Summary
Ectodomain shedding is a proteolytic process that regulates the levels and functions of membrane proteins. Dysregulated shedding is linked to severe diseases, including cancer and Alzheimer's disease. However, the exact cleavage sites of shedding substrates remain largely unknown. Here, we explore the landscape of ectodomain shedding by generating large-scale, cell-type-specific maps of shedding cleavage sites. By means of N- and C-terminal peptide enrichment and quantitative mass spectrometry, we quantified protein termini in the culture media of 10 human cell lines and identified 489 cleavage sites on 163 membrane proteins whose proteolytic terminal fragments are downregulated in the presence of a broad-spectrum metalloprotease inhibitor. A major fraction of the presented cleavage sites was identified in a cell-type-specific manner and mapped onto receptors, cell adhesion molecules, and protein kinases and phosphatases. We confidently identified 86 cleavage sites as metalloprotease substrates by means of knowledge-based scoring.
Subject areas: Molecular Biology, Cell Biology, Omics, Proteomics
Graphical abstract
Highlights
-
•
Secretomes across 10 human cell lines were investigated by protein terminomics
-
•
Cell-type-specific maps of shedding cleavage sites were generated
-
•
Most of the cleavage sites were identified in a cell-type-specific manner
-
•
Knowledge-based scoring enabled prediction of responsible sheddases
Molecular Biology; Cell Biology; Omics; Proteomics
Introduction
Membrane proteins have critical physiological roles, and their abundance and functions are tightly controlled through multiple mechanisms, including ectodomain shedding (shedding), which is a form of limited proteolysis that liberates the extracellular domain of membrane proteins (Huovila et al., 2005; Lichtenthaler et al., 2018; Weber and Saftig, 2012). Shedding contributes to cellular interactions with the environment by releasing active cytokines, growth factors or other mediators from their membrane-bound precursors, or conversely, by reducing the levels of receptors and adhesion proteins at the cell surface (Huovila et al., 2005; Weber and Saftig, 2012). Shedding often triggers further proteolysis within the transmembrane domain – known as regulated intramembrane proteolysis (RIP) – thereby releasing the intracellular domain into the cytoplasmic region. These processes enable bidirectional signal transduction (Beard et al., 2019; Reiss and Saftig, 2009; Weber and Saftig, 2012).
A protease involved in shedding is specifically referred to as a sheddase. Distinct sheddases may cleave the same substrate at different sites, generating multiple proteoforms that may exhibit different biological functions and activities (Niedermaier and Huesgen, 2019). For instance, the difference of cleavage sites of amyloid precursor protein (APP) by a disintegrin and metalloprotease 10 (ADAM10) and by beta-site APP cleaving enzyme 1 (BACE1) is critical for the pathogenesis of Alzheimer's disease (Chow et al., 2010). Therefore, it is important to identify cleavage sites in order to achieve a precise understanding of the physiological roles of shedding.
Discovery and analysis of large numbers of proteoforms is the exclusive domain of liquid chromatography/tandem mass spectrometry (LC/MS/MS)-based proteomics (Aebersold et al., 2018; Olsen and Mann, 2013). For the identification of shedding substrates, it is preferable to investigate the proteins secreted into the cell culture media, rather than those remaining in the membrane, because the secretome is much less complex than the membrane proteome. Additionally, the fragments remaining in the membrane are often unstable due to further proteolytic events such as RIP and lysosomal degradation (Güner and Lichtenthaler, 2020; Merilahti and Elenius, 2019). By using established hydrazide chemistry, 18 metalloprotease substrates in the culture media were identified (Tsumagari et al., 2017); most of the membrane proteins are glycosylated (Apweiler et al., 1999). However, this approach did not lead to precise identification of the cleavage sites. In fact, knowledge of the cleavage sites of shedding substrates is limited, despite its importance. In standard shotgun proteomics, only peptides generated in accordance with the specificity of the employed digestive enzymes at both N-and C-termini are considered. On the other hand, it is essential for identifying shedding-originated peptide termini to employ semi-specific searches in which peptides may result from specific cleavage by the employed digestive enzymes at only one terminus, while the other terminus may be generated by a non-specific cleavage event, such as shedding (Niedermaier and Huesgen, 2019). Thus, terminal peptide enrichment from secreted proteins coupled with semi-specific search should be employed in order to efficiently identify shedding cleavage sites.
Several methods have been reported for protein terminomics. Prudova et al. employed a method for N-terminal peptide enrichment called TAILS (terminal amine isotopic labeling of substrates) (Kleifeld et al., 2010), and identified 201 and 19 cleavage products formed by recombinant MMP-2 and MMP-9, respectively (Prudova et al., 2010). An alternative strategy is to utilize an engineered enzyme to selectively label free protein N-termini with biotin-tags that enable positive selection of N-terminal peptides (Mahrus et al., 2008; Weeks and Wells, 2018). However, there are still obstacles to the large-scale detection of cleavage sites generated by endogenous sheddases in living cells. First, terminal peptide enrichment strategies generally consist of multiple steps including some chemical reactions, and therefore relatively large amounts of samples are needed. Second, the protein amounts in the culture media are much smaller than those obtained from cell lysates, and thus it is hard to prepare sufficiently large samples for terminal peptide enrichment. Finally, C-terminomics specifically remains a challenging task because the enrichment efficiency is greatly inferior to that of the N-terminal counterpart due to the difficulty in chemically modifying the carboxy group (Niedermaier and Huesgen, 2019).
Recently, our group developed a simple and rapid methodology for N-terminal peptide enrichment, in which N-terminal peptides of TrypN digests are isolated by strong cation exchange (SCX) chromatography at low pH (Chang et al., 2021). Earlier reports have demonstrated that C-terminal peptides generated by trypsin digestion are eluted first in SCX, together with acetylated N-terminal peptides (Alpert et al., 2010; Gauci et al., 2009; Helbig et al., 2010), and we discovered here this feature enables the identification and quantification of C-terminal peptides on a comparable scale to that of the N-terminal counterparts with the same amount of input (Figures S1A and S1B). Here, we applied these sensitive terminomics methodologies to conduct the first large-scale study of shedding cleavage sites targeted by endogenous metalloproteases, which have emerged as the major sheddase family. We quantitatively identify putative metalloprotease-regulated ectodomain shedding cleavage sites based on the results of broad-spectrum metalloprotease inhibitor treatment, and provide an overview of their positional and functional landscape.
Results
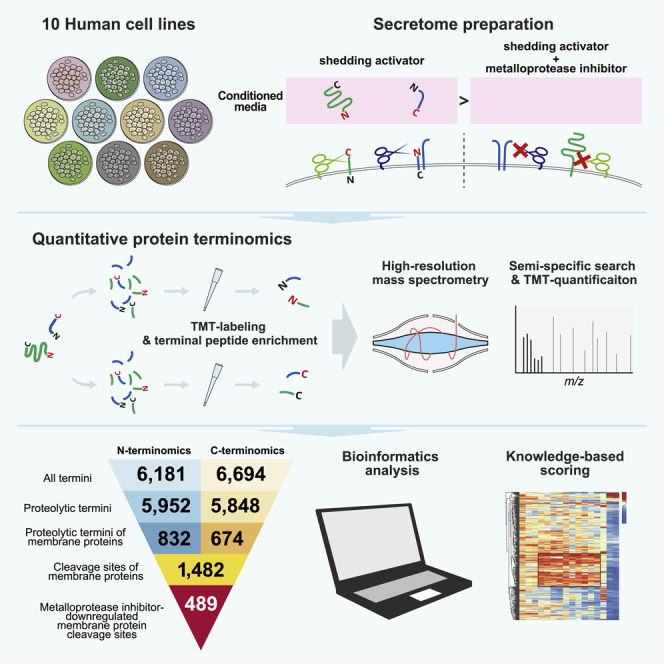
Sensitive terminomics workflow enables efficient identification and reproducible quantification of N- and C-termini in the secretome
Protein shedding can be transiently upregulated by certain agonists, such as phorbol 12-myristate 13-acetate (PMA), activating mainly ADAM17 (Huovila et al., 2005). To achieve large-scale detection of membrane protein cleavage sites by members of the metalloprotease family, we employed a quantitative terminomics workflow consisting of shedding activation with PMA, broad-spectrum metalloprotease inhibitor BB-94 (batimastat) treatment, SCX-based terminal peptide enrichment (Alpert et al., 2010; Chang et al., 2021; Helbig et al., 2010), TMT labeling and nanoLC/MS/MS measurement (Figures S1A–S1D).
