Abstract
Upper respiratory and pulmonary diseases are the primary manifestations of coronavirus disease 2019 (COVID-19). However, kidney involvement has also been recognized and extensively described. A large percentage of affected patients present with acute kidney injury (AKI). However, specific phenotypic aspects of AKI or other renal manifestations of COVID-19 remain sparsely characterized. Many reports indicate that proteinuria can be detected in AKI associated with COVID-19 (CoV-AKI) despite CoV-AKI being largely described as a form of acute tubular injury. On the other hand, individuals of African ancestry with the high-risk APOL1 genotype are uniquely at risk of developing collapsing glomerulopathy when they are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the entity now known as COVID-19-associated nephropathy (COVAN). Patients with COVAN typically present with nephrotic-range proteinuria. The exact incidence of proteinuria in COVID-19 is unclear due to heterogeneity in the frequency with which proteinuria has been assessed in cases of COVID-19, as well as methodological differences in the way proteinuria is measured and/or reported. In this review we discuss the current evidence of proteinuria as a manifestation of COVID-19 and elaborate on potential pathophysiological mechanisms associated with it.
Keywords: AKI, albuminuria, nephrotic, protein-to-creatinine, SARS-CoV-2
INTRODUCTION
The pandemic of coronavirus disease 2019 (COVID-19) caused by the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to millions of cases and fatalities worldwide, with a clinical spectrum ranging from a mild upper respiratory tract infection to critical illness, respiratory failure and death [1]. Kidney dysfunction is also relatively common among hospitalized patients with COVID-19 and may range from the presence of isolated proteinuria and/or hematuria to full-blown acute kidney injury (AKI) requiring renal replacement therapy (RRT) [2]. In a systematic review and meta-analysis of AKI in COVID-19, the prevalence of AKI across 20 cohorts was 17%, with a range of 0.5–80% [3]. Similarly, the incidence of proteinuria in COVID-19 with or without concomitant AKI has been reported in a wide range of 28–84% [4–8].
While proteinuria has been reported in the absence of AKI in COVID-19 and may denote a subclinical insult, the widely described observation is the presence of proteinuria in cases of Kidney Disease: Improving Global Outcomes–defined AKI. In order to examine the existing evidence indicating that proteinuria is a manifestation of AKI associated with COVID-19 (CoV-AKI), it is essential to understand the mechanisms of disease leading to AKI in COVID-19. The overwhelming majority of reports strongly support the notion that acute tubular injury (ATI) is the primary lesion driving CoV-AKI. The available evidence comes from clinical cohorts with granular phenotypic characterization and urinary sediment microscopy, premortem biopsy series and postmortem pathological reports [4, 5, 9, 10]. As seen in other infectious diseases that progress to systemic inflammatory response syndrome, endothelial dysfunction and shock [11–15], the majority of the CoV-AKI cases are preceded by gradual or sudden hemodynamic decompensation or occur with temporal alignment with respiratory decompensation and acute respiratory distress syndrome, thus exhibiting a clinical course greatly suggestive of an ischemic form of ATI [4, 16, 17]. Positive pressure mechanical ventilation, often utilized in this setting, has also been identified as an independent risk factor for AKI [18, 19]. In addition, as seen in other viral infections, evidence of rhabdomyolysis has been reported in a small fraction of cases of CoV-AKI [4, 17]. Moreover, critically ill patients with COVID-19 are occasionally exposed to nephrotoxic antibiotics. Therefore a component of toxic ATI may be the predominant insult in CoV-AKI or coexist with an ischemic acute tubular insult.
Although the majority of cases of CoV-AKI appear to correspond to forms of ATI, a syndrome characterized by nephrotic-range proteinuria and AKI caused by a pathological lesion of collapsing glomerulopathy has been described in susceptible individuals of African ancestry and carriers of the high-risk apolipoprotein L1 (APOL1) genotype. This entity has been termed COVID-19-associated nephropathy (COVAN) [20–23]. Thus, when a patient of African descent is infected with SARS-CoV- 2 and presents with concomitant AKI and new-onset nephrotic-range proteinuria, COVAN must be suspected. While the exact pathogenesis of COVAN has not been fully elucidated, it appears to resemble the “two-hit” model of collapsing glomerulopathy described with other viral infections, such as human immunodeficiency virus (HIV) and parvovirus B19 (PVB19), among others, where activation of the interferon (IFN)–chemokine pathway under the background of a mutant APOL1 gene expression and function triggers podocyte damage and a distinct glomerular lesion [23, 24].
