Abstract:
Amiodarone is an anti-arrhythmic agent that is frequently used to treat tachycardias in critically ill adults and children. Because of physicochemical properties of amiodarone, extracorporeal membrane oxygenation (ECMO) circuits are expected to extract amiodarone from circulation, increasing the risk of therapeutic failure. The present study seeks to determine amiodarone extraction by the ECMO circuit. Amiodarone was administered to three ex vivo circuit configurations (n = 3 per configuration) to determine the effect of each circuit component on drug extraction. The circuits were primed with human blood; standard amiodarone doses were administered; and serial samples were collected over 24 hours. Additional circuits were primed with crystalloid fluid to analyze the effect of blood on extraction and to investigate circuit saturation by drug. The crystalloid circuits were dosed multiple times over 72 hours, including a massive dose at 48 hours. For both setups, the flow was set to 1 L/min. Drug was added to separate tubes containing the prime solution to serve as controls. Drug concentrations were quantified with a validated assay, and drug recovery was calculated for each sample. Mean recovery for the circuits and controls were compared to correct for drug degradation over time. Amiodarone was heavily extracted by all ECMO circuit configurations. Eight hours after dosing, mean recovery in the blood prime circuits was 13.5–22.1%. In the crystalloid prime circuits, drug recovery decreased even more rapidly, with a mean recovery of 22.0% at 30 minutes. Similarly, drug recovery decreased more quickly in the crystalloid prime controls than in the blood prime controls. Saturation was not achieved in the crystalloid prime circuits, as final amiodarone concentrations were at the lower limit of quantification. The results suggest that amiodarone is rapidly extracted by the ECMO circuit and that saturation is not achieved by standard doses. In vivo circuit extraction may cause decreased drug exposure.
Keywords: drug extraction, extracorporeal membrane oxygenation, amiodarone, pharmacokinetics
Amiodarone is frequently used to treat ventricular and supraventricular tachycardias in critically ill adults and children (1,2). Patients with refractory arrhythmias may also require support with extracorporeal membrane oxygenation (ECMO) (3). During ECMO support, drugs such as amiodarone interact with the different components of the ECMO circuit. Interactions with the tubing, oxygenator, and hemofilter can dramatically alter drug exposure because of nonspecific binding between the drug and these components (4). This adsorption results in the extraction of drug from circulation. The extent to which each circuit component extracts drug depends on the component material and physicochemical properties of the drug. Generally, highly lipophilic and highly protein-bound drugs are heavily extracted by the ECMO circuit; however, this is not universally true (5,6). In addition, some investigators believe that the drug-binding sites in the circuit can become saturated by drug over time (7). Consequently, optimal dosing of amiodarone in patients on ECMO is unknown.
To determine optimal dosing on ECMO, it is important to first understand how a drug interacts with the different components of the circuit. These interactions can be studied using ex vivo environments, in which an isolated ECMO circuit is connected to a reservoir, instead of a patient. Drug is injected into the isolated circuit, and its concentration is measured over time. Changes in drug concentration are due to either circuit extraction or drug degradation. Prior studies have used this setup to investigate the effect of ECMO on the pharmacokinetics (PK) of antimicrobials and sedatives (8–19).
Amiodarone is highly lipophilic (logP 7.2) and protein-bound (96%) and likely to be extracted by the ECMO circuit. Limited clinical data from case reports suggest higher doses are needed to achieve therapeutic exposure (1,20–22). Based on these physicochemical properties, its exceptionally long half-life, and its frequency of use in conjunction with ECMO, we designed a study to investigate the extraction of amiodarone by each component of an ECMO circuit.