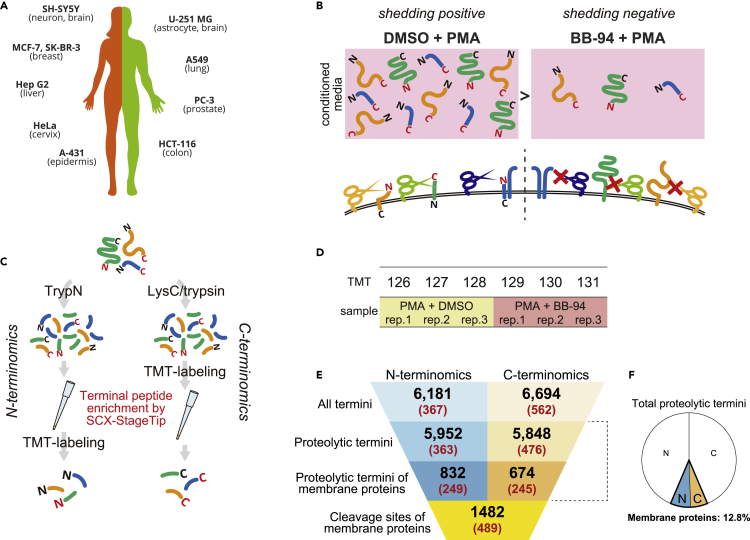
We investigated secreted protein fractions across a panel of ten human cell lines stimulated with PMA following BB-94 treatment (Figures 1A and 1B). Samples were prepared in triplicate for each condition. The digest of 10 μg protein per replicate was subjected to terminal peptide enrichment: thus, in total only 1.2 mg of protein was utilized in the whole of this study. Terminal peptide enrichment was performed by SCX-StageTip (Adachi et al., 2016; Rappsilber et al., 2007). Briefly, for N-terminal peptide enrichment, TrypN is first employed to generate protein N-terminal peptides without Lys or Arg. Then, the N-terminal peptides can be separated from the internal peptides by SCX due to their weaker retention (Figures S1A and S1B). Similarly, LysC and trypsin are used to generate C-terminal peptides without Lys or Arg for isolation of C-terminal peptides (Figures S1A and S1B). Among trypsin/LysC-digested peptides, C-terminal peptides and acetylated N-terminal peptides have similar charge distributions (Figure S1A), and therefore these terminal peptides are isolated together (Figure S1E). We eliminated acetylated N-terminal peptides generated by trypsin/LysC and TrypN from the identification list in this study, since peptides cleaved by sheddase should have unmodified termini. Note that N-terminal peptides were TMT-labeled after enrichment, since the TMT tag affects the enrichment efficiency (Figure 1C). Also note that acetylated N-terminal peptides generated by TrypN cannot be quantified in our workflow because these peptides do not have TMT-reactive sites (Figure S1A). For C-terminomics, the flow-through and the 0.5% TFA-eluted fraction were separately collected and subjected to LC/MS/MS (Figure S1D). C-Terminal peptides were identified with the selectivity of 38% and 13% on average for the flow-through and the 0.5% TFA-eluted fractions, respectively (Figure S1E). Triplicates of controls and of BB-94-treated samples were multiplexed using 6-plexed TMT reagents (Figure 1D). We analyzed the TMT-labeled terminal peptides by high-resolution Orbitrap mass spectrometry considering a wide search space in semi-specific search mode (Niedermaier and Huesgen, 2019). Semi-specific search was performed using the Andromeda search engine on MaxQuant (Cox and Mann, 2008; Cox et al., 2011) against the SwissProt human protein database, including isoform sequences. TMT-reporter intensities were normalized by the trimmed mean of M values (TMM) method (Robinson and Oshlack, 2010). We excluded peptides with missed cleavages for further analyses in order to simplify the relationship between the cleavage sites and the corresponding peptides.
Figure 1.
Experimental design and quantitative terminomics workflow for large-scale analysis of cleavage sites by metalloproteases
(A) List of investigated cultured cell lines (10 human cancer cell lines).
(B) Experimental design. Cells were treated with DMSO or BB-94 for 1 hr, followed by PMA treatment for 1 hr. Triplicate samples were prepared for each condition.
(C) Workflow of sample preparation. Proteins were digested with either TrypN or LysC and trypsin. Note that N-terminal peptides were TMT-labeled after terminal peptide enrichment, while C-terminal peptide enrichment was performed following TMT-labeling. See Figures S1C and S1D for details.
(D) Triplicate shedding-positive samples (PMA + DMSO) and shedding-negative samples (PMA + BB-94) were labeled as shown with TMT channels and form a 6-plexed TMT.
(E) Summary of the number of quantified termini and resulting cleavage sites. The parenthesized numbers show those significantly downregulated by BB-94. All termini: the total numbers of N- or C- termini (native protein termini and proteolytic termini). Proteolytic termini: the numbers of N- or C-termini that are presumably generated by endogenous proteases. Proteolytic termini on membrane proteins: the numbers of proteolytic N- or C-termini that are mapped on membrane proteins. Cleavage sites of membrane proteins: the total number of cleavage sites presented by proteolytic N- and C-termini on membrane proteins.
(F) Pie chart depicting the ratio of proteolytic termini mapped on membrane proteins in total proteolytic termini. The white areas show non-membrane proteins, and the areas highlighted with colors show membrane proteins. N, N-termini; C, C-termini.
Our strategy led to the quantification of 6,181 N-termini and 6,694 C-termini in total (Figure 1 E and Tables S1 and S2). In order to find peptides containing cleavage sites targeted by endogenous proteases, we extracted peptides with termini not cleaved by the spiked protease used for sample preparation, so-called “semi-specific peptides” (Figure S1F). Such terminal peptides would be derived from endogenous proteolysis events, such as shedding. In this study, we refer to such termini, presumably generated by endogenous proteolysis, as “proteolytic termini” and the terminal peptides presenting the proteolytic termini as “proteolytic peptides” (Figure S1F). Finally, our dataset included 5,952 proteolytic N-termini and 5,848 proteolytic C-termini (Figure 1E and Tables S1 and S2). Of identified proteolytic termini, 832 N- and 674 C-termini were derived from membrane proteins (14.0% and 11.5% of the respective proteolytic terminome), which were defined using the UniProtKB Keywords “transmembrane” or “GPI-anchor”. Figure S1G shows the protein yields for each cell line to perform N, C-terminomics, and Figures S2 and S3 summarize the reproducibility in triplicate analyses. For individual conditions, there was excellent reproducibility in the peak intensity of identified peptides, with Pearson correlation coefficients in the ranges of R > 0.93 (0.98 on average) for N-terminomics and R > 0.97 for C-terminomics (0.99 on average). We created volcano plots with truncation at the false discovery rate of 0.05 and artificial within-groups variance (S0) of 0.1 (default parameters) using Perseus (Tyanova et al., 2016) (Figures S4 and S5), which yielded 363 proteolytic N-termini and 476 proteolytic C-termini that were significantly downregulated upon BB-94 treatment (Figure 1E). Importantly, these included 249 proteolytic N-termini (68.6% of downregulated proteolytic N-termini) and 245 proteolytic C-termini (51.5% of downregulated proteolytic C-termini) mapped on membrane proteins, affording a total of 489 cleavage sites (Table S3). Note that five downregulated membrane protein cleavage sites were presented by both proteolytic N- and C-terminal peptides. As our results show (Figure 1F), proteolytic terminal peptides derived from membrane proteins account for a small fraction of the total proteolytic terminal peptides, which is one reason why they are so difficult to detect with MS. Our workflow enabled efficient identification and quantification of both proteolytic N- and C-termini of membrane proteins, highlighting a major strength of our methodology for achieving deep analysis of membrane protein cleavage sites with a limited amount of material.
BB-94 selectively downregulates proteolytic termini of membrane proteins in the secretome
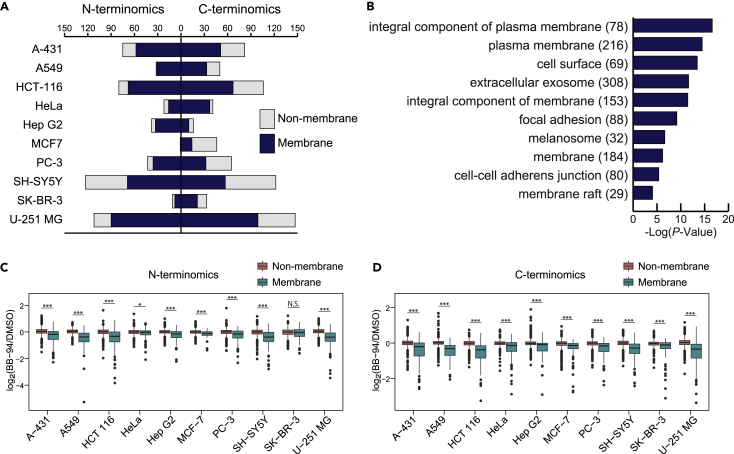
The number of downregulated termini varied among investigated cell lines (Figure 2A). U-251 MG cells have the highest number of downregulated termini, while MCF-7 has the lowest number. First, we examined the subcellular distribution of downregulated proteins using Gene Ontology (GO) term enrichment analysis by DAVID (v6.8; https://david.ncifcrf.gov/) (Huang et al., 2009). We found strong enrichment for terms that evoke membrane proteins, such as plasma membrane and cell surface (Figure 2B). In addition, in most samples, except the N-terminome of SK-BR-3, proteolytic termini of membrane proteins were downregulated by BB-94, while the abundances of proteolytic termini of non-membrane proteins were not changed (Figures 2C and 2D). These results indicate that BB-94 broadly and selectively targets the cleavage of membrane proteins, which would be useful for studying metalloprotease-dependent shedding. In addition, the provided cleavage site information in living cells should facilitate an understanding of the mechanism of action of BB-94.
Figure 2.
Overview of the BB-94-downregulated proteolytic terminome in the supernatant
(A) Distribution of the number of downregulated proteolytic termini mapped on membrane proteins and non-membrane proteins in the respective cell lines.
(B) Enrichment analysis on GO term cellular components performed by DAVID (v8.0). The top ten significant terms are shown, with Bonferroni-adjusted p values. The parenthesized numbers show the number of proteins.
(C and D) The log2-transformed ratios (BB-94/DMSO) of proteolytic termini are compared between membrane proteins and non-membrane proteins in the respective cell lines. The p values were calculated with the Wilcoxon rank-sum test and a Bonferroni adjustment was applied. ∗∗∗, p < 0.005; ∗∗, p < 0.001; ∗, p < 0.05; N.S., not significant.
