Introduction
Sepsis-associated acute kidney injury (S-AKI) is a common, life-threatening complication in hospitalized and critically ill patients. S-AKI increases in-hospital mortality six to eight fold,1 and the risk of developing chronic kidney disease (CKD) three fold.2,3 Furthermore, up to a quarter of patients with S-AKI will require renal replacement therapy (RRT).4
The kidney is one of the earliest injured organs during sepsis. Acute kidney injury (AKI) develops in about two thirds of patients with septic shock,5,6 and in half of them, AKI develops before presenting to the emergency department.1 Therefore, it is reasonable to consider AKI as an early sign of sepsis. Importantly, patients who recover from S-AKI have similar 1-year mortality than patients with sepsis who never developed AKI in the first place, suggesting that the pathophysiologic processes leading to S-AKI are reversible to a certain extent.
The early presentation of S-AKI limits the impact of preventive interventions, but opens the door to the development of therapeutic strategies focusing on reversing cell injury and promoting adaptive repair. To embrace this change in paradigm, the mechanisms by which renal tubular epithelial cells (TEC) are injured during sepsis, the defense strategies that TEC employ to defend from such injury and the mechanisms by which defense strategies may become maladaptive need to be better understood.
In this issue of the Clinics, we provide an overview of the current understanding of S-AKI, with emphasis on pathophysiology and biomarker development, and finalize with remarks on current preventive and therapeutic approaches.
Definitions
In 2016, sepsis was re-defined as a ‘life-threatening organ dysfunction caused by a dysregulated host immune response to infection.’7 This new definition emphasizes the importance of organ dysfunction in the pathophysiology of sepsis, and underscores the preponderant role that organ dysfunction plays in mortality by sepsis. Despite broad tools to assess organ dysfunction, such as the sequential organ failure assessment (SOFA) score,8,9 or more precise tools such as the Kidney Disease: Improving Global Outcomes (KDIGO) criteria for AKI, sepsis remains a clinical diagnosis, one that relies heavily on the experience of the clinician.
Similar to sepsis, ongoing efforts to unify the definition of AKI have yielded the three largest classification systems developed in the last two decades: the Risk, Injury, Failure, Loss of kidney function, and End stage kidney disease (RIFLE) criteria proposed by the Acute Dialysis Quality Initiative (ADQI)10, the Acute Kidney Injury network (AKIN) criteria and the most recent KDIGO criteria (Table 1).11 All three classification systems rely on an increase in serum creatinine (sCr) and/or a decrease in urinary output (UO) to establish the diagnosis of AKI.11 Despite this, current sepsis guidelines still recommend the use of the SOFA score to define AKI, which is problematic because SOFA neither distinguishes between chronic and acute kidney disease, nor considers demographic differences in baseline sCr.
Table 1.
KDIGO Criteria for Acute Kidney Injury (AKI)
| KDIGO Criteria for Acute Kidney Injury (AKI) | ||
|---|---|---|
| Stage | Serum Creatinine (sCr) | Urine Output (UO) |
| 1 | 1.5–1.9x baseline OR ≥ 0.3mg/dl (>26.5 μmol/l) increase |
<0.5 ml/kg/h for 6–12 h |
| 2 | 2.0–2.9x baseline | <0.5 ml/kg/h for 12 h |
| 3 | 3x baseline OR Increase in SrCr ≥ 4.0 mg /dl (353.6 μmol/l) OR Initiation of RRT OR In patients < 18 years, decrease in eGFR to <35 ml/min per 1.73m2 |
<0.3 ml/kg/h for ≥ 24 h OR Anuria for ≥ 12 h |
Adopted and adapted from: Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clinical practice 120, c179–184, doi:10.1159/000339789 (2012).11
In the absence of a consensus definition and based on current clinical and pathophysiologic understanding, it is reasonable to define S-AKI as a clinical syndrome characterized by an abrupt deterioration of renal function manifested by an increase in sCr, oliguria, or both, in the presence of sepsis without other meaningful explaining factors.4,12
Epidemiology
Current estimates show that S-AKI affects 10% to 67% of septic patients.13,14 More specifically, up to two thirds of patients with sepsis or septic shock will develop S-AKI.6,15 With approximately 19 million cases of sepsis occurring globally every year,16 it is reasonable to estimate that up to 11 million patients will develop S-AKI every year. Additionally, in comparison to AKI of other etiologies in critically ill patients, S-AKI carries an increased risk of death, fewer free-ventilator days and longer hospital stays.17,18 An important feature is S-AKI is an early event in the progression of sepsis. Half of the patients with septic shock develop AKI before presenting to the Emergency Department.15 In this context, AKI can play a fundamental role as a sepsis-defining event. Analogous to the canaries that would alert coal miners about the presence of lethal toxins in the air, AKI may be an early sign alerting of the presence of sepsis.
Pathophysiology of S-AKI
Limitations to a better understanding of the pathophysiology of S-AKI
Advancing the understanding of S-AKI pathophysiology faces multiple limitations. The first limitation is establishing temporality in human S-AKI. Because more than 50% of patients with septic shock develop AKI before receiving medical attention,15 it is difficult to establish which one came first.19 This not only detracts from developing effective preventive therapies, but hinders the possibility of establishing temporality as a scientific principle of causality. Second, because patients with sepsis, and more so with S-AKI, are often in critical condition, the risk of obtaining tissue biopsies largely outweighs the benefit of establishing a pathologic diagnosis, and therefore data on the pathologic evolution of S-AKI is lacking. Furthermore, although some real-time monitoring techniques have been proposed, applicability remainslimited.20 Third, despite recent progress made to unify the diagnosis of S-AKI, reliance on sCr and UO poses significant limitations to the timely diagnosis of AKI and provides no information about the specific cause of injury. The discovery of novel biomarkers of tubular injury21 and non-invasive techniques to assess renal blood flow (RBF),20 bare the promise of improving the diagnosis of S-AKI and hint toward possible causal mechanisms.
Because of these limitations, progress in understanding the mechanisms leading to S-AKI has relied largely on translational, in vitro and in vivo animal models. Although these studies have and continue to provide valuable mechanistic insight, there is a translational barrier that prevents direct extrapolation to human sepsis. Studies using post-mortem biopsies of patients dying with S-AKI have helped overcome this barrier and have revolutionized the understanding of the pathophysiology of S-AKI, as will be described in the next section. However, these studies focus on very late stages, and provide no insight into earlier stages of the disease. The Kidney Precision Medicine Project (KPMP) may offer a solution to further the understanding of human S-AKI and other forms of AKI. KPMP is a project created by The National Institutes of Health in the US that aims to ethically obtain and evaluate kidney biopsies from patients with AKI and CKD, therefore providing an unprecedented opportunity to investigate the evolution of human S-AKI. It is clear that a combination of research strategies that can move knowledge between the bench (in vitro and in vivo models) and the bedside (i.e., KPMP, observational and clinical trials) will be the most efficient approach to understand the mechanisms by which sepsis induces AKI.
Disruptive notions that have challenged existing paradigms
Although sublethal hypoperfusion may still play a role in certain cases of S-AKI, the concept that lethal cellular hypoxia leading to necrosis (i.e., acute tubular necrosis or ATN), like during ischemia reperfusion injury animal models or after aortic cross clamping during an abdominal aneurysm repair, causes S-AKI has been challenged. In an ovine model of gram-negative septic shock, Langenberg et al.22 showed that S-AKI can occur in the setting of normal or increased RBF suggesting that decreased global perfusion to the kidney was not necessary for S-AKI to occur. In a similar model, Meiden et al23. confirmed this finding, showing that S-AKI occurred without changes in RBF, oxygen delivery or renal histology. This is relevant to human sepsis because Prowle et al.20 showed that patients with septic shock with preserved RBF still developed S-AKI, and Murugan et al.24 demonstrated that a quarter of septic patients who never presented signs of hemodynamic instability still developed AKI. Importantly, Takasu and Hotchkiss25 demonstrated in postmortem biopsies of patients dying with sepsis that S-AKI develops in the absence of overt TEC necrosis or apoptosis (less than 5% of renal tubules examined). Based on this, it is clear that S-AKI can occur in the absence of overt signs of global renal hypoperfusion and/or macrohemodynamic instability,26 that S-AKI is not equivalent to ATN,19,23,27 and that mechanisms other than hypoperfusion must be at play.