MATERIALS AND METHODS
Circuit Configurations
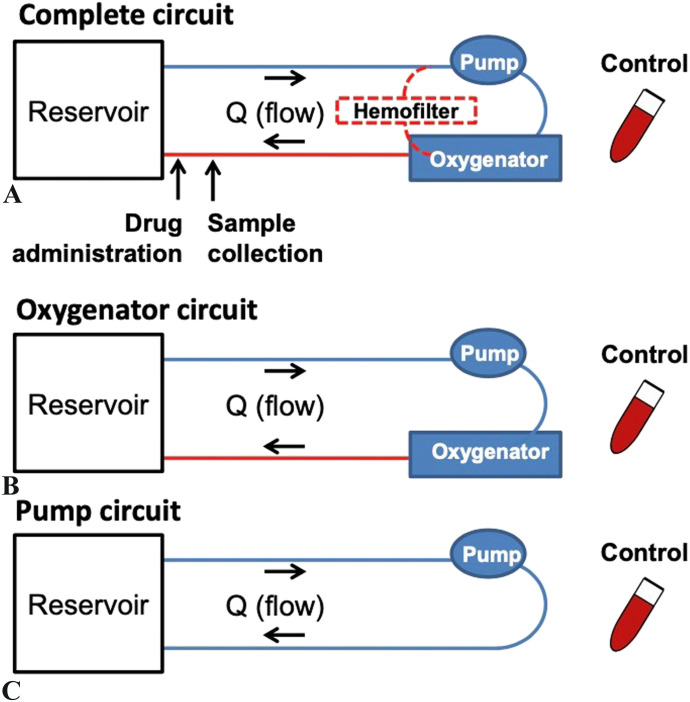
Three different ECMO circuit configurations were used to determine the contribution of each circuit component to drug extraction (Figure 1). The complete circuit included a 10-fr Bio-Medicus arterial cannula (Medtronic, Dublin, Ireland), phosphorylcholine-coated polyvinyl chloride (PVC) Smart Tubing (Sorin, Saluggia, Italy), a Revolution centrifugal pump (Sorin), a DHF0.2 hemofilter (Sorin), and a Quadrox iD polymethylpentene adult oxygenator (Getinge, Gothenburg, Sweden). The oxygenator circuit was identical, except that it lacked a hemofilter. Consequently, any difference in extraction between the complete and oxygenator circuits was attributed to the hemofilter. Similarly, the pump circuit lacked an oxygenator and a hemofilter. So, any difference between the oxygenator and pump circuits was due to the oxygenator. Each configuration was tested in triplicate.
Figure 1.
ECMO circuit configurations.
Circuit Setup
The circuits were assembled according to standard clinical practice. Both blood prime and crystalloid prime circuits were used. The blood prime for the complete and oxygenator circuits consisted of 1 unit of packed human red blood cells (∼350 mL), .5 units of human fresh human frozen plasma (∼175 mL), and Plasma-Lyte A crystalloid (Baxter Healthcare, Deerfield, IL) (500 mL). When possible, recently expired blood products were used to limit the effect on the hospital supply of banked blood. In addition, heparin sodium (500 units), sodium bicarbonate (7 mEq), tromethamine (2 g), calcium gluconate (650 mg), and albumin (12.5 g) were added to prevent coagulation and to mimic physiologic conditions. All prime solution components added to the pump circuits were scaled down to 2/3 of what was used in the other circuit configurations because the oxygenator itself holds ∼1/3 of the prime solution volume. In summary, a total of nine blood prime circuits were tested: three in the complete configuration, three in the oxygenator configuration, and three in the pump configuration.
The three crystalloid prime circuits were assembled according to the oxygenator configuration. The prime solution consisted of Plasma-Lyte A (1,000 mL), sodium bicarbonate (7 mEq), tromethamine (2 g), calcium gluconate (650 mg), and albumin (12.5 g). Similarly, all volumes were scaled down 2/3 for the pump circuit.
The circuits were completed with a reservoir, which was a double-spiked IV bag with sufficient volume to prevent air from entering the circuit. As is typical for a 10-kg child, ECMO flow was set to 1 L/min, which was measured post-oxygenator with an ultrasonic flowmeter (Sorin). A Cincinnati Sub-Zero Hemotherm (Terumo Cardiovascular, Ann Arbor, MI) was used to maintain a constant temperature of 37°C within the setup for the complete and oxygenator circuits. In the pump circuit, heating pads were wrapped around the reservoir and tubing and adjusted to hold a constant 37°C. pH and temperature were monitored in real time using a CDI Blood Parameter Monitoring System (Terumo Cardiovascular). Sodium bicarbonate via the drug administration port and/or carbon dioxide via the sweep gas were added to maintain physiologic pH (7.2–7.5).
