Abstract
Background
During COVID-19 pandemic, school closure has been mandated in analogy to its effect against influenza, but it is unclear whether schools are early COVID-19 amplifiers.
Methods
We performed a cross-sectional and prospective cohort study in Italy during the second COVID-19 wave (from September 30, 2020 until at least February 28, 2021). We used databases from the Italian Ministry of Education, the Veneto region systems of SARS-CoV-2 cases notification and of schools’ secondary cases tracing to compare SARS-CoV-2 incidence in students/school staff and general population and incidence across age groups. Number of tests, secondary infections by type of index case and ratio cases/ tests per school were estimated using an adjusted multivariable generalized linear regression model. Regional reproduction numbers Rt were estimated from Italian Civil Protection daily incidence data with a method of posterior distribution using a Markov Chain Monte Carlo algorithm.
Findings
SARS-CoV-2 incidence among students was lower than in the general population. Secondary infections at school were <1%, and clusters of ≥2 secondary cases occurred in 5–7% of the analysed schools. Incidence among teachers was comparable to the population of similar age (P = 0.23). Secondary infections among teachers were rare, occurring more frequently when the index case was a teacher than a student (37% vs. 10%, P = 0.007). Before and around the date of school opening in Veneto, SARS-CoV-2 incidence grew maximally in 20–29- and 45–49-years old individuals, not among students. The lag between school opening dates in Italian regions and the increase in the regional COVID-19 Rt was not uniform. Finally, school closures in two regions where they were implemented before other measures did not affect Rt decrease.
Interpretation
This analysis does not support a role for school opening as a driver of the second COVID-19 wave in Italy, a large European country with high SARS-CoV-2 incidence.
Funding
Fondazione MITE.
Research in context.
Evidence before this study
The role of schools and at large of children as amplifiers of the COVID-19 pandemics was debated. Despite biological and epidemiological evidence that children play a marginal role in SARS-CoV-2 spread, policies of school closures have been predicated, mostly based on the temporal coincidence between school reopening in certain countries and COVID-19 outbreaks. Whether schools contributed to the so called “second COVID-19 wave” was uncertain. Italy's regional calendar of school reopening and databases of positivity at school allowed to estimate the impact of schools on the increase of SARS-CoV-2 incidence that occurred in autumn 2020.
Added value of this study
We found that incidence among students was lower than in the general population and that incidence among teachers was comparable to that among individuals of the same age bracket. Moreover, secondary infections and clusters at school were rare. When the secondary case was a teacher, the index case was more frequently a teacher than a student. In Veneto Region, during the first phase of the second wave, incidence among school age individuals was low as opposed to the sustained incidence among individuals of 45–49 years. Finally, the time lag between school opening and Rt increase was not uniform across Italian regions with different school opening dates, with lag times shorter in regions where schools opened later. Thus, SARS-CoV-2 infections rarely occur at school and transmission from students to teachers is infrequent. Moreover, a role for school age individuals and school openings as a driver of the COVID-19 second wave is not supported.
Implications of the available evidence
Our findings could inform policy initiatives of school openings during the current COVID-19 pandemic.
Alt-text: Unlabelled box
1. Introduction
School closures represent a widespread nonpharmacological intervention (NPI) in the context of the current Coronavirus Disease 2019 (COVID-19) pandemic. In Italy, schools have been closed for half of the 2019–2020 school year and, during the second COVID-19 wave, high schools have been closed again, students switching to “integrated digital learning” nationwide since November 6, 2020. The rationale for such a NPI has mostly been drawn from the reported beneficial effect of school closure during influenza pandemics [1], even if the debate was still open [2]. However, while children's immune system is naïve to influenza antigens, making them a known reservoir of influenza infection, they do not appear to be as affected by COVID-19 as adults, representing a small fraction of documented COVID-19 cases. Like SARS-CoV and MERS-CoV, SARS-CoV-2 indeed affects children less, causing fewer symptoms, a less severe disease and much lower case-fatality rates [3], [4], [5].
Several biological factors might contribute to the reduced COVID-19 risk in children: first, children express significantly fewer ACE2 receptors – the entry point of SARS-CoV-2 into human cells – compared to adults [6]; second, they are commonly exposed to other seasonal coronaviruses and develop both humoral and cellular cross-immunity [7]. Children appear therefore less susceptible to the infection, and when infected may have a preformed arsenal of neutralizing cross-reactive antibodies that might reduce the likelihood of transmitting the virus. This biological evidence is mirrored in several epidemiological studies. A meta-analysis of 32 studies from different countries suggests that children are less susceptible to SARS-CoV-2 infection compared with adults [8]. An age-structured mathematical model applied to epidemic data from China, Italy, Japan, Singapore, Canada and South Korea estimates that individuals younger than 20 years of age display half the chance of being infected than adults [9]. In the context of households (the most common route of secondary infection), chances of transmission from children to adults are low and the spread seldom starts from children. In a large study including 15,771 children (age 1–18) living in Germany, almost two-thirds of children living with virus-positive family members were negative for SARS-CoV-2 antibodies and virus tests, suggesting that transmission to children is infrequent [10]. The child represented the index case in only three families (9.7%) among 31 household transmission chains that involved children in China, Singapore, USA, Vietnam, and South Korea [11]. In a meta-analysis of all contact-tracing studies up to May 16 2020, children were 56% less susceptible to SARS-CoV-2 than adults [Pooled OR=0.44 (95%CI 0.29, 0.69)] [8]. In the Italian town of Vo’ Euganeo, where 70% of the population was screened twice and 2.6% of the population resulted positive, no child below 10 years of age was found positive, even if these children lived in the same household with a positive individual [12]. In a large cohort study on 12 million people in the UK, the risk of infecting and becoming infected with SARS-COV-2 grew with age [13]. In the same study, risk of contracting SARS-CoV-2 for >9 million adults living with children up to 11 years of age was not higher than that of the rest of the population. The risk increased slightly for those who lived with adolescents aged 12 to 18, but this risk did not correspond to a greater lethality in case of infection. Indeed, there was no significant effect of the school closure on the epidemic trend in the families analysed, when compared to the rest of the population [13].
Despite evidence indicating a marginal role for children in COVID-19 pandemic, school openings (or re-openings) have been considered as potential drivers of surges of cases in the general population [14]. This concept has been based on clinical, epidemiological, modeling studies and by systematic reviews that however show conflicting results on whether school closures efficaciously curtailed the incidence of infection [15, 16]. Adolescents were reported to spread the virus as likely as adults [17], and in one study, levels of SARS-CoV-2 genetic material in the upper respiratory tract of children <5 year old with mild to moderate COVID-19 were higher than in children 6–17 year old and adults [18]. Furthermore, in a COVID-19 outbreak at a summer camp in Georgia, children of all ages were found to be highly susceptible to infection: 51% of the 6–10 years old campers tested positive, as did 44% of those aged 11 to 17 [19]. In Israel, schools fully reopened on May 17, 2020 and ten days later a major outbreak of COVID-19 occurred in a high school; temporal correlation between school openings and the second wave was interpreted as a causal link [20]. By extension, policymakers (as well as the lay public) attribute to school openings a key role in amplifying infection rates in the general population [14]. This opinion is particularly widespread in Italy, where schools remained closed from February 25, 2020 in Northern Italian regions (from March 9, 2020 nationwide) until September, when they reopened in different days across the 20 different Italian regions and two autonomous Provinces.
According to the Italian National Statistical Institute (ISTAT), 9,150,518 students attended the different school cycles in 2019 in Italy. These cycles include kindergarten (scuola dell'infanzia, attended by 3–5 years old children), elementary (scuola primaria attended by 6–10 years old children), middle (scuola secondaria di primo grado attended by 11–13 years old children) and high school (scuola secondaria di secondo grado attended by 14–18 years old children). Education is compulsory from 6 to 16 years of age. Pre-elementary school education that includes kindergarten as well as nurseries (asili nido, attended by children 0–2 years old) is not compulsory. On average, students represented 15% of the population of each of the 20 Italian regions and two autonomous Provinces (range: 10.7%−19%; Table 1). In 2020, while kindergartens and nurseries started nationwide on September 1st, the calendarized opening day of all other schools differed among regions. In most regions, schools started on September 14; in a second group of regions, schools opened on September 24; in two other regions, on September 16 or 22 (Table 2). The Italian Government mandated a protocol to minimize risk of COVID-19 diffusion that followed most of the strictest recommendations [21]. Measures included non-compulsory temperature control and hand hygiene at the school entrance; unidirectional flows of students; mask mandate for all personnel and students in common areas and for high school students also when seated at their desks (and always for teachers, combined with face-shields in certain settings; this mask mandate was then extended to students also when seated starting from November 6, 2020), compulsory 1 m seat to seat distance, frequent classroom natural ventilation, ban or reduction of school sports and music, reduced duration of school hours and reduced school duration [22]. In case staff members are diagnosed as COVID-19 positive, they must promptly inform the school Principal. Similarly, parents must promptly report to the schools any case of COVID-19 positivity in their children, and Principals must coordinate with local units of the National Health System to perform secondary screenings among staff/students, or to mandate quarantine for 14 days with a swab to all quarantined students/personnel before re-admitting them to the school premises. From October 13, 2020 quarantine was reduced to 10 days with a negative swab or remained of 14 days if a swab was not performed. Notwithstanding these rules, school opening has been accounted as the driver of the second COVID-19 wave by the popular press, as well as by opinion makers and their closure has been predicated by several data analysts [14]. Consequently, high schools nationwide and, in certain regions, the second and third year of middle schools have been closed since November 6. In other regions (Campania and Apulia), closure of all schools including elementary and kindergarten has been mandated since October 16 and 30, respectively. In Lombardy, high schools have been closed since October 26. However, whether school openings played a crucial role in the second wave of COVID-19 infections remains to be ascertained. Italy was in a privileged position to investigate this possibility: school calendars are regional and starting dates are staggered among different regions by up to 17 days.
