Abstract
Crouch gait, a pathological pattern of walking characterized by excessive knee flexion, is one of the most common gait disorders observed in children with cerebral palsy (CP). Effective treatment of crouch during childhood is critical to maintain mobility into adulthood, yet current interventions do not adequately alleviate crouch in most individuals. Powered exoskeletons provide an untapped opportunity for intervention. The multiple contributors to crouch, including spasticity, contracture, muscle weakness, and poor motor control make design and control of such devices challenging in this population. To our knowledge, no evidence exists regarding the feasibility or efficacy of utilizing motorized assistance to alleviate knee flexion in crouch gait. Here, we present the design of and first results from a powered exoskeleton for extension assistance as a treatment for crouch gait in children with CP. Our exoskeleton, based on the architecture of a knee-ankle-foot orthosis, is lightweight (3.2 kg) and modular. On board sensors enable knee extension assistance to be provided during distinct phases of the gait cycle. We tested our device on one 6 year old male participant with spastic diplegia from CP. Our results show that the powered exoskeleton improved knee extension during stance by 18.1° while total knee range of motion improved 21.0°. Importantly, we observed no significant decrease in knee extensor muscle activity, indicating the user did not rely solely on the exoskeleton to extend the limb. These results establish the initial feasibility of robotic exoskeletons for treatment of crouch and provide impetus for continued investigation of these devices with the aim of deployment for long term gait training in this population.
Keywords: Exoskeleton, Rehabilitation Robotics, Crouch Gait, Orthosis, Knee
I. Introduction
Cerebral palsy (CP) is a group of motor disabilities caused by a perinatal non-progressive injury to the central nervous system. CP is the most common pediatric motor disorder, affecting between 1–4 per 1000 live births [1–3]. Individuals with CP present a variety of disabilities including spasticity, rigidity and diminished coordination and motor control [4]. Individuals with CP are often partitioned into subgroups based on the type and distribution of motor impairments. One of the most common subgroups is spastic diplegia [2, 3], in which pathologic gait patterns are frequently observed [5]. Crouch gait, which is characterized by excessive stance phase knee flexion and may be accompanied by other deficits at the hip and/or ankle, is one of the most frequently observed gait deviations [6]. Crouch gait can lead to joint pain [7] and degenerative arthritis [8] due to elevated joint contact forces [9], bony deformities [10], and increased risk for falls from inadequate foot clearance [11]. Additionally, crouch gait is less efficient than normal walking patterns [12] and many experience deterioration of walking ability with age [13], leading to a loss of ambulation in a large portion of the population [14].
The most common treatments for crouch gait include surgery, botulinum toxin injections, physical therapy/strengthening, and orthotic interventions. Outcomes from surgical interventions, which typically target the hamstrings, are variable [15, 16]. Recently, distal femoral extension osteotomy procedures have shown improvement of knee extension in short-term follow up, particularly when combined with patellar tendon advancement [17], offering a potentially effective but more invasive solution. One reason for the mixed surgical outcomes may be heterogeneity underlying the crouch gait pattern in diplegic CP. In addition to hamstring spasticity, anti-gravity (extensor) weakness clearly contributes to the deterioration of walking capabilities as a function of age. However, like surgery, muscle strengthening programs have inconsistent outcomes [18, 19]. Achieving and maintaining adequate strength levels in individuals with motor disabilities is a challenging problem that is exacerbated because ambulatory children with CP are less active than children without disabilities [20]. Orthotic bracing, aimed to increase mobility of individuals with crouch gait, may block or restrict motion at the ankle and or the knee joint to provide passive weight support or to suppress unwanted motions. Traditional and floor reaction ankle-foot orthoses (AFO and FRAFO, respectively) have been shown to temporarily improve knee extension and spatiotemporal gait parameters while worn [21, 22]. However, long term use can lead to greater weakness in the restricted muscle groups over time. Treadmill based robotic assisted therapy offers the advantage of repetitive practice. Improved function has been reported in pilot studies of treadmill based training in CP [23, 24], but their overall effectiveness compared to traditional therapies of equal intensity is equivalent [25, 26]. Furthermore, these strategies are limited in the environment and duration in which they can be applied and have not been explicitly studied for remediation of crouch gait. Functional electrical stimulation (FES) devices have been applied to treat crouch gait in children with CP with limited success using percutaneous [27] and surface [28] electrodes to stimulate extensor muscles; however, the intensity of stimulation required to extend the flexed knee can be difficult to reach.
There is a clear need for more effective interventions which can preserve or augment strength on a continuous basis for those with crouch gait from CP. The recent surge in powered exoskeleton technology [29, 30] provides one avenue for exploration. To date, the primary design focus of these wearable robotic devices has been on restoring lost function due to paralysis, e.g. from spinal cord injury [31, 32] or stroke [33], to assist with load carriage [34], and enhance normal walking efficiency [35]. Transfer of these wearable technologies to pediatric populations has been limited. Challenges also exist for designing an exoskeleton specifically to treat crouch gait. The conditions that accompany or contribute to crouch, such as spasticity, contracture, instability, poor motor control, and muscle weakness make the effects and efficacy of introducing motorized assistance unclear in this population. Here, we present a new powered exoskeleton specifically designed for rehabilitation of crouch gait in children with CP. Our design is modular and can optionally incorporate surface electrical stimulation or accommodate the replacement of the powered motor assistance with a passive damper at the knee. The exoskeleton is designed to provide powered assistance to knee extension during stance and/or swing phases of gait. The overall goal of the exoskeleton project is twofold. First, we seek to investigate the effects of powered knee assistance as a treatment strategy for children with crouch gait from CP. Second, we plan to utilize the powered exoskeleton as a test bed to enhance current understanding of the neuromuscular causes underlying crouch gait with an eye towards improved design of future interventions. In addition to design description, this paper presents an initial functional and clinical evaluation of our device as a rehabilitation tool in a single participant with crouch gait from CP.
II. Methods
A. Design Concept
The primary objective of our research was to build an assistive device to alleviate persistent knee flexion arising from crouch gait in children with CP. Our design was conceptualized as a wearable robot that provides on-demand assistive torque at the knee joint to facilitate knee extension during walking while preserving (or enhancing) muscle activity of the user. The choice to focus on the knee joint was not trivial. While pronounced knee flexion is the hallmark symptom of crouch gait in CP, the musculoskeletal system is a complex linkage with muscles spanning or influencing motion at multiple joints; thus, virtually all lower extremity muscles can induce accelerations at the ankle, knee, and hip and therefore have an impact on the flexed posture observed in this population. Previous studies have shown that the capacity of hip and knee extensor muscles to extend the limb is reduced in a crouched posture while the flexion accelerations induced by gravity are increased [36]. The result is persistent activity of extensors throughout stance phase and transference of responsibility for forward progression to more proximal muscle groups [37]. These observations suggest that dynamically changing the posture during walking could enhance the muscles’ ability to extend the limbs. Taken together with clinical evidence suggesting that targeted strength training of knee extensors can increase knee extension during walking [38], particularly in the absence of hamstring spasticity [19], we believe that a powered exoskeleton that provides dynamic extension assistance at the knee could reduce excessive knee flexion and facilitate more normal, but appropriate knee extensor activity. When worn for an extended period of time, such a device may have therapeutic benefit in terms of muscle strengthening and improved motor coordination, ultimately eliminating the need to wear the exoskeleton.
