google Scholar]
- 22.Jang JJ, Danik S, Goldman M. Primary cardiac lymphoma: diagnosis and treatment guided by transesophageal echocardiogram perfusion imaging. J Am Soc Echocardiogr 2006; 19: 1073.e7-9. [DOI] [PubMed] [Google Scholar]
- 23.Morillas P, Quiles J, Nunez D, et al. Complete regression of cardiac non-Hodgkin lymphoma. Int J Cardiol 2006; 106: 426–427. [DOI] [PubMed] [Google Scholar]
- 24.Hirabayashi T, Tanabe M, Onishi K, et al . Cardiac malignant lymphoma with atrial arrhythmias. Int J Cardiol 2007; 114: 42–44. [DOI] [PubMed] [Google Scholar]
- 25.Piccaluga PP, Vigna E, Placci A, et al. Primary cardiac non-Hodgkin lymphoma presenting with atrial flutter and pericardial effusion. Br J Haematol 2006; 134: 356. [DOI] [PubMed] [Google Scholar]
- 26.Nonami A, Takenaka K, Kamezaki K, et al. Successful treatment of primary cardiac lymphoma by rituximab-CHOP and high-dose chemotherapy with autologous peripheral blood stem cell transplantation. Int J Hematol 2007; 85: 264–266. [DOI] [PubMed] [Google Scholar]
- 27.Kaul P, Javangula K. Burkitt lymphoma masquerading as cardiac tamponade. J Cardiothorac Surg 2007; 2: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang YY, Fan K, Lam YM, et al. Primary cardiac lymphoma presenting with right heart mass and bradycardia. Ann Hematol 2007; 86: 685–686. [DOI] [PubMed] [Google Scholar]
- 29.Knowles JW, Elliott AB, Brody J. A case of complete heart block reverting to normal sinus rhythm after treatment for cardiac invasive Burkitt's lymphoma. Ann Hematol 2007; 86: 687–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakagawa Y, Ikeda U, Hirose M, et al. Successful treatment of primary cardiac lymphoma with monoclonal CD20 antibody (rituximab). Circ J 2004; 68: 172–173. [DOI] [PubMed] [Google Scholar]