Abstract
Mounting evidence suggests that obesity, parameters of metabolic syndrome, and asthma are significantly associated. Interestingly, these conditions are also associated with microbiome dysbiosis, notably in the airway microbiome for patients with asthma and in the gut microbiome for patients with obesity and/or metabolic syndrome. In this review, since improvements in asthma control, lung function and airway hyperresponsiveness are often reported after bariatric surgery, we investigated the potential role of bacterial gut and airway microbiome changes after bariatric surgery in ameliorating asthma symptoms. Rapid and persistent gut microbiota alterations were reported post-surgery, some of which can be sustained for years. The gut microbiome is thought to modulate airway cellular responses via short-chain fatty acids and inflammatory mediators, such that increased propionate and butyrate levels following surgery may aid in reducing asthma symptoms. In addition, increased prevalence of Akkermansia muciniphila after Roux-en-Y gastric bypass and sleeve gastrectomy may confer protection against airway hyperreactivity and inflammation. Metabolic syndrome parameters also improved following bariatric surgery, and whether weight loss-independent metabolic changes affect airway processes and asthma pathobiology merits further research. Fulfilling knowledge gaps outlined in this review could facilitate the development of new therapeutic options for patients with obesity and asthma.
Keywords: Obesity, microbiome, metabolic syndrome, bariatric surgery
Introduction
Asthma is a heterogeneous disease characterized by bronchial inflammation, airway hyperresponsiveness (AHR) and airway remodeling resulting in dyspnea.1 The observed heterogeneity has contributed to the current understanding that asthma treatments are most effective in specific subsets of patients based on asthma phenotype, and further research of asthma pathophysiology is needed in order to appropriately manage the multidimensionality of asthma. Multiple large cluster analyses of patients with asthma have identified various asthma endotypes with incidence being dependent on age of asthma onset, sex, body mass index (BMI), or inflammatory profiles, among others.2–4 Of these clusters, patients with early-onset asthma—asthma that presents between 0 to 12 years of age —typically exhibit atopic symptoms with a male predominant population; however, post-puberty, the population is female predominant.1
In addition to the early-onset atopic asthma endotype, cluster analyses identified a significant number of patients with late-onset asthma concurrent with obesity consisting predominantly of adult females.2–4 This latter population of mostly female patients with late-onset asthma and obesity exhibited less evidence of atopic inflammation and reported more frequent and severe exacerbations than atopic patients with asthma.4 While evidence has shown that increasing BMI is associated with severe asthma,5,6 the underlying mechanism is poorly understood, and few treatment options are available.
Interestingly, patients with obesity and asthma undergoing bariatric surgery experience a significant increase in lung function 12 months post-operation, with greater improvement in patients with late-onset non-atopic asthma and obesity.7 Such findings support the use of surgical intervention as a possible treatment for this asthma endotype.8 In addition, patients with normal serum immunoglobulin E (IgE) experienced a greater post-operative improvement in AHR following methacholine challenge when compared to their high IgE counterparts.7 These findings were supported by another study that reported patients with asthma who underwent bariatric surgery experienced an increase in small airway function and asthma control, but no improvement in the ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC), an indicator of airway obstruction.9 These studies suggested that obesity is associated with irreversible airway remodeling in asthma.
The prevalence of obesity is also associated with metabolic syndrome, and recent studies have identified a relationship between metabolic syndrome and asthma incidence.10 Although the definition of metabolic syndrome is debated, most clinicians follow the modified National Cholesterol Education Program (NCEP) Adults Treatment Panel III (ATP III) guidelines set forth by the American Heart Association and the National Heart, Lung and Blood Institute.11 According to the modified NCEP ATP III, in order to be diagnosed with metabolic syndrome the patient must exhibit at least 3 of the 5 following criteria presented in Table 1,11,12 all of which were found to be significantly associated with asthma incidence.10,13–21 Such findings support the hypothesis that diagnosis of metabolic syndrome can be used to identify individuals at higher risk for developing asthma. By considering obesity and metabolic syndrome in patients with asthma, clinicians may be able to implement early prevention management strategies, such as improved nutrition education, dietary changes, exercise regimens and weight loss programs to slow the progression of airway remodeling.22
Table 1.
Modified NCEP ATP III Values of Inclusion for Metabolic Syndrome, Adapted from Huang.12
| Criteria | Criteria Definition | Association with Asthma |
|---|---|---|
| Abdominal Adiposity | Waist Circumference > 40 inches for Males (M) > 35 inches for Females (F) |
Yes10,13,14 |
| Dyslipidemia | Triglycerides ≥ 150 mg/dl or prescription for treatment | Yes15,16 |
| Low Blood High Density Lipoprotein (HDL) | HDL < 40 mg/dl (M) < 50 mg/dl (F) or prescription for treatment |
Yes15–17 |
| Insulin Resistance/Hyperglycemia | Fasting Glucose ≥ 100 mg/dl or prescription for treatment | Yes10,18,19 |
| Hypertension | Systolic Pressure ≥ 130 mmHg Diastolic Pressure ≥ 85 mmHg or prescription for treatment |
Yes20,21 |
To explore how bariatric surgery impacts obesity and asthma outcomes, this review investigates the potential role of postoperative microbiota alterations in modulating asthma and metabolic syndrome symptoms. The microbiota refers to the collection of microorganisms living within and on us. The genomes and genes in the microbiota are collectively termed the metagenome, as obtained from shotgun sequencing. The microbiota composition can also be estimated using next generation sequencing of marker genes such as 16S ribosomal ribonucleic acid gene for prokaryotes. The term microbiome refers to the microbiota, its metagenome, and the surrounding abiotic environmental conditions.23 The reduction of cost in sequencing and the development of metagenomic analytical pipelines have increasingly allowed researchers to study the microbiome without the limitations of culture-dependent methodologies.
The role the gut microbiome plays in regulating human health has garnered researchers’ attention. The gut microbiota is incredibly diverse and distinct; in a global dataset (n = 3,948) of gut microbiomes, a total of 664 genera were discovered, of which only 14 were shared by more than 95% of individuals.24 Most gut microbiota belong to phyla Firmicutes and Bacteroidetes, followed by Actinobacteria, Proteobacteria and Verrucomicrobia.25 Gut microbiome dysbiosis, a phenomenon that occurs when the microbial community composition is altered to create an imbalance in the community, is associated with obesity (see Microbiomes in Obesity and Metabolic Syndrome Section), hypertension, diabetes, and cancer, among other diseases.26 One known mechanism through which the gut microbiome affects human health is via the production of short-chain fatty acids (SCFAs). Microbes produce SCFAs such as butyrate, formate, acetate, and propionate through fermentation of complex carbohydrates. SCFAs can regulate inflammatory immune response and also partake in maintaining colonic epithelial integrity, appetite regulation, lipid metabolism amongst others.27 The intricate relationships between the gut microbiome, asthma and/or obesity are further discussed later in this review.