Microcirculatory dysfunction
Sepsis causes alterations in regional microcirculatory flow characterized by an increase in heterogeneity of blood flow, a decrease in the proportion of capillaries carrying stopped or intermittent (non-nurturing) blood flow and a decrease in the proportion of capillaries carrying sluggish and continuous (nurturing) flow.26,28–30 This pattern of microcirculatory dysfunction is present in septic humans and in animal models across every vital organ, is independent of changes in macrohemodynamic parameters28–34 and is associated with the development of organ dysfunction and worse outcome. Based on this, microcirculatory dysfunction has been proposed to be a key mechanism in the causal pathway of organ injury.26
Multiple mechanisms have been proposed that may lead to microcirculatory dysfunction. Endothelial injury, autonomic dysfunction, shedding of the glycocalyx, and activation of the coagulation cascade result in increased leucocyte and platelet rolling and adhesion, reduction in blood flow velocity, and microthrombi formation, ultimately disrupting microvascular flow (Figure 1).17,29,35 As a consequence of altered renal peritubular capillary flow, the release damage associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) in the vicinity of TEC by slow moving leukocytes and platelets, may induce significant tubular injury.36 Furthermore, microcirculatory dysfunction can lead to altered regional blood flow distribution potentially resulting in patchy areas of ischemia and loss of autoregulation, aggravating TEC injury and dysfunction.26,37–39 In the kidney, peritubular capillary dysfunction can result in direct tubular epithelial injury. Tubular epithelial dysfunction can result in the loss of glomerular filtration rate (GFR) through the activation of the tubuloglomerular feedback by increasing non-reabsorbed chloride concentration to the macula densa. In this way, peritubular capillary dysfunction leading to renal tubular injury can result in decreased GFR and UO, and in increased sCr.
Fig. 1.
Inflammatory response and microcirculatory dysfunction. Pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) are inflammatory mediators derived from bacteria and host immune cells, respectively. These inflammatory mediators bind to pattern recognition receptors (PRRs) expressed on the surface of innate immune cells, endothelial cells, and renal TECs initiating a downstream cascade of signals. This cascade increases the synthesis of proinflammatory cytokines, reactive oxygen species (ROS), oxidative stress, and endothelial activation by nitric oxide and nitric oxide synthase (iNOS) upregulation. During inflammation, DAMPs and PAMPs are filtered in the glomeruli. Once in the tubule, these bind the Toll-like receptor (TLRs) present in the apical membrane of the TEC. In addition, some evidence suggests that TECs are also exposed to the inflammatory mediators present in the peritubular circulation, creating a double-hit effect. Moreover, the inflammatory response can also injure the TECs by increasing the oxidative stress and producing ROS. Microcirculatory dysfunction is the result of a series of events that lead to an impaired delivery of oxygen and nutrients to the tissue. Endothelial activation provoked by the inflammatory response results in a cascade of events that lead to shedding of the glycocalyx, increased leukocyte migration, and endothelial permeability. In addition, microcirculatory dysfunction is characterized by a heterogeneous flow, reduced number of capillaries with continuous flow, with an associated increase of capillaries with sluggish or no flow. Sluggish and no flow, a result of the increased expression of adhesion molecules on the inflammatory and endothelial cells, facilitate the migration of neutrophils and macrophage to the interstitial space. Furthermore, the areas with sluggish flow have increased production of ROS and oxidative stress, manifested by TEC apical vacuolization.16,35,49 APC, antigen-presenting cell; RBC, red blood cell. (Adapted from: Peerapornratana, S., Manrique-Caballero, C. L., Gó mez, H. & Kellum, J. A. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment.
Kidney international 96, 1083–1099, https://doi.org/10.1016/j.kint.2019.05.026 (2019).)
Loss of GFR during sepsis can also occur as a consequence of impaired microcirculatory hemodynamics at the glomerular level. Under normal physiologic states, GFR is tightly regulated to maintain a constant filtration rate over a wide blood pressure range, through dilation and contraction of the afferent and efferent arterioles. However, during sepsis, GFR control is impaired by at least two mechanisms. First, simultaneous constriction of the afferent arteriole and dilation of the efferent arteriole decreases glomerular hydrostatic pressure, and thereby GFR. Second, constriction of the afferent arteriole results in intrarenal shunting through extraglomerular capillaries, which bypass the glomerulus altogether and result in decreased GFR.20,26,40–42
Inflammatory Response
The inflammatory response is the host’s primary defense mechanism against infections, and is critical to initiating and mediating repair processes necessary to recover function after injury. However, a dysregulated inflammatory response may cause further injury and result in maladaptive repair. During sepsis, the recognition of released PAMPs and DAMPs43 by pattern recognition receptors (i.e., Toll-like receptors or TLR) expressed on the surface of immune cells and renal TEC initiate intracellular molecular cascades that manifest phenotypically as the inflammatory response to infection.44 In TEC, binding of DAMPs/PAMPs to TLRs (i.e. TLR2 and TLR4) triggers a downstream signaling cascade that activates nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB), which upregulates the gene expression of inflammatory cytokines and is necessary for immune cell recruitment to the site of injury and bacterial clearance.45 However, the exposure to these inflammatory mediators and the activation of innate immunity in TEC results in increased oxidative stress, reactive oxygen species production and mitochondrial injury, all of which exacerbate the TEC injury.44,46–48 Released blood-borne PAMPs and DAMPs in renal peritubular capillaries can gain access to the interstitial space and the vicinity of the basolateral membrane of TEC. In addition, PAMPs/DAMPs can be filtered through the glomerulus, and can be recognized TLR4 receptors in the apical membrane of TEC initiating inflammatory responses and inducing inflammatory and oxidative injury (Figure 1). This double hit mechanism makes proximal TECs especially susceptible to injury. A critical question that remains unanswered is whether the shutdown of tubular function is an adaptive mechanism of the kidney to avoid further injury and cell death, or just an epiphenomenon of TEC injury.
Metabolic Reprogramming
Metabolic reprograming is a conserved defense mechanism that cells use to optimize and reprioritize energy expenditure,17,49,50 and adapt to environmental or intracellular danger signals while preventing cell death.25,50–52 In sepsis, this has been better characterized in T-cells and monocytes. In response to inflammatory signals, monocytes and T-cells characteristically shift metabolism from oxidative phosphorylation (OXPHOS) toward aerobic glycolysis during the acute phase of the syndrome, reminiscent of the switch toward Warburg metabolism in cancer cells.50 Importantly, this metabolic shift is necessary for T-cells and monocytes to differentiate into pro-inflammatory phenotypes like Th-17 and M1 macrophages, respectively, and to mount an appropriate inflammatory response (Figure 2).53 Inflammatory cells shift back to OXPHOS in order to ‘turn off’ inflammation, and return to an anti-inflammatory phenotype.54,55 This switch back to OXPHOS is also necessary for animals to survive sepsis and recover organ function, because persistence of glycolysis results in increased death, and in survivors, in chronic inflammation, fibrotic repair and CKD.56,57
Figure 2. Metabolic Reprogramming.
In the early metabolic response to S-AKI, renal tubular epithelial cells undergo a proinflammatory phase (acute anabolic phase) metabolism in which the Akt/mammalian target of rapamycin complex 1 (mTORC1)/Hypoxia Inducible Factor (HIF)-1α complex drives the induction of aerobic glycolysis by increasing the expression of glycolytic enzymes (e.g. lactate dehydrogenase [LDH], PKM2 and pyruvate dehydrogenase kinase [PDHK]). HIF-1α promotes the conversion of pyruvate to lactate and, along with PDHK, inhibit the conversion of lactate into acetyl-CoA hindering the induction of the Krebs cycle and decreasing OXPHOS. In the late anti-inflammatory (adaptive catabolic phase) OXPHOS, metabolic pathways are reestablished. This is driven by adenosine monophosphate-activated protein kinase (AMPK) activation, Sirt1 and Sirt6. AMPK activates Sirt1 and Sirt6. Sirt6 will block the activity of HIF-1α switching back from aerobic glycolysis to OXPHOS. is induced by the decrease in ATP levels. AMPK activates peroxisome proliferator-activated receptor (PPAR) γ coactivator-1α (PGC)-1α and with CPT-1 will stimulate fatty acid oxidation and oxidative metabolism. Furthermore, PGC1α along with AMPK will induce mitochondrial biogenesis.17,36,50
Adopted and adapted from: Gómez, H., Kellum, J. A. & Ronco, C. Metabolic reprogramming and tolerance during sepsis-induced AKI. Nature Reviews Nephrology 13, 143, doi:10.1038/nrneph.2016.186 (2017).