Control
For both the blood prime and crystalloid prime setups, three controls were analyzed to determine natural drug degradation over time. The controls consisted of a PVC test tube filled with 30 mL of the ECMO prime solution. Collection of the prime solution from the ECMO circuit occurred before drug administration but after ≥5 minutes of ECMO circulation to ensure adequate mixing. Thus, the prime solution in the control was identical to that in the ECMO circuit. The control tubes were capped and held in a 37°C water bath for the duration of the experiment.
Drug Administration and Sample Collection
Amiodarone was dosed into the ECMO circuit via a port in the arterial cannula to achieve a therapeutic concentration of 3 µg/mL (therapeutic range for amiodarone 1.5–4 µg/mL) (23,24). All doses were proportionally adjusted by volume to achieve the same drug concentration in the circuits and controls. Drug injection occurred at time = 0. For the controls, drug was introduced into the test tube at time = −5 minutes, after which the tubes were immediately capped and placed in a gentle rotator. At time = 0, the tubes were returned to the 37°C water bath for the remainder of the experiment.
Samples were collected at 1, 5, 15, and 30 minutes and 1, 2, 3, 4, 8, 12, and 24 hours for the blood prime circuits and controls. The three separate crystalloid prime circuits and controls were tested for 72 hours, with multiple dosing time points. Standard dosing to 3 µg/mL occurred at 0, 24, 30, 36, and 42 hours, and a large dose that is 10 times the standard dose, which aimed for a circuit concentration of amiodarone of 30 µg/mL, was administered at 48 hours to investigate the possibility of saturating the circuit. Samples were collected at 1, 5, 15, and 30 minutes and 1, 2, 3, 4, 8, 12, and 24 hours; 24 hours 1 minute and 5 minutes; 25, 28, 29, and 30 hours; 30 hours 1 minute; 36 hours; 36 hours 1 minute; 42 hours; 42 hours 1, 5, and 30 minutes; 43, 46, 47, 48 hours; 48 hours 1, 5, 15, and 30 minutes; and 49, 50, 51, 52, 56, 60, and 72 hours. At times when dosing and sampling overlapped, the sample was collected immediately before dose administration.
After sample collection in the blood prime circuits, the blood was centrifuged at 3,000 g for 10 minutes. The plasma was then pipetted into a cryovial, which was immediately placed into a −80°C freezer for storage. For the crystalloid experiments, centrifugation was not performed, and the samples were placed directly into a cryovial for storage at −80°C.
Analysis
Drug concentrations were measured by ARUP Laboratories (Salt Lake City, UT) using high-performance liquid chromatography (HPLC) along with ultraviolet (UV) spectrophotometry (25). Samples were prepared with .25 mL of plasma, 20 µL of an internal standard (2 ethyl-3-[3,5-dibromo-4-di-n-propylaminopeopoxybenzoyl]benzothiophene), and .2 mL of 1 M sodium phosphate monobasic. After mixing by a vortex, the samples were diluted with .5 mL of HPLC-grade methyl-t-butyl-ether and centrifuged. The mobile phase was composed of acetonitrile/methanol/.05M ammonium acetate at 40:56:3 v/v. The analytes were separated on a 50 × 4.6-mm PhenoSphere Cyano 3-µ particle column (Phenomenex, Torrance, CA) with a flow rate of .9 mL/min. Amiodarone concentration was determined by UV spectrophotometry at 242 nm. The quantitation range was .3–6.0 µg/mL.
Because of differences in the ECMO circuit volume, amiodarone concentrations varied slightly between experiments. Thus, the percentage of drug recovery was calculated for analysis using the following equation:
where is the concentration at time = t and is the initial concentration at time = 1 minute. Data are reported as mean and 95% confidence interval.
Ethics
The Duke University Medical Center Institutional Review Board provided a waiver of review because the protocol met the definition of research not involving human subjects.
