Abstract
Objective:
Self-monitoring is critical for weight management, but little is known about lapses in the use of digital self-monitoring. The objectives were to examine whether lapses in self-weighing and wearing activity trackers are associated with weight and activity outcomes and to identify objective predictors of lapses.
Methods:
Participants (N=160, BMI=25.2±3.1 kg/m2, 32.5±4.9 years) were drawn from a sample of young adults in the SNAP-E weight gain prevention trial. Analyses evaluated associations between weighing and tracker lapses and changes in weight and steps/day during the first 90 days after receiving a smart scale and activity tracker.
Results:
On average, participants weighed 49.6% of days and wore activity trackers 75.2% of days. Every 1-day increase in a weighing lapse was associated with a 0.06-lb. gain. Lapses in tracker wear were not associated with changes in steps/day or weight between wear days. Weight gain predicted a higher likelihood of starting a lapse in weighing and tracker wear, while lower steps predicted a higher likelihood of a tracker lapse.
Conclusions:
Weight gain may discourage adherence to self-monitoring. Future research could examine just-in-time supports to anticipate and reduce the frequency or length of self-monitoring lapses.
Keywords: intervention, young adults, physical activity, weighing, adherence
Introduction
Previous research has proven self-monitoring to be a key strategy for both weight loss and maintenance (1, 2), though participant adherence to self-monitoring during weight management interventions generally declines over time (3-5). Whereas prior weight loss interventions required paper monitoring, the emergence of connected health tools, such as smart scales and activity trackers, has the potential to reduce the burden of self-monitoring weight and physical activity.
Behavioral weight loss interventions recommend frequent self-weighing, a self-monitoring behavior that gives individuals objective feedback about how their eating and exercise behaviors are affecting their weight (6, 7). Data from randomized controlled trials and observational studies have demonstrated that daily self-weighing is associated with better short-term weight loss outcomes than less frequent weighing (8-11) and that after initial weight loss, a decline in self-weighing frequency is associated with greater weight gain (12).
There is evidence to suggest that using activity trackers to automatically track physical activity can lead to increased self-monitoring engagement (13) and in some cases, increases in objectively-measured moderate-to-vigorous physical activity (14-16). Activity trackers increase access to activity data, provide positive reinforcement, and allow for self-awareness and accountability (17, 18). However, existing studies have noted that tracker wear declines over time, with approximately 20-25% of participants ceasing tracker wear in the first 1-3 months (14, 19). Thus, there is a need to explore patterns of tracker wear when device use is at its highest to identify early predictors of nonadherence.
Previous research has examined self-reported predictors of self-monitoring, including qualitative evidence suggesting that barriers to daily self-weighing include forgetting, motivation, lack of knowledge of the benefits (20, 21), and problems with charging and tracker functionality (22). A recent analysis found that greater adherence to paper self-monitoring of diet, weight, and activity was associated with concurrent weight change and that lower weight losses were associated with lower self-monitoring in the following month (23), but few studies have evaluated day-to-day objective predictors of nonadherence to monitoring.
The use of data from digital tools such as smart scales and activity trackers allows for the measurement of temporal lapses, or periods of nonadherence, in self-monitoring. Two recent intervention studies, one with African American breast cancer survivors and an 8-week worksite wellness program, analyzed data from participants’ digital smart scales and found small, but significant weight gain for each additional day of nonadherence to self-weighing (24, 25). Periods of daily self-weighing and daily tracker use were associated with weight losses of 0.028 kg and 0.017 kg, respectively (25). One internet-based weight loss intervention found that an increase in caloric intake on one day was a predictor of non-adherence to weighing the following day (26). It is not yet known if similar patterns occur among young adults, a population that gains 1-2 pounds per year (27). Though research has shown that overall frequency of self-weighing is associated with weight gain prevention and weight loss in this population (28, 29), identifying daily predictors and outcomes of digital self-monitoring lapses will be critical for supporting continued adherence. Therefore, the objectives of the current study were: 1) to describe the frequency and length of lapses in self-weighing and wearing an activity tracker, examine the association between lapses in self-weighing and wearing an activity tracker, and corresponding changes in weight and activity among young adults participating in a weight gain prevention intervention, and 2) to examine weight- and activity-related predictors of self-weighing and activity tracker lapses.
Methods
Participants, Study Procedures, and Data
This secondary data analysis included a sub-sample of young adults enrolled in an ongoing weight gain prevention study (30). The SNAP (Study of Novel Approaches to Prevention) study randomized 599 young adults recruited from August 2010 to February 2012 to one of three groups, a small changes intervention (SC), a large changes intervention (LC), or a self-guided control group (SG). Details on methods and the Consolidated Standards of Reporting Trials diagram for the trial have been published (30). Participants were ages 18-35, BMI 21.0 to 30.9 kg/m2, had Internet access, and were recruited primarily using mass mailings and emails. The SC and LC interventions included 10 in-person group meetings across 4 months with a supplemental website, followed by 4-week Internet-based refresher courses offered two times per year. The SC group focused on making small, 100-calorie changes in eating and activity to result in gradual weight loss over time. The goal of LC was to create an initial 5- to 10-pound weight loss to “buffer” against gradual weight gain through a reduction in caloric intake of 500-1000 kcals/day and increasing activity to 250 minutes or more of moderate-to-vigorous physical activity (MVPA) per week. Both groups emphasized daily self-weighing and encouraged daily recording, on paper, of their weight, diet, and activity. After 4 months, LC participants were encouraged to continue with 250+ minutes of weekly MVPA but did not track calories unless they experienced weight gain, at which point they returned to a 1200-1800 calorie diet. SC participants were encouraged to continue with their small changes throughout the program. At 3 years the LC and SC groups had reduced weight gain compared to the SG group (−2.37 kg, −0.56 kg, and +0.26 kg, respectively), and LC was more effective than SC (30).
