Abstract:
New cardiopulmonary bypass device techniques emerge and are reported in the scientific literature. The extent to which they are actually adopted into clinical practice is not well known. Since 1989, we have periodically surveyed pediatric cardiac centers to ascertain practice patterns. In December 2016, a 186-question perfusion survey was distributed to pediatric cardiac surgery centers all over the world using a Web-based survey tool. Responses were received from 93 North American (NA) centers (the United States and Canada) and 67 non–NA (NNA) centers, representing 19,645 cumulative annual procedures in NA and 27,776 in NNA centers on patients <18 years. Wide variation in practice was evident across geographic regions. However, the most common pediatric circuit consisted of a hard-shell (open) venous reservoir, an arterial roller pump, and a hollow-fiber membrane oxygenator with a separate or integrated arterial filter. Compared with our previous surveys, there was increased utilization of all types of safety devices. The use of an electronic perfusion record was reported by 50% of NA centers and 31% of NNA centers. There was wide regional variation in cardioplegia delivery systems and cardioplegia solutions. Seventy-nine percent of the centers reported the use of some form of modified ultrafiltration. The survey demonstrated that there remains variation in perfusion practice for pediatric patients. Future surveys will be useful to evaluate the adoption of emerging perfusion practice guidelines.
Keywords: international survey, pediatric perfusion, survey, cardiopulmonary bypass
The conduct of neonatal, infant, and pediatric cardiopulmonary bypass (CPB) is continuously evolving as new devices and innovative new techniques are introduced. Periodically, surveys have been undertaken to document practices in specific countries and geographic regions of the United Kingdom, Japan, France, and North America (NA) (1–8). Surveys provide perspectives on practice patterns during the time periods they were administered. Historically, surveys have identified regional differences in practices. For example, the survey by Elliot (1) from 1993 found only 31% of the 16 centers in the United Kingdom used an arterial line filter, and a survey of 127 NA centers conducted during the same time period reported over 90% of centers used an arterial line filter (4).
Surveys are of value in studying the congruence of current practice with current evidence and current standards of care (9). Current practice patterns may also be of some value when evaluating standards of practice. In December of 2016, the survey task force sent out a global survey to obtain up-to-date information on perfusion practice (Appendix A). Although the questions in the current survey largely followed those in previous surveys, the questionnaire was updated as needed.
MATERIALS AND METHODS
The 2011 International Pediatric Perfusion Survey was assessed and revised by the survey task force and resulted in an updated 186-question survey (8). This survey research proposal was reviewed and approved by the Institutional Review Board of Maine Medical Center (IRB Groom-4566NR_4566NR_5.4.15). The survey was created, and study data were collected and managed using an electronic data capture tool hosted at Nationwide Children’s Hospital (NCH) Research Electronic Data Capture (REDCap) (10–11). REDCap is a secure, Web-based software platform designed to support data capture for research studies, providing an intuitive interface for validated data capture, audit trails for tracking data manipulation, and export procedures, automated export procedures for seamless data downloads to common statistical packages, and procedures for data integration and interoperability with external sources (12). The survey form was beta-tested and edited by the coauthors. The survey was then distributed by email to the perfusion department directors at pediatric cardiothoracic programs all over the world.
The questionnaire requested demographic data including case volume for the years 2014, 2015, and 2016. The unit of analysis for the survey was the pediatric cardiothoracic surgery center. The survey consisted of 186 questions covering the topics of disposables, device and equipment usage, techniques used, medications administered, blood product usage, physiologic parameters, prime components, perfusion procedural record type (electronic or paper), circulatory assist devices, multidisciplinary staffing levels, and inquiries related to the conduct of CPB.
Responses were stratified into one of the following geographic regions: NA, which comprises the United States and Canada; South America (SA); Asia (ASIA); Europe (EU); and Oceania (OA), which comprises Australia and New Zealand. Results were tabulated as proportions or percentage of total respondents for categorical variables and as median for continuous variables and stratified by geographic area. Questions relating to the technique and equipment were reported as frequency relative to the number of total responding centers and evaluated accordingly.
In an effort to maximize the response rate, an invitation email was sent in advance of the survey to outline the importance of receiving one completed survey response from each facility. Surveys were sent by email on November 10, 2015 with subsequent repeat electronic mailings made to non-respondents. Surveys and reminders were sent by the NCH REDCap administrator to a list of perfusionists and other key stakeholders which were compiled by the authors and colleagues. Non-respondents and undeliverable contacts were reverified, and in some cases, contact information was updated, or contact by phone was made to increase the response rate. Some respondents did not answer every question. For each question, the number of responses for that particular question is listed as “n.”
Once these avenues were exhausted, the survey was closed for further data entry. The responses were analyzed for completeness and for duplicates. The completed survey demographic and response information was initially tabulated using REDCap analytic tools but was later exported to Microsoft Excel® (Redmond, WA) for further analysis. The results were then analyzed by regional subsets: NA, SA, Asia, Europe (EU), and OA.
RESULTS
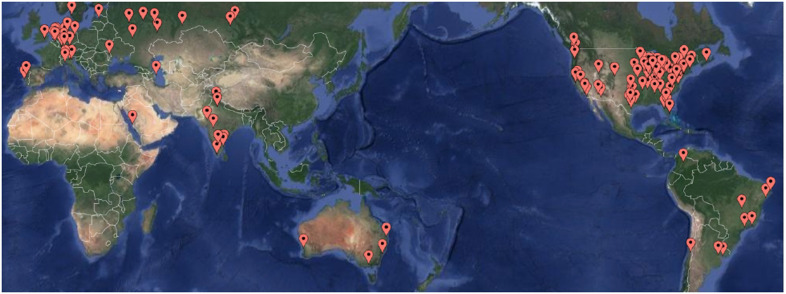
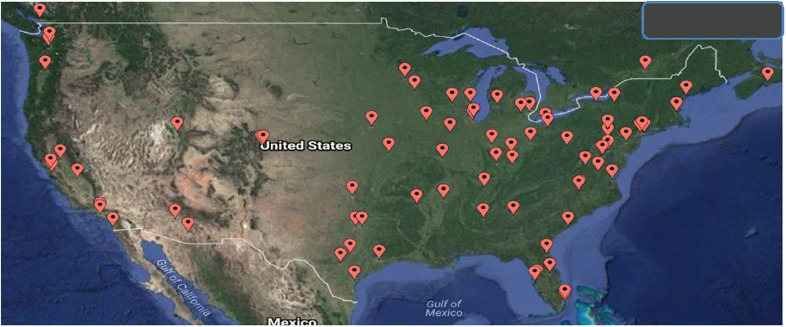
Program demographic responses were received from 160 of the 252 centers for an overall response rate of 63%. Figure 1 shows overall responses by location, and likewise, Figure 2 shows NA response by location. Table 1 summarizes the response rate for the five geographic regions. The average annual pediatric caseload (<18 years of age) for responding centers in 2016 was 137 cases per year per center (median 185 cases/year/center; range, 12–3,080 cases/year/center). The total reported pediatric case count in 2014, 2015, and 2016 was 38,361, 45,956 and 47,421, respectively.
Figure 1.
World map of respondents.
Figure 2.
Map of NA respondents.
Table 1.
Surveyed regions and response rate.
| NA | SA | Asia | OA | EU | Total | |
|---|---|---|---|---|---|---|
| Percent of total responses (%) | 58 | 6 | 10 | 3 | 23 | 100 |
| Surveys sent (n) | 113 | 12 | 33 | 5 | 89 | 252 |
| Responses (n) | 93 | 10 | 16 | 4 | 37 | 160 |
| Regional responses (%) | 82 | 83 | 48 | 80 | 42 | 63 |
Staffing Levels and Competency
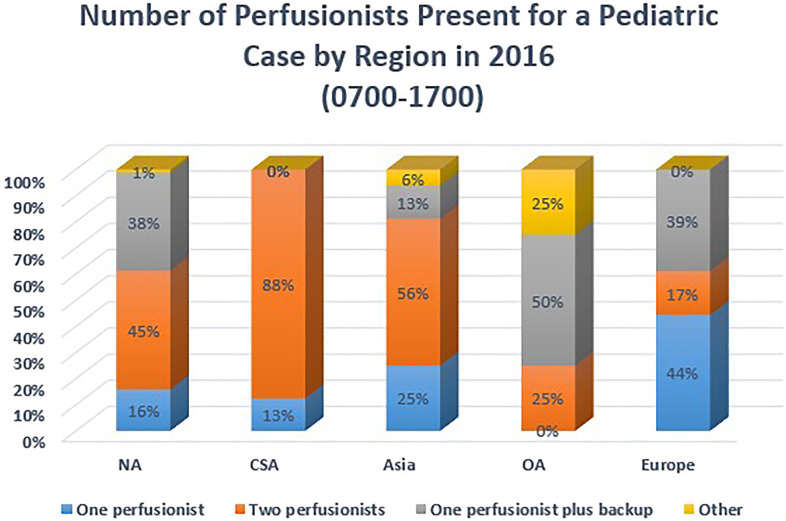
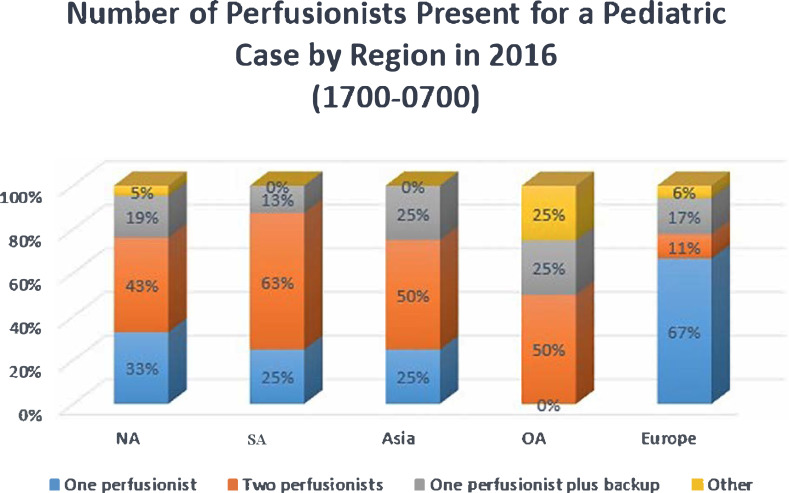
Perfusionist staffing levels for CPB procedures were by region and by the time of day that procedures occurred. Figure 3 summarizes staffing levels during typical working hours of 07:00–17:00, and Figure 4 summarizes staffing levels from 17:00–07:00. Asia and SA both reported that the majority of their centers staff each pediatric case with two perfusionists from 07:00 to 17:00; however, this percentage decreases for the evening hours for urgent and emergent cases. EU reported 44% of responding centers staff one perfusionist per case from 07:00 to 17:00, and 67% report this same staffing model overnight (17:00–07:00). Respondents were asked about their opinion on the number of annual pediatric cases they considered necessary for a perfusionist to be considered competent. There were 154 responses to this question. Eighteen percent responded that a minimum of less than 40 cases/year was sufficient. Fifty-nine percent indicated 40 to 59 cases/year was sufficient, and 23% were of the opinion that 70 cases/year was the minimum number of cases a perfusionist should perform to be considered competent as a pediatric perfusionist.