B. Hardware and Electronics
The varying postures and physical deformities present in children with CP, combined with the heterogeneous causes of crouch gait, necessitate the design of a human-machine interface which can be customized to each individual. We based our design around the architecture of a knee-ankle-foot orthosis (KAFO) containing custom molded thermoplastic shells for the foot, shank, and thigh segments connected by rigid uprights mounted on the lateral side of the leg. KAFOs employ joint mechanisms in parallel with anatomical joint centers to allow (or control) segment motion.
To generate torque at the knee joint, we designed a custom motor assembly (Figure 1) that was light weight, low-profile, and capable of providing a reasonable level of assistance in most children and young adults with crouch gait. Previously, we found that internal knee extensor moment scales with the degree of crouch and body mass [39], but rarely exceeds 50 Nm for individuals that would be candidates for the proposed device (i.e. children with mild-moderate crouch). As such, peak torque output was specified to provide 1/3rd of the internal demand on knee extensors for mild-moderate crouch (~16 Nm). Using a transmission to increase torque output comes at the cost of reduced speed capabilities. The motor transmission system was designed to achieve a no-load speed similar to peak angular velocity during swing phase knee extension, which can exceed 300 °/sec.
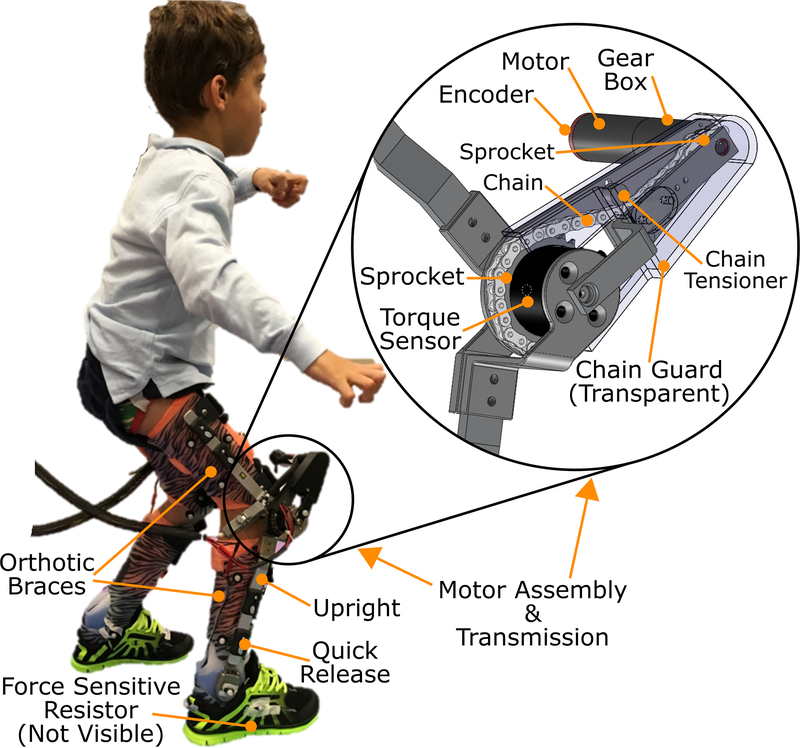
Fig. 1.
Photograph and schematic of the robotic exoskeleton for knee extension assist to alleviate crouch gait from cerebral palsy.
The motor assembly was comprised of a back-drivable 24 V, 90 W brushless motor with a 3-stage, 89:1 reduction planetary gear head and an embedded quadrature encoder (EC-powermax, Maxon Motors, Fall River MA). A second stage, chain sprocket transmission with a 3.5:1 speed reduction transmits torque from the inline shaft of the motor to the knee center of rotation. The maximum rated output torque of the assembly is approximately 16.1 Nm with a 3.82 A maximum continuous current draw. A torque sensor (Transducer Techniques, Temecula, CA) is mounted on the drive shaft at the knee. The assembly is modular such that it can be attached to the lateral upright of any KAFO. To facilitate unrestricted knee motion within the physiological range, the motor is mounted anterior and superior to the knee joint (Figure 1). The motor assembly unit is connected to the lower (shank) and upper (thigh) uprights by custom designed brackets. Each upright connects to the thermoplastic shell via quick release attachments to facilitate speedy removal and attachment after they have been donned by the user.
A commercially available, adjustable dynamic response (ADR; Ultraflex Systems, Pottstown PA) ankle joint mechanism mounted on the lateral upright connects the foot and shank components. The ADR device can be freed to allow unencumbered ankle motion, locked to restrict motion, or adjusted to provide dynamic assistance. A force sensitive resistor (FSR, Interlink Electronics, Westlake Village, CA) mounted on the foot of the brace provides information regarding foot-floor contact. The thermoplastic foot plate is worn inside the shoe. The mass properties of the exoskeleton are provided in Table I.
TABLE I.
Mass Properties of the Exoskeleton
| Segment | Weight (kg) | Ixx (kg·m2) | Iyy (kg·m2) | Izz (kg·m2) |
|---|---|---|---|---|
| Foot | 0.11 | 0.0003 | 0.0007 | 0.0008 |
| Shank | 0.31 | 0.0147 | 0.0006 | 0.0145 |
| Thigh | 1.16 | 0.0103 | 0.0075 | 0.0098 |
Total exoskeleton weight per limb = 1.58 kg. Control box weight = 1.96 kg. The axes are defined as follows: x: anterior-posterior; y: superior-inferior; z: medial-lateral.
The exoskeleton control box contains custom designed circuitry that provides power and signal conditioning for sensors as well as feedback control of the motors. Commercially available servocontrollers (ESCON, Maxon Motors) enable closed loop current (torque) control of the exoskeleton motors and knee joint position feedback based on encoder quadrature. Knee angle, FSR, and joint torque signals are input into a feedback control system implemented in a single board computer (Cool RoadRunner LX800, LiPPERT Embedded Computers, Vista, CA) running Debian Linux with Xenomai kernel for real-time control. Signals are transmitted to and from the exoskeleton via a multifunction A/D data acquisition board (PC /104, Sensoray, Portland, OR). The exoskeleton can be tethered to a DC power source for extended use in the lab setting or powered by a 22.2 V lithium-ion battery. The total weight of the control box is 1.96 kg.