In the fall of 2015, all SNAP participants were offered the opportunity to continue in SNAP-E, an Internet-based extension of the study to determine if the intervention effects could be maintained out to 6 years (31). Details about SNAP-E have been published (31). All participants who re-consented into SNAP-E received a cellular-connected scale and an activity tracker to automatically transmit weight and activity data to study servers. The control group received no specific recommendations. SC and LC participants continued following the recommendations given in SNAP, including daily self-weighing, were asked to wear their trackers every day, and received monthly email feedback on their weight. Participants were offered optional bi-annual 4-week refresher courses conducted through email, text messages, and mail that focused on one to two behavioral topics. Each Monday, participants received email feedback about their progress on the refresher behaviors. In the first refresher in October 2015, the first weekly email included either reinforcement for weighing at least once in the past 7 days or a reminder to weigh, and the second weekly email provided similar feedback based on Fitbit wear. Participants did not report dietary tracking information during SNAP-E. Approval for SNAP and SNAP-E was obtained by the Institutional Review Board of the University of North Carolina at Chapel Hill.
Participants included in this analysis were those who were randomized to SC or LC and re-consented to the SNAP-E study by October 31, 2015. This is the time point by which most participants had re-consented and received devices; after this point re-enrollment occurred slowly throughout and after the holidays. Out of the 170 participants who received their smart scales and trackers by October 31, we included a sample of 160 participants who had at least five observations of self-monitoring data from both the smart scale and activity tracker within the first 90 days (N = 85 from LC, N = 75 from SC; Table 1). We chose to limit data to the initial 3 months in order to capture adherence to self-weighing and activity tracker use while the devices were new to the participants, and then to capture breaks, or lapses, in self-monitoring during a period when device use frequently drops off (14, 19).
Table 1.
Baseline characteristics of SNAP-E participants included in current analyses (N = 160) and entire SNAP-E sample (N = 504)
| Current Analysis Mean ± SD/ n (%) N = 160 |
SNAP-E Sample Mean ± SD/ n (%) N = 504 |
|
|---|---|---|
| Age (years) | 33.1 (4.6) | 32.7 (4.3) |
| BMI (kg/m2) | 25.5 (3.3) | 26.0 (3.6) |
| Weight (lbs.) | 157.4 (26.2) | 161.4 (28.0) |
| Female | 80.6% | 77.4% |
| Race | ||
| Black non-Hispanic | 11.3% | 10.1% |
| White non-Hispanic | 75.6% | 73.8% |
| Other | 13.1% | 16.1% |
| Individual Income ≥ $50,000 | 61.6% | 58.9% |
| Education (college degree or more) | 93.7% | 91.5% |
Measures
Demographics.
Participants reported demographic characteristics including age, gender, race, education, and income at each assessment. Data used in this analysis was pulled from the SNAP assessment that occurred closest in time to each participant’s enrollment in SNAP-E (this varied depending by cohort).
Weight, Height, and BMI.
Objective weight and height data from the SNAP assessment closest in time to participants’ enrollment in SNAP-E was used to calculate body mass index (BMI) in kg/m2. Measurements were taken by trained, blinded study staff.
Self-Weighing Data.
Throughout the SNAP-E study, participants self-weighed on cellular-connected wireless scales (BodyTrace) and weights were automatically saved to the SNAP-E server. Each day of the 90-day interval was categorized as a weighing day or a non-weighing day. If a participant weighed more than once on any given day, the first weight of the day was used. A weighing lapse was defined as one or more consecutive days without weighing. The length of weighing lapse was the number of days in between consecutive weight measurements, or the number of days that passed before the participant weighed again. The weight change outcome of interest was weight change between two consecutive weight measurements. Weight values were also used to calculate prior 7-day weight change (weight today minus earliest prior weight during the last 7 days).
Physical Activity Data.
Participants wore Fitbit Charge (San Francisco, CA) activity trackers to self-monitor their physical activity. Participants synced their tracker with the Fitbit app on their smartphone (or a Bluetooth device on their computer), which then synced to the study server. A tracker lapse was defined as one or more consecutive days without wearing the tracker (i.e. < 1,000 steps per day, suggesting limited or no wear time, based on prior studies) (25). The length of tracker lapse was the number of consecutive days that the tracker was not worn. Because within-participant step variability is high, day-to-day step variability was operationalized as the difference between steps on any given day and the participants’ average steps over the 90-day interval (calculated as steps per day averaged across the entire 90-day interval). The activity outcome of interest was each day’s steps as a difference from participants’ average 90-day steps (calculated as current day’s steps – average 90-day steps), hereafter referred to as steps from average. Additional variables included steps as percent of average, defined as ([current day’s steps minus average 90-day steps]/average 90-day steps), and average 7-day steps as percent of average, defined as the average daily steps over the last 7 days as a percent of average 90-day steps ([average 7-day steps minus average 90-day steps]/average 90-day steps).