Topological analysis of downregulated membrane proteins and positional analysis of the cleavage sites
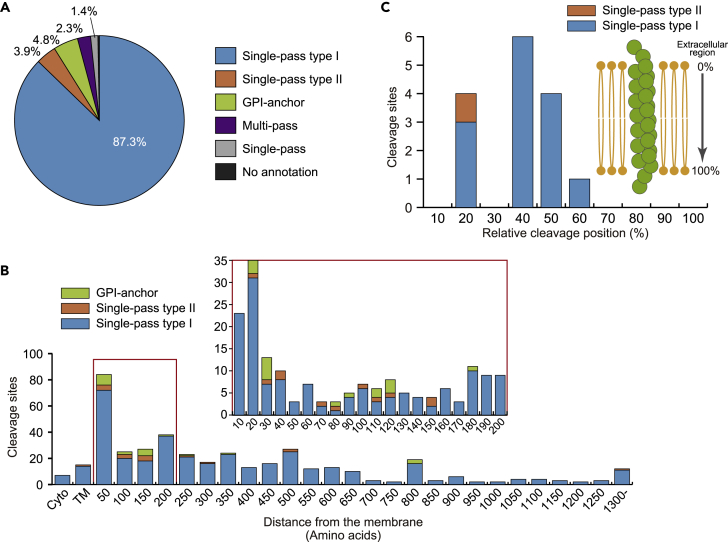
We found that some of the downregulated membrane protein cleavage sites are localized at or around signal peptide cleavage sites (Figure S6A). Note that the sequences around the signal peptide cleavage sites showed overrepresention of AxA at P3-P1 (three to one amino acids upstream of the cleavage sites) (Figure S6B), which is the motif of canonical signal peptidases (Paetzel et al., 2002). Because it is not clear whether these cleavages occurred as metalloprotease-dependent shedding, signal peptidases or the combined events, we excluded 56 sites within ±5 amino acids from the signal peptide cleavage sites for further analyses in this study. Topological analysis of the remaining 433 sites based on the UniProtKB (https://www.uniprot.org/) annotation revealed that single-pass type I membrane proteins, which have their N-termini in the extracellular region, are the majority (378 sites, 87.3%), followed by GPI-anchor proteins (21 sites, 4.8%), single-pass type II membrane proteins, which have the C-termini in the extracellular region (17 sites, 3.9%), and multi-pass membrane proteins, which span the membrane more than once (10 sites, 2.3%) (Figure 3A).
Figure 3.
Topological analysis of downregulated membrane proteins and positional analysis of the cleavage sites
(A) Percentages of the various topologies of membrane proteins identified with downregulated cleavage sites. The categories of membrane proteins follow the notation in UniProt.
(B) The distribution of the distance (number of amino acids) from the transmembrane domain or GPI-anchor site to the cleavage site is depicted for single-pass type-I and type-II membrane proteins and GPI-anchored proteins. The section surrounded with a red box is shown in detail above. TM: cleavage sites within the transmembrane domain. Cyto: cleavage sites within the cytoplasmic domain.
(C) Relative cleavage positions of the cleavage sites within the transmembrane domain of single-pass type I and type II membrane proteins. Values represent the length (number of amino acids) from the extracellular region to the cleavage site, divided by the total length (number of amino acids) of the transmembrane domain.
We examined the cleavage positions of single-pass type I and type II membrane proteins and GPI-anchor proteins. Of the 416 cleavage sites, 394 sites (94.7%) were distributed on the extracellular domain, confirming that BB-94 targets “ectodomain shedding” (Figure 3B). Notably, we found that metalloprotease-regulated proteolysis occurred in close proximity to the cell surface: 174 sites (44.2%) were within 200 amino acids from the transmembrane domain or GPI-anchor site, which evidently suggests that large portions of the extracellular domains are released by shedding. This trend is consistent with previous findings on individual shedding substrates (Hinkle et al., 2004; Shirakabe et al., 2011; Zheng et al., 2004), and thus validates our results. On the other hand, 15 BB-94-downregulated sites were mapped within the transmembrane domain (Figure 3B), including three APP cleavage sites considered to be cleaved by γ-secretase (corresponding to amyloid β 37, 38, and 40) (Takami et al., 2009) (Figure S6C), and this suggests that our dataset includes cleavage sites generated downstream of metalloprotease-dependent shedding (Brown et al., 2000; Edwards et al., 2008; Reiss and Saftig, 2009). Interestingly, these downregulated cleavage sites inside the transmembrane domain were likely to be located at the extracellular side (Figure 3C). γ-Secretase cleaves APP in a stepwise manner, in which APP is first cleaved at the membrane-cytoplasm boundary, followed by successive tri- or tetrapeptide trimming (Takami et al., 2009). Consequently, the resulting terminus may be positioned at the extracellular side. Notably, in addition to APP, we identified five downregulated proteins (ALCAM in A431 and HCT-116; GLG1 in HCT-116; PIGR in A431; SDC1 in A431, A-549, HCT-116, HeLa, PC-3, SH-SY5Y, SK-BR-3, and U-251 MG; SDC4 in HCT-116) with cleavage sites on the extracellular domain and inside the transmembrane domain in the same cell line (Table S3). These proteins could have roles in bidirectional signal transduction across the cell membrane (Beard et al., 2019; Reiss and Saftig, 2009; Weber and Saftig, 2012). It is noteworthy that we found only 7 sites (2 sites by N-terminomics and 5 sites by C-terminomics) were mapped on the cytoplasmic domain (Figure 3B), which might originate from dead cells.
We identified 10 BB-94-downregulated cleavage sites on 7 multi-pass membrane proteins (Table S3), and investigated their localization using the TMHMM Server (v2.0; http://www.cbs.dtu.dk/services/TMHMM-2.0/). We found 7 sites on 4 proteins (PLP2, GPR126, IGSF1, CELSR2) were mapped on their extracellular domains (Table S3). Among them, 6 sites were localized at the extracellular region with only one transmembrane domain, as exemplified by CELSR2 F815↓L816 (Figure S6D). These single cleavages can lead to ectodomain shedding. On the other hand, the cleavage site of G-protein coupled receptor 126 (F840↓T841) is located on the extracellular region between two membrane-spanning domains (Figure S6E). This site was identified with C-terminal proteolytic peptides, indicating that this ectodomain was shed as a result of additional cleavage at the N-terminal side of F840. Of the cleavage sites on multi-pass membrane proteins, 3 sites were mapped on their cytoplasmic domains. As mentioned above, these sites might be artifacts. Overall, in our terminomics, most of the BB-94-downregulated sites on membrane proteins (416 sites (97.7%) out of 426 membrane protein cleavage sites) were mapped on their extracellular domains or transmembrane domains, supporting the reliability of our data set.
Functional characterization of ectodomain shedding
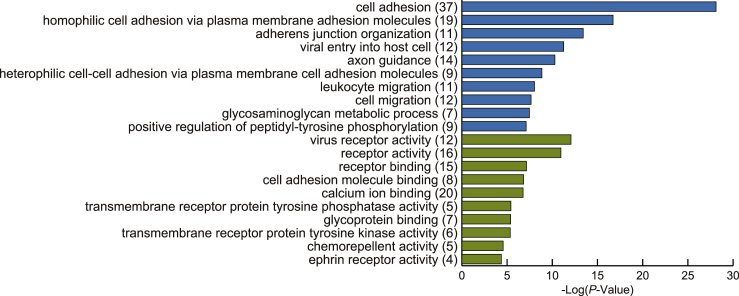
We functionally characterized the downregulated cleavage sites on the extracellular domain as metalloprotease-regulated shedding cleavage sites (394 cleavage sites on 119 proteins; Figure 3B). DAVID enrichment analysis (Huang et al., 2009) for GO term biological processes revealed significant enrichment for cell adhesion proteins (Figure 4), which is consistent with a previous study (Tsumagari et al., 2017). In addition, we found enrichment of proteins related to cell migration, including CD44, whose shedding is important for CD44-dependent cell migration (Nagano and Saya, 2004). Analysis of GO terms of molecular function showed that shedding targets central components of signal transduction, such as receptors and protein tyrosine kinases/phosphatases. Importantly, we observed that shedding liberates a large portion of the extracellular domain (Figure 3B), which is the main functional region of membrane proteins, on a proteomic scale. Thus, overall, our results indicate that shedding regulates fundamental biological events by modulating membrane protein functions.
Figure 4.
Functional analysis of metalloprotease-regulated shedding substrates
DAVID enrichment analysis of metalloprotease-regulated shedding substrates according to GO term biological processes (blue) and molecular functions (green). The top ten significant terms are shown, with Bonferroni-adjusted p values. The parenthesized numbers show the numbers of proteins.
Cell-type-specificity of shedding
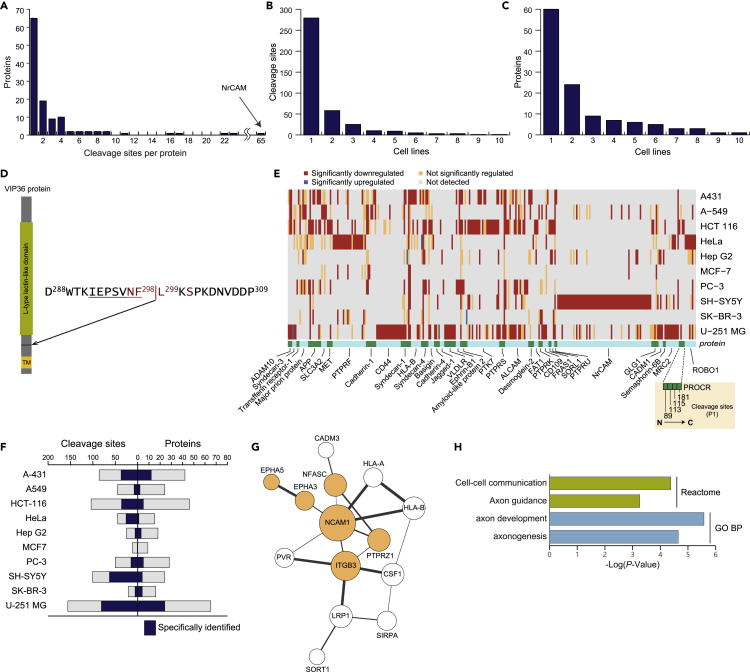
Differently processed proteoforms can exhibit distinct physiological properties (Chow et al., 2010; Hu et al., 2006; La Marca et al., 2011; Willem et al., 2006). Whereas most BB-94-downregulated membrane proteins contain one cleavage site, interestingly no less than 65 cleavage sites were identified on neuronal cell adhesion molecule (NrCAM) (Figure 5A). This indicates that proteolysis can produce an impressive number of proteoforms and may critically contribute to the complexity of the human proteome. A large population of the identified shedding substrates was cell-type-specific rather than globally identified, at both the cleavage site and protein levels (Figures 5B and 5C), suggesting that shedding occurs depending on the individual functions of the cells. Sixty proteins were found in one cell line, while only one protein, 36-kDa vesicular integral-membrane protein VIP36 coded by the LMAN2 gene, was found commonly in all investigated cell lines. VIP36 is a validated shedding substrate (Shirakabe et al., 2011). Intriguingly, the VIP36 protein was cleaved at the same site (F298↓L299) across all analyzed cell lines. In the previous study, western blotting in combination with amino acid substitution at expected cleavage sites failed to identify the precise cleavage site of VIP36 (Shirakabe et al., 2011), but the site we identified here is positioned just within the expected region (Figure 5D), which again validates our result and underscores the strength of our strategy. VIP36 is a lectin domain-containing transmembrane protein that functions as a cargo receptor transporting glycoproteins. While shedding of the VIP36 protein plays a critical role in phagocytosis in Raw 264.7 cells (Shirakabe et al., 2011), the role of VIP36 shedding in other cell types is unknown.
