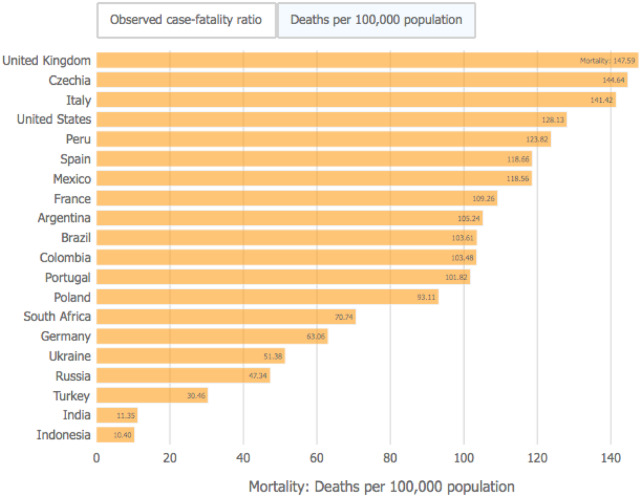
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the coronavirus that causes the infection known as coronavirus disease 2019 (COVID-19), has spread rapidly causing a pandemic that has not only seriously affected human health, but also restricted daily life activities and weakened the world economy to unprecedented levels. Most people who are COVID-19-positive fortunately present with mild symptoms, but older people and those with comorbidities may present with severe respiratory complications, often requiring care in intensive medical units. As of the end of January 2021, mortality worldwide attributable to COVID-19 has been estimated at 2 150 000. The death rate per 100 000 population as reported by the John Hopkins Coronavirus Resource Centre is depicted in Figure 1. The impact of COVID-19 on the nephrology community has been important at different levels: (i) patients with end-stage kidney disease on dialysis and those with kidney transplants are at risk for COVID-19 complications and the mortality is high; (ii) acute kidney injury (AKI) ascribed to COVID-19 is a frequent complication of severe cases and moreover is associated with high mortality; and (iii) there is COVID-19-related glomerular involvement, characterized in some cases by collapsing glomerulopathy and tubular damage in other cases.
FIGURE 1:
COVID-19 deaths per 100 000 population. Extracted from John Hopkins Coronavirus Resource Center (date: 25 January 2021).
All of this COVID-19-related kidney pathology adds greatly to the already high global burden of kidney disease in terms of human suffering and costs to the medical systems. It is estimated that the number of people that suffer from kidney disease in the world exceeds 850 million, which is twice the estimated number of people with diabetes (422 million). This places kidney disease among one of the most common of all diseases around the world [1]. In-hospital patients seen by nephrologists, moreover, are the more complex, with the highest morbidity and mortality [2]. We do not know yet the long-term outcomes of COVID-19-related kidney disease in terms of recovery of kidney function or persistence of proteinuria, which could add additional need for nephrology care. Taking all of this into consideration, the impact of COVID-19 in the nephrology community and our patients could be potentially devastating. An analysis of over 17 million adults found almost 11 000 COVID-19-related deaths [3]. Advanced chronic kidney disease (CKD) was identified as one of the most important risk factors for death in COVID-19 patients in this order: dialysis patients [adjusted Hazard Ratio (aHR) 3.69], transplant recipients (aHR 3.53) and CKD (estimated glomerular filtration rate <30 mL/min/1.73 m2) (aHR 2.52) [4]. The COVID-19 mortality risk in CKD patients is actually greater than the risk observed in people with diabetes and chronic heart disease, suggesting priority for clinical trials, vaccinations and future action plans in our nephrology field [4]. Results from the European Renal Association COVID-19 Database (ERACODA) revealed a 28-day mortality in kidney transplant and dialysis patients with COVID-19 as high as 21.3 and 25%, respectively [5]. Hypertension, diabetes mellitus, coronary artery disease, heart failure and chronic lung disease were not identified as independent risk factors [6]. In concordance with these results, a study from Dialysis Clinic Inc., a national not-for-profit dialysis provider caring for >15 000 maintenance dialysis patients at 260 outpatient dialysis clinics, showed that dialysis patients are at high risk of developing COVID-19, and 90-day mortality ascribed to COVID-19 approaches 25% [7]. Among dialysis patients, residence in a long-term care facility, Black race, male sex, diabetes, receipt of in-centre dialysis and treatment at an urban dialysis centre were identified as risk factors for COVID-19 infection. In dialysis patients with COVID-19 disease, older age, hypertension, congestive heart failure and peripheral vascular disease as well as wheelchair use, were associated with higher risk of death [7].
