Abstract
Sexual selection produces extravagant male traits, such as colorful ornaments, via female mate choice. More rarely, in mating systems in which males allocate mating effort between multiple females, female ornaments may evolve via male mate choice. Females of many anthropoid primates exhibit ornaments that indicate intraindividual cyclical fertility, but which have also been proposed to function as interindividual quality signals. Rhesus macaque females are one such species, exhibiting cyclical facial color variation that indicates ovulatory status, but in which the function of interindividual variation is unknown. We collected digital images of the faces of 32 rhesus macaque adult females. We assessed mating rates, and consortship by males, according to female face coloration. We also assessed whether female coloration was linked to physical (skinfold fat, body mass index) or physiological (fecal glucocorticoid metabolite [fGCM], urinary C-peptide concentrations) condition. We found that redder-faced females were mated more frequently, and consorted for longer periods by top-ranked males. Redder females had higher fGCM concentrations, perhaps related to their increased mating activity and consequent energy mobilization, and blood flow. Prior analyses have shown that female facial redness is a heritable trait, and that redder-faced females have higher annual fecundity, while other evidence suggests that color expression is likely to be a signal rather than a cue. Collectively, the available evidence suggests that female coloration has evolved at least in part via male mate choice. Its evolution as a sexually selected ornament attractive to males is probably attributable to the high female reproductive synchrony found in this species.
Keywords: coloration, ornaments, sexual selection, signaling
Sexual selection produces traits that are advantages in competition, or that are attractive to the opposite sex. We studied the bright red facial coloration of 32 female rhesus monkeys. We found that males preferred females with more colorful faces. When combined with other lines of evidence, our study suggests that the colorful face of female rhesus monkeys may have evolved by male mate choice.
INTRODUCTION
Sexual selection explains the prevalence of traits that influence the reproductive rates of carriers, sometimes at a detriment to their survival (Darwin 1872; Andersson 1994). This evolutionary process is thought to be usually stronger on males than on females because their reproductive rate is less constrained by gamete production and parental investment (Bateman 1948; Trivers 1972). This typically produces extravagant male traits, such as armaments via direct male–male competition, and ornaments via female mate choice (Clutton-Brock and McAuliffe 2009). However, stronger sexual selection on males is not always the case, and females of some species exhibit extravagant conspicuous sexually selected traits (Clutton-Brock 2009). This occurs most commonly in species with sex-role reversal, where extensive paternal care limits the reproductive success of males, leading males to be highly choosy in their mate choices, and to female traits which males use to select mates (Clutton-Brock 2009; Edward and Chapman 2011).
Female ornamentation can also occur without any sex-reversal. For example, many Anthropoid primates exhibit conspicuous signals in females, despite the absence of sex-role reversal (Dixson 1983; Dixson 2012)—indeed, anthropoid females are thought to expend some of the highest levels of maternal investment in the animal kingdom (Lee 1987). One set of anthropoid traits that have long been of interest to evolutionary biologists is the colorful red skin ornaments exhibited by females of several anthropoid species, which includes sexual swellings of the anogenital area of species such as baboons (Domb and Pagel 2001; Higham et al. 2008; Fitzpatrick et al. 2014) and chimpanzees (Emery and Whitten 2003; Deschner et al. 2004), the red facial coloration of Japanese (Fujita 2010; Rigaill et al. 2015; Rigaill et al. 2019), and rhesus macaques (Dubuc et al. 2009; Dubuc, Winters, et al. 2014), and the red chest patch of geladas (Bergman et al. 2009) (reviewed in Dixson [2012] Chapter 7). Most hypotheses and empirical studies surrounding such female signals focus on the function of conspicuous sexual swellings, but may also be applied to signals such as facial and chest coloration too. There are many hypotheses related to the function of sexual swellings, which try to explain three types of variation: 1) intracycle variation, in which swelling sizes change across the menstrual cycle; 2) intrafemale intercycle variation, in which swelling sizes differ between cycles of the same female; and 3) interfemale variation, in which swelling sizes differ between different females (Zinner et al. 2002). One well-studied hypothesis to explain intracycle variation, proposes that this correlates with conceptive probability across the cycle, with swellings being larger when conceptive probability is high, and small when it is low (the graded signal hypothesis, Nunn 1999). Studies of multiple species have generally supported this hypothesis, although there is a great variation in the extent of to which swelling size correlates with conceptive probability among anthropoids (e.g., macaques, Higham and Dubuc 2015). One well-studied hypothesis that proposes to explain interindividual signal variation is the reliable indicator hypothesis (Pagel 1994), which proposes that sexual swelling expression represents variation in female quality, allowing males to choose between females of different quality by choosing the female with the largest swelling (i.e., exhibiting the greatest signal expression).
Under the reliable indicator hypothesis (Pagel 1994), the conspicuous traits exhibited by anthropoid primate females have evolved via male mate choice, paralleling the function of male conspicuous traits that are selected via intersexual selection. Nonetheless, a weakness of the hypothesis is that it did not address the benefits that females might accrue, such as paternal care, which would be necessary for selection to act on the signaler (Alberts and Fitzpatrick 2012). Although many studies have tested whether such signals covary with intracycle differences in fertility (e.g., long-tailed macaques, Engelhardt et al. [2005]; chimpanzees, Deschner et al. [2004]; olive baboons, Higham et al. [2008]), very few studies of sexual swellings have tested whether such signals are potentially informative about interindividual differences in female quality, as proposed by the reliable indicator hypothesis. While one study of olive baboons claimed that sexual swelling size did indeed indicate female reproductive value (Domb and Pagel 2001), the study was criticized for a flawed analysis (Zinner et al. 2002). A study of chacma baboon sexual swellings suggested that swellings may have initially evolved as signals of intraindividual fertility, and then have secondarily evolved into interindividual quality signals over time (Huchard et al. 2009). Similar analyses have not been conducted in other anthropoid genera, and it remains unclear whether signals that are attractive to males within cycles might also be used by males to select among different females. While these hypotheses were formulated for sexual swellings, they may nonetheless also be applied to analogous female fertility signals exhibited on other parts of the body, such as the red chest patches of female gelada (Dixson 2012).