Experimental data suggests that a similar reprioritization of metabolism may occur in TEC during S-AKI.49,58 Furthermore, this reprioritization might be the explanation of the dissociation that exists between function deterioration and structural changes. Using gas chromatography/mass spectrometry, we showed a decrease in the substrate flux through the tricarboxylic acid cycle, and a shift of metabolism toward glycolysis in kidneys of C57BL/6 mice 8 hours after inducing sepsis by performing cecal-ligation and puncture (CLP).49 This supports the idea that, during the early phase of sepsis, TECs switch from a highly efficient (i.e. OXPHOS) to a less efficient energy producing mechanism (i.e. aerobic glycolysis).50 Similar results have been demonstrated in in vitro studies, in which human kidney 2 cells exposed to Lipopolysaccharide (LPS) show an early increase in drivers of aerobic glycolysis and a switch back to OXPHOS.59
Metabolic reprogramming during sepsis may result in the reprioritization of TEC functions and in a decrease in ATP synthetic capacity. In support of this, CLP and human sepsis induce a decrease in ATP levels in different tissues and organs, including the kidney.60–62 Inflammatory stimuli from cytokines or PAMPs results in the downregulation of the expression of TEC ion transporters and in shut down of tubular ionic transport,17,63–67 thereby sacrificing ‘non-vital’ functions for cell survival (Figure 3). Pharmacologic manipulation of metabolic reprogramming impacts renal function and survival during sepsis in experimental models. For instance, stimulation of OXPHOS through pharmacologic activation of the metabolic master regulator AMP activated protein kinase (AMPK), results in prevention of AKI and increased survival after CLP.59 Stimulation of other OXPHOS regulators such as Sirt1 or the peroxisome proliferator-activated receptor (PPAR)-γ coactivator-1α (PGC-1α)68 also decrease mortality, supporting the notion of a protective effect of OXPHOS during sepsis.69–71 Conversely, pharmacologic inhibition of AMPK during experimental sepsis increases mortality and may impair metabolic flexibility by limiting the capacity of TEC to recruit OXPHOS and glycolysis (i.e., metabolic fitness).59 Whether protection is a direct consequence of restoring OXPHOS or other effects of these regulators on mitochondrial quality control processes such as mitophagy (recycling of dysfunctional mitochondria) or biogenesis (synthesis of new mitochondria), or on interference of cell signaling pathways like the mTORC1/HIF-1α pathway is still unclear72. Regardless, the availability of functional mitochondria is an essential component of cell metabolism, OXPHOS and metabolic reprogramming72, and therefore it is possible that the benefits of promoting OXPHOS may be secondary to the effects of OXPHOS regulators on mitochondrial function.
Figure 3. Metabolic Adaptive Response to Sepsis: Prioritizing Cell Survival.
As consequence of the early metabolic reprogramming that TEC undergoes during sepsis, non-vital functions such as cell replication, protein synthesis and ion transportation are put in ‘stand-by’, and the limited amount of ATP available is redirected toward vital functions, prioritizing cell survival at the expense of cell function. Active transport pumps such as Na+/K+ ATPase are engulfed by the cell to limit the spend of ATP. Additionally, one of the most cell energy-consuming processes is cell replication. TEC have intrinsic mechanisms that allow to detect if the cell possesses enough ATP to undergo a complete cell cycle. If this is not the case it would shut down cell replication, resulting in elevation of cycle arrest biomarkers (i.e. IGFBP7 and TIMP2). Finally, to restore TEC oxidative metabolism and normal TEC metabolism, a healthy pool of mitochondria is required. During sepsis, mitochondrial population is severely injured. As a protective mechanism, mitochondrial quality control processes such as mitophagy and biogenesis are activated as a mechanism to restore the mitochondrial pool and switch back to OXPHOS.
Adopted and adapted from: Peerapornratana, S., Manrique-Caballero, C. L., Gómez, H. & Kellum, J. A. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney international 96, 1083–1099, doi:10.1016/j.kint.2019.05.026 (2019).
Diagnosis
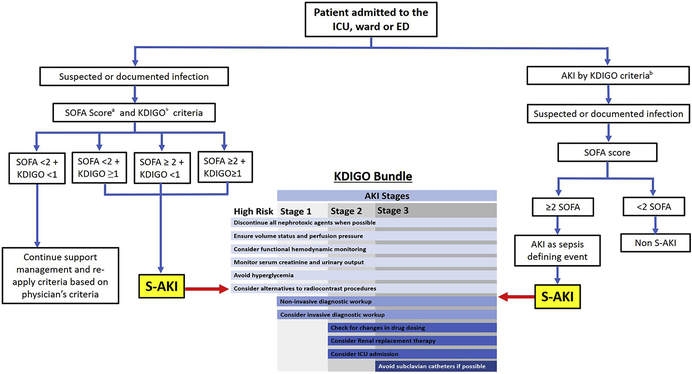
The diagnosis of S-AKI is based on sCr levels and UO in the framework of the KDIGO criteria in a patient with sepsis (Table 1).11 However, the definition of sepsis is based on SOFA score, which is problematic when evaluating AKI. First, the renal SOFA score does not account for underlying CKD and thus, can’t differentiate between new-onset AKI, underlying CKD or acute or chronic AKI. Second, SOFA relies on a discrete data point of creatinine, which is a delayed marker of renal dysfunction and provides no information about course. The assessment of renal function using the KDIGO criteria overcomes some of these limitations by detecting AKI earlier using urine output criteria, and by discriminating AKI from CKD by using the change in creatinine from baseline.11 For instance, UO may be more sensitive than spot sCr levels in detecting changes in renal function during sepsis, as changes in UO can be detected as early as every 3–5 hours. 73,74 Furthermore, changes in UO and sCr are associated with the severity of injury and with short- and long-term outcomes like the need for dialysis and mortality75, and rigorous monitoring of UO is associated with an improved survival.76 Therefore, we propose an evaluation of renal function in patients with suspected or documented infection based on KIDGO criteria and not SOFA (Figure 4).
Fig. 4.
Proposed diagnostic approach to S-AKI and AKI as a sepsis-defining event. aSepsis-3 Q18 criteria7: patient with suspected or documented infection who has a total SOFA score greater than or equal to 2. bKDIGO criteria.11 Stage 1 AKI: increase in serum creatinine level 1.5 to 1.9 times baseline or increase in serum creatinine level greater than or equal to 0.3 mg/dL within 48 hours or urine output less than 0.5 mL/kg/h for 6 to 12 hours. Stage 2 AKI: increase in serum creatinine level 2.0 to 2.9 times baseline or urine output less than 0.5 mL/kg/h for greater than or equal to 12 hours. Stage 3 AKI: increase in serum creatinine level greater than or equal to 3.0 times baseline, or increase in serum creatinine level greater than or equal to 4.0 mg/dL, or urine output less than 0.3 mL/kg/h for greater than or equal to 24 hours, or anuria for greater than or equal to 12 hours, or need for initiation of RRT. ED, emergency department; ICU, intensive care unit.