Table 1.
Demographics of Italian Regions and autonomous Provinces. Data are from the National Statistical Institute (ISTAT). In Italy, elementary school starts at 6, middle school at 11, high school at 14 years of age.
| Region | Population | Preschool students (%) | Elementary school students (%) | Middle school students (%) | High school students (%) | Students/ population (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Abruzzo | 1,305,770 | 48,397 | 3.7% | 55,893 | 4.3% | 34,881 | 2.7% | 58,308 | 4.5% | 15.1% |
| Apulia | 4,008,296 | 181,674 | 4.5% | 178,761 | 4.5% | 115,152 | 2.9% | 205,348 | 5.1% | 17.0% |
| Basilicata | 556,934 | 19,710 | 3.5% | 14,110 | 2.5% | 14,696 | 2.6% | 26,640 | 4.8% | 13.5% |
| Bolzano | 532,080 | 27,742 | 5.2% | 27,592 | 5.2% | 17,097 | 3.2% | 28,846 | 5.4% | 19.0% |
| Calabria | 1,924,701 | 80,534 | 4.2% | 85,450 | 4.4% | 54,642 | 2.8% | 77,850 | 4.0% | 15.5% |
| Campania | 5,785,861 | 254,097 | 4.4% | 232,042 | 4.0% | 183,729 | 3.2% | 324,049 | 5.6% | 17.2% |
| Emilia-Romagna | 4,467,118 | 207,566 | 4.6% | 203,083 | 4.5% | 87,735 | 2.0% | 200,680 | 4.5% | 15.6% |
| Friuli-Venezia Giulia | 1,211,357 | 34,169 | 2.8% | 50,546 | 4.2% | 22,584 | 1.9% | 43,230 | 3.6% | 12.4% |
| Lazio | 5,865,544 | 239,656 | 4.1% | 223,071 | 3.8% | 168,949 | 2.9% | 270,075 | 4.6% | 15.4% |
| Liguria | 1,543,127 | 59,214 | 3.8% | 48,338 | 3.1% | 38,327 | 2.5% | 64,141 | 4.2% | 13.6% |
| Lombardy | 10,103,969 | 256,204 | 2.5% | 475,220 | 4.7% | 208,087 | 2.1% | 477,029 | 4.7% | 14.0% |
| Marche | 1,518,400 | 66,271 | 4.4% | 66,740 | 4.4% | 29,095 | 1.9% | 68,507 | 4.5% | 15.2% |
| Molise | 302,265 | 12,214 | 4.0% | 11,544 | 3.8% | 7484 | 2.5% | 10,903 | 3.6% | 13.9% |
| Piedmont | 4,341,375 | 137,009 | 3.2% | 151,981 | 3.5% | 117,142 | 2.7% | 156,974 | 3.6% | 13.0% |
| Sardinia | 1,630,474 | 51,318 | 3.1% | 63,957 | 3.9% | 40,501 | 2.5% | 19,189 | 1.2% | 10.7% |
| Sicily | 4,968,410 | 247,970 | 5.0% | 190,547 | 3.8% | 147,430 | 3.0% | 259,111 | 5.2% | 17.0% |
| Tuscany | 3,722,729 | 135,146 | 3.6% | 130,853 | 3.5% | 101,638 | 2.7% | 135,178 | 3.6% | 13.5% |
| Trento | 542,739 | 19,206 | 3.5% | 26,771 | 4.9% | 16,483 | 3.0% | 27,833 | 5.1% | 16.6% |
| Umbria | 880,285 | 37,363 | 4.2% | 31,048 | 3.5% | 24,520 | 2.8% | 39,075 | 4.4% | 15.0% |
| Valle D'Aosta | 125,501 | 4647 | 3.7% | 5740 | 4.6% | 3662 | 2.9% | 4758 | 3.8% | 15.0% |
| Veneto | 4,907,704 | 225,722 | 4.6% | 223,780 | 4.6% | 142,348 | 2.9% | 233,716 | 4.8% | 16.8% |
| Italy | 60,244,639 | 2345,829 | 4% | 2497,067 | 4% | 1576,182 | 3% | 2731,440 | 5% | 15% |
Table 2.
Dates of School opening in the 21 Italian Regions and autonomous Provinces (Trento and Bolzano).
| School Opening | Sept. 7 | Sept. 14 | Sept. 16 | Sept. 22 | Sept. 24 |
|---|---|---|---|---|---|
| Region/Autonomous Province | Bolzano | Emilia-Romagna | Friuli Venezia Giulia | Sardinia | Abruzzo |
| Lazio | Apulia | ||||
| Liguria | Basilicata | ||||
| Lombardy | Calabria | ||||
| Marche | Campania | ||||
| Molise | |||||
| Piedmont | |||||
| Sicily | |||||
| Tuscany | |||||
| Trento | |||||
| Umbria | |||||
| Valle D'Aosta | |||||
| Veneto |
The aims of this study were to investigate the overall incidence of SARS-CoV-2 infection among students and teachers, as well as whether there was an association between the increase in transmissibility of SARS-CoV-2 (measured as reproduction number Rt) and dates of school openings in different Italian Regions. We also estimated the incidence of SARS-CoV-2 by age in Veneto and the incidence of SARS-CoV-2 positive students, teachers, and non-teaching staff members in public and private schools in two weeks between the end of November and beginning of December in the Italian regions. We calculated the rate of secondary infections per number of swab tests and frequency of clusters identified during contact tracing activity in a large sample of Italian Schools. We also estimated the frequency of secondary infections in teachers by type of index case (student, teacher, or non-teaching staff member).
2. Methods
2.1. Study design
This was a cross-sectional and prospective cohort study. The cross-sectional cohort study [23] was designed to compare incidence of COVID-19 among students and teaching and non-teaching school staff versus that in the general population. We used the following cohorts: students, teachers, non-teaching school staff and general population, stratified by class of age where indicated. In these cohorts, we calculated SARS-CoV-2 incidence in the September 12 to November 8, 2020 period.
The prospective cohort studies were designed to address four questions: (i) whether concomitant to school opening COVID-19 incidence increased earlier among students than in the general population; (ii) whether COVID-19 positive students or school staff (teaching and non-teaching) resulted in COVID-19 outbreaks in schools; (iii) whether secondary cases in school settings were predominantly associated with student index cases; (iv) whether the increase in regional SARS-CoV-2 reproduction number Rt followed the different school opening dates at a constant time interval. As for the first question, we stratified incidence of newly reported COVID-19 cases for age from August 28 to October 24, 2020 by analysing datasets extracted from the Veneto Region system of SARS-CoV-2 cases notification. As for the second question, we analysed data collected by the Italian Ministry of Education (Ministero dell'Istruzione - MI) from contact tracing in monitored schools from November 23 to December 5, 2020. As for the third question, we extracted information from the province of Verona (Veneto Region) database of secondary infections among students, teachers, and non-teaching staff in 339 schools in the November 25 to December 21, 2020 period. Last, as for the fourth question, we calculated the transmission number Rt, in each Italian region from the new daily cases in the period August 6 to December 2, 2020.
2.2. Databases
2.2.1. Calculation of SARS-CoV-2 incidence among students, school staff and general population
For the calculation of incidence among students and teaching and non-teaching staff, we accessed data collected within the comprehensive, national reporting system put in place by MI. This database gathers information from school Principals every week for each comprehensive private and state institute and contains the number of new positive SARS-CoV-2 cases per school per week (Monday to Sunday) from September 12 (two days before school openings in most regions) to November 8, 2020. This database reports the incidence in the first (kindergarten, elementary and middle school) and second cycle of education (high school) by region. Data (available as supplementary material) were retrieved from 7976 public school institutes (97% of total), accounting for 7,376,698 students, 775,451 teachers and 206,120 non-teaching staff members. We also analysed data of SARS-CoV-2 incidence in schools in the period 23–28 November 2020 in a sample of 6827 public institutes (81.6% of the total) and 7035 private institutes (55.6% of the total institutes). SARS-CoV-2 incidence rates were calculated irrespective of whether the infection was acquired within or outside the educational setting. Attendance denominators for educational settings were obtained from the MI open database (https://dati.istruzione.it/opendata/ accessed on December 3, 2020). For incidence rates calculations, denominators were drawn from MI enrolment figures.