One strong candidate for a female signal that could be sexually selected as both an intra and interfemale signal of reproductive value, is the red facial coloration of female rhesus macaques. In parous adult females, facial skin intensity varies over the course of the ovarian cycle with the greatest expression around the timing of ovulation, such that it is covaries with intracycle variation in fertility (Dubuc et al. 2009; Higham et al. 2010). Such variation is perceived by males (Waitt et al. 2006; Higham et al. 2011). Rhesus facial and hindquarter skin redness is under the control of targeted estrogen receptors only present in bare skin areas, and not found in adjacent skin (Rhodes et al. 1997), which suggests that there has been selection on the color change for its communicative value—that is, that the color change is a signal rather than a cue. Interfemale red-faced coloration is heritable, and females with redder faces exhibit higher reproductive rates (Dubuc, Winters, et al. 2014). Behavioral evidence shows that males of this species are selective (reviewed in Paul 2002). For instance, higher-ranking males appear to prefer higher-ranking and parous females. Male selectivity is likely to be a response to the high level of synchrony in female mating activity in this seasonally breeding species forming large multimale–multifemale groups (Higham and Maestripieri 2014). While these are necessary conditions for intersexual selection to act on the trait, there is as yet no observational evidence of whether males prefer to mate with redder-faced faces—a key piece of evidence that is required to argue that female coloration itself is under sexual selection via male mate choice.
There are also other mechanisms aside from sexual selection that could be invoked to explain how such female traits might evolve. For instance, the trait may be exhibited in females because it is exhibited in males, and it may be coexpressed in females without being selected against as long as the cost to its expression in females is low (Kraaijeveld et al. 2007). Evidence that rhesus macaque facial coloration is under sexual selection in males is quite strong. There is both observational (Dubuc, Allen, et al. 2014) and experimental (Dubuc et al. 2016) evidence that dark redder-faced males are preferred by females, and the trait is heritable (Dubuc, Winters, et al. 2014), with selection gradients demonstrating that higher-ranked males with dark redder faces have higher fecundity (Dubuc, Winters, et al. 2014). However, since females with redder facial skin color have higher fecundity (Dubuc, Winters, et al. 2014), males starting to select females with redder faces in the population would start to outcompete males without this preference. When combined with the other existing data on female facial redness outlined above, evidence for such a preference would suggest facial redness is under intersexual selection in females in addition to males, but such evidence is currently lacking.
Understanding the extent to which underlying physiological condition may be associated with trait variation is also informative for understanding the selection pressures that might be acting on the trait, in addition to the extent to which it might be considered a signal versus a cue, influencing male behavior. Because skin color is determined by blood flow in the capillaries, the degree of expression of coloration might be influenced by blood oxygenation (skin redness) and flow (skin darkness), and hence directly related to physiological condition (Changizi et al. 2006; Stephen et al. 2009). Intensity of signal expression may also be limited by the consequence of male behaviors: female attractiveness in mammals is often accompanied with harassment and coercion as well as high mating activity, which could lead to increased energetic expenditure. As such, redder females may be in better physical or physiological condition overall, but also may be in worse energetic condition due to increased mating and other activity, leading us to make no prediction as to whether redder females are in better or worse physical or physiological condition.
In this study, our objectives are to ask: 1) Do males prefer females with redder and/or darker faces? And 2) Is face redness or darkness linked to physical or physiological condition?, in the Cayo Santiago free-ranging population of rhesus macaques (Macaca mulatta). Due to large group sizes and hence a large number of cycling females per group, combined with breeding seasonality, this population shows a high degree of synchrony in female mating activity (e.g., Dubuc et al. 2011), requiring males to choose between multiple mating females. Large group sizes and seasonal breeding are thought to be found among truly wild rhesus populations, too (Southwick and Siddiqi 2011).
METHODS
Study population and subjects
The study took place on the free-ranging population of Cayo Santiago, a 15.2 ha island located 1km off the East coast of Puerto Rico, which is managed by the Caribbean Primate Research Center (CPRC), and which was established in 1938. We studied group R, which consisted of 82 adult females (≥3 years old), 42–45 adult males (≥5.5 years old), 11 subadult males (3–5 years old), and 120 immatures. Data collection focused on the 32 females who had given birth in the previous mating season, that is, that all had offspring of similar age at the beginning of the study. Subject females varied in age (mean ± SEM 7.69 ± 0.07 years). Thirty out of the 32 females in our sample conceived during the study period, precluding us from conducting any analyses focused on color and conception likelihood. Subjects were captured during the yearly trapping period (mid-January to mid-March 2012) immediately prior to the mating season (mid-February to mid-June), and all noninvasive data (behavior, urine, and fecal samples) were collected 5 days per week at the peak of the mating season (11 March to 18 May 2012). The investigation was approved by the IACUC of the University of Puerto Rico, Medical Sciences Campus (protocol No. A0100108).
Assessment of skin redness and darkness
Images were collected two to three times per week for all 32 females (mean ± SEM 19.81 ± 1.01 images per female). Details of image collection and measurements can be found in Dubuc et al. (2014). Briefly, multiple images of males and a color standard (X-rite ColorChecker passport) were captured in RAW format from 1 to 3 m away from subjects using a calibrated Canon EOS Rebel T2i camera with an 18 megapixel CMOS APS-sensor and an EFS55-250mm f/4–5.6 IS lens. Immediately after the capture of an image, we took a second photograph of the color standard placed in the same location and photographed under the same lighting and camera settings as the subject (i.e., the “sequential method”: Higham 2006; Bergman and Beehner 2008; Stevens 2009). Facial skin color was quantified by measuring color values from images converted to 16-bit TIFF files using DCRAW (Coffin 2008). We first took average red (R), green (G), and blue (B) measurements (reflecting the camera sensor stimulation) from a portion of the face which included the bridge of the nose and all skin between the nostrils and the corners of the eyes, avoiding areas of dirt, dappled light, or shadows, and the neutral gray patches of the color standard. Landmarks selected around this area were joined using cubic spline interpolation using a customized MATLAB function. RGB (red, green, and blue) values were transformed to rhesus color space via a polynomial colorspace transformation (Stevens et al. 2007), corrected for variation in lighting by applying the Von-Kries transformation (Ives 1912; reprinted in Brill 1995) using the white patch of the color checker. Measurements from multiple successive face images taken of the same subject with multiple corresponding standard images were averaged to yield that subject’s overall facial skin color phenotype for that day. We calculated female facial redness as (LW-MW)/(LW+MW) (the Red-Green Opponency Channel, hereafter “Redness”) and luminance as (LW+MW)/2, hereafter “Darkness” (Osorio and Vorobyev 2005). We describe higher Red-Green Opponency values as “redder,” and lower luminance values as “darker.” We present summary statistics for Redness and Darkness values in Table 1.
Table 1.