Figure 3.
Number of perfusionists present (07:00–17:00) for a pediatric case by region.
Figure 4.
Number of perfusionists present (17:00–07:00) for a pediatric case by region.
Safety Devices and Circuit Components
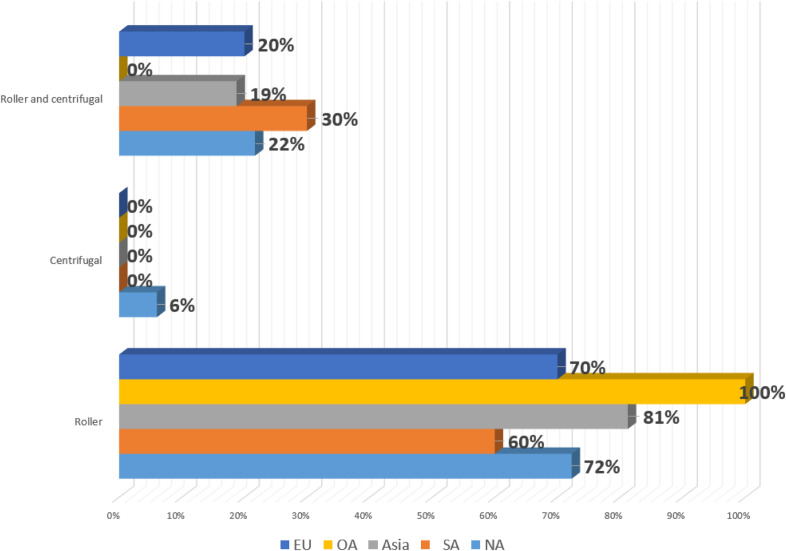
Utilization of safety devices and circuit components is summarized in Table 2. Ninety percent of all responding centers use a level detector. Ninety-four percent of NA centers report using a bubble detector on their cases, whereas 0% and 27% from SA and EU reported use, respectively. A one-way valve in the left ventricular or aortic root vent line was used in 88% of responding centers in NA but 0% in those responding from SA. A gas supply oxygen analyzer was used in 50% or less of all responding centers. Most centers reported use of a roller-type pump for propulsion of blood through the CPB circuit. A small number of centers reported the use of some of both roller-type and centrifugal pumps (presumably centrifugal pumps in older, larger patients). Arterial pump use by region is summarized in Figure 5. Open reservoirs (hard-shell) are used in 72% of responding centers. Vacuum-assisted venous drainage (VAVD) is reportedly used on 74% of all responding centers, whereas this technique is predominantly used in NA and SA with 89% and 78% utilization, respectively. This is in comparison to utilization in Asia, OA, and EU (44%, 50%, 58%, respectively). Pre-bypass filters are used in all responding OA centers, along with 82% in NA. The utilization of pre-bypass filters remains lower in responding centers from SA (22%), Asia (25%), and EU (25%). The overall use of arterial line filters in neonates (aged <30 days), infants (aged 30 days–1 year), and pediatrics (aged 1–18 years) is 68%, 64%, and 64%, respectively. Crystalloid cardioplegia filters are used in one-quarter of all responding centers. Table 3 shows the changes in the use of safety devices and continuous monitoring devices by NA centers over time. Ultrafiltration usage is summarized in Table 4. Ninety-four percent of NA centers reported use of ultrafiltration on >90% of their pediatric cases, with the next closest region being OA at 75%, and overall, 72% of responding centers used ultrafiltration >90% of cases.
Table 2.
Safety devices and circuit components by region.
| NA (%) | SA (%) | Asia (%) | OA (%) | EU (%) | Total (%) | |
|---|---|---|---|---|---|---|
| (n = 85) | (n = 10) | (n = 16) | (n = 4) | (n = 37) | (n = 152) | |
| Safety devices | ||||||
| Level detector | 98 | 50 | 63 | 100 | 95 | 90 |
| Bubble detector | 94 | 0 | 44 | 100 | 27 | 66 |
| One-way valve in vent line | 88 | 0 | 19 | 50 | 62 | 68 |
| One-way purge line | 66 | 30 | 88 | 75 | 65 | 66 |
| Gas supply oxygen analyzer | 41 | 10 | 25 | 50 | 49 | 39 |
| Arterial pump | ||||||
| Roller | 72 | 60 | 81 | 100 | 70 | 64 |
| Centrifugal | 6 | 0 | 0 | 0 | 0 | 3 |
| Roller and centrifugal | 22 | 30 | 19 | 0 | 20 | 18 |
| Components/techniques | ||||||
| Open reservoir (hard-shell) | 95 | 0 | 75 | 100 | 70 | 72 |
| Vacuum assist venous drainage | 89 | 78 | 44 | 50 | 58 | 74 |
| Pre-bypass filter | 82 | 22 | 25 | 100 | 25 | 58 |
| Arterial line filter-neonates | 87 | 30 | 63 | 100 | 65 | 68 |
| Arterial line filter-infants | 86 | 20 | 44 | 75 | 65 | 64 |
| Arterial line filter-pediatrics | 81 | 40 | 63 | 75 | 55 | 69 |
| Cardioplegia filter | 28 | 25 | 13 | 50 | 26 | 25 |
| Ultrafiltration device use | ||||||
| Ultrafiltration used <50% of cases | 1 | 10 | 33 | 25 | 34 | 13 |
| Ultrafiltration used 50–89% of cases | 5 | 30 | 33 | 0 | 9 | 11 |
| Ultrafiltration used >90% of cases | 94 | 60 | 33 | 75 | 57 | 76 |
Figure 5.
Arterial pump type by region.
Table 3.
Safety device usage over time.
| Year surveyed | 1989 (%) | 1994 (%) | 1999 (%) | 2004 (%) | 2011 (%) | 2016 (%) |
|---|---|---|---|---|---|---|
| Level sensor | 65 | 67 | 79 | 90 | 100 | 99 |
| Bubble sensor | 72 | 85 | 88 | 83 | 96 | 100 |
| One-way valve (vent) | 52 | 66 | 69 | 75 | 87 | 89 |
| One-way valve (purge) | 72 | 90 | 92 | 92 | 85 | 70 |
| Oxygenator gas supply analyzer | 28 | 49 | 47 | 48 | 40 | 40 |
| Arterial blood gases | 67 | 86 | 71 | 77 | 83 | 90 |
| Arterial blood O2 saturation | 9 | 26 | 26 | 19 | 37 | |
| Venous blood gases | 48 | 52 | 31 | 21 | 25 | 17 |
| Venous blood O2 saturation | 48 | 78 | 88 | 94 | 90 | 96 |
North America trends of safety devices and circuit continuous monitoring use (% use).
Table 4.