Statistical Analysis
Means and frequencies were used to describe baseline characteristics of the SNAP-E participants included in this analysis. All analyses collapsed across treatment groups given the similar recommendations for daily self-weighing and tracker wear. Descriptive statistics, including means and medians, were used to describe weighing and tracker lapses across the 90-day interval. For descriptive purposes, generalized estimating equations (GEE) models evaluated the likelihood of weighing and tracker wear as a function of day of the week. Weighing and tracker lapses were classified by length into four categories (0 days, 1-3 days, 4-6 days, 7+ days), and one-sample t-tests evaluated the weight changes and steps from average associated with each lapse length category.
GEE models accounting for repeated observations within individuals were used to evaluate confounders of the relationship between length of weighing and tracker lapses and weight change/steps from average, including demographic variables such as age, income, race, education, gender, and treatment group. Increasing age was associated with shorter weighing lapses and greater weight gain per day during lapses; thus, age was included in all weighing GEE models. GEE models were used to evaluate associations between a weighing lapse length and change in weight, and a tracker lapse length and steps from average.
Separate GEE models evaluated objective weight- and activity-related predictors of the start of a weighing or tracker lapse, including weight change since last weight observation, weight change in the last 7 days, current day’s steps as percent of average, and average 7-day steps as percent of average. All statistical analyses were completed using SAS 9.3 (Cary, NC).
Results
The percentage of participants that weighed and wore their tracker across the 90-day interval is depicted in Figure 1. Out of 90 days, participants weighed a median of 41 days (51.3%; IQR=25.0-65.5) and wore their tracker a median of 80 days (80%; IQR=50.5-89.0). Descriptive data on weighing and tracker lapses can be found in Table 2, calculated across all 14,400 observations (160 participants across 90 days; also referred to as participant-days) and also calculating the average of participants’ mean and median length of lapses. All 160 participants had at least one weighing lapse, but 45 participants had no tracker lapses (i.e., they wore their tracker all 90 days). When averaged across all observations, the mean lapse length was 3.3 ± 4.5 days for weighing and 3.3 ± 6.1 days for tracker wear. The median weighing lapse was 2 days (IQR=1-3) and median tracker lapse was 1 day (IQR=1-3). When averaged by participant, the median weighing lapse was 3 days (IQR=1.9-4.4) and the median tracker lapse among 115 participants that had at least one lapse was 2 days (IQR=1-4). Figure 2 displays the percent of participants weighing and wearing a tracker across each day of the week.
Figure 1.
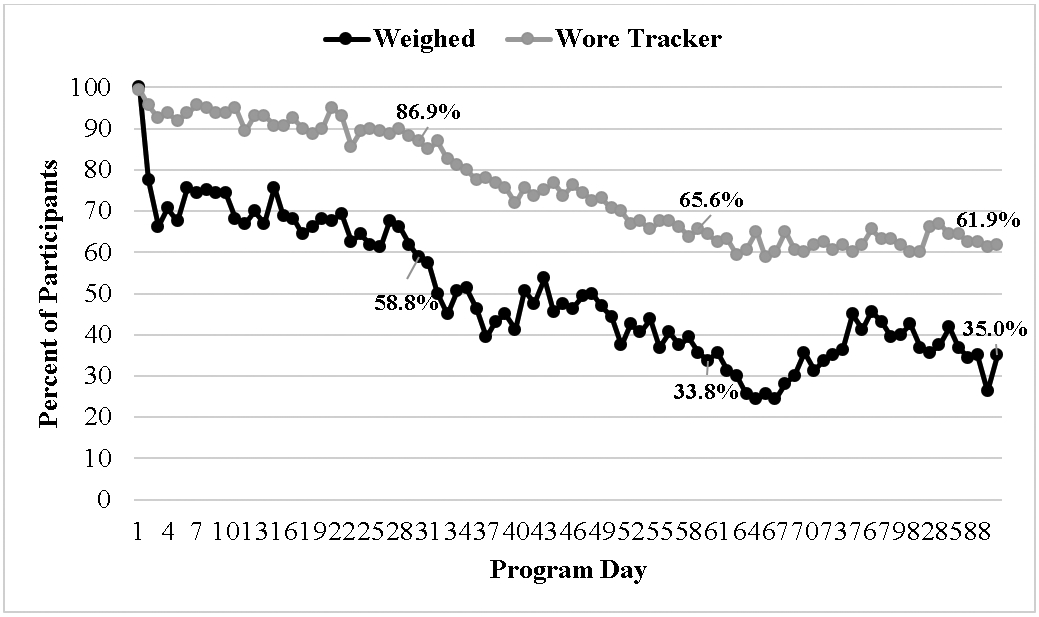
Percent of participants that weighed and wore tracker each day in the first 90 days, with percentages included at Days 30, 60, and 90
Table 2.