Because of the expression of the membrane-bound peptidase angiotensin-converting enzyme 2 (ACE2) in tubular epithelia and podocytes [25–27] and the known property of ACE2 as a facilitator of cell entry for SARS-CoV-2 [28–32], it has been proposed that direct viral infection into the kidneys may account for some of the AKI pathogenesis for both CoV-AKI and COVAN. However, the existing evidence is sparse, inconsistent and not conclusive at this time [9, 17, 20, 33]. To date, studies strongly suggest that systemic host responses are largely responsible for the pathogenesis of AKI in COVID-19, including that of proteinuria.
In summary, the two dominant syndromes of AKI in COVID-19 are a form of ATI (CoV-AKI) and a distinct glomerulopathy (COVAN). Depending on the severity and timing of presentation as well as the presence or absence of a pre-existing comorbidity, the magnitude and persistence of proteinuria expected to be encountered in each syndrome may vary, and that variability may explain the wide discrepancy in the reported incidence. Therefore, optimal understanding of the pitfalls around measurement and interpretation of proteinuria in a patient with COVID-19 is required.
ASSESSMENT OF PROTEINURIA IN COVID-19
Detection and quantification of urinary protein excretion is central to the diagnosis and management of acute and chronic kidney disease (CKD) [34]. Detection of urinary albumin excretion with urine dipstick testing offers semiquantitative information and is primarily viewed as a screening test that requires confirmatory testing with direct quantification of urinary albumin or protein excretion [35]. Quantification of proteinuria is important in that a greater degree of proteinuria is well-recognized as a manifestation of glomerular diseases, and glomerular diseases often require medical management that may substantially differ from that recommended for non-glomerular kidney diseases. Furthermore, proteinuria is associated with a greater risk for progression of proteinuric CKD and is used to monitor the response to therapy [36–38]. Urine protein:creatinine ratio (UPCR) in single-voided urine samples correlates well with measurements of 24-hour urinary protein when properly interpreted by taking into consideration the different rates of creatinine excretion [39–43]. However, there are important considerations to take into account when proteinuria is assessed during AKI.
First, it is imperative to determine if a patient who is found to have proteinuria during a case of COVID-19 had prior documentation of proteinuria before the acute viral illness. The notion of ‘baseline serum creatinine’ is widely pursued and utilized in clinical settings. Arguably, knowing the baseline status of proteinuria can be equally important in the assessment of AKI in a hospitalized patient. Unfortunately, in many instances, no prior medical records are available to ascertain if the detection of proteinuria is a new finding or a chronic feature. Thus when proteinuria is detected in a patient with COVID-19, caution is advised before it is assumed that it necessarily corresponds to the acute illness itself. This is particularly relevant in CoV-AKI because advanced CKD has been demonstrated to be a risk factor for CoV-AKI RRT [44]. Thus it is conceivable that a fair number of patients with preexisting CKD who develop CoV-AKI may have preexisting proteinuria even though it may not be documented in prior medical records.
Second, UPCR is a test that adequately performs during steady state, meaning in the context of steady urinary excretion of creatinine, something that clearly does not occur in AKI. Besides, UPCR was developed as a test in CKD, not in the context of oliguria, when urinary concentration of protein and creatinine can exhibit large variability [34]. Consequently, detection and quantification of proteinuria in CoV-AKI should be performed with careful consideration for the state of AKI and the volume of urinary output. Perhaps the combination of urinary dipstick and serial UPCR measurements can provide more reliable information than one isolated test. Notably, while there are limitations to interpret a UPCR in AKI, UPCR does offer valuable information during cases of COVID-19. In fact, all of the reported cases of COVAN who presented with nephrotic-range proteinuria were detected by urinary dipstick or UPCR and were later corroborated to be valid by repeated testing and ultimately, a glomerular lesion was confirmed by tissue diagnosis. In summary, UPCR and urinary dipstick proteinuria may be used in the assessment of proteinuria in COVID-19, but one must remain cognizant of their limitations and interpret them with caution.
IS PROTEINURIA A FEATURE OF COV-AKI?
Multiple observational studies have reported the presence of proteinuria in CoV-AKI [4, 5, 16, 43, 45, 46] (Table 1). However, there is significant heterogeneity across studies in the way data of proteinuria were extracted, examined and reported. Urinalysis may represent a routine test during hospital admission in some centers, whereas it may only be ordered in the context of AKI in others. Therefore it is cumbersome to generate a clear conclusion. In our cohort of 161 patients with CoV-AKI, we defined significant proteinuria as ≥2+ dipstick or UPCR ≥0.5 g/g and found it to be present in 112 (69%) patients. In an effort to determine if the proteinuria was part of the acute illness, we examined for evidence of prior documentation of proteinuria in the medical records. Of those 112 patients with CoV-AKI and proteinuria, the findings revealed that 25 (22%) had pre-existing proteinuria, whereas proteinuria was confirmed to be de novo in 63 (56%) of them. The baseline status of proteinuria was unknown in 24 (21%; 15% of the entire CoV-AKI cohort). Similarly, we defined nephrotic-range proteinuria as 3+ dipstick and UPCR ≥3.0 g/g and found it to be present in 14 (9%) patients in the entire cohort (13% of those with proteinuria). Among them, 7 (4%) had pre-existing nephrotic-range proteinuria, whereas 6 (4%) had de novo nephrotic-range proteinuria (which included 3 cases of COVAN) and only 1 patient with unknown baseline [4]. When we compared this cohort with a cohort of patients with AKI before the COVID-19 era, the incidence of proteinuria in CoV-AKI was not significantly different than that observed in pre-COVID-19 AKI [46]. Nonetheless, the significant fraction of patients with unknown baseline status of proteinuria hampers our ability to draw definite conclusions.