Ultrafiltration utilization by region.
| NA (%) | SA (%) | Asia (%) | OA (%) | EU (%) | Total (%) | |
|---|---|---|---|---|---|---|
| (n = 86) | (n = 13) | (n = 18) | (n = 7) | (n = 51) | (n = 175) | |
| Ultrafiltration used >90% | 88 | 38 | 22 | 43 | 31 | 59 |
| Ultrafiltration used 50–89% | 9 | 54 | 50 | 43 | 45 | 72 |
| Ultrafiltration used <50% | 2 | 8 | 28 | 14 | 24 | 12 |
Monitoring Devices
The utilization of real-time, in-line monitoring devices during pediatric CPB cases is reported in Table 5. An electroencephalogram is used in less than 5% of responding centers. Ninety-two percent of centers report the use of arterial pressure monitoring by a catheter. Cerebral oximetry monitoring was reported in 93%, 96%, and 100% of centers in NA, EU, and OA, respectively, whereas 40% and 38% were reported in Asia and SA, respectively. Ninety-three percent of centers responded that they monitor the circuit line pressure during CPB, and 86% reported monitoring the cardioplegia system pressure. Arterial in-line blood gas monitoring was used in 89% of centers responding from NA, compared with 10% in SA. Venous in-line blood gas monitoring was lower in all responding centers, with 0% in OA, however 32% in EU. Electronic medical records (EMR) were used in 43% of all responding centers. EU documented a median automated data capture rate of 20 seconds (range 1–60 seconds), OA also reported a median rate of 20 seconds (range 15–20 seconds), whereas the data were more diversified in NA, with a median rate of 18 seconds (range 1–300 seconds). In NA respondents, 37% were using the LivaNova PLC (Arvada, CO), 28% were using the Spectrum Medical (Gloucester, United Kingdom) Viper EMR, 14% EPIC (Verona, WI), Terumo Cardiovascular (Ann Arbor, MI), and other represented 9% of respondents each, and Getinge JoCAP (Gotenborg, Sweden) was documented in 2%. Twenty-five percent of respondents from OA reported use JoCAP, and 75% reported use of the LivaNova system.
Table 5.
Real-time monitoring utilization by region.
| NA (%) | SA (%) | Asia (%) | OA (%) | EU (%) | Total (%) | |
|---|---|---|---|---|---|---|
| (n = 92) | (n = 10) | (n = 16) | (n = 4) | (n = 37) | (n = 159) | |
| Electroencephalogram | 3 | 0 | 0 | 0 | 14 | 5 |
| Arterial pressure by cuff | 30 | 40 | 25 | 0 | 30 | 30 |
| Arterial pressure by catheter | 99 | 80 | 69 | 100 | 89 | 92 |
| Pulmonary artery pressure | 12 | 10 | 38 | 0 | 11 | 14 |
| Left atrial pressure | 15 | 20 | 38 | 0 | 24 | 19 |
| Central venous pressure | 87 | 80 | 94 | 75 | 97 | 89 |
| CPB arterial line pressure | 96 | 60 | 100 | 100 | 92 | 93 |
| Cardioplegia infusion pressure | 96 | 30 | 88 | 100 | 76 | 86 |
| Pulse oximetry | 76 | 90 | 69 | 100 | 86 | 79 |
| Cerebral oximetry | 93 | 40 | 38 | 100 | 78 | 81 |
| EMR | 52 | 0 | 13 | 100 | 40 | 43 |
| Arterial blood gas in-line | 89 | 10 | 31 | 75 | 65 | 72 |
| Venous blood gas-in-line | 16 | 20 | 25 | 0 | 32 | 21 |
| Venous saturation in-line | 96 | 60 | 44 | 100 | 81 | 85 |
| Other | 7 | 10 | 19 | 0 | 14 | 9 |
Extracorporeal Life Support Devices
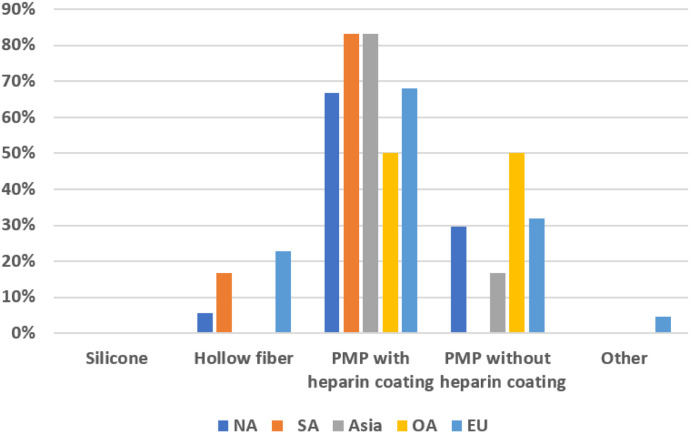
Table 6 summarizes the type of extracorporeal life support available by region. The most common type of ventricular assist device (VAD) support at responding centers was a centrifugal pump. Extracorporeal membrane oxygenation (ECMO) was used in 58% of centers. Roller pumps were used for VAD support by 19% of NA respondents and at 44% of Asian centers, along with 11% of EU. OA reporting centers responded with availability for support with ECMO (50%), centrifugal VAD (100%), pneumatic VAD (25%), and 0% to all other available devices. When looking primarily at ECMO pumps used, in NA, CSA, OA and EU, centrifugal pumps were used primarily in 50% or greater of the responding centers. Most responding centers from all regions were using polymethylpentene (PMP) oxygenators. Figure 6 shows the differences between PMP oxygenators with heparin versus those without. All regions preferred heparin-coated PMP oxygenators (≥67%), with the exception of OA where it was a 50% split between heparin-coated and non–heparin-coated PMP oxygenators.
Table 6.
Extracorporeal support used by region.
| NA (%) | SA (%) | Asia (%) | OA (%) | EU (%) | Total (%) | |
|---|---|---|---|---|---|---|
| Type of support | (n = 88) | (n = 12) | (n = 16) | (n = 4) | (n = 35) | (n = 155) |
| ECMO | 58 | 75 | 38 | 50 | 63 | 58 |
| Centrifugal pump VAD | 84 | 83 | 75 | 100 | 80 | 83 |
| Pneumatic VAD | 50 | 42 | 0 | 25 | 46 | 43 |
| Intra-aortic balloon pump | 22 | 33 | 38 | 0 | 31 | 26 |
| Roller pump | 19 | 33 | 44 | 0 | 11 | 21 |
| Other | 3 | 8 | 0 | 0 | 14 | 6 |
| Do not use VAD | 7 | 8 | 6 | 0 | 23 | 10 |
*Percentages do not add up to 100%.
Figure 6.
Type of extracorporeal membrane oxygenator used by region.
Techniques
Circuit prime and blood products
Common prime constituents are listed in Table 7. Eighty-nine percent and 100% of centers in NA and OA, respectively, used Plasma-Lyte A (Baxter Corporation, Deerfield, IL) as the crystalloid priming solution for their circuits. Lactated Ringer’s solution (Hospira Inc, Lake Forest, IL) is predominantly used as the priming solution in SA (80%). Nearly all (90%) centers in NA add 25% albumin (Talecris Biotherapeutics, Inc., Research Triangle Park, NC) to the prime, whereas only 50% of those responding from SA and Asia used it in the prime. EU reported use of 5% and 25% albumin in only 24% and 30% of responding centers, respectively. Hetastarch (Hospira, Inc., Lake Forest, IL) is used in 25% of centers from Asia. Heparin sodium (Sagent Pharmaceuticals, Schaumburg, IL) and sodium bicarbonate (Hospira, Inc., Lake Forest, IL) were used in a majority of all responding centers. Mannitol (Hospira, Inc, Lake Forest, IL) was not used in any of the responding centers from OA. Calcium chloride is used in the prime in 75% of centers from OA, whereas it was reportedly used at 81% of centers from EU. Steroids are not used at all in centers responding from OA but 29%, 30%, and 25% of NA, SA, and Asia. Antifibrinolytics are used in 60% of NA centers and 38% of Asian centers.
Table 7.
Prime and drug additives by region.
| NA (%) | SA (%) | Asia (%) | OA (%) | EU (%) | Total (%) | |
|---|---|---|---|---|---|---|
| (n = 92) | (n = 10) | (n = 16) | (n = 4) | (n = 37) | (n = 159) | |
| Plasma-Lyte A | 89 | 30 | 69 | 100 | 27 | 69 |
| Normosol-R | 10 | 0 | 0 | 0 | 19 | 10 |
| Lactated Ringer’s | 2 | 80 | 44 | 0 | 19 | 15 |
| Other | 3 | 10 | 38 | 0 | 46 | 17 |
| 5% albumin | 7 | 30 | 25 | 0 | 24 | 14 |
| 25% albumin | 90 | 50 | 50 | 75 | 30 | 69 |
| Hetastarch | 0 | 0 | 25 | 0 | 5 | 4 |
| 5% plasma protein | 2 | 0 | 19 | 0 | 8 | 5 |
| Pentastarch | 0 | 0 | 0 | 0 | 0 | 0 |
| Gelatin | 0 | 10 | 0 | 0 | 19 | 5 |
| Heparin | 99 | 100 | 100 | 100 | 97 | 99 |
| Sodium bicarbonate | 88 | 70 | 94 | 100 | 73 | 84 |
| Mannitol | 68 | 90 | 81 | 0 | 81 | 72 |
| Calcium chloride | 34 | 20 | 6 | 75 | 11 | 26 |
| Steroids | 29 | 30 | 25 | 0 | 5 | 23 |
| Antibiotics | 33 | 40 | 19 | 0 | 22 | 28 |
| Furosemide | 11 | 0 | 19 | 0 | 11 | 11 |
| THAM | 0 | 0 | 0 | 0 | 8 | 2 |
| Lidocaine | 0 | 10 | 6 | 0 | 0 | 1 |
| Antifibrinolytics | 60 | 30 | 38 | 0 | 22 | 45 |
Retrograde autologous priming was used in 6% of neonatal patients (aged <30 days), 19% of infant patients (aged >31 days–1 year), 50% of pediatric patients (aged >1 year–18 years), and 53% of adult patients (>18 years) among all centers. The average patient body weight at which all respondents would consider initiating CPB with a bloodless prime is 11 kg. The blood priming component was nearly split among all centers for neonates and infants. Fifty-six percent of neonatal (<30 days) CPB circuits are primed with irradiated packed red blood cells, where infant (aged 31 days–1 year) circuits are primed with citrated packed red cells in 54% of centers. Twenty-eight percent of centers use an autotransfusion device to wash red blood cells before adding them to the prime, and 44% washed the packed red blood cells perioperatively just before administering to a patient.