To calculate regional SARS-CoV-2 incidence, we used the public national database of COVID-19 positivity determined as SARS-CoV-2 RT-PCR swab positivity and available at https://github.com/pcm-dpc/COVID-19 (accessed on December 3, 2020), from September 12 to November 8, 2020. Regional population was estimated from the Office for National Statistics (Istituto Nazionale di Statistica, ISTAT, http://demo.istat.it/ accessed on December 3, 2020).
2.2.2. Calculation of SARS-CoV-2 incidence in Veneto region students and general population
We used datasets extracted from the Veneto Region system of SARS-CoV-2 cases notification. We stratified incidence of newly reported COVID-19 cases for age from August 28 to October 24, 2020, when overall COVID-19 incidence in Veneto increased from ~2/10,000 to ~35/10,000. We stratified incidence of newly reported COVID-19 cases for age by using the classic demographic brackets (we used one single group of 75+ years old individuals as we did not find differences in incidence in groups above age 75) and calculated daily incidence of newly reported cases in these age categories. Denominators were from ISTAT, http://demo.istat.it/ (accessed on December 3, 2020).
2.2.3. Analysis of contact tracing
We analysed data collected by MI from contact tracing in the monitored schools (from November 23 to December 5, 2020). Information was retrieved from 5971 (45%) public and private institutes in the week 23–28 November 2020, and 7035 (55.6%) institutes in the week 30 November-5 December 2020, accounting for 423,516 and 496,289 students in the first and second week, respectively. For outbreaks, direction of transmission from the index case to secondary cases was inferred based on the date of symptom onset for symptomatic individuals and date of testing for asymptomatic individuals. We evaluated associations between event measures in educational settings, regional COVID-19 incidence, and other regional characteristics to identify possible predictors for cases and outbreaks. When Institutes suspect or identify a case or outbreak of COVID-19, they must inform the Department of Prevention of the local unit (AULSS) of the National Health System responsible for contact tracing and the MI. AULSS then performs risk assessment and decides on any additional investigation and infection control measure, based on factors such as the number of new positive subjects, disease severity, and potential of transmission at school. AULSS records each event in an online national database of public health management. MI and AULSS have legal permission to process these information (https://istruzioneveneto.gov.it/wp-content/uploads/2020/10/Informativa-sul-trattamento-dei-dati-Test-screening.pdf).
To determine whether secondary cases in school settings were predominantly associated with student index cases, we extracted information regarding secondary infections among students, teachers, and non-teaching staff in 339 schools in the province of Verona (Veneto Region) from November 25 to December 21, by type of index case.
2.2.4. Calculation of SARS-CoV-2 transmission number Rt
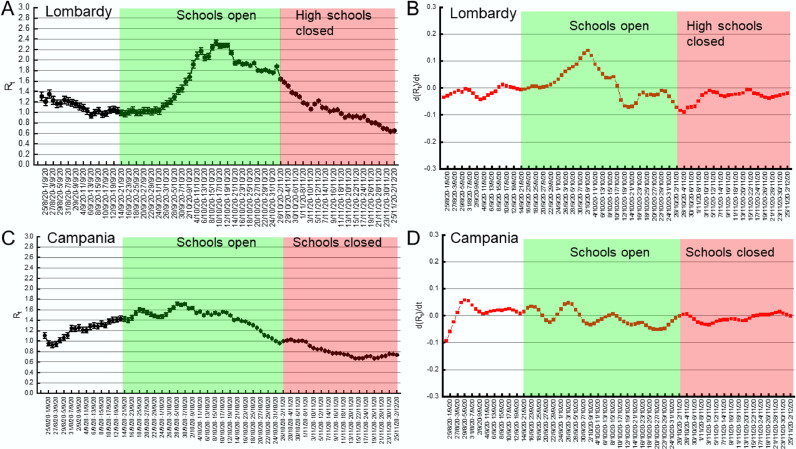
To calculate the regional transmission number Rt, new daily SARS-CoV-2 cases in the period August 6 to December 2, 2020 were retrieved from the database of the Italian Civil Protection (https://github.com/pcm-dpc/COVID-19). The period August 6 to December 2, 2020 was chosen to include in the analysis the new daily cases one month before the earliest school openings (Bolzano, September 7, 2020) and until this paper was prepared. Because of the stability (i.e., lack of recalculations) of the data communicated by Campania and Lombardy, in Fig. 5 we could estimate Rt on the positives at a RT-PCR for SARS-CoV-2 in swabs prescribed by a physician (sospetto diagnostico, i.e., clinical indication). Of note, no qualitative difference was found with Rt estimated from all new daily SARS-CoV-2 cases in these two regions in the timeframe of our analysis.
Fig. 5.
School closures do not affect Rt decrease in Lombardy and Campania.
(A, C) Median Rt in the indicated 7 days periods (±5–95% Credible Intervals) in Lombardy (A) and Campania (C). Days of school opening and closure are indicated.
(B, D) First order derivative of Rt in Lombardy (B) and Campania (D). Days of school opening and closure are indicated.
2.3. Statistical methods
Mean (standard deviations), median values (inter-quartile ranges), and boxplots for continuous variables and absolute and relative frequencies for categorical variables are presented. Differences among groups for continuous variables were tested by means of the non-parametric Wilcoxon-rank sum test and differences for categorical variables were tested by means of the Chi-square test.
Rates of secondary infections were defined as number of cases/number of tests occurring the same week after a SARS-COV-2 positive student or teacher was found. Least Square means (LSM), 95% confidence intervals (CI) and P-values of rate of secondary infections and number of positive tests per institute and week are estimated with a multivariable generalized linear regression model adjusted for week of test and density of the region, weighted for the number of tests released in each institute to trace close contacts. Square root transformations were carried out to achieve normality of residuals of full models.
Incidence rates were calculated as the sum of all new positives in each week, divided by the size of the population. We work out the cases per 10,000 (a standard epidemiological way of presenting incidence) by dividing the number of cases by the population in each age group (estimates are from ISTAT, 2019).
To generate the incidence heatmap, a matrix of the weekly incidence referred to individual age ranges was calculated. By using Excel, individual cells were color-coded in a 3-color scale (green-beige-red) of increasing weekly incidence rate. To generate the heatmap of distance between age brackets, the same matrix was fed to the Heatmapper algorithm (www.heatmapper.ca) and we selected to calculate the distance between rows and columns using the Euclidean Distance Measurement Method.
Transmissibility was measured by the reproduction number Rt, as the average number of secondary cases caused by an infected individual. We estimated Rt over the months incorporating uncertainty in the distribution of the serial interval (the time between the onset of symptoms in a primary case and the onset of symptoms in secondary cases) [24]. Rt was computed by using EpiEstim [24] with parameters from the first COVID-19 wave in Italy as defined by Merler and co-workers [25] (serial interval: 6.6, gamma: 4.9). Rt was computed using the number of new cases/day in each region. In all graphs, Rt values are reported as median values for a 7-day posterior timeframe with 95% credible intervals. When an NPI was introduced and school opening occurred, their effect on Rt was referred to the first day of the corresponding 7-day period. For example, if schools opened on September 14, their effect on Rt was introduced from the period September 14–20.
We computed the cross-correlation analysis between time series of incidence in the population of students 6–13 and 14–18 years old, as well as in the general population using the cross-correlation function of OriginPro 2021 (OriginLab, Northampton, MA, USA)
All statistical analyses were performed with Statistical Analysis System Version 9.4 (SAS Institute, Cary, NC, USA) except those in Figs. 4 and 5 that were performed with OriginPro 2021 (OriginLab, Northampton, MA, USA).
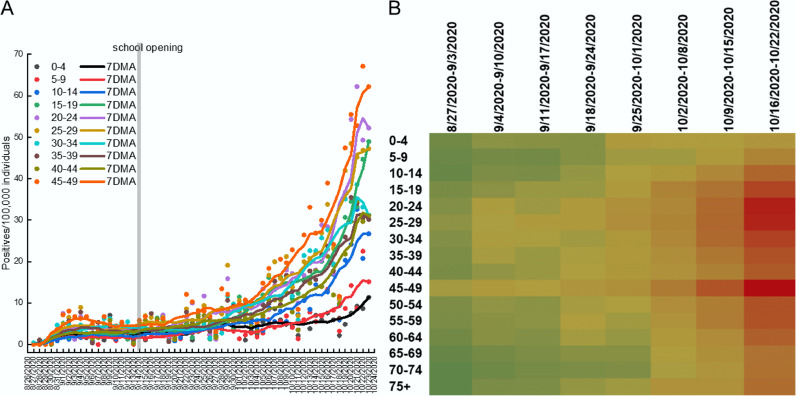
Fig. 4.
During the second COVID-19 wave incidence of SARS-CoV-2 rises initially among young adults and 45–49 years old individuals in Veneto region.
(A, B) Daily incidence and 7 days adjacent average (7DMA) of SARS-CoV-2 positivity among individuals of the indicated age range. Consistency of the population in each age bracket was from ISTAT and is detailed in Table 6.