However, even with KDIGO criteria, the reliance on sCr and UO is problematic because of their lack of sensitivity and specificity and other several limitations.77 For instance, UO is difficult to track outside of the ICU, where a significant proportion of patients with early sepsis are usually admitted.78 In addition, aggressive fluid resuscitation of the septic patient may result in dilution of sCr, resulting in the underdiagnosis of S-AKI. Furthermore, decreased skeletal muscle perfusion during sepsis results in decreased creatinine production, leading once again to an underrepresentation of the alterations in glomerular filtration and tubular injury by sCr.79 In addition, ascertaining baseline sCr values is sometimes difficult, making it challenging to define real changes from baseline and determining the presence of chronic renal injury prior to the septic insult. In contrast, diagnosing AKI may not be sufficient, however, because treatment options will depend on defining and identifying the mechanisms leading to AKI. For instance, differentiating contrast-induced AKI from S-AKI is important and influences therapeutic decision making as well as prognostication of outcome. Several techniques have been put forth to address the origin of renal injury. The score based on the number of renal tubular cells and casts found in the urinary sediment has been suggested to differentiate S-AKI from other causes of AKI. Using RIFLE criteria and the need for RRT as reference standards, a score ≥ 3 was associated with higher urine Neutrophil Gelatinase-Associated Lipocalin (NGAL) levels and with increased severity of S-AKI with a sensitivity and specificity of 67.0% and 95.0%.80 Another example is the presence of de novo dipstick albuminuria within the first 24h of hospital admission, which has been associated with the development of S-AKI after adjusting for comorbidities, critical illness parameters, baseline renal function, demographics and exposure to nephrotoxins (Odds ratio [OR]:1.87, 95% Confidence Interval [CI] 1.21–2.89, p<0.01).81
A promising strategy to improve the diagnosis of S-AKI is the development of better kidney injury biomarkers. The ideal S-AKI biomarker should predict or diagnose S-AKI early in the disease course, provide information about the mechanism and location of renal injury, serve as a monitor for progression and recovery, and predict outcome. Although it is unlikely that a single biomarker will fit this profile, it is plausible that a combination of injury and functional biomarkers could fulfill these characteristics.82 The most relevant available biomarkers, their physiologic function and performance in the diagnosis of S-AKI are summarized in (Table 2) and will be described below.
Table 2.
Biomarkers for S-AKI
| Biomarker | Sample Source | Primary tubular release location | Physiologic Function | Use in S-AKI |
|---|---|---|---|---|
| NGAL | Plasma/Urine | Thick ascending limb and collecting duct | Anti-inflammatory and antiapoptotic protein that is involved in the synthesis and transport of iron into the renal tubular epithelium. 21,125,126 NGAL confers a bacteriostatic effect limiting bacterial iron uptake. 126 | Urine NGAL is more specific than plasma NGAL.87,127 However, plasma NGAL has been shown to predict S-AKI recovery.128 |
| KIM-1 | Plasma/Urine | Proximal tubules | Type 1 transmembrane glycoprotein that has an anti-inflammatory effect on the kidney. Participates in renal recovery and tubular regeneration 21 | In one prospective study, KIM-1 in the first 24h after admission had an AUC of 0.91 for the diagnosis of S-AKI. Non survivors had higher level of urinary KIM-1 at 24 and 48 hours than survivors.91 |
| L-FABP | Urine | Proximal tubules | From the lipocalin family, involved in binding and transportation of longchain fatty acids to the peroxisome and mitochondria to be metabolized. Plays a role as antioxidant reducing cellular oxidative stress due to the binding of fatty acid oxidation products.92 | In a cohort of 145 patients with S-AKI, urinary levels of L-FABP at admission were higher in non survivors with S-AKI and had a higher AUC score than APACHE II and SOFA score.93 It has also shown to be a predictor of mortality in septic children.129 |
| TIMP 2-IGFBP7 | Urine | Proximal tubules | Both proteins regulate cell growth and apoptosis. In the presence of cell injury, TIMP 2 and IGFBP7 are upregulated and may lead to G1 cell cycle arrest through the induction of p27 and p21, respectively.21,94,95 | FDA approved biomarker for risk assessment tool of AKI in sepsis. Urine TIMP2/IGFBP7 has the highest specificity for renal injury, as there is minimal elevation in the presence of other organ injury.97 High TIMP2 and IGFBP7 levels in the early phase of septic shock are independent risk factors for progression to severe AKI in the next 24h.98 |
Neutrophil Gelatinase-Associated Lipocalin (NGAL)
NGAL is expressed in many cell types, including prostate, uterus, salivary gland, lung, trachea, stomach, colon and kidney.83 NGAL functions as an iron transporter into the renal tubular cells, and it is released in the serum primarily by TEC in the presence of ischemia.84,85 Bagshaw et al, demonstrated that plasma and urinary NGAL levels are higher in S-AKI than in other AKI etiologies86, however, despite similar sensitivity (plasma NGAL: 83.0% and urinary NGAL: 80.0% ), urine NGAL has higher specificity than plasma NGAL for S-AKI (80.0% vs 57.0%).87
Kidney Injury Molecule-1 (KIM-1)
KIM-1 is a type 1 glycoprotein expressed in the membrane of proximal renal tubules upon ischemic or inflammatory injury. A meta-analysis of 11 clinical studies suggested that urinary KIM-1 had a sensitivity of 74.0% (95% CI, 61.0–84.0%), a specificity of 86.0% (95% CI, 74.0–93.0%) and an AUC of 0.86 (95% CI, 0.83–0.89) for the diagnosis of AKI.88 Despite its good performance in AKI, the evidence supporting its role specifically in S-AKI is scarce. A sepsis model in zebrafish found higher transcriptional levels of KIM-1 on the nephritic tubule at 24h in septic compared to non-septic fish.89 In a cross-sectional study of 102 patients with different AKI etiologies including contrast induced and nephrotoxins, urinary KIM-1 levels were higher in S-AKI. 90 The performance of urinary KIM-1 in S-AKI has been evaluated in one prospective study in 150 patients with sepsis, which showed that urinary KIM-1 measured within the first 24h of admission had an AUC of 0.91 for the diagnosis of S-AKI.91
Liver-type fatty acid binding protein (L-FABP)
L-FABP is a part of the lipocalin protein family and is in charge of binding free fatty acid on the cytoplasm and transporting them to the mitochondria and peroxisomes for their metabolism.92 Data on the performance of L-FABP in S-AKI is limited, in part because L-FABP is not expressed in mice, but it has shown promise in predicting severity of S-AKI. One study including 145 septic patients, demonstrated that a high urinary L-FABP level at admission to the ICU was associated with higher mortality, with higher AUC for predicting mortality than APACHE II or SOFA ( 0.99 vs 0.92 and vs. 0.81, respectively).93
Tissue Inhibitor of metalloproteinase 2 (TIMP2) and Insulin-Like Growth Factor Binding Protein 7 (IGFBP7)
TIMP2 and IGFBP7 are proteins involved in the induction of G1 cell cycle arrest, and the regulation of cell growth and apoptosis.21,94,95 In the discovery and validation studies for TIMP2 and IGFBP7, which included 522 and 744 critically ill patients respectively, the combination of urine TIMP2 and IGFBP7 had the highest sensitivity and specificity for the prediction of AKI in comparison with any other biomarker including urine KIM-1, NGAL, L-FABP, and IL-18.94,96 Importantly, the diagnostic performance of TIMP2/IGFBP7 was better in septic patients (AUC for any AKI: 0.80 vs S-AKI 0.84) and increased minimally in the presence of other causes of organ dysfunction.94,971 In addition, an elevation in TIMP2 and IGFBP7 levels early in the course of septic shock was an independent predictor of the progression from mild/moderate (KDIGO stage 1 or 2) to severe AKI (KDIGO stage 3) over the next 24h.98
Recovery from S-AKI and long-term follow-up
The recognition that cell death alone is insufficient to explain the profound loss of renal function during S-AKI,25 and that organ dysfunction may be a manifestation of cellular adaptive defense strategies,59 has led to the consideration that S-AKI may be reversible. The progression of renal dysfunction and failure to recover has been attributed to maladaptive repair. Disordered regeneration in the tubular, vascular and interstitial compartments of the kidney in response to AKI results in vascular insufficiency, glomerular hypertension, and interstitial fibrosis leading to progression to CKD99,100. The evaluation of renal recovery after AKI has many pitfalls,101 the most significant of which is the lack of a definition of recovery, until recently. The ADQI 16 consensus group recently defined AKI recovery as the absence of sCr and UO criteria (by KDIGO) within 7 days of AKI diagnosis.102
A prospective observational study including 1753 patients, found that S-AKI is associated with higher risk of death and longer hospital stay than non-septic AKI18. However, this study also showed that patients with S-AKI had lower sCr levels at hospital discharge compared to patients with non-septic AKI (median: 1.2 Interquartile range (IQR) [0.83–1.79] vs 1.37 IQR [1–2.08] mg/dl; p=0.01 ), suggesting that patients with S-AKI may have higher rates of recovery. Importantly, recovery from S-AKI improves short- and long-term survival of patients with sepsis. Kellum et al. 103 and Fiorentino et al. showed that patients who recover from S-AKI have similar one-year and three-year mortality as patients who never developed AKI in the first place1,103, supporting the theory that organ dysfunction during sepsis is not permanent.