C. Software Based Controller
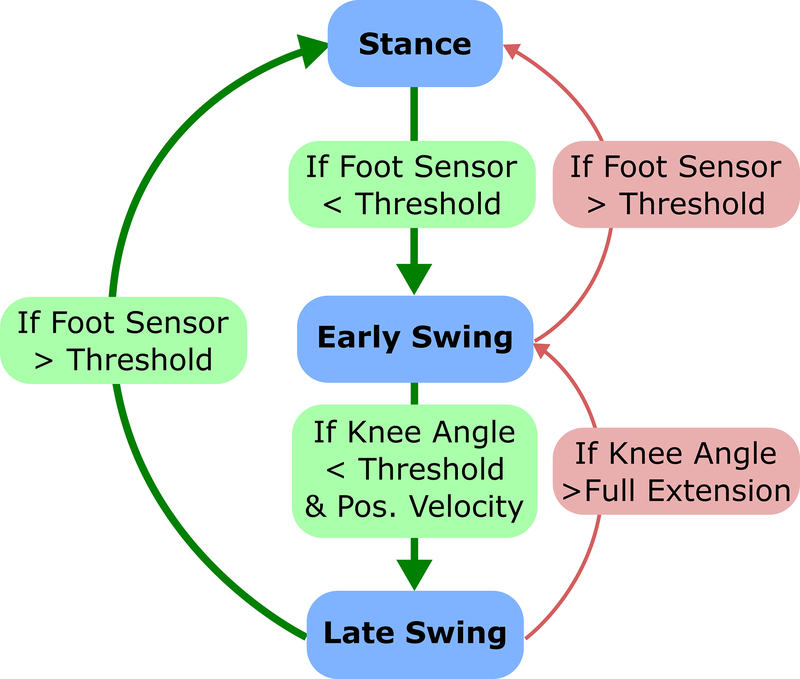
Rule-based hierarchical control, as implemented in a finite state machine, has been previously deployed for control of prosthetics, FES systems, controllable orthotics, and powered exoskeletons which restore or assist walking [40–43]. Splitting the gait cycle into discrete states based on detected gait events provides enhanced consistency and robustness to an inherently variable process and allows implementation of lower level controllers within each state. To control our powered exoskeleton, we developed a finite state machine (FSM) which divided the gait cycle into three discrete phases: stance, early swing, and late swing (Figure 2). FSM thresholds were based on previously available data from children with mild-moderate crouch [36], and were verified and adjusted (if necessary) during the first walking session. The FSR was used to govern the transition between stance and swing states based on foot ground contact. Knee angle position and velocity, computed as first derivative, were used to detect transition between early and late swing phases. A proportional-integral-derivative (PID) control scheme was used to achieve the desired torque output at the knee within each state. The PID error value was computed as the difference between the measured and desired torque. The PID gains were tuned manually during bench-top testing prior to the participant donning the device. They were adjusted so torque output reached a stable response with minimal chatter and latency. The gains were the same for all experimental sessions. The torque set point remained constant during a given state based on the implemented control scheme as discussed below. When the set-point was zero, as in early swing, the PID controller compensated for the inherent friction of the motor-transmission assembly, allowing for the knee joint to be freely articulated (i.e. frictionless).
Fig. 2.
Diagram of the FSM used to specify the desired knee extensor assistance at different phases across the gait cycle. The states included stance, early swing, and late swing. The stance state was identified by a reading from the foot sensor greater than 60% of the value measured during quiet standing. The early swing state was identified by a foot sensor reading less than 60% of the value measured during quiet standing and/or if the knee angle surpassed full extension while the foot sensor remained below the threshold (indicating swing). The late swing state was identified by a positive angular velocity of the exoskeleton knee joint (extension) when the knee was flexed greater than 30 degrees.
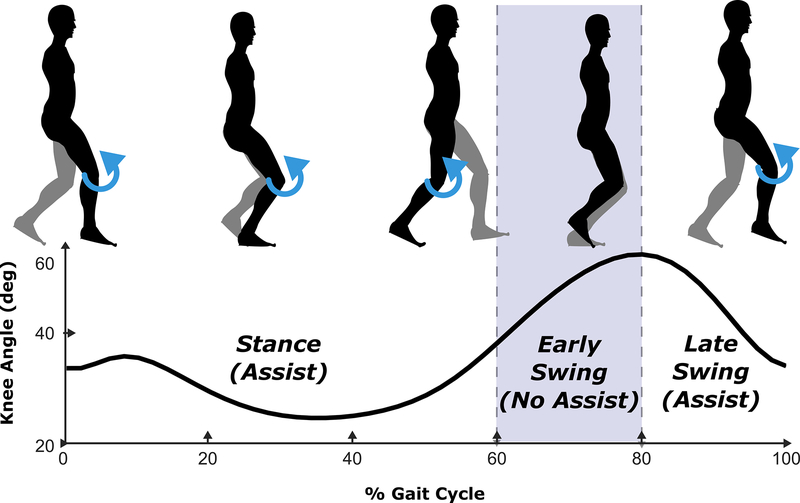
For this initial study, we investigated a straightforward control strategy that provides knee extension assistance at different intervals within the gait cycle (Figure 3). The exoskeleton provided extension torque at the knee during stance phase to assist body weight support and during the late-swing phase to assist knee extension for foot placement. The control strategy implemented a frictionless (zero torque) period during early swing to facilitate knee flexion for toe-clearance.
Fig. 3.
Diagram of the assistance (blue) provided by the exoskeleton relative to different phases of the gait cycle. The motor controller operated in a free (frictionless) state during the early-swing phase to allow for natural knee flexion required for toe-clearance during swing.
D. Device Validation and Clinical Evaluation
To validate the functional performance and preliminary efficacy of our exoskeleton, one male participant diagnosed with crouch gait from CP was recruited. He was classified level II on the gross motor function classification system (GMFCS) and was able to walk for at least 30 feet without assistance. At the time of data collection, he was six years old, height of 118 cm, and weight of 20.0 kg. Prior to data collection, we obtained informed consent and assent from the parent and participant, respectively. This study was approved by the Institutional Review Board at the National Institutes of Health.
The participant completed 5 visits at the NIH. The first visit included a clinical assessment of lower limb strength and spasticity, over-ground gait assessment of his baseline gait condition while wearing prescribed AFOs, and casting for fabrication of custom orthotic braces. The initial visit was followed by three practice sessions, during which the participant walked with the powered exoskeleton over-ground and on a treadmill, and a final visit for data collection. When using the exoskeleton, the participant was instructed to walk in a manner similar to their normal walking habits.
During the practice visits, the exoskeleton torque output was adjusted upwards from 1Nm at 0.5 Nm increments. Final values of 3.5 Nm for stance phase and 2.625 Nm (75% of the stance value) for swing were established based on participant preference and visual feedback of participant comfort and walking stability. Total walking time in the exoskeleton was 18 minutes across the four sessions. Frequent short breaks were given to reduce fatigue and maintain participant attention and focus on the walking task. Total walking time does not include experimental setup, device tuning and calibration, or walking in control conditions (i.e. without powered exoskeleton assistance).
Several precautions were implemented to insure patient safety. An unweighted safety line that connected from an overhead track to a gait belt around the torso was in place in case of a fall. An emergency stop button providing the ability to cut power to the device was held by a researcher at all times. The motor controller also incorporated several safety measures that limited the maximum torque output and prevented knee hyperextension.
The fifth visit was for experimental data collection with the exoskeleton under two conditions: a free knee joint (no motor) and the assistive mode with extensor torque set at 3.5 Nm for stance phase and 2.625 Nm (75% of the stance value) for swing (Figure 3). The purpose of these conditions was to assess the effect of the brace and motor on gait separately. The ankle joint was set for free rotation in both conditions. The participant completed 3 successful overground walking bouts along a 5.5 m pathway in each mode. Researchers held the control box of the exoskeleton, which was tethered to a power supply to assure uninterrupted operation during lab testing. Kinematic data were collected at 100 Hz using 10 motion capture cameras (Vicon Motion Systems, Oxford, UK) and a custom marker set similar to [44]. Specifically, we placed 3 markers on the foot, clusters comprised of 4 non-collinear markers on the shank and thigh segments, 4 markers on the pelvis, and 3 on the trunk. Markers were also placed on the medial and lateral aspects of the ankle and knee joints. Three markers were placed on each shank and thigh segment of the orthotic brace. Muscle activity was collected bilaterally from rectus femoris, vastus lateralis, semitendinosus, and medial gastrocnemius using a wireless EMG system (Trigno, Delsys, Boston, MA) recorded at 1000 Hz. EMG data were band-pass filtered at 15–380 Hz, full-wave rectified, and low-pass filtered at 7 Hz to create a linear envelope [45]. At the beginning of each trial, a pulse was sent from the exoskeleton control box to the Vicon system to synchronize motion capture data (marker trajectories, ground reaction forces, and EMG) with data from the exoskeleton sensors (torques, angles, and controller states). Lower-extremity joint angles were computed from marker trajectories using inverse kinematics implemented in Visual 3-D software (C-Motion, Gaithersburg, MD). Experimental data were time normalized to each gait cycle using linear interpolation, and averaged across the gait cycles for each walking condition.