For respiratory illnesses, the role of airway/lung microbiome has received particular interest. Contrary to traditional belief, the lungs are not sterile: Sze et al.28 reported that approximately 20–1,252 bacterial cells exist for every 1,000 somatic cells in the lung. The most common bacterial phyla include Firmicutes and Proteobacteria, followed by Actinobacteria and Bacteroidetes.29 In healthy individuals, the lung microbiome resembles the mouth microbiome, and lower and upper respiratory tract microbiomes are compositionally similar but the lower respiratory tract harbors less bacterial biomass.30 Among other diseases, dysbiosis in the lung microbiome is associated with chronic obstructive pulmonary disease28 and asthma (see Microbiomes and Asthma Section).
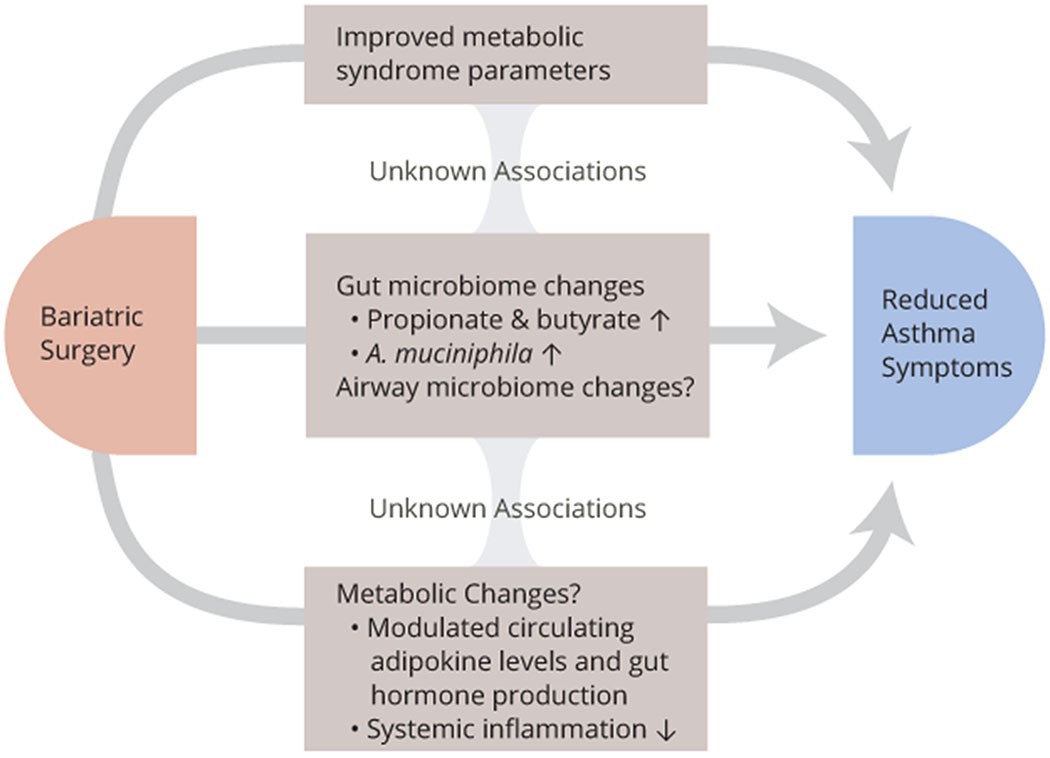
In this review, given the role of airway/lung and gut microbiomes on human health, we first explore how these microbiomes affect asthma as well as obesity and metabolic syndrome. Then we discuss the microbiota changes associated with bariatric surgery. Finally, we present the microbiome in patients with obesity and asthma in relation to previous sections to draw conclusions on whether bariatric surgery affects airway and gut microbiomes to reduce symptoms of asthma, obesity, and metabolic syndromes. An overview of mechanisms through which bariatric surgery may improve asthma pathobiology are presented in Figure 1.
Figure 1:
Mechanisms through which bariatric surgery may contribute to reduced asthma symptoms, including airway inflammation, remodeling and AHR, in addition to specific weight-loss effects. While postsurgical gut microbiome changes and possibly airway microbiome changes could impact metabolic syndrome and/or metabolic changes, more studies that explore their associations and possible causality are needed.
Microbiomes and Asthma
In recent years, exploring the role of the gut and lung microbiomes in asthma has gained traction. For reference, the taxonomy hierarchy of microbiota is as follows, from most inclusive to most specific: domain, kingdom, phylum, class, order, family, genus, and species. In the gut microbiome, children at risk of asthma had significantly lower relative abundances of genera Faecalibacterium, Lachnospira, Rothia, and Veillonella (FLVR) at 3 months of age. Supplementing germ-free mice with live FLVR significantly reduced proinflammatory cytokines interleukin (IL)-17A, IL-6, and tumor necrosis factor-alpha (TNFα) associated with severe asthma.31 Further, lower gut microbiome diversity at 1 month old32 and slower diversification of gut microbiota during infants’ first year33 were related to asthma. For children with asthma at 5 years of age (n = 60), increased risk of asthma was associated with higher abundance of Veillonella and lower abundances of Roseburia, Alistipes and Flavonifractor at 1-year of age, though the association was only significant for children with asthmatic mothers.34 The contradictory role of Veillonella may be due to the depth of sequencing analysis; identification at the species level may be needed to fully elucidate the role of genus Veillonella in asthma. Similarly, the genus Clostridium was shown to be protective of wheezing in infants35 though colonization of the species Clostridium difficile in infancy (1 month) is associated with increased risk of wheezing and asthma at 6 to 7 years old.36
In the airway microbiome, several studies reported the presence of Streptococcus pneumoniae, Haemophilus influenzae,37 Moraxella catarrhalis37,38 and Haemophilus spp.38,39 in patients with asthma. Proteobacteria were associated with worsening Asthma Control Questionnaire scores [as defined by Juniper et al.40] and increased expression of inflammation genes related to T-helper cell 17 (Th17)-directed pathways in patients with severe asthma.41 Moreover, Proteobacteria were enriched in airway39 microbiota of patients with asthma, and Proteobacteria families (Pasteurellaceae, Enterobacteriaceae, Neisseriaceae, Burkholderiaceae, and Pseudomonadaceae) correlated with increased AHR in patients with sub-optimally controlled asthma.42 In particular, Gammaproteobacteria were more abundant in the sputum of individuals with asthma compared to healthy counterparts.43 In comparison, Prevotella39,44 and Veillonella44 were less abundant in patients with asthma. Additionally, an increase in lower airway bacterial populations compared to healthy controls was observed in patients with sub-optimally controlled or severe asthma.41,42
Airway microbiota trends varied based on sputum inflammatory cell profiles: neutrophilic asthma was associated with enriched Proteobacteria,45 Moraxella, Haemophilus,46 and H. influenzae.45 Further, reduced sputum bacterial diversity characterized neutrophilic asthma as compared to other asthma phenotypes,45,46 with lower prevalence of Streptococcus, Gemella, and Porphyromonas observed.46 In comparison, patients with asthma and high sputum eosinophils had sputum enrichments of Tropheryma whipplei,45 Granulicatella adiacens, Streptococcus parasanguinis, S. pneumoniae, Veillonella rogosae, Haemophilus parainfluenzae, and Neisseria perflava.47
In the gut microbiome, diet can induce rapid microbiota changes. Thus, whether diet can influence lung and gut microbiomes and hence, airway pathobiology and asthma symptoms, was considered. One study reported that lifetime supplementation of vitamin D increased an Acinetobacter operational taxonomic unit (OTU) in female mice lungs. However, allergic airway disease induced via ovalbumin caused substantially greater alterations in the lung microbiota, signifying that the microbiota-modulating role of vitamin D may be limited.48 Other studies involved the gut microbiome in explaining how diet affects asthma. In mice, a high-fiber diet led to protection against allergen-induced peribronchial inflammation, AHR and airway mucus production through increased SCFA levels in blood. In particular, propionate reduced Th2-related proinflammatory cytokines, IL-13, IL-4 and IL-5 and Th17-directed IL-17A in the lungs in a mechanism requiring signaling through the SCFA receptor, G-protein coupled receptor 41 (GPR41).49 Also, Sun et al.50 demonstrated in mice that SCFAs promoted Th1 cell production of an immunosuppressive cytokine, IL-10, by signaling through another SCFA receptor, GPR43. Finally, Zhang et al.51 correlated high fiber intake in mice with reduced allergic responses, lower serum IgE and IL-4 levels, and a higher IL-10 level, though higher proinflammatory interferon gamma (IFN-γ) levels were also observed.