Because of the importance to long term outcomes and the association with the development of life-threatening complications such as CKD, cardiovascular disease, bone fractures and proteinuria amongst others,104,105 it is recommended that recovery from S-AKI is monitored during the hospital stay and beyond discharge if absent.4,11,106
Prevention and treatment of S-AKI
Most therapies for S-AKI remain reactive and non-specific, focusing on preventing secondary sources of injury like pre-renal injury, venous congestion and the use of nephrotoxins, and rely on the ability of the clinician to approach each individual case. Furthermore, due to the difficulties of establishing precise timing of injury, it has been challenging to develop preventive therapies for patients admitted with new onset sepsis. However, preventive strategies may still prove useful for hospitalized patients in whom timely diagnosis can be established.
The KDIGO Bundle
The KDIGO guidelines have suggested a bundle of selected supportive strategies to prevent AKI (Figure 4). This strategy, despite not being specific to any mechanism, appears promising as the application of the KDIGO bundle in patients undergoing cardiothoracic surgery has already demonstrated benefit in reducing the frequency and severity of AKI.107 To our knowledge, the only randomized clinical trial addressing the effectiveness of the KDIGO bundle in patients with sepsis is underway in Alicante, Spain, and started recruitment in January 2020 (ClinicalTrials.gov, NCT04222361). This study will specifically assess if the implementation of the KDIGO bundle can reduce the occurrence and severity of AKI in high-risk abdominal post-surgical septic patients.
Antibiotics, source control and nephrotoxins
Whether infection is suspected, or sepsis is diagnosed, early and appropriate initiation of antibiotic treatment and identification of septic source is crucial to prevent AKI and reduce mortality. Delays in initiating appropriate antimicrobial therapy from the time of onset of hypotension in septic shock are associated with early AKI development.108,109 However, caution should be used when prescribing and monitoring antibiotic therapy, as many of the antibiotics used to treat the infection leading to sepsis are also nephrotoxic. Medications like vancomycin, particularly in combination with other antimicrobials like piperacillin-tazobactam, aminoglycosides or amphotericin B, or with other nephrotoxins like intravenous radiocontrast media should be used with caution.11
Types of Intravenous Fluids
The evidence is now clear that in critically ill and especially in septic patients, the use of hydroxyethyl starch and gelatin based solutions increases the risk of AKI and mortality110,111, and that balanced crystalloids are the fluid of choice112,113. Furthermore, the use of saline should be abandoned based on large RCTs that have now confirmed the findings of observational114,115 studies showing that the use of fluids with high chloride concentration increases the risk of AKI.
The most recent SALT-ED 116 and SMART112 trials were carried out to compare balanced crystalloids against 0.9% saline on different clinical outcomes in non-critically and critically ill patients, respectively. Both studies favored the use of balanced crystalloids demonstrating a protective effect major kidney adverse events (MAKE) at 30 days. Furthermore, the SMART trial demonstrated a larger protective effect of balanced crystalloids in septic patients than in the general population (OR: 0.80 (95%CI) vs 0.90 (95%CI), respectively.113
Although albumin-based solutions have been shown to be safe in recent multicenter RCTs117,118 dissipating concerns about renal toxicity, albumin has not been found to be superior to balanced crystalloids117 and thus, current recommendations still favor the use of balanced crystalloids for the resuscitation of patients with sepsis.
Hemodynamic Support
Fluid resuscitation followed by vasopressor agents is the cornerstone treatment in septic shock. In 2001, Rivers et al.119 published a landmark study demonstrating that Early Goal Directed Therapy (EGDT) decreased mortality of patients with septic shock. Although this was a single center trial and had limitations that have been eagerly criticized throughout the past two decades, this study changed the approach to the resuscitation of the septic patient, setting a new standard of care and probably saving many lives. This may be one of the reasons why more recent trials analyzing the effect of EGDT, have shown no benefit in terms of S-AKI, use of RRT or mortality.120
The SEPSISPAM multicenter, RCT showed that, demonstrated that the mean arterial (MAP) pressure target in sepsis must be 65–70 mmHg, because except for patients with underlying hypertension, a higher MAP (80 – 85 mmHg) did not improve survival.121 Currently, norepinephrine is the recommended first-line agent for treatment of septic shock122, whereas the use of vasopressin has been discouraged based on its cost and the confirmation in a multicenter RCT and patient-level meta-analysis that although safe, vasopressin does not improve survival as compared to norepinephrine.123,124
Conclusions
S-AKI is defined as the abrupt renal function deterioration in the presence of sepsis, is an early, common, life-threatening complication and an independent risk factor for mortality. The early presentation of renal dysfunction in the course of sepsis suggests AKI may well be one of the earliest markers of the presence of sepsis and in the context of the new definition of sepsis, a sepsis-defining event. Although the pathophysiology of S-AKI remains incompletely understood, it is clear that S-AKI is not equivalent to ATN, and that, in addition to hypoperfusion, other mechanisms are at play. The interplay between microcirculatory dysfunction, inflammation and metabolic reprogramming of the TEC in response to sepsis are candidate mechanisms that, if better understood, can open doors to specific therapies to prevent or reverse S-AKI. In parallel to understanding specific mechanisms, the identification of better biomarkers to enhance early and mechanism-sensitive detection of S-AKI remains a critical step in improving outcomes.
Synopsis.
Sepsis associated acute kidney injury (S-AKI) is a common and life-threatening complication in hospitalized and critically ill patients. It is characterized by rapid deterioration of renal function associated with sepsis. The pathophysiology of S-AKI remains incompletely understood and thus, most therapies remain reactive and non-specific. Several pathogenic mechanisms have been described to explain S-AKI, such as microcirculatory dysfunction, a dysregulated inflammatory response and cellular metabolic reprogramming. Additionally, several biomarkers have been developed in an attempt to improve diagnostic sensitivity and specificity of S-AKI. In this clinic, we will provide an overview of the current understanding of S-AKI, with particular attention to recent advances in the pathophysiology and biomarkers development and finalize with remarks on current preventive and therapeutic approaches.
Key Points.
Sepsis-associated acute kidney injury (S-AKI) is a life-threatening complication characterized by an abrupt deterioration of renal function, manifested by increased serum creatinine, oliguria, or both, associated to infection or sepsis.
Sepsis is the most common cause of AKI, and AKI is a sepsis-defining event by virtue of being one of the earliest manifestations of sepsis.
Although the pathophysiology of S-AKI remains incompletely understood, the interplay of microcirculatory dysfunction, dysregulated inflammation and metabolic reprogramming in the context of tubular dysfunction are important contributors to S-AKI.
Novel biomarkers of tubular stress and damage recently validated for risk prediction and early diagnosis of AKI, promise to provide the next step in the evolution of strategies to diagnose and monitor therapy.
Recovery from S-AKI is possible, and it is associated with a decline in mortality. Thus, unraveling mechanisms that promote renal recovery and restore function should be a priority in developing treatment strategies for S-AKI.
Clinics Care Points.
Diagnosis and definition of S-AKI primarily rely on the KDIGO criteria and SOFA score; however, these tools present many limitations and pitfalls. Therefore, a high clinical suspicion of S-AKI is needed for early diagnosis and treatment initiation.
Newer kidney injury biomarkers, with higher sensitivity and specificity, are necessary for the early diagnosis and prevention of S-AKI.
S-AKI is considered a sepsis-defining event and AKI may be one of the earliest complications of sepsis. Therefore, sepsis must be suspected in AKI with unknown origin.
Therapies for S-AKI remain reactive and non-specific. Early initiation of antibiotics, adequate hemodynamic support and avoidance of nephrotoxins remain as pillars of therapy.
Acknowledgements:
The authors thank Dr. John A. Kellum, for the many insightful, enriching and foresight discussions about sepsis and AKI, and the role of AKI as a sepsis defining event.
Funding: This work was supported in part by National Institutes of Health (NIH) Grant 1K08GM117310-01 and the University of Pittsburgh School of Medicine Dean’s Faculty Advancement Award
Disclosure: HG received research grant from TES pharma to study mechanisms of AKI in sepsis, HG is site PI of an industry sponsored grant (AM Pharma) to study the effect of recombinant alkaline phosphatase in sepsis induced AKI.