E. Statistical Analysis
One-factor ANOVAs were used to compare differences in knee joint kinematics and EMG across exoskeleton assistive modes. Post-hoc comparisons were made using the Tukey-Kramer method if a significant main effect was found.
III. Results
A. Exoskeleton Performance Validation
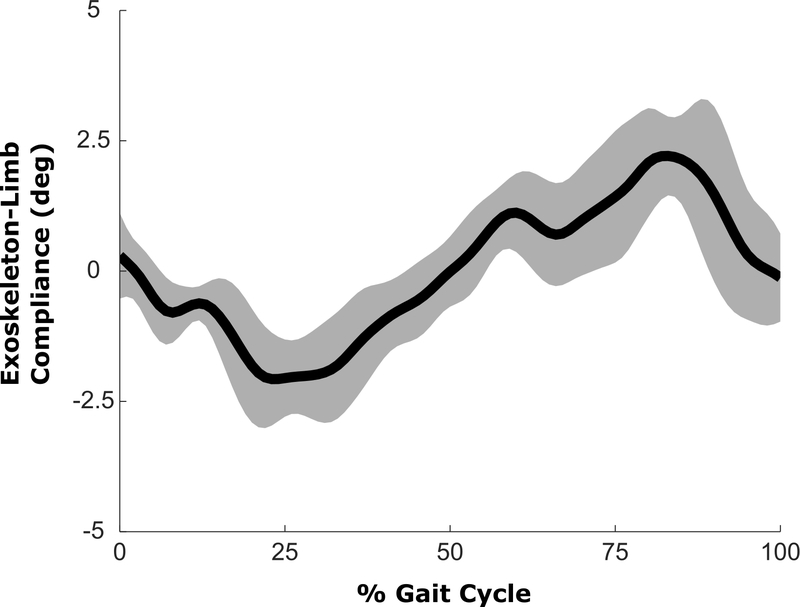
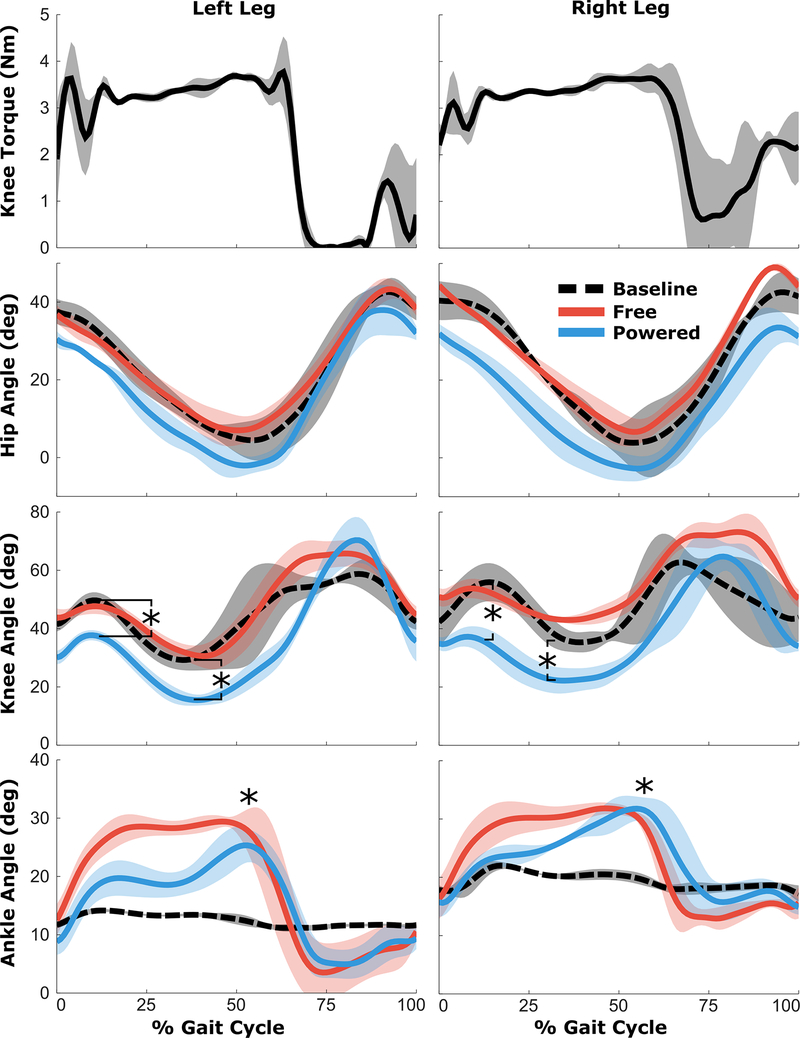
We measured the latency of the robotic exoskeleton by attaching the uprights to a solid extruded aluminum frame, creating an isometric condition under which timing of torque application could be quantified. The on-off and off-on latency was measured as the time between when the controller triggered the transition and when torque measured by the onboard sensor reached the target value. The average latency, across 10 trials, was 53 ± 8 ms for activation and 57 ± 7 ms for deactivation. The compliance between the brace and limb was evaluated by comparing sagittal plane knee joint angle computed from the exoskeleton markers with the biological knee angle computed from markers placed directly on the skin. The difference in knee angle was not significant (p > 0.99) indicating minimal motion between the body and exoskeleton (Figure 4). The torque provided by the powered exoskeleton was consistent with the design specifications (Figure 5). Swing phase assistance was provided only after volitional knee extension began. Swing phase knee extension was delayed in the left leg compared to the right leg, which resulted in a reduced interval during which swing extension assistance could be applied to the left limb prior to heel-strike.
Fig. 4.
Mean exoskeleton angular compliance, computed as the difference between the knee joint angle measured from the exoskeleton and the knee joint angle measured from the biological segments during the powered assistance condition. The shaded regions represent ±1SD from the mean.
Fig. 5.
Top Row) Mean knee extensor torques across the gait cycle. Bottom Rows) Mean sagittal plane hip, knee, and ankle joint angles across the gait cycle during the baseline condition (black-dashed), the free knee condition (red), and the powered exoskeleton (blue) conditions. The shaded regions represent ±1SD from the mean. * Indicates significant difference between the respective exoskeleton condition and baseline (p<0.05).
B. Joint Kinematics & Spatiotemporal Measures
The experimental biomechanics data from overground walking were screened to ensure that only complete gait cycles free from adverse events (e.g. toe-drag) were included in the subsequent analyses. The total number of analyzed gait cycles for each condition is shown in Table II.
TABLE II.