Summary statistics for facial redness and darkness
| ID | Mean redness (min – max) | Mean darkness (min – max) | CV redness (%) | CV darkness (%) |
|---|---|---|---|---|
| 1 | 0.095 (0.082–0.110) | 0.252 (0.196–0.335) | 7.425 | 12.349 |
| 2 | 0.087 (0.065–0.110) | 0.283 (0.206–0.431) | 14.079 | 21.949 |
| 3 | 0.082 (0.050–0.101) | 0.362 (0.233–0.489) | 15.019 | 20.124 |
| 4 | 0.091 (0.060–0.127) | 0.287 (0.175–0.591) | 15.030 | 28.788 |
| 5 | 0.081 (0.062–0.104) | 0.295 (0.238–0.403) | 14.545 | 14.439 |
| 6 | 0.092 (0.076–0.115) | 0.285 (0.208–0.405) | 12.304 | 15.766 |
| 7 | 0.072 (0.057–0.082) | 0.337 (0.285–0.447) | 8.349 | 12.260 |
| 8 | 0.089 (0.073–0.110) | 0.311 (0.252–0.411) | 12.216 | 13.716 |
| 9 | 0.081 (0.066–0.097) | 0.289 (0.220–0.396) | 11.708 | 13.990 |
| 10 | 0.091 (0.074–0.113) | 0.292 (0.195–0.475) | 12.247 | 23.030 |
| 11 | 0.084 (0.055–0.112) | 0.252 (0.186–0.338) | 16.441 | 16.894 |
| 12 | 0.091 (0.068–0.113) | 0.293 (0.213–0.401) | 9.318 | 16.910 |
| 13 | 0.084 (0.060–0.105) | 0.319 (0.251–0.483) | 12.553 | 22.799 |
| 14 | 0.099 (0.083–0.126) | 0.221 (0.160–0.282) | 10.862 | 18.621 |
| 15 | 0.110 (0.095–0.129) | 0.284 (0.182–0.363) | 9.930 | 18.296 |
| 16 | 0.089 (0.060–0.127) | 0.300 (0.215–0.412) | 19.469 | 15.202 |
| 17 | 0.084 (0.060–0.108) | 0.316 (0.241–0.429) | 19.095 | 20.942 |
| 18 | 0.084 (0.066–0.114) | 0.278 (0.185–0.389) | 13.374 | 19.179 |
| 19 | 0.074 (0.065–0.084) | 0.309 (0.224–0.353) | 8.481 | 13.239 |
| 20 | 0.075 (0.056–0.094) | 0.303 (0.233–0.462) | 11.254 | 19.569 |
| 21 | 0.090 (0.079–0.100) | 0.281 (0.188–0.370) | 6.762 | 14.923 |
| 22 | 0.084 (0.069–0.098) | 0.305 (0.216–0.501) | 10.874 | 28.885 |
| 23 | 0.089 (0.073–0.114) | 0.244 (0.187–0.304) | 12.232 | 11.967 |
| 24 | 0.084 (0.062–0.105) | 0.299 (0.231–0.398) | 12.214 | 15.284 |
| 25 | 0.087 (0.073–0.112) | 0.315 (0.253–0.505) | 11.720 | 18.695 |
| 26 | 0.071 (0.047–0.091) | 0.320 (0.208–0.416) | 18.032 | 20.770 |
| 27 | 0.085 (0.073–0.098) | 0.285 (0.215–0.361) | 8.056 | 14.013 |
| 28 | 0.092 (0.079–0.113) | 0.258 (0.156–0.324) | 11.443 | 16.990 |
| 29 | 0.087 (0.072–0.108) | 0.277 (0.206–0.340) | 9.594 | 13.813 |
| 30 | 0.081 (0.075–0.084) | 0.284 (0.248–0.348) | 4.331 | 14.689 |
| 31 | 0.081 (0.052–0.099) | 0.320 (0.173–0.584) | 13.108 | 28.047 |
| 32 | 0.077 (0.057–0.097) | 0.328 (0.233–0.422) | 14.217 | 16.568 |
| Total | 14.964 | 20.470 |
Behavioral data collection and definitions
Behavioral data were collected by two trained observers who have collected behavioral data together on multiple studies (Dubuc, Hughes, et al. 2012; Dubuc, Muniz, et al. 2012; Dubuc, Allen, et al. 2014). Data collection focused on male–female socio-sexual interactions. All occurrences of male–female interactions were recorded (Altmann 1974). We recorded female proceptive behaviors (presentation of hindquarters and hand slaps (Carpenter 1942; Michael and Zumpe 1970; Wallen et al. 1984; Dixson 2012), plus all mounts and intromissions. We considered as proceptive females that were: observed mating, exhibiting a sperm plug, receiving or emitting sexual solicitations, or consorting with a male. Consecutive proceptive days (with gaps of 1–2 days) were considered as being part of the same proceptive period or behavioral estrus. Mating was considered to take place when a mount with penetration and pelvic thrusts occurred outside the context of social tension and conflicts. Since rhesus macaques are multiple-mounters, two observed mounts were considered part of the same mating series if they took place ≤30 min apart from each other, unless an ejaculation pause or a new sperm plug was observed (Dubuc et al. 2011). Consortships were defined as sexual associations where a male and a female maintained proximity and synchronized their activity, regardless of who was mainly in charge of such maintenance. No evidence of mating between the male and the female was required for them to be considered as forming a consortship, but the female did need to be considered to be behaving proceptively.
Dominance rank
For females, we used previously calculated dominance ranks from Mandalaywala et al. (2014), which were determined based on the outcome of dyadic agonistic interactions between females in Group R. Dyadic interactions were placed into a winner-loser matrix and MatMan was used to generate linear dominance hierarchies (cf. Higham and Maestripieri 2014). Following 10,000 iterations, significant linear hierarchies were produced (linearity test using Landau’s linearity index corrected for unknown relationships, P = 0.03). For males, we used previously calculated dominance ranks from Dubuc et al. (2013, 2014). Briefly, male dominance ranks were calculated for all males resident in group R in 2011 using agonistic interactions; this hierarchy was then modified to include three males that immigrated into group R in 2012. Since rhesus macaque males “queue” for dominance, such that males enter a new group at the bottom of the hierarchy and rise in rank over time (Manson 1995), all three of these new males were assigned the lowest rank in the hierarchy. We defined top-ranked males as the males with the four highest dominance ranks. In studies of species with largely asynchronous female fertile phases, it is common to independently assess the mate choice preferences of the alpha male (e.g., Bradley et al. 2005; Setchell et al. 2005), who should have priority of access to the fertile female on any given day (Altmann 1962). However, due to the high female reproductive synchrony in our study population, assessing the choices of several top-ranked males seems more appropriate. In our sample, 2.48 females from the 32 study subjects were seen in consort with males per day (range 0–9) across all observation days. This translates to 3.49 females in consort on an average observation day across the whole study group (45 females). We therefore reasoned that the mate choices of the four top-ranked males were important to assess independently of those of all males.
Female attractiveness and male mating effort
We used two measures to assess female attractiveness to males: 1) the number of mating series in which females were involved and 2) the dominance rank of male consortship partners, that is, the ones that have priority of access to females. We calculated each measure for each observation day of the study (i.e., for the entire dataset).
