Abstract
Decades of research indicate that individuals exposed to childhood adversity are at risk for poor physical and mental health across their lifespan. More recently, intergenerational transmission of trauma and prenatal programming frameworks suggest an even longer reach for adverse childhood experiences (ACEs), with consequences that extend to subsequent generations. Beyond the individual-level consequences typically observed by empirical studies of ACEs, mothers’ experiences of early adversity may also compromise the maternal-child dyadic relationship. We propose a conceptual model whereby mothers’ ACEs impact maternal-infant dyadic functioning and later biobehavioral health outcomes through heightened perinatal psychosocial risk. We provide support for the proposed paths and mechanistic processes in our model with data drawn from Las Madres Nuevas, a longitudinal study of low-income Mexican-origin families who participated in a series of home and laboratory visits from the prenatal period through early childhood. Higher ACEs exposure among Las Madres Nuevas participants was associated with numerous perinatal psychosocial risk factors, which predicted poorer mother-infant dyadic functioning. Compromised dyadic functioning during infancy was associated with later maternal mental health and child behavior problems. We conclude with discussion of prevention and treatment strategies that can buffer against proposed risk pathways, including perinatal assessment of maternal ACEs and psychosocial risk, perinatal treatment of maternal distress, and mother-infant therapy in the postpartum period. It is our hope that the proposed conceptual model will serve as a guide for future research to examine the lasting consequences of childhood adversities within and across generations among high-risk populations.
Keywords: adverse childhood experiences, prenatal programming, intergenerational transmission of trauma, mother-child dyadic relationship, transactional
Since Felitti et al.’s (1998) groundbreaking study of Adverse Childhood Experiences (ACEs), research has consistently – and convincingly – documented negative physical and mental health consequences of such exposures across the lifespan. As originally conceptualized, ACEs refer to 10 categories of adversities across three domains (abuse, neglect, and household dysfunction) experienced prior to age 18 (Felitti et al., 1998). Within clinical settings, ACEs are commonly evaluated by a checklist (i.e., occurred/did not occur). However, the past two decades of empirical research have borne witness to significant variability in the definition and measurement of childhood adversity (McLaughlin, 2016). While some studies examine a count or “cumulative exposure” of adverse experiences, others focus on one specific form of adversity (e.g., child maltreatment) and conduct in-depth assessments of age of onset, severity, and chronicity of the exposure.
Beyond robust evidence of individual-level risks associated with ACEs, emerging research indicates that the impact may be intergenerationally transmitted from parents to offspring. Limited research has explored the potential for intergenerational transmission processes during the prenatal period, possibly due to the additional vulnerability of pregnant women (Schwerdtfeger & Nelson Goff, 2007). Yet, it is for this reason that empirical studies during this life stage are critical: The prenatal period can be a time of increased susceptibility to the development of mental health issues among women with a history of childhood adversity (O’Connor et al., 2019), which can have a lasting negative impact on maternal and child health.
Research conducted within a framework of prenatal programming is distinct from that of intergenerational transmission of trauma; however, both are tethered conceptually by the idea that the impact of mothers’ experiences of adversity may extend to offspring. The present paper proposes a conceptual model that integrates the intergenerational transmission of trauma and prenatal programming frameworks in order to understand the long-term legacy of maternal ACEs on mothers and their children (see Figure 1). Specifically, we theorize that maternal ACEs increase perinatal psychosocial risk, which in turn, is associated with disruptions to mother-infant coregulatory, dyadic functioning (ways in which mothers and their offspring regulate each other’s biological, emotional, and behavioral processes). Dyadic functioning is proposed to influence subsequent mother and child biobehavioral health, which affect each other through transactional relations over time. Our focus on mother-infant dyadic processes extends prior ACEs research that tends to focus on individual-level outcomes. This conceptual model may hold applicability to all women with a history of traumatic exposures and to these women’s offspring. However, exposure to childhood adversity is more frequent among underrepresented individuals with sociodemographic risk factors (e.g., racial/ethnic minority, low socioeconomic status; Merrick et al., 2018), and its consequences may be exacerbated among groups with few resources for amelioration or prevention of subsequent adversity. For illustrative purposes, we provide empirical support for the proposed pathways in the model, where available, using a sample at elevated sociodemographic risk: impoverished, Mexican-origin women and their children participating in a longitudinal study, Las Madres Nuevas.
Figure 1.
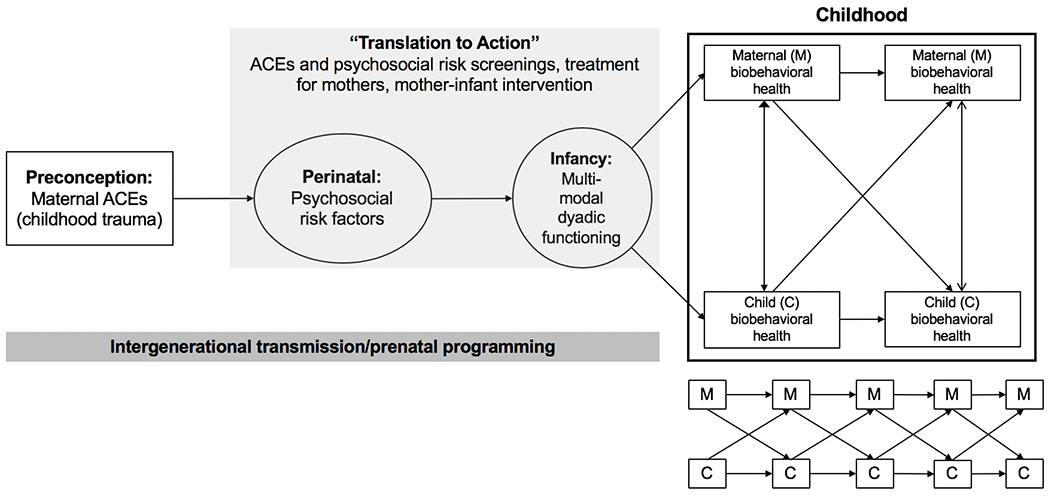
Conceptual model of the intergenerational transmission of maternal ACEs to offspring
The lasting consequences of maternal trauma exposure on offspring
Empirical support for intergenerational transmission of trauma, or the hypothesis that offspring are affected by parental trauma or stress exposure, has been largely demonstrated among populations that experienced war, genocide, and other forms of major life trauma (Bowers & Yehuda, 2016; Sangalang & Vang, 2016). Maternal childhood maltreatment and cumulative exposure to family-based, relational adversities can also increase risk for psychological problems among offspring (e.g., Fredlan et al., 2017). Theorists have proposed multiple indirect and direct mechanisms to account for the transmission of trauma consequences from parents to their offspring. Indirect social and emotional mechanisms include social learning (e.g., direct modeling of interpersonal and behavioral problems), as well as ineffective coping strategies, secondary traumatization through hearing stories of parental trauma, poor parental mental health, and maladaptive parental styles and family functioning (Bowers & Yehuda, 2016). For example, parents who experienced war-related trauma are more likely to adopt a role-reversing parental style with their children (i.e., parentification), leading to disrupted parent-child dyadic processes and poor child adjustment (Field et al., 2013). In addition to social and emotional consequences, a biological “scar” may persist after a traumatic experience has passed, reflected in epigenetic effects, changes to gametes, and alterations of the gestational uterine environment and fetoplacental interactions that have widespread downstream effects on offspring’s functioning (Bowers & Yehuda, 2016; Sosnowski et al., 2018; Yehuda & Lehrner, 2018).