Kinematic, Muscle Activity, and Spatio-temporal Parameters from the Clinical Evaluation of the Exoskeleton
| Baseline AFO | Free | Powered | ||||
|---|---|---|---|---|---|---|
| R | L | R | L | R | L | |
| Number of Gait Cycles | 3 | 4 | 4 | 5 | 4 | 4 |
| Max Knee Flexion Angle Stance (°) | 56.0 (6.5) | 49.7 (2.8) | 54.1 (3.4) | 48.1 (2.6) | 37.4 (3.6) | 37.8 (1.8) |
| Peak Knee Extension Angle Stance (°) | 41.3 (5.3) | 31.4 (3.4) | 42.6 (1.7) | 29.8 (3.2) | 23.2 (4.7) | 19.9 (3.0) |
| Knee Range of Motion (°) | 32.3 (10.1) | 34.4 (6.5) | 32.5 (5.8) | 36.5 (7.6) | 47.6 (4.4) | 55.4 (8.9) |
| Max Hip Extension Angle (°) | 2.0 (5.4) | 3.6 (3.7) | 6.3 (3.1) | 6.1 (3.7) | −3.4 (3.3) | −2.3 (2.4) |
| Max Ankle Dorsi-Flexion Angle (°) | 22.1 (0.6) | 14.2 (0.3) | 32.4 (1.1) | 30.3 (1.5) | 41.4 (2.7) | 26.0 (2.4) |
| Step Length (cm) | 32.9 (1.8) | 35.0 (6.1) | 25.5 (4.5) | 25.8 (6.5) | 22.3 (5.3) | 27.3 (3.4) |
| Step Width (cm) | 18.4 (4.3) | 21.3 (7.9) | 16.8 (2.1) | 16.3 (4.1) | 20.6 (2.6) | 19.6 (3.0) |
| Cadence (Steps/min) | 152 (3) | 135 (6) | 108 (17) | 106 (7) | 113(14) | 98 (34) |
Values are mean (SD). Bold indicates a significant difference from the baseline condition (p<0.05).
To assess the effects of motorized extension assistance on the participant’s gait, kinematics (joint angles) and spatiotemporal parameters were calculated under three conditions (Figure 5): shod + AFO (baseline), walking with the exoskeleton brace with a free knee joint (free), and walking with assistance from the powered exoskeleton (powered). There was a significant increase in total knee range of motion and knee extension during stance phase when walking with the powered exoskeleton compared to the free knee and baseline conditions (Table II). Compared to baseline, the powered exoskeleton increased peak knee extension during stance by 18.1° and 11.5° in the right and left leg, respectively. Maximum knee flexion during stance was decreased in the exoskeleton condition by similar levels (18.6° and 11.9°) compared to baseline. There was no significant difference in the hip angle between the baseline, free knee, and powered exoskeleton conditions. However, peak ankle dorsiflexion was increased significantly for both the free knee and powered conditions (Table II) due to the free ankle joint compared to the rigid AFO at baseline. Yet, there was no significant difference in knee extension during stance or knee range of motion between the baseline and free conditions. There were no significant differences in step length or step width between walking conditions. Cadence was significantly reduced in both the free knee and powered exoskeleton conditions compared to baseline.
C. Muscle Activity
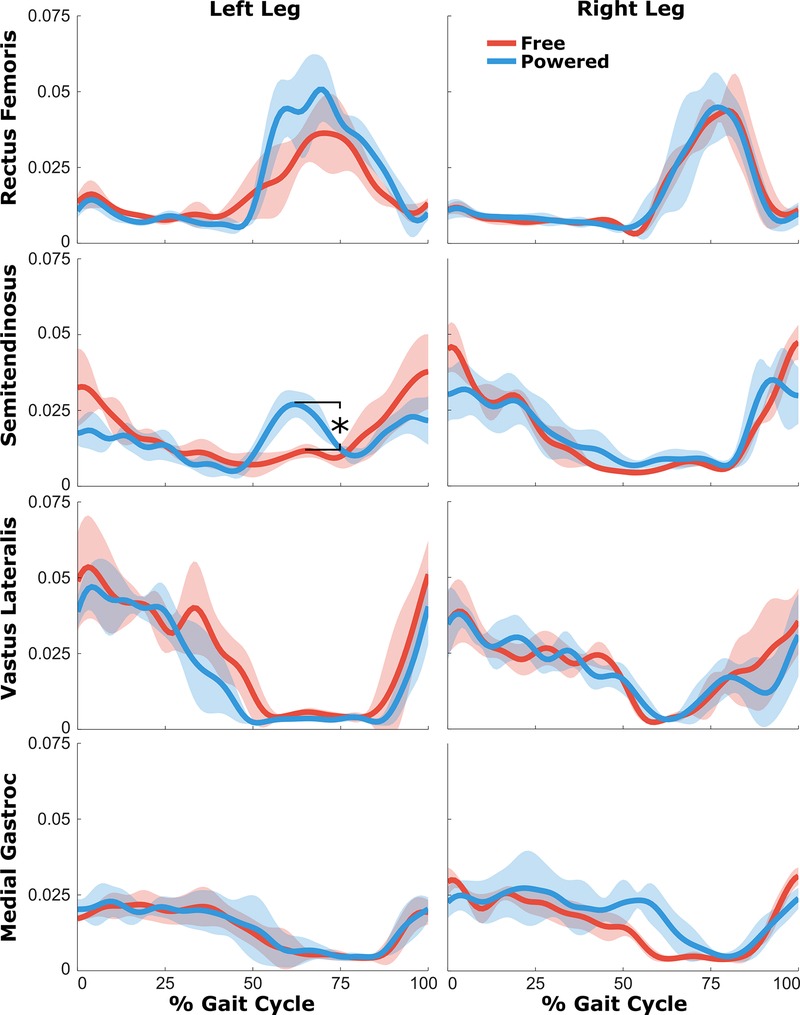
Walking with the powered exoskeleton affected EMG activity of several muscles compared to walking with the brace with a free knee joint (Figure 6). There was elevated activity during late stance phase in both rectus femoris and semitendinosus in the powered condition. Post-hoc tests showed a significant increase in peak semitendinosus linear envelope during late stance (p < 0.001). Importantly, the robotic exoskeleton did not have a large effect on the vastus lateralis activity, which maintained similar levels during late-swing and stance phases when assistance was provided. Likewise, no differences in medial gastrocnemius EMG were observed between the free knee and powered conditions.
Fig. 6.
Mean EMG linear envelopes (in mV) across the gait cycle during the free knee (red) and powered exoskeleton (blue) conditions. The shaded regions represent ±1SD from the mean.
IV. Discussion
We report here the design and first application of a powered exoskeleton during walking for treatment of crouch gait in children with CP. During this preliminary evaluation we examined the effects of relatively straightforward knee extension assistance during stance and late-swing phase. Our analysis shows that our FSM control strategy activated and deactivated the assistance during walking with latencies of similar duration to those of biological processes (e.g. muscle contractions), and therefore may be well-suited for adequate human-machine interaction. One potential challenge in exoskeleton design is relative motion between the limbs and the brace when assistive torque is applied. Our data indicate that our exoskeleton design and implementation suitably mitigated this issue as we observed no significant motion of the exoskeleton relative to the underlying limb at the knee joint in one individual. Owing to the patient’s weight (20.0 kg), our target torque levels were small but sufficient to demonstrate the functional capacity of our device to elicit the desired outcomes (i.e., increased knee extension during stance). The torque set-point during each assistive phase was held constant at 3.5 Nm for stance and 2.6 Nm for swing, respectively. Even though the torque was constant for each state, the resulting kinematic profile maintained relatively normal waveforms, including continuous knee extension for foot placement and an early stance loading response. While more sophisticated control methods are possible [39], the primary goal of this study was to assess the feasibility of injecting additional energy into the gait cycle to alleviate crouch.
