Abstract:
Modified ultrafiltration (MUF) is still used after pediatric cardiopulmonary bypass (CPB) in some pediatric cardiac surgery centers to decrease transfusion requirements. Other potential benefits of MUF include clearance of inflammatory markers and improvement in myocardial function. Our hypothesis is that MUF will hemoconcentrate coagulation factors and improve thromboelastography (TEG) parameters after pediatric CPB. Patients younger than 6 months were prospectively enrolled over a year. TEG was carried out before MUF, after MUF, and after protamine administration. Paired t tests were conducted to compare values pre-MUF and post-MUF as well as post-MUF and post-protamine administration. Thirty patients were enrolled in the study, with 20 (67%) neonates in the cohort. Seven arterial switch operations and nine Norwood procedures were found to be performed among the cohort. Reaction time (R), angle (α), and maximum amplitude (MA) were significantly worse post-MUF compared with pre-MUF (p < .001). They improved significantly after protamine administration compared with post-MUF (p < .001). The amount of fluid removal was significantly associated with a worse post-MUF R, angle, and MA and worse post-protamine administration, angle, and MA but with no effect on post-protamine R. MUF caused worsening of TEG parameters that is reversed by protamine administration.
Keywords: congenital heart diseases, cardiopulmonary bypass, anticoagulation, blood coagulation, thromboelastography
Thromboelastography (TEG) is a hemostatic assay that measures the global viscoelastic properties of whole blood clot formation under low shear stress. It shows the interaction of platelets with the coagulation cascade (aggregation, clot strengthening, fibrin cross-linking, and fibrinolysis). It therefore provides an evaluation of the functional integrity of the coagulation system from initial clot formation to clot retraction/dissolution (1).
Patients undergoing congenital heart surgeries require the use of cardiopulmonary bypass (CPB). Extracorporeal circulation exposes circulating blood cells to the non-physiologic surface of the bypass circuit, which starts a systemic inflammatory reaction by activation of cytokines and other cellular and humoral factors, which may cause organ dysfunction, particularly in the lungs, heart, and brain (2).
Modified ultrafiltration (MUF) is performed after weaning from CPB. Although MUF removes intravascular fluid and interstitial fluid from the lungs and decreases the need for blood transfusion, other beneficial effects that result in decreased post-operative morbidity and improve end-organ function, have been described (3). MUF has been shown to decrease early morbidity and lower blood transfusion requirements in adult patients undergoing cardiac surgery (4).
The effects of MUF on coagulation factors as demonstrated by TEG after pediatric CPB have been reported in infants with suggestion of improved hemostasis after MUF (5). The objective of this study was to analyze the effects of MUF on TEG parameters in infants undergoing repair of congenital heart diseases using CPB.
METHODS
Study-Specific Methodology
The study was approved by the Advocate Institutional Review Board. Consent was obtained from the parents before the surgical procedure. Thirty consecutive patients (aged less than 6 months) undergoing repair of congenital heart diseases using CPB were enrolled prospectively over a period of 6 months. The inclusion criteria included all patients younger than 6 months undergoing cardiac surgery using CPB. All patients underwent MUF after weaning from CPB. Blood samples for TEG were obtained at three time points: before MUF after weaning off CPB (pre-MUF), after MUF (post-MUF), and after protamine administration (post-protamine). Packed red blood cells were added on CPB selectively to keep the hematocrit level higher than 24% while on CPB. If a patient needed blood products at the end of surgery, they were given after the post-protamine TEG sample is withdrawn.
Cardiopulmonary Bypass Strategy
CPB was instituted using aortic and bicaval cannulation with a target flow of 2.5 L/min/m2. Systemic temperature was lowered to 32°C in most patients and to 28°C in more complex cases such as Norwood, arterial switch, and repair of total anomalous pulmonary venous return (TAPVR). Cooling and warming were carried out using a pH stat strategy, during which the carbon dioxide level was allowed to increase in the CPB circuit to maintain a normal temperature-corrected pH. A target hematocrit of 24% was used. A 300 units/kg loading dose of heparin was given for a target-activated clotting time greater than 480 seconds. Del Nido cardioplegia solution was used to induce cardiac arrest. A 20 mL/kg induction dose was administered initially with subsequent 10 mL/kg doses given every 45 minutes. Steroids were given routinely (10 mg/kg Solu-Medrol intra-venous) on induction of anesthesia. Aminocaproic acid (Amicar) was also used for all neonates and patients having redo surgery.