Ultrafiltration
Overall, 97% of centers reported the routine use of ultrafiltration during CPB. Multiple types and timing of ultrafiltration were used before, during, and after CPB, as summarized in Table 8. The majority of centers using modified ultrafiltration (MUF) conduct arteriovenous (A-V) MUF (70%), and the cumulative total of those using A-V and venovenous (V-V) MUF among all centers is 79%. And, 57% of centers used some form of pre-BUF, although only 18% of centers used an ultrafilter to concentrate the residual extracorporeal circuit volume post-CPB.
Table 8.
Types of ultrafiltration by region.
| NA (%) | SA (%) | Asia (%) | OA (%) | EU (%) | Total (%) | |
|---|---|---|---|---|---|---|
| (n = 89) | (n = 8) | (n = 16) | (n = 4) | (n = 35) | (n = 152) | |
| Never | 0 | 0 | 0 | 0 | 3 | 1 |
| Pre-CPB | 64 | 38 | 69 | 100 | 31 | 57 |
| A-V MUF | 69 | 63 | 63 | 100 | 74 | 70 |
| V-V MUF | 7 | 0 | 13 | 0 | 17 | 9 |
| Post-CPB | 20 | 13 | 19 | 25 | 14 | 18 |
| During CPB | 99 | 100 | 100 | 100 | 91 | 97 |
Myocardial protection
The majority of responding centers documented using del Nido cardioplegia solution (54%). In NA, the use of del Nido solution was reported by 74% of the centers. Centers from OA reported exclusive use (100%) of a high potassium solution only. Twenty-two percent of all respondents used Custodiol histidine-tryptophan-ketoglutarate (HTK), and 39% used a high potassium solution. In NA, the utilization of Custodiol HTK solution was the lowest (8%). Table 9 summarizes the cardioplegia delivery systems and cardioplegia solution use stratified by region.
Table 9.
Cardioplegia solutions and delivery systems by region.
| NA (%) | SA (%) | Asia (%) | OA (%) | EU (%) | Total (%) | |
|---|---|---|---|---|---|---|
| Cardioplegia Solution | (n = 89) | (n = 8) | (n = 16) | (n = 4) | (n = 36) | (n = 153) |
| Hyperpolarizing (Custodiol® HTK) | 8 | 50 | 6 | 0 | 58 | 22 |
| Depolarizing (High Potassium) | 22 | 50 | 69 | 100 | 56 | 39 |
| Modified Depolarizing (del Nido) | 74 | 25 | 69 | 0 | 11 | 54 |
| Cardioplegia Delivery System | ||||||
| Recirculating | 30 | 25 | 31 | 25 | 40 | 32 |
| Single Pass | 70 | 50 | 69 | 75 | 31 | 60 |
| Syringe | 0 | 50 | 0 | 0 | 9 | 3 |
| Do not use | 0 | 0 | 0 | 0 | 6 | 1 |
| Other | 0 | 13 | 0 | 0 | 14 | 4 |
Recirculating, prepared in a batch and recirculated through a reservoir system. Singe pass, solution and blood passes one time through the cardioplegia heat exchanger. Syringe, drawing up in a syringe and injected directly.
Patient Management
Heparin management
Heparin remains the primary anticoagulant used in all regions during CPB for pediatric patients. OA, EU, and Asia all used a fixed protocol based on patients’ weight in greater than 90% of centers, whereas NA (72%) and CSA (75%) had more mixed approaches that included the use of a dose–response curve with the HMS Hepcon device (Medtronic, Minneapolis, MN). Seventeen percent of all centers reported administration of plasma antithrombin III (Grifols Therapeutics Inc., Research Triangle Park, NC) during CPB.
Hypothermia technique
When comparing cooling gradients of water bath to blood temperature, the average gradient observed was 9°C, compared with 8°C for rewarming. Of the responding centers (90.5%) that reported monitoring a maximum arterial temperature during rewarming, 36.9°C was the average maximum temperature. Retrograde selective cerebral perfusion (SCP) was used in 5% of respondents; antegrade SCP was used in 86% of centers; and 11% reported not using any form of SCP. The average target temperature used for SCP was 22°C. Fifty-seven percent of centers add some mixture of carbon dioxide to the oxygenator sweep gas during hypothermia. During SCP, 38% of centers place ice packs on the head. Thirty-five percent of centers tested for cold agglutinins before CPB surgery.
Blood gas management
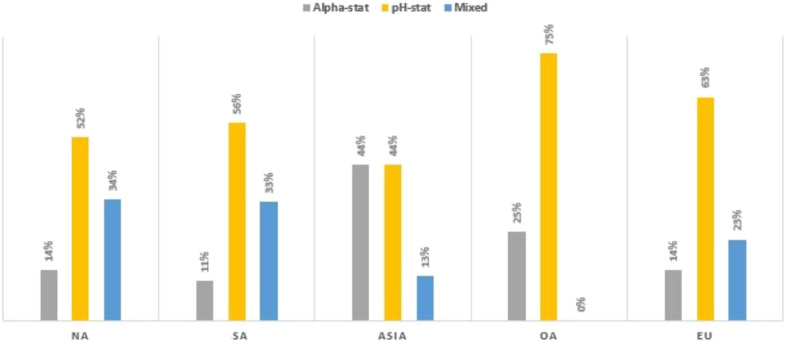
The pH stat blood gas management strategy was used in 54% of all responding centers (Figure 7). Asia reported using pH stat 44%, with 44% use alpha stat strategy management and 13% reporting use of a mixed (alpha and pH stat) approach. NA, EU, and SA have a low utilization of the alpha stat management strategy at 14%, 14%, and 11%, respectively.
Figure 7.
Blood gas management strategy during hypothermia by region.
DISCUSSION
This is the sixth in a series of pediatric perfusion practice surveys conducted approximately every 5 years since 1989 (4–8). The first four surveys were limited to NA centers, and the two most recent surveys targeted clinicians worldwide. The aim of the first survey was to determine the extent to which certain techniques and devices were used in NA. There remains a significant variation in practice across regions. Furthermore, some of the respondents commented that there was variation within their center related to individual surgeon’s preferences. Collectively, the six surveys document trends in practice in the regions surveyed along with a few other publications regarding staffing and incidents on CPB (13,14). For example, Table 3 shows the changes in the use of safety devices and continuous monitoring devices by NA centers.
Decisions related to the use of extracorporeal technology to support pediatric patients during cardiac surgery are critical because they may influence survival and long-term outcome for young patients. Variation in clinical practice may be related to a number of factors. Rodgers described a theoretical framework for explaining the diffusion of innovation (15). Five characteristics, namely, relative advantage, compatibility, complexity, trial ability, and observe ability influence the rate of change. He further described five categories of user adopters of new ideas: innovators (quickest to adopt), early adopters, the early majority, the late majority, and laggards (last to adopt). Each group adds a uniquely valuable perspective. The latter adds historical context, and although clinicians in this group delay the rate of change, they add value by insisting on strong evidence before making a change. They are typically less deliberate but more cautious. Stammers and colleagues conducted a perfusion practice survey in 2000 and reported that economics also played a role in some equipment decisions (16).
Ideally, practice should not be determined by proxy, but rather by published evidence. It is likely that areas of practice where there is wide variability are those areas where evidence is lacking. A survey can identify gaps between the published literature and the clinical practice. Over the years, more contemporary research findings have helped to inform teams about some practices. For example, MUF emerged as a technique after it was first described in 1991 (17). The use of MUF has been studied in numerous randomized controlled trials, systematic reviews, and meta-analyses (18–21). This technically challenging therapy has been steadily increased in use to nearly 80% of respondents in the most recent survey reported herein. The emergence and adoption of the use of del Nido cardioplegia has been an area of dramatic change. The use of del Nido started as an NA phenomenon; however, its use is gradually spreading to other regions. One of the barriers to its spread has been the availability of Plasma-Lyte solution in some parts of the world. However, there are recent studies examining the use of substitute electrolyte solutions in its formulation (22). More recent randomized controlled trials that examined different cardioplegia solutions have been published (23–28). Despite survey results showing widespread use of some practices, evidence is still lacking for many techniques. For example, Hirsch and colleagues conducted a systematic review of cerebral protective strategies for pediatric patients undergoing cardiac surgery and concluded that except for avoiding extreme hemodilution, there is insufficient evidence to recommend use of any specific neuromonitoring or neuroprotective strategy during CPB (29). They further pointed out the need for more randomized trials and long-term outcome studies to inform the development of practice standards and guidelines.