Descriptive statistics for lapse length (in days) across SNAP-E participants included in current analyses (N=160)
| Length of Lapse (in days) | |||||
|---|---|---|---|---|---|
| Across all observations (participant-day level) | |||||
| N | Number of Lapses |
Mean (SD) |
Median (IQR 25-75) |
Range | |
| Weighing lapses | 160 | 1718 | 3.3 (4.5) | 2 (1-3) | 1-56 |
| Activity tracker lapses | 115 | 510 | 3.3 (6.1) | 1 (1-3) | 1-61 |
| Mean number and length by participant | |||||
| N | Mean Number of Lapses (SD) |
Mean (SD) |
Median (IQR 25-75) |
||
| Weighing lapses | 160 | 10.74 (4.94) | 3.77 (3.34) | 3 (1.9-4.4) | N/A |
| Activity tracker lapsesa | 115 | 4.43 (3.31) | 3.45 (4.17) | 2 (1-4) | N/A |
| Activity tracker lapsesb | 160 | 3.19 (3.45) | N/A | N/A | N/A |
Results include N=115 participants with at least one tracker lapse.
Results include all 160 participants.
Figure 2.
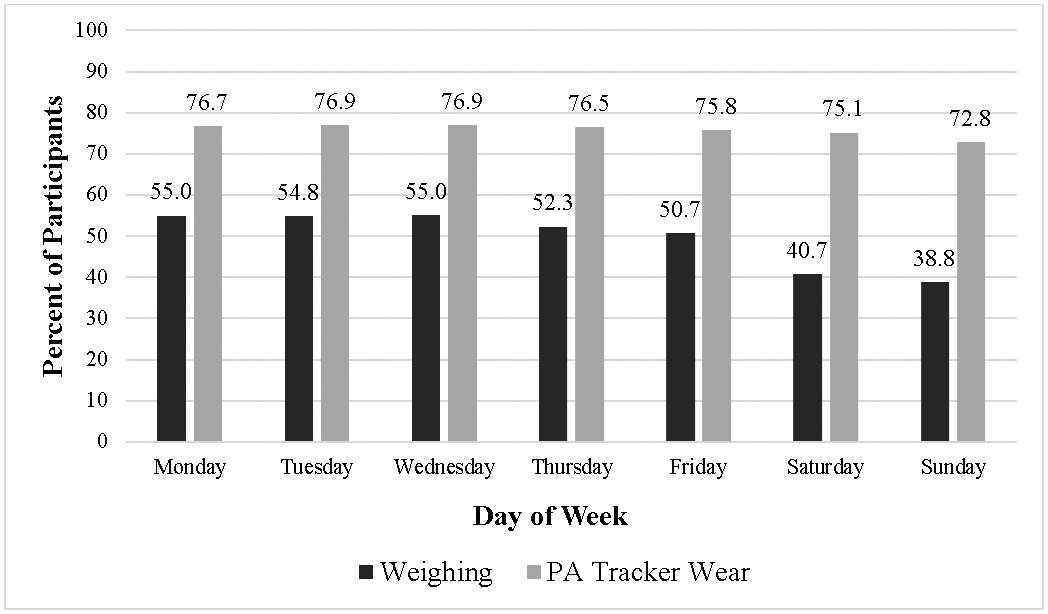
Percent of participants that weighed and wore tracker by day of the week
Day of the Week
There was a decrease in odds of weighing as the week proceeded from Monday through Sunday (β = −0.117, 95% CI: −0.136, −0.098; p<.0001; OR = 0.890; 95% CI: 0.873, 0.907). A similar relationship was found for tracker wear, though the upper bound of the CI was close to 1 (β = −0.008, 95% CI: −0.011, −0.004; p<.0001; OR = 0.992; 95% CI: 0.989, 0.996).
Lapse Length and Corresponding Weight/Activity Changes
Table 3 includes mean weight changes and steps from average for weighing and tracker lapse lengths of 0 days, 1-3 days, 4-6 days, and 7 days or longer. Notably, a mean weight loss of 0.09 lbs. occurred when there was no weighing lapse, (i.e., weighing two consecutive days), but weight gain occurred with a weighing lapse of any length. On the day following a tracker lapse of any length, steps were significantly lower compared to participants’ 90-day average.
Table 3.
Mean changes in weight and steps/day by length of lapse at the observation (participant-day) level and averaged across participants
| Lapse Length |
Weight Change (lb) | Steps from Average (steps/day) | ||
|---|---|---|---|---|
| Across all observations (participant-day level) | ||||
| n | Mean Change (95% CI) | n | Mean Change (95% CI) | |
| No Lapse | 5240 | −0.088 (−0.119, −0.057)**** | 10165 | 38.1 (−32.4, 108.6) |
| 1-3 Days | 1392 | 0.135 (0.043, 0.228)** | 432 | −762.4 (−1085.8, −439.0)**** |
| 4-6 Days | 164 | 0.786 (0.481, 1.092)**** | 36 | −1635.1 (−2827.9, −442.3)** |
| 7+ Days | 162 | 0.614 (0.263, 0.964)** | 42 | −787.4 (−1529.1, −45.74)* |
| Averaged across participants | ||||
| N | Mean Change (95% CI) | N | Mean Change (95% CI) | |
| No Lapse | 157 | −0.103 (−0.161, −0.045)*** | 160 | 46.9 (20.3, 73.5)*** |
| 1-3 Days | 158 | 0.158 (0.038, 0.278)* | 107 | −834.2 (−1284.8, −383.6)*** |
| 4-6 Days | 91 | 1.197 (0.775, 1.618)*** | 28 | −1652.0 (−3026.5, −277.5)* |
| 7+ Days | 92 | 0.742 (0.392, 1.093)** | 32 | −901.8 (−1734.8, −68.8)* |
NOTE. Mean changes in weight and steps/day occurred during the corresponding time period of a lapse (not per day).