Physical condition
All subjects except one were captured and anesthetized with ketamine by trained CPRC employees. Body mass was measured using a hanging scale. Body length (top of head to tail base) and skinfold fat above and below the navel were measured with electronic calipers by CD. Each measurement was taken two times and averaged. Body mass index (BMI) was calculated as kg/m2 (Campbell and Gerald 2004) and skinfold fat as the average of both measurements.
Physiological condition
Fecal samples were collected opportunistically during fieldwork for measurement of glucocorticoid metabolites, break-down products of metabolic hormones associated with energy allocation in response to the physical and social environment. Special effort was invested in collecting samples from females for which no recent (within the last 1–2 weeks) samples had been collected, if needed. Samples were kept in a cooler with ice while in the field and then put in a −20 °C freezer upon return from the field. Fecal samples were shipped to the German Primate Center (DPZ) on ice for analysis using a previously validated glucocorticoid metabolite assay (Hoffman et al. 2011). All fecal samples arrived at the DPZ frozen, and were subsequently prepared for enzyme-immuno assay (EIA) by being lyophilized and pulverized. An aliquot (50–70 mg) of the resulting fecal powder was extracted with 3 mL of 80% methanol by vortexing for 15 min (Heistermann et al. 1995). Samples were analyzed for 11β-hydroxyetiocholanolone, a major metabolite of cortisol in primate feces (e.g., Heistermann et al. 2006) using an EIA that has been biologically validated for use in rhesus macaques (see Hoffman et al. 2011 for validation, and Heistermann et al. 2004 for a detailed description of the EIA). High and low concentration standards were assayed across plates to assess measurement variation, which demonstrated interassay variation of below 15%, and intra-assay variation of below 10%. Assay results are standardized for differences in fecal weight and are expressed as fecal glucocorticoid metabolite (fGCM) concentration (ng) per dry fecal weight.
Urine samples were collected opportunistically for measurement of urinary C-peptide (UCP) of insulin. This biomarker is a measure of energetic status, and in rhesus macaques has been shown to be primarily influenced by whether an individual is in overall positive or negative energy balance (Girard-Buttoz et al. 2011). Sampling effort was again invested in collecting samples from females when no samples had been collected in the previous 1–2 weeks. Samples were not collected if contamination with feces or blood was suspected. In order to remove substrate from the sample, we allowed each sample to settle and pipetted off the clean urine from the top, transferring the urine into a new tube every minute or so until the sample was completely clean (Higham, Heistermann, et al. 2011). Samples were kept in a cooler on ice while in the field and then put in a −20 °C freezer upon return from the field. Samples were shipped to New York University (NYU) for analyses using a previously validated assay (Girard-Buttoz et al. 2011; Higham, Girard-Buttoz, et al. 2011; Higham, Heistermann, et al. 2011). High and low concentration standards assayed across plates to assess measurement variation demonstrated interassay variation of below 15%, and intra-assay variation of below 10%. Assay results were indexed for differences in urine concentration by creatinine measurement via the Jaffe reaction.
Statistical analyses
All statistical analyses were performed in R version 4.0.1 (R Development Core Team 2020). We removed outlying values prior to analyses (n = 3 fGCM measures). These data points were greater than three standard deviations above the mean and may represent methodological errors. Some fecal samples contain large amounts of undigested fecal matter. Removing the undigested matter leads to a low fecal dry weight, and results in a very high hormone-per-weight concentration. While fGCM concentrations can increase and decrease, it is unlikely that a fGCM measure, which is an integrated measure of fGCM production over a few days, should show very high one-off spikes, leading us to question whether strongly outlying values were likely to be physiologically valid. In addition, we also reasoned that we were in any case more interested in baseline effects than one-off responses to acute stressors. We did not remove any other values from the dataset.
Do males prefer females with redder and/or darker faces?
First, we explored whether female color predicted patterns of investment among males using generalized linear mixed models (GLMMs). We ran a total of eight models. First, we examined patterns of male investment across our entire dataset (four models). We tested whether redder (one model) and/or darker females (a second model) were involved in more mating series per day using GLMMs with a negative binomial error structure, setting the number of mating series as the response variable, with color, age, and rank (fixed effects), and female ID and study week (to control for changes across the mating season) (random effects) as predictors, and the number of observations per female per day as an offset. Age and rank were coded as numerical predictors, and study week was coded as a categorical predictor. We also tested whether on a daily basis, the four top-ranked males were more likely to consort redder (one model) and/or darker females (a second model) using GLMMs with a binomial error structure, setting whether or not a female was consorted by a top male as the response variable, with color, age, and rank (fixed effects), and female ID and study week (random effects) as predictor variables, and the number of observations per female per day as an offset. As above, we coded age and rank as numerical predictors and study week as a categorical predictor. Including female ID as a random effect allowed us to control for uneven sampling across females and generate more robust estimates for each female. Nevertheless, to further ensure to that our analyses focused on interindividual variation, we ran a second set of analyses (four models) using only data collected on females when they were recorded as exhibiting proceptive behaviors toward males (hereafter: proceptive-only dataset), using the same model structures outlined above. We log-transformed the number of observations (offset) in all models. Several females were observed in consort but were not formally focaled on a given day. We excluded these cases from the models (n = 32 for redness, n = 33 for darkness) because zeroes (for the observation time offset) cannot be log-transformed. Proceptive-only dataset models that analyzed whether color influenced consortship by top-ranked males did not meet assumptions regarding the distribution of residuals as originally structured, so we also ran a second set of these models, dropping the random ID term representing the study week. Results were qualitatively the same, so we present results from models including the week random ID term. All GLMM analyses were run in the R package “glmmTMB” (Brooks et al. 2017), and residuals were visualized using the R package “DHARMa” (Hartig 2020).
Is face redness or darkness linked to physical or physiological condition?
Next, we used two approaches to test whether skin redness or darkness was linked to physical or physiological condition (estimated based on BMI, skinfold fat, fGCM concentrations, and UCP concentrations). Because we had only one measure of BMI and skinfold fat from just before the mating season, we first conducted analyses across the whole dataset using color values averaged across the mating season and single measurements of BMI and skinfold fat, using GLMs, controlling for age and sex (fixed effects). GLMs were implemented using the glm function using log-transformed measures for all variables. Second, since we had more regular fGCM and UCP measurements, we used LMMs to test for the effect of fGCM and UCP by week. We ran four models in total. We set color (either Redness or Darkness) as the response variable, and either fGCM or UCP as a numerical predictor variable, controlling for dominance rank and age (fixed effects) and female ID (random effect) in all models. Dominance rank and age were coded as continuous predictor variables. In our UCP models, we included collection time, since exploratory analysis revealed a strong effect of collection time on UCP concentrations. We log-transformed dominance rank and age, but we used untransformed values for redness and darkness because weekly averaged values of redness and darkness were normally distributed. LMMs were run in the R package “lme4” (Bates et al. 2015), and significance of fixed effects was tested using type II Wald chi-square tests implemented using the R package “car” (Fox and Weisberg 2011). For all models, we visually assessed residual plots to verify that residuals were normally distributed and uncorrelated with fitted values.