The CPB circuit prime volume was 225 mL. It consisted of 175 mL of PlasmaLyte-A, 50 mL of 25% albumin, 20 meq/L sodium bicarbonate, and heparin. For patients weighing more than 5 Kg, the prime volume of the CPB circuit was 240cc. A hemoconcentrator (DHF02 LivaNova, London, United Kingdom) was used on all cases. Zero-balance ultrafiltration during CPB and MUF was performed routinely on all patients.
The hemoconcentrator is placed as a bridge between the arterial and the venous line (Figure 1). The access is posterior to the arterial line filter, and return is directly into the venous line. Besides performing zero-balance ultrafiltration during CPB, MUF is routinely performed at our center after weaning from CPB. During MUF, the patient’s arterial blood pressure passively drives blood flow through the hemoconcentrator from the arterial cannula and back to the patient through the venous cannula. As volume is removed through the effluent line, blood from the pump reservoir is pumped forward through the circuit to avoid a hypovolemic state. MUF is discontinued when all circuit blood is returned to the patient. Residual blood volume in the venous and arterial lines is chased back to the patient before taking the arterial and venous cannulas out. The filter used for MUF allows passage of molecules smaller than a molecular weight (MW) of 65,000 Da.
Figure 1.
Diagram showing the location of the hemoconcentrator in the CBP circuit. The arrows reflect the blood flow during modified ultrafiltration. X reflects clamping the venous line and opening the bridge toward the hemoconcentrator.
Thromboelastography
TEG machines (TEG 5000, Haemonetics Corporation, Boston, MA) are available in the operating room. A small sample of whole blood (.36 mL) is collected and placed in a cup on the TEG analyzer within 2 minutes. A torsion wire and pin are suspended in the whole blood sample as the cup rotates. As the clot begins to form, it binds the cup and pin. Time to clot, maximum clot strength, and clot breakdown are measured and analyzed. A heparinase cup, which reverses 6 units/mL of heparin, is used for pre-MUF and post-MUF samples. The post-protamine sample uses a non-heparinase cup to rule out residual active heparin in the patient’s blood.
Statistical Analyses
It was estimated that 30 subjects would be needed to detect as small as a 2-second difference in R pre- versus post-MUF at a significance level of .05 with 80% power. Data were summarized using means/standard deviations, medians/interquartile ranges, or frequencies/proportions based on the level of measurement and normality. Pre- and post-MUF TEG parameters were compared using paired t tests or Wilcoxon signed rank tests, as appropriate. Pearson correlation and Student t tests were applied to examine the associations between blood product utilization and changes in TEG measures. Statistical significance was confirmed with a p value of < .05.
RESULTS
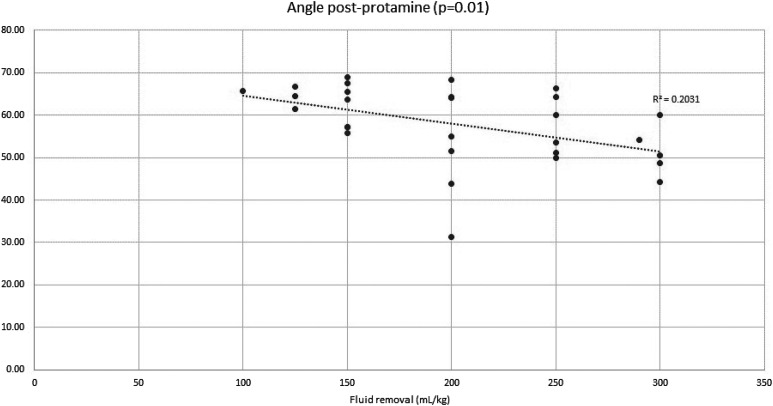
Thirty consecutive patients were enrolled over a period of 6 months. Patients’ demographics are shown in Table 1. Twenty patients (67%) were neonates. The most common surgical procedures performed were Norwood operation and the arterial switch operation (Table 2). The average volume of fluid removed during MUF was 57.28 ± 19.5 mL/kg, with a range of 22.7–100 mL/kg. TEG results are summarized in Tables 3 and 4. Post-MUF TEG parameters were significantly worse than pre-MUF TEG parameters, as evidenced by a statistically significant decrease in the angle and maximum amplitude (MA) with a concomitant significant increase in the reaction time (R). After protamine administration, all TEG parameters improved significantly compared with post-MUF results (Table 3). The amount of fluid removed was significantly associated with a worse post-MUF R (Figure 2) but did not affect the post-protamine R (Figure 3). Similarly, the amount of fluid removed during MUF was associated with significant worsening of post-MUF and post-protamine angle and MA (Figures 4–7).