The powered exoskeleton significantly altered lower extremity kinematics and reduced the amount of crouch compared to the baseline condition. The subject walked in a more extended, upright posture; we observed significant increases in our primary outcome variable, maximum knee extension during stance phase in both the left and right legs. The improvement (12–19°) was similar to the range of long-term improvement reported from invasive surgical treatment (10–20°) [46]. Peak stance phase knee flexion was also significantly reduced in both legs when walking with the robotic exoskeleton. The participant displayed baseline asymmetry with slightly less crouch during stance in the left leg. Our results suggest that the assistive torque, set to the same value in both legs, had a larger effect on stance posture in the more affected limb. Future work will focus on more sophisticated methods for delivery of assistive torque, including varying levels of assistance between limbs and adjusting the amount of torque applied within each phase in real-time.
The overall knee range of motion for the entire gait cycle was significantly increased during walking with the powered exoskeleton, resulting in a trajectory that was closer to that of normal waking. While there were gains in knee extension during stance, we also observed an increase in knee flexion during swing phase with both the powered exoskeleton and the free knee condition. One possible explanation for the increased swing phase knee flexion is the additional mass of the exoskeleton bracing, which may have resulted in increased knee flexor activity to assure toe clearance in the novel walking task. Our results at the knee highlight the complex interactions between the different joints of the lower limbs during walking. A full evaluation of these interactions and more detailed assessment of the exoskeleton effects on full lower extremity kinematics and muscle activity are planned in the future.
Interestingly, the increased knee ROM during the exoskeleton walking condition may have mitigated the kinematic benefit of extension assistance provided by the motor. A more flexed knee during swing increases the late swing extension requirement as the limb must articulate through a larger ROM. The transition from swing phase flexion to swing phase extension was delayed in the left leg compared to the right, limiting the time available to provide swing phase assistance from the exoskeleton. Peak swing knee extension velocity in the left leg was approximately 350°/sec, resulting in motor shaft velocity of 18,160 revolutions per minute (RPM), which exceeded the no load speed of the motor (16,300 RPM or 314°/sec) and therefore limited the ability to provide extension assistance in this phase (Figure 5). Swing phase knee extension velocities in the right leg were less than 300°/sec. In future implementations, a higher power motor with a lower gear reduction may be deployed at the expense of additional weight and energy usage. Our torque set-points were also relatively small and selected according to patient preference and visual observation of walking stability. We were able to slowly increase the torque levels over the 3 practice sessions. Still, the participant walked with reduced cadence while using the exoskeleton compared to their baseline gait, a somewhat expected finding given the novelty of the task that may be improved with additional time for accommodation to the exoskeleton. Considering the limited time the participant walked with the exoskeleton, it is likely that continued use would result in increasing comfort levels, the opportunity to increase the assistance, and further kinematic improvements.
We observed few differences in EMG during walking with the powered exoskeleton. In the left leg, elevated hip and knee flexor activity at the end of stance phase and in early swing (50–75% of the gait cycle) was observed. The knee extensor torque provided by the motor at the end of stance may have triggered this undesirable antagonistic flexion response. Importantly, we observed no differences in knee extensor activity during stance or swing phase extension assistance in either leg. At the end of the stance phase in the left leg, vastus lateralis activity ceased earlier when using the powered exoskeleton compared to the free knee. This pattern of activity more closely resembles that of normal walking, and offers the possibility that long term use of the exoskeleton may help facilitate elimination of the persistent extensor activity during stance phase of crouch.
A critical, but still unanswered, question exists regarding the effect of robotic assistance during walking on muscle activity. Previous studies of have demonstrated that the addition of robotic assistance during treadmill walking in neurologically intact populations does not diminish muscle activity, and may in fact increase it [47]. The same effect has been observed in lower extremity exoskeletons which provide assistance to multiple joints [48] and which target the knee specifically [49]. However, studies of individuals with neurological insults resulting in diminished function suggest the opposite effect; namely that EMG activity is decreased when robotic assistance is applied [50]. While further investigation is needed, our results offer preliminary evidence that children with crouch gait from CP do not significantly reduce their knee extensor activity when walking with powered extension assistance from an exoskeleton in the short-term. The participant did not simply allow the motors to take over control of knee extension responsibilities and kinematic improvements were observed. Careful analysis of all lower extremity EMG over an extended duration and across multiple individuals will be paramount going forward, because maintaining the user’s volitional muscle activity is a crucial element for the long-term success of these devices as a rehabilitation tool. Another potential barrier to use of exoskeletons in children is device size and weight, however our results from the free knee and powered exoskeleton conditions show that additional inertia from the exoskeleton did not impede ambulation. These results are in agreement with recent studies showing gait function in children with CP is robust to moderate increases in limb masses from assistive devices [51].
Several limitations warrant discussion. First, a single participant was included in this analysis to evaluate the function and performance of our exoskeleton in a clinical setting. As a result of this successful evaluation, we are conducting a larger, ongoing study, designed to investigate the effects of powered knee assistance as a treatment strategy for children with crouch gait from CP with a broader range of size and severity. Another limitation was that the participant did not carry the control box and we used a tethered power supply. In this initial study, we prioritized evaluation of motorized knee extensor assistance in this population prior to adding additional burden. Under studied conditions, a 22.2V, 2500mAh battery weighing 0.75 kg could operate the exoskeleton for over an hour. Future work will prioritize reducing device weight and power requirements, and streamlining embedded electronics to enable device use outside the laboratory setting. An additional challenge for long-term use of the device in children is growth over time. While custom-molded braces were used in this study, future versions may contain orthotics that will not require custom molding to be individually adaptable. This may have the added benefit of reduced cost and longer-term adaptability.
In conclusion, this study demonstrated the potential for a powered exoskeleton to reduce excessive knee flexion during crouch gait from CP without a significant reduction in volitional EMG activity. Owing to the heterogeneity of crouch and CP in general, our future work will focus on assessment of exoskeletons in a broad cohort. A major advantage of the exoskeleton as a therapeutic intervention is that the control strategy can be tuned to optimize the assistance for each individual. Our modular design allows future integration of additional modes of assistance, including passive support at the ankle and surface electrical stimulation. We anticipate eventual deployment of this exoskeleton as a wearable training device outside of the lab, providing the opportunity to increase dosage levels of gait therapy far beyond those in existing treadmill based paradigms.
Acknowledgment
This research was supported by the Intramural Research Program of the NIH Clinical Center (protocol # 13-CC-0210). We also acknowledge the contribution of Mark DeHarde of Ultraflex Systems (Pottsville, PA) for providing the braces used in this study, his expertise optimizing the fit and settings of the orthosis, and constructive feedback on the exoskeleton design.
Biography

Zachary F. Lerner received the Ph.D. degree in biomedical engineering from Colorado State University, Fort Collins, CO in 2015. He is currently a postdoctoral fellow in the Functional & Applied Biomechanics Section of the Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD.

Diane L. Damiano received the Ph.D. degree in research methods/biomechanics from the University of Virginia, Charlottesville, in 1993. She is currently the Chief of the Functional and Applied Biomechanics Section in the Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD.

Hyung-Soon Park (M’05) received the Ph.D. degree in mechanical engineering from the Korea Advanced Institute of Science and Technology, Daejeon, Korea. From 2009–2013, he was a staff scientist with the Rehabilitation Medicine Department at the National Institutes of Health, Bethesda, MD, USA. He is now an Associate Professor in the Mechanical Engineering Department, Korea Advanced Institute of Science and Technology, Daejeon, Korea. His current research interests are application of robotics and control technology for effective neuro-rehabilitation, and study of neuromuscular impairments post brain injury.

Andrew J. Gravunder received the B.S. and M.S. degrees in biomedical engineering from Catholic University, Washington DC. He is currently a Research Engineer in the Functional and Applied Biomechanics Section in the Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD.