RESULTS
Do males prefer redder and/or darker females?
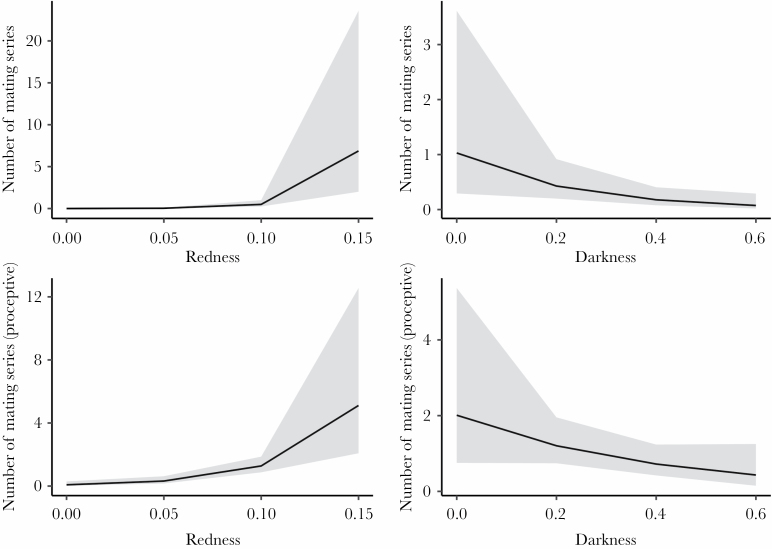
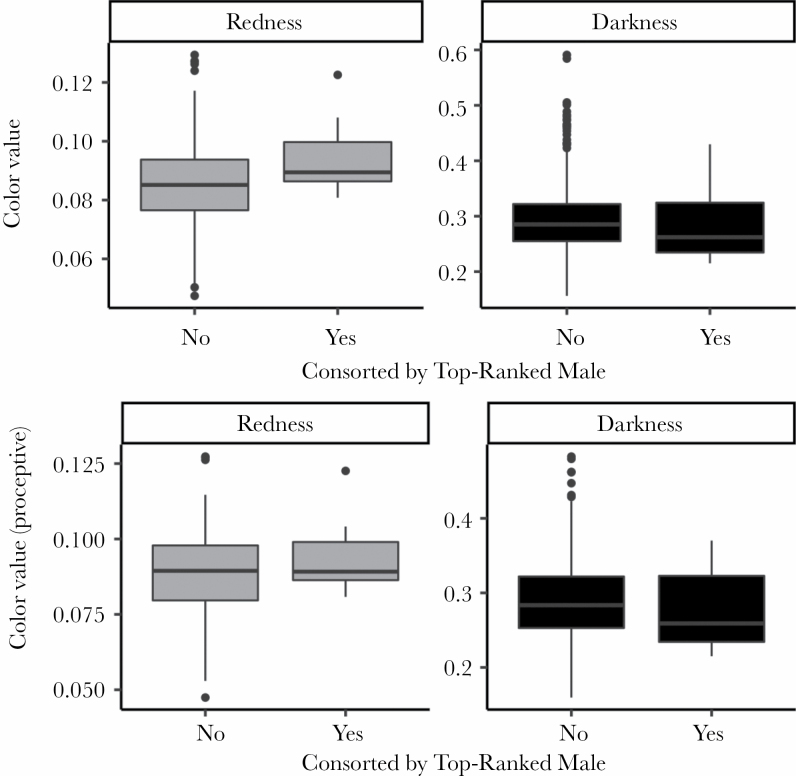
Across the entire dataset, the females who engaged in the highest number of mating series across all males were both darker and redder (Table 2; Figure 1). However, when only the proceptive period of the ovarian cycle is considered, only redder (but not darker) females engaged in more mating series (Table 3; Figure 1). Models of daily data revealed that top-ranked males formed consortships with redder, but not darker females more often across the whole dataset (Table 4; Figure 2); this difference is not detected in the proceptive-only dataset (Table 5; Figure 2). Overall, results suggest that males bias mating effort toward redder females.
Table 2.
Results of negative binomial GLMMs testing the effects of redness or darkness, age, and rank on the number of mating series in which a female participated, controlling for female ID and study week, with an offset for the number of observations per female per day. Analyses shown are across the whole dataset
| Estimate | Standard error | z-value | P | |
|---|---|---|---|---|
| Number of mating series (redness; daily) n = 602 female days | ||||
| Intercept | −7.569 | 0.978 | −7.737 | <0.001 |
| Redness | 52.602 | 8.790 | 5.984 | <0.001 |
| Age | −0.126 | 0.065 | −1.932 | 0.053 |
| Rank | 0.011 | 0.008 | 1.336 | 0.182 |
| Number of mating series (darkness; daily) n = 602 female days | ||||
| Intercept | −1.811 | 0.810 | −2.236 | 0.025 |
| Darkness | −4.394 | 1.884 | −2.333 | 0.020 |
| Age | −0.086 | 0.051 | −1.668 | 0.095 |
| Rank | 0.009 | 0.007 | 1.397 | 0.163 |
Bold values indicate statistical significance (P < 0.05).
Figure 1.
Marginal effects of Redness (left column) and Darkness (right column) values on the number of mating series per day for all females (top row, n = 602 female days for redness, 602 female days for darkness) and for proceptive females (bottom row, n = 252 female days for both redness and darkness). The shaded area represents 95% confidence bands of mean-fitted values.
Table 3.
Results of negative binomial GLMMs testing the effects of redness or darkness, age, and rank on the number of mating series in which a female participated, controlling for female ID and study week, with an offset for the number of observations per female per day. Analyses shown are for proceptive females only
| Estimate | Standard error | z-value | P | |
|---|---|---|---|---|
| Number of mating series (redness; daily) n = 252 female days | ||||
| Intercept | −4.793 | 0.678 | −7.068 | <0.001 |
| Redness | 27.756 | 7.065 | 3.929 | <0.001 |
| Age | −0.076 | 0.033 | −2.314 | 0.021 |
| Rank | 0.013 | 0.004 | 3.289 | 0.001 |
| Number of mating series (darkness; daily) n = 252 female days | ||||
| Intercept | −1.680 | 0.605 | −2.776 | 0.006 |
| Darkness | −2.562 | 1.590 | −1.611 | 0.107 |
| Age | −0.053 | 0.031 | −1.716 | 0.086 |
| Rank | 0.012 | 0.004 | 3.057 | 0.002 |
Bold values indicate statistical significance (P < 0.05).