The first survey, published in 1989, was conducted at a time when there were no published practice guidelines. Practice patterns were more related to where the leadership at a program had trained and what they had been taught by their mentors. There are a growing number of practice guidelines that synthesize the evidence and expert opinions to provide recommendations (30). Surveys, such as this one, provide a snapshot of clinical practice at a point in time. It has been said that science tells us what is possible, guidelines tell us what we should do, and a registry informs us about what we actually do. Similarly, our surveys provide a summary of actual clinical practice.
Standards and Guidelines for Pediatric and Congenital Perfusion Practice have been adopted by the American Society of Extracorporeal Technology and published in 2019 (31). Additional guidelines for temperature management, anticoagulation, and blood management have been published since this survey was taken. It will be interesting to monitor over time how well these guidelines are actually adopted into clinical practice. Surveys, such as this one, may provide important information from which one can make some inferences about the effects of standards and guidelines on actual practices locally and regionally.
There are a number of weaknesses to this study. Although we sought to obtain as many responses as possible, there were centers and regions of the world that did not participate in the survey. We are delighted that this survey included centers from Russia, SA, and India. We hope in the future to get more global penetration across and within regions.
Another source of potential error is related to how well some respondents understood a particular question. Although the questionnaire was beta tested, it was evident from respondents’ comments that some questions were skipped, or answered incorrectly possibly because of regional colloquialisms and limited response options. The goal of this survey was to gather information from individual pediatric cardiothoracic programs and determine how emerging techniques, technologies, and equipment have been incorporated into clinical practice norms across this group of respondents.
A large amount of information was collected in this 186-question survey; however, there is a limit to what can be reported in a single publication.
With the publication of new standards and guidelines for practice subsequent to this survey, future surveys are warranted to measure the extent to which the new guidelines and standards are adopted. We are indebted to each perfusionist that completed the survey, making it possible for us to compile this collective experience.
ACKNOWLEDGMENTS
The authors thank Rachel Fonseca for her assistance in managing the database, validation of survey responses, and correspondence with the survey task force.
Appendix A
Pediatric Perfusion
Please complete the survey below.
Please answer all questions that state “*must provide value.” If you do not, you will not be able to submit the survey at the end.
At any time, you may click the “Save and Return Later” button at the bottom of the survey. If you notice any issues with the survey, please leave feedback at the bottom.
Thank you!
Hospital name:
City/town:
State/province:
Country:
Which of the following best describes your institution?
Community hospital (for profit)
Community hospital (not for profit)
University-affiliated hospital (non-VA).
University hospital.
Veterans administration hospital.
How many pediatric cardiothoracic (CT) surgeons are at your institution?
How many full-time pediatric cardiac anesthesiologists are at your institution?
Do you have a congenital surgical fellowship?
Yes.
No.
How many adult (≥18 years of age) CPB cases did your institution perform in
2014.
2015.
2016.
How many pediatric (1 year - < 18 years) CPB cases did your institution perform in
2014.
2015.
2016.
How many neonatal and infant (<1 year) CPB cases did you perform in
2014.
2015.
2016.
What is the crystalloid component of your circuit prime?
Lactated ringer’s solution.
5% dextrose and lactated ringer’s.
Plasma-Lyte A solution.
Normosol R.
9% sodium chloride (saline).
Other.
Please specify.
Which of the following colloid additives do you use in your prime?
5% albumin
25% albumin.
5% plasma protein.
6% hetastarch.
Petastarch
Gelatin.
Other.
None.
Please specify.
What other additives are routinely added to the “non-blood” circuit prime?
Mannitol.
Heparin.
Calcium chloride.
Furosemide.
Lidocaine.
Steroid.
Antibiotics.
Sodium bicarbonate.
THAM
Magnesium sulfate.
Tranexamic acid.
Calcium gluconate.
Amicar.
Other.
None.
Please specify.
What is the static prime milliliter volume (with minimum operating level) of your neonatal circuit? (including cardioplegia if you use blood cardioplegia).
Which of the following blood products do you use to prime the bypass circuit for the following age-groups?
Neonatal (0–30 days).
Infant (31 days–1 year).
Pediatric (>1 year–<18 years).
Adult (≥18 years).
Fresh heparinized whole blood.
Irradiated red blood cells.
Citrated whole blood.
Citrated packed red cells.
Fresh frozen plasma.
Platelets.
Crystalloid prime.
Other.
Please specify (neonatal).
Please specify (infant).
Please specify (pediatric).
Please specify (adult).
Does perfusion use an autotransfusion device to wash packed red blood cells before adding them to the prime? Administering to a patient while in the OR?
Yes/No.
Do you process the circuit prime through an ultrafiltrator (pre-buf)?
Yes/No.
According to your perfusion protocol, what is the minimal acceptable hematocrit during procedures requiring the following hypothermia?
| Mild (32–35°C) | |
|---|---|
| Moderate (26–31°C) | |
| Deep (20–25°C) | |
| Profound (<20°C) |
Just before the termination of bypass for patients presenting with:
| Cyanotic defects (%) | |
|---|---|
| Acyanotic defects (%) |
Which arterial pumps do you use for pediatric cardiac procedures for each of the following?
Roller pump system.
Centrifugal pump system.
Neonatal (0–30 days).
Infant (31 days–<1 year).
Pediatric (1 year–<18).
Adult (≥18 year).
Roller manufacturer (infant).
Roller manufacturer (neonatal).
Roller manufacturer (pediatric).
Roller manufacturer (adult).
Centrifugal manufacturer (infant).
Centrifugal manufacturer (neonatal).
Centrifugal manufacturer (pediatric).
Centrifugal manufacturer (adult).
What type of reservoir and oxygenator system do you typically use for CPB?
Open reservoir/closed reservoir.
Hollow fiber.
Bubble.
Silicone.
Diffusion.
PMP.
Which of the following safety devices do you use level detector.
Air bubble detector (outlet of venous reservoir).
Air bubble detector (pre-oxygenator).
Air bubble detector (post-arterial line filter).
Air bubble detector (arterial line, pre-arterial line filter).
One-way valve in vent line.
One-way valve in sucker line.
One-way purge line (arterial filter).
Oxygen analyzer in the gas line.
Auto clamp.
Venous clamp.
Cardioplegia air bubble detector.
Other.
Please specify.
Do you use a pre-bypass filter/recirculation filter during priming of the extracorporeal circuit?
Yes/No.
Filter micron size.
Which arterial line filters do you use in the extracorporeal circuit?
Neonates (0–30 days)
Infants (31 days–<1 year)
Pediatrics (1 year–18 years).
Adults (≥18 years).
Capiox/Terumo AF02.
Capiox/Terumo AF125X.
Capiox/Terumo AF200X.
Capiox/Terumo AL6X.
Capiox/Terumo AL8X.
Capiox/Terumo FX series integrated filter.
Medtronic affinity pixie.
Medtronic affinity.
Medtronic affinity AF100.
Maquet quart.
Maquet quadrox-i integrated filter.
Pall AL3.
Pall AL6.
Pall AL8.
Pall leukoguard six.
Pall autovent SV
Dideco/Liva Nova D130.
Dideco/Liva Nova D131.
Dideco/Liva Nova D731.
Dideco/Liva Nova D732.
Dideco/Liva Nova D733.
Dideco/Liva Nova D734.
Dideco/Liva Nova inspire with integrated filter.
Dideco/Liva Nova synthesis.
We do not use an arterial line filter.
What type of cases do you use a leukodepletion filter in your circuit?
Transplants.
All cardiac cases.
Do not use a leukodepletion filter.
Other.
Please specify.
Do you use VAVD during CPB?
Yes/No.
What (%) of cases is VAVD utilized?
0%
100%
(Place a mark on the scale above).
What is the MAXIMUM negative pressure (in mmHg) in your protocol during VAVD?
Do you use biopassive/coated circuit components?
Yes/No.
What coating(s) do you use?
Bio-line.
Physio.
Safeline.
Smart.
Trillium.
X-coating.
Carmeda.
Other.
Please specify (tubing).
Please specify (oxygenator).
Do you use a retrograde autologous priming (RAP)?
Neonatal (0–30 days)
Infant (31 days–<1 year)
Pediatric (1 year–<18 years)
Adult (≥18 years).
Yes/No.
What is the lowest patient weight (kg) you would _________ consider a bloodless initiation of bypass?
Which parameters do you monitor routinely on bypass?
Electrocardiogram (ECG).
Electroencephalogram (EEG).
Arterial pressure by cuff.
Arterial pressure by in-dwelling catheter.
Pulmonary artery pressure.
Central venous pressure.
Left atrial pressure.
Intracranial pressure.
Pulse oximetry.
Pump circuit arterial line pressure.
Cardiolegia infusion pressure.
Near infrared spectroscopy (cerebral).
Near infrared spectroscopy (somatic).
Myocardial pH.
Right atrial pressure.
Other.
What is the lowest patient weight (kg) you would consider a bloodless initiation of bypass?
Which parameters do you monitor routinely on bypass?
Electrocardiogram (ECG)
Electroencephalogram (EEG)
Arterial pressure by cuff.
Arterial pressure by in-dwelling catheter.
Pulmonary artery pressure.
Central venous pressure.
Left atrial pressure.
Intracranial pressure.
Pulse oximetry.
Pump circuit arterial line pressure.
Cardiolegia infusion pressure.
NIRS (cerebral).
NIRS (somatic).