p < .05
p < .01
p < .001
p < .0001
Results of the weight change GEE model indicated that every 1-day increase in weighing lapse length was associated with a 0.057-lb. increase in weight since the prior weight observation (β = 0.057; 95% CI: 0.038, 0.076; p < .0001; Table 4). Using the prediction equation, a 17.5-day lapse in self-weighing would lead to a weight gain of 1 pound. Results of the steps change GEE model indicated that every 1-day increase in activity tracker lapse length was associated with a decrease of 57.3 steps compared to average steps (β = −57.316; 95% CI: −97.328, −17.305; p = .005), but was not associated with changes in weight.
Table 4.
GEE models predicting concurrent weight change and steps change from weighing and tracker lapse lengths
| Weight Changea (lb) | Steps from Average | |||
|---|---|---|---|---|
| β (95% CI) | p value | β (95% CI) | p value | |
| Self-weighing | ||||
| Intercept | 0.020 (−0.076, 0.119) | .67 | 115.542 (0.537, 230.547) | .049 |
| Weighing lapse length | 0.057 (0.038, 0.076) | < .0001 | −46.611 (−92.339, −0.884) | .046 |
| Activity tracking | ||||
| Intercept | 0.085 (−0.030, 0.201) | .15 | 5.908 (−4.755, 16.571) | .28 |
| Activity tracker lapse length | −0.012 (−0.043, 0.020) | .47 | −57.316 (−97.328, −17.305) | .005 |
GEE models controlling for age.
Predictors of Weighing and PA Tracker Lapses
Predictors of a weighing lapse included weight change from the prior weight observation and weight change over the last 7 days (Table 5). These findings suggest that for every 1-lb. increase in weight since the last weight observation, the odds of starting a weighing lapse the following day increased by 8.0% (Odds Ratio (OR) = 1.080; 95% CI: 1.039, 1.123). For every 1-lb. increase in weight over the last 7 days, the odds of starting a weighing lapse the following day increased by 6.7% (OR = 1.067; 95% CI: 1.037, 1.098). Current day steps as a percent of average steps and 7-day average steps were not associated with the start of a weighing lapse.
Table 5.
Predictors of the start of weighing and tracker lapse
| Weighing Lapsea | Tracker Lapse | |||
|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Weight change since last weight | 1.080 | 1.039, 1.123 | 1.039 | 0.948, 1.138 |
| 7-Day weight change | 1.067 | 1.037, 1.098 | 1.073 | 1.103, 1.135 |
| Current day’s steps as percent of average | 0.922 | 0.774, 1.099 | 0.418 | 0.305, 0.572 |
| 7-Day average steps as percent of average | 0.997 | 0.994, 1.001 | 0.986 | 0.981, 0.991 |
GEE models controlling for age.
Predictors of a tracker lapse included current day steps as percent of average and prior 7-day average steps as percent of average. For every 1% increase in steps on any given day (as percent of average 90-day steps), the odds of starting a tracker lapse the next day decreased by 58.2% (OR = 0.418; 95% CI: 0.305, 0.572). In addition, for every 1% increase in average steps over the last 7 days as (as percent of average 90-day steps), the odds of starting a tracker lapse decreased by 1.4% (OR = 0.986; 95% CI: 0.981, 0.991). Every 1-lb. increase in weight over the last 7 days was associated with a 7.3% increase in the odds of starting a tracker lapse the following day (OR = 1.073; 95% CI: 1.103, 1.135).
Discussion
This secondary analysis of data from a long-term weight gain prevention trial among young adults suggests that lapses in weighing and tracker wear are related to concurrent changes in behavioral outcomes. Periods of daily self-weighing were associated with weight loss, while a weighing lapse of any length was associated with weight gain. The longer the lapse in weighing, the greater the amount of weight gained, such that a 17.5-day lapse in self-weighing would be associated with a 1-lb. weight gain. Given the small number of lapses of 4 days or more relative to those with 3 or fewer, future research will be needed to determine if the association is indeed linear. Nonetheless, these findings are commensurate with the association between greater self-weighing frequency and greater weight loss (8-10, 32, 33), including behavioral interventions showing that weighing at least 6 days per week leads to greater weight loss than weighing weekly (8, 10). Moreover, it is similar to the findings of Martin et al. who found that every 1-day increase in nonadherence to weighing among African American breast cancer survivors was associated with a weight increase of 0.031 kg, which would be equal to a 1-lb weight gain after 14.6 days (25). Frequent, short lapses (< 3 days) may be relatively inconsequential given their limited proximal effect on weight; however, a 1-lb. weight gain in 17 days is clinically relevant, given that it outpaces the average 1-2 lbs. of weight gained by young adults each year (27), and that small weight gains are not easily lost, even among successful weight losers (34).
In the current study, a tracker lapse of any length was associated with attaining fewer steps than average the next day the tracker was worn, such that each day without tracker wear was associated with a decrease of 57 steps, but was not associated with concurrent weight change. Steps were not significantly different than average on consecutive days of tracker wear, but this is expected given that steps are being compared to participants’ average steps over the 90-day period. These results suggest that minimal changes in activity occur during tracker lapses that may result in lower energy expenditure over time but may not contribute to weight gain.