Figure 5.
Cell-type-specificity of shedding
(A) The distribution of the number of cleavage sites per protein is depicted for single-pass type-I and type-II transmembrane proteins and GPI-anchored proteins.
(B and C) Histograms depicting the number of shedding substrate identified in 1–10 cell lines at the cleavage site level (B) and at the protein level (C).
(D) VIP36 cleavage site. The amino acids that were previously suggested to be essential for cleavage are highlighted in red (Shirakabe et al., 2011). The sequence of the peptide identified in this study is underlined. TM, transmembrane domain.
(E) Heatmap depicting membrane proteins shed by cleavage at differential sites in different cell lines (36 proteins). Each gray, yellow, red, or blue box designates a cleavage site. Cleavage sites are arranged in the order from N- to C-terminus from left to right in each protein, as the example of PROCR shows. At the bottom, to visualize the boundaries of proteins, green and light-blue boxes are alternately arranged in alphabetical order of UniProt accession from left to right.
(F) Distribution of the number of metalloprotease-regulated cleavage sites in the respective cell lines. The number of cleavage sites specifically identified in each cell type is highlighted in color.
(G and H) Protein interaction network of shedding substrate proteins specifically identified in U-251 MG cells (G). Proteins without any interactions are excluded. Significantly enriched reactome and GO terms of interest are shown with the Benjamini-adjusted p values (H), and the proteins annotated with these terms are highlighted in color (F). All analyses were performed by STRING (v11). The network was visualized using Cytoscape (v3.8.0). Node size reflects the number of connections (direct edges), and edge line width reflects the combined score calculated by STRING.
We found 36 proteins were cleaved at different sites depending on the cell lines (Figure 5E). Of these, 5 proteins have proteolytic peptides upregulated upon BB-94 treatment, indicating these peptides might be generated by multiple proteolytic events (see later discussion for syndecan-1).
We identified cell-line-specific shedding substrate proteins in nine cell lines, except for MCF-7. Astrocytoma-derived U-251 MG cells yielded the highest number of substrates (Figure 5F), and 24 proteins were uniquely identified in U-251 MG cells. To investigate the functions of these U-251 MG cell-specific substrates, we investigated protein-protein interactions of these substrate proteins using the STRING database (v11.0; https://string-db.org/) (Szklarczyk et al., 2019), which resulted in significantly more interactions than would be expected for a random protein set of similar size with a p value of 6.13 × 10−11 (calculated by STRING) (Figure 5G). Interestingly, enrichment analysis revealed that these U-251 MG cell-specific substrates are related to typical functions of neurons, such as axon development and axon guidance, rather than those of astrocytes (Figure 5H). This may indicate that shedding contributes to cellular communication across different cell types in the central nervous system.
Evaluation of metalloprotease-regulated shedding cleavage sites using PWM scoring
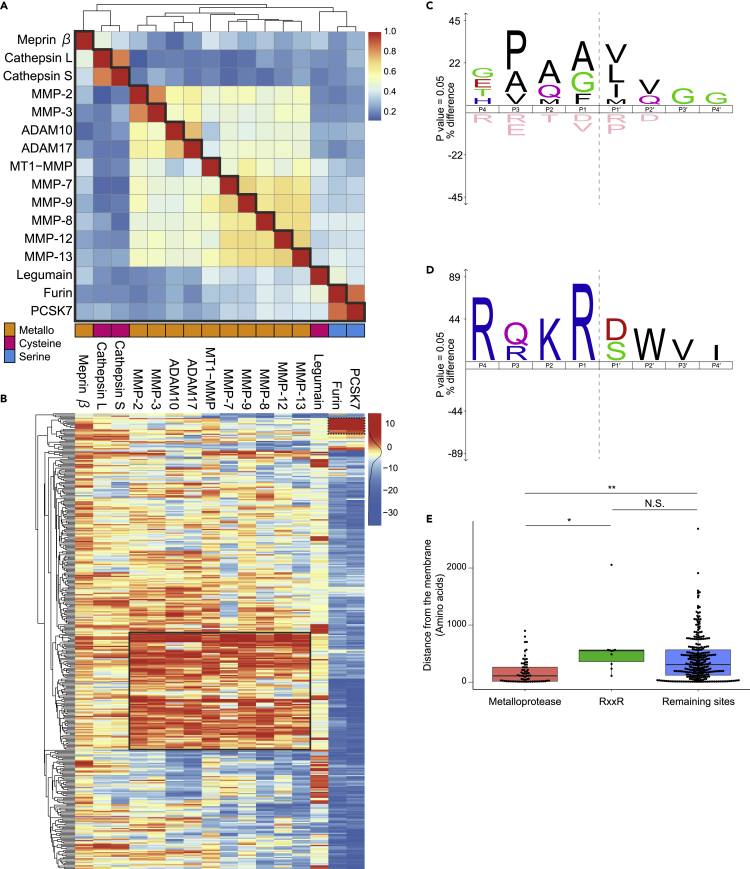
As mentioned above, our results include cleavage sites downstream of the initial metalloprotease cleavages. Therefore, to identify the direct cleavage sites by metalloproteases with greater confidence, we evaluated the identified ectodomain cleavage sites of type-I membrane proteins and GPI-anchored proteins, whose N-terminal regions are located on the extracellular surface, using position weight matrix (PWM) scoring (Imamura et al., 2017) with the accumulated substrate information in the MEROPS protease database (v.12.1, https://www.ebi.ac.uk/merops/index.shtml) (Rawlings et al., 2018). Although ADAM17 and ADAM10 are major sheddases (Huovila et al., 2005), the numbers of their registered substrate cleavage sites are much fewer than those of other metalloproteases due to the relative absence of studies on their cleavage sites, despite their physiological importance. We concatenated in vitro substrate information to the MEROPS-registered substrates (Tucher et al., 2014), which yielded 225 and 381 cleavage site sequences for ADAM10 and ADAM17, respectively. In addition we further selected 14 sheddases (matrix metalloprotease (MMP) −2, −3, −7, −8, −9, −12, −13, MT1-MMP, legumain, meprin β, cathepsin S, cathepsin L, furin and proprotein convertase subtilisin/kexin 7 (PCSK7)) from various families, based on following criteria: described as canonical or part-time sheddases in a recent review (Lichtenthaler et al., 2018); having a number of registered substrates in MEROPS greater than one hundred.
We computed substrate sequence PWMs for these individual sheddases (Figures 6A and S7). Importantly, individual sheddases showed distinct substrate preference signatures. Overall, most metalloproteases, except meprin β, commonly exhibit a preference for hydrophobic residues such as Leu and Ile at the P1′ position (one amino acid downstream of the cleavage site), and for Pro and other hydrophobic residues at the P3 position (three amino acids upstream of the cleavage sites); these trends are consistent with previous reports (Eckhard et al., 2016; Tucher et al., 2014). ADAM10 characteristically exhibits a preference for bulky hydrophobic residues such as Tyr, Trp and Phe, which reflects their structural differences (Seegar et al., 2017). Furin and PCSK7 showed known RxxR or RxKR cleavage motifs at P4-P1 (Seidah et al., 2013). Legumain and meprin β showed preferences for Asp/Asn at P1 and acidic residues at P1′, respectively. Given that the PWMs are validated, they can provide a basis for scoring identified putative shedding sites of the respective sheddases.
Figure 6.
PWM scoring of downregulated cleavage sites
(A) Heatmap depicting the cosine similarity between PWMs for 16 selected sheddases shown in Figure S7. High similarity is highlighted in red, while low similarity is highlighted in blue. Sheddases are ordered by clustering based on the cosine distances (1 – cosine similarity).
(B) Hierarchical clustering analysis of PWM scores. High-scored sites are highlighted in red, while low-scored sites are highlighted in blue. A cluster of cleavage sites high-scored for metalloproteases (metalloprotease cluster) and a cluster for furin and PCSK7 (RxxR cluster) are enclosed with solid-line and dashed-line boxes, respectively.
(C and D) Sequence logos of cleavage sites in the metalloprotease cluster (C) and in the RxxR cluster (D) generated by iceLogo (https://iomics.ugent.be/icelogoserver/). Dashed line shows the cleavage site.
(E) The distances from the transmembrane domain or GPI-anchor site to the cleavage site are compared between the metalloprotease cluster, the RxxR motif cluster, and the remaining sites. The p values were calculated with the Wilcoxon rank-sum test and Bonferroni adjustment was applied. ∗∗, p = 1.26 × 10−7; ∗, p = 8.82 × 10−4; N.S., not significant.
We calculated PWM scores between more than 6000 relationships (16 sheddases x 378 cleavage sites) (Figure 6B, Table S4). Clustering analysis revealed a group of 86 cleavage sites high-scored for metalloproteases, including the α cleavage site of APP mediated by ADAM10 (Figures 6B and 6C; Table 1). This cluster also included the VIP36 cleavage site that was commonly identified in the analyzed cultured cells (Figure 5D). Notably, although these proteins were previously known to undergo shedding, in many cases, the exact cleavage sites that we identified here have not been reported. We also found another cluster, which involving the RxxR motif of furin and PCSK7 (Figures 6B and 6D). These serine proteases are considered as part-time sheddases, which mainly function as proprotein convertases, but can additionally participate in shedding (Lichtenthaler et al., 2018). The downregulation of these cleavages upon BB-94 treatment might be a secondary or later event following the inhibition of metalloproteases, rather than being due to direct inhibition by BB-94.