Similar, but conceptually distinct from intergenerational transmission of trauma, the prenatal programming framework emphasizes that plasticity occurring during fetal development may allow for responsiveness to changing environmental conditions as experienced by the mother during pregnancy. Evolutionarily, such plasticity is thought to enhance offspring survival potential in the idiosyncratic environments into which offspring are born (Glover, 2011). This framework is frequently applied to explain the impact of significant stressors experienced by the mother during pregnancy on offspring risk of long-term physical and mental health problems. Evidence to support prenatal programming of risk has been demonstrated across a wide range of prenatal stressors (e.g., maternal psychological distress, acute or chronic environmental stressors) and offspring outcomes (e.g., depression, anxiety, Type 2 diabetes, obesity, cognitive and intellectual function; see review by Entringer et al., 2015).
Animal research and limited human studies further suggest that preconception stress can increase offspring risk of adverse birth outcomes, social behavior problems, affective behavior problems, autism spectrum disorder, and ADHD (Class et al., 2013, 2014; Li, 2010), with increased risk that extends into adulthood (Khashan et al., 2011). It is in this expansion of a prenatal programming framework to preconception stress that theoretical and empirical overlap with intergenerational transmission of trauma is readily apparent. Similar to research on intergenerational transmission of trauma, prenatal programming effects on offspring health and development function, in part, through epigenetic mechanisms and altered placental glucocorticoid exposure during fetal development of the hypothalamic-pituitary-adrenal (HPA) axis (Davis & Sandman, 2012). For example, prior trauma exposure may influence mothers’ psychological state during pregnancy, which may impact the gestational uterine environment and fetoplacental interactions (Cortessis et al., 2012). Preconception stress may also have direct effects on placental-fetal stress physiology (Moog et al., 2016). Parental preconception trauma may expose the fetus to higher levels of glucocorticoids, with implications for regulatory functioning of offspring (Yehuda & Lehrner, 2018).
A stressful prenatal environment, whether stemming from stress newly experienced during pregnancy or early childhood adversity, is likely to carry over into the postnatal environment, affecting maternal behavior in ways that negatively impact an offspring’s physical and mental health. For example, mothers’ experiences of childhood maltreatment may compromise her provision of postnatal care and disrupt development of children’s biological regulatory systems (Thompson-Booth et al., 2018). Similarly, maternal prenatal stress and depression that extend into the postpartum period can impair parenting behaviors and the quality of mother-infant interactions (Goodman & Gotlib, 1999), with implications for delayed cognitive, behavioral, and emotional development of offspring (Murray et al., 2010).
An overview of Las Madres Nuevas
We illustrate empirical support, where available, for the pathways linking maternal ACEs, perinatal risk, dyadic functioning, and maternal/infant biobehavioral health shown in Figure 1 with new statistical analyses and previously published findings from the ongoing longitudinal study, Las Madres Nuevas. The study follows 322 low-income Mexican-American mother-infant dyads from the prenatal period through 9 years of age. Mexican-origin women were recruited from prenatal clinics in Maricopa County, Arizona. Eligibility criteria included language fluency in Spanish or English, poverty-level income status, and an anticipated healthy, singlet birth (see Luecken et al., 2015 for full eligibility criteria). At study entry, women ranged in age from 18-42 years (mean 27.8, SD 6.5), 86% were born in Mexico, 41% graduated high school, and the median family income was $10,000-$15,000. Rates of self-reported ACEs are shown in Table 1.
Table 1:
Adverse Childhood Experiences (ACE) and Correlations with Prenatal Psychosocial Risk Factors
| Prenatal psychosocial risk factors | |||||||
|---|---|---|---|---|---|---|---|
| Negative Life Events2 | Economic hardship3 | Depressive symptoms4 | Family stress5 | Perceived stress6 | Daily hassles7 | Pregnancy hassles8 | |
| ACE1; Range = 0-5 M =1.2 (SD=1.2) 1 or more: 65.5% |
r = .31* | r =.21* | r =.35* | r =.40* | r =.24* | r =.27* | r =.21* |
p < .001
Maltreatment (scored above recommended cutoff for physical, sexual, or emotional abuse); N = 75 (23.3%)
Parent died; N = 16 (5%)
Parents divorced; N = 80 (24.8%)
Parent with mental health problem; N = 102 (31.7%)
Parent with drug/alcohol abuse; N = 92 (28.6%)
Parental abandonment; N = 16 (5%)
Left home before 16; N = 9 (2.8%)
Was in foster care; N = 2 (.6%)
Childhood Trauma Questionnaire (Bernstein, Ahluvalia, Pogge, & Handelsman, 1997) and Composite International Diagnostic Interview (CIDI) Childhood items (Kessler & Ustün, 2004).
PRAMS Negative Life Events; Centers for Disease Control and Prevention (2014) Pregnancy Risk Assessment Monitoring System; www.cdc.gov/PRAMS
Economic Hardship Scale; Barrera, M., Caples, H., & Tein, J. (2001). The psychological sense of economic hardship: Measurement models, validity, and cross-ethnic equivalence for urban families. American Journal of Community Psychology, 29, 493-517
EPDS; Cox, J.L., Holden, J.M., & Sagovsky, R. (1987) Detection of postnatal depression. British Journal of Psychiatry, 150, 782-786
Family Negative Support Scale; Turner, R.J.,& Marino, F. (1994) Social support and social structure: a descriptive epidemiology. Journal of Health and Social Behavior, 35,193–212.
Perceived Stress Scale; Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385-396.
Parenting Daily Hassles Scale; Crnic, K.A., & Greenberg, M.T. (1990). Minor parenting stresses in young children. Child Development, 61, 1628-1637
The Pregnancy Experience Scale; DiPietro, J.A., Christensen, A.L., & Costigan, K.A. (2008). The Pregnancy Experience Scale – Brief Version. Journal of Psychosomatic Obstetrics and Gynaecology, 29, 262-267
Participation in Las Madres Nuevas included five home visits (prenatal, 6 weeks, 12 weeks, 18 weeks, and 24 weeks postpartum), and lab visits at ages 1 year, 1.5 years, 2 years, 3 years, 4.5 years, and 6 years (with ongoing data collection at 7.5 and 9 years). A host of variables were assessed at these visits, including survey measures of maternal ACEs, prenatal and postpartum stress, depression, and economic hardship; biological measures of mother and infant physiological functioning, including respiratory sinus arrhythmia (RSA) and cortisol; observational measures of mother-child interaction and child self-regulation; and maternal report of child developmental competencies and behavior problems.