Table 4.
Results of binomial GLMMs testing the effects of redness or darkness, age, and rank on the likelihood of a female being consorted by a top-ranked male, controlling for female ID and study week, with an offset for the number of observations per female per day. Analyses shown are across the whole dataset
| Estimate | Standard error | z-value | P | |
|---|---|---|---|---|
| Consort by top-ranked male (redness; daily) n = 602 female days | ||||
| Intercept | −28.067 | 6.870 | −4.085 | <0.001 |
| Redness | 116.735 | 43.369 | 2.692 | 0.007 |
| Age | 0.106 | 0.383 | 0.278 | 0.780 |
| Rank | 0.026 | 0.054 | 0.480 | 0.631 |
| Consort by top-ranked male (darkness; daily) n = 602 female days | ||||
| Intercept | −13.523 | 4.467 | −3.027 | 0.002 |
| Darkness | −7.912 | 9.095 | −0.870 | 0.384 |
| Age | 0.130 | 0.342 | 0.379 | 0.705 |
| Rank | 0.012 | 0.046 | 0.268 | 0.789 |
Bold values indicate statistical significance (P < 0.05).
Figure 2.
Differences in redness (left) and darkness (right) values for females when they were consorted by top-ranked males. Smaller values indicate darker skin color. Top-ranked males consorted redder females more often (P = 0.007, n = 634 female days, but not darker females P = 0.384, n = 634 female days) across the full dataset (top row), but not the proceptive-only dataset (redness P = 0.498, n = 257 female days, or darkness P = 0.783, n = 257 female days) (bottom row). Note that sample sizes for the figures produced here directly from the raw data are slightly larger than for the models (Tables 4 and 5, see Methods for details).
Table 5.
Results of binomial GLMMs testing the effects of color (redness or darkness), age, and rank on the likelihood of a female being consorted by a top-ranked male, controlling for female ID and study week, with an offset for the number of observations per female per day. Analyses shown are for proceptive females only
| Estimate | Standard error | z-value | P | |
|---|---|---|---|---|
| Consort by top-ranked male (redness; daily) n = 252 female days | ||||
| Intercept | −42.774 | 13.271 | −3.223 | 0.001 |
| Redness | 64.671 | 95.369 | 0.678 | 0.498 |
| Age | 0.065 | 0.585 | 0.110 | 0.912 |
| Rank | 0.014 | 0.100 | 0.143 | 0.886 |
| Consort by top-ranked male (darkness; daily) n = 252 female days | ||||
| Intercept | −34.803 | 13.947 | −2.495 | 0.013 |
| Darkness | −6.702 | 24.345 | −0.275 | 0.783 |
| Age | 0.060 | 0.586 | 0.103 | 0.918 |
| Rank | 0.008 | 0.101 | 0.082 | 0.935 |
Bold values indicate statistical significance (P < 0.05).
Is face redness or darkness linked to physical or physiological condition?
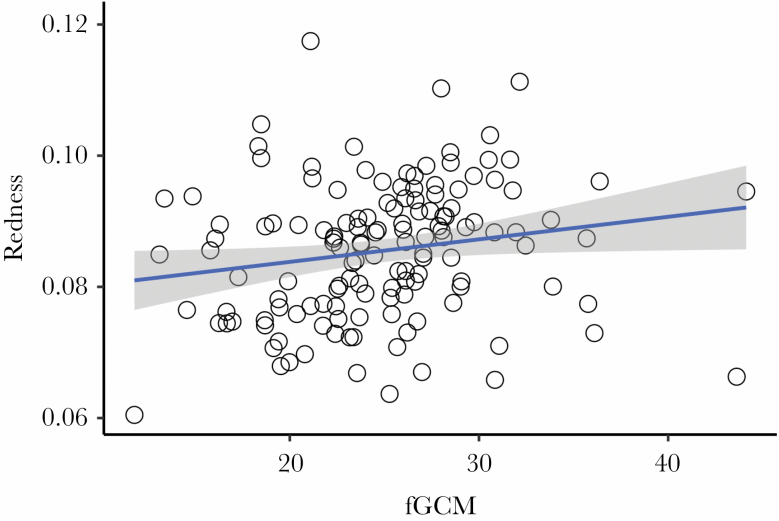
There was a significant positive association between female redness and fGCM concentrations, but not between female darkness and fGCM concentrations (Table 6; Figure 3). No association was found for UCP concentrations (Table 6), BMI (Table 7), or skinfold fat (Table 7). Results suggest that, overall, redder females have higher concentrations of fGCM during the mating season.
Table 6.
Results of LMMs for weekly noninvasive measurements of skin color and physiological measurements, controlling for female ID as a random effect. In UCP models, we also included collection time as a fixed effect
| Estimate | Standard error | chi-square (df) | P | |
|---|---|---|---|---|
| fGCM (redness) n = 137 female weeks | ||||
| Intercept | 0.065 | 0.009 | ||
| fGCM | <0.001 | <0.001 | 5.270 (1) | 0.022 |
| Age | 0.008 | 0.004 | 4.289 (1) | 0.038 |
| Rank | −0.001 | 0.001 | 0.448 (1) | 0.503 |
| fGCM (darkness) n = 137 female weeks | ||||
| Intercept | 0.325 | 0.040 | ||
| fGCM | <0.001 | <0.001 | <0.001 (1) | 0.989 |
| Age | −0.016 | 0.016 | 0.978 (1) | 0.322 |
| Rank | −0.001 | 0.006 | 0.031 (1) | 0.861 |
| UCP (redness) n = 79 female weeks | ||||
| Intercept | 0.074 | 0.013 | ||
| UCP | <0.001 | <0.001 | 0.073 (1) | 0.787 |
| Sample collection time | <−0.005 | 0.012 | 0.150(1) | 0.699 |
| Age | 0.007 | 0.005 | 2.312 (1) | 0.129 |
| Rank | −0.001 | 0.002 | 0.099 (1) | 0.753 |
| UCP (darkness) n = 79 female weeks | ||||
| Intercept | 0.336 | 0.045 | ||
| UCP | 0.003 | 0.002 | 3.154 (1) | 0.076 |
| Sample collection time | <−0.011 | 0.051 | 0.046 (1) | 0.830 |
| Age | −0.027 | 0.016 | 2.924 (1) | 0.087 |
| Rank | 0.004 | 0.007 | 0.345 (1) | 0.557 |
Bold values indicate statistical significance (P < 0.05).
Figure 3.
Weekly measures of fGCM and redness values (n = 137).
Table 7.