Table 1.
List of cleavage sites grouped into the metalloprotease cluster
| UniProt acc.a | Gene namea | Protein namea | Cleavage windowa | P1a | ADAM10 PWM scoreb | ADAM17 PWM scoreb |
|---|---|---|---|---|---|---|
| P55283 | CDH4 | Cadherin-4 | TIGA↓VAAA | 724 | 5.10 | 8.23 |
| Q9H3T3 | SEMA6B | Semaphorin-6B | GPGR↓LTPA | 55 | 3.64 | 2.35 |
| P04156 | PRNP | Major prion protein | GAAA↓AGAV | 117 | 4.54 | 5.51 |
| P04156 | PRNP | Major prion protein | AAAG↓AVVG | 119 | 3.74 | 4.51 |
| P04156 | PRNP | Major prion protein | AGAA↓AAGA | 116 | 4.24 | 4.67 |
| P04156 | PRNP | Major prion protein | AAGA↓VVGG | 120 | 6.91 | 9.05 |
| P55291 | CDH15 | Cadherin-15 | GAAA↓LLAG | 594 | 8.07 | 6.95 |
| Q96NY8 | PVRL4 | Nectin-4 | DPQE↓DSGK | 337 | −6.80 | −4.09 |
| P18827 | SDC1 | Syndecan-1 | GPKE↓GEAV | 101 | −6.35 | −5.73 |
| P98172 | EFNB1 | Ephrin-B1 | GASG↓GSSG | 224 | −0.33 | −2.67 |
| P10586 | PTPRF | Receptor-type tyrosine-protein phosphatase F | GPFQ↓EVDG | 364 | −4.41 | −2.74 |
| Q13308 | PTK7 | Inactive tyrosine-protein kinase 7 | VPEE↓SEGP | 689 | −6.23 | −1.29 |
| Q13308 | PTK7 | Inactive tyrosine-protein kinase 7 | HPAS↓EAEI | 136 | −6.89 | −4.66 |
| P30466 | HLA-B | HLA class I histocompatibility antigen, B-18 alpha chain | ISVG↓YVDG | 50 | 2.44 | −0.77 |
| P05067 | APP | Amyloid beta A4 protein | HHQK↓LVFF | 687 | 6.61 | 1.80 |
| P22223 | CDH3 | Cadherin-3 | EVQR↓LTVT | 348 | 2.66 | 3.67 |
| P0C7U0 | ELFN1 | Protein ELFN1 | YAAE↓VVGP | 258 | 2.81 | 7.02 |
| P09603 | CSF1 | Macrophage colony stimulating factor 1 | KAFL↓LVQD | 87 | 5.31 | 4.01 |
| P04156 | PRNP | Major prion protein | HMAG↓AAAA | 114 | 3.98 | 5.00 |
| Q5ZPR3 | CD276 | CD276 antigen | VSLQ↓VAAP | 137 | 3.27 | 5.51 |
| Q8WVN6 | SECTM1 | Secreted and transmembrane protein 1 | GAEP↓QSAP | 138 | 1.12 | −0.59 |
| P05067 | APP | Amyloid beta A4 protein | VLAN↓MISE | 579 | 2.09 | 0.35 |
| O60462 | NRP2 | Neuropilin-2 | PISA↓FAGE | 806 | 3.02 | 2.37 |
| P29317 | EPHA2 | Ephrin type-A receptor 2 | QVQA↓LTQE | 509 | 3.96 | 3.43 |
| Q13308 | PTK7 | Inactive tyrosine-protein kinase 7 | TPAG↓SIEA | 488 | 2.16 | 1.41 |
| Q9BRK3 | MXRA8 | Matrix-remodeling-associated protein 8 | AAAG↓SSVV | 33 | 1.45 | 1.31 |
| P98172 | EFNB1 | Ephrin-B1 | ASGG↓SSGD | 225 | 0.27 | −0.01 |
| O75056 | SDC3 | Syndecan-3 | GSSA↓AQLP | 371 | 0.53 | 0.62 |
| Q14126 | DSG2 | Desmoglein-2 | VLEG↓MVEE | 279 | −2.71 | −3.98 |
| Q7Z5N4 | SDK1 | Protein sidekick-1 | AVSA↓QVEA | 1998 | 1.79 | −1.62 |
| P27824 | CANX | Calnexin | VVGQ↓MIEA | 472 | 0.93 | −0.12 |
| P16422 | EPCAM | Epithelial cell adhesion molecule | DVAY↓YFEK | 214 | 0.56 | −1.84 |
| P09758 | TACSTD2 | Tumor-associated calcium signal transducer 2 | DAAY↓YFER | 224 | −0.05 | −1.47 |
| P55287 | CDH11 | Cadherin-11 | NAEA↓YILN | 608 | 2.10 | 3.07 |
| P18827 | SDC1 | Syndecan-1 | EGEA↓VVLP | 104 | −0.61 | 3.95 |
| Q92823 | NRCAM | Neuronal cell adhesion molecule | FVPY↓LIKV | 817 | 2.38 | 3.18 |
| Q9Y6N7 | ROBO1 | Roundabout homolog 1 | VIPF↓LVPG | 841 | 1.12 | 0.81 |
| Q8N126 | CADM3 | Cell adhesion molecule 3 | PTAM↓IRPD | 236 | 1.10 | 2.31 |
| P30480 | HLA-B; HLA-A | HLA class I histocompatibility antigen, B-7 alpha chain | TLQS↓MYGC | 121 | −1.28 | −1.22 |
| P22223 | CDH3 | Cadherin-3 | EPVC↓VYTA | 460 | −1.00 | 2.00 |
| P04626 | ERBB2 | Receptor tyrosine-protein kinase erbB-2 | TACP↓YNYL | 300 | 2.36 | −1.11 |
| Q13740 | ALCAM | CD166 antigen | EADE↓ISDE | 513 | −4.86 | −1.57 |
| Q9Y6N7 | ROBO1 | Roundabout homolog 1 | PQPA↓IFWR | 381 | −2.50 | −2.20 |
| Q6YHK3 | CD109 | CD109 antigen | PSEA↓ISLS | 1330 | −1.14 | 1.18 |
| Q08174 | PCDH1 | Protocadherin-1 | NAEL↓VYSL | 541 | 0.24 | 4.81 |
| Q9Y624 | F11R | Junctional adhesion molecule A | TSNA↓VRME | 226 | −1.04 | 0.15 |
| P78504 | JAG1 | Protein jagged1 | HPCY↓NSGT | 792 | 1.70 | −0.26 |
| P10586 | PTPRF | Receptor-type tyrosine-protein phosphatase F | QPNT↓EYSF | 1071 | −8.26 | −4.91 |
| P16070 | CD44 | CD44 antigen | DIYP↓SNPT | 170 | −3.85 | −4.47 |
| Q15262-2 | PTPRK | Receptor-type tyrosine-protein phosphatase kappa | PDPA↓KQTD | 745 | −5.87 | −4.31 |
| Q92896 | GLG1 | Golgi apparatus protein 1 | SDLA↓MQVM | 1135 | −3.57 | −1.88 |
| Q13308 | PTK7 | Inactive tyrosine-protein kinase 7 | NSCN↓IKHT | 671 | −6.94 | −1.51 |
| P98155 | VLDLR | Very low-density lipoprotein receptor | ICIN↓LKGG | 412 | −0.49 | −2.39 |
| Q92823 | NRCAM | Neuronal cell adhesion molecule | LIIN↓IMSE | 107 | −2.44 | −3.91 |
| Q92823 | NRCAM | Neuronal cell adhesion molecule | APQN↓LVLS | 369 | 2.19 | 1.59 |
| Q13332 | PTPRS | Receptor-type tyrosine-protein phosphatase S | DPQP↓IVDG | 1275 | 5.02 | 4.15 |
| Q92823 | NRCAM | Neuronal cell adhesion molecule | HPNG↓ILTE | 980 | 0.61 | 0.51 |
| P12830 | CDH1 | Cadherin-1 | GAAG↓VCRK | 693 | −7.43 | −0.27 |
| P22223 | CDH3 | Cadherin-3 | DPAG↓WLAM | 484 | −0.57 | 1.40 |
| P18827 | SDC1 | Syndecan-1 | ATGA↓SQGL | 242 | 0.99 | 3.08 |
| P30480 | HLA-C | HLA class I histocompatibility antigen, Cw-7 alpha chain | DLRS↓WTAA | 156 | −3.91 | 1.61 |
| Q14574 | DSC3 | Desmocollin-3 | TPAA↓QYVR | 475 | 2.20 | 0.76 |
| Q14517 | FAT1 | Protocadherin Fat 1 | PPFF↓FTIV | 3477 | 2.92 | 2.73 |
| P32926 | DSG3 | Desmoglein-3 | TPMF↓LLSR | 209 | 3.29 | 3.82 |
| Q13740 | ALCAM | CD166 antigen | NVSA↓ISIP | 502 | 2.41 | 4.33 |
| Q92896 | GLG1 | Golgi apparatus protein 1 | LAMQ↓VMTS | 1137 | 2.46 | 3.50 |
| Q92823 | NRCAM | Neuronal cell adhesion molecule | PAMA↓SRQV | 1158 | 1.61 | 4.36 |
| Q14210 | LY6D | Lymphocyte antigen 6D | PSYT↓LQGQ | 72 | 0.27 | −0.23 |
| P35613 | BSG | Basigin | EPMG↓TANI | 211 | −0.01 | 2.09 |
| P58658 | EVA1C | Protein eva-1 homolog C | DPSG↓SKVL | 294 | −2.14 | −0.98 |
| P10586 | PTPRF | Receptor-type tyrosine-protein phosphatase F | TPAQ↓QQEE | 1254 | −0.07 | −4.15 |
| Q12907 | LMAN2 | Vesicular integral-membrane protein VIP36 | SVNF↓LKSP | 298 | 3.04 | 3.67 |
| P18827 | SDC1 | Syndecan-1 | ASQG↓LLDR | 245 | 0.55 | −1.12 |
| P35052 | GPC1 | Glypican-1 | HPQL↓LLPD | 182 | 3.64 | 0.73 |
| Q14126 | DSG2 | Desmoglein-2 | EIQF↓LISD | 570 | 5.13 | 4.76 |
| P15151 | PVR | Poliovirus receptor | EVQK↓VQLT | 153 | 1.30 | 3.26 |
| Q14210 | LY6D | Lymphocyte antigen 6D | LQGQ↓VSSG | 76 | 0.94 | 3.05 |
| P98172 | EFNB1 | Ephrin-B1 | GPGA↓SGGS | 222 | 1.06 | 2.28 |
| P78504 | JAG1 | Protein jagged1 | AVAE↓VRVQ | 1054 | −1.10 | 3.13 |
| Q92823 | NRCAM | Neuronal cell adhesion molecule | EVSG↓TQTT | 702 | −1.68 | 0.85 |
| P05067 | APP | Amyloid beta A4 protein | ELLP↓VNGE | 608 | 1.35 | 3.06 |
| Q92823 | NRCAM | Neuronal cell adhesion molecule | TPEG↓VPSA | 950 | −0.12 | 3.10 |
| P30508 | HLA-C | HLA class I histocompatibility antigen, Cw-12 alpha chain | EPRF↓IAVG | 46 | 0.07 | 4.02 |
| P30480 | HLA-C | HLA class I histocompatibility antigen, Cw-7 alpha chain | EPRF↓ISVG | 46 | −1.41 | 2.74 |
| Q08174 | PCDH1 | Protocadherin-1 | KKYF↓LQTT | 457 | −0.81 | −2.64 |
| P08581 | MET | Hepatocyte growth factor receptor | GFMF↓LTDQ | 228 | 6.58 | 1.36 |
The cleavage sites grouped into the metalloprotease cluster shown in Figure 6B are listed. The order from top to bottom is the same to that of the cluster.