Intergenerational transmission of trauma and prenatal programming: An integrated conceptual model
Pathway from maternal ACEs to perinatal psychosocial risk
The intergenerational transmission of trauma framework suggests that although ACEs are specific to the childhood and adolescent period, their legacy is not. Stressful experiences during childhood may act as catalysts for subsequent traumatic and stressful exposures that further deplete the resources of vulnerable individuals (Nurius, Green, Logan-Green, & Borja, 2015). Although the impending birth of a child may be a joyous time, it can also be one of heightened stress. Pregnant women who have witnessed or experienced interpersonal traumas within their families of origin are at risk of experiencing elevated distress given memories of their own painful childhoods (Schwerdtfeger & Nelson Goff, 2007). In the study Las Madres Nuevas, women across the sample reported a high frequency of negative life events during pregnancy (e.g., partner’s loss of a job [34%], death of someone close [23%] and homelessness [9%]). Compared to women with higher socioeconomic status in the majority culture, low-income, immigrant, and ethnic minority women experience more frequent and severe stressors and also encounter qualitatively different exposures. Illustratively, women in Las Madres Nuevas frequently endorsed culturally relevant stressors during pregnancy (e.g., separated from family due to money or immigration issues; frequent arguments with family due to different customs).
As shown in Table 1, Las Madres Nuevas participants with high exposure to ACEs experience a multitude of major and minor prenatal psychosocial risk factors. The impact of childhood adversity may persist into the postpartum period; in our sample, maternal ACEs are also associated with higher levels of depressive symptoms, more daily hassles, less partner support, and poorer maternal sleep following childbirth (Ciciolla et al., 2013; Gress-Smith et al., 2012; Luecken et al., 2016).
Pathways from maternal ACEs and perinatal psychosocial risk to dyadic functioning and maternal/child health
The chain of women’s stress exposure that begins in childhood and persists into the perinatal period can have lasting negative consequences for mothers and their children. Evidence from Las Madres Nuevas consistently associates prenatal maternal distress to individual-level maternal and child biobehavioral health. For example, even after controlling for postnatal stress, mothers who report more prenatal stress have offspring who demonstrate more temperamental negativity in infancy and more behavior problems at 18 months (Lin et al., 2017). Higher prenatal stress also predicts elevated maternal postpartum depressive (PPD) symptoms (Coburn et al., 2016). A particularly novel component of our model, however, is its focus on maternal-child dyadic functioning as a key intermediate mechanism linking maternal ACEs and perinatal psychosocial risk to later maternal and child outcomes.
Dyadic processes are conditional and bidirectional, capturing the ways in which mothers respond to the signals of their children and how children, in turn, respond to the signals of their mother (Bronfenbrenner & Morris, 1998). Mutual regulatory processes establish the dyad as its own measurable regulatory system, distinct from the regulation (or dysregulation) of the individuals who comprise it (Tronick, 2007). Dyadic measures provide predictive information above and beyond what is captured by individual mother and child measures (i.e., Lunkenheimer et al., 2013). For example, models of preschool children’s self-regulation improved when dyadic measures of parent-child contingencies supplemented measures of maternal and child behavior (Lunkenheimer et al., 2013). Global coding systems with dyadic components and microcoded timeseries analyses are common methods for indexing the degree of affective synchrony and reciprocal exchange between mother and child, the rigidity in interactions, and the smoothness of behavioral and affective transitions (i.e., Feldman, 2010). Dyadic functioning can be coded as parents and offspring engage in standardized, laboratory-based tasks or unstructured play. Tasks that include a challenge can capture the dyad’s ability to navigate through difficult situations and recover from task-induced frustration.
The importance of adaptive dyadic functioning for mothers and offspring cannot be overstated. Children’s biobehavioral regulatory capacities are predominantly “other-regulated” during the early years of life, as children rely on the support of primary caregivers to mount responses to environmental challenges (Perry et al., 2012). Dysregulated dyadic interactions are detrimental to the development of children’s self-regulatory strategies, with corresponding risk for physiological dysregulation (Feldman, 2007) and behavior problems (Lunkenheimer et al., 2013). Mothers are also at risk; dyadic dysregulation has been associated with indicators of maternal chronic physiological stress (Tarullo et al., 2017) and poor postpartum adjustment (Luecken et al., 2019). Moreover, the quality of dyadic functioning that begins in the postpartum may persist through early adolescence (Feldman, 2010), suggesting long-term adverse consequences of poor dyadic functioning for both mothers and their offspring.
To measure dyadic functioning in Las Madres Nuevas, mothers and infants were observed during home visits as they engaged in a structured interaction protocol that included free play, frustration, soothing, teaching, and “peek-a-boo” episodes, and during laboratory visits as they engaged in free play, clean-up, blowing bubbles together, and teaching tasks intended to illicit mild frustration in both members of the dyad. These observational episodes were coded for dyadic reciprocity and dyadic dysregulation. Dyadic reciprocity was coded during free play using the Coding Interactive Behavior rating system (Feldman, 1998). The dyadic interaction was considered highly reciprocal if mother and child were engaging in “give-and-take” play, meaning they both attended and responded to each other’s cues. The dyadic interaction was not considered reciprocal if the mother-child exchanges were disjointed and at least one member was withdrawn or overriding the other. Dyadic dysregulation was coded using a standardized coding system that reflected the extent to which mother-infant pairs were able to modulate their collective emotional, attentional, and behavioral arousal (adapted from Hoffman et al., 2006). Dyads unsuccessful at modulating their collective arousal, evidenced by frequent, intense, labile emotional swings, were considered highly dysregulated. Dyads with minimal dysregulation were able to negotiate through their collective arousal and recover quickly.
Among Las Madres Nuevas participants, the detrimental influence of maternal ACEs on dyadic reciprocity operates via an indirect pathway of elevated perinatal psychosocial risk. As shown in Table 1, maternal reports of ACEs predict perinatal psychosocial risk. A composite measure of perinatal psychosocial risk predicts lower dyadic reciprocity at 24 weeks postpartum (p = .014; Curci et al., 2020). Similarly, prenatal negative life events predicted dyadic dysregulation at 12 weeks; dyadic dysregulation, in turn, was associated with higher infant cortisol and elevated maternal PPD symptoms (Luecken et al., 2019). It is important to evaluate these findings within extant empirical research on prenatal programming: Prenatal stress has emerged as a robust predictor of individual-level outcomes, including infant hypothalamic-pituitary-adrenal (HPA) axis functioning (Glover et al., 2010; Luecken et al., 2015). Results from Las Madres Nuevas extend this work by identifying mother-infant dyadic dysregulation as an important (and potentially modifiable) mechanism through which prenatal risk may affect later maternal and infant well-being.