Table 1.
Published cohorts reporting the incidence of proteinuria in COVID-19
| Study | COVID cases, n | AKI status (n) or elevated baseline SCr | Proteinuria |
Method of assessment | ||
|---|---|---|---|---|---|---|
| Any degree, n (%) | Significant, n (%) | Nephrotic/ heavy, n (%) | ||||
| Pei et al. [43] | 467 (333 tested for renal involvement) | AKI (35) | 31 (88) | 7 (20) | UDS | |
| No AKI (298) | 188 (63) | 30 (10) | ||||
| Cheng et al. [5] | 701 | AKI (36), elevated baseline SCr (53) | 37/53 (70) | 16/53 (30) | UDS | |
| No AKI (665) 389 tested | 157 (40) | 29 (8) | ||||
| Mohamed et al. [4] | 644 | AKI (161) (147 tested for proteinuria) | 126 (78) | 112 (69)a | 14 (9) | UDS, UPCR |
| No AKI (483) | NA | NA | NA | |||
| Li et al. [47] | 193 (147 tested for proteinuria) | AKI (55) | 88/147 (60) | 15/147b [10] | 3/147 (2) | UDS |
| No AKI (138) | ||||||
| Chaudhri et al. [6] | 300 | AKI (82) | 84 (28) | NA | NA | UDS |
| No AKI (218) | ||||||
| Liu et al. [48] | 119 | NA | 34 (29) | NA | NA | UDS |
| Chan et al. [7] | 3993 (656 tested for proteinuria) | AKI (1835) | 365 (84) | NA | NA | UDS, UPCR |
| No AKI (2158) | 159 (72) | NA | NA | |||
| Justine et al.[49] | 239 (153 tested for proteinuria) | NA | 132 (86) | 68 (44)c | 2 (1.3) | UPCR, SDVS |
| Fisher et al. [8] | 3345 (2590 tested for proteinuria) | AKI (1903) | NA | 1128 (95)d | 55 (4.6) | UPCR, UDS |
| No AKI (1442) | NA | 633 (98)d | 9 (1.1) | |||
| Lee et al. [50] | 1002 | AKI (294) (269 tested for proteinuria) | 207 (77) | NA | NA | UDS |
| No AKI (708) (479 tested for proteinuria) | 298 (62) | NA | NA | |||
| Hirsch et al. [16] | 5449 | AKI (1993) (646 tested for proteinuria | 478 (74) | 272 (42) | NA | UDS |
| No AKI (3456) | NA | NA | NA | |||
| Portolés et al. [51] | 1603 | AKI (336) | 160 (48) | 160 (48)e | NA | UPCR |
| No AKI (1267) | 405 (32) | 405 (32) | NA | |||
SCr, serum creatinine; UDS, urine dipstick test; UPCR, urine protein-to-creatinine ratio; SDVS, Siemens Dimension Vista instrument to assess α1-microglobulin, β2-microglobulin; NA, not available.
Significant proteinuria defined as ≥2+ protein by dipstick or UPCR ≥0.5 g/g.
Significant proteinuria defined as not defined, only reported 15 patients with 2+ and 3 patients with 3+.
Significant proteinuria defined as UPCR >0.5 g/g.
Significant proteinuria defined as ‘heavy’ proteinuria >0.5 g/g.
Significant proteinuria defined as >0.3 g/L.
Other studies have reported the incidence of proteinuria in COVID-19 with or without AKI. In a study of 701 patients with COVID-19, 1+ dipstick proteinuria was found in 128 of 389 (33%) patients without AKI and 21 of 53 (40%) patients with AKI, whereas 2+–3+ dipstick proteinuria was found in 29 of 389 (8%) patients without AKI and 16 of 53 (30%) patients with AKI [5]. In another report including 3345 patients with COVID-19, UPCR <30 mg/g was found in 5 of 1903 (0.2%) patients with AKI compared with 7 of 1442 without AKI (0.4%), 30–500 mg/g in 59% of those with AKI and 44% of those without AKI and >500 mg/g in 3% of those with AKI and 0.6% of those without AKI [8]. These studies suggest that low-grade proteinuria can be detected during COVID-19 irrespective of the copresence of AKI, whereas only in cases of AKIis overt proteinuria more frequent in CoV-AKI than in COVID-19 without AKI. These data could be interpreted as possible subclinical kidney involvement during COVID-19, but not sufficient to cause an increase in serum creatinine. Alternatively, it could be seen as potential noise caused by the acute illness, inadequate sampling or artifacts and of insignificant clinical relevance. In fact, Pei et al. [43] reported resolution of proteinuria within a median duration of 12 days in 69% of patients with COVID-19 who had presented with detected proteinuria, highlighting the fact that transient detection may not carry short-term consequences.