RESULTS
Blood Prime Circuits
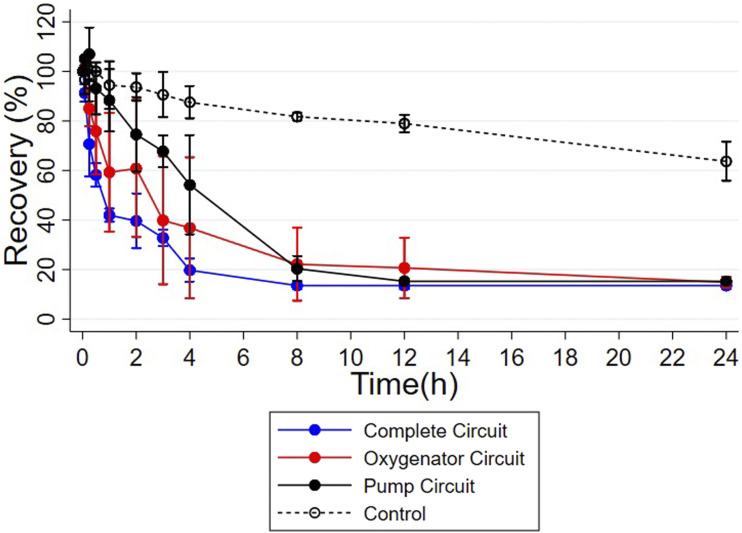
All blood prime circuits showed low amiodarone recovery starting at 8 hours: The mean (95% confidence interval) recovery in the three complete circuits was 13.5% (11.8, 15.2; n = 3); mean recovery in the three oxygenator circuits was 22.1% (5.4, 38.8; n = 3); and mean recovery in the three pump circuits was 18.6% (12.3, 24.9; n = 3) (Figure 2). These levels are much lower than recovery in the controls at 8 hours (81.2% [79.3, 83.1]; n = 3). Over 24 hours, drug recovery decreased steadily in the controls.
Figure 2.
Recovery of amiodarone by blood prime circuit configuration. Values are represented by mean, and error bars show 95% confidence interval.
Crystalloid Prime Circuits
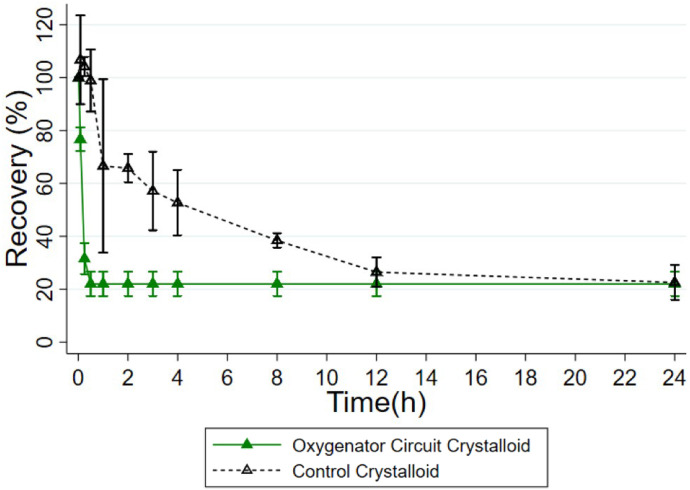
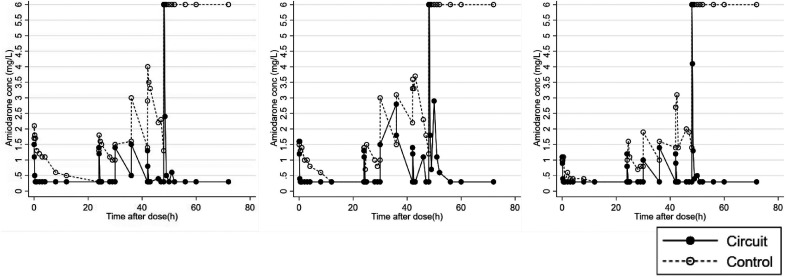
There was comparably low recovery in the crystalloid prime circuits. At 30 minutes, drug concentrations neared the lower limit of quantification (LLOQ) at .3 µg/mL in all circuits, which corresponds to a mean recovery of 22.0% (16.8, 27.2; n = 3) (Figure 3). After additional doses, drug concentrations continued to drop to the LLOQ in the circuits (Figure 4). This remained true following the massive dose, which was 10 times the standard dose, administered at 48 hours.