Accessions, gene names, protein names, cleavage window sequences and P1 positions (the first upstream amino acid of the cleavage site) follow those in the SwissProt database utilized for database search. Cleavage sites are indicated with “↓”.
PWM scores for ADAM10 and ADAM17 shown in Figure 6B.
Comparison of the distances from the transmembrane domain to the cleavage site in the metalloprotease cluster, the RxxR cluster, and the remaining sites revealed that the cleavage sites in the metalloprotease cluster are located closer to the cell surface (Figure 6E). Furthermore, the cleavage sites close to the membrane (<200 amino acids in Figure 3B) are significantly enriched in metalloprotease cluster cleavage sites with the p value of 2.5 × 10−3 (Fisher's exact test). These results confirm that metalloproteases shed their substrates at positions close to the membrane.
Syndecan-1 with multiple cleavages
Of the 378 sites subjected to PWM scoring, 18 sites on 13 proteins were confirmed as proteolytic sites by the registered data in MEROPS (Table S3). For further validation, we focused on syndecan-1, and compared the quantitative terminomics results (BB-94/DMSO) with the expression profiles of the putative responsible sheddases in MEROPS with PWM scores. We obtained the mRNA expression profiles of these sheddases for nine of ten cell lines, except HCT-116, from The Human Protein Atlas (https://www.proteinatlas.org/).
We found three MEROPS-registered cleavage sites, R230↓N231, A242↓S243 and G245↓L246 (Manon-Jensen et al., 2013) of syndecan-1, in our dataset (Figures S8B and S8C). Notably, MT-MMP1 and MMP-7 gave high PWM scores (>2) to G245↓L246, and these sheddases have been reported as responsible sheddases for this site (Figure S8C). Cleavage at A242↓S243, possibly by ADAM17, MMP-9 and MMP-12 based on PWM scores>2, was observed across all analyzed cell lines, and was significantly downregulated in 9 cell lines including HCT-116, but not Hep G2 (Figure S8B). In addition to these downregulated cleavages, we found the cleavage site at R230↓N231 on syndecan-1, which has been reported to be mediated by plasmin (Manon-Jensen et al., 2013) was upregulated (Figures 5E and S8A). Interestingly, significant upregulation of the cleavage at R230↓N231 was observed only in Hep G2 cells (Figure S8A). Moreover, plasmin is expressed only in Hep G2 cells, indicating that the cleavage at R230↓N231 in Hep G2 cells would be mediated by plasmin. Furthermore, the proteolytic peptide having this upregulated site harbors two identified metalloprotease-dependent cleavage sites (Figure S8D). This proteolytic peptide (231-248) was predominantly secreted in Hep G2 cells in the presence of BB-94 (Figure S8E), indicating that the suppression of double cleaved peptides (231-242 or 231-245) by BB-94 results in the generation of the plasmin-cleaved peptide (231-248).
In vitro assay verifying the cleavage prediction by PWM scores
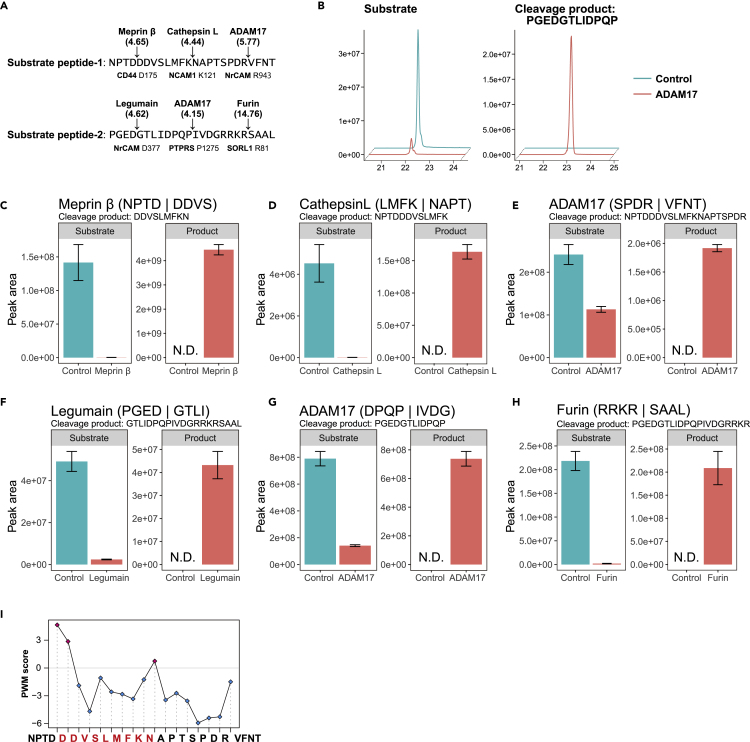
Finally, to demonstrate the accuracy of prediction based on our PWM scoring, we performed in vitro assay using peptide substrates and recombinant sheddases. We selected and tested 6 cleavage site-sheddase pairs with high PWM scores and prepared two synthetic peptides containing 3 cleavage sites for each (Figure 7A). As expected, all tested sites were successfully cleaved by the corresponding sheddases (Figures 7B–7H). Legumain has a clear D/N motif at the P1 position (Figure S7), and we also confirmed that legumain cleaved substrate peptide-2 at other D sites by quantifying peptides such as PGEDGTLIDPQPIVD, GRRKRSAAL, and GTLIDPQPIVD, in addition to the expected peptide (Figure 7F). Meprin β incubated with substrate peptide-1 generated abundant DDVSLMFKN peptide (Figure 7C) rather than DDVSLMFKNAPTSPDRVFNT, the expected product. To evaluate the cleavage selectivity, we computed PWM scores at every site of substrate peptide-1 for meprin β. We found the MFKN↓APTS sequence has a relatively higher score (Figure 7I), in accordance with the observation that the major cleavage product was DDVSLMFKN. Notably, we also detected the DVSLMFKN peptide corresponding to the second highest score (PTDD↓DVSL) (Figure 7I). Overall, these experimental results strongly validate our data set and support the accuracy of the scoring method.
Figure 7.
Validation of identified cleavage sites by in vitro assay
(A) Sequences of utilized synthetic substrate peptides. The substrates were designed as the flanking ±4 residues surrounding each cleavage site. Three substrate sequences were concatenated and synthesized as a 24-mer peptide. Arrows show the tested cleavage sites and sheddases. Parenthesized numbers show PWM scores. Under the sequences, protein names are shown with the P1 amino acid number, respectively.
(B) Detection of substrate and cleavage product by LC/MS is illustrated with their extracted ion chromatograms for ADAM17 and the DPQPIVDG sequence of PTPRS as an example.
(C–H) The peak areas of the substrate and the cleavage product peptides are calculated. The sequence of cleavage product utilized for quantification is shown above. The experiments were done in triplicate, and the data are presented as mean ± standard error of the mean (SEM). N.D., not detected.
(I) PWM scores for meprin β at respective positions of substrate peptide-1. Positive scores are highlighted in magenta and negative scores are highlighted in blue. The sequence of the cleavage product utilized for the quantification of cleavage by meprin β is highlighted in red on the x axis.
Discussion
Here, we have demonstrated that SCX-based N- and C-terminal peptide enrichment in combination with high-resolution mass spectrometry allows reproducible quantification of thousands of protein termini derived from membrane proteins in culture supernatant, with limited amounts of materials. One major advantage of our workflow is that only a very short conditioning time (1 hr) is needed for collecting the secreted proteins, which would reduce undesired alterations arising from elimination of serum (Eichelbaum et al., 2012), whereas many previous shedding studies have employed longer incubation times (Johnson et al., 2017; Tsumagari et al., 2017). To avoid non-physiological alterations caused by the serum-free condition, smart technologies have been developed that enable shedding substrate identification in the presence of serum, such as hiSPECS (Tüshaus et al., 2020). Application of our sensitive terminal peptides enrichment workflow to the shedding substrate protein samples obtained by hiSPECS might make it possible to more reliably identify physiological cleavage sites. As our protocol of N, C-terminomics consists of simple steps, including isobaric tagging and tip-based SCX, it should prove to be a practically useful platform not only for identification of shedding substrates in the supernatant, but also for conventional N, C-terminomics of cells and tissues.