Results of GLMs testing relationships between average redness or darkness, BMI, and skinfold fat (n = 31 females)
| Estimate | Standard error | t-value | P | |
|---|---|---|---|---|
| BMI (redness) | ||||
| Intercept | −2.736 | 0.426 | −6.423 | <0.001 |
| BMI | 0.044 | 0.116 | 0.379 | 0.708 |
| Age | 0.078 | 0.046 | 1.711 | 0.099 |
| Rank | −0.009 | 0.017 | −0.516 | 0.610 |
| BMI (darkness) | ||||
| Intercept | −1.835 | 0.467 | −3.933 | <0.001 |
| BMI | 0.209 | 0.127 | 1.649 | 0.111 |
| Age | −0.039 | 0.050 | −0.774 | 0.446 |
| Rank | −0.017 | 0.019 | −0.915 | 0.368 |
| Skinfold fat (redness) | ||||
| Intercept | −2.576 | 0.101 | −25.389 | <0.001 |
| Skinfold Fat | −0.007 | 0.021 | −0.359 | 0.722 |
| Age | 0.081 | 0.047 | 1.715 | 0.098 |
| Rank | −0.008 | 0.017 | −0.501 | 0.621 |
| Skinfold fat (darkness) | ||||
| Intercept | −1.100 | 0.114 | −9.680 | <0.001 |
| Skinfold fat | 0.028 | 0.023 | 1.191 | 0.244 |
| Age | −0.064 | 0.053 | −1.212 | 0.236 |
| Rank | −0.015 | 0.019 | −0.765 | 0.451 |
Bold values indicate statistical significance (P < 0.05).
DISCUSSION
Our results show that female skin redness and darkness are associated with male mating activity. Specifically, males mated more with females with redder and darker faces, and the top-ranked males, who have priority of access, preferentially consort redder-faced females. The fact that only skin redness, but not darkness, is associated with interfemale variation in sexual activity when the analyses are limited to proceptive periods of the ovarian cycles, suggest that color (i.e., blood oxygenation; Changizi et al. 2006; Stephen et al. 2009) rather than darkness (i.e., blood flow; Changizi et al. 2006; Stephen et al. 2009) is the more important variable in determining male preference.
Cumulative evidence strongly suggests that facial skin coloration is most likely to be a signal under sexual selection in both females and males in rhesus macaques. Results to date do not support the view that trait expression in females could be a nonadaptive by-product of the evolution in males: the trait is heritable in females, is related to higher reproductive success, with redder-faced females having higher annual fecundity (Dubuc, Winters, et al. 2014), and males prefer to consort and mate with these females (this study). Our conclusion that the trait is a sexually selected signal rather than a cue is further supported by what is known about the mechanism by which the facial skin reddens. The redness of rhesus macaque facial skin is caused by the binding of estrogen to estrogen receptors, which are expressed in far greater concentration in the face than in adjacent skin on the body (Rhodes et al. 1997). Targeted tissue-specific estrogen-receptor expression in the bare skin the facial and hindquarter area, which is not present in adjacent areas of the skin covered in fur, shows that facial skin has become specialized and targeted to create dark red color, and hence the visual signaling effect. As such, it is unlikely that facial skin color could be a mere cue of female quality that correlated directly with female physiological condition and hence fecundity, while not being under selection for its communicative value. Even if a redder face was originally a cue to female fecundity, perhaps initially because it is linked to the flow of oxygenated blood and indicates female health, then any male that started using facial redness to choose females - a preference we found in the present study would start to do better than males not doing so. This preference for the trait among males will start to select for greater expression of it in females for its communicative value, as long as females gain fitness benefits from increasing expression and their attractiveness to males (see below).
Collectively then, our results are consistent with the idea that female facial coloration acts as an ornament, indicating a female’s reproductive potential and value as a mate, with females being preferentially selected by males according to their degree of color expression. This would suggest that interfemale color variation is a classical intersexually selected ornament, but of an unusual kind, being exhibited by females of a large mammal species in the absence of sex-role reversal. Combined with results of prior studies, these results suggest that interfemale differences in redness may reflect interfemale differences in quality, while skin darkness is more linked to intrafemale variation in the probability of conception (Higham et al. 2010).
One key question to address relates to the fitness benefits that females might obtain by signaling their quality, since such benefits must manifest for the signal to be selected. Often, such benefits are framed in the context of “good genes” explanations, but the theoretical basis for expecting such effects, and the extent of the supporting empirical evidence available, continues to be the source of much debate (Møller and Alatalo 1999; Alonzo and Servedio 2019). In our system, males are highly variable in their reproductive success (Dubuc, Ruiz-Lambides, et al. 2014) and males who combine both high dominance rank and dark red face coloration have the greatest annual fecundity (Dubuc, Winters, et al. 2014). Females are preferentially proceptive to such males—those with redder, darker, facial coloration (Dubuc, Allen, et al. 2014), and prefer to look at such males when tested experimentally (Dubuc et al. 2016). This evidence is consistent with the idea that females do exhibit mate choice preferences. In a context where females exhibit mate choice preferences for specific males, and in which many females are synchronously fertile and sexually active at the same time such that males must choose which female to mate with (Dubuc et al. 2011) selection should favor signals in females that increase mating interest from desirable males. Because such males sire more offspring, this provides mechanisms by which females accrue benefits from mating with such males—females may themselves have increased fecundity by mating with such males, and will also give birth to sons who may inherit these favorable traits from their fathers.
We expect an interfemale quality ornament to be particularly likely to evolve in a species such as rhesus macaques, for multiple reasons. Rhesus macaques live in very large groups (as many as >400 individuals) on Cayo Santiago, and are thought to live at least facultatively in very large groups in the wild too (Southwick and Siddiqi 2011). They are also seasonal breeders; large group sizes and seasonal breeding are typically considered to be the two primary factors determining the likelihood of female reproductive synchrony (Kutsukake and Nunn 2006; Ostner et al. 2008; Gogarten and Koenig 2013). Consistent with this, the Cayo Santiago rhesus macaque population has an unusually high degree of female fertile phase and birth synchrony (Dubuc et al. 2011; Hernández‐Pacheco et al. 2016). This high degree of fertile phase synchrony means that there are often multiple females fertile on the same day. Given low degrees of body and canine size dimorphism in this species (Plavcan 2004), males cannot monopolize multiple females. Moreover, sperm competition is extremely strong, as indicated by large relative testis volume (Bercovitch and Rodriguez 1993), and despite increased investment in production, sperm may be a limited resource for promiscuously mating males. Collectively, these factors should select for male choosiness when selecting mates, and when allocating mating effort. Males may be able to parse out variation in different dimensions of female coloration—intracycle versus interfemale color variation. In support of this, males who are familiar with specific females prefer looking at images of those females when they exhibit their red fertile faces, while the same preferences are not shown across all males (Higham, Hughes, et al. 2011). Mathematical modeling has shown that learning the intracycle variation for specific females has significant fitness benefits for males (Ma and Higham 2018).