Figure 3.
Recovery of amiodarone by crystalloid prime circuits following the first dose. Values are represented by mean, and error bars show 95% confidence interval.
Figure 4.
Drug concentration in the three crystalloid prime circuits in the oxygenator configuration dosed multiple times over 72 hours. A massive dose was administered at time = 48 hours. Of note, the lower and upper limits of quantitation were .3 and 6 mg/L, respectively.
The crystalloid controls also experienced a loss in drug recovery, but this occurred more slowly than that in the circuits (Figure 3). Drug concentrations continued to drop following additional doses in the controls (Figure 4). However, following the massive dose at 48 hours, drug concentrations remained at the upper limit of quantification (ULOQ) at 6.0 µg/mL.
DISCUSSION
In vivo ECMO impacts drug exposure through its effect on the body and by direct interactions with the drug. It affects the body by increasing the volume of distribution because of circuit priming with large volumes of exogenous blood or crystalloid and physiologic changes such as inflammation (26,27). The ECMO circuit can also directly clear drug, as in hemofiltration (28,29). Lastly, nonspecific binding between the drug and circuit components can lead to drug extraction. This mainly depends on the physicochemical properties of the drug and circuit materials (30). These effects are further complicated by organ dysfunction in critically ill patients. The present ex vivo study demonstrates substantial amiodarone extraction in ECMO. These findings are expected becuase of amiodarone’s high lipophilicity (logP 7.6) and protein binding (96%), which are properties previously associated with drug extraction (7,9,22,31).
Notably, the rates at which amiodarone concentration in the three blood prime configurations reached the LLOQ were not uniform. At 8 hours, drug extraction was highest in the complete circuit and lowest in the pump circuit. Because the complete circuit has all three circuit components (the hemofilter, oxygenator, and tubing) and the pump circuit only has the ECMO tubing, these data suggest that all three components contribute to amiodarone extraction. Importantly, drug loss was also noted in the blood prime controls. But, it was substantially less than in the circuit configurations. There are a few possible reasons for drug loss in the controls, including 1) adsorption to the PVC of the control tubes, 2) plasma metabolism of amiodarone, and 3) drug degradation. Adsorption to the PVC is suggested by the observed extraction in the pump circuit, of which the only component is PVC tubing. However, nonspecific binding to the tubes should occur quickly, and the observed decrease in drug concentration in the controls occurs at a slow rate throughout the duration of the experiments (32–34). This suggests that there may be another element at play. Amiodarone has an incredibly long half-life in the human body (up to 100 days). In addition, it is primarily eliminated via hepatic clearance, and it is not known to undergo plasma metabolism (21). Thus, in an ex vivo configuration, blood metabolism is not likely to have a significant influence on amiodarone concentration. Finally, amiodarone is known to degrade in light, and the control tubes were not protected from room lighting. Therefore, light degradation was likely the primary contributor to the observed drug loss (35). Future studies should address amiodarone extraction in light and dark environments.
The rapid drops in amiodarone concentration following each dose in the crystalloid prime circuits occurred much faster than those in the blood prime circuits. This suggests that the presence of blood protects amiodarone from extraction. This may be because of differences in the concentrations of the primary protein-binding partner of amiodarone, albumin. In a separate study, small increases in albumin concentrations were shown to protect micafungin, another highly protein-bound drug, from extraction (8). Although equivalent amounts of exogenous albumin were added to both the blood prime and crystalloid prime circuits in this study, the blood also contained endogenous albumin. Thus, albumin concentrations in the crystalloid prime circuits were likely lower than those in the blood prime circuits, which would result in more unbound amiodarone in the crystalloid prime circuits. This increased amount of unbound drug further exposes it to extraction by the ECMO circuit, which helps explain the extremely rapid drops in amiodarone concentrations. Future studies should vary albumin concentrations to isolate its protective effects on amiodarone extraction.