Our research has observed consequences of maternal prenatal adversity on dyadic functioning beyond the infancy period. In Las Madres Nuevas, prenatal risk factors, including maternal prenatal depressive symptoms and family economic stress, predict lower dyadic reciprocity at 1 and 2 years, which predicts poorer child self-regulation at 3 years, with persistent effects at 4.5 years (Curci et al., 2020). Similarly, dyadic dysregulation at 2 years predicts more child behavior problems and maternal depressive symptoms at 36 months, above and beyond individual-level risk factors that are associated with children’s developmental outcomes and maternal mental health (Winstone et al., 2020). The negative consequences of dyadic dysregulation on children’s behavioral problems may emerge due to children’s reduced exposure to parental models of adaptive regulatory strategies. Mothers may also experience heightened distress and less parenting self-efficacy when interactions with their child are lower quality. This may be particularly true in the context of Mexican cultural values in which the maternal role and positive, supportive familial relations are highly valued (Gress-Smith et al. 2013).
Maternal-infant dyadic functioning encompasses a complex set of processes that benefit from an advanced analytic approach. Dynamic systems methods offer a complementary perspective to the previously described observational methods by exploring how the dyad functions as a unit in synchronous/asynchronous or stable/unstable ways, as opposed to what the dyad is doing (Feldman, 2007). In an application of dynamic systems methods to Las Madres Nuevas data, Coburn, Crnic, and Ross (2015) coded mothers and their 12-week-old infants during a mildly frustrating teaching task. Microanalytic coding (Tronick & Weinberg, 1990) and global behavior rating scales (Feldman, 1998) captured dynamic dyadic behavior, including states in which mother-infant pairs displayed mutual, co-regulated engagement in a positive interaction and states in which one or both members became negative, reflecting co-dysregulation. Dyads with mothers who reported more prenatal depressive symptoms were characterized by shorter durations in positive, mutually engaged states and longer durations in negative, co-dysregulated states. Results persisted after adjusting for PPD symptoms, suggesting that the dyadic relationship is uniquely sensitive to prenatal distress (Coburn et al., 2015).
Transactional relations between maternal and infant biobehavioral health
As illustrated by our conceptual model, dyadic functioning affects subsequent maternal and child biobehavioral health. However, rather than “endpoints” in our model, maternal and child biobehavioral outcomes are more appropriately conceptualized as components of a transactional process through which mothers and their offspring influence each other over time. Although child-directed effects on parental functioning have a long theoretical history, models of parent-directed effects on child functioning continue to predominate in empirical literature (Paschall & Mastergeorge, 2016). Similarly, within intergenerational transmission of trauma and prenatal programming frameworks, parent functioning typically predicts child outcomes with little exploration of how child characteristics affect parent functioning.
Extant research on transactional relations typically focuses on the ways in which parenting affects child behavior and how child behavior, in turn, elicits varied parenting behaviors. For example, Patterson’s coercive model describes a process whereby harsh parenting styles contribute to children’s oppositional behaviors (Patterson, 1982). Parents’ attention to children’s defiance serves as inadvertent reinforcement that increases the frequency of deviant child behavior, leading to increased parental frustration and harsh practices (Patterson, 2002). Evidence from Las Madres Nuevas suggests several other examples of transactional relations. Higher maternal prenatal depressive symptoms, for example, predict elevated cortisol among infants (Luecken et al., 2015) and elevated infant cortisol predicts higher subsequent maternal PPD symptoms (Luecken et al., 2019). Mothers’ depressive symptoms may be sensitive to other biobehavioral indicators of infant functioning, including RSA (Somers et al., 2019) and temperamental negativity (Luecken et al., 2015). In another ethnically diverse, economically disadvantaged sample, Roubinov, Epel, Adler, Laraia, & Bush (2019) observed cross-lagged associations between maternal and child mental health from toddlerhood through preschool. Higher maternal depressive symptoms during toddlerhood were associated with more internalizing symptoms among preschool age children. In a parallel fashion, higher toddler internalizing symptoms were associated with increases in maternal depressive symptoms when offspring were preschool-age. Similar studies of reciprocal relations between maternal depression and child socioemotional problems suggest that child effects on parent mental health may be stronger than parent effects on child mental health (Baker et al., 2019).
In the preceding sections, we reported findings from Las Madres Nuevas (and to a lesser degree, related research from similar populations) to illustrate empirical support for our conceptual model. However, due to the complex and expansive longitudinal framework of our model, such support is largely derived through tests of discrete paths. For example, we provide evidence of the association of maternal ACEs to perinatal psychosocial risk and from perinatal psychosocial risk to dyadic functioning; however, comprehensive tests of mediation remain an important direction for future research. Similarly, we describe findings of the relation between dyadic functioning and later maternal or child outcomes; effects of dyadic indicators on cross-lagged or transactional relations between mothers and infants remain to be tested.
Translation to Action
Our conceptual model is not intended to imply that psychosocial risk is deterministic; we acknowledge extensive empirical evidence of resilient individuals who do not develop clinical levels of psychopathology even in the context of significant risk. Although the forthcoming section focuses on formal efforts to prevent and/or ameliorate the consequences of ACEs, credence must be given to other factors that facilitate a natural desistence from potentially maladaptive pathways. For example, resilience may be fostered through religious or spiritual resources, connection with cultural traditions, or creation of community-based social support networks (Lehrner & Yehuda, 2018). Longitudinal studies suggest secure attachment and high-quality parent-child relationships are among the most potent predictors of long-term resilience following childhood adversity exposure (Werner, 2005). Notably, resilience often develops in a continuous, cascading manner across the lifespan, with resilience-promoting resources early in life (e.g., positive parenting) begetting protective factors in adolescence and adulthood (e.g., academic competence, self-esteem; Luecken & Gress, 2009; Masten et al., 1999;).
Preventive interventions can contribute to the development of resilience following childhood adversity. For mothers at risk, our conceptual model suggests the importance of preventive interventions during the perinatal period. Practitioners, researchers, and policy makers promote early intervention during childhood as a “best-practice” in the prevention and treatment of ACEs (Burke Harris et al., 2017). We propose the following for all families, particularly those at higher sociodemographic risk: (1) assessment of maternal ACEs and psychosocial risk during the perinatal period, (2) treatment of maternal distress during the perinatal period, and (3) mother-infant therapy in the early postpartum period to promote high quality dyadic interactions.
Assessment of maternal ACEs and psychosocial risk during the perinatal period
Screening pregnant women for ACEs is the recommended standard in prenatal care (Kilpatrick et al., 2017). Given the regularity of prenatal care visits, obstetricians are well positioned to implement ACEs screening and identify families at risk for intergenerational transmission of adversity. A recent study suggests that the inclusion of ACEs screening during prenatal care visits is feasible and acceptable, and such visits are often the first opportunity for disclosure to a healthcare provider (Flanagan et al., 2018). Group prenatal care models (i.e., small groups of women with similar due dates; Ickovics et al., 2007), have also been proposed as a platform through which to conduct risk screenings and provide education and social support to women as they prepare for motherhood (American College of Obstetricians and Gynecologists, 2018). Clinician discomfort with ACEs questioning has been identified as a barrier to screening, but providing education about how to inquire sensitively and respond to disclosure of ACEs can facilitate screening procedures (Flanagan et al., 2018). Clinician training is especially important given that women with a history of ACEs, particularly sexual abuse, may feel uncomfortable with aspects of prenatal care such as vaginal examinations (Leeners et al., 2006). The California Department of Health Care Services (2020) recently created the first-in-nation statewide effort to screen children for ACEs during healthcare visits. Future policy efforts may also consider screening parents regarding their own ACEs.