Abbreviations
- AKI
Acute kidney injury
- ATN
Acute tubular necrosis
- CKD
Chronic kidney disease
- DAMPs
Damage-associated molecular patterns
- GFR
Glomerular filtration rate
- IGFBP7
Insulin-Like Growth Factor Binding Protein 7
- ICU
Intensive care unit
- KDIGO
Kidney Disease: Improving Global Outcomes
- KIM-1
Kidney Injury Molecule-1
- L-FABP
Liver-type Fatty Acid Binding Protein
- NGAL
Neutrophil Gelatinase-Associated Lipocalin
- OXPHOS
Oxidative phosphorylation
- PAMPs
Pattern-associated Molecular Patterns
- RBF
Renal blood flow
- RRT
Renal replacement therapy
- S-AKI
Sepsis-associated acute kidney injury
- sCr
Serum creatinine
- TEC
Tubular epithelial cells
- TIMP2
Tissue inhibitor of metalloproteinase 2
- TLR
Toll-like receptors
- UO
Urinary output
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kellum JA et al. The Effects of Alternative Resuscitation Strategies on Acute Kidney Injury in Patients with Septic Shock. American journal of respiratory and critical care medicine 193, 281–287, doi: 10.1164/rccm.201505-0995OC (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wald R et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. Jama 302, 1179–1185, doi: 10.1001/jama.2009.1322 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Coca SG, Yusuf B, Shlipak MG, Garg AX & Parikh CR Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation 53, 961–973, doi: 10.1053/j.ajkd.2008.11.034 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godin M, Murray P & Mehta RL Clinical approach to the patient with AKI and sepsis. Seminars in nephrology 35, 12–22, doi: 10.1016/j.semnephrol.2015.01.003 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoste EA et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive care medicine 41, 1411–1423, doi: 10.1007/s00134-015-3934-7 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Uchino S et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294, 813–818, doi: 10.1001/jama.294.7.813 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Singer M et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810, doi: 10.1001/jama.2016.0287 %J JAMA (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Churpek MM et al. Quick Sepsis-related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores for Detecting Clinical Deterioration in Infected Patients outside the Intensive Care Unit. American journal of respiratory and critical care medicine 195, 906–911, doi: 10.1164/rccm.201604-0854OC (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao Q et al. Multicentre validation of a sepsis prediction algorithm using only vital sign data in the emergency department, general ward and ICU. 8, e017833, doi: 10.1136/bmjopen-2017-017833 %J BMJ Open (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellomo R et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical care (London, England) 8, R204–R212, doi: 10.1186/cc2872 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khwaja A KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clinical practice 120, c179–184, doi: 10.1159/000339789 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Bellomo R et al. Acute kidney injury in sepsis. Intensive care medicine 43, 816–828, doi: 10.1007/s00134-017-4755-7 (2017). [DOI] [PubMed] [Google Scholar]
- 13.White LE et al. Acute kidney injury is surprisingly common and a powerful predictor of mortality in surgical sepsis. The journal of trauma and acute care surgery 75, 432–438 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagshaw SM, George C & Bellomo R Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care 12, R47, doi: 10.1186/cc6863 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellum JA et al. The Effects of Alternative Resuscitation Strategies on Acute Kidney Injury in Patients with Septic Shock. American journal of respiratory and critical care medicine 193, 281–287, doi: 10.1164/rccm.201505-0995OC (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adhikari NK, Fowler RA, Bhagwanjee S & Rubenfeld GD Critical care and the global burden of critical illness in adults. Lancet (London, England) 376, 1339–1346, doi: 10.1016/s0140-6736(10)60446-1 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peerapornratana S, Manrique-Caballero CL, Gómez H & Kellum JA Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney international 96, 1083–1099, doi: 10.1016/j.kint.2019.05.026 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagshaw SM et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clinical journal of the American Society of Nephrology : CJASN 2, 431–439, doi: 10.2215/CJN.03681106 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Rosen S & Heyman SN Difficulties in understanding human “acute tubular necrosis”: limited data and flawed animal models. Kidney international 60, 1220–1224, doi: 10.1046/j.1523-1755.2001.00930.x (2001). [DOI] [PubMed] [Google Scholar]
- 20.Prowle JR, Molan MP, Hornsey E & Bellomo R Measurement of renal blood flow by phase-contrast magnetic resonance imaging during septic acute kidney injury: a pilot investigation. Critical care medicine 40, 1768–1776, doi: 10.1097/CCM.0b013e318246bd85 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Srisawat N & Kellum JA The Role of Biomarkers in Acute Kidney Injury. Critical care clinics 36, 125–140, doi: 10.1016/j.ccc.2019.08.010 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Langenberg C, Wan L, Egi M, May CN & Bellomo R Renal blood flow in experimental septic acute renal failure. Kidney international 69, 1996–2002, doi: 10.1038/sj.ki.5000440 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Maiden MJ et al. Structure and Function of the Kidney in Septic Shock. A Prospective Controlled Experimental Study. American journal of respiratory and critical care medicine 194, 692–700, doi: 10.1164/rccm.201511-2285OC (2016). [DOI] [PubMed] [Google Scholar]
- 24.Murugan R et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney international 77, 527–535, doi: 10.1038/ki.2009.502 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takasu O et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. American journal of respiratory and critical care medicine 187, 509–517, doi: 10.1164/rccm.201211-1983OC (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Post EH, Kellum JA, Bellomo R & Vincent JL Renal perfusion in sepsis: from macro- to microcirculation. Kidney international 91, 45–60, doi: 10.1016/j.kint.2016.07.032 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Kosaka J, Lankadeva YR, May CN & Bellomo R Histopathology of Septic Acute Kidney Injury: A Systematic Review of Experimental Data. Critical care medicine 44, e897–903, doi: 10.1097/ccm.0000000000001735 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Seely KA et al. Hemodynamic changes in the kidney in a pediatric rat model of sepsis-induced acute kidney injury. American journal of physiology. Renal physiology 301, F209–217, doi: 10.1152/ajprenal.00687.2010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Backer D et al. Microcirculatory alterations: potential mechanisms and implications for therapy. Annals of intensive care 1, 27–27, doi: 10.1186/2110-5820-1-27 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verdant CL et al. Evaluation of sublingual and gut mucosal microcirculation in sepsis: a quantitative analysis. Critical care medicine 37, 2875–2881, doi: 10.1097/CCM.0b013e3181b029c1 (2009). [DOI] [PubMed] [Google Scholar]
- 31.De Backer D, Creteur J, Preiser JC, Dubois MJ & Vincent JL Microvascular blood flow is altered in patients with sepsis. American journal of respiratory and critical care medicine 166, 98–104 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Tiwari MM, Brock RW, Megyesi JK, Kaushal GP & Mayeux PR Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. American journal of physiology. Renal physiology 289, F1324–1332, doi: 10.1152/ajprenal.00124.2005 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Holthoff JH, Wang Z, Seely KA, Gokden N & Mayeux PR Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney international 81, 370–378, doi: 10.1038/ki.2011.347 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ince C Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care 19 Suppl 3, S8, doi: 10.1186/cc14726 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verma SK & Molitoris BA Renal endothelial injury and microvascular dysfunction in acute kidney injury. Seminars in nephrology 35, 96–107, doi: 10.1016/j.semnephrol.2015.01.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez H et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock (Augusta, Ga.) 41, 3–11, doi: 10.1097/shk.0000000000000052 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dyson A et al. Microvascular and interstitial oxygen tension in the renal cortex and medulla studied in a 4-h rat model of LPS-induced endotoxemia. Shock (Augusta, Ga.) 36, 83–89, doi: 10.1097/SHK.0b013e3182169d5a (2011). [DOI] [PubMed] [Google Scholar]
- 38.Almac E, Siegemund M, Demirci C & Ince C Microcirculatory recruitment maneuvers correct tissue CO2 abnormalities in sepsis. Minerva anestesiologica 72, 507–519 (2006). [PubMed] [Google Scholar]