Interestingly, weight gain in the prior 7 days predicted lapses in tracker wear. Evidence from cross-sectional surveys and focus groups suggests that adults disengage from tracker use primarily due to not liking the look or feel of trackers (35, 36) and perceiving activity data to be inaccurate or unreliable (35-37). Facilitators to tracker use include the social functionality of trackers and their apps (35) and simple visualizations of activity data (38). With the knowledge that other factors, such as weight gain, may discourage tracker use, further research should explore the day-to-day factors that encourage or discourage tracker wear, and why, in order to inform unique messaging or behavioral strategies for intervention.
Overall, tracker wear was higher than self-weighing and median tracker lapses were lower than those for self-weighing, which suggests there may be greater barriers to daily self-weighing. Qualitative research has identified barriers to self-weighing such as forgetting, motivational problems, and lack of knowledge of its benefits (20, 21), and a prior analysis identified increased caloric intake as a predictor of lower likelihood to self-weigh the next day (26). The current study identified recent weight gain as a potential barrier to self-weighing. Perhaps when participants are on a weight gain trajectory, seeing the number on the scale causes anxiety or frustration. This has been suggested by data indicating that self-weighing is less helpful and more frustrating during a period of weight maintenance that follows initial weight loss (39).
There may be other practical barriers to self-weighing that are not barriers to tracker wear, such as the ability to wear trackers but not take their scale while traveling. This is suggested by the results that weighing is less likely to occur as the week progresses from Monday to Sunday, while tracker wear did not vary by day of the week. Previous research showed that participants were most likely to skip weighing on weekend days, and greater caloric intake relative to one’s average intake was associated with a lower likelihood of weighing the following day (26). It is possible that greater caloric intake on weekends leads to lower weighing frequency given evidence that caloric intake is significantly higher on weekend days compared to weekdays (40), though that cannot be confirmed with this data and is an important topic for further study.
This is one of the first studies to examine outcomes and objective predictors of lapses in self-monitoring of weight and activity. The findings that self-monitoring lapses among young adults are associated with concurrent weight gain, and that weight gain appears to discourage self-monitoring, have implications for future intervention development. Digital health data can now be used to inform highly tailored message algorithms, with the goal of delivering the right message at the right time, and only when needed, instead of relying on pre-determined messages and timepoints (41-43). These results suggest that while lapses in self-weighing occur frequently and are associated with weight gain, median lapses are only 1-2 days. Given that some participants experience message fatigue and report that frequent messaging can be annoying (44, 45), waiting to deliver a message until a self-monitoring lapse becomes “high risk” (i.e., after at least 3-4 days when the risk for weight gain increases, or 3+ days when a tracker lapse may continue unless “interrupted”) may be ideal for continued adherence.
Despite the strengths of this study, this is a sample of young adults who had already participated in 3-4 years of a weight gain prevention trial and had experience with self-monitoring and other behavioral skills that support weight maintenance. Thus, the results may not be generalizable to participants with overweight or obesity who enroll in a behavioral weight loss intervention with specific weight loss goals. In addition, the current sample is a subsample of SNAP-E participants. Those who consented after Fall 2015 were not included in this analysis due to slow, gradual enrollment after that time and the need to exclude participants who received devices during or immediately after the holidays. When divided into three monthly intervals, in which days 31-60 included most of the holiday period, both weighing and tracker wear were highest in days 1-30 and lowest in days 61-90, suggesting that the holidays did not impact adherence beyond typical declines in device usage (results not reported). However, it is possible that devices provided at another time of year may result in different patterns of initial adherence. A small number of participants may have owned a tracker prior to SNAP-E (data not available), but the use of smart scales and activity trackers was new to most participants and allowed for the opportunity to examine use among new users. There are likely additional predictors of lapses in self-monitoring, such as dietary intake (26), that were not measured or collected during SNAP. Future research should examine diet as well as other time-varying predictors such as psychosocial factors (e.g., mood) and other behavioral factors (e.g., sleep, social environment). In addition, predictors of lapses in long-term usage of digital devices may differ compared to initial usage. In this study, participants received reminders to weigh and wear their tracker via weekly email feedback during the first refresher, which may have impacted initial adherence. An important next step is analyzing self-monitoring data over a longer time frame and testing the effect of highly tailored messages to develop strategies for increasing participant engagement and weight loss success.
Study Importance Questions.
- What is already known about this subject?
- Self-monitoring is a key strategy for weight loss and weight maintenance, but participant adherence to self-monitoring during weight management interventions generally declines over time.
- Little is known about the predictors and outcomes of day-to-day lapses in self-monitoring.
- What are the new findings in your manuscript?
- Greater self-weighing lapse lengths are associated with increased concurrent weight gain.
- There may be greater barriers to daily self-weighing compared to wearing activity trackers and recent weight gain may discourage adherence to self-monitoring.
- How might your results change the direction of research or the focus of clinical practice?
- Self-monitoring lapses are expected, but digital health data can be used in computer-tailored interventions to send messages that prevent lapses or that interrupt lapses before they result in significant weight gain.