Myocardial pH.
Right atrial pressure.
Other.
Please specify.
Which of the following laboratory values (non-calculated) do you routinely monitor during bypass?
Glucose.
Ionized calcium.
Platelet count.
Colloid osmotic pressure.
Lactate.
Anti-Xa level.
AT3.
Arterial blood gas.
Venous blood gas.
Other.
Please specify.
Which of the following variables do you monitor arterial blood gases continuously using in-line sensors in the perfusion venous blood gases circuit?
Arterial saturation (measured, not calculated).
Venous saturation (measured, not calculated).
Oxygen delivery (DO2).
Hematocrit/hemoglobin.
Expired CO2.
Other.
Please specify.
What pediatric VADs are available at your institution?
Centrifugal pump.
Roller pump.
Intra-aortic balloon pump (IABP) Extra corporeal life support (ECLS)
Berlin Heart.
HeartWare.
Heart mate II.
Tandem heart.
Total artificial heart.
Impella.
Do not use.
Ventricular assist.
Other.
None.
Please specify.
How many times in 2016 did you use a VAD for pediatric open-heart post-cardiotomy support?
What oxygenation do you use for ECLS?
Silicone (non-micro porous).
Hollow fiber.
PMP with heparin coating.
PMP without heparin coating.
Other.
Please specify.
What type of pump do you use during ECLS?
Roller pump.
Centrifugal pump.
Which temperature do you routinely monitor during pediatric open-heart procedures? (check all that apply)
Rectal.
Nasopharyngeal.
Esophageal.
Tympanic.
Arterial blood.
Venous blood.
Cardioplegia solution.
Myocardial.
Bladder.
Deep muscle.
Other.
Please specify.
What percent of time do you use pulsatile flow during pediatric bypass procedures?
0%
100%
Do you or your lab test your patients for cold agglutins before surgery?
Yes.
No.
Have you ever modified your hypothermia technique due to cold agglutinin test results?
Yes.
No.
Do you ventilate the oxygenator with a carbon dioxide mixture during hypothermia?
Yes.
No.
Do you use surface cooling for your patients (ice packs or cooling blanket) after anesthesia induction?
Yes.
Rarely.
Only on circulatory arrest procedures.
No.
When do you pack the patient’s head in ice?
During all procedures.
When circulatory arrest is used.
During SCP.
Never.
Other.
(Place a mark on the scale above).
What % of cases do you use a profoundly hypothermic prime (<20°C) to initiate bypass?
0%
100%
(Place a mark on the scale above).
To what core temperature do you typically cool the patient when you use the following:
Circulatory arrest (°C).
SCP (°C).
Low flow (°C).
Do you perform SCP?
Retrograde.
Antegrade.
None.
What is your target blood flow rate? (mL/kg/min).
What is your target temperature? (°C).
Do you use SCP for procedures that involve arch reconstruction?
Always.
Sometimes.
Never.
Which blood gas management strategy do you use during deep hypothermia?
Alpha-stat (blood gases measured at 37°C).
pH-stat (blood gases corrected to patient temperature).
Mixed (alpha-stat/pH-stat on same patient).
During bypass, what is considered the optimal Pa02 (mmHg) for the following:
100–199
200–299
300–399
>400.
Acyanotic patient, during normothermia, and full flow?
Cyanotic patient, during normothermia, and full flow.
During hypothermia and prior to circulatory arrest.
How do you calculate blood flow rate?
mL/kg/min.
L/min/m2.
When using the following ranges of hypothermia, what flow rate do you use?
Mild (32–35°C) (mL/kg/min).
Moderate (26–31°C) (mL/kg/min).
Deep (20–25°C) (mL/kg/min).
Profound (<20°C) (mL/kg/min).
Mild (32–35°C) (L/min/m2).
Moderate (26–31°C) (L/min/m2).
Deep (20–25°C) (L/min/m2).
Profound (<20°C) (L/min/m2).
Do you observe a temperature gradient (°C) guideline when:
Cooling.
Rewarming.
Yes/No.
Cooling water-to-blood temperature range:
Rewarming water-to-blood temperature range:
Is there a maximum inflow blood temperature that you will NOT exceed during rewarming?
Yes/No.
Maximum temperature:
To what temperature (°C) do you rewarm the patient before terminating CPB for each of the following?
Rectal.
Nasopharyngeal.
Oral.
Bladder.
Other.
Do you ever administer Antithombin III concentrate on CPB?
Yes/No.
What dose and indications?
How do you determine the loading dose of heparin for pediatric patients?
Dose–response curve by activated clotting time (Hepcon HMS).
Fixed protocol by patient weight.
Other.
Please specify.
Do you monitor ACT’s during CPB?
Yes/No.
What do you consider an adequate Activated Clotting Time (ACT) during CPB (in seconds)?
Which device do you use to monitor ACTs?
How often (in minutes) do you monitor ACT times during the following:
Hypothermia.
Rewarming.
How is the protamine dose determined? Calculation based on total heparin administered Bull curve.
Fixed dose by patient weight.
Automated dose–response curve (Hepcon).
Do you administer antifibrinolytics?
Yes - Aminocaproic acid.
Yes - Tranexamic acid.
Yes - Aprotinin.
Yes - Other.
No - None.
Please specify.
How are they applied?
Bolus to patient CPB prime.
Continuous infusion.
Which of the following vapor anesthetic agents do you halothane administer via the oxygenator?
Ethrane.
Isoforane.
Desflurane.
Sevoflurane.
Do not use.
Other.
Please specify.
During CPB, what is your target mean arterial blood pressure (mmHg)?
< 20 20–29 30–39 40–49 50 or more
Do not treat hypotension during CPB?
Neonatal (0–30 days).
Infant (31 days–< 1 year).
Pediatric (1 year–< 18 years).
Adult (≥18 years).
Which vasoactive drugs do you ROUTINELY administer during CPB?
Epinephrine.
Phenylephrine (Neosynephrine).
Levophed (Norepinephrine).
Phentolamine (Regitine).
Nitroprusside (Nipride).
Prostaglandin E.
Nitroglycerin.
Other.
Please specify.
At what blood glucose level (mg/dL) do you administer insulin during bypass?
100–150.
150–200.
200–250.
250–300.
>300.
Only treat adult glucose levels.
Do not treat glucose during bypass.
In what percentage of cases do you use a cell salvage device?
0%
100%
How do you process the residual pump volume after bypass?
Cell salvage.
Direct infusion.
Ultrafiltrate and bag.
We do not collect or process residual pump volume.
(Place a mark on the scale above).
Other.
Please specify.
What cardioplegia solution do you use?
Crystalloid only.
Microplegia - cold.
Microplegia - warm.
Oxygenated crystalloid.
Warm continuous blood cardioplegia.
Blood and Crystalloid mixed.
How do you administer cardioplegia?
Roller pump Pressure bag.
Syringe by surgeon.
Quest MPS system.
Microplegia (blood and drugs via infusion pump).
Other.
Please specify.
Which of the following BEST describes your single-pass cardioplegia system?
Recirculating.
Syringe injection.
Do not use cardioplegia.
Other.
Please specify.
What is the ratio of blood to crystalloid in the single-pass system?
Parts Blood.
Parts Crystalloid.
Do you give a dose of warm substrate-enhanced cardioplegia before the cross-clamp is removed?
Yes.
No.
Sometimes.
What is the percentage of time you infuse cardioplegia?
Antegrade only (%)
Retrograde only (%)
Antegrade and retrograde (same case) (%)
What is your target delivery pressure, flow, and temperature parameters for cardioplegia dosing?
Temperature (C).
Initial flow rate (mL/kg/min).
Initial dose (mL/kg).
Maintenance dose (mL).
Maintenance dose (mL/kg).
What is the indication for maintenance cardioplegic doses?
Time.
Myocardial temperature.
Observed electrical/mechanical activity.
Do not re-infuse.
Time (in minutes).
Myocaridal temp (C).
What is the Potassium concentration of your cardioplegia?
Induction (meq/L).
Maintenance (meq/L).
What type of cardioplegia solution do you use?
Hyperpolarizing (ex. Potassium).
Depolarizing (ex. Custodiol)
Modified Depolarizing (ex. Del nido).
Do you use any of the following in your cardioplegia solution?
Lidocaine.
Adenosine.
Icorandil.
Pinacidil.
Cromakalim.
Minoxidil.
Aprillkalim.
Loprazolam.
Magnesium Sulfate.
Dextrose.
Mannitol.
Procainimide.
Other.
Please specify.
Do you filter the crystalloid component of your cardioplegia?
Yes/No.
Micron size:
What % of cases do you use some type of ultrafiltration in conjunction with CPB (0% if never)
0%
100%
When do you use the ultrafiltration device?
(Place a mark on the scale above).
Pre-bypass to buffer and concentrate the prime.
During bypass.
Post-bypass by arteriovenous method (A-V MUF).
Post-bypass by venovenous method (V-V MUF).
Used to concentrate the circuit only after CPB.
Never.
Do you use an electronic perfusion record?
Yes/No.
What is the automated data capture rate? (seconds).
What software do you use?
Do you perform hybrid procedures (some combination of cardiac surgical and interventional radiology) at your institution?
Yes/No.
Where are they performed?
Hybrid operating room.
Catherization laboratory.
Traditional operating room.
Other.
Please specify.