Screening for perinatal psychosocial risk factors is also warranted, particularly among low-income, ethnic minority women. The American College of Obstetricians and Gynecologists recommends that obstetric care providers screen women for depression and anxiety symptoms at least once during pregnancy (ACOG, 2018). An additional period of unmet maternal healthcare needs occurs during the first three months following birth, or the “fourth trimester” (Tully et al., 2017). Similar recommendations have been set forth by the American Academy of Pediatrics for routine maternal PPD screenings during well-child visits (Earls et al., 2019). For women of color, experiences of discrimination during pregnancy have been associated with PPD symptoms, higher pregnancy distress, and lower birth weight (Earnshaw et al., 2013), yet prenatal assessments of discrimination are rare (Dominguez et al,, 2008). Among Las Madres Nuevas participants, stressors related to discrimination and immigration during pregnancy predicted more PPD symptoms (Coburn et al., 2016). Given low rates of prenatal care among low-income, ethnic minority women and the scarcity of culturally valid assessment tools, early screening and referral opportunities may be limited among those at highest risk (Luecken et al., 2009; Tully et al., 2017). For these women, screening after childbirth or during the early postpartum period may be an alternative opportunity to identify mothers with significant ACEs and/or perinatal distress.
Treatment of maternal distress during the perinatal period
Treatment of maternal distress is critical to promoting maternal and child health in the perinatal period and beyond, particularly for mothers with elevated ACEs scores (Ångerud, et al., 2018). The U.S. Preventive Services Task Force’s recent meta-analysis found counseling-based interventions (primarily cognitive behavioral therapy [CBT] and interpersonal therapy [IPT]) to be effective in preventing perinatal depression (O’Connor et al., 2019). Perinatal depression interventions from a CBT or IPT framework have been associated with lower incidence of clinical levels of PPD symptoms (Tandon et al., 2011) and lower maternal cortisol levels (Urizar & Muñoz, 2011). Mindfulness-based interventions may also reduce maternal depression, anxiety, and stress during the perinatal period (Taylor et al., 2016). Within a high-risk sample of low-income, ethnic minority women, a prenatal mindfulness intervention was associated with lower risk of maternal depressive symptoms through 18 months postpartum (Felder et al., 2017) and with reduced healthcare utilization for infants of moderately depressed women (Roubinov et al., 2018). Lasting effects of perinatal mental health counseling-based interventions have been reported on other offspring outcomes, including lower infant cortisol (Urizar & Muñoz, 2011), better parent-child attachment (Handley et al., 2017), and improved infant temperament (Chan, 2014). A recent systematic review found evidence to suggest that prenatal depression interventions may improve child functioning, particularly with respect to reduced child dysregulation (Goodman et al., 2018). Preliminary evidence suggests large-scale health system interventions (e.g., universally implemented interventions; Brugha et al., 2011), physical activity interventions (Perales et al., 2015) and peer counseling (Dennis, 2003) may also promote positive maternal and child outcomes.
Additional research is needed to understand the impact of interventions that target potential protective factors during pregnancy. Postpartum depression affects (and is affected by) women’s social and familial support (Letourneau et al., 2012), but women with histories of childhood abuse may experience lower support or a continuation of abuse during the perinatal period (Jackson et al., 2015; Luecken et al., 2016). Promoting mother’s social support is a core component of optimal postpartum care (ACOG, 2018). Home visiting programs (Sweet & Appelbaum, 2004) and family therapy (Cluxton-Keller & Bruce, 2018) may be effective strategies to increase social support among new mothers. Family support may be particularly relevant to Mexican-origin women in the United States who subscribe to traditional Mexican cultural values such as familismo and marianismo that emphasize the importance of the family and the expectation that women will be self-sacrificial mothers (Castillo et al., 2010). Among women in Las Madres Nuevas, higher prenatal expectations for partner and family support in the postpartum period were associated with fewer prenatal depressive symptoms (Gress-Smith et al., 2013). Somers and colleagues (2019) demonstrated the health-promotive effects of maternal social support during the postpartum period on later infant behavioral problems among Las Madres Nuevas families.
Mother-infant dyadic therapy in the early postpartum period
Individual treatment of maternal mental health during the perinatal period may not be sufficient in promoting healthy mother-child interactions (Forman et al., 2007). Relationship-based, dyadic interventions have the potential to improve maternal and child health but are implemented less frequently in the early postpartum period. Infant or Child-Parent Psychotherapy (IPP or CPP; infancy up to 5 years of age), Parent-Child Interaction Therapy (PCIT; 2 through 12 years of age), and Attachment and Biobehavioral Catch-up (ABC; 6 to 48 months of age) are among the most empirically-supported and widely-disseminated dyadic interventions that promote secure mother-child attachment, regulate mother-child interactions, reduce children’s behavioral problems, and reduce maternal stress (Cicchetti et al., 2006; Grube & Liming, 2018; Lieberman et al., 2005). Of these interventions, only CPP addresses mothers’ ACEs and history of trauma (Lieberman et al., 2015). Dyadic processes are likely at the heart of documented bidirectional relations among maternal and child mental health that emerge early in development (Baker et al., 2019) such that even when one partner in the dyad is the primary target of treatment, the other may benefit. For example, when children’s depressive symptoms were the primary target of intervention, a dyadic parent-child psychotherapy intervention reduced child depressive symptoms, parenting stress, and parental depressive symptoms (Luby et al., 2018).
Conclusions
Converging evidence across neurobiological, psychological, and health policy research underscores the necessity of efforts to ameliorate the long-term negative consequences of adverse exposures during childhood. The consequences of ACEs are not limited to the exposed individual but extend to subsequent generations and parent-offspring dyadic functioning. In the current paper, we integrate the intergenerational transmission of trauma and prenatal programming frameworks to propose a model whereby mothers’ ACEs compromise maternal-infant dyadic functioning through elevations in perinatal psychosocial risk. Dysregulation within the dyad, in turn, has implications for maladaptive maternal and offspring biobehavioral health. Commensurate with the complex pathways through which ACEs exert their effects, our model spans multiple developmental periods, individual and dyadic levels, and biological and behavioral outcomes. However, in order to discuss such components with appropriate depth, additional contributing factors were not modeled; future research should consider genetic/epigenetic factors, varied maternal and paternal parenting styles, and other sociocultural influences.
By uncovering various intra- and inter-individual mechanisms of risk, we highlight opportunities for prevention and intervention that include (individual-level) ACEs screening recommendations and (individual-, dyadic- and family-level) interventions. Although empirical support for our model was primarily drawn from a longitudinal study of low-income Mexican-origin women and their children, we believe the imperative to address these issues applies across cultural and socioeconomic boundaries. It is our hope that future research will use this conceptual model as a guide for research among other populations, with consideration of a broader range of risk and protective factors that may uniquely shape the lasting implications of maternal ACEs. By rising to the challenge of developing comprehensive and holistic models of the implications of early adverse exposures, we may be better positioned to implement robust, empirically-supported interventions for at-risk populations.