The results were validated by the observation of membrane-protein-selective downregulation by BB-94. Broadly speaking, shedding occurs in the vicinity of the cell surface, thus liberating a large portion of the extracellular domain. Also, shedding targets the main components of signal transduction including protein tyrosine kinases/phosphatases and receptors. We successfully identified the cleavage site of VIP36, which could not be fully clarified by amino acid substitution and western blotting studies (Shirakabe et al., 2011), and interestingly this was the only cleavage site commonly identified across the 10 investigated cell lines. The importance of investigating diverse cell types is highlighted by the substantial differences of shedding pattern across the analyzed cell lines. We focused on the function of U-251 MG cell-unique shedding and found that the released proteins are important for the environment, rather than the cells themselves. Overall, our results provide further support for the idea that shedding contributes to cellular communication. Many of the identified putative substrate proteins were previously reported to undergo shedding, and here we have unveiled the exact cleavage sites. We wish to emphasize that this is the first study in which native shedding is overviewed at the cleavage site level on a proteome-wide scale across multiple cell types.
Our data set contains cleavage sites generated downstream of metalloprotease-dependent shedding. In addition, all fragments may be further processed by a wide range of extracellular endo- and exopeptidases. We therefore evaluated identified metalloprotease-regulated cleavage sites by PWM scoring, which we previously employed to evaluate protein kinase-phosphorylation site relationships (Imamura et al., 2017). We successfully found cleavage sites high-scored for metalloproteases, which could represent the direct substrates of the metalloproteases. In addition, we found a cluster high-scored for furin and PCSK7, which have a clear cleavage motif, RxxR. Proteolytic processing by these proteases is mainly involved in the maturation of membrane proteins. These proteases might be activated as a result of drastic upregulation of membrane protein turnover by PMA-activated shedding. We observed distinct signatures of PWMs even for ADAMs and MMPs, which have extremely similar preferences. Importantly, we demonstrated that identified shedding site sequences with high PWM scores can be indeed cleaved, at least in vitro. In the future, accumulating knowledge of cleavage sites should enable even more powerful approaches, such as machine learning, for confident prediction of sheddase-cleavage site relationships.
Our data set provides a useful resource to support hypothesis-driven studies on shedding. It seems clear that a technological breakthrough in proteomic identification of shedding cleavage sites is needed, not only from a scientific viewpoint, but also for therapeutic purposes, given the involvement of shedding in various diseases, including cancer, cardiovascular disease, inflammation, and neurodegeneration (Edwards et al., 2008; Herrlich and Herrlich, 2017; Weber and Saftig, 2012). It has been suggested that cells shed different membrane proteins depending upon external stimulation (Dang et al., 2013; Huovila et al., 2005), i.e., cells selectively shed substrates in response to different stimuli. However, how shedding is made specific has long been enigmatic. Most shedding is mediated by ADAM10 and ADAM17, and these sheddases share many substrates (Gooz, 2010; Huovila et al., 2005). In addition, sheddases generally have broad amino acid preferences, and do not require a specific amino acid at any position surrounding the cleavage site. Consequently, it is extremely difficult to understand the basal mechanisms that determine shedding targets. Our strategy presented here enables systematic investigation of changes of shedding at the site level and should facilitate work to identify critical factors that regulate shedding by means of proteomics studies.
Limitations of the study
First, termini that are not accessible by TrypN or LysC/trypsin, e.g. due to too short or too long terminal peptides, would not be captured in our methodology. Second, we have not performed biochemical validation of the specificity of spiked proteases utilized for sample preparation. Thus, there is a possibility that identified semi-specific peptides may have been generated by digestion at unpredicted sites during sample preparation; however, this seems unlikely, as previous studies have demonstrated extremely high specificity, particularly for trypsin (Olsen et al., 2004; Wilson et al., 2020). Third, we utilized a short pulse of PMA to stimulate shedding, which is an artificial stimulus, so that physiological cleavage sites of the identified proteins may be different from the identified sites. Forth, we only used in vitro peptide assay by MS to validate the sheddase-substrate relationship since we and others have experienced that traditional western blotting coupled with or without single amino acid substitution at expected cleavage sites of the substrates did not work due to the unexpected cleavage selectivity (Shirakabe et al., 2011; Tsumagari et al., 2017). Finally, as already discussed, serum-free conditions are required, which may lead to non-physiological changes, even though the duration is short in our method.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Yasushi Ishihama (yishiham@pharm.kyoto-u.ac.jp).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The raw LC/MS/MS data generated during this study have been deposited with the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the jPOST partner repository (http://jpost.org) with the data set identifier PXD021378 (JPST000632).
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
We thank Eito Yamamoto (Kyoto University) for assisting in the development of the C-terminal peptide enrichment protocol and Atsuko Sehara-Fujisawa (Kyoto University) for providing SH-SY5Y cells. K.T. was supported by a fellowship for young scientists from the Japan Society for the Promotion of Science (JSPS). This work was supported by the JST Strategic Basic Research Program, CREST (grant No. 18070870), and by a JSPS Grant-in-Aid for Scientific Research (No. 17H05667).
Author contributions
Conceptualization, K.T. and Y.I.; methodology, K.T. and C-H.C.; formal analysis, K.T.; investigation, K.T.; resources, K.T.; writing – original draft, K.T.; writing – review & editing, Y.I.; visualization, K.T.; supervision, Y.I.; project administration, Y.I.; funding acquisition, K.T. and Y.I.
Declaration of interests
The authors declare no competing interests.
Published: April 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102259.
Supplemental information
All quantified N-terminal peptides and C-terminal peptides are listed in Tables S1 and S2 respectively.
All quantified N-terminal peptides and C-terminal peptides are listed in Tables S1 and S2 respectively.
References
- Adachi J., Hashiguchi K., Nagano M., Sato M., Sato A., Fukamizu K., Ishihama Y., Tomonaga T. Improved proteome and phosphoproteome analysis on a cation exchanger by a combined acid and salt gradient. Anal. Chem. 2016;88:7899–7903. doi: 10.1021/acs.analchem.6b01232. [DOI] [PubMed] [Google Scholar]
- Aebersold R., Agar J.N., Amster I.J., Baker M.S., Bertozzi C.R., Boja E.S., Costello C.E., Cravatt B.F., Fenselau C., Garcia B.A. How many human proteoforms are there? Nat. Chem. Biol. 2018;14:206–214. doi: 10.1038/nchembio.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert A.J., Petritis K., Kangas L., Smith R.D., Mechtler K., Mitulović G., Mohammed S., Heck A.J.R. Peptide orientation affects selectivity in ion-exchange chromatography. Anal. Chem. 2010;82:5253–5259. doi: 10.1021/ac100651k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apweiler R., Hermjakob H., Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta. 1999;1473:4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- Beard H.A., Barniol-Xicota M., Yang J., Verhelst S.H.L. Discovery of cellular roles of intramembrane proteases. ACS Chem. Biol. 2019;14:2372–2388. doi: 10.1021/acschembio.9b00404. [DOI] [PubMed] [Google Scholar]
- Brown M.S., Ye J., Rawson R.B., Goldstein J.L. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- Chang C.-H., Chang H.-Y., Rappsilber J., Ishihama Y. Isolation of acetylated and unmodified protein N-terminal peptides by strong cation exchange chromatographic separation of TrypN-digested peptides. Mol. Cell. Proteomics. 2021;20:100003. doi: 10.1074/mcp.TIR120.002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow V.W., Mattson M.P., Wong P.C., Gleichmann M. An overview of APP processing enzymes and products. Neuromolecular Med. 2010;12:1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J., Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- Cox J., Neuhauser N., Michalski A., Scheltema R.A., Olsen J.V., Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011;10:1794–1805. doi: 10.1021/pr101065j. [DOI] [PubMed] [Google Scholar]
- Dang M., Armbruster N., Miller M.A., Cermeno E., Hartmann M., Bell G.W., Root D.E., Lauffenburger D.A., Lodish H.F., Herrlich A. Regulated ADAM17-dependent EGF family ligand release by substrate-selecting signaling pathways. Proc. Natl. Acad. Sci. U S A. 2013;110:9776–9781. doi: 10.1073/pnas.1307478110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhard U., Huesgen P.F., Schilling O., Bellac C.L., Butler G.S., Cox J.H., Dufour A., Goebeler V., Kappelhoff R., Auf dem Keller U. Active site specificity profiling of the matrix metalloproteinase family: proteomic identification of 4300 cleavage sites by nine MMPs explored with structural and synthetic peptide cleavage analyses. Matrix Biol. 2016;49:37–60. doi: 10.1016/j.matbio.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Edwards D.R., Handsley M.M., Pennington C.J. The ADAM metalloproteinases. Mol. Aspects Med. 2008;29:258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelbaum K., Winter M., Diaz M.B., Herzig S., Krijgsveld J. Selective enrichment of newly synthesized proteins for quantitative secretome analysis. Nat. Biotechnol. 2012;30:984–990. doi: 10.1038/nbt.2356. [DOI] [PubMed] [Google Scholar]