Other studies have reported that the incidence of proteinuria in COVID-19 is greater when AKI coexists. In a cohort of 1002 patients with COVID-19, simply using dipstick proteinuria, proteinuria was more common in patients with AKI compared with those without AKI (77% versus 62%) [50]. Coexistence of proteinuria and hematuria has also been examined. Chaudhri et al. [6] described 300 patients with COVID-19 and reported, at admission, 61 (20%) had proteinuria, 18 (6%) had hematuria and 13 (4%) had combined proteinuria and hematuria. During the hospitalization, 23 (8%), 38 (13%) and 15 (5%) patients developed proteinuria, hematuria and combined proteinuria and hematuria, respectively. During hospitalization, de novo proteinuria was significantly associated with an increased risk of death [odds ratio 8.9 (95% confidence interval 1.2–11.4), P = 0.04].
MECHANISMS OF PROTEINURIA IN COV-AKI
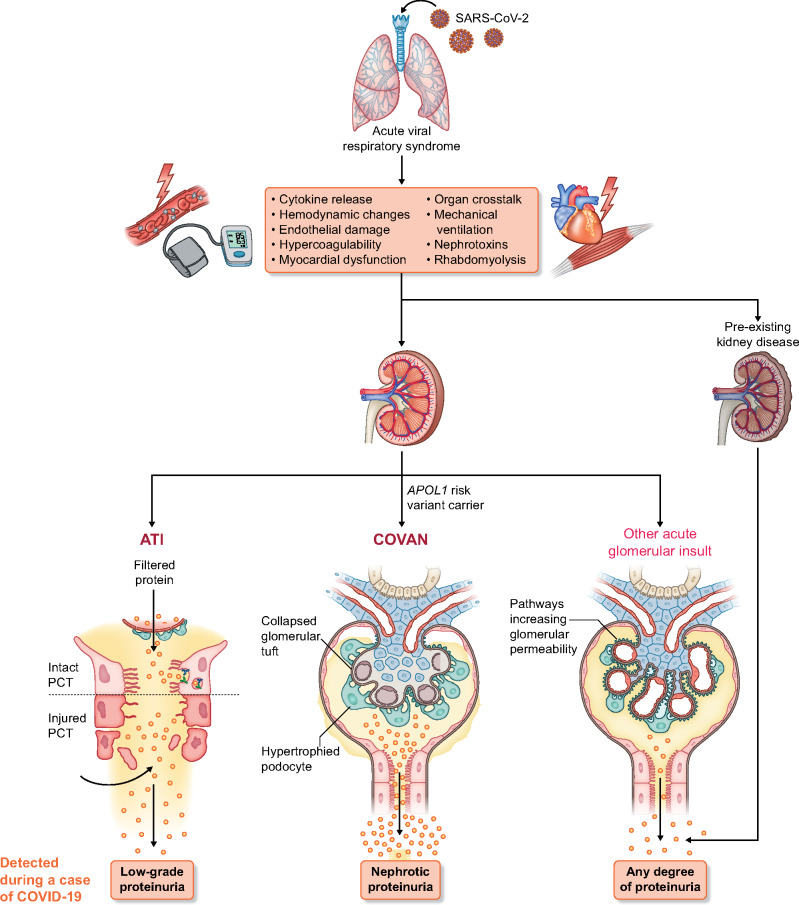
Proteinuria is a reflection of glomerular membrane barrier permeability dysfunction or impaired reabsorption by epithelial cells of the proximal tubuli [34, 52, 53]. In physiological conditions, all low molecular weight proteins (e.g. α1-microglobulin, β2-microglobulin) and a fraction of intermediate molecular weight proteins, mainly albumin, cross the glomerular barrier and are completely reabsorbed by proximal tubular epithelial cells [53–55]. In the context of COVID-19, disruption of the glomerular capillary wall can certainly occur in COVAN, whereas impairment in tubular reabsorption during ATI is also plausible (Figure 1).