(C) Heatmap of weekly incidence of SarsCoV2 in individuals of the indicated age ranges in the Veneto region during the indicated timeframe. The color scale goes from green (low incidence) to beige (medium incidence) and to red (high incidence). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.4. Role of the funding source
The Italian Ministry of Health with Ricerca Corrente and 5 × 1000 funds (to SG) did not support study design, data collection, data analysis, interpretation, and writing of the report. Fondazione MITE funds (to SG, FC, and LS) had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
2.5. Declaration of interest
LS received advisory honoraria on behalf of Astellas Pharmaceuticals and sits on the advisory board of Mitochondria in Motion, Inc.
3. Results
3.1. Incidence of COVID-19 among students is lower than in the general population
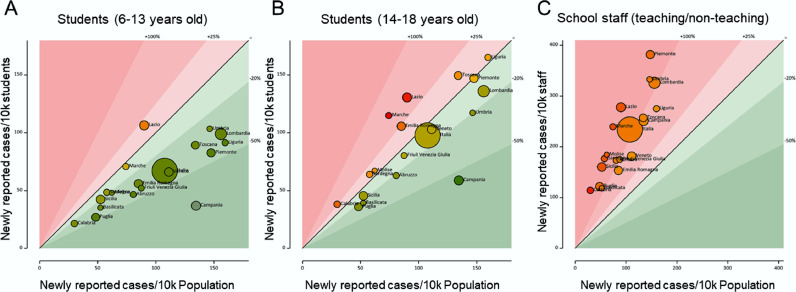
To first gain insight into the diffusion of COVID-19 in Italian Schools, we compared the incidence of new SARS-CoV-2 positives in the period and per week among students, teachers, and non-teaching staff members of elementary, middle, and high schools to the incidence of SARS-CoV-2 positivity in the general population for each region. The incidence of positives among students was lower than that in the population (overall incidence: 108/10,000), irrespective of whether we analysed elementary and middle schools (incidence: 66/10,000), or high schools (incidence: 98/10,000). Incidence of new positives among elementary and middle school students was on average 38.9% lower than in the general population in all Italian regions but Lazio (Fig. 1A). In the case of high schools, incidence of new positives among the students was 9% lower to that of the general population (Fig. 1B). In the three regions of Lazio, Marche, and Emilia-Romagna, it was higher than in the general population. Among teachers and non-teaching staff incidence was 2-fold higher than that observed in the general population (approx. 220/10,000, Fig. 1C). These data indicate that students are largely protected from SARS-CoV-2 infection, irrespective of their school cycle. Conversely, infection appears to be more widespread among teachers and non-teaching staff members of schools than in the general population. Of note, while teachers share classrooms for several hours with students, non-teaching staff members include administrative personnel and janitors who seldom interact with students.
Fig. 1.
Incidence of SARS-CoV-2 is lower among students than in the general population.
Bubble graphs of SARS-CoV-2 incidence between September 12 and November 7 among 6–13 years old (A) and 14–18 years old (B) students and among teaching and non-teaching staff members (C) in Italian regions and autonomous provinces compared to the incidence in the general population. Size of bubbles is proportional to the measured incidence in the analysed school populations. The 45° line indicates equivalence between general population and school population incidence. Bubbles are color-coded in a green-yellow-red gradient proportional to the value of the ratio between the analysed population and the general population. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We next used a second database in which MI collected the number of new cases in the period 23–28 November. This database offers a snapshot of the distribution of new cases in a limited timeframe during the peak of the second COVID-19 wave. New positive subjects were found mostly among teachers and non-teaching staff members: SARS-CoV-2 positives were 0.32% of students, 1.52% of teachers and 1.96% of non-teaching staff members (Table S1, Fig. S1). The highest rate was found in Molise and the lowest in Calabria. Incidences of new cases in kindergarten were 0.21% in pupils and 2.35% among teachers (P<0.001); in elementary schools were 0.35% among children and 1.83% among teachers (P<0.001). In middle schools, 0.45% students and 1.60% teachers were found positive (P<0.001, Tables S2 and S3). Similar incidence rates were found in private schools (Tables S4-S6), except for a slightly lower rate among non-teaching staff members (1.67%, Table S4). This database allowed us to also investigate how often the communication of a positive case elicited quarantine for students/staff members. A quarantine period was requested for 1.92% of students, 2.30% of teachers and 2.56% of non-teaching staff members of the analysed public schools (Table S7). In private schools, rates of quarantines were very similar, except for a slightly higher rate for children (2.65%, Table S8). These data indicate that even during the peak of the second COVID-19 wave, students were less infected than adults in school establishments, and that -overall, the quarantine system was widespread, vis-à-vis a very low rate of positivity among students.
Finally, to compare the degree of infection transmission from students and teachers to their close contacts, we analysed data collected by MI from contact tracing in the monitored schools from November 23 to December 5, 2020. The Least Square Means (LSM) estimates of the incidence of secondary cases over the number of tests carried out on close contacts of a positive subjects in school was less than 1% per school and week for teachers and students, in kindergarten, elementary and middle schools. Estimates of rates when the index case was a student or a teacher were not statistically different (P = 0.81 in Kindergartens, P = 0.22 in elementary schools, P = 0.50 in middle schools; Table 3). The number of tests per institute per week ranged from an average of 7 in kindergarten to 18 in middle schools (Table 4), even though the distribution was very skewed and reached up to 100–200 swab tests. Twenty-seven schools carried out more than 100 tests in a week. We did not notice any difference in the number of tests per school if the index case was a student or a teacher (Fig. S2). Clusters, defined as >2 SARS-CoV-2 positive subjects identified in one week following contact tracing of index cases, were found in 5% to 7% of schools (Fig. S3). On average, 49%−56% of all close contacts of a positive student or teacher were placed in quarantine for 10 days, with the need of a negative swab at the end of the period to be readmitted at school.
Table 3.
Rates of secondary infections identified by contact tracing in Italian Schools (from November 23 to December 5, 2020). We calculated rates of secondary infections as number of cases over the number of tests performed up to a week after a SARS-COV-2 positive student or teacher was found. LSM, 95% Confidence Interval (95%CI) and P-values of secondary infections rates per institute and week were estimated with a multivariable generalized linear regression model. P-value refers to Student vs. Teachers as index case.
| Student as index case LSM 95%CI |
Teacher as index case LSM 95%CI |
P-value | |
|---|---|---|---|
| Kindergarten | 0.78% (0.45%, 1.20%) | 0.71% (0.33%, 1.22%) | 0.81 |
| Elementary school | 0.68% (0.48%, 0.91%) | 0.98% (0.64%, 1.39%) | 0.22 |
| Middle school | 0.74% (0.53%, 0.97%) | 0.90% (0.51%, 1.40%) | 0.50 |
Table 4.
Activity of contact tracing following a positive case among students and teachers in Italian Schools (from 23 of November to 5 of December 2020). Mean and standard deviation of number of tests per institute.
| Type of school | n. of schools | Mean number of tests | Standard deviation | Absolute range | |
|---|---|---|---|---|---|
| Student Index case | Kindergarten | 531 | 9 | 13 | 0–87 |
| Elementary | 873 | 16 | 20 | 0–150 | |
| Middle schools | 753 | 17 | 21 | 0–87 | |
| Teacher Index case | Kindergarten | 465 | 7 | 15 | 0–180 |
| Elementary | 540 | 13 | 25 | 0–232 | |
| Middle schools | 338 | 12 | 26 | 0–117 |
3.2. Increases in Rt in Italian regions with different school opening dates
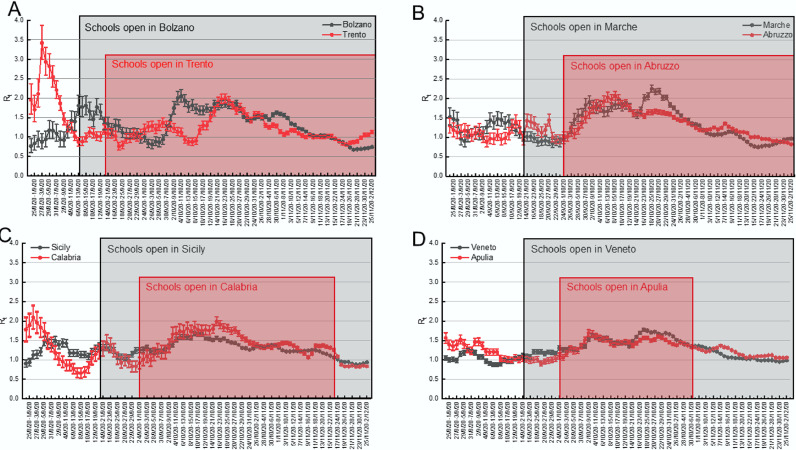
We reasoned that if school openings had played a role in the second wave of COVID-19 in Italy, the reproduction number Rt shall have increased earlier in the regions where schools started earlier. We first tested this hypothesis by analysing the case of the two provinces of Bolzano, where schools started on September 7, and Trento, where they started on September 14 (Table 2). Given the similarities between these two alpine territories in terms of orography, population density (72 inhabitants/km² in Bolzano; 87 in Trento), climate and lifestyle, they represent a very useful case scenario to investigate the role of schools in the local spread of COVID-19. We computed Rt 25 on the incidence of the positives at a RT-PCR for SARS-CoV-2 genetic material test from an oro/nasopharyngeal swab. Notwithstanding that schools in Trento opened 7 days later than in Bolzano, the increase in Rt (defined as an increase sustained for >3 moments and leading to Rt >1) occurred in Trento from the period September 23–30, whereas in Bolzano Rt started to increase from the period September 29-October 6, suggesting that there was no temporal relation between schools opening and surge in Rt (Fig. 2A).