Table 1.
Patients’ demographics.
| Mean ± Standard Deviation | Minimum | Maximum | |
|---|---|---|---|
| Gender | – | – | |
| Male | 19 (63.3%) | ||
| Female | 11 (36.7%) | ||
| Gestational age at birth (weeks) | 37.47 ± 1.833 | 31 | 40 |
| Age (months) | 1.53 ± 1.95 | 0.1 | 5.50 |
| Weight (kg) | 3.71 ± 1.18 | 2.40 | 6.50 |
| Height (cm) | 50.1 ± 6.04 | 32.5 | 63.0 |
| BSA (m2) | .23 ± .04 | .20 | .30 |
Table 2.
Surgical procedures performed.
| Type of surgery | n | % |
|---|---|---|
| Arterial switch operation | 7 | 23.3 |
| Norwood–Sano | 9 | 30.0 |
| AVSD repair | 3 | 10.0 |
| Bidirectional Glenn | 2 | 6.7 |
| TAPVR repair | 2 | 6.7 |
| VSD repair | 2 | 6.7 |
| Other | 5 | 16.7 |
| Total | 30 | 100.0 |
Table 3.
Pre-MUF and post-MUF TEG parameters.
| TEG Component | Normal TEG Values | Pre-MUF | Post-MUF | p Value |
|---|---|---|---|---|
| Angle | 59–74° | 44.81 ± 11.68 | 29.69 ± 14.40 | <.001 |
| R | 4–9 minute | 10.26 ± 5.05 | 15.59 ± 7.10 | <.001 |
| MA | 55–74 mm | 48.37 ± 7.73 | 41.54 ± 11.68 | <.001 |
Table 4.
Post-MUF and Post-protamine TEG parameters.
| TEG Component | Normal TEG Values | Post-MUF | Post-Protamine | p Value |
|---|---|---|---|---|
| Angle | 59–74° | 29.69 ± 14.40 | 57.77 ± 8.94 | <.001 |
| R | 4–9 minute | 15.59 ± 7.10 | 7.70 ± 2.62 | <.001 |
| MA | 55–74 mm | 41.54 ± 11.68 | 53.83 ± 8.18 | <.001 |
Figure 2.
Post-MUF R and total volume of fluid removed during MUF. R2 represents the magnitude of the correlation.
Figure 3.
Post-protamine R and total volume of fluid removed during MUF. R2 represents the magnitude of the correlation.
Figure 4.
Post-MUF angle and total volume of fluid removed during MUF. R2 represents the magnitude of the correlation.
Figure 7.
Post-protamine MA and total volume of fluid removed during the MUF. R2 represents the magnitude of the correlation.
Figure 5.
Post-protamine angle and total volume of fluid removed during MUF. R2 represents the magnitude of the correlation.
Figure 6.
Post-MUF MA and total volume of fluid removed during MUF. R2 represents the magnitude of the correlation.
DISCUSSION
Our study demonstrated that the TEG parameters worsened with an increase in total volume of fluid removed during MUF. The worsening in post-MUF TEG parameters can be explained by a relative increase in heparin concentration post-MUF. This theory is supported by the fact that all TEG parameters improved significantly after reversal of heparin with protamine. Because the heparinase cup reverses up to 6 units/kg of heparin, the heparin concentration must have been much higher than 6 units/kg in the post-MUF TEG results. One may check for this theory as well by running anti-Xa level or heparin concentration post-MUF to demonstrate an increase in heparin activity due to higher heparin concentration after MUF.
Several studies have demonstrated that MUF reduces the amount of circulating inflammatory markers and is associated with an improvement in hemodynamic variables including myocardial function, blood pressure, and cardiac index (6–9). MUF is also associated with decrease blood loss, chest tube drainage, and blood transfusion requirements (2). MUF is carried out routinely at our institution after pediatric cardiac surgery.