FIGURE 1:
Pathogenesis of proteinuria in COVID-19. Individuals infected with SARS-CoV-2 develop an acute respiratory illness that is followed by multiple pathological mechanisms that may lead to the development of AKI. Low-grade proteinuria can be observed in COVID-19 presenting with a subclinical form of AKI, i.e. without elevation of the serum creatinine concentration. The most common type of AKI in COVID-19 is ATI. In cases of ATI, low-grade proteinuria can also be detected, presumably resulting from damage to the proximal convoluted tubule, when detached and sloughed tubular cells cannot reabsorb the filtered protein that is normally reclaimed through the apical endocytic apparatus. Susceptible individuals of African ancestry who are carriers of the APOL1 high-risk genotype can acquire collapsing glomerulopathy when they are infected with COVID-19 (i.e. COVAN) and present with nephrotic-range proteinuria and AKI. Systemic inflammatory response has been linked to an increase in glomerular permeability. Other acute glomerular insults can occur in patients with COVID-19 (e.g. thrombotic microangiopathy). Preexisting CKD can lead to detection of proteinuria in a patient with COVID-19 and the magnitude of the proteinuria in those cases could be of any degree, stable or worse than its baseline value.
Evidence of impaired renal tubular handling of protein in ATI: applicable to CoV-AKI
Reabsorption of filtered proteins occurs predominantly in the pars convoluta, in the S1 and S2 segments of the proximal tubule and to a lesser extent in the pars recta (late S2 and S3 segments). The epithelial cells of these segments contain an extensive apical endocytic apparatus [53] that is responsible for the receptor-mediated endocytosis of proteins. In this apparatus there are two separate receptors characterized by different degrees of affinity for albumin, megalin (low affinity) and cubulin (high affinity) [56, 57] located in clathrin-coated pits where they bind albumin [58–60]. After endocytosis, albumin can be directed to endocytic vesicles for transcytosis via the retrieval pathway or to lysosomes for degradation [61]. In the retrieval pathway, up to 400–500 g/day of albumin return to the vascular space without degradation [62, 63]. Also, endosomes containing segregated proteins fuse with lysosomes where proteins are hydrolyzed and the resulting amino acids return to the circulation or are excreted into the urinary space [64]. As a result, the immunochemical assay for albumin depends on whether the proteins are intact or in fragments [65], as only intact albumin is measured by this assay and ∼90–95% of urinary albumin is broken into fragments [56, 66]. In ATI, the structural and functional alterations in proximal tubular epithelial cells depend upon the severity and duration of the injury [67, 68]. Profound and sustained ATI associated with detachment of renal tubular epithelial cells from the basement membrane, sloughing of cells into the tubular lumen and effacement and loss of the brush border in proximal epithelia would be expected to damage the apical endocytic apparatus [65, 69], resulting in albuminuria. ATI could underestimate the albuminuria, as it leads to fragmentation and/or oxidation of filtered albumin that will not be detected by the standard immunochemical assay [70].
Evidence of impaired glomerular filtration barrier permeability dysfunction: applicable to COVAN or other acute glomerular insults
Collapsing glomerulopathy is a variant of focal segmental glomerulosclerosis (FSGS), defined as the presence of segmental capillary tuft collapse and wrinkling and folding in at least one glomerulus in association with podocyte hypertrophy and/or hyperplasia with pseudo-crescent formation [71]. It is an aggressive disease that classically manifests with massive proteinuria and rapidly progressive kidney disease [20–23, 72]. While collapsing glomerulopathy has been linked to various conditions (HIV, PVB19, cytomegalovirus, Epstein–Barr virus and medications such as pamidronate and IFN-α) [23], it has now been shown to also be the pathological lesion of COVAN. In a case series, six patients with COVID-19 presented with AKI and nephrotic-range proteinuria (UPCR ranging from 3.6 to 25 g/g) and were subsequently found to have COVAN. Importantly, there was no evidence of viral detection in the kidney infection by in situ hybridization and all patients tested positive for the high-risk APOL1 gene variant [20]. Blockade of the renin–angiotensin system is an established therapy for proteinuric glomerulopathies, including forms of focal segmental glomerulosclerosis. Therefore it seems prudent to recommend the use of ACE inhibitors and/or angiotensin receptor blockers in patients with COVAN who are not dialysis dependent and have reached a plateau phase with relatively stable serum creatinine. Beyond that, it is perhaps premature to provide additional treatment recommendations regarding antiviral or immunosuppressive therapy for COVAN. Anecdotal reports vary between complete spontaneous remission and development of end-stage kidney disease. Therefore we must remain vigilant for future studies emerging with more detailed descriptions of the prognosis of COVAN.
While other glomerular diseases, such as thrombotic microangiopathy [10], have been described as kidney-related manifestations in patients with COVID-19, they have not been reported consistently [4, 33]. Thus it remains unclear if those cases of non-COVAN glomerulopathies are merely coincidental. However, it should be recognized that experimental models of sepsis or systemic inflammatory response syndrome have demonstrated that glomerular permeability and glycocalyx integrity can be altered by circulating cytokines in that setting, potentially leading to some degree of proteinuria [73–75] (Figure 1).