At your institution, which of the following parameters determines tubing set diameter and combination?
Patient Weight (kg)
Calculated blood flow (mL/min)
Cooling temperature (°C).
Operative procedure.
Other.
Please specify.
What are the MAXIMUM weight cut-offs (kg weight) for each of the following tubing combinations? Enter N/A if you do not use a specified tubing combination.
1/8″ × 3/16″
1/8″ × 1/4″
3/16″ × 3/16″
3/16″ × 1/4″
5/32″ × 1/4″
1/4″ × 1/4″
1/4″ × 5/16″
1/4″ × 3/8″
3/8″ × 3/8″
3/8″ × 1/2″
What are the MAXIMUM blood flow cut-offs (mL/min) for each of the following tubing combinations? Enter N/A if you do not use a specified tubing combination.
1/8″ × 3/16″
1/8″ × 1/4″
3/16″ × 3/16″
3/16″ × 1/4″
5/32″ × 1/4″
1/4″ × 1/4″
1/4″ × 5/16″
1/4″ × 3/8″
3/8″ × 3/8″
3/8″ × 1/2″
What are the MAXIMUM temperatures (°C) for each of the following tubing combinations? Enter N/A if you do not use a specified tubing combination.
1/8″ × 3/16″
1/8″ × 1/4″
3/16″ × 3/16″
3/16″ × 1/4″
5/32″ × 1/4″
1/4″ × 1/4″
1/4″ × 5/16″
1/4″ × 3/8″
3/8″ × 3/8″
3/8″ × 1/2″
What determines each of the following tubing combinations? Enter N/A if you do not use a specified tubing combination.
1/8″ × 3/16″
1/8″ × 1/4″
3/16″ × 3/16″
3/16″ × 1/4″
5/32″ × 1/4″
1/4″ × 1/4″
1/4″ × 5/16″
1/4″ × 3/8″
3/8″ × 3/8″
3/8″ × 1/2″
How many perfusionists are present for each case during conventional work days (example: 07:00–17:00)?
One perfusionist in the operating room pediatric/neonatal.
Two perfusionists in the operating room.
One perfusionist per operating room case plus a backup perfusionist in the hospital.
Other.
Please specify.
How many perfusionists are present for each case during conventional work days (example: 17:00–07:00)?
One perfusionist in the operating room pediatric/neonatal.
Two perfusionists in the operating room.
One perfusionist per operating room case plus a backup perfusionist in the hospital.
Other.
Please specify.
How many perfusionists are present for each case during non-conventional work days (example: weekends)?
One perfusionist in the operating room pediatric/neonatal.
Two perfusionists in the operating room.
One perfusionist per operating room case plus a backup perfusionist in the hospital.
Other.
Please specify.
What is the minimum number of pediatric pump cases you believe a perfusionist should do annually to qualify as a competent pediatric perfusionist?
0–20.
21–30.
31–40.
41–50.
51–60.
61–70.
>70.
Would you like to receive a copy of the survey results?
Yes/No.
First and last name:
Email address:
Please provide any feedback on the survey itself here (i.e., what didn’t make sense, what didn't work correctly, etc.).
Appendix B
| 1 | In neonates or preterms, we also use the following tubing combination: 1/8–1/8 |
| 2 | The part about the cardioplegia do not include the hyperpotassic cold blood cardiolpegia; for this reason, my answer is not clear |
| 3 | Great job! |
| 4 | … |
| 5 | Good job! Really:) |
| 6 | We use mI/kg/min and CI. We use flow rate necessary to close aortic valve with close attention to delivery pressure, ECG, etc. and communication to the surgeon during plegia. We use a range for HCT depending on the case and anomaly, rather than temperature, but temperature is always considered with circulatory arrest or SCP. The questions worded what determines combination of tubing needed further |
| 7 | One or two questions could be improved. “What time” in the CPG section was completely undefinable? |
| 8 | Cardioplegia dosing should have maximum amount and maximum flow rate or pressure, etc. for induction and maintenance |
| 9 | Flows during hypothermia are based on adequacy of perfusion markers, not just temp. Rewarming temperature for us is venous temperature, do not care what other temperatures read. We have found inaccurate temperatures when using rectal/bladder. Survey could be more complete/accurate if allowed to enter information, not just numbers in some fields. Some answers need an explanation |
| 10 | For the CPB-related questions, specify with/without cross-clamp for pressure monitoring. Please reach out to me for any clarification |
| 11 | There were questions on the FT staff of pediatric surgeons and anesthesiologists, but not percussionists. I would have liked to see these data included in the survey too |
| 12 | Our HCT protocol is not based on what temperature we cool to, but rather patient age and pathology. Patients younger than 1 year of age: Minimal HCT is 30% on CPB and 35% weaning off CPB. Greater than 1 year minimal HCT is generally 24% but will consider SVO2, NIRS, ABP, etc before pulling the trigger. Single ventricle patients: Minimal HCT is 30% on CPB and 35% weaning off CPB, regardless of age |
| 13 | With four surgeons, we use three different cardioplegia delivery techniques, from Plegisol, del Nido, Custodiol HTK, and so on. My responses were mostly for the del Nido delivery because it asked about blood/crystalloid ratio |
| 14 | Too many required fields. Flaw in the survey is that there is no way to enter the different surgeon protocols at same instant. I have 3 surgeons doing things 3 different ways! 2 years until i retire |
| 15 | Temperature fields that are not used by our institution should have N/A option, instead of forced value. Same for selective cerebral perfusion area. |
| 16 | Our definition of prime is the circuit volume which hemodilutes patient blood when going on CPB. Because the cardioplegia in our circuit does not, we object to this survey definition of pump prime calculation |
| 17 | Great survey. I look forward to seeing the results. A lot of these questions relate to topics of disagreement among colleagues or even other institutions. Thank you! |
| 18 | Difficult to answer flow rate questions because they are based on age/weight, not temperature |
| 19 | No selection for multiple surgeons who do things differently |
| 20 | We use equal St. Thomas and del Nido CPG, which could only select one set of parameters, so we could not provide information regarding our del Nido CPG |
| 21 | Under ultrafiltration, we do V-AMUF (SMUF). Our data of capture rate are 1 sec, but our record is captured in 1-m intervals |
| 22 | Percentage of time you infuse cardioplegia: I took this to mean percent of cases using cardioplegia. Phasing out measuring routine Lactate levels as we migrate to the iSTAT and occasionally using the Dideco D736 arterial filter for neonates. Great job!! Sorry this was so late |
| 23 | I used “0” in numeric fields as N/A. Also, I do not have the neo vs. pediatric breakouts |
| 24 | For some items, it would have been naive to put ranges (e.g., temperatures, hematocrits). What was the “time” portion of the cardioplegia asking—time for redoes, time over which the initial does was given |
| 25 | Most of the clinical/protocol questions are surgeon-specific versus protocol-driven, that is, Hgb/HCT for various temperatures, CPG flow, pressures, blood priming, etc.—there should be a caveat to explain this in the selection above |
| 26 | We have two surgeons who use very different parameters for blood flow and temperature limits in regard to circulatory arrest. Because of the limitations of the answer field, I was only able to answer in reference to one surgeon, that is, 18°C or 18°C or 28°C |
| 27 | What is the lowest patient weight (kg) you would consider a bloodless initiation of bypass? I answered 6 kg—decision based more on calculated dilutional HCT, degree of cooling, and patient lesion |
| 28 | Thank you very much for this survey. I might suggest fewer mandatory fields |
| 29 | Difficult to commit to hard number of sometimes for “limits,” as patients vary. Well put together, survey is an excellent work |
| 30 | Often a lot of times, our temperatures are more on a range depending on patient and their operative course. Also, flows vary on many other factors like BP, NIRS, lactate, and venous saturation; often, we flow much more than our BSA guideline recommends, or less during profound cooling, anesthesia treats glucose |
| 31 | Cardioplegia subsequent dose wrong label. Temperatures should have had arterial vs ventral temperature in-line sensors says measured not calc....but all are calc. neonate prime blood-can be both irradiated and citrated. Overall good survey! |
| 32 | Was there supposed to be a question regarding cardioplegia delivery pressure? It is mentioned in the section heading, but there was no specific question. I did not like the question of cardioplegia initial flow in ml/kg/min |
| 33 | Protocols vary from a 1-year-old to an 18-year-old, resulting in inconsistent response (i.e., blood prime) is the cardioplegia K+concentration that of the crystalloid component or delivered solution? |
| 34 | Cardioplegia additives: We use Custodial. Flow rates: 2.6 cardiac index used for patient over 10 kg. Do not monitor oral/other temperatures |
| 35 | I was unsure of the question asking how many times VAD was used in 2016 for post-cardio to my support. We only use ECMO support post-cardiotomy. I figured you might have meant for ECMO to be included, but also thought if you wanted ECMO support, it would have been worded “VAD/ECMO.” We did 7 VAD implants in 2016, but none were post-cardiotomy, so I answered 0. We probably initiated 7 ECMO supports post-cardiotomy in 2016 |