Thomas C. Bulea (S’10-M’12) received the B.S. in mechanical engineering from The Ohio State University, Columbus, OH and the M.S. and Ph.D. degrees in biomedical enagineering from Case Western Reserve University, Cleveland, OH. He completed a post-doctoral fellowship at the National Institutes of Health and a visiting post-doctoral fellowship at University of Houston. He is currently a staff scientist in the Functional and Applied Biomechanics Section of the Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD. His research focuses on integration of neural interfacing and rehabilitation robotics to develop new therapeutic tools and interventions for treatment of movement disorders and paralysis.
Contributor Information
Zachary F. Lerner, Functional & Applied Biomechanics Section of the Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD 20892
Diane L. Damiano, Functional & Applied Biomechanics Section of the Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD 20892
Hyung-Soon Park, Department of Mechanical Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, South Korea.
Andrew J. Gravunder, Functional & Applied Biomechanics Section of the Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD 20892
Thomas C. Bulea, Functional & Applied Biomechanics Section of the Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD 20892.
References
- [1].Himpens E, Van den Broeck C, Oostra A, Calders P, and Vanhaesebrouck P, “Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: a meta-analytic review,” Dev Med Child Neurol, vol. 50, pp. 334–40, May 2008. [DOI] [PubMed] [Google Scholar]
- [2].Odding E, Roebroeck ME, and Stam HJ, “The epidemiology of cerebral palsy: incidence, impairments and risk factors,” Disabil Rehabil, vol. 28, pp. 183–91, February 28 2006. [DOI] [PubMed] [Google Scholar]
- [3].Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, and Durkin MS, “Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration,” Pediatrics, vol. 121, pp. 547–54, March 2008. [DOI] [PubMed] [Google Scholar]
- [4].Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, and Jacobsson B, “A report: the definition and classification of cerebral palsy April 2006,” Dev Med Child Neurol Suppl, vol. 109, pp. 8–14, February 2007. [PubMed] [Google Scholar]
- [5].Damiano DL, Alter KE, and Chambers H, “New clinical and research trends in lower extremity management for ambulatory children with cerebral palsy,” Phys Med Rehabil Clin N Am, vol. 20, pp. 469–91, August 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wren TA, Rethlefsen S, and Kay RM, “Prevalence of specific gait abnormalities in children with cerebral palsy: influence of cerebral palsy subtype, age, and previous surgery,” J Pediatr Orthop, vol. 25, pp. 79–83, Jan-Feb 2005. [DOI] [PubMed] [Google Scholar]
- [7].Jahnsen R, Villien L, Aamodt G, Stanghelle JK, and Holm I, “Musculoskeletal pain in adults with cerebral palsy compared with the general population,” J Rehabil Med, vol. 36, pp. 78–84, March 2004. [DOI] [PubMed] [Google Scholar]
- [8].Opheim A, Jahnsen R, Olsson E, and Stanghelle JK, “Walking function, pain, and fatigue in adults with cerebral palsy: a 7-year follow-up study,” Dev Med Child Neurol, vol. 51, pp. 381–8, May 2009. [DOI] [PubMed] [Google Scholar]
- [9].Steele KM, Demers MS, Schwartz MH, and Delp SL, “Compressive tibiofemoral force during crouch gait,” Gait Posture, vol. 35, pp. 556–60, April 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kerr Graham H and Selber P, “Musculoskeletal aspects of cerebral palsy,” J Bone Joint Surg Br, vol. 85, pp. 157–66, March 2003. [DOI] [PubMed] [Google Scholar]
- [11].van der Krogt MM, Bregman DJ, Wisse M, Doorenbosch CA, Harlaar J, and Collins SH, “How crouch gait can dynamically induce stiff-knee gait,” Ann Biomed Eng, vol. 38, pp. 1593–606, April 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rose J, Gamble JG, Burgos A, Medeiros J, and Haskell WL, “Energy expenditure index of walking for normal children and for children with cerebral palsy,” Dev Med Child Neurol, vol. 32, pp. 333–40, April 1990. [DOI] [PubMed] [Google Scholar]
- [13].Bell KJ, Ounpuu S, DeLuca PA, and Romness MJ, “Natural progression of gait in children with cerebral palsy,” J Pediatr Orthop, vol. 22, pp. 677–82, Sep-Oct 2002. [PubMed] [Google Scholar]
- [14].Bottos M and Gericke C, “Ambulatory capacity in cerebral palsy: prognostic criteria and consequences for intervention,” Dev Med Child Neurol, vol. 45, pp. 786–90, November 2003. [DOI] [PubMed] [Google Scholar]
- [15].De Mattos C, Patrick Do K, Pierce R, Feng J, Aiona M, and Sussman M, “Comparison of hamstring transfer with hamstring lengthening in ambulatory children with cerebral palsy: further follow-up,” J Child Orthop, vol. 8, pp. 513–20, December 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ounpuu S, Solomito M, Bell K, DeLuca P, and Pierz K, “Long-term outcomes after multilevel surgery including rectus femoris, hamstring and gastrocnemius procedures in children with cerebral palsy,” Gait Posture, vol. 42, pp. 365–72, September 2015. [DOI] [PubMed] [Google Scholar]
- [17].Stout JL, Gage JR, Schwartz MH, and Novacheck TF, “Distal femoral extension osteotomy and patellar tendon advancement to treat persistent crouch gait in cerebral palsy,” J Bone Joint Surg Am, vol. 90, pp. 2470–84, November 2008. [DOI] [PubMed] [Google Scholar]
- [18].Damiano DL, Vaughan CL, and Abel MF, “Muscle response to heavy resistance exercise in children with spastic cerebral palsy,” Dev Med Child Neurol, vol. 37, pp. 731–9, August 1995. [DOI] [PubMed] [Google Scholar]
- [19].Steele KM, Damiano DL, Eek MN, Unger M, and Delp SL, “Characteristics associated with improved knee extension after strength training for individuals with cerebral palsy and crouch gait,” J Pediatr Rehabil Med, vol. 5, pp. 99–106, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bjornson KF, Belza B, Kartin D, Logsdon R, and McLaughlin JF, “Ambulatory physical activity performance in youth with cerebral palsy and youth who are developing typically,” Phys Ther, vol. 87, pp. 248–57, March 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brehm MA, Harlaar J, and Schwartz M, “Effect of ankle-foot orthoses on walking efficiency and gait in children with cerebral palsy,” J Rehabil Med, vol. 40, pp. 529–34, July 2008. [DOI] [PubMed] [Google Scholar]
- [22].Rogozinski BM, Davids JR, Davis RB 3rd, Jameson GG, and Blackhurst DW, “The efficacy of the floor-reaction ankle-foot orthosis in children with cerebral palsy,” J Bone Joint Surg Am, vol. 91, pp. 2440–7, October 2009. [DOI] [PubMed] [Google Scholar]
- [23].Dodd KJ and Foley S, “Partial body-weight-supported treadmill training can improve walking in children with cerebral palsy: a clinical controlled trial,” Dev Med Child Neurol, vol. 49, pp. 101–5, February 2007. [DOI] [PubMed] [Google Scholar]
- [24].Borggraefe I, Schaefer JS, Klaiber M, Dabrowski E, Ammann-Reiffer C, Knecht B, Berweck S, Heinen F, and Meyer-Heim A, “Robotic-assisted treadmill therapy improves walking and standing performance in children and adolescents with cerebral palsy,” Eur J Paediatr Neurol, vol. 14, pp. 496–502, November 2010. [DOI] [PubMed] [Google Scholar]