Another common concern in ECMO is drug saturation to the circuit binding sites, after which additional doses would not be extracted (7). This concern is compounded when other lipophilic drugs are co-administered. However, because the rapid decrease in drug concentration continued following each additional amiodarone dose, particularly the massive dose given at 48 hours, saturation was not observed in the present study. Interestingly for the crystalloid prime controls, amiodarone concentrations remained at the ULOQ following the massive dose at 48 hours. There are two possible explanations for this: 1) saturation was achieved in the control tubes or, more likely, 2) there was not enough time for drug extraction to lower drug concentration within the limits of quantification.
There are several limitations to this study. First, the ULOQ prevented a thorough explanation of drug PK in the crystalloid prime controls following the massive dose. Second, the presence of the hemofilter shunt slightly alters flow in the complete circuits vs the oxygenator and pump circuits, which lack the shunt. The shunt has an ∼10% recirculation rate and exposes circuit components to ∼10% more flow in a complete circuit, which could contribute to the observed faster extraction rates in this configuration. A third limitation relates to the dosing regimen. Clinically, amiodarone is delivered to patients on ECMO via continuous infusions and intermittent boluses. However, in the present study, amiodarone was administered in discrete doses. A fourth limitation relates to other materials to which amiodarone may adhere. ECMO is known to induce cell fragmentation because of shear stress in the oxygenator (36). This fragmentation, and other potential phospholipid debris, may provide additional binding sites to which amiodarone may adhere. Additional work should analyze whether amiodarone is able to bind to such materials and to what extent this contributes to amiodarone bioavailability. Finally, previous studies have shown that different surface coatings of the circuit, which are added to increase biocompatibility, can have differing effects on drug extraction (37). Although the surface coating in this study was phosphorylcholine, several other types of coatings are used in standard practice. Thus, different surface coatings should be investigated to increase the clinical relevance of the study.
In summary, the present study suggests that the ECMO circuit heavily extracts amiodarone from circulation. This may result in significantly less drug exposure in vivo and necessitate dosing modifications to achieve a therapeutic effect. However, this study alone does not provide sufficient data to accurately predict optimal amiodarone dosing in vivo as drug disposition in critically ill patients on ECMO is multifactorial. Physiologically based PK (PBPK) models can translate ECMO ex vivo results into bedside dosing recommendations (38). PBPK models are mathematical tools that integrate drug-specific (e.g., metabolism and protein binding) and population-specific (e.g., organ size and blood flow) information to predict the effect of different factors (e.g., age, genetic variants, and disease) on drug exposure (39,40). These models are structured in a physiologically relevant manner with virtual organ compartments connected by blood flow. Each virtual “organ” is parameterized with mass-balance differential equations characterizing the disposition of drug within the compartment. To model drug exposure in populations on ECMO, an ECMO “organ” can be linked to the PBPK model and parameterized using data from ex vivo studies. By defining the volume, blood flows, and drug clearance of the ECMO compartment, the impact of ECMO on drug disposition can be predicted in vivo, and the ECMO PBPK model can be used to determine optimal dosing in this vulnerable population.
ACKNOWLEDGMENTS
We are grateful to Mike Lowe for technical support during the experiments. None of the authors has any conflict of interest to report for the present study. This work was supported by the National Institutes of Health (1R01HD097775).