Public Significance Statement:
The current paper integrates the intergenerational transmission of trauma and prenatal programming frameworks to understand how maternal adverse childhood experiences (ACEs) affect mothers and their children. Our results suggest maternal ACEs may compromise the mother-child dyadic relationship, with negative consequences on the health of mothers and their children. We highlight perinatal prevention and intervention efforts as particularly important to reduce the long-term deleterious effects of ACEs within and across generations.
Footnotes
Conflicts of Interest and Source of Funding: The authors have no conflicts of interest to report. The study was funded by the National Institute of Mental Health (R01 MH083173-01 and R01 MH083173-01A1S). The first author is supported by NIMH 5K23MH113709. The fourth author is supported by a Graduate Research Fellowship from the National Science Foundation (Fellow ID: 2016228976).
References
- American College of Obstetricians and Gynecologists. (2018). ACOG committee opinion no. 736: optimizing postpartum care. Obstetrics and Gynecology, 131(5), e140–e150. [DOI] [PubMed] [Google Scholar]
- Ångerud K, Annerbäck EM, Tydén T, Boddeti S, & Kristiansson P (2018). Adverse childhood experiences and depressive symptomatology among pregnant women. Acta Obstetricia et Gynecologica Scandinavica, 97(6), 701–708. [DOI] [PubMed] [Google Scholar]
- Baker CE, Brooks-Gunn J, & Gouskova N (2019). Reciprocal relations between maternal depression and child behavior problems in families served by Head Start. Child Development. [DOI] [PubMed] [Google Scholar]
- Bowers M, Yehuda R (2016). Intergenerational transmission of stress in humans. Neuropsychopharmacology, 41, 232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U, & Morris PA (1998). The ecology of developmental processes. In Damon W & Lerner RM (Eds.), Handbook of child psychology: Theoretical models of human development (p. 993–1028). John Wiley & Sons Inc. [Google Scholar]
- Brugha TS, Morrell CJ, Slade P, & Walters SJ (2011). Universal prevention of depression in women postnatally: cluster randomized trial evidence in primary care. Psychological Medicine, 41(4), 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Department of Healthcare Services (2020, February 26). California launches “ACEs Aware” Initiative to address the public health crisis of toxic stress from childhood trauma. Retrieved from: https://www.dhcs.ca.gov/Documents/ACEs-AWARE-INITIATIVE.pdf
- Castillo LG, Perez FV, Castillo R, & Ghosheh MR (2010). Construction and initial validation of the Marianismo Beliefs Scale. Counselling Psychology Quarterly, 23(2), 163–175. [Google Scholar]
- Chan KP (2014). Prenatal meditation influences infant behaviors. Infant Behavior and Development, 37(4), 556–561. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, & Toth SL (2006). Fostering secure attachment in infants in maltreating families through preventive interventions. Development and Psychopathology, 18(3), 623–649. [DOI] [PubMed] [Google Scholar]
- Ciciolla L, Panza K, Crnic K, Luecken L, & Gonzales N (April, 2013). History of childhood trauma and postpartum functioning: Affective, biological, and psychosocial associations. Poster presented at the Society for Research in Child Development Biennial Meeting, Seattle, WA. [Google Scholar]
- Class QA, Abel KM, Khashan AS, Rickert ME, Dalman C, Larsson H & D’Onofrio BM (2014). Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychological Medicine, 44(1), 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Khashan AS, Lichtenstein P, Långström N, & D’Onofrio BM (2013). Maternal stress and infant mortality: the importance of the preconception period. Psychological Science, 24(7), 1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluxton-Keller F, & Bruce ML (2018). Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. Clinical Child and Family Psychology Review, 18(4), 395–412. [DOI] [PubMed] [Google Scholar]
- Coburn SS, Gonzales NA, Luecken LJ, & Crnic KA (2016). Multiple domains of stress predict postpartum depressive symptoms in low-income Mexican American women: the moderating effect of social support. Archives of Women’s Mental Health, 19(6), 1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn SS, Crnic KA, & Ross EK (2015). Mother-infant dyadic state behavior: Dynamic systems in the context of risk. Infant and Child Development, 24, 274–297. [Google Scholar]
- Cortessis VK, Thomas DC, Levine AJ, Breton CV, Mack TM, Siegmund KD, Haile RW, & Laird PW (2012). Environmental epigenetics: prospects for studying epigenetic mediation of exposure–response relationships. Human Genetics, 131, 1565–1589. 10.1007/s00439-012-1189-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curci SG, Winstone LK, Crnic K, Ross EK, van Huisstede L, & Luecken LJ (2020). A developmental and contextual approach to the emergence of self-regulation among low-income, Mexican American children. Poster presentation accepted to the International Congress for Infant Studies, Glasgow, Scotland. [Google Scholar]
- Davis EP & Sandman CA (2012). Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology, 37(8), 1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis CL (2003). The effect of peer support on postpartum depression: a pilot randomized controlled trial. The Canadian Journal of Psychiatry, 48(2), 115–124. [DOI] [PubMed] [Google Scholar]
- Dominguez TP, Dunkel-Schetter C, Glynn LM, Hobel C, & Sandman CA (2008). Racial differences in birth outcomes: the role of general, pregnancy, and racism stress. Health Psychology, 27(2), 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls MF, & Committee on Psychosocial Aspects of Child and Family Health. (2010). Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics, 126(5), 1032–1039. [DOI] [PubMed] [Google Scholar]