CONCLUSION
In summary, proteinuria is a reported feature of COVID-19. While somewhat frequent, the reported incidence might be overestimated due to significant variability in the frequency and mode of assessment. Proteinuria in cases of COVID-19 without AKI is often transient and of unclear long-term implications. Because most cases of CoV-AKI are driven by a form of ATI that can cause impairment in tubular reabsorption of filtered proteins, it is expected that low-grade proteinuria will be observed in that setting and in many of those cases proteinuria is expected to be transient. A more overt degree of proteinuria in a patient of African ancestry, particularly nephrotic range, should prompt suspicion for COVAN, whereas rare cases of nephrotic-range proteinuria in patients of other ethnicities should be approached as is routinely done outside the context of COVID-19. Although new-onset proteinuria may develop during the course of COVID-19, it is advised to investigate for previous documentation of proteinuria, particularly in patients presenting with pre-existing CKD, to avoid incorrect assertions regarding the precise onset of proteinuria. More studies are needed to better determine the true incidence of de novo proteinuria in cases of COVID-19 and to gain a better understanding of the long-term consequences (Box 1).
Box 1
Areas for further investigation in COVID-19-associated proteinuria
True incidence of de novo proteinuria
Long-term implications
Role of direct tubular injury or alterations in glomerular permeability
Long-term outcomes of patients with COVAN
Ascertainment of a direct link between COVID-19 and thrombotic microangiopathy
Spectrum of proteinuria in ambulatory cases of COVID-19
FUNDING
This article is part of a supplement supported by Fresenius Medical Care without any influence on its content.
CONFLICT OF INTEREST STATEMENT
J.C.Q.V has participated in consulting with Mallinckrodt Pharmaceuticals and Bayer, advisory boards with Mallinckrodt Pharmaceuticals and Retrophin and speakers bureau with Otsuka Pharmaceuticals.
REFERENCES
- 1. Zhu N, Zhang D, Wang W. et al. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China. N Engl J Med 2020; 382: 727–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farouk SS, Fiaccadori E, Cravedi P. et al. COVID-19 and the kidney: what we think we know so far and what we don’t. J Nephrol 2020; 33: 1213–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robbins-Juarez SY, Qian L, King KL. et al. Outcomes for patients with COVID-19 and acute kidney Injury: a systematic review and meta-analysis. Kidney Int Rep 2020; 5: 1149–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mohamed MM, Lukitsch I, Torres-Ortiz AE. et al. Acute kidney injury associated with coronavirus disease 2019 in urban New Orleans. Kidney360 2020; 1: 614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng Y, Luo R, Wang K. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020; 97: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaudhri I, Moffitt R, Taub E. et al. Association of proteinuria and hematuria with acute kidney injury and mortality in hospitalized patients with COVID-19. Kidney Blood Press Res 2020; 45: 1018–1032 [DOI] [PubMed] [Google Scholar]
- 7. Chan L, Chaudhary K, Saha A. et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 2021; 32: 151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fisher M, Neugarten J, Bellin E. et al. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol 2020; 31: 2145–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Santoriello D, Khairallah P, Bomback AS. et al. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol 2020; 31: 2158–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akilesh S, Nast CC, Yamashita M. et al. Multicenter clinicopathologic correlation of kidney biopsies performed in COVID-19 patients presenting with acute kidney injury or proteinuria. Am J Kidney Dis 2021; 77: 82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zarbock A, Gomez H, Kellum JA.. Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies. Curr Opin Crit Care 2014; 20: 588–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goyal P, Choi JJ, Pinheiro LC. et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020; 382: 2372–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guan WJ, Ni ZY, Hu Y. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen N, Zhou M, Dong X. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen G, Wu D, Guo W. et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130: 2620–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirsch JS, Ng JH, Ross DW. et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020; 98: 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sharma P, Uppal NN, Wanchoo R. et al. COVID-19-associated kidney injury: acase series of kidney biopsy findings. J Am Soc Nephrol 2020; 31: 1948–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van den Akker JP, Egal M, Groeneveld AB.. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care 2013; 17: R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Darmon M, Schortgen F, Leon R. et al. Impact of mild hypoxemia on renal function and renal resistive index during mechanical ventilation. Intensive Care Med 2009; 35: 1031–1038 [DOI] [PubMed] [Google Scholar]