REFERENCES
- 1.Watt K, Li JS, Benjamin DK Jr, et al. Pediatric cardiovascular drug dosing in critically ill children and extracorporeal membrane oxygenation. J Cardiovasc Pharmacol. 2011;58:126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramesh Iyer V. Drug therapy considerations in arrhythmias in children. Indian Pacing Electrophysiol J. 2008;8:202–10. [PMC free article] [PubMed] [Google Scholar]
- 3.Kendrick JG, Macready JJ, Kissoon N. Amiodarone treatment of junctional ectopic tachycardia in a neonate receiving extracorporeal membrane oxygenation. Ann Pharmacother. 2006;40:1872–5. [DOI] [PubMed] [Google Scholar]
- 4.Dzierba AL, Abrams D, Brodie D. Medicating patients during extracorporeal membrane oxygenation: The evidence is building. Crit Care (London, England). 2017;21:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shekar K, Roberts JA, McDonald CI, et al. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: Results from an ex vivo study. Crit Care. 2015;19:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherwin J, Heath T, Watt K. Pharmacokinetics and dosing of anti-infective drugs in patients on extracorporeal membrane oxygenation: A review of the current literature. Clin Therapeut. 2016;38:1976–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buck ML. Pharmacokinetic changes during extracorporeal membrane oxygenation: Implications for drug therapy of neonates. Clin Pharmacokinet. 2003;42:403–17. [DOI] [PubMed] [Google Scholar]
- 8.Watt KM, Cohen-Wolkowiez M, Williams DC, et al. Antifungal extraction by the extracorporeal membrane oxygenation circuit. J Extra Corpor Technol. 2017;49:150–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Wildschut ED, Ahsman MJ, Allegaert K, et al. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36:2109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallefeld SH, Sherwin J, Zimmerman KO, et al. Dexmedetomidine extraction by the extracorporeal membrane oxygenation circuit: Results from an in vitro study. Perfusion. 2020;35:209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harthan AA, Buckley KW, Heger ML, et al. Medication adsorption into contemporary extracorporeal membrane oxygenator circuits. J Pediatr Pharmacol Therapeut. 2014;19:288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahsman MJ, Hanekamp M, Wildschut ED, et al. Population pharmacokinetics of midazolam and its metabolites during venoarterial extracorporeal membrane oxygenation in neonates. Clin Pharmacokinet. 2010;49:407–19. [DOI] [PubMed] [Google Scholar]
- 13.Shekar K, Roberts JA, McDonald CI, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care. 2012;16:R194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemaitre F, Hasni N, Leprince P, et al. Propofol, midazolam, vancomycin and cyclosporine therapeutic drug monitoring in extracorporeal membrane oxygenation circuits primed with whole human blood. Crit Care. 2015;19:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahsman MJ, Wildschut ED, Tibboel D, et al. Pharmacokinetics of cefotaxime and desacetylcefotaxime in infants during extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 2010;54:1734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta NM, Halwick DR, Dodson BL, et al. Potential drug sequestration during extracorporeal membrane oxygenation: Results from an ex vivo experiment. Intensive Care Med. 2007;33:1018–24. [DOI] [PubMed] [Google Scholar]
- 17.Wildschut ED, de Hoog M, Ahsman MJ, et al. Plasma concentrations of oseltamivir and oseltamivir carboxylate in critically ill children on extracorporeal membrane oxygenation support. PLoS One. 2010;5:e10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Vorst MM, Wildschut E, Houmes RJ, et al. Evaluation of furosemide regimens in neonates treated with extracorporeal membrane oxygenation. Crit Care. 2006;10:R168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cies JJ, Moore WS 2nd, Giliam N, et al. Oxygenator impact on ceftolozane and tazobactam in extracorporeal membrane oxygenation circuits. Pediatr Crit Care Med. 2020;21:276–82. [DOI] [PubMed] [Google Scholar]