Fig. 2.
Increases in Rt are not univocally correlated with school opening times in different Italian territories.
Pairwise comparison of median Rt in the indicated 7 days periods (±5–95% Credible Intervals) in the provinces of Bolzano and Trento (A) and in the indicated regions (B-D). The periods of school opening are highlighted by a box shaded in the same color of the respective province or region.
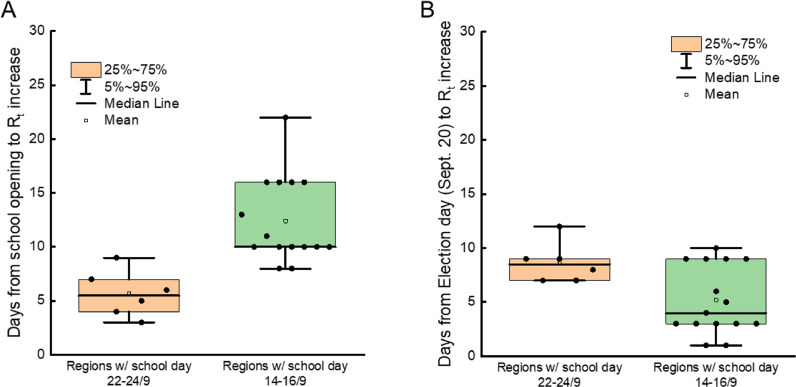
We extended our analyses to larger areas of the country, by applying them to different pairs of Regions, where schools opened on different days. We thus compared the temporal distribution of Rt in Abruzzo and Marche, two bordering regions of central-eastern Italy. In Marche, schools opened on September 14, in Abruzzo on September 24. In both regions, Rt started to increase from the 25/9–2/10 period (Fig. 2B). We repeated the same exercise for the pair Sicily-Calabria, where schools started on September 14 and 24, respectively. Again, we found no difference in the period when Rt started to increase (Fig. 2C). Finally, even in the case of the pair Veneto-Apulia, where schools opened on September 14 and 24 respectively, we did not appreciate any difference in the period when Rt started to increase (Fig. 2D). Altogether, these data indicate that the increase in SARS-CoV-2 reproduction number in different Italian regions occurred indeed after school openings, but that at the same time the delay between school opening and Rt rise was not constant as it would be expected if it were the only driver of COVID-19 diffusion. Indeed, this lag time appeared shorter in those regions where schools opened on September 24, and longer in those regions where schools opened on September 14. We further corroborated this finding by calculating the number of days from the date of the school opening to the Rt increase across all Italian regions (Fig. S4). The average delay from school opening to Rt increase was 5.7 days (CI95%: 3.4–8.0) in regions where schools opened on September 22 or 24, 12.4 days (CI95%: 10.2–14.6) in regions where schools opened on September 14 or 16 (Fig. 3A, P<0.05 in a Kolmogorov-Smirnov test). Conversely, the average delay between the Rt rise and the national election day held on September 21 was comparable in all regions: the mean was 8.6 (CI95%: 6.7–10.6) in regions where schools opened on September 22/24 and 5.2 (CI95%: 3.4–7.0) in regions where schools opened on September 7 or 14/16 (Fig. 3B). In conclusion, we did not find an unequivocally constant delay between school opening and Rt rise.
Fig. 3.
Increases in Rt are not univocally correlated with school opening times across Italian regions.
Box plots of the indicated quantiles for the days of delay between school openings (A) and September 20–21 national election day (B) and Rt increase in Italian regions clustered by their school opening dates. Date of Rt increase was calculated as the first day of the period when median Rt started an increase sustained in time (>3 consecutives periods).
3.3. Early increase in COVID-19 incidence among adults, not school age individuals during the second wave in the Veneto region
Because we did not find a strong temporal relation between school openings and the second COVID-19 wave in Italy, we decided to explore whether SARS-CoV-2 positivity circulated early in individuals different than children. To this end, we performed a prospective study on datasets extracted from the Veneto Region system of SARS-CoV-2 cases notification from August 28 to October 24, 2020, when overall COVID-19 incidence in Veneto increased from ~2/10,000 to ~35/10,000. In the period August 28- September 6, 2020 incidence increased among individuals 45 to 49-year-old and 25 to 39-year-old, albeit to a lower extent. Conversely, incidence remained very low in the other analysed age groups. Incidence increased again in the last decade of September in the age groups 45–49 and to a lower extent in the age groups 20–24 and 25–29 (Fig. 4A). These data suggested that at least in Veneto the earliest increase in SARS-CoV-2 positivity occurred in adults, followed by younger individuals, but not in adolescents that were often deemed as potential spreaders because of their high number of social contacts and their presumed laxity in adhering to the infection risk mitigation protocols. We therefore further inspected the temporal distribution of incidence among age classes. Visual inspection of a heatmap of the incidence of COVID-19 cases in every age group in the 8 weeks under consideration confirmed that the earliest increase in incidence occurs not among children or adolescents, but among individuals 20–49 years of age. These individuals appeared to be the drivers of the second wave, as incidence then propagated to individuals of other age categories (Fig. 4B). Indeed, by applying a Euclidean distance algorithm to the same matrix used to generate the heatmap, we found that children and adolescents are ranked as the groups closest to the least affected groups by this second COVID-19 wave (60–64 and 65–69 years of age). Conversely, individuals 20 to 29, and 45 to 49 years old are the most distant from the protected 60–69 years old individuals (Fig. S5).
We also compared the incidence of SARS-CoV-2 from September 19 to October 18 among teachers and among the general population of the age group 25–65 in Veneto. We selected this age group because teachers’ age is comprised between these two extremes, given the required tertiary education to be enrolled, and the legal retirement age of teachers. Interestingly, incidence among teachers started to increase after the general population of the same age; moreover, at the end of the period under consideration, incidence among teachers and among the general population aged 25–65 was not significantly different (12/10,000 vs. 11.1/10,000, P = 0.36, Fig. S6).
Finally, we investigated the frequency of secondary infections at schools in Verona and province from November 25 to December 21 on datasets extracted from the Veneto Region system of SARS-CoV-2 cases notification. We found 380 students, 30 non-teaching staff members and 114 teachers index cases in 339 schools for which contact tracing was performed. From this contact tracing and testing, a total of 76 secondary cases were identified (Table 5). The frequency of secondary cases was higher among students than among teachers and non-teaching staff members (71%, 22.4% and 6.6%, respectively). A secondary case among teachers was more frequent when the index case was a teacher than when it was a student (37% vs. 10%, P = 0.007, Fig. S7). Secondary cases among non-teaching staff members were exclusively due to contacts with other non-teaching staff members.
Table 6.
Population distribution per age in Veneto. Data are from ISTAT.
| Age bracket | Population |
|---|---|
| 0–4 | 184,725 |
| 5–9 | 217,931 |
| 10–14 | 236,205 |
| 15–19 | 234,882 |
| 20–24 | 239,341 |
| 25–29 | 245,517 |
| 30–34 | 256,481 |
| 35–39 | 281,868 |
| 40–44 | 343,714 |
| 45–49 | 223,416 |
| 50–54 | 418,076 |
| 55–59 | 385,088 |
| 60–64 | 321,876 |
| 65–69 | 283,649 |
| 70–74 | 268,762 |
| 75+ | 540,129 |
Table 5.
Index and secondary cases in 339 schools of the Province of Verona (from November 25 to December 21, 2020). Note that in the case of one teacher index case, 2 secondary cases among other teachers were identified. Frequency of teachers and students is significantly different by index case: P = 0.007 students vs. teachers.
| Index cases | Secondary cases |
||||
|---|---|---|---|---|---|
| Total | Students | Teachers | Staff | ||
| Students | 355 | 60 (100%) |
54 (90%) | 6 (10%) |
0 (0%) |
| Students <13 years old | 38 (100%) |
33 (87%) | 5 (13%) |
0 (0%) |
|
| Students 13–18 years old | 22 (100%) |
21 (95%) | 1 (5%) |
0 (0%) |
|
| Teachers | 112 | 16 (100%) |
10 (63%) | 6 (37%) |
0 (0%) |
| Non-teaching Staff members | 25 | 5 (100%) |
0 (0% |
0 (0%) |
5 (100%) |
| Total | 492 | 81 | 64 | 12 | 5 |
Altogether, these analyses indicate that in the Italian Veneto region, children and adolescents were not early drivers of the second wave, which was conversely associated with an early increase in incidence among 20–29- and 45–49-years old individuals. Importantly, teachers were not at greater risk of SARS-CoV-2 infection than the age matched general population. Finally, even when teachers were infected at school, infections were mainly due to other teachers.