- 20. Wu H, Larsen CP, Hernandez-Arroyo CF. et al. AKI and collapsing glomerulopathy associated with COVID-19 and APOL1 high-risk genotype. J Am Soc Nephrol 2020; 31: 1688–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peleg Y, Kudose S, D’Agati V. et al. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep 2020; 5: 940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Larsen CP, Bourne TD, Wilson JD. et al. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep 2020; 5: 935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Velez JCQ, Caza T, Larsen CP.. COVAN is the new HIVAN: the re-emergence of collapsing glomerulopathy with COVID-19. Nat Rev Nephrol 2020; 16: 565–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beckerman P, Bi-Karchin J, Park AS. et al. Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat Med 2017; 23: 429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamming I, Timens W, Bulthuis ML. et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203: 631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Velez JC, Bland AM, Arthur JM. et al. Characterization of renin-angiotensin system enzyme activities in cultured mouse podocytes. Am J Physiol Renal Physiol 2007; 293: F398–F407 [DOI] [PubMed] [Google Scholar]
- 27. Ye M, Wysocki J, William J. et al. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol 2006; 17: 3067–3075 [DOI] [PubMed] [Google Scholar]
- 28. Su H, Yang M, Wan C. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020; 98: 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farkash EA, Wilson AM, Jentzen JM.. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol 2020; 31: 1683–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Remmelink M, De Mendonça R, D’Haene N. et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care 2020; 24: 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Braun F, Lütgehetmann M, Pfefferle S. et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 2020; 396: 597–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lu R, Zhao X, Li J. et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395: 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kudose S, Batal I, Santoriello D. et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol 2020; 31: 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nguyen MT, Maynard SE, Kimmel PL.. Misapplications of commonly used kidney equations: renal physiology in practice. Clin J Am Soc Nephrol 2009; 4: 528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Polkinghorne KR. Detection and measurement of urinary protein. Curr Opin Nephrol Hypertens 2006; 15: 625–630 [DOI] [PubMed] [Google Scholar]
- 36. Ying T, Clayton P, Naresh C. et al. Predictive value of spot versus 24-hour measures of proteinuria for death, end-stage kidney disease or chronic kidney disease progression. BMC Nephrol 2018; 19: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gansevoort RT, Matsushita K, van der Velde M. et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80: 93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen CH, Wu HY, Wang CL. et al. Proteinuria as a therapeutic target in advanced chronic kidney disease: a retrospective multicenter cohort study. Sci Rep 2016; 6: 26539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwab SJ, Christensen RL, Dougherty K. et al. Quantitation of proteinuria by the use of protein-to-creatinine ratios in single urine samples. Arch Intern Med 1987; 147: 943–944 [PubMed] [Google Scholar]
- 40. Ginsberg JM, Chang BS, Matarese RA. et al. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med 1983; 309: 1543–1546 [DOI] [PubMed] [Google Scholar]
- 41. Houser M. Assessment of proteinuria using random urine samples. J Pediatr 1984; 104: 845–848 [DOI] [PubMed] [Google Scholar]
- 42. Price CP, Newall RG, Boyd JC.. Use of protein:creatinine ratio measurements on random urine samples for prediction of significant proteinuria: a systematic review. Clin Chem 2005; 51: 1577–1586 [DOI] [PubMed] [Google Scholar]
- 43. Pei G, Zhang Z, Peng J. et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 2020; 31: 1157–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gupta S, Coca SG, Chan L. et al. AKI treated with renal replacement therapy in critically Ill patients with COVID-19. J Am Soc Nephrol 2021; 32: 161–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Werion A, Belkhir L, Perrot M. et al. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int 2020; 98: 1296–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Varghese V, Mohamed M, Velez JCQ.. Incidence of new-onset proteinuria in AKI associated with COVID-19 is not greater than it is in AKI from other causes. J Am Soc Nephrol 2020; 31: TH-PO0673 [Google Scholar]
- 47. Li Z, Wu M, Yao J. et al. Caution on kidney dysfunctions of COVID-19 patients. medRxiv2020; doi: 10.1101/2020.02.08.20021212
- 48. Liu R, Ma Q, Han H. et al. The value of urine biochemical parameters in the prediction of the severity of coronavirus disease 2019. Clin Chem Lab Med 2020; 58: 1121–1124 [DOI] [PubMed] [Google Scholar]