Acknowledgments
This project was supported by funding from the National Institutes of Health (NHLBI 5R01HL127341, U01HL090864, U01090875) and a Gillings Innovation Laboratory award funded by the 2007 Gillings Gift to the UNC Gillings School of Global Public Health. This work was supported in part by UNC’s Connected Health Applications & Interventions Core through a grant from NIH (DK056350) to the UNC Nutrition Obesity Research Center. We thank the SNAP-E participants for their important contributions. We gratefully acknowledge the staff and students of the UNC Weight Research Program for their valuable support, including Karen Hatley, Kristen Polzien, and Molly Diamond, in addition to Derek Hales and Katelyn Garcia for guidance provided.
Footnotes
CLINICAL TRIAL REGISTRATION: clinicaltrials.gov NCT01183689
DISCLOSURE: Drs. Tate, Espeland, and Wing received funding from NHLBI during the conduct of this study. Dr. Tate is a member of the Scientific Advisory Board for WW International. The other authors declare no conflict of interest.
References
- 1.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc 2011;111:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shieh C, Knisely MR, Clark D, Carpenter JS. Self-weighing in weight management interventions: A systematic review of literature. Obes Res Clin Pract 2016;10:493–519. [DOI] [PubMed] [Google Scholar]
- 3.Burke LE, Styn MA, Sereika SM, et al. Using mHealth technology to enhance self-monitoring for weight loss: a randomized trial. Am J Prev Med 2012;43:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey J, Krukowski R, Priest J, West D. Log Often, Lose More: Electronic Dietary Self-Monitoring for Weight Loss. Obesity (Silver Spring) 2019;27:380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conroy MB, Yang K, Elci OU, et al. Physical activity self-monitoring and weight loss: 6-month results of the SMART trial. Med Sci Sports Exerc 2011;43:1568–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGuire MT, Wing RR, Klem ML, Hill JO. Behavioral strategies of individuals who have maintained long-term weight losses. Obes Res 1999;7:334–341. [DOI] [PubMed] [Google Scholar]
- 7.Boutelle K. Weighing the evidence: benefits of regular weight monitoring for weight control. J Nutr Educ Behav 2006;38:131. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel-Hodge C, Ward DS. The efficacy of a daily self-weighing weight loss intervention using smart scales and e-mail. Obesity (Silver Spring) 2013;21:1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linde JA, Jeffery RW, French SA, Pronk NP, Boyle RG. Self-weighing in weight gain prevention and weight loss trials. Ann Behav Med 2005;30:210–216. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y, Burke LE, Danford CA, Ewing LJ, Terry MA, Sereika SM. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes 2016;40:1392–1396. [DOI] [PubMed] [Google Scholar]
- 11.Patel ML, Brooks TL, Bennett GG. Consistent self-monitoring in a commercial app-based intervention for weight loss: results from a randomized trial. J Behav Med 2020;43:391–401. [DOI] [PubMed] [Google Scholar]
- 12.Butryn ML, Phelan S, Hill JO, Wing RR. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity (Silver Spring, Md) 2007;15:3091–3096. [DOI] [PubMed] [Google Scholar]
- 13.Kim JY, Wineinger NE, Taitel M, et al. Self-Monitoring Utilization Patterns Among Individuals in an Incentivized Program for Healthy Behaviors. J Med Internet Res 2016;18:e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelstein EA, Haaland BA, Bilger M, et al. Effectiveness of activity trackers with and without incentives to increase physical activity (TRIPPA): a randomised controlled trial. Lancet Diabetes Endocrinol 2016;4:983–995. [DOI] [PubMed] [Google Scholar]
- 15.Brickwood K-J, Watson G, O’Brien J, Williams AD. Consumer-Based Wearable Activity Trackers Increase Physical Activity Participation: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2019;7:e11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartman SJ, Nelson SH, Weiner LS. Patterns of Fitbit Use and Activity Levels Throughout a Physical Activity Intervention: Exploratory Analysis from a Randomized Controlled Trial. JMIR Mhealth Uhealth 2018;6:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips SM, Courneya KS, Welch WA, et al. Breast cancer survivors’ preferences for mHealth physical activity interventions: findings from a mixed methods study. J Cancer Surviv 2019;13:292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindgren T, Hooper J, Fukuoka Y. Perceptions and Experiences of Women Participating in a Digital Technology-Based Physical Activity Intervention (the mPED Trial): Qualitative Study. JMIR Public Health Surveill 2019;5:e13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermsen S, Moons J, Kerkhof P, Wiekens C, De Groot M. Determinants for sustained use of an activity tracker: observational study. JMIR Mhealth Uhealth 2017;5:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Wal MHL, Jaarsma T, Moser DK, van Gilst WH, van Veldhuisen DJ. Qualitative examination of compliance in heart failure patients in The Netherlands. Heart Lung 2010;39:121–130. [DOI] [PubMed] [Google Scholar]
- 21.Schweitzer RD, Head K, Dwyer JW. Psychological factors and treatment adherence behavior in patients with chronic heart failure. J Cardiovasc Nurs 2007;22:76–83. [DOI] [PubMed] [Google Scholar]