Butyrate and propionate were shown to increase differentiation of regulatory T cells (Tregs).52 Tregs suppress Th2 cell functions via cell-to-cell contact or immunosuppressive cytokines IL-10 and transforming growth factor beta, thereby reducing allergic responses.53 In mice, both in vivo and in vitro propionate treatments were shown to increase expression of IL10 and Tregs transcription factor Foxp3.54 Taken together, diet, especially fiber and SCFA production, can ultimately affect airway inflammation in allergic asthma via reducing Th2- or Th17-related proinflammatory cytokines while increasing immunosuppressive Tregs and IL-10.
Current research data suggest an interplay between diet, gut microbiome, and airway inflammation through SCFAs and pro- or anti-inflammatory cytokines, but more studies that directly compare changes in the lung microbiome with altered diet could elucidate whether reduced allergic or asthma symptoms are due solely to gut microbiota changes. Moreover, since observational human studies are associative, murine models focusing on causal links between microbiome dysbiosis and airway pathobiology would aid in developing potential prebiotic or probiotic treatments. In addition, since gut microbiome studies on asthma have hitherto emphasized gut microbiome dysbiosis in infancy, studying whether adult patients with different asthma endotypes have specific gut microbiome dysbiosis could provide valuable information in developing targeted pre- or probiotic treatments.
Microbiomes in Obesity and Metabolic Syndrome
Microbiota alterations in the gut are associated with obesity. At the phylum level, some studies suggested that obesity is associated with increased Firmicutes/Bacteroidetes (F/B) ratio in humans.55,56 However, conflicting evidence exists,57 including a meta-analysis that did not find any statistically significant correlation between F/B ratio and BMI.58 Studies vary greatly on which microbes are most affected by obesity. Microbiota dysbiosis in patients with obesity includes greater presence of Prevotellaceae, Veillonellaceae,55 Lachnospiraceae,59 Lactobacillus spp., Bacteroides fragilis,60 Roseburia sp., Faecalibacterium sp.,59 Megamonas spp., and a Prevotella spp.-dominated enterotype.61 Obesity was also associated with lower gene richness,62 lower microbiota diversity, and reduced presence of Odoribacteraceae, Clostridaceae,55 Oscillospiraceae,61 Succinivibrio sp., and Oscillospira sp.59 Bifidobacterium spp. were inversely correlated with BMI, whereas B. fragilis and Lactobacillus spp. were positively correlated.60 Patients with obesity also had higher serum concentrations of proinflammatory cytokines IL-6 and TNFα62 and increased gut permeability than lean subjects.55
SCFAs are identified as modulators of asthma-related cytokines (Microbiomes and Asthma Section). Their role in obesity is more complicated due to their contradictory effects: SCFAs provide an extra energy source, and thus, may promote obesity, but simultaneously stimulate appetite and satiety regulating hormones to help prevent obesity. In humans, higher fecal acetate, butyrate,56 propionate,57 and total SCFAs56,57 were observed with obesity, and levels of SCFAs are correlated with hypertension, obesity, and gut microbiota dysbiosis.63 Conversely, a study reported reduced fecal propionic and butyric acids concentrations in children with obesity59 and in another, inulin-propionate ester supplementation increased satiety hormones peptide YY and glucagon like peptide-1 to achieve reduced weight gain as compared to inulin controls.64 In murine studies, supplementation of acetate, propionate, butyrate or their mixture conferred protection against diet-induced obesity,65,66 insulin resistance,66 and reduced increases in triglycerides and IL-6.65 Thus, the complex interplay between gut microbiota, SCFA production, obesity and metabolic syndrome merits further research if SCFAs or their microbial producers are to be considered for obesity and/or asthma therapies.
Several human cohort studies identified significant associations between gut microbiota dysbiosis and changes in parameters of metabolic syndrome. Fu et al.67 noted, after correcting for age and sex, that gut bacterial richness was negatively correlated with both BMI and serum levels of triglycerides and positively correlated with serum HDL levels. This study estimated that as high as 6% of the variation in blood lipid levels may be attributed to gut microbiota composition, independent of age, sex and genetic factors. Similarly, Le Roy et al.68 demonstrated that, in older females, gut biodiversity impacted visceral fat mass accumulation, either independently of, or in synergy with, diet, suggesting that a complex mechanism influenced by gut microbiota composition governs the development of abdominal adiposity. Gut microbiota dysbiosis was further associated with the pathogenesis of hypertension69 and insulin resistance.70 Palmu et al.,69 among others, identified an increased risk of hypertension in individuals with poor gut microbial diversity stemming from changes in dietary intake. Furthermore, Kayser et al.70 associated lower microbial gene richness with insulin resistance through ceramide accumulation. Taken together, current literature suggests an association between the parameters of metabolic syndrome and gut microbiome composition; however, further research is needed to better understand gut microbial diversities influence on metabolic syndrome development. While a correlation exists between the gut microbiome, obesity, and parameters of metabolic syndrome, the same cannot be said for the lung microbiome. Aside from sections of studies that explore the airway/lung microbiomes of patients with obesity and asthma (in Bariatric Surgery, Microbiome Alterations, and Asthma Concurrent with Obesity Section), all research studies identified through our literature search focused exclusively on gut microbiome alterations in relation to obesity. A large knowledge gap exists as to whether obesity, unassociated with asthma, alters the airway/lung microbiome, and if the change in microbiota composition leads to increased susceptibility to asthma. Furthermore, there is a dearth of literature correlating metabolic syndrome parameters and alterations in the airway microbiome. Given the associations between metabolic syndrome parameters and asthma (Table 1), this merits further attention to enhance our understanding of the multifaceted modulators of airway microbiome and asthma severity.