The nephrology community throughout the world has responded well to the challenges posed by COVID-19 and the high demand for kidney replacement therapies for patients with AKI and those already on dialysis.
In this issue, Dr Cozollino et al. describe strategies employed to avoid COVID-19 infection in patients with end-stage kidney disease and the important role of promoting home dialysis and peritoneal dialysis [8]. In situations of medical crisis, epidemiological studies for assessing mortality and outcomes in our dialysis and transplant programmes are of the upmost importance, as described by Dr Noordzij et al. Nephrologists have been mainly trained to take care of patients, and yet the interpretation of emerging new studies is of crucial importance for the progress of our specialty. Dr Noordzij et al. offer clues for interpreting epidemiological papers in the context of COVID-19 and kidney disease. The authors bring up the need for awareness of the differences in the study design, outcome definitions and applied methods that should help future and young investigators to better interpret and design epidemiological studies in the nephrology field [9]. Dr Toapanta et al. discuss COVID-19 in kidney transplants, a topic of great importance since mortality in kidney transplant recipients with COVID-19 infection has been estimated to be as high as 20%. Of note, incidence of AKI and the need of renal replacement therapy in hospitalized kidney transplant patients with COVID-19 infection is high. Whereas in the general population, the variability of the reported AKI-related COVID-19 infection is high and varies depending on the country and type of study, studies performed in kidney transplant patients showed consistent results with an incidence of AKI around 45%. The occurrence of AKI risk in this specific CKD population is discussed by Toapanta et al. [10].
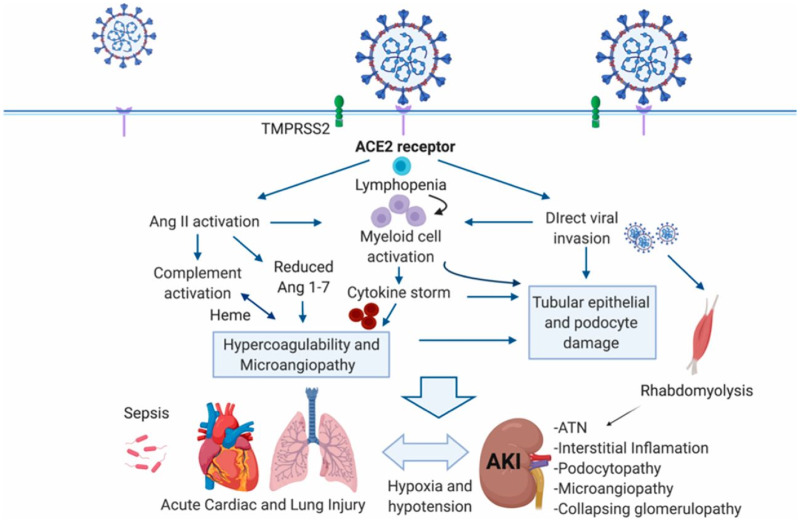
The impact of COVID-19 disease affects not only patients with pre-existing kidney disease or kidney transplantation. De novo kidney disease is commonly seen in hospitalized patients with COVID-19, particularly those with severe disease that require intensive care [11, 12]. In such patients, the incidence of AKI is very high and is associated with high mortality [13–15]. The pathophysiology of this form of AKI is not well understood, but several factors including activation of the innate immune system, complement activation, Angiotensin (Ang) II overactivity and the development of a hypercoagulable state have been incriminated (Figure 2) [16]. In many patients, however, AKI is simply attributable to hypotension and sepsis. Another entity that has been frequently described in patients with COVID-19 is collapsing glomerulopathy, usually associated with AKI and in African Americans with APOL risk alleles [17–19].
FIGURE 2:
Targeting of ACE2 by SARS-CoV-2 may result in Ang dysregulation, innate and adaptive immune pathway activation and hypercoagulation to result in organ injury and AKI associated with COVID-19. Organ crosstalk between the injured lungs, the heart and the kidney may further propagate injury. CD8+ T cells and natural killer cells can restrain macrophage activation and are potential targets for SARS-CoV-2. ATN, acute tubular necrosis; TMPRSS2, transmembrane protease, serine 2. From Batlle et al. J Am Soc Nephrol 2020; 31: 1380–1383 [16] (with permission).
Despite the intensity of medical care required to take care of these sick patients with COVID-19 by the nephrology community, there has also been an effort to convey the new information that has been rapidly accrued during the past year. In this issue of CKJ, Sharma et al. discuss AKI in COVID-19 patients, reviewing the histopathological changes based on kidney biopsies and autopsies [20]. Drs Mohamed and Velez discuss the importance of proteinuria including the assessment and methods for its detection, the mechanisms involved and the significance of ‘de novo’ proteinuria in COVID-19 patients [21].