- Earnshaw VA, Rosenthal L, Lewis JB, Stasko EC, Tobin JN, Lewis TT, & Ickovics JR (2013). Maternal experiences with everyday discrimination and infant birth weight: A test of mediators and moderators among young, urban women of color. Annals of Behavioral Medicine, 45(1), 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, & Wadhwa PD (2015). Prenatal stress, development, health and disease risk: A psychobiological perspective – 2015 Curt Richter Award Paper. Psychoneuroendocrinology, 62, 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder JN, Roubinov D, Bush NR, Coleman-Phox K, Vieten C, Laraia B, & Epel E (2018). Effect of prenatal mindfulness training on depressive symptom severity through 18-months postpartum: A latent profile analysis. Journal of Clinical Psychology, 74(7), 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R (2010). The relational basis of adolescent adjustment: trajectories of mother–child interactive behaviors from infancy to adolescence shape adolescents’ adaptation. Attachment & Human Development, 12(1–2), 173–192. [DOI] [PubMed] [Google Scholar]
- Feldman R, 2007. Parent–Infant synchrony: Biological foundations and developmental outcomes. Current Directions in Psychological Science 16(6), 340–345. [Google Scholar]
- Feldman R (1998). Coding interactive behavior (CIB). Ramat-Gan: Bar-Ilan University. [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, … Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Field NP, Muong S, & Sochanvimean V (2013). Parental styles in the intergenerational transmission of trauma stemming from the Khmer Rouge regime in Cambodia. American Journal of Orthopsychiatry, 83(4), 483–494. [DOI] [PubMed] [Google Scholar]
- Flanagan T, Alabaster A, McCaw B, Stoller N, Watson C, & Young-Wolff KC (2018). Feasibility and acceptability of screening for adverse childhood experiences in prenatal care. Journal of Women’s Health, 27(7), 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman DR, O’Hara MW, Stuart S, Gorman LL, Larsen KE, & Coy KC (2007). Effective treatment for postpartum depression is not sufficient to improve the developing mother–child relationship. Development and Psychopathology, 19(2), 585–602. [DOI] [PubMed] [Google Scholar]
- Fredlan N, McFarlane J, Symes L, & Maddoux J (2017). Exploring the association between maternal adverse childhood experiences with maternal health and child behavior following intimate partner violence. Journal of Women’s Health, 27(1), 64–71. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Cullum KA, Dimidjian S, River LM, & Kim CY (2018). Opening windows of opportunities: Evidence for interventions to prevent or treat depression in pregnant women being associated with changes in offspring’s developmental trajectories of psychopathology risk. Development and Psychopathology, 30(3), 1179–1196. [DOI] [PubMed] [Google Scholar]
- Gress-Smith Tanaka, R. Luecken LJ, Crnic K, & Gonzales N (2012). The effects of postpartum depression and childhood adversity on maternal and infant sleep in low-income Mexican American families. Paper presented at the International Conference on Infant Studies, Minneapolis, MN. [Google Scholar]
- Glover V, O’Connor TG, & O’Donnell K (2010). Prenatal stress and the programming of the HPA axis. Neuroscience & Biobehavioral Reviews, 35(1), 17–22. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, & Spencer HG (2005). Predictive adaptive responses and human evolution. Trends in Ecology & Evolution, 20(10), 527–533. [DOI] [PubMed] [Google Scholar]
- Goodman SH & Gotlib IH (1999). Risk for psychopathology in the children of depressedmothers: A developmental model for understanding mechanisms of transmission. Psychological Review, 106(3), 458–490. [DOI] [PubMed] [Google Scholar]
- Gress-Smith JL, Roubinov DS, Tanaka R, Crnic K, Gonzales N, Enders C, & Luecken LJ (2013). Prenatal expectations in Mexican American women: development of a culturally sensitive measure. Archives of Women’s Mental Health, 16(4), 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube WA, & Liming KW (2018). Attachment and Biobehavioral Catch-up: A systematic review. Infant Mental Health Journal, 39(6), 656–673. [DOI] [PubMed] [Google Scholar]
- Handley ED, Michl-Petzing LC, Rogosch FA, Cicchetti D, & Toth SL (2017). Developmental cascade effects of interpersonal psychotherapy for depressed mothers: Longitudinal associations with toddler attachment, temperament, and maternal parenting efficacy. Development and Psychopathology, 29(2), 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NB, Marques SS, Oh D, Bucci M, & Cloutier M (2017). Prevent, screen, heal: collective action to fight the toxic effects of early life adversity. Academic Pediatrics, 17(7), S14–S15. [DOI] [PubMed] [Google Scholar]
- Hoffman C, Crnic KA, & Baker JK (2006). Maternal depression and parenting: Implications for children’s emergent emotion regulation and behavioral functioning. Parenting: Science and Practice, 6(4), 271–295. [Google Scholar]
- Ickovics JR, Kershaw TS, Westdahl C, Magriples U, Massey Z, Reynolds H, & Rising SS (2007). Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstetrics and Gynecology, 110(2 Pt 1), 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CL, Ciciolla L, Crnic KA, Luecken LJ, Gonzales NA, & Coonrod DV (2015). Intimate partner violence before and during pregnancy: Related demographic and psychosocial factors and postpartum depressive symptoms among Mexican American women. Journal of Interpersonal Violence, 30, 659–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khashan AS, McNamee R, Henriksen TB, Pedersen MG, Kenny LC, Abel KM, & Mortensen PB (2011). Risk of affective disorders following prenatal exposure to severe life events: a Danish population-based cohort study. Journal of Psychiatric Research, 45(7), 879–885. [DOI] [PubMed] [Google Scholar]
- Kilpatrick SJ, Papile LA, & Macones GA (2017). Guidelines for perinatal care. American Academy of Pediatrics. [Google Scholar]
- Leeners B, Richter-Appelt H, Imthurn B, & Rath W (2006). Influence of childhood sexual abuse on pregnancy, delivery, and the early postpartum period in adult women. Journal of Psychosomatic Research, 61(2), 139–151. [DOI] [PubMed] [Google Scholar]
- Letourneau NL, Dennis CL, Benzies K, Duffett-Leger L, Stewart M, Tryphonopoulos PD & Watson W (2012). Postpartum depression is a family affair: addressing the impact on mothers, fathers, and children. Issues in Mental Health Nursing, 33(7), 445–457. [DOI] [PubMed] [Google Scholar]
- Li J, Olsen J, Vestergaard M, & Obel C (2010). Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: a nationwide follow-up study in Denmark. European Child & Adolescent Psychiatry, 19(10), 747–753. [DOI] [PubMed] [Google Scholar]
- Lieberman AF, Ippen CG, & Van Horn P (2015). ”Don’t Hit My Mommy!”: A Manual for Child-parent Psychotherapy with Young Children Exposed to Violence and Other Trauma. Zero to Three.