Microbiomes and Bariatric Surgery
In addition to reduced caloric intake, a potential explanation for weight reduction following bariatric surgery is through gut microbiota alterations. The two most common bariatric surgery procedures are Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG); both physically restrict stomach size, and RYGB is also malabsorptive.71 From literature review, taxa that exhibited statistically significant change in two or more human studies as compared to pre-RYGB and/or SG operation conditions are summarized below (Table 2). Both surgery types induce changes in the microbiome, but RYGB induces greater microbiota alterations as compared to laparoscopic adjustable gastric banding72 and to SG.73 Gut microbiota alterations were observed as early as 1 month after surgery.74 Significant shifts were observed at 3 months73,75–79 and at 6 months.77,80 Interestingly, at 3 months, two studies reported an increase in microbiota commonly found in oral cavity explained by an increase of gastrointestinal pH after RYGB.73,77 This increase was a transient change attenuated by 6 months,77 whereas other microbiota changes were sustained through 12 months.75,76,80 These findings suggest that bariatric surgery induces rapid changes in gut microbiota, some of which are persistent.
Table 2.
Bariatric Surgery-Induced Gut Microbiota Changes in Humans Compared to Preoperative Conditions
| Increase | Decrease | |
|---|---|---|
| RYGB | Bacteroidetes71 Firmicutes81 Fusobacteria73,75 Proteobacteria73,75,78 Akkermansia79,80 Alistipes82 Bacteroides82 Citrobacter78,80 Fusobacterium73 Granulicatella73,80 Klebsiella79,80 Streptococcus76,79,80,83 Veillonella73,76,78,80 Alistipes shahii75,79 Streptococcus parasanguinis75,79 Streptococcus salivarius75 Streptococcus thermophilus75,79 Veillonella dispar75,78 Veillonella parvula75,78 |
Bacteroidetes81 Firmicutes78 Bifidobacteriaceae73 Anaerostipes78 Bacteroides79 Bifidobacterium73,82 Blautia76,80,82 Faecalibacterium78,83 Coprococcus comes78 Faecalibacterium prausnitzii75,78 Streptococcus salivarius83 |
| SG | Bacteroidetes81 Fusobacteria74 Akkermansia73,79 Alistipes83 Fusobacterium74 Klebsiella79 Streptococcus79,83 Alistipes shahii79 Faecalibacterium prausnitzii84 Streptococcus parasanguinis79 Streptococcus salivarius83 Streptococcus thermophilus79 |
Firmicutes74 Bifidobacteriaceae73,74 Anaerostipes73 Bacteroides79 Bifidobacterium73 Coprococcus comes84 |
Additional studies demonstrate that alterations to gut microbiota remain after 1 year81 to and even up to a decade85 after bariatric surgery. The following studies compared gut microbiota of non-operated controls to patients who had undergone RYGB, and thus are limited by inter-individual differences such as diet, lifestyle, and genetic differences. Fouladi et al.86 demonstrated that 2-5 years after RYGB, subjects that received surgery had increased Micrococcales, Lactobacillales, Rothia and Streptococcus. Similarly, Tremaroli et al.87 reported increases in Proteobacteria genera Escherichia, Klebsiella, and Pseudomonas that were observed 9 years after surgery. Furthermore, 10.6 years after RYGB, those in the surgery group had more Verrucomicrobiaceae and Streptococcaceae, but less Bacteroidaceae.85 Overall, these studies suggest that RYGB may induce a varied and persistent microbiota change.
In order to reduce confounding variables, rodent models were used to study the effects of bariatric surgery. In mice, SG increased relative abundance of Bacteroidetes and reduced Firmicutes, which was observed even after co-housing with sham-operated mice.88 Haange et al.89 compared post-RYGB rats to sham-operated, body weight matched rats and reported RYGB-induced increases in Proteobacteria and Actinobacteria and a decrease in Firmicutes independent of weight loss. While these increases agree with human studies in Table 2, they also observed an increase of Bifidobacteriaceae,89 contradicting human studies.73,74 Murine models were also used to verify functional changes in gut microbiota. Colonization of mice with feces from post-RYGB human subjects (9.4 years post-surgery) attenuated body fat gain as compared to mice colonized with fecal samples from post-vertical banded gastroplasty subjects or from subjects with obesity.87 Furthermore, mice colonized with feces from post-RYGB subjects (2-5 years post-surgery) who successfully lost weight (% excess weight loss (EWL) > 50%) gained less weight compared to mice colonized with feces from post-RYGB subjects that did not lose weight. Since the two human cohorts’ gut microbiota composition did not differ significantly, this difference suggests that long-term gut microbiota functionality changes may also affect weight loss after bariatric surgery.86
Some patients who have undergone bariatric surgery also reported improvements in metabolic syndrome parameters including reduced waist circumference,62,71,73,76,84 reduced triglycerides,73,76,84 reduced fasting glucose,62,75 increased HDL,76 and reduced blood pressure.62,73,84 Interestingly, some of the microbiota changes were also associated with metabolic syndrome parameters. Veillonella increase after RYGB73,76,78,80 is inversely correlated with waist circumference73 and positively with percent EWL.72 Escherichia, Akkermansia, Enterococcus and Carnobacterium were also positively correlated with percent EWL, whereas Bifidobacterium and Sutterella were negatively correlated.72 Following SG, reduction in waist circumference correlated negatively with Proteobacteria.71 Verrucomicrobia, particularly Akkermansia, tended to correlate positively with HDL cholesterol.73 Nonetheless, whether the microbiota changes are directly responsible for metabolic syndrome improvements remain unclear. This highlights a need for studies that elucidate mechanistic pathways through which gut microbiota may modulate metabolic syndrome.
Finally, whether bariatric surgery-induced microbiota changes differed from diet-induced changes were explored. Unlike bariatric surgery, medical weight loss (diet and physical activity) did not induce major changes in microbiota.71,90 Increased prevalence of Roseburia90 and Akkermansia were reported in association with medical weight loss.91 While transient changes were observed at 3 months after medical weight loss, the gut microbiota composition had a tendency to return to baseline composition after 1 year (except for an increase in Akkermansia), which differs from long-term changes observed with bariatric surgery.91 One study reported more significant diet-induced changes including increased F/B ratio.84 Another explored the effect of crash diet prior to RYGB or SG, which resulted in reduced Shannon diversity [a measure of species diversity in a community that takes into account both the abundance and evenness of a particular species in the overall community92], Streptococcaceae and Ruminococcaceae relative abundance, and increased Rikenellaceae prevalence. Following bariatric surgery, Shannon diversity was restored to baseline levels.93 Since a restrictive diet is often recommended before bariatric surgery, this observation raises the question of whether the comparison to a baseline after a restrictive diet in studies analyzing postoperative microbiota changes is appropriate.
Evidence currently suggests that bariatric surgery induces human gut microbiota alterations which may last for years after surgery. However, because studies discerning long-term effects of bariatric surgery compared microbiota to non-operated controls,85,87 similar studies that compare the same cohort to pre-bariatric surgery baselines are recommended to reduce inter-individual differences. Human studies are also limited by low sample size, some with less than 10 patients who underwent bariatric surgery.74,78,80,84,90 There is a clear need for more studies that consider the effect of a restrictive diet before surgery if one was employed. Finally, despite growing evidence that bariatric surgery could be beneficial for patients with asthma,7,9,94,95 to the best of our knowledge no study has explored whether the airway/lung microbiome changes after bariatric surgery.