- 20.Perry JC, Fenrich AL, Hulse JE, et al. Pediatric use of intravenous amiodarone: Efficacy and safety in critically ill patients from a multicenter protocol. J Am Coll Cardiol. 1996;27:1246–50. [DOI] [PubMed] [Google Scholar]
- 21.Latini R, Tognoni G, Kates RE. Clinical pharmacokinetics of amiodarone. Clin Pharmacokinet. 1984;9:136–56. [DOI] [PubMed] [Google Scholar]
- 22.Lalloz MR, Byfield PG, Greenwood RM, et al. Binding of amiodarone by serum proteins and the effects of drugs, hormones and other interacting ligands. J Pharm Pharmacol. 1984;36:366–72. [DOI] [PubMed] [Google Scholar]
- 23.Stäubli M, Bircher J, Galeazzi RL, et al. Serum concentrations of amiodarone during long term therapy. Relation to dose, efficacy and toxicity. Eur J Clin Pharmacol. 1983;24:485–94. [DOI] [PubMed] [Google Scholar]
- 24.Dayal D. We urgently need guidelines for managing COVID-19 in children with comorbidities. Acta Paediatr. 2020;109:1497–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juenke JM, Brown PI, McMillin GA, et al. A rapid procedure for the monitoring of amiodarone and N-desethylamiodarone by HPLC-UV detection. J Anal Toxicol. 2004;28:63–6. [DOI] [PubMed] [Google Scholar]
- 26.Yamasaki Y, Hayashi T, Nakatani T, et al. Early experience with low-prime (99 ml) extracorporeal membrane oxygenation support in children. ASAIO J. 2006;52:110–4. [DOI] [PubMed] [Google Scholar]
- 27.Millar JE, Fanning JP, McDonald CI, et al. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit Care. 2016;20:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith AH, Hardison DC, Worden CR, et al. Acute renal failure during extracorporeal support in the pediatric cardiac patient. ASAIO J. 2009;55:412–6. [DOI] [PubMed] [Google Scholar]
- 29.Zwiers AJ, de Wildt SN, Hop WC, et al. Acute kidney injury is a frequent complication in critically ill neonates receiving extracorporeal membrane oxygenation: A 14-year cohort study. Crit Care. 2013;17:R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dagan O, Klein J, Gruenwald C, et al. Preliminary studies of the effects of extracorporeal membrane oxygenator on the disposition of common pediatric drugs. Ther Drug Monit. 1993;15:263–6. [DOI] [PubMed] [Google Scholar]
- 31.Raffaeli G, Pokorna P, Allegaert K, et al. Drug disposition and pharmacotherapy in neonatal ECMO: From fragmented data to integrated knowledge. Frontiers in Pediatrics. 2019;7:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preston TJ, Hodge AB, Riley JB, et al. In vitro drug adsorption and plasma free hemoglobin levels associated with hollow fiber oxygenators in the extracorporeal life support (ECLS) circuit. J Extra Corpor Technol. 2007;39:234–7. [PMC free article] [PubMed] [Google Scholar]
- 33.Palmgren JJ, Monkkonen J, Korjamo T, et al. Drug adsorption to plastic containers and retention of drugs in cultured cells under in vitro conditions. Eur J Pharm Biopharm. 2006;64:369–78. [DOI] [PubMed] [Google Scholar]
- 34.Unger JK, Kuehlein G, Schroers A, et al. Adsorption of xenobiotics to plastic tubing incorporated into dynamic in vitro systems used in pharmacological research--limits and progress. Biomaterials. 2001;22:2031–7. [DOI] [PubMed] [Google Scholar]
- 35.Kawabata K, Sugihara K, Sanoh S, et al. Photodegradation of pharmaceuticals in the aquatic environment by sunlight and UV-A, -B and -C irradiation. J Toxicol Sci. 2013;38:215–23. [DOI] [PubMed] [Google Scholar]
- 36.Williams DC, Turi JL, Hornik CP, et al. Circuit oxygenator contributes to extracorporeal membrane oxygenation-induced hemolysis. ASAIO J. 61:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preston TJ, Ratliff TM, Gomez D, et al. Modified surface coatings and their effect on drug adsorption within the extracorporeal life support circuit. J Extra Corpor Technol. 2010;42:199–202. [PMC free article] [PubMed] [Google Scholar]
- 38.Watt KM, Cohen-Wolkowiez M, Barrett JS, et al. Physiologically based pharmacokinetic approach to determine dosing on extracorporeal life support: Fluconazole in children on ECMO. CPT Pharmacometrics Syst Pharmacol. 2018;7:629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45:1013–34. [DOI] [PubMed] [Google Scholar]
- 40.Zhao P, Zhang L, Grillo JA, et al. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther. 2011;89:259–67. [DOI] [PubMed] [Google Scholar]