- Lieberman AF, Van Horn P, & Ippen CG (2005). Toward evidence-based treatment: Child-parent psychotherapy with preschoolers exposed to marital violence. Journal of the American Academy of Child & Adolescent Psychiatry, 44(12), 1241–1248. [DOI] [PubMed] [Google Scholar]
- Lin B, Crnic KA, Luecken LJ, & Gonzales NA (2017). Ontogeny of emotional and behavioral problems in a low-income, Mexican American sample. Developmental Psychology, 53(12), 2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Barch DM, Whalen D, Tillman R, & Freedland KE (2018). A randomized controlled trial of parent-child psychotherapy targeting emotion development for early childhood depression. American Journal of Psychiatry, 175(11), 1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken LJ, Crnic KA, Gonzales NA, Winstone LK, & Somers JA (2019). Mother infant dyadic dysregulation and postpartum depressive symptoms in low-income Mexican-origin women. Biological Psychology, 147, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken LJ, & Gress J (2009). Early adversity and resilience in emerging adulthood. In Reich J, Zautra A, & Hall J (Eds). Handbook of Adult Resilience. Guilford Publications: New York, pp. 238–257. [Google Scholar]
- Luecken LJ, MacKinnon DP, Jewell SA, Crnic KA, & Gonzales NA (2015). Effects of prenatal factors and temperament on infant cortisol regulation in low-income Mexican American families. Developmental Psychobiology, 57(8), 961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken LJ, Jewell SL, & MacKinnon DP (2016). Prediction of postpartum weight in low income Mexican-origin women from childhood experiences of abuse and family conflict. Psychosomatic Medicine, 78(9), 1103–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken LJ, Purdom CL, & Howe R (2009). Prenatal care initiation in low-income Hispanic women: Risk and protective factors. American Journal of Health Behavior, 33(3), 264–275. [DOI] [PubMed] [Google Scholar]
- Lunkenheimer ES, Albrecht EC, & Kemp CJ (2013). Dyadic flexibility in early parent–child interactions: Relations with maternal depressive symptoms and child negativity and behaviour problems. Infant and Child Development, 22(3), 250–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Hubbard JJ, Gest SD, Tellegen A, Garmezy N, & Ramirez M (1999). Competence in the context of adversity: Pathways to resilience and maladaptation from childhood to late adolescence. Development and Psychopathology, 11, 143–169. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA (2016). Future directions in childhood adversity and youth psychopathology. Journal of Clinical Child & Adolescent Psychology, 45(3), 361–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick MT, Ford DC, Ports KA, & Guinn AS (2018). Prevalence of adverse childhood experiences from the 2011–2014 behavioral risk factor surveillance system in 23 states. JAMA Pediatrics, 172(11), 1038–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog NK, Buss C, Entringer S, Shahbaba B, Gillen DL, Hobel CJ, & Wadhwa PD(2016). Maternal exposure to childhood trauma is associated during pregnancy with placental-fetal stress physiology. Biological Psychiatry, 79(10), 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Halligan S, & Cooper P (2010). Effects of postnatal depression on mother–infant interactions and child development. The Wiley-Blackwell Handbook of Infant Development, 2, 192–220. [Google Scholar]
- Nurius PS, Green S, Logan-Greene P, & Borja S (2015). Life course pathways of adverse childhood experiences toward adult psychological well-being: A stress process analysis. Child Abuse & Neglect, 45, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor E, Senger CA, Henninger ML, Coppola E, & Gaynes BN (2019). Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA, 321(6), 588–601. [DOI] [PubMed] [Google Scholar]
- Paschall KW, & Mastergeorge AM (2016). A review of 25 years of research in bidirectionality in parent–child relationships: An examination of methodological approaches. International Journal of Behavioral Development, 40(5), 442–451. [Google Scholar]
- Patterson GR (2002). The early development of coercive family process. In Reid J, Patterson GR, & Snyder J (Eds.), Antisocial behavior in children and adolescents: A developmental analysis and model for intervention (pp. 25–44). Washington, DC: American Psychological Association. [Google Scholar]
- Patterson GR (1982). A social learning approach: Coercive family process (Vol. 3). Eugene, OR: Castalia Publishing Company. [Google Scholar]
- Perales M, Refoyo I, Coteron J, Bacchi M, & Barakat R (2015). Exercise during pregnancy attenuates prenatal depression: a randomized controlled trial. Evaluation & the Health Professions, 38(1), 59–72. [DOI] [PubMed] [Google Scholar]
- Perry NB, Calkins SD, Nelson JA, Leerkes EM, & Marcovitch S (2012). Mothers’ responses to children’s negative emotions and child emotion regulation: The moderating role of vagal suppression. Developmental Psychobiology, 54(5), 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubinov DS, Epel ES, Adler NE, Laraia BA, & Bush NR (2019). Transactions between maternal and child depressive symptoms emerge early in life. Journal of Clinical Child & Adolescent Psychology, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubinov DS, Felder JN, Vieten C, Coleman-Phox K, Laraia B, Adler N, & Bush NR (2018). Maternal depressive symptoms and infant healthcare utilization: The moderating role of prenatal mindfulness. General Hospital Psychiatry, 53, 82–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangalang CC & Vang C (2017). Integenerational trauma in refugee families: A systematicreview. Journal of Immigrant and Minority Health, 19, 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerdtfeger KL, & Goff BSN (2007). Intergenerational transmission of trauma: Exploring mother–infant prenatal attachment. Journal of Traumatic Stress, 20(1), 39–51. [DOI] [PubMed] [Google Scholar]
- Somers JA, Curci SG, & Luecken LJ (2019). Infant vagal tone and maternal depressive symptoms: A bottom-up perspective. Journal of Clinical Child & Adolescent Psychology, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers JA, Jewell SL, Hanna Ibrahim M, & Luecken LJ. (2019). Infants’ biological sensitivity to the effects of maternal social support: Evidence among Mexican American families. Infancy, 24(2), 275–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnowski DW, Booth C, York TP, Amstadter AB, Kliewer W (2018). Maternal prenatal stress and infant DNA methylation: A systematic review. Developmental Psychobiology, 60, 127–139. [DOI] [PubMed] [Google Scholar]
- Sweet MA, & Appelbaum MI (2004). Is home visiting an effective strategy? A meta-analytic review of home visiting programs for families with young children. Child Development, 75(5), 1435–1456. [DOI] [PubMed] [Google Scholar]
- Tandon SD, Perry DF, Mendelson T, Kemp K, & Leis JA (2011). Preventing perinatal depression in low-income home visiting clients: a randomized controlled trial. Journal of Consulting and Clinical Psychology, 79(5), 707. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, John AMS, & Meyer JS (2017). Chronic stress in the mother-infant dyad: Maternal hair cortisol, infant salivary cortisol and interactional synchrony. Infant Behavior and Development, 47, 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BL, Cavanagh K, & Strauss C (2016). The effectiveness of mindfulness-based interventions in the perinatal period: a systematic review and meta-analysis. PloS one, 11(5), e0155720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Booth C, Viding E, Puetz VB, Rutherford HJ, Mayes LC, & McCrory EJ (2018). Ghosts in the nursery: An experimental investigation of a parent’s own maltreatment experience, attention to infant faces, and dyadic reciprocity. Emotion, 19, 1093–1102. [DOI] [PubMed] [Google Scholar]
- Tronick E, (2007). The neurobehavioral and social-emotional development of infants and children. W.W. Norton & Company. [Google Scholar]
- Tronick EZ, & Weinberg MK (1990). The infant regulatory scoring system (IRSS). Unpublished manuscript, Children’s Hospital/Harvard Medical School, Boston. [Google Scholar]
- Tully KP, Stuebe AM, & Verbiest SB (2017). The fourth trimester: a critical transition period with unmet maternal health needs. American Journal of Obstetrics and Gynecology, 217(1), 37–41. [DOI] [PubMed] [Google Scholar]
- Urizar GG Jr, & Muñoz RF (2011). Impact of a prenatal cognitive-behavioral stress management intervention on salivary cortisol levels in low-income mothers and their infants. Psychoneuroendocrinology, 36(10), 1480–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E (2005). What can we learn about resilience from large-scale longitudinal studies?. In Goldstein S, Sam & Brooks RB (Eds.), Handbook of resilience in children (pp. 91–105). New York: Kluwer Academic/Plenum Publishers. [Google Scholar]
- Winstone LK, Curci SG, & Crnic KA (2020). Pathways to Maternal and Child Well Being: Stability and Transaction across Toddlerhood. Parenting, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R & Lehrner A (2018). Intergenerational transmission of trauma effects: Putative role of epigenetic mechanisms. World Psychiatry, 17(3), 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]