Bariatric Surgery, Microbiome Alterations, and Asthma Concurrent with Obesity
Bariatric surgery is associated with improvements in asthma control7,95,96 and in AHR,7 though this protective effect was not observed in patients with metabolic syndrome.96 Provided the relationships between the airway and gut microbiomes with obesity, asthma and bariatric surgery as presented in Figure 2, we questioned if the microbiomes contribute to bariatric surgery-associated protection against asthma. However, only a few studies could be identified that analyze microbiomes of patients with obesity and asthma, rendering a comparison between pre- and post-surgery microbiomes difficult.
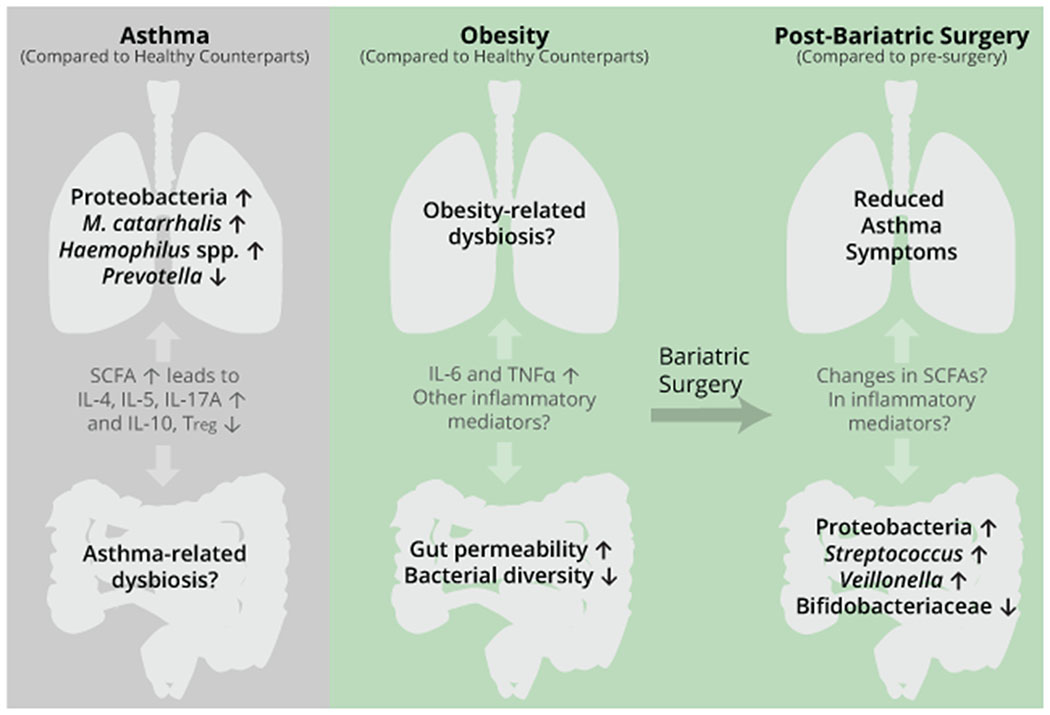
Figure 2:
Microbiome dysbiosis in patients with obesity or asthma compared to healthy counterparts and postsurgical changes following bariatric surgery. Microbial taxa were chosen because they were amongst the most reported changes in literature. Full taxa changes are available in the Microbiomes and Asthma Section for asthma and in Table 2 for bariatric surgery. Short chain fatty acids (SCFAs) and inflammatory mediators such as interleukins (IL), regulatory T cells (Tregs) and tumor necrosis factor alpha (TNFα) are key signaling features of the gut/lung axis, and knowledge gaps are marked with a question mark.
Huang et al.41 reported that patients with obesity and severe asthma had increased prevalence of taxa belonging to Prevotellaceae, Mycoplasmataceae, Lachnospiraceae (Clostridium), and Spirochaetaceae (Treponema). Furthermore, a quantitative polymerase chain reaction analysis positively correlated Prevotella spp. with increased BMI. Similarly, taxa in Bacteroidetes and Firmicutes could be significantly correlated with BMI.41 Michalovich et al.97 compared gut and airway microbiomes between patients with obesity, patients with both obesity and asthma, patients with asthma and healthy controls. In the lower respiratory tract, decreases of Enterococcaceae, Aeromonadaceae, Paraprevotella, Phascolarctobacterium, and Megasphaera were reported that were unique to patients with obesity and asthma, and a decrease of Parabacteroides and Coriobacteriaceae in both patients with obesity and all patients with asthma compared to healthy counterparts. Unfortunately, to the best of our knowledge, no study has reported lung or airway microbiome changes after bariatric surgery; thus, future research is needed to elucidate whether relative abundances of taxa associated with patients with obesity and asthma are altered following surgery.
In a murine model, administration of antibiotics to db/db mice with genetic obesity reduced ozone-induced AHR. Furthermore, fecal transplantation from db/db mice to lean germ-free mice resulted in ozone-induced AHR. Since ozone is a common non-atopic asthma trigger, this suggests that the gut microbiota associated with obesity may contribute to augmented AHR in nonatopic asthma.98 In gut microbiota of patients with obesity and asthma, Michalovich et al.97 reported an increased prevalence of Tissierellaceae (genus 1-68) and decreased Serratia compared to healthy counterparts; further, a reduction in Clostridiales was observed as with other patients with obesity. However, over 70% of the patients with asthma (with and without obesity) in this study had allergy. Thus, analyses of gut microbiome in patients with asthma and obesity that are stratified by atopic status or Th2 inflammatory gene signatures may reveal important differences in the influence of gut microbiome on clinical outcomes in specific asthma phenotypes.
Current data on bacterial gut microbiota changes following bariatric surgery and their relationships to asthma are insufficient, although one species shows promise. In a single study, fecal A. muciniphila levels were negatively correlated with asthma severity independent of BMI, and both acute and chronic murine models suggested that A. muciniphila conferred protection against airway hyperreactivity and inflammation.97 A. muciniphila’s role in airway health merits attention as this species was reported to increase after RYGB and SG.75,77,79 Lower abundances of Alistipes,34 Veillonella, and Faecalibacterium31 were associated with increased risk of asthma in infants. Of these, Alistipes82,83 and Veillonella73,76,78,80 were reported to increase following bariatric surgery; in comparison, decreases in Faecalibacterium were reported.78,83 Given the physiological differences between an infant and an adult, and the conflicting evidence that Veillonella is positively correlated with increased risk of asthma,34 whether any gut microbiota changes following bariatric surgery is associated with altered airway pathobiology and improved clinical outcomes in asthma remains a knowledge gap aside from possible beneficial effects of increased presence of A. muciniphila.
A possible link between gut microbiota alterations and improved asthma symptoms includes increased production of SCFAs, particularly butyrate and propionate. Following RYGB, increases of propionate/acetate and butyrate/acetate ratios were reported in humans,72,80 though another study reported a postsurgical tendency of SCFAs to decrease after RYGB.87 While SCFAs have a conflicting role in obesity, butyrate50,52 and propionate49,50,52,54 were associated with reduced proinflammatory cytokines and/or increased immunosuppressive cytokines which could in turn modulate the pathobiology of asthma concurrent with obesity. Thus, the role of increased propionate and butyrate levels and their impact on asthma-related cytokines is another knowledge gap. This is particularly important because postsurgical improvements in AHR paradoxically coincided with increases of proinflammatory TNFα and IL-6 cytokines, among others, both in humans7 and in mice.99 Hence, another question remains about which beneficial effects, if any, of SCFA-related cytokines could mitigate harmful effects of increased proinflammatory cytokines.