| 36 | Some choices for blood prime, flow rates, etc. are surgeon- and case-dependent, but I did my best to describe the most common scenarios. I was forced to enter values for temperatures that we do not monitor, so I entered 0 |
| 37 | For the question, “When using the following ranges of hypothermia, what flow rate do you use?,” mL/kg/min is not constant between neonates and adults. Possibly revise |
| 38 | 1. No ACTs on bypass, heparin concentration measured with ABGs every 20–30 minutes. ACT only pre- and post-bypass and following heparin does prior to bypass. |
| 2. CP flow rate is pressure-dependent. | |
| 3. “Lowest patient weight (kg) you would consider a bloodless initiation of bypass?” depends on the procedure being done and calculated hemodilution. | |
| 4. HCT prior to termination varies and does not account for final HCT after minimum of 10 minutes of V-VMUF | |
| 39 | Very nice survey. There are a feq “it depend” questions that do not allow for flexibility. for example, blood product use depends on the HCT and procedure. Flow rates (at temperature)...we use an age-adjusted scale for flow calculations. CPG depends on the case and surgeon. Only one pathway available once selected. I assume it is implied to report the most common. Difference in conventional and non-conventional work days. CPG additives, not a none option. Not able to capture all the things people are doing, but a very nicely designed survey. Thank you |
| 40 | We used 3 VADs for support to transplant and 1 for support to home |
| 41 | Some answers could change by patient size, for example, flow rate calculation/temperature section. Patient flow does decrease by size. I answered using neo/peds, However, we use flow high on all patients. Circulation is preferred over nought |
| 42 | When using “low flow” bypass, we roughly follow the degree of hypothermia for flow rate |
| 43 | We are a satellite program of Seattle Children’s. Until 2016, we were able to prime our smaller circuits with citrated whole blood; it was great! Currently, we routinely blood prime for all children less than 10 kg. We attempt clear primes on kids greater than 10 kg |
| 44 | Under VAD types you included ECLS; is ECLS included in the question about post-cardiotomy VAD usage? This was unclear |
| 45 | I think each subset has a comments section just to address some answers that did not fit exactly into the provided answers |
| 46 | When asking questions about flow at certain temperatures, we should specify what weight/age-group as flows for neonates at 32°C which would be different to flows for 14-year-olds at 32°C. Also minimum weight to consider bloodless prime depends on patients stating hematocrit as well as weight |
| 47 | We use body surface area (m2) for plegia |
| 48 | To what core temperature, do you typically cool the patient when you use the following: Low flow depends on how long you are taking for low flow. |
| 49 | To what core temperature do you typically cool the patient when you use the following: Low flow depends on how long you are taking for low flow |
REFERENCES
- 1.Elliot M. Current paediatric perfusion practice in the UK. Perfusion. 1993;8:7–25. [DOI] [PubMed] [Google Scholar]
- 2.Itoh I, Pouard SS. Pediatric perfusion in Japan: 2010 practice survey. Perfusion. 2012;27:72–7. [DOI] [PubMed] [Google Scholar]
- 3.Charrie’re J, Pelissie’ M, Verd C, et al. Survey: Retrospective survey of monitoring/safety devices and incidents of cardiopulmonary bypass for cardiac surgery in France. J Extra Corpor Technol. 2007;39:142–57. [PMC free article] [PubMed] [Google Scholar]
- 4.Groom RC, Hill AG, Kurusz M, et al. Current paediatric perfusion practice in North America. Perfusion. 1993;8:27–38. [DOI] [PubMed] [Google Scholar]
- 5.Groom R, Hill A, Kurusz M. Pediatric perfusion practice in North America; an update. Perfusion. 1995;10:393–401. [DOI] [PubMed] [Google Scholar]
- 6.Cecere G, Groom R, Forest R. A 10-year review of pediatric perfusion practice in North America. Perfusion. 2002;17:83–9. [DOI] [PubMed] [Google Scholar]
- 7.Groom R, Froebe S, Martin J, et al. Update on pediatric perfusion practice in North America: 2005 survey. J Extra Corpor Technol. 2005;37:343–50. [PMC free article] [PubMed] [Google Scholar]
- 8.Harvey B, Shann K, Fitzgerald D, et al. International pediatric perfusion practice: 2011 survey results. J Extra Corpor Technol. 2012;44:186–93. [PMC free article] [PubMed] [Google Scholar]
- 9.Kurusz M. Lessons from perfusion surveys. Perfusion. 1997;12:221–7. [DOI] [PubMed] [Google Scholar]
- 10.Harris P, Taylor B, Thielkec R, et al. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris P, Taylor R. Minor B, et al. ; REDCap Consortium. The REDCap consortium: Building an international community of software partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Research Electronic Data Capture Consortium. REDCap Citations. Nashville, TN: Vanderbilt University. Available at: http://projectredcap.org/resources/citations/. Accessed May 9, 2019. [Google Scholar]
- 13.Confliffe J, Riley JB, Clutter J, et al. A report of perfusion staffing survey: Decision factors that influence staffing of perfusion teams. J Extra Corpor Technol. 2007;39:249–53. [PMC free article] [PubMed] [Google Scholar]
- 14.Stammers A, Mejak B. An update on perfusion safety: Does the type of perfusion practice affect the rate of incidents related to cardiopulmonary bypass? Perfusion 2001;16:189–98. [DOI] [PubMed] [Google Scholar]
- 15.Rodgers E. Diffusion of Innovations, 4th ed. New York, NY: Free Press; 1995:7–31. [Google Scholar]
- 16.Stammers A, Mejak B, Rauch E, et al. Factors affecting perfusionists’ decisions on equipment utilization: Results of a United States survey. J Extra Corpor Technol. 2000;32:4–10. [PubMed] [Google Scholar]
- 17.Naik SK1, Knight A, Elliott MJ. A successful modification of ultrafiltration for cardiopulmonary bypass in children. Perfusion. 1991;6:41–50. [DOI] [PubMed] [Google Scholar]
- 18.Kuratani N, Bunsangjaroen P, Srimueang T, et al. Modified versus conventional ultrafiltration in pediatric cardiac surgery: A meta-analysis of randomized controlled trials comparing clinical outcome parameters. J Thorac Cardiovasc Surg. 2011;142:861–7. [DOI] [PubMed] [Google Scholar]
- 19.Boodhwani M, Williams K, Babaev A, et al. Ultrafiltration reduces blood transfusions following cardiac surgery: A meta-analysis. Eur J Cardio Thorac Surg. 2006;30:892–7. [DOI] [PubMed] [Google Scholar]
- 20.Bierer J, Stanzel R, Henderson M, et al. Ultrafiltration in pediatric cardiac surgery review. World J Pediatr Congenit Heart Surg. 2019;10:778–88. [DOI] [PubMed] [Google Scholar]
- 21.Xing W, Liu YL, Yang KH, et al. Clinical effects of modified ultrafiltration during pediatric cardiac surgery: A systematic review. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:1665–9. [PubMed] [Google Scholar]
- 22.Kantathut N, Cherntanomwong P, Khajarern S, et al. Lactated Ringer’s as a base solution for del Nido Cardioplegia. J Extra Corpor Technol. 2019;51:153–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Talwar S, Bhoje A. Comparison of del Nido and St. Thomas cardioplegia solutions in pediatric patients: A prospective randomized controlled trial. Semin Thorac Cardiovasc Surg. 2017;29:366–74. [DOI] [PubMed] [Google Scholar]
- 24.Mishra P, Jadhav RB, Mohapatra CK, et al. Comparison of del Nido cardioplegia and St. Thomas hospital solution - two types of cardioplegia in adult cardiac surgeryKardiochir. Torakochirurgia Pol. 2016;13:295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talwar S, Chatterjee S, Sreenivas V, et al. Comparison of del Nido and histidine-tryptophan-ketoglutarate cardioplegia solutions in pediatric patients undergoing open heart surgery: A prospective randomized clinical trial. J Thorac Cardiovasc Surg. 2019;157:1182–92. [DOI] [PubMed] [Google Scholar]
- 26.Negi SL, Mandal B, Singh RS, et al. Myocardial protection and clinical outcomes in tetralogy of Fallot patients undergoing intracardiac repair: A randomized study of two cardioplegic techniques. Perfusion. 2019:34:495–502. [DOI] [PubMed] [Google Scholar]
- 27.Gorjipour F, Dehaki MG, Totonchi Z, et al. Inflammatory cytokine response and cardiac troponin I changes in cardiopulmonary bypass using two cardioplegia solutions; del Nido and modified St. Thomas’: A randomized controlled trial. Perfusion. 2017;32:394–402. [DOI] [PubMed] [Google Scholar]
- 28.Drury NE, Yim I, Patel AJ, et al. Cardioplegia in paediatric cardiac surgery: A systematic review of randomized controlled trials. Interact Cardiovasc Thorac Surg. 2019;28:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirsch JC, Jacobs ML, Andropoulos D, et al. Protecting the infant brain during cardiac surgery: A systematic review. Ann Thorac Surg. 2012;94:1365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hessel E, Groom RC. Guidelines for conduct of cardiopulmonary bypass. An editorial. J Cardiothorac Vasc Anesth. 2020;35:P1–17. [DOI] [PubMed] [Google Scholar]
- 31.American Society of ExtraCorporeal Technology Standards and Guidelines For Pediatric and Congenital Perfusion Practice, 2019. Chicago, IL: AmSECT. Available at: http://www.amsect.org/page/standards-and-guidelines-for-pediatric-and-congenital-perfusion-practice. Accessed May 17, 2020. [DOI] [PubMed] [Google Scholar]