- 39.Rajendram R & Prowle JRJCC Venous congestion: are we adding insult to kidney injury in sepsis? 18, 104, doi: 10.1186/cc13709 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martensson J & Bellomo R Sepsis-Induced Acute Kidney Injury. Critical care clinics 31, 649–660, doi: 10.1016/j.ccc.2015.06.003 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Singh P & Okusa MD The role of tubuloglomerular feedback in the pathogenesis of acute kidney injury. Contributions to nephrology 174, 12–21, doi: 10.1159/000329229 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Calzavacca P, Evans RG, Bailey M, Bellomo R & May CN Cortical and Medullary Tissue Perfusion and Oxygenation in Experimental Septic Acute Kidney Injury. Critical care medicine 43, e431–439, doi: 10.1097/ccm.0000000000001198 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Jang HR & Rabb H Immune cells in experimental acute kidney injury. Nat Rev Nephrol 11, 88–101, doi: 10.1038/nrneph.2014.180 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Fry DE Sepsis, systemic inflammatory response, and multiple organ dysfunction: the mystery continues. The American surgeon 78, 1–8 (2012). [PubMed] [Google Scholar]
- 45.Novak ML & Koh TJ Macrophage phenotypes during tissue repair. J Leukoc Biol 93, 875–881, doi: 10.1189/jlb.1012512 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hotchkiss RS & Karl IE The pathophysiology and treatment of sepsis. N Engl J Med 348, 138–150, doi: 10.1056/NEJMra021333 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Kalakeche R et al. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. Journal of the American Society of Nephrology : JASN 22, 1505–1516, doi: 10.1681/ASN.2011020203 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dellepiane S, Marengo M & Cantaluppi VJCC Detrimental cross-talk between sepsis and acute kidney injury: new pathogenic mechanisms, early biomarkers and targeted therapies. 20, 61, doi: 10.1186/s13054-016-1219-3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waltz P, Carchman E, Gomez H & Zuckerbraun B Sepsis results in an altered renal metabolic and osmolyte profile. The Journal of surgical research 202, 8–12, doi: 10.1016/j.jss.2015.12.011 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Gómez H, Kellum JA & Ronco C Metabolic reprogramming and tolerance during sepsis-induced AKI. Nature Reviews Nephrology 13, 143, doi: 10.1038/nrneph.2016.186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hotchkiss RS et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Critical care medicine 27, 1230–1251 (1999). [DOI] [PubMed] [Google Scholar]
- 52.Singer M, De Santis V, Vitale D & Jeffcoate W Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet 364, 545–548, doi: 10.1016/s0140-6736(04)16815-3 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Frauwirth KA et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Cheng SC et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol 17, 406–413, doi: 10.1038/ni.3398 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Cheng SC et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345, 1250684, doi: 10.1126/science.1250684 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bataille A et al. Increased Fatty Acid Oxidation in Differentiated Proximal Tubular Cells Surviving a Reversible Episode of Acute Kidney Injury. Cell Physiol Biochem 47, 1338–1351, doi: 10.1159/000490819 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Lan R et al. Mitochondrial Pathology and Glycolytic Shift during Proximal Tubule Atrophy after Ischemic AKI. J Am Soc Nephrol 27, 3356–3367, doi: 10.1681/asn.2015020177 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith JA, Stallons LJ & Schnellmann RG Renal cortical hexokinase and pentose phosphate pathway activation through the EGFR/Akt signaling pathway in endotoxin-induced acute kidney injury. American journal of physiology. Renal physiology 307, F435–F444, doi: 10.1152/ajprenal.00271.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin K et al. Activation of AMP-activated protein kinase during sepsis/inflammation improves survival by preserving cellular metabolic fitness. FASEB J, doi: 10.1096/fj.201901900R (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brealey D et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360, 219–223, doi: 10.1016/s0140-6736(02)09459-x (2002). [DOI] [PubMed] [Google Scholar]
- 61.Brealey D et al. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol 286, R491–497, doi: 10.1152/ajpregu.00432.2003 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Patil NK, Parajuli N, MacMillan-Crow LA & Mayeux PR Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: mitochondria-targeted antioxidant mitigates injury. American journal of physiology. Renal physiology 306, F734–743, doi: 10.1152/ajprenal.00643.2013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mandel LJ & Balaban RS Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. The American journal of physiology 240, F357–371, doi: 10.1152/ajprenal.1981.240.5.F357 (1981). [DOI] [PubMed] [Google Scholar]
- 64.Bhargava P & Schnellmann RG Mitochondrial energetics in the kidney. Nature Reviews Nephrology 13, 629, doi: 10.1038/nrneph.2017.107 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidt C, Hocherl K, Schweda F & Bucher M Proinflammatory cytokines cause down-regulation of renal chloride entry pathways during sepsis. Critical care medicine 35, 2110–2119 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Good DW, George T & Bruns A, Watts I Lipopolysaccharide directly alters renal tubule transport through distinct TLR4-dependent pathways in basolateral and apical membranes. 297, F866–F874, doi: 10.1152/ajprenal.00335.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsiao HW et al. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock (Augusta, Ga.) 37, 289–296, doi: 10.1097/SHK.0b013e318240b52a (2012). [DOI] [PubMed] [Google Scholar]
- 68.Haden DW et al. Mitochondrial Biogenesis Restores Oxidative Metabolism during Staphylococcus aureus Sepsis. American journal of respiratory and critical care medicine 176, 768–777, doi: 10.1164/rccm.200701-161OC (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang L et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nature communications 5, 4436, doi: 10.1038/ncomms5436 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Escobar DA et al. Adenosine monophosphate-activated protein kinase activation protects against sepsis-induced organ injury and inflammation. The Journal of surgical research 194, 262–272, doi: 10.1016/j.jss.2014.10.009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Opal SM et al. PHARMACOLOGICAL SIRT1 ACTIVATION IMPROVES MORTALITY AND MARKEDLY ALTERS TRANSCRIPTIONAL PROFILES THAT ACCOMPANY EXPERIMENTAL SEPSIS. Shock (Augusta, Ga.) 45, 411–418, doi: 10.1097/shk.0000000000000528 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Sun J et al. Mitochondria in Sepsis-Induced AKI. J Am Soc Nephrol 30, 1151–1161, doi: 10.1681/asn.2018111126 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leedahl DD et al. Derivation of urine output thresholds that identify a very high risk of AKI in patients with septic shock. Clinical journal of the American Society of Nephrology : CJASN 9, 1168–1174, doi: 10.2215/cjn.09360913 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prowle JR et al. Oliguria as predictive biomarker of acute kidney injury in critically ill patients. Crit Care 15, R172, doi: 10.1186/cc10318 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kellum JA et al. Classifying AKI by Urine Output versus Serum Creatinine Level. J Am Soc Nephrol 26, 2231–2238, doi: 10.1681/ASN.2014070724 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin K et al. Intensive Monitoring of Urine Output Is Associated With Increased Detection of Acute Kidney Injury and Improved Outcomes. Chest 152, 972–979, doi: 10.1016/j.chest.2017.05.011 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Waikar SS, Betensky RA, Emerson SC & Bonventre JV Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol 23, 13–21, doi: 10.1681/ASN.2010111124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szakmany T et al. Sepsis Prevalence and Outcome on the General Wards and Emergency Departments in Wales: Results of a Multi-Centre, Observational, Point Prevalence Study. PLoS One 11, e0167230, doi: 10.1371/journal.pone.0167230 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doi K et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol 20, 1217–1221, doi: 10.1681/ASN.2008060617 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bagshaw SM et al. A prospective evaluation of urine microscopy in septic and non-septic acute kidney injury. Nephrol Dial Transplant 27, 582–588, doi: 10.1093/ndt/gfr331 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Neyra JA et al. Association of de novo dipstick albuminuria with severe acute kidney injury in critically ill septic patients. Nephron. Clinical practice 128, 373–380, doi: 10.1159/000368902 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murray PT et al. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney international 85, 513–521, doi: 10.1038/ki.2013.374 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Friedl A, Stoesz SP, Buckley P & Gould MN Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem J 31, 433–441, doi: 10.1023/a:1003708808934 (1999). [DOI] [PubMed] [Google Scholar]