To advance the field, the baseline lung and gut microbiota composition of individuals with asthma and obesity before surgery needs to be established, and secondly, how microbiome changes affect postsurgical improvements in asthma symptoms should be evaluated. In particular, we have identified that studying the relationship between improvements in asthma symptoms based on asthma phenotypes and microbiota changes may aid in developing personalized pre- or probiotic treatment. In addition, there is a dearth of information on whether weight-independent metabolic changes following bariatric surgery result in remediation of asthma symptoms. At present, some postsurgical changes in the gut microbiome are correlated with parameters of metabolic syndrome. Furthermore, reduced asthma medication usage95 and gut microbiome alterations were reported as early as 30 days post-surgery,74 which is likely too soon for weight loss to have a large impact. Thus, a central question is whether this improvement in asthma control is due to metabolic changes and whether these changes are caused by microbiome alterations. Given the association between metabolic syndrome parameters and asthma (Table 1), if changes in gut microbiome can be strongly associated with improvement of asthma outcomes through metabolic syndrome remission, new microbiome-based therapeutics may be devised for patients with obesity and asthma.
Conclusion
Microbiome dysbiosis is a contributing factor in metabolic syndrome, obesity and asthma. Herein, we considered the role of bariatric surgery and subsequent microbiota changes for patients with obesity and asthma. We did not review the impact of viruses, archaea, and fungi on asthma. We also did not examine antibiotic treatment for asthma, though azithromycin appears to reduce severe asthma exacerbations while improving quality of life for patients with persistent symptomatic asthma.100 All these factors will affect post-surgical outcomes and should ultimately be considered.
Bariatric surgery results in long-term bacterial gut microbiota changes that may influence airway inflammation, AHR and lung function through improvements in metabolic syndrome parameters and SCFA production. By studying changes in the lung/airway microbiomes following bariatric surgery, researchers can further untangle the complex interplay between obesity, asthma, and the microbiome. Advancing research will help determine whether bariatric surgery converts the airway and/or gut microbiome of patients with asthma to the same state as lean patients with asthma or as lean subjects without asthma, and elucidate how the altered microbiomes affect asthma pathobiology in patients with obesity. By filling the knowledge gaps highlighted in this review, we are hopeful that new therapeutic options will become available to patients with obesity and asthma.
Study Importance Questions.
What is already known about this subject?
Parameters of metabolic syndrome and increasing body mass index (BMI) are significantly associated with asthma.
The gut microbiome not only influences obesity and metabolic syndrome but also affects airway inflammation via short chain fatty acid production and modulation of cytokine levels.
Significant improvements in asthma control and airway hyperresponsiveness are reported following bariatric surgery.
What are the new findings in your manuscript?
Bariatric surgery induces rapid yet persistent changes in gut microbiota composition that may influence asthma pathobiology through increased prevalence of Akkermansia muciniphila and altered short chain fatty acid production.
Major knowledge gaps include lung microbiome composition of adult patients with asthma before and after bariatric surgery and the impact of weight-independent metabolic changes following bariatric surgery on asthma pathobiology.
How might your results change the direction of research or the focus of clinical practice?
For patients with obesity and asthma, bariatric surgery could become a promising treatment for symptoms of asthma and underlying airway inflammation.
Fulfilling knowledge gaps outlined in this review can help develop new therapeutic options for patients with asthma and obesity by targeting the gut and/or the lung microbiome.
Acknowledgments
The authors thank Dr. Eric Monson from the Duke University Center for Data and Visualization Sciences with his guidance on the figures.
Funding: This research was supported by NIH 5R01HL130234 (JLI) and NIH/NIEHS supported Duke University Superfund Research Center P42ES010356 (YJK and CKG).
Disclosure: Dr. Ingram reports grants from NIH/NHLBI, during the conduct of the study; Yeon Ji Kim reports grants from NIH/NIEHS, during the conduct of the study; Dr. Gunsch reports grants from NIH/NIEHS, during the conduct of the study.
Abbreviations
- AHR
Airway hyperresponsiveness
- BMI
Body mass index
- IgE
Immunoglobulin E
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- NCEP
National Cholesterol Education Program
- ATP III
Adults Treatment Panel III
- HDL
High density lipoprotein
- SCFA
Short-chain fatty acids
- FLVR
Faecalibacterium, Lachnospira, Rothia, and Veillonella
- IL
Interleukin
- TNFα
Tumor necrosis factor alpha
- Th
T-helper cell
- OTU
Operational taxonomic unit
- GPR
G-protein coupled receptor
- IFN-γ
Interferon gamma
- Treg
regulatory T cell
- F/B ratio
Firmicutes/Bacteroidetes ratio
- RYGB
Roux-en-Y gastric bypass
- SG
Sleeve gastrectomy
- EWL
Excess weight loss
References
- 1.Trivedi M, Denton E. Asthma in Children and Adults–What Are The Differences and What Can They Tell Us About Asthma? Front Pediatr. 2019;7:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lefaudeux D, De Meulder B, Loza MJ, et al. U-BIOPRED clinical adult asthma clusters linked to a subset of sputum omics. J Allergy Clin Immunol. 2017;139(6):1797–1807. [DOI] [PubMed] [Google Scholar]
- 4.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang E, Wechsler ME, Tran TN, et al. Characterization of Severe Asthma Worldwide: Data From the International Severe Asthma Registry. Chest. 2020;157(4):790–804. [DOI] [PubMed] [Google Scholar]
- 6.Gibeon D, Batuwita K, Osmond M, et al. Obesity-associated severe asthma represents a distinct clinical phenotype: analysis of the British Thoracic Society Difficult Asthma Registry Patient cohort according to BMI. Chest. 2013;143(2):406–414. [DOI] [PubMed] [Google Scholar]
- 7.Dixon AE, Pratley RE, Forgione PM, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128(3):508–515. e502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaaban TA. Bariatric surgery: a potential cure for asthma? Eur Respir Rev. 2019;28(152):190003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Huisstede A, Rudolphus A, Cabezas MC, et al. Effect of bariatric surgery on asthma control, lung function and bronchial and systemic inflammation in morbidly obese subjects with asthma. Thorax. 2015;70(7):659–667. [DOI] [PubMed] [Google Scholar]
- 10.Brumpton BM, Camargo CA, Romundstad PR, Langhammer A, Chen Y, Mai X-M. Metabolic syndrome and incidence of asthma in adults: the HUNT study. Eur Respir J. 2013;42(6):1495–1502. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw Cardiol. 2005;4(4):198–203. [DOI] [PubMed] [Google Scholar]
- 12.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv N, Xiao L, Camargo CA Jr., et al. Abdominal and general adiposity and level of asthma control in adults with uncontrolled asthma. Ann Am Thorac Soc. 2014;11(8):1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goudarzi H, Konno S, Kimura H, et al. Impact of Abdominal Visceral Adiposity on Adult Asthma Symptoms. The Journal of Allergy and Clinical Immunology: In Practice. 2019;7(4):1222–1229.e1225. [DOI] [PubMed] [Google Scholar]
- 15.Fenger RV, Gonzalez-Quintela A, Linneberg A, et al. The relationship of serum triglycerides, serum HDL, and obesity to the risk of wheezing in 85,555 adults. Respir Med. 2013;107(6):816–824. [DOI] [PubMed] [Google Scholar]