- 22.Wu HS, Gal R, van Sleeuwen NC, et al. Breast cancer survivors’ experiences with an activity tracker integrated into a supervised exercise program: qualitative study. JMIR Mhealth Uhealth 2019;7:e10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein SP, Goldstein CM, Bond DS, Raynor HA, Wing RR, Thomas JG. Associations between self-monitoring and weight change in behavioral weight loss interventions. Health Psychol 2019;38:1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helander EE, Vuorinen A-L, Wansink B, Korhonen IKJ. Are breaks in daily self-weighing associated with weight gain? PLoS One 2014;9:e113164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin CL, Tate DF, Valle CG. Nonadherence to daily self-weighing and activity tracking is associated with weight fluctuations among African American breast cancer survivors. PLoS One 2018;13:e0199751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanenbaum ML, Ross KM, Wing RR. Overeat today, skip the scale tomorrow: An examination of caloric intake predicting nonadherence to daily self-weighing. Obesity (Silver Spring) 2016;24:2341–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Truesdale KP, Stevens J, Lewis CE, Schreiner PJ, Loria CM, Cai J. Changes in risk factors for cardiovascular disease by baseline weight status in young adults who maintain or gain weight over 15 years: the CARDIA study. Int J Obes 2006;30:1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levitsky DA, Garay J, Nausbaum M, Neighbors L, Dellavalle DM. Monitoring weight daily blocks the freshman weight gain: a model for combating the epidemic of obesity. Int J Obes 2006;30:1003–1010. [DOI] [PubMed] [Google Scholar]
- 29.Rosenbaum DL, Espel HM, Butryn ML, Zhang F, Lowe MR. Daily self-weighing and weight gain prevention: a longitudinal study of college-aged women. J Behav Med 2017;40:846–853. [DOI] [PubMed] [Google Scholar]
- 30.Wing RR, Tate DF, Espeland MA, et al. Innovative Self-Regulation Strategies to Reduce Weight Gain in Young Adults: The Study of Novel Approaches to Weight Gain Prevention (SNAP) Randomized Clinical Trial. JAMA Intern Med 2016;176:755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wing RR, Espeland MA, Tate DF, et al. Weight gain over 6 years in young adults: the study of novel approaches to weight gain prevention randomized trial. Obesity (Silver Spring) 2020;28:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanWormer JJ, Martinez AM, Martinson BC, et al. Self-weighing promotes weight loss for obese adults. Am J Prev Med 2009;36:70–73. [DOI] [PubMed] [Google Scholar]
- 33.Gokee-Larose J, Gorin AA, Wing RR. Behavioral self-regulation for weight loss in young adults: a randomized controlled trial. Int J Behav Nutr Phys Act 2009;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phelan S, Hill JO, Lang W, Dibello JR, Wing RR. Recovery from relapse among successful weight maintainers. Am J Clin Nutr 2003;78:1079–1084. [DOI] [PubMed] [Google Scholar]
- 35.Harrison D, Marshall P, Bianchi-Berthouze N, Bird J. Activity tracking: Barriers, workarounds and customisation. In: Proceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing - UbiComp' ' ’15. New York, New York, USA: ACM Press, 2015, pp 617–621. [Google Scholar]
- 36.Lewis ZH, Pritting L, Picazo A-L, JeanMarie-Tucker M. The utility of wearable fitness trackers and implications for increased engagement: An exploratory, mixed methods observational study. Digit Health 2020;6:2055207619900059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alley S, Schoeppe S, Guertler D, Jennings C, Duncan MJ, Vandelanotte C. Interest and preferences for using advanced physical activity tracking devices: results of a national cross-sectional survey. BMJ Open 2016;6:e011243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rapp A, Cena F. Personal informatics for everyday life: How users without prior self-tracking experience engage with personal data. Int J Hum Comput Stud 2016;94:1–17. [Google Scholar]
- 39.Fahey MC, Klesges RC, Kocak M, Wayne Talcott G, Krukowski RA. Changes in the Perceptions of Self-weighing Across Time in a Behavioral Weight Loss Intervention. Obesity (Silver Spring) 2018;26:1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.An R. Weekend-weekday differences in diet among U.S. adults, 2003-2012. Ann Epidemiol 2016;26:57–65. [DOI] [PubMed] [Google Scholar]
- 41.Thomas JG, Bond DS. Behavioral response to a just-in-time adaptive intervention (JITAI) to reduce sedentary behavior in obese adults: Implications for JITAI optimization. Health Psychol 2015;34S:1261–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klasnja P, Smith S, Seewald NJ, et al. Efficacy of Contextually Tailored Suggestions for Physical Activity: A Micro-randomized Optimization Trial of HeartSteps. Ann Behav Med 2019;53:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forman EM, Goldstein SP, Crochiere RJ, et al. Randomized controlled trial of OnTrack, a just-in-time adaptive intervention designed to enhance weight loss. Transl Behav Med 2019;9:989–1001. [DOI] [PubMed] [Google Scholar]
- 44.Oppezzo MA, Stanton MV, Garcia A, Rigdon J, Berman JR, Gardner CD. To text or not to text: electronic message intervention to improve treatment adherence versus matched historical controls. JMIR Mhealth Uhealth 2019;7:e11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baseman JG, Revere D, Painter I, Toyoji M, Thiede H, Duchin J. Public health communications and alert fatigue. BMC Health Serv Res 2013;13:295. [DOI] [PMC free article] [PubMed] [Google Scholar]