3.4. School closures did not alter the rate of Rt decline in Lombardy and Campania
Since we did not find a correlation between school opening and the rise in Rt, we wished to understand whether the opposite, i.e., school closures, impacted on Rt. Again, the territorial differences in the mandate of different NPI in Italy offered a useful paradigm to investigate this possibility. We considered the two cases of Lombardy, where the President of the Region mandated closure of high schools from October 26; and Campania, where the closure of all school grades (including kindergartens) was mandated from October 16. Lombardy and Campania together account for 25% of Italy's population, being the first and second most populous regions. These school closures occurred before the national Government implemented a regional risk stratification system to modulate lockdown, according to the local epidemiological and hospital stress status (November 6), but after the mandate for universal mask wearing outside home (October 14) and, in the case of Lombardy, after the closure of restaurants, cafes, and bars at 6PM with a nationwide curfew at 10PM (October 23). Interestingly, Rt decline started before high school closures in both regions: in Lombardy in the period October 8–15 (Fig. 5A for absolute Rt values and 5B for its first order derivative); in Campania, in the period September 30-October 7 (Fig. 5C for absolute Rt values and 5D for its first order derivative). Noteworthy, the same pattern was observed if we analysed Rt computed over total SARS-CoV-2 positivity albeit, in the case of Campania, Rt decline started only three periods before implementation of school closures (Fig. S3, red lines in the plots of Campania and Lombardy). In the case of Campania, we could also extend our analysis to the overall incidence among students and general population. We found that, while incidence dropped among students, probably because they were no longer attending schools and therefore tested, incidence in the general population continued to increase (Fig. S8A), reflecting the fact that Rt remained >1 until the period 5–11 November. Moreover, a cross-correlation analysis between the time series of incidence among students and the general population confirmed that incidence increased simultaneously among students and general population (Fig. S8B). Altogether, these data indicate that school closures did not impact on the speed of Rt decline in Lombardy and Campania. Furthermore, the increasing trend of COVID-19 incidence in the general population observed in Campania was concomitant to that observed among students and not curtailed by school closures.
4. Discussion
Whether school reopening contributed to the second wave of COVID-19 in Italy was unclear. Here, by analysing data from Italian regions and schools, we did not find a significant association between school opening and rise of infection in the general population. Our conclusion is based (i) on the finding of lower incidence of SARS-CoV-2 positivity among students than in general population; (ii) on the lack of a fixed temporal association between school reopening dates in different Italian regions and Rt increase in the same region; (iii) on the analysis of the temporal changes in incidence among different age classes in the Veneto region during the initial phases of the second wave.
At variance with influenza, in which younger individuals seem to represent a reservoir of virus and contribute to its propagation to general population, [26], [27], [28], [29], [30] SARS-CoV-2 seems to spare school age children and adolescents: clinically, they are mostly paucisymptomatic [5]; from the epidemiology of infection perspective, they are very rarely accounted for as the index case [11], indicating that not only they are largely spared from the clinical consequences of the infection, but they also are less likely to transmit it. Overall, these data suggest that spread of COVID-19 within school settings may be limited [31, 32]. Indeed, our data indicate that infection incidence is lower in students of any education cycle, compared to the general population. Moreover, at least in the case of elementary school children, contact tracing in schools confirms that they are less likely to transmit the virus to adults, as evidenced by a 73% lower number of secondary cases among teachers when the index case is a student (10%), compared to secondary cases elicited by a teacher index case (37%). These epidemiological data are in line with the finding that children harbor antibodies against the other common coronaviruses, and that these antibodies are cross reactive and neutralizing against SARS-CoV-2 [7]. Our findings are also consistent with several other reports of very limited spread of COVID-19 between children and from children to adults. In Australia (New South Wales), following COVID-19 positivity of 9 students in primary and high schools and 9 staff members, only 2 of the 735 students, and 0 of the 128 staff members with whom they had contact were identified as secondary cases [33]. In Ireland, during the first wave, 6 COVID-19 cases were identified in schools (three children and three adults). Among their 1155 school contacts, zero infections were recorded [34]. In the Netherlands, 10 COVID-19 cases aged <18 had 43 contacts, but nobody was infected, whereas 221 patients older than 18 were associated with 8.3% of infections [35].
Of note, we found higher rates of incidence in teachers and non-teaching staff members compared to the general population. One possible explanation for this finding is that teachers might become infected at school because of their prolonged proximity to students. However, by judging from contact tracing activity in schools of the populous province of Verona (Veneto region), secondary infections at school are rare: only 13 teachers were identified as secondary cases from 524 traced index cases. Among these rare events, frequency of secondary infections among teachers was higher when the index case was a teacher rather than a student. In the Campania region, where schools were open for 17 days (from September 24 to October 16; school week of 5 days), incidence among teachers and non-teaching staff members in the period September 12-November 7 was still higher than that in the general population. It would be difficult to ascribe this difference to 17 days of school over a total of 56 days. We also performed an important, often overlooked normalization and compared incidence among teachers from the Veneto region with incidence in the general population of similar age: incidences were comparable, and differences not significant. Thus, while incidence among teachers is similar to that in the age—matched general population, teachers are allegedly perceived at greater risk. Perhaps this perception stems from the fact that in Italy the school environment is meticulously and continuously controlled, as confirmed by our finding of very high number of tests performed for each positive case, especially when the index case is a student. This remarkable system of monitoring unveils a large proportion of perhaps asymptomatic infections among teachers, resulting in the apparently higher incidence among this type of workers. It cannot be argued that teachers and non-teaching staff members are more susceptible to infection than the general population. In fact, this increase in the incidence of test positives is not mirrored by an increase in mortality-morbidity that would mark a more susceptible population [36]. In sum, our analysis of data collected by the MI indicates that in Italy students are less infected than the general population and the overall protocols for contact tracing work well, questioning whether schools played a role as amplifiers of the second COVID-19 wave.
Decision makers, popular press and public opinion in Italy ascribed the second wave of COVID-19 to school reopening [14]. This was often accompanied by deprecating comments on “individual behavior” of adolescents especially, who would not follow the strict rules at school or outside them. However, our data suggest that this common sentiment is not evidence-based, but perhaps grounded on the temporal correlation between school opening (in September) and second wave (in October-November). Rather, our data do not identify a constant temporal association between school reopening and rise in Rt analysed on a regional basis. Because of the staggered school reopening calendar in Italy, we were well positioned to address whether there was such an association between the date of school opening and the date of reproduction number increase. Conversely, a constant association was present when we analysed the temporal distance between Rt rise and the election day, held in Italy on September 20 (and morning of 21), 2020.
Interestingly, other reports are in line with our findings: in Great Britain, incidence among staff members was higher than among students (27 cases [95% CI 23–32] per 100,000 per day among staff; 18 cases [14–24] in early-year students, 6.0 cases [4.3–8.2] in primary schools students, and 6.8 cases [2.7–14] in secondary school students); further, most cases linked to outbreaks were in staff members (154 [73%] staff vs. 56 [27%] children of 210 total cases). The median number of secondary cases in outbreaks was one (IQR 1–2) for student index cases and one (1–5) for staff index cases [37]. In Spain, the evolution of the global incidence does not suggest significant effects of school reopening. In most cases, there was slight if any increase in pediatric cases, consistent with the diagnostic efforts in schools [38]. In Germany, data collected from 53,000 schools and day-cares in autumn indicate that only circa 32 schools had more than two positives per week [39]. Finally, a recent report by the ECDC summarizes the available knowledge and reaches conclusions very similar to ours. While ECDC concludes with high confidence that transmission of SARS-CoV-2 can occur within school, they also note with moderate confidence that prevalence of COVID-19 within schools is influenced by the community prevalence especially when community transmission is sustained. Most importantly, transmission in schools account for a minority of all COVID-19 cases in a given country and school staff are generally at no higher risk of infection than other occupations [40]. ECDC recommends a variety of NPI to mitigate the risk of school COVID-19 transmission [40] that are even less stringent than the rules currently implemented in Italy. For example in Italy children from 6 years of age must always wear face masks at school including when sitting at their desk or playing in outdoor playgrounds [22], irrespective of the local epidemiological condition that WHO [41] and ECDC [40] take into consideration when advising on schools NPIs.
A current concern is that the SARS-CoV-2 variant B.1.1.7, becoming largely diffuse and predicted to display a greater Rt 42, might be more transmissible especially among children It shall be noted that the possibility that this variant become predominant because of a greater susceptibility of school age individuals (0–19) was duly took into consideration. However, modeling predicts that individuals of this age group should be twice as susceptible to the B.1.1.7 variant as compared to the wild-type virus to support its observed widespread diffusion [42]. Furthermore, transmission of this variant by school age individuals appears to be lower also in the real world. The most recent Public Health England report on the transmissibility of the variants of concern contains datasets of contact tracing activity performed on individuals infected with wild-type and B.1.1.7 SARS-CoV-2. The report concludes that transmissibility of B.1.1.7 is 30–35% higher than that of wild-type SARS-CoV-2 [43]. From this report, we extrapolated secondary infection rates stratified by age of the index case (0–19 or 20+). In the case of 0–19 years old index cases carrying wild-type SARS-CoV-2, secondary cases were reported in 279 of the 3479 contacts (8.0%) and in 317 of the 3004 contacts of an index case carrying the B.1.1.7 SARS-CoV-2 variant (10.6%). These proportions were respectively 14.1% (891 secondary cases out of 6298 contacts) and 19.7% (968 out of 4920) when the index case was 20 years and older. Thus, the increase in transmissibility of the B.1.1.7 SARS-CoV-2 variant is 39.7% if the index case is 20 years or older, and 32.5% if the person is 0–19 years old. Even with this variant, transmission by school age individuals remains therefore 46% lower than by older persons. Thus, while we were not able to investigate the role of school opening and closure in a time of widespread diffusion of B.1.1.7 SARS-CoV-2 variant, these real-world data on lower transmissibility by school age individuals support that again, 0–19 years old individuals are less prone to transmit it forward than adults.