- 16.Barochia AV, Gordon EM, Kaler M, et al. High density lipoproteins and type 2 inflammatory biomarkers are negatively correlated in atopic asthmatics. J Lipid Res. 2017;58(8):1713–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rastogi D, Jung M, Strizich G, et al. Association of systemic inflammation, adiposity, and metabolic dysregulation with asthma burden among Hispanic adults. Respir Med. 2017;125:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardet JC, Ash S, Kusa T, Camargo CA Jr., Israel E. Insulin resistance modifies the association between obesity and current asthma in adults. Eur Respir J. 2016;48(2):403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karampatakis N, Karampatakis T, Galli-Tsinopoulou A, et al. Impaired glucose metabolism and bronchial hyperresponsiveness in obese prepubertal asthmatic children. Pediatr Pulmonol. 2017;52(2):160–166. [DOI] [PubMed] [Google Scholar]
- 20.Sun D, Li X, Heianza Y, et al. History of Asthma From Childhood and Arterial Stiffness in Asymptomatic Young Adults. Hypertension. 2018;71(5):928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee KH, Lee HS. Hypertension and diabetes mellitus as risk factors for asthma in Korean adults: the Sixth Korea National Health and Nutrition Examination Survey. Int Health. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoodley I, Williams L, Thompson C, Scott H, Wood L. Evidence for lifestyle interventions in asthma. Breathe. 2019;15(2):e50–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchesi JR, Ravel J. The vocabulary of microbiome research: a proposal. Microbiome. 2015;3(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science. 2016;352(6285):560–564. [DOI] [PubMed] [Google Scholar]
- 25.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding R-x, Goh W-R, Wu R-n, et al. Revisit gut microbiota and its impact on human health and disease. J Food Drug Anal. 2019;27(3):623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7(3):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sze MA, Dimitriu PA, Hayashi S, et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(10):1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Bafadhel M, Haldar K, et al. Lung microbiome dynamics in COPD exacerbations. Eur Respir J. 2016;47(4):1082–1092. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ES, Bittinger K, Haas AR, et al. Topographical Continuity of Bacterial Populations in the Healthy Human Respiratory Tract. Am J Respir Crit Care Med. 2011;184(8):957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arrieta M-C, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152–307ra152. [DOI] [PubMed] [Google Scholar]
- 32.Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44(6):842–850. [DOI] [PubMed] [Google Scholar]
- 33.Durack J, Kimes NE, Lin DL, et al. Delayed gut microbiota development in high-risk for asthma infants is temporarily modifiable by Lactobacillus supplementation. Nat Commun. 2018;9(1):707–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stokholm J, Blaser MJ, Thorsen J, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun. 2018;9(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verhulst SL, Vael C, Beunckens C, Nelen V, Goossens H, Desager K. A Longitudinal Analysis on the Association Between Antibiotic Use, Intestinal Microflora, and Wheezing During the First Year of Life. J Asthma. 2008;45(9):828–832. [DOI] [PubMed] [Google Scholar]
- 36.van Nimwegen FA, Penders J, Stobberingh EE, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128(5):948–955. e943. [DOI] [PubMed] [Google Scholar]
- 37.Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357(15):1487–1495. [DOI] [PubMed] [Google Scholar]
- 38.Green BJ, Wiriyachaiporn S, Grainge C, et al. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One. 2014;9(6):e100645–e100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578–e8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juniper E, O’Byrne P, Guyatt G, Ferrie P, King D. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. [DOI] [PubMed] [Google Scholar]
- 41.Huang YJ, Nariya S, Harris JM, et al. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J Allergy Clin Immunol. 2015;136(4):874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang YJ, Nelson CE, Brodie EL, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127(2):372–381.e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013;131(2):346–352.e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denner DR, Sangwan N, Becker JB, et al. Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J Allergy Clin Immunol. 2016;137(5):1398–1405.e1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson JL, Daly J, Baines KJ, et al. Airway dysbiosis: Haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur Respir J. 2016;47(3):792–800. [DOI] [PubMed] [Google Scholar]
- 46.Taylor SL, Leong LEX, Choo JM, et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J Allergy Clin Immunol. 2018;141(1):94–103.e115. [DOI] [PubMed] [Google Scholar]
- 47.Son J-H, Kim JH, Chang HS, Park J-S, Park C-S. Relationship of Microbial Profile With Airway Immune Response in Eosinophilic or Neutrophilic Inflammation of Asthmatics. Allergy Asthma Immunol Res. 2020;12(3):412–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roggenbuck M, Anderson D, Barfod KK, et al. Vitamin D and allergic airway disease shape the murine lung microbiome in a sex-specific manner. Respir Res. 2016;17(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trompette A, Gollwitzer ES, Yadava K, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–166. [DOI] [PubMed] [Google Scholar]
- 50.Sun M, Wu W, Chen L, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9(1):3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z, Shi L, Pang W, et al. Dietary Fiber Intake Regulates Intestinal Microflora and Inhibits Ovalbumin-Induced Allergic Airway Inflammation in a Mouse Model. PLoS One. 2016;11(2):e0147778–e0147778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8(3):218–230. [DOI] [PubMed] [Google Scholar]
- 54.Smith PM, Howitt MR, Panikov N, et al. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science. 2013;341(6145):569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serena C, Ceperuelo-Mallafré V, Keiran N, et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018;12(7):1642–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rahat-Rozenbloom S, Fernandes J, Gloor GB, Wolever TMS. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int J Obes (Lond). 2014;38(12):1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwiertz A, Taras D, Schäfer K, et al. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity. 2010;18(1):190–195. [DOI] [PubMed] [Google Scholar]
- 58.Sze MA, Schloss PD. Looking for a Signal in the Noise: Revisiting Obesity and the Microbiome. mBio. 2016;7(4):e01018–01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murugesan S, Ulloa-Martínez M, Martínez-Rojano H, et al. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur J Clin Microbiol Infect Dis. 2015;34(7):1337–1346. [DOI] [PubMed] [Google Scholar]
- 60.Ignacio A, Fernandes MR, Rodrigues VAA, et al. Correlation between body mass index and faecal microbiota from children. Clin Microbiol Infect. 2016;22(3):258.e251–258.e258. [DOI] [PubMed] [Google Scholar]
- 61.Maya-Lucas O, Murugesan S, Nirmalkar K, et al. The gut microbiome of Mexican children affected by obesity. Anaerobe. 2019;55:11–23. [DOI] [PubMed] [Google Scholar]
- 62.Liu R, Hong J, Xu X, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23(7):859–868. [DOI] [PubMed] [Google Scholar]