- 84.Wang K et al. Biomarkers of Sepsis-Induced Acute Kidney Injury. Biomed Res Int 2018, 6937947, doi: 10.1155/2018/6937947 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Charlton JR, Portilla D & Okusa MD A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant 29, 1301–1311, doi: 10.1093/ndt/gft510 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bagshaw SM et al. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive care medicine 36, 452–461, doi: 10.1007/s00134-009-1724-9 (2010). [DOI] [PubMed] [Google Scholar]
- 87.Zhang A et al. Diagnosis and prognosis of neutrophil gelatinase-associated lipocalin for acute kidney injury with sepsis: a systematic review and meta-analysis. Crit Care 20, 41, doi: 10.1186/s13054-016-1212-x (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shao X et al. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: a meta-analysis. PLoS One 9, e84131, doi: 10.1371/journal.pone.0084131 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wen X et al. A zebrafish model of infection-associated acute kidney injury. American journal of physiology. Renal physiology 315, F291–f299, doi: 10.1152/ajprenal.00328.2017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vaidya VS et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clinical and translational science 1, 200–208, doi: 10.1111/j.1752-8062.2008.00053.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tu Y et al. Urinary netrin-1 and KIM-1 as early biomarkers for septic acute kidney injury. Ren Fail 36, 1559–1563, doi: 10.3109/0886022X.2014.949764 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Pelsers MM, Hermens WT & Glatz JF Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta 352, 15–35, doi: 10.1016/j.cccn.2004.09.001 (2005). [DOI] [PubMed] [Google Scholar]
- 93.Doi K et al. Urinary L-type fatty acid-binding protein as a new biomarker of sepsis complicated with acute kidney injury. Critical care medicine 38, 2037–2042, doi: 10.1097/CCM.0b013e3181eedac0 (2010). [DOI] [PubMed] [Google Scholar]
- 94.Kashani K et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 17, R25, doi: 10.1186/cc12503 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vijayan A et al. Clinical Use of the Urine Biomarker [TIMP-2] × [IGFBP7] for Acute Kidney Injury Risk Assessment. American journal of kidney diseases : the official journal of the National Kidney Foundation 68, 19–28, doi: 10.1053/j.ajkd.2015.12.033 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang D, Yuan Y, Guo L & Wang Q Comparison of urinary TIMP-2 and IGFBP7 cut-offs to predict acute kidney injury in critically ill patients: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 98, e16232, doi: 10.1097/MD.0000000000016232 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Honore PM et al. Urinary Tissue Inhibitor of Metalloproteinase-2 and Insulin-Like Growth Factor-Binding Protein 7 for Risk Stratification of Acute Kidney Injury in Patients With Sepsis. Critical care medicine 44, 1851–1860, doi: 10.1097/CCM.0000000000001827 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maizel J et al. Urinary TIMP2 and IGFBP7 Identifies High Risk Patients of Short-Term Progression from Mild and Moderate to Severe Acute Kidney Injury during Septic Shock: A Prospective Cohort Study. Dis Markers 2019, 3471215, doi: 10.1155/2019/3471215 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Basile DP et al. Progression after AKI: Understanding Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. J Am Soc Nephrol 27, 687–697, doi: 10.1681/ASN.2015030309 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chawla LS, Eggers PW, Star RA & Kimmel PL Acute kidney injury and chronic kidney disease as interconnected syndromes. The New England journal of medicine 371, 58–66, doi: 10.1056/NEJMra1214243 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Forni LG et al. Renal recovery after acute kidney injury. Intensive care medicine 43, 855–866, doi: 10.1007/s00134-017-4809-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chawla LS et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nature reviews. Nephrology 13, 241–257, doi: 10.1038/nrneph.2017.2 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Fiorentino M et al. Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery. PLoS One 13, e0198269, doi: 10.1371/journal.pone.0198269 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Noble RA, Lucas BJ & Selby NM Long-Term Outcomes in Patients with Acute Kidney Injury. Clinical journal of the American Society of Nephrology : CJASN 15, 423–429, doi: 10.2215/CJN.10410919 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.See EJ et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney international 95, 160–172, doi: 10.1016/j.kint.2018.08.036 (2019). [DOI] [PubMed] [Google Scholar]
- 106.Odutayo A et al. AKI and Long-Term Risk for Cardiovascular Events and Mortality. J Am Soc Nephrol 28, 377–387, doi: 10.1681/asn.2016010105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Meersch M et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive care medicine 43, 1551–1561, doi: 10.1007/s00134-016-4670-3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kumar A et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Critical care medicine 34, 1589–1596, doi: 10.1097/01.Ccm.0000217961.75225.E9 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Kumar A Optimizing antimicrobial therapy in sepsis and septic shock. Crit Care Clin 25, 733–751, viii, doi: 10.1016/j.ccc.2009.08.004 (2009). [DOI] [PubMed] [Google Scholar]
- 110.Perner A et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. The New England journal of medicine 367, 124–134, doi: 10.1056/NEJMoa1204242 (2012). [DOI] [PubMed] [Google Scholar]
- 111.Pisano A, Landoni G & Bellomo R The risk of infusing gelatin? Die-hard misconceptions and forgotten (or ignored) truths. Minerva anestesiologica 82, 1107–1114 (2016). [PubMed] [Google Scholar]
- 112.Semler MW et al. Balanced Crystalloids versus Saline in Critically Ill Adults. The New England journal of medicine 378, 829–839, doi: 10.1056/NEJMoa1711584 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Semler MW et al. Balanced Crystalloids versus Saline in the Intensive Care Unit. The SALT Randomized Trial. Am J Respir Crit Care Med 195, 1362–1372, doi: 10.1164/rccm.201607-1345OC (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yunos NM et al. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intensive care medicine 41, 257–264, doi: 10.1007/s00134-014-3593-0 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Yunos NM et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. Jama 308, 1566–1572, doi: 10.1001/jama.2012.13356 (2012). [DOI] [PubMed] [Google Scholar]
- 116.Self WH et al. Balanced Crystalloids versus Saline in Noncritically Ill Adults. N Engl J Med 378, 819–828, doi: 10.1056/NEJMoa1711586 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Caironi P et al. Albumin replacement in patients with severe sepsis or septic shock. The New England journal of medicine 370, 1412–1421, doi: 10.1056/NEJMoa1305727 (2014). [DOI] [PubMed] [Google Scholar]
- 118.Finfer S et al. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive care medicine 37, 86–96, doi: 10.1007/s00134-010-2039-6 (2011). [DOI] [PubMed] [Google Scholar]
- 119.Rivers E et al. Early Goal-Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. 345, 1368–1377, doi: 10.1056/NEJMoa010307 (2001). [DOI] [PubMed] [Google Scholar]
- 120.Angus DC et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive care medicine 41, 1549–1560, doi: 10.1007/s00134-015-3822-1 (2015). [DOI] [PubMed] [Google Scholar]
- 121.Asfar P et al. High versus low blood-pressure target in patients with septic shock. The New England journal of medicine 370, 1583–1593, doi: 10.1056/NEJMoa1312173 (2014). [DOI] [PubMed] [Google Scholar]
- 122.Rhodes A et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive care medicine 43, 304–377, doi: 10.1007/s00134-017-4683-6 (2017). [DOI] [PubMed] [Google Scholar]
- 123.Gordon AC et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. Jama 316, 509–518, doi: 10.1001/jama.2016.10485 (2016). [DOI] [PubMed] [Google Scholar]
- 124.Nagendran M et al. Vasopressin in septic shock: an individual patient data meta-analysis of randomised controlled trials. Intensive care medicine 45, 844–855, doi: 10.1007/s00134-019-05620-2 (2019). [DOI] [PubMed] [Google Scholar]
- 125.Li JY et al. Detection of intracellular iron by its regulatory effect. Am J Physiol Cell Physiol 287, C1547–1559, doi: 10.1152/ajpcell.00260.2004 (2004). [DOI] [PubMed] [Google Scholar]
- 126.Mori K et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. The Journal of clinical investigation 115, 610–621, doi: 10.1172/JCI23056 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mårtensson J et al. Neutrophil gelatinase-associated lipocalin in adult septic patients with and without acute kidney injury. Intensive care medicine 36, 1333–1340, doi: 10.1007/s00134-010-1887-4 (2010). [DOI] [PubMed] [Google Scholar]
- 128.Srisawat N et al. Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney international 80, 545–552, doi: 10.1038/ki.2011.160 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yoshimatsu S et al. Urinary L-FABP as a mortality predictor in <5-year-old children with sepsis in Bangladesh. Pediatr Int 58, 185–191, doi: 10.1111/ped.12765 (2016). [DOI] [PubMed] [Google Scholar]