A different question is whether closing schools is efficacious in curtailing viral spread. In some Italian regions analysed here, school closure was mandated by local authorities and eventually in certain regions by the National Government. However, this closure had no effect on the incidence of COVID-19 in the general population or in Rt decline, which had started before the mandated school closure and that continued with the same speed, irrespective of school closures in Lombardy (partial) and Campania (total). This finding is in line with a literature review of all available studies (n = 16) on the efficacy of school closures and other social distancing practices in schools in China and Hong Kong, where the rapidly implemented school closures did not substantially contribute to the control of the spread [16]. In Australia, by comparing data from 25 schools of different grades with those of the general population, it was found that students and school staff did not contribute to the spread of the virus more than the general population [44]. On the other hand, an analysis of the impact of different NPI on the reproduction number Rt across 131 countries found that school closures alone could reduce Rt by 15% (R ratio: 0.85, 95%CI: 0.66–1.10), whereas school reopening could increase it by 24% (R ratio: 1.24, 95%CI: 1.00–1.52) twenty-eight days after their implementation. However, these measured Rt changes are not statistically significant, as evidenced by the very large and overlapping confidence intervals of the R ratios [45]. Moreover, authors warn on the limitations of their estimates: for example, they could not consider the different precautions related to the reopening of schools taken by some countries, such as physical distancing within classrooms and masking procedures; they did not consider the impact of school holidays and the effect of reopening different school levels (e.g., elementary and middle schools). Finally, authors analysed the impact of given NPIs by comparing Rt from two arbitrarily drawn periods before and after the implementation of the given NPI [45]. While this approach might be more practical when comparing multiple countries, it is less informative than our analysis, performed over the whole Rt curve.
In our analyses, Rt started declining even before the implementation of any NPI in all regions analysed. These results, while perhaps surprising, are in line with findings from the group of Merler [46] who analysed the impact of the national March-May strict lockdown on Rt in Italy. While they concluded that this lockdown reduced Rt and brought it below 1, they admitted that the decline in Rt had started well before the national lockdown was implemented. Indeed, visual inspection of their published Rt curves confirms that this NPI did not affect the slope of Rt decline. Whether our findings can be generalized to other countries, in which the use of NPI might be less extensive than in Italy, remains unclear and admittedly requires further studies.
Of the highest importance, our study is strengthened by the several sources of data used. Longitudinal data of regional incidence of SARS-COV-2 positives subjects deposited in the public repository of the Italian Civil Protection, incidence from the Veneto Region system of COVID-19 case notification with information by age, and incidence in schools from MI with information for students, teachers and non-teaching staff members. A systematic review investigated sources of bias in observational studies trying to assess the role of school closures in the reduction of COVID-19 community transmission [47]. Several studies were found at risk of confounding factors and collinearity from other NPI implemented around the time of school closures. We believe that our study is a low risk of bias because we compared community transmission of SARS-CoV-2 before and after school closure/re-opening in single geographical units (regions and provinces). This approach, as commented by the authors of this review, controls for confounding from population sociodemographic factors [47]. We also compared transmission in different regions opening schools at different dates and this analysis is not confounded by inclusion of other NPIs because while school calendar in Italy is regionalized, NPIs are mandated nationwide, in schools and outside schools. Furthermore, we analysed several prospective cohorts. This type of study design reduces the risk of bias, as opposed to the cross-sectional study design of previous publications on this topic that analysed data at a single cut-off date. Indeed, Walsh and colleagues essentially conclude that while most studies show effects, higher quality studies tend not to [47], probably a consequence of the strong study design in the latter.
The limitations of our study include: (i) Information on SARS-CoV-2 positive individuals in schools are retrieved by school Principals and can be partial; (ii) these data represent a global snapshot of the whole school, not of individual classes; (iii) data on number of SARS-COV-2 positives subjects deposited in the public repository of the Italian Civil Protection might suffer from delays in reporting or -even worse, from differences in reporting criteria by different regions; (iv)comparisons across regions of the impact of school opening dates on Rt changes suffer from ecological bias. However, it shall be noted that the nationwide Rt computed on the total positives and that on the cases by diagnostic suspicion are very similar and that their temporal trends are superimposable, thus reinforcing the strength of the analysis presented here.
In conclusion, our analysis does not find an association in Italy between dates of school opening and the increase in SARS-CoV-2 Rt. Reciprocally, school closures did not affect the rate of Rt decline. Also, the incidence of SARS-CoV-2 among students is lower than that in the general population; In addition, the incidence among teachers is comparable to that recorded in the general population of the same age. Finally, contact tracing in schools resulted in very low frequency of secondary infections found per test, and low frequency of clusters despite a high number of tests every week. Our analysis provides evidence that school openings are not to be considered as a relevant factor influencing the spread of the COVID-19 epidemics and that school closures did not improve the already occurring decline in the reproduction number of COVID-19, at least in two populous Italian regions. Closure of schools has dire consequences on children and adolescents motor activity [48], social interaction, psychological well-being [49, 50] and psychopathological problems [51, 52], on the risk of obesity [53] and screen addiction [54], on the protection from situations of domestic abuse [55], and on learning performance. Our data add further support to the consolidating notion that risks of school closures are not outweighed by benefits. They moreover suggest that the conclusion that school openings favoured COVID-19 spread is correlative at best, and hence it does not help in the identification of the best NPIs to curtail SARS-CoV-2 diffusion.
Author Contributions
SG, FC and LS designed the study. MLI, LS and SG collected data. SG, MR, MLI and LS performed data analysis. SG, FC and LS led manuscript writing. SG, FB, FC, and LS reviewed the literature. All authors contributed to final draft.
Declaration of Interests
LS received SAB honoraria from Astellas Pharmaceuticals and sits on the SAB of Mitochondria in Motion, Inc. All other authors have no interests to declare.
Acknowledgments
Acknowledgments
We thank Dr. Guido Silvestri (Emory University School of Medicine) for his support, Dr. Sabrina Brigadoi (Depts. Of Developmental Psychology and Information Engineering, University of Padua) for helpful discussions. SG acknowledges the support by the Italian Ministry of Health with Ricerca Corrente and 5 × 1000 funds. Federica Bellerba is a PhD student at the European School of Molecular Medicine (SEMM), Milan, Italy.
Data sharing statement
Sources of publicly searchable databases are indicated in the paper or available as supplementary material. The remaining data may be made available from the corresponding authors upon reasonable request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100092.
Contributor Information
Sara Gandini, Email: sara.gandini@ieo.it.
Luca Scorrano, Email: luca.scorrano@unipd.it.