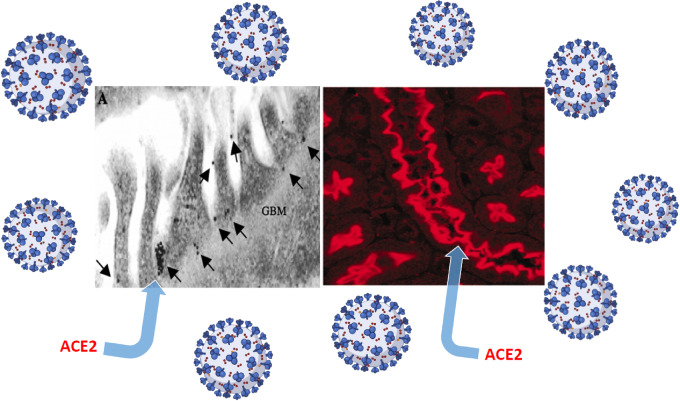
Ang-converting enzyme 2 (ACE2) is the main receptor for SARS-CoV-2 viral cell entry [22, 23]. In the kidney, ACE2 is present mainly in the apical border of the proximal tubular cells and, to a lesser extent, in podocytes (Figure 3) [24]. It is still a matter of some debate whether kidney SARS-CoV-2 invasion causes, in part, the manifestations of COVID-19 described above. There are a few studies that have documented well the presence of SARS-CoV-2 in the glomerulus and in proximal tubular cells [25].
FIGURE 3:
A depiction of ACE2, the main receptor of SARS-CoV-2, in glomerular podocytes using immunogold labelling (left) and proximal tubular cells by immunofluorescence (right). GBM, glomerular basement membrane. Data modified from Ye et al. J Am Soc Nephrol 2006; 17: 3067–3075 [24].
Following the recognition in January 2020 that ACE2 is the receptor for SARS-CoV-2, like SARS-CoV, concerns were raised that some drugs such as the renin–Ang system (RAS) blockers could be deleterious in COVID-19 patients [26]. The concern was based on studies prior to COVID-19, most of them in experimental animals, showing that ACE2 could be upregulated in the heart, kidney vasculature and other organs [27, 28]. Recent work has shown, however, that in the lungs, the site of SARS-CoV-2 entry, ACE2 expression is not increased [29]. After the initial concern, several publications and position statements by scientific specialty societies reassured the medical community that there were no grounds to abandon these medications pending studies addressing this issue [30–32]. In this issue, Cohen et al. provide a critical review of the physiologic and epidemiologic evidence of the interactions between RAS blockade and COVID-19 that, in our opinion, brings this ongoing discussion towards an end [33]. One year later, after all the concerns were raised and with multiple publications addressing this issue, it seems quite clear that the use of RAS blockers is generally safe in the context of COVID-19.
In summary, this issue of CKJ covers current knowledge of COVID-19 disease and its impact on the kidney. It is hoped that the various contributions will provide the nephrology community with valuable information that is useful for the care of patients with underlying kidney disease and those with the pathologies that have emerged related to the COVID-19 pandemic.
FUNDING
This article is part of a supplement supported by Fresenius Medical Care without any influence on its content.
CONFLICT OF INTEREST STATEMENT
D. Batlle is coinventor of patents entitled ‘Active Low Molecular Weight Variants of Angiotensin Converting Enzyme 2', ‘Active low molecular weight variants of Angiotensin Converting Enzyme 2 (ACE2) for the treatment of diseases and conditions of the eye' and ‘Shorter soluble forms of Angiotensin Converting Enzyme 2 (ACE2) for treating and preventing coronavirus infection.' D. Batlle is founder of Angiotensin Therapeutics Inc. D. Batlle has received consulting fees from AstraZeneca, Relypsa, and Tricida, all unrelated to this work. D. Batlle received unrelated support from a grant from AstraZeneca. M.J. Soler has no conflict of interest to declare related to this work.