Our results demonstrate significant worsening of the TEG parameters post-MUF with significant improvement after protamine administration. The fact that improvement after protamine administration occurred before transfusion of any blood products suggests that initial worsening could be due to a relative increase in heparin activity and concentration post-MUF, rather than a change in clotting factor concentration, which is consistent with previous literature (10–12). Williams et al. (10) showed an increase in heparin after ultrafiltration (2.0 ± .6 U/mL to 2.5 ± .8 U/mL). Despotis et al. (11) showed a significant increase in plasma anti-Xa heparin activity after hemofiltration (3.9 ± 1.7 U/mL before hemofiltration to 5.0 ± 1.8 U/mL after hemofiltration, p = .003). The increase in heparin activity was directly related to the ultrafiltrate volume. Our results showed similar pattern. The amount of fluid removed was significantly associated with a worse post-MUF R (reflecting higher heparin concentration) that was reversed with protamine administration. The fact that the volume of fluid removed was associated with a worse post-MUF and post-protamine angle, and MA is due to low clotting factors and platelets, which is common after pediatric CPB. The filter we used for MUF allows passage of molecules smaller than an MW of 65,000 Da. The MW of majority of clotting factors is greater than 65,000 and therefore are not filtered out, but rather are consumed during CPB. Although the average MW of low-MW heparin is 5,500–6,500 Da, some heparin may not be filtered (possibly due to negative charge “binding” it to plasma protein).
There are limitations to the current study. First, this is a single-center study. Practices regarding perfusion and anesthesia strategies for pediatric congenital heart surgery using CPB differ across institutions. Differences in these practices can impact the coagulation profiles of patients. In addition, varying filters used during CPB can also impact the current findings. Thus, the degree of change in TEG parameters may change from center to center. The direction of change seems unlikely to change, however. Moreover, because of the low numbers of patients in this study, we were unable to do regression analysis for other clinical factors, and anti-Xa levels were not available to correlate with the data here.
CONCLUSION
MUF caused worsening of TEG parameters that is reversed by protamine administration. Worsening of TEG parameters was proportional to the amount of fluid removed during MUF.
REFERENCES
- 1.Mallett SV, Cox DJ. Thrombelastography. Br J Anaesth. 1992;69:307–13. [DOI] [PubMed] [Google Scholar]
- 2.Ziyaeifard M, Alizadehasl A, Massoumi G. Modified ultrafiltration during cardiopulmonary bypass and postoperative course of pediatric cardiac surgery. Res Cardiovasc Med. 2014;3:e17830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziyaeifard M, Alizadehasl A, Massoumi G. Modified ultrafiltration during cardiopulmonary bypass and postoperative course of pediatric cardiac surgery. Res Cardiovasc Med. 2014;3:217830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollinger I, Shore-Lesserson L, Vela-Cantos F, et al. Modified ultrafiltration in infants: Influence on thromboelastograpgy parameters. Anesth Analgesia. 1999;88:30SCA. [Google Scholar]
- 5.Luciani GB, Menon T, Vecchi B, et al. Modified ultrafiltration reduces morbidity after adult cardiac operations: A prospective, randomized clinical trial. Circulation. 2001;104:I253–9. [DOI] [PubMed] [Google Scholar]
- 6.Papadopoulos N, Bakhtiary F, Grun V, et al. The effect of normovolemic modified ultrafiltration on inflammatory mediators, endotoxins, terminal complement complexes and clinical outcome in high-risk cardiac surgery patients. Perfusion. 2013;28:306–14. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi RR, Shore DF, White PA, et al. Modified ultrafiltration improves global left ventricular systolic function after open-heart surgery in infants and children. Eur J Cardio Thorac Surg 1999;15:742–6. [DOI] [PubMed] [Google Scholar]
- 8.Hodges UM, Berg S, Naik SK, et al. Filtration of fentanyl is not the cause of the elevation of arterial blood pressure associated with post-bypass ultrafiltration in children. J Cardiothorac Vasc Anesth 1994;8:653–7. [DOI] [PubMed] [Google Scholar]
- 9.Naik SB, Elliott, M.J. Modified ultrafiltration improves hemodynamics after cardiopulmonary bypass in children. J Am Coll Cardiol 1993;56:1518–22. [Google Scholar]
- 10.Williams GD, Ramamoorthy C, Totzek FR, et al. Comparison of the effects of red cell separation and ultrafiltration on heparin concentration during pediatric cardiac surgery. J Cardiothorac Vasc Anesth 1997;11:840–4. [DOI] [PubMed] [Google Scholar]
- 11.Despotis GJ, Levine V, Filos KS, et al. Hemofiltration during cardiopulmonary bypass: The effect on anti-xa and anti-IIa heparin activity. Anesth Analg 1997;84:479–83. [DOI] [PubMed] [Google Scholar]
- 12.Barron MEW A, Perryman R. Adverse hemostatic effects of modified ultrafiltration as assessed by thromboelastography. Anesth Analgesia 1998;86:24SCA. [Google Scholar]