- Gauci S., Helbig A.O., Slijper M., Krijgsveld J., Heck A.J.R., Mohammed S. Lys-N and trypsin cover complementary parts of the phosphoproteome in a refined SCX-based approach. Anal. Chem. 2009;81:4493–4501. doi: 10.1021/ac9004309. [DOI] [PubMed] [Google Scholar]
- Gooz M. ADAM-17: the enzyme that does it all. Crit. Rev. Biochem. Mol. Biol. 2010;45:146–169. doi: 10.3109/10409231003628015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güner G., Lichtenthaler S.F. The substrate repertoire of γ-secretase/presenilin. Semin. Cell Dev. Biol. 2020;105:27–42. doi: 10.1016/j.semcdb.2020.05.019. [DOI] [PubMed] [Google Scholar]
- Helbig A.O., Gauci S., Raijmakers R., Van Breukelen B., Slijper M., Mohammed S., Heck A.J.R. Profiling of N-acetylated protein termini provides in-depth insights into the N-terminal nature of the proteome. Mol. Cell. Proteomics. 2010;9:928–939. doi: 10.1074/mcp.M900463-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlich P., Herrlich A. ADAM metalloprotease-released cancer biomarkers. Trends Cancer. 2017;3:482–490. doi: 10.1016/j.trecan.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Hinkle C.L., Sunnarborg S.W., Loiselle D., Parker C.E., Stevenson M., Russell W.E., Lee D.C. Selective roles for tumor necrosis factor α-converting enzyme/ADAM17 in the shedding of the epidermal growth factor receptor ligand family. The juxtamembrane stalk determines cleavage efficiency. J. Biol. Chem. 2004;279:24179–24188. doi: 10.1074/jbc.M312141200. [DOI] [PubMed] [Google Scholar]
- Hu X., Hicks C.W., He W., Wong P., MacKlin W.B., Trapp B.D., Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- Huang D.W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huovila A.-P.J., Turner A.J., Pelto-Huikko M., Kärkkäinen I., Ortiz R.M. Shedding light on ADAM metalloproteinases. Trends Biochem. Sci. 2005;30:413–422. doi: 10.1016/j.tibs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Imamura H., Wagih O., Niinae T., Sugiyama N., Beltrao P., Ishihama Y. Identifications of putative PKA substrates with quantitative phosphoproteomics and primary-sequence-based scoring. J. Proteome Res. 2017;16:1825–1830. doi: 10.1021/acs.jproteome.7b00087. [DOI] [PubMed] [Google Scholar]
- Johnson N., Březinová J., Stephens E., Burbridge E., Freeman M., Adrain C., Strisovsky K. Quantitative proteomics screen identifies a substrate repertoire of rhomboid protease RHBDL2 in human cells and implicates it in epithelial homeostasis. Sci. Rep. 2017;7:7283. doi: 10.1038/s41598-017-07556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleifeld O., Doucet A., Auf Dem Keller U., Prudova A., Schilling O., Kainthan R.K., Starr A.E., Foster L.J., Kizhakkedathu J.N., Overall C.M. Isotopic labeling of terminal amines in complex samples identifies protein N-termini and protease cleavage products. Nat. Biotechnol. 2010;28:281–288. doi: 10.1038/nbt.1611. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler S.F., Lemberg M.K., Fluhrer R. Proteolytic ectodomain shedding of membrane proteins in mammals—hardware, concepts, and recent developments. EMBO J. 2018;37:e99456. doi: 10.15252/embj.201899456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrus S., Trinidad J.C., Barkan D.T., Sali A., Burlingame A.L., Wells J.A. Global sequencing of proteolytic cleavage sites in apoptosis by specific labeling of protein N termini. Cell. 2008;134:866–876. doi: 10.1016/j.cell.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manon-Jensen T., Multhaupt H.A.B., Couchman J.R. Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. FEBS J. 2013;280:2320–2331. doi: 10.1111/febs.12174. [DOI] [PubMed] [Google Scholar]
- La Marca R., Cerri F., Horiuchi K., Bachi A., Feltri M.L., Wrabetz L., Blobel C.P., Quattrini A., Salzer J.L., Taveggia C. TACE (ADAM17) inhibits Schwann cell myelination. Nat. Neurosci. 2011;14:857–865. doi: 10.1038/nn.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilahti J.A.M., Elenius K. Gamma-secretase-dependent signaling of receptor tyrosine kinases. Oncogene. 2019;38:151–163. doi: 10.1038/s41388-018-0465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano O., Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004;95:930–935. doi: 10.1111/j.1349-7006.2004.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermaier S., Huesgen P.F. Positional proteomics for identification of secreted proteoforms released by site-specific processing of membrane proteins. Biochim. Biophys. Acta. 2019;1867:140138. doi: 10.1016/j.bbapap.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Olsen J.V., Mann M. Status of large-scale analysis of posttranslational modifications by mass spectrometry. Mol. Cell. Proteomics. 2013;12:3444–3452. doi: 10.1074/mcp.O113.034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J.V., Ong S.E., Mann M. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol. Cell. Proteomics. 2004;3:608–614. doi: 10.1074/mcp.T400003-MCP200. [DOI] [PubMed] [Google Scholar]
- Paetzel M., Karla A., Strynadka N.C.J., Dalbey R.E. Signal peptidases. Chem. Rev. 2002;102:4549–4580. doi: 10.1021/cr010166y. [DOI] [PubMed] [Google Scholar]
- Prudova A., Auf dem Keller U., Butler G.S., Overall C.M. Multiplex N-terminome analysis of MMP-2 and MMP-9 substrate degradomes by iTRAQ-TAILS quantitative proteomics. Mol. Cell. Proteomics. 2010;9:894–911. doi: 10.1074/mcp.M000050-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappsilber J., Mann M., Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007;2:1896–1906. doi: 10.1038/nprot.2007.261. [DOI] [PubMed] [Google Scholar]
- Rawlings N.D., Barrett A.J., Thomas P.D., Huang X., Bateman A., Finn R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018;46:D624–D632. doi: 10.1093/nar/gkx1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss K., Saftig P. The “A Disintegrin and Metalloprotease” (ADAM) family of sheddases: physiological and cellular functions. Semin. Cell Dev. Biol. 2009;20:126–137. doi: 10.1016/j.semcdb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Robinson M.D., Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11:R25. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegar T.C.M., Killingsworth L.B., Saha N., Meyer P.A., Patra D., Zimmerman B., Janes P.W., Rubinstein E., Nikolov D.B., Skiniotis G. Structural basis for regulated proteolysis by the α-secretase ADAM10. Cell. 2017;171:1638–1648.e7. doi: 10.1016/j.cell.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah N.G., Sadr M.S., Chrétien M., Mbikay M. The multifaceted proprotein convertases: their unique, redundant, complementary, and opposite functions. J. Biol. Chem. 2013;288:21473–21481. doi: 10.1074/jbc.R113.481549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakabe K., Hattori S., Seiki M., Koyasu S., Okada Y. VIP36 protein is a target of ectodomain shedding and regulates phagocytosis in macrophage Raw 264.7 cells. J. Biol. Chem. 2011;286:43154–43163. doi: 10.1074/jbc.M111.275586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami M., Nagashima Y., Sano Y., Ishihara S., Morishima-Kawashima M., Funamoto S., Ihara Y. γ-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of β-carboxyl terminal fragment. J. Neurosci. 2009;29:13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumagari K., Shirakabe K., Ogura M., Sato F., Ishihama Y., Sehara-Fujisawa A. Secretome analysis to elucidate metalloprotease-dependent ectodomain shedding of glycoproteins during neuronal differentiation. Genes Cells. 2017;22:237–244. doi: 10.1111/gtc.12466. [DOI] [PubMed] [Google Scholar]
- Tucher J., Linke D., Koudelka T., Cassidy L., Tredup C., Wichert R., Pietrzik C., Becker-Pauly C., Tholey A. LC-MS based cleavage site profiling of the proteases ADAM10 and ADAM17 using proteome-derived peptide libraries. J. Proteome Res. 2014;13:2205–2214. doi: 10.1021/pr401135u. [DOI] [PubMed] [Google Scholar]
- Tüshaus J., Müller S.A., Kataka E.S., Zaucha J., Sebastian Monasor L., Su M., Güner G., Jocher G., Tahirovic S., Frishman D. An optimized quantitative proteomics method establishes the cell type-resolved mouse brain secretome. EMBO J. 2020;39:e105693. doi: 10.15252/embj.2020105693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyanova S., Temu T., Sinitcyn P., Carlson A., Hein M.Y., Geiger T., Mann M., Cox J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods. 2016;13:731–740. doi: 10.1038/nmeth.3901. [DOI] [PubMed] [Google Scholar]
- Weber S., Saftig P. Ectodomain shedding and ADAMs in development. Development. 2012;139:3693–3709. doi: 10.1242/dev.076398. [DOI] [PubMed] [Google Scholar]
- Weeks A.M., Wells J.A. Engineering peptide ligase specificity by proteomic identification of ligation sites. Nat. Chem. Biol. 2018;14:50–57. doi: 10.1038/nchembio.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willem M., Garratt A.N., Novak B., Citron M., Kaufmann S., Rittger A., DeStrooper B., Saftig P., Birchmeier C., Haass C. Control of peripheral nerve myelination by the β-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- Wilson J.P., Ipsaro J.J., Del Giudice S.N., Turna N.S., Gauss C.M., Dusenbury K.H., Marquart K., Rivera K.D., Pappin D.J. Tryp-N: a thermostable protease for the production of N-terminal argininyl and lysinyl peptides. J. Proteome Res. 2020;19:1459–1469. doi: 10.1021/acs.jproteome.9b00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Saftig P., Hartmann D., Blobel C. Evaluation of the contribution of different ADAMs to tumor necrosis factor α (TNFα) shedding and of the function of the TNFα ectodomain in ensuring selective stimulated shedding by the TNFα convertase (TACE/ADAM17) J. Biol. Chem. 2004;279:42898–42906. doi: 10.1074/jbc.M403193200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All quantified N-terminal peptides and C-terminal peptides are listed in Tables S1 and S2 respectively.
All quantified N-terminal peptides and C-terminal peptides are listed in Tables S1 and S2 respectively.
Data Availability Statement
The raw LC/MS/MS data generated during this study have been deposited with the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the jPOST partner repository (http://jpost.org) with the data set identifier PXD021378 (JPST000632).