REFERENCES
- 1. Jager KJ, Kovesdy C, Langham R. et al. A single number for advocacy and communication—worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant 2019; 34: 1803–1805 [DOI] [PubMed] [Google Scholar]
- 2. Tonelli M, Wiebe N, Manns BJ. et al. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open 2018; 1: e184852-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williamson EJ, Walker AJ, Bhaskaran K. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584: 430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ERA-EDTA Council, ERACODA Working Group. Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant 2021; 36: 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hilbrands LB, Duivenvoorden R, Vart P. et al. ERACODA Collaborators. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant 2020; 35: 1973–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noordzij M, Duivenvoorden R, Pena MJ et al. collaborative ERACODA authors. ERACODA: the European database collecting clinical information of patients on kidney replacement therapy with COVID-19. Nephrol Dial Transplant 2020; 35: 2023–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsu CM, Weiner DE, Aweh G. et al. COVID-19 Infection among US dialysis patients: risk factors and outcomes from a national dialysis provider. Am J Kidney Dis 2021; S0272-6386(21)00025-1. doi: 10.1053/j.ajkd.2021.01.003. Online ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cozzolino M, Conte F, Zappulo F. et al. COVID-19 pandemic era: is it time to promote home dialysis and peritoneal dialysis? Clin Kidney J 2021; 14 (Suppl 1): i6–i13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noordzij M, Vart P, Duivenvoorden R. et al. Pitfalls when comparing COVID-19-related outcomes across studies—lessons learnt from the ERACODA collaboration. Clin Kidney J 2021; 14 (Suppl 1): i14–i20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toapanta N, Torres IB, Sellarés J. et al. Kidney transplantation and COVID-19 renal and patient prognosis. Clin Kidney J 2021; 14 (Suppl 1): i21–i29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng Y, Luo R, Wang K. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney international 2020; 97: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang X, Yu Y, Xu J. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respirat Med 2020; 8: 475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pei G, Zhang Z, Peng J. et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 2020; 31: 1157–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fisher M, Neugarten J, Bellin E. et al. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol 2020; 31: 2145–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirsch JS, Ng JH, Ross DW. et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020; 98: 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Batlle D, Soler MJ, Sparks MA. et al. ; on behalf of the COVID-19 and ACE2 in Cardiovascular, Lung, and Kidney Working Group. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol 2020; 31: 1380–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Velez JCQ, Caza T, Larsen CP.. COVAN is the new HIVAN: the re-emergence of collapsing glomerulopathy with COVID-19. Nat Rev Nephrol 2020; 16: 565–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu H, Larsen CP, Hernandez-Arroyo CF. et al. AKI and collapsing glomerulopathy associated with COVID-19 and APOL1 high-risk genotype. J Am Soc Nephrol 2020; 31: 1688–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shetty AA, Tawhari I, Safar-Boueri L. et al. COVID-19-associated glomerular disease. J Am Soc Nephrol 2021; 32: 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma P, Ng JH, Bijol V. et al. Pathology of COVID-19-associated acute kidney injury. Clin Kidney J 2021; 14 (Suppl 1): i30–i39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mohamed MMB, Velez JCQ. Proteinuria in COVID-19. Clin Kidney J 2021; 14 (Suppl 1): i40–i47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shang J, Ye G, Shi K. et al. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020; 581: 221–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davidson AM, Wysocki J, Batlle D.. Interaction of SARS-CoV-2 and other coronavirus with ACE (angiotensin-converting enzyme)-2 as their main receptor: therapeutic implications. Hypertension 2020; 76: 1339–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ye M, Wysocki J, William J. et al. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol 2006; 17: 3067–3075 [DOI] [PubMed] [Google Scholar]
- 25. Puelles VG, Lütgehetmann M, Lindenmeyer MT. et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 2020; 383: 590–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fang L, Karakiulakis G, Roth M.. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respirat Med 2020; 8: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferrario CM, Jessup J, Chappell MC. et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005; 111: 2605–2610 [DOI] [PubMed] [Google Scholar]
- 28. Soler MJ, Ye M, Wysocki J. et al. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol 2009; 296: F398–F405 [DOI] [PubMed] [Google Scholar]
- 29. Wysocki J, Lores E, Ye M. et al. Kidney and lung ACE2 expression after an ACE inhibitor or an Ang II receptor blocker: implications for COVID-19. J Am Soc Nephrol 2020; 31: 1941–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Danser AJ, Epstein M, Batlle D.. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension 2020; 75: 1382–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sparks MA, South A, Welling P. et al. Sound science before quick judgement regarding RAS blockade in COVID-19. Clin J Am Soc Nephrol 2020; 15: 714–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vaduganathan M, Vardeny O, Michel T. et al. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020; 382: 1653–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohen JB, South AM, Shaltout HA. et al. Renin–angiotensin system blockade in the COVID-19 pandemic. Clin Kidney J 2021; 14 (Suppl 1): i48–i59 [DOI] [PMC free article] [PubMed] [Google Scholar]