- 63.De la Cuesta-Zuluaga J, Mueller NT, Álvarez-Quintero R, et al. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients. 2019;11(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chambers ES, Viardot A, Psichas A, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64(11):1744–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K. Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota. Sci Rep. 2016;6(1):37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin HV, Frassetto A, Kowalik EJ, Jr., et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7(4):e35240–e35240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu J, Bonder MJ, Cenit MC, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. 2015;117(9):817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Le Roy CI, Bowyer RC, Castillo-Fernandez JE, et al. Dissecting the role of the gut microbiota and diet on visceral fat mass accumulation. Sci Rep. 2019;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palmu J, Salosensaari A, Havulinna AS, et al. Association Between the Gut Microbiota and Blood Pressure in a Population Cohort of 6953 Individuals. Journal of the American Heart Association. 2020;9(15):e016641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kayser BD, Prifti E, Lhomme M, et al. Elevated serum ceramides are linked with obesity-associated gut dysbiosis and impaired glucose metabolism. Metabolomics. 2019;15(11):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Medina DA, Pedreros JP, Turiel D, et al. Distinct patterns in the gut microbiota after surgical or medical therapy in obese patients. PeerJ. 2017;5:e3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ilhan ZE, DiBaise JK, Isern NG, et al. Distinctive microbiomes and metabolites linked with weight loss after gastric bypass, but not gastric banding. ISME J. 2017;11(9):2047–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sánchez-Alcoholado L, Gutiérrez-Repiso C, Gómez-Pérez AM, García-Fuentes E, Tinahones FJ, Moreno-Indias I. Gut microbiota adaptation after weight loss by Roux-en-Y gastric bypass or sleeve gastrectomy bariatric surgeries. Surg Obes Relat Dis. 2019;15(11):1888–1895. [DOI] [PubMed] [Google Scholar]
- 74.Sanmiguel CP, Jacobs J, Gupta A, et al. Surgically Induced Changes in Gut Microbiome and Hedonic Eating as Related to Weight Loss: Preliminary Findings in Obese Women Undergoing Bariatric Surgery. Psychosom Med. 2017;79(8):880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palleja A, Kashani A, Allin KH, et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016;8(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al Assal K, Prifti E, Belda E, et al. Gut Microbiota Profile of Obese Diabetic Women Submitted to Roux-en-Y Gastric Bypass and Its Association with Food Intake and Postoperative Diabetes Remission. Nutrients. 2020;12(2):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palmisano S, Campisciano G, Silvestri M, et al. Changes in Gut Microbiota Composition after Bariatric Surgery: a New Balance to Decode. J Gastrointest Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 78.Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13(6):514–522. [DOI] [PubMed] [Google Scholar]
- 79.Yu D, Shu X-O, Howard EF, Long J, English WJ, Flynn CR. Fecal metagenomics and metabolomics reveal gut microbial changes after bariatric surgery. Surg Obes Relat Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ilhan ZE, DiBaise JK, Dautel SE, et al. Temporospatial shifts in the human gut microbiome and metabolome after gastric bypass surgery. NPJ Biofilms Microbiomes. 2020;6(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murphy R, Tsai P, Jüllig M, Liu A, Plank L, Booth M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes Surg. 2017;27(4):917–925. [DOI] [PubMed] [Google Scholar]
- 82.Kong L-C, Tap J, Aron-Wisnewsky J, et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98(1):16–24. [DOI] [PubMed] [Google Scholar]
- 83.Wang FG, Bai RX, Yan WM, Yan M, Dong LY, Song MM. Differential composition of gut microbiota among healthy volunteers, morbidly obese patients and post-bariatric surgery patients. Exp Ther Med. 2019;17(3):2268–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Damms-Machado A, Mitra S, Schollenberger AE, et al. Effects of Surgical and Dietary Weight Loss Therapy for Obesity on Gut Microbiota Composition and Nutrient Absorption. Biomed Res Int. 2015;2015:806248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mabey JG, Chaston JM, Castro DG, Adams TD, Hunt SC, Davidson LE. Gut microbiota differs a decade after bariatric surgery relative to a nonsurgical comparison group. Surg Obes Relat Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fouladi F, Brooks AE, Fodor AA, et al. The Role of the Gut Microbiota in Sustained Weight Loss Following Roux-en-Y Gastric Bypass Surgery. Obes Surg. 2019;29(4):1259–1267. [DOI] [PubMed] [Google Scholar]
- 87.Tremaroli V, Karlsson F, Werling M, et al. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015;22(2):228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jahansouz C, Staley C, Bernlohr DA, Sadowsky MJ, Khoruts A, Ikramuddin S. Sleeve gastrectomy drives persistent shifts in the gut microbiome. Surg Obes Relat Dis. 2017;13(6):916–924. [DOI] [PubMed] [Google Scholar]
- 89.Haange S-B, Jehmlich N, Krügel U, et al. Gastric bypass surgery in a rat model alters the community structure and functional composition of the intestinal microbiota independently of weight loss. Microbiome. 2020;8(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee CJ, Florea L, Sears CL, et al. Changes in Gut Microbiome after Bariatric Surgery Versus Medical Weight Loss in a Pilot Randomized Trial. Obes Surg. 2019;29(10):3239–3245. [DOI] [PubMed] [Google Scholar]
- 91.Louis S, Tappu R-M, Damms-Machado A, Huson DH, Bischoff SC. Characterization of the Gut Microbial Community of Obese Patients Following a Weight-Loss Intervention Using Whole Metagenome Shotgun Sequencing. PLoS One. 2016;11(2):e0149564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spellerberg IF, Fedor PJ. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index. Global Ecology and Biogeography. 2003;12(3):177–179. [Google Scholar]
- 93.Paganelli FL, Luyer M, Hazelbag CM, et al. Roux-Y Gastric Bypass and Sleeve Gastrectomy directly change gut microbiota composition independent of surgery type. Sci Rep. 2019;9(1):10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maniscalco M, Zamparelli AS, Vitale DF, et al. Long-term effect of weight loss induced by bariatric surgery on asthma control and health related quality of life in asthmatic patients with severe obesity: A pilot study. Respir Med. 2017;130:69–74. [DOI] [PubMed] [Google Scholar]
- 95.Guerron AD, Ortega CB, Lee H-J, Davalos G, Ingram J, Portenier D. Asthma medication usage is significantly reduced following bariatric surgery. Surg Endosc. 2019;33(6):1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Forno E, Zhang P, Nouraie M, et al. The impact of bariatric surgery on asthma control differs among obese individuals with reported prior or current asthma, with or without metabolic syndrome. PLoS One. 2019;14(4):e0214730–e0214730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Michalovich D, Rodriguez-Perez N, Smolinska S, et al. Obesity and disease severity magnify disturbed microbiome-immune interactions in asthma patients. Nat Commun. 2019;10(1):5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tashiro H, Cho Y, Kasahara DI, et al. Microbiota Contribute to Obesity-related Increases in the Pulmonary Response to Ozone. Am J Respir Cell Mol Biol. 2019;61(6):702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ather JL, Chung M, Hoyt LR, et al. Weight Loss Decreases Inherent and Allergic Methacholine Hyperresponsiveness in Mouse Models of Diet-Induced Obese Asthma. Am J Respir Cell Mol Biol. 2016;55(2):176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. The Lancet. 2017;390(10095):659–668. [DOI] [PubMed] [Google Scholar]