Appendix. Supplementary materials
References
- 1.Jackson C., Vynnycky E., Hawker J., Olowokure B., Mangtani P. School closures and influenza: systematic review of epidemiological studies. BMJ Open. 2013;3(2) doi: 10.1136/bmjopen-2012-002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cauchemez S., Ferguson N.M., Wachtel C. Closure of schools during an influenza pandemic. Lancet Infect Dis. 2009;9(8):473–481. doi: 10.1016/S1473-3099(09)70176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snape M.D., Viner R.M. COVID-19 in children and young people. Science. 2020:eabd6165. doi: 10.1126/science.abd6165. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39(5):355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoang A., Chorath K., Moreira A. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunyavanich S., Do A., Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323(23):2427. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng K.W., Faulkner N., Cornish G.H. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science. 2020;370(6522):1339–1343. doi: 10.1126/science.abe1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viner R.M., Mytton O.T., Bonell C. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies N.G., Klepac P., Liu Y. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 10.Hippich M., Holthaus L., Assfalg R., et al. A public health antibody screening indicates a 6-fold higher SARS-CoV-2 exposure rate than reported cases in children. Med. [DOI] [PMC free article] [PubMed]
- 11.Zhu Y., Bloxham C.J., Hulme K.D. A meta-analysis on the role of children in SARS-CoV-2 in household transmission clusters. medRxiv. 2020 doi: 10.1093/cid/ciaa1825. 2020.03.26.20044826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavezzo E., Franchin E., Ciavarella C. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo'. Nature. 2020;584(7821):425–429. doi: 10.1038/s41586-020-2488-1. [DOI] [PubMed] [Google Scholar]
- 13.Forbes H., Morton C.E., Bacon S. Association between living with children and outcomes from COVID-19: an OpenSAFELY cohort study of 12 million adults in England. medRxiv. 2020 doi: 10.1136/bmj.n628. 2020.11.01.20222315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sebastiani G., Palù G. COVID-19 and school activities in Italy. Viruses. 2020;12(11) doi: 10.3390/v12111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee B., Hanley J.P., Nowak S., Bates J.H.T., Hébert-Dufresne L. Modeling the impact of school reopening on SARS-CoV-2 transmission using contact structure data from Shanghai. BMC Public Health. 2020;20(1):1713. doi: 10.1186/s12889-020-09799-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viner R.M., Russell S.J., Croker H. School closure and management practices during coronavirus outbreaks including COVID-19: a rapid systematic review. Lancet Child Adolesc Health. 2020;4(5):397–404. doi: 10.1016/S2352-4642(20)30095-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park Y.J., Choe Y.J., Park O. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465–2468. doi: 10.3201/eid2610.201315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heald-Sargent T., Muller W.J., Zheng X., Rippe J., Patel A.B., Kociolek L.K. Age-related differences in nasopharyngeal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) levels in patients with mild to moderate coronavirus disease 2019 (COVID-19) JAMA Pediatr. 2020;174(9):902–903. doi: 10.1001/jamapediatrics.2020.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szablewski C.M., Chang K.T., Brown M.M. SARS-CoV-2 transmission and infection among attendees of an overnight camp - Georgia, June 2020. Morb Mortal Wkly Rep. 2020;69(31):1023–1025. doi: 10.15585/mmwr.mm6931e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein-Zamir C., Abramson N., Shoob H. A large COVID-19 outbreak in a high school 10 days after schools’ reopening, Israel, May 2020. Eurosurveillance. 2020;25(29) doi: 10.2807/1560-7917.ES.2020.25.29.2001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lordan R., Fitzgerald G.A., Grosser T. Reopening schools during COVID-19. Science. 2020;369(6508):1146. doi: 10.1126/science.abe5765. [DOI] [PubMed] [Google Scholar]
- 22.Gruppo di Lavoro ISS MdS, Ministero dell'Istruzione, INAIL, Fondazione Bruno Kessler, Regione Emilia-Romagna, Regione Veneto. Indicazioni operative per la gestione di casi e focolai di SARS-CoV-2 nelle scuole e nei servizi educativi dell'infanzia. 2020. http://www.salute.gov.it/imgs/C_17_pubblicazioni_2944_allegato.pdf. Accessed March 12, 2020.
- 23.Hudson J.I., Pope H.G., Jr., Glynn R.J. The cross-sectional cohort study: an underutilized design. Epidemiology. 2005;16(3) doi: 10.1097/01.ede.0000158224.50593.e3. [DOI] [PubMed] [Google Scholar]
- 24.Cori A., Ferguson N.M., Fraser C., Cauchemez S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am J Epidemiol. 2013;178(9):1505–1512. doi: 10.1093/aje/kwt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cereda D., Tirani M., Rovida F. The early phase of the COVID-19 outbreak in Lombardy, Italy. Popul Evol. 2020 [Google Scholar]
- 26.Worby C.J., Chaves S.S., Wallinga J., Lipsitch M., Finelli L., Goldstein E. On the relative role of different age groups in influenza epidemics. Epidemics. 2015;13:10–16. doi: 10.1016/j.epidem.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King J.A., Whitten T.A., Bakal J.A., McAlister F.A. Symptoms associated with a positive result for a swab for SARS-CoV-2 infection among children in Alberta. Can Med Assoc J. 2020 doi: 10.1503/cmaj.202065. cmaj.202065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riccardo F., Ajelli M., Andrianou X.D. Epidemiological characteristics of COVID-19 cases in Italy and estimates of the reproductive numbers one month into the epidemic. medRxiv. 2020 doi: 10.2807/1560-7917.ES.2020.25.49.2000790. 2020.04.08.20056861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Götzinger F., Santiago-García B., Noguera-Julián A. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prevention CfDCa. Flu & young children. 2020. https://www.cdc.gov/flu/highrisk/children.htm. Accessed October 12, 2020.
- 31.Lewis D. Why schools probably aren't COVID hotspots. Nature. 2020;587(7832):17. doi: 10.1038/d41586-020-02973-3. [DOI] [PubMed] [Google Scholar]
- 32.Organization WH. Coronavirus disease (COVID-19): schools. . 2020. https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-schools.
- 33.Macartney K., Quinn H.E., Pillsbury A.J. Transmission of SARS-CoV-2 in Australian educational settings: a prospective cohort study. Lancet Child Adolesc Health. 2020;4(11):807–816. doi: 10.1016/S2352-4642(20)30251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heavey L., Casey G., Kelly C., Kelly D., McDarby G. No evidence of secondary transmission of COVID-19 from children attending school in Ireland, 2020. Eurosurveillance. 2020;25(21) doi: 10.2807/1560-7917.ES.2020.25.21.2000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.NRLM. Children and Covid19. 2020. https://www.rivm.nl/en/novel-coronavirus-covid-19/children-and-covid-19.
- 36.Windsor-Shellard BB, Asim. Coronavirus (COVID-19) related deaths by occupation, England and wales: deaths registered between 9 March and 25 May 2020. 2020.
- 37.Ismail S.A., Saliba V., Lopez Bernal J., Ramsay M.E., Ladhani S.N. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis. [DOI] [PMC free article] [PubMed]
- 38.Sabaté M.C., Iglesias P.J.C., Soler C., et al. Analysis and prediction of COVID-19 for EU-EFTA-UK and other countries. 2020; 2020.
- 39.Corona-KiTa-Dashboard.https://experience.arcgis.com/experience/7520318455c24d0e84e47e5be3c3a61d. Accessed December 12, 2020.
- 40.ECDC. COVID-19 in children and the role of school settings in transmission - first update. Stockholm, 2020.
- 41.WHO. Advice on the use of masks for children in the community in the context of COVID-19, 2020.
- 42.Davies N.G., Abbott S., Barnard R.C. Estimated transmissibility and severity of novel SARS-CoV-2 variant of concern 202012/01 in England. medRxiv. 2021 2020.12.24.20248822. [Google Scholar]
- 43.England P.H. Investigation of SARS-CoV-2 variants of concern - England. Tech Brief. 2021;6 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/961299/Variants_of_Concern_VOC_Technical_Briefing_6_England-1.pdf Accessed March 3, 2021. [Google Scholar]
- 44.Surveillance NCfIRa. COVID-19 in schools and early childhood education and care services – the term 3 experience in NSW 2020.
- 45.Li Y., Campbell H., Kulkarni D. The temporal association of introducing and lifting non-pharmaceutical interventions with the time-varying reproduction number (<em>R</em>) of SARS-CoV-2: a modelling study across 131 countries. Lancet Infect Dis. 2021;21(2):193–202. doi: 10.1016/S1473-3099(20)30785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guzzetta G., Riccardo F., Marziano V. Impact of a Nationwide Lockdown on SARS-CoV-2 Transmissibility, Italy. Emerg Infect Dis. 2020;27(1) doi: 10.3201/eid2701.202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh S., Chowdhury A., Russell S. Do school closures reduce community transmission of COVID-19? A systematic review of observational studies. medRxiv. 2021 doi: 10.1136/bmjopen-2021-053371. 2021.01.02.21249146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.López-Bueno R., López-Sánchez G.F., Casajús J.A. Health-related behaviors among school-aged children and adolescents during the Spanish Covid-19 confinement. Front Pediatr. 2020;8:573. doi: 10.3389/fped.2020.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang G., Zhang Y., Zhao J., Zhang J., Jiang F. Mitigate the effects of home confinement on children during the COVID-19 outbreak. Lancet. 2020;395(10228):945–947. doi: 10.1016/S0140-6736(20)30547-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie X., Xue Q., Zhou Y. Mental health status among children in home confinement during the coronavirus disease 2019 outbreak in Hubei Province, China. JAMA Pediatr. 2020;174(9):898–900. doi: 10.1001/jamapediatrics.2020.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Golberstein E., Wen H., Miller B.F. Coronavirus disease 2019 (COVID-19) and mental health for children and adolescents. JAMA Pediatr. 2020;174(9):819–820. doi: 10.1001/jamapediatrics.2020.1456. [DOI] [PubMed] [Google Scholar]
- 52.Duan L., Shao X., Wang Y. An investigation of mental health status of children and adolescents in china during the outbreak of COVID-19. J Affect Disord. 2020;275:112–118. doi: 10.1016/j.jad.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunton G.F., Do B., Wang S.D. Early effects of the COVID-19 pandemic on physical activity and sedentary behavior in children living in the U.S. BMC Public Health. 2020;20(1):1351. doi: 10.1186/s12889-020-09429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong H., Yang F., Lu X., Hao W. Internet addiction and related psychological factors among children and adolescents in China during the coronavirus disease 2019 (COVID-19) epidemic. Front Psychiatry. 2020;11(751) doi: 10.3389/fpsyt.2020.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silliman Cohen R.I., Bosk E.A. Vulnerable youth and the COVID-19 pandemic. Pediatrics. 2020;146(1) doi: 10.1542/peds.2020-1306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.