- [25].Willoughby KL, Dodd KJ, Shields N, and Foley S, “Efficacy of partial body weight-supported treadmill training compared with overground walking practice for children with cerebral palsy: a randomized controlled trial,” Arch Phys Med Rehabil, vol. 91, pp. 333–9, March 2010. [DOI] [PubMed] [Google Scholar]
- [26].Dobkin BH and Duncan PW, “Should body weight-supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate?,” Neurorehabil Neural Repair, vol. 26, pp. 308–17, May 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bertoti DB, Stanger M, Betz RR, Akers J, Maynahon M, and Mulcahey MJ, “Percutaneous Intramuscular Functional Electrical Stimulation as an Intervention Choice for Children with Cerebral Palsy,” Pediatr Phys Ther, vol. 9, pp. 123–127, 1997. [Google Scholar]
- [28].Khamis S, Martikaro R, Wientroub S, Hemo Y, and Hayek S, “A functional electrical stimulation system improves knee control in crouch gait,” J Child Orthop, vol. 9, pp. 137–43, April 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ferris DP, Sawicki GS, and Daley MA, “A PHYSIOLOGIST’S PERSPECTIVE ON ROBOTIC EXOSKELETONS FOR HUMAN LOCOMOTION,” Int J HR, vol. 4, pp. 507–528, September 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dollar A and Herr H, “Lower Extremity Exoskeletons and Active Orthoses:Challenges and State-of-the-Art,” IEEE Trans Robotics, vol. 24, pp. 144–158, February 2008. [Google Scholar]
- [31].Esquenazi A, Talaty M, Packel A, and Saulino M, “The ReWalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury,” Am J Phys Med Rehabil, vol. 91, pp. 911–21, November 2012. [DOI] [PubMed] [Google Scholar]
- [32].Farris RJ, Quintero HA, Murray SA, Ha KH, Hartigan C, and Goldfarb M, “A preliminary assessment of legged mobility provided by a lower limb exoskeleton for persons with paraplegia,” IEEE Trans Neural Syst Rehabil Eng, vol. 22, pp. 482–90, May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bortole M, Venkatakrishnan A, Zhu F, Moreno JC, Francisco GE, Pons JL, and Contreras-Vidal JL, “The H2 robotic exoskeleton for gait rehabilitation after stroke: early findings from a clinical study,” J Neuroeng Rehabil, vol. 12, p. 54, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zoss AB, Kazerooni H, and Chu A, “Biomechanical Design of the Berkeley Lower Extremity Exoskeleton (BLEEX),” IEEE/ASME Trans Mechatron, vol. 11, pp. 128–138, 2006. [Google Scholar]
- [35].Mooney LM, Rouse EJ, and Herr HM, “Autonomous exoskeleton reduces metabolic cost of human walking,” J Neuroeng Rehabil, vol. 11, p. 151, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hicks JL, Schwartz MH, Arnold AS, and Delp SL, “Crouched postures reduce the capacity of muscles to extend the hip and knee during the single-limb stance phase of gait,” J Biomech, vol. 41, pp. 960–7, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Steele KM, Seth A, Hicks JL, Schwartz MS, and Delp SL, “Muscle contributions to support and progression during single-limb stance in crouch gait,” J Biomech, vol. 43, pp. 2099–105, August 10 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Damiano DL, Arnold AS, Steele KM, and Delp SL, “Can strength training predictably improve gait kinematics? A pilot study on the effects of hip and knee extensor strengthening on lower-extremity alignment in cerebral palsy,” Phys Ther, vol. 90, pp. 269–79, February 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lerner Z, Damiano D, and Bulea T, “Estimating the Mechanical Behavior of the Knee Joint During Crouch Gait: Implications for Real-Time Motor Control of Robotic Knee Orthoses,” IEEE Trans Neural Syst Rehabil Eng, in press,April 14 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sweeney PC, Lyons GM, and Veltink PH, “Finite state control of functional electrical stimulation for the rehabilitation of gait,” Med Biol Eng Comput, vol. 38, pp. 121–6, March 2000. [DOI] [PubMed] [Google Scholar]
- [41].Hugh H and Ari W, “User‐adaptive control of a magnetorheological prosthetic knee,” Industrial Robot: An International Journal, vol. 30, pp. 42–55, 2003. [Google Scholar]
- [42].Bulea TC, Kobetic R, Audu ML, Schnellenberger JR, and Triolo RJ, “Finite state control of a variable impedance hybrid neuroprosthesis for locomotion after paralysis,” IEEE Trans Neural Syst Rehabil Eng, vol. 21, pp. 141–51, January 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].del-Ama AJ, Gil-Agudo A, Pons JL, and Moreno JC, “Hybrid FES-robot cooperative control of ambulatory gait rehabilitation exoskeleton,” J Neuroeng Rehabil, vol. 11, p. 27, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kwon S, Park HS, Stanley CJ, Kim J, and Damiano DL, “A practical strategy for sEMG-based knee joint moment estimation during gait and its validation in individuals with cerebral palsy,” IEEE Trans Biomed Eng, vol. 59, pp. 1480–7, May 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lerner ZF, Board WJ, and Browning RC, “Pediatric obesity and walking duration increase medial tibiofemoral compartment contact forces,” J Orthop Res, August 13 2015. [DOI] [PubMed] [Google Scholar]
- [46].Dreher T, Vegvari D, Wolf SL, Klotz M, Muller S, Metaxiotis D, Wenz W, Doderlein L, and Braatz F, “Long-term effects after conversion of biarticular to monoarticular muscles compared with musculotendinous lengthening in children with spastic diplegia,” Gait Posture, vol. 37, pp. 430–5, March 2013. [DOI] [PubMed] [Google Scholar]
- [47].Hidler JM and Wall AE, “Alterations in muscle activation patterns during robotic-assisted walking,” Clin Biomech (Bristol, Avon), vol. 20, pp. 184–93, February 2005. [DOI] [PubMed] [Google Scholar]
- [48].Sylos-Labini F, La Scaleia V, d’Avella A, Pisotta I, Tamburella F, Scivoletto G, Molinari M, Wang S, Wang L, van Asseldonk E, van der Kooij H, Hoellinger T, Cheron G, Thorsteinsson F, Ilzkovitz M, Gancet J, Hauffe R, Zanov F, Lacquaniti F, and Ivanenko YP, “EMG patterns during assisted walking in the exoskeleton,” Front Hum Neurosci, vol. 8, p. 423, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Knaepen K, Beyl P, Deurinck S, Hagman F, Lefeber D, and Meeusen R, “Human-Robot Interaction: Kinematics and Muscle Activity Inside a Powered Compliant Knee Exoskeleton,” IEEE Trans Neural Syst Rehabil Eng, vol. 22, pp. 1128–37, 2014 2014. [DOI] [PubMed] [Google Scholar]
- [50].Israel JF, Campbell DD, Kahn JH, and Hornby TG, “Metabolic costs and muscle activity patterns during robotic- and therapist-assisted treadmill walking in individuals with incomplete spinal cord injury,” Phys Ther, vol. 86, pp. 1466–78, November 2006. [DOI] [PubMed] [Google Scholar]
- [51].Rossi S, Colazza A, Petrarca M, Castelli E, Cappa P, and Krebs HI, “Feasibility study of a wearable exoskeleton for children: is the gait altered by adding masses on lower limbs?,” PLoS One, vol. 8, p. e73139, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]