- 49. Justine H, Antoine B, Laurence L. et al. Proteinuria in COVID-19: prevalence, characterization and prognostic role. J Nephrol2021; doi: 10.1007/s40620-020-00931-w [DOI] [PMC free article] [PubMed]
- 50. Lee JR, Silberzweig J, Akchurin O. et al. Characteristics of acute kidney injury in hospitalized COVID-19 patients in an urban academic medical center. Clin J Am Soc Nephrol 2020; 16: 284–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Portolés J, Marques M, López-Sánchez P. et al. Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol Dial Transplant 2020; 35: 1353–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Russo LM, Sandoval RM, McKee M. et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int 2007; 71: 504–513 [DOI] [PubMed] [Google Scholar]
- 53. D’mico G, Bazzi C.. Pathophysiology of proteinuria. Kidney Int 2003; 63: 809–825 [DOI] [PubMed] [Google Scholar]
- 54. Oken DE, Kirschbaum BB, Landwehr DM.. Micropuncture studies of the mechanisms of normal and pathologic albuminuria. Contrib Nephrol 1981; 24: 1–7 [DOI] [PubMed] [Google Scholar]
- 55. Tojo A, Endou H.. Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol 1992; 263: F601–F606 [DOI] [PubMed] [Google Scholar]
- 56. Osicka TM, Houlihan CA, Chan JG. et al. Albuminuria in patients with type 1 diabetes is directly linked to changes in the lysosome-mediated degradation of albumin during renal passage. Diabetes 2000; 49: 1579–1584 [DOI] [PubMed] [Google Scholar]
- 57. Park CH, Maack T.. Albumin absorption and catabolism by isolated perfused proximal convoluted tubules of the rabbit. J Clin Invest 1984; 73: 767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brunskill NJ, Nahorski S, Walls J. et al. Characteristics of albumin binding to opossum kidney cells and identification of potential receptors. Pflugers Arch 1997; 433: 497–504 [DOI] [PubMed] [Google Scholar]
- 59. Kerjaschki D, Farquhar MG.. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci USA 1982; 79: 5557–5561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Christensen EI, Birn H, Verroust P. et al. Megalin-mediated endocytosis in renal proximal tubule. Ren Fail 1998; 20: 191–199 [DOI] [PubMed] [Google Scholar]
- 61. Marinó M, Andrews D, Brown D. et al. Transcytosis of retinol-binding protein across renal proximal tubule cells after megalin (gp 330)-mediated endocytosis. J Am Soc Nephrol 2001; 12: 637–648 [DOI] [PubMed] [Google Scholar]
- 62. Eppel GA, Osicka TM, Pratt LM. et al. The return of glomerular-filtered albumin to the rat renal vein. Kidney Int 1999; 55: 1861–1870 [DOI] [PubMed] [Google Scholar]
- 63. Russo LM, Bakris GL, Comper WD.. Renal handling of albumin: a critical review of basic concepts and perspective. Am J Kidney Dis 2002; 39: 899–919 [DOI] [PubMed] [Google Scholar]
- 64. Christensen EI, Nielsen S.. Structural and functional features of protein handling in the kidney proximal tubule. Semin Nephrol 1991; 11: 414–439 [PubMed] [Google Scholar]
- 65. West BL, Picken MM, Leehey DJ.. Albuminuria in acute tubular necrosis. Nephrol Dial Transplant 2006; 21: 2953–2956 [DOI] [PubMed] [Google Scholar]
- 66. Greive KA, Balazs ND, Comper WD.. Protein fragments in urine have been considerably underestimated by various protein assays. Clin Chem 2001; 47: 1717–1719 [PubMed] [Google Scholar]
- 67. Ashworth SL, Molitoris BA.. Pathophysiology and functional significance of apical membrane disruption during ischemia. Curr Opin Nephrol Hypertens 1999; 8: 449–458 [DOI] [PubMed] [Google Scholar]
- 68. Siegel NJ, Devarajan P, Van Why S.. Renal cell injury: metabolic and structural alterations. Pediatr Res 1994; 36: 129–136 [DOI] [PubMed] [Google Scholar]
- 69. Basile DP, Anderson MD, Sutton TA.. Pathophysiology of acute kidney injury. Compr Physiol 2012; 2: 1303–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Agarwal R. On the nature of proteinuria with acute renal injury in patients with chronic kidney disease. Am J Physiol Renal Physiol 2005; 288: F265–F2671 [DOI] [PubMed] [Google Scholar]
- 71. D’Agati VD, Fogo AB, Bruijn JA. et al. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis 2004; 43: 368–382 [DOI] [PubMed] [Google Scholar]
- 72. Gaillard F, Ismael S, Sannier A. et al. Tubuloreticular inclusions in COVID-19-related collapsing glomerulopathy. Kidney Int 2020; 98: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Adembri C, Sgambati E, Vitali L. et al. Sepsis induces albuminuria and alterations in the glomerular filtration barrier: a morphofunctional study in the rat. Crit Care 2011; 15: R277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Axelsson J, Rippe A, Venturoli D. et al. Effects of early endotoxemia and dextran-induced anaphylaxis on the size selectivity of the glomerular filtration barrier in rats. Am J Physiol Renal Physiol 2009; 296: F242–F248 [DOI] [PubMed] [Google Scholar]
- 75. Sverrisson K, Axelsson J, Rippe A. et al. Acute reactive oxygen species (ROS)-dependent effects of IL-1β, TNF-α, and IL-6 on the glomerular filtration barrier (GFB) in vivo. Am J Physiol Renal Physiol 2015; 309: F800–F806 [DOI] [PubMed] [Google Scholar]