We made no prediction about whether females who were redder and/or darker would be in better or worse physiological condition with respect to our available measures (i.e., via measures of energetic status, such as body fat and BMI; urinary C-peptide of insulin concentrations (Girard-Buttoz et al. 2011; Higham, Girard-Buttoz, et al. 2011); and glucocorticoids (Dallman et al. 1993; Beehner and Bergman 2017). Our finding that females who are redder have higher fGCM concentrations could be explained in two different ways. Firstly, since redder-faced females have higher fecundity, they may differ in energetic condition, energy mobilization, and blood flow relative to other females. Under this scenario, these females are in different physical or physiological condition, which manifests itself in both the expression of redder-faces, indicating higher rates of oxygenated blood-flow, and higher fecundity. A second, nonmutually exclusive alternative is that the relationship is not directly related to underlying properties, but instead reflects the higher energetic burden of a lot of mating and constant attention, coercion, and harassment by males. This additional mating and other associated physical activities, may lead to higher levels of energy mobilization among these females, who hence exhibit higher fGCM concentrations. This seems likely to be the case in rhesus macaques, because proceptive females appear restless, and active (J. Higham, personal observation). Further studies will be needed to examine the extent to which skin color is directly affected by levels of physical activity.
The data presented here, combined with our results from prior studies showing that female signal expression indicates female fecundity (Dubuc, Winters, et al. 2014), are consistent with the reliable indicator hypothesis (Pagel 1994). Originally developed for understanding the function of female sexual swellings, we suggest it can also explain the function of interindividual variation in female face coloration in rhesus macaques, a species in which sexual swellings are absent. While it remains unclear whether sexual swelling size is acting as a reliable indicator of female quality in most species (though see Huchard et al. [2009] for chacma baboons) our results shed new light on the comparative function of sexual signals in primates. Indeed, sexual swellings are found in species where females are aseasonal breeders (Nunn 1999), and among Papionins have their strongest degree of expression in species with the least amount of female reproductive synchrony, such as crested macaques (Higham et al. 2012). It therefore seems less likely that the reliable indicator hypothesis will apply to sexual swellings compared to signals found in species in which many females are fertile at the same time, and in which males choose between multiple fertile females. There may also be substantive differences in the function of sexual swellings across species given extensive comparative variation in the socioecology and mating systems of primates that exhibit such swellings (for baboons, see Petersdorf et al. [2019], especially given that sexual swellings have evolved at least twice independently (Nunn 1999).
Our results support adding interindividual variation in female rhesus macaque facial coloration to the list of signals thought to represent female ornaments used by males in mate choice. Other examples of evidence for such ornaments include: male tropidurid lizards, which prefer females with white over red throat patches (Watkins 1997); house finches, in which males prefer females with redder plumage (Hill 1993); bluethroats, in which males prefer females with more colorful throat patches (Amundsen et al. 1997); and blue-footed boobies, in which males prefer females with bluer feet (Torres and Velando 2005). However, rhesus macaque female coloration has some elements of added complexity, in that it is also an intraindividual signal of variation in fertility and conceptive probability. It may represent an example of an intraindividual signal of fertility, which has secondarily evolved to become an interindividual signal of quality, as outlined in the evolutionary model of Huchard et al. (2009). It is also one of the only known color ornaments functioning as an interindividual signal indicator for females of any large mammal species. This is likely to be attributable to the relatively unusual (for a large mammal) sexual selection pressures experienced by rhesus macaques, in which large group sizes, reproductive seasonality, and a polygynandrous mating system cause reduced direct, and increased indirect, forms of male–male competition, while increasing female–female competition and direct female mate choice for the best males.
Acknowledgement
We thank the staff of the Caribbean Primate Research Center for their logistical support. We thank Sean Coyne, Greg Ruber, and Jesus Madrid, for help collecting samples and for sharing information on animal whereabouts in the field. We thank Andrea Heistermann for analysis of the laboratory samples, and Paul Brown for assistance with image measurements. We thank the Associate Editor Louise Barrett and two anonymous reviewers for comments that improved a previous version of this manuscript. The content of this publication does not represent the official views of NCRR, ORIP, or NIH.
FUNDING
This project was made possible by intramural funds from New York University (to J.H.), a Fonds de recherche du Québec – Société et culture fellowship (to C.D.), as well as the National Center for Research Resources and Office of Research Infrastructure Programs of NIH (grant number 2P40RR03640-25) to C.P.R.C.
Data availability: Analyses reported in this article can be reproduced using the data provided by Higham et al. (2020).
REFERENCES
- Alberts SC, Fitzpatrick CL. 2012. Paternal care and the evolution of exaggerated sexual swellings in primates. Behav Ecol. 23:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo SH, Servedio MR. 2019. Grey zones of sexual selection: why is finding a modern definition so hard? Proc R Soc B Biol Sci. 286(1909):20191325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann SA. 1962. A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Ann N Y Acad Sci. 102:338–435. [DOI] [PubMed] [Google Scholar]
- Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour. 49:227–267. [DOI] [PubMed] [Google Scholar]
- Amundsen T, Forsgren E, Hansen LTT. 1997. On the function of female ornaments: male bluethroats prefer colourful females. Proc R Soc Lond B Biol Sci. 264(1388):1579–1586. [Google Scholar]
- Andersson MB. 1994. Sexual selection. Princeton (NJ): Princeton University Press. [Google Scholar]
- Bateman AJ. 1948. Intra-sexual selection in Drosophila. Heredity. 2(Pt. 3):349–368. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw. 67:1–48. [Google Scholar]
- Beehner JC, Bergman TJ. 2017. The next step for stress research in primates: To identify relationships between glucocorticoid secretion and fitness. Horm Behav. 91:68–83. [DOI] [PubMed] [Google Scholar]
- Bercovitch FB, Rodriguez JF. 1993. Testis size, epididymis weight, and sperm competition in rhesus macaques. Am J Primatol. 30:163–168. [DOI] [PubMed] [Google Scholar]
- Bergman TJ, Beehner JC. 2008. A simple method for measuring colour in wild animals: validation and use on chest patch colour in geladas (Theropithecus gelada). Biol J Linn Soc. 94(2):231–240. [Google Scholar]
- Bergman TJ, Ho L, Beehner JC. 2009. Chest color and social status in male geladas (Theropithecus gelada). Int J Primatol. 30(6):791–806. [Google Scholar]
- Bradley BJ, Robbins MM, Williamson EA, Steklis HD, Steklis NG, Eckhardt N, Boesch C, Vigilant L. 2005. Mountain gorilla tug-of-war: silverbacks have limited control over reproduction in multimale groups. Proc Natl Acad Sci USA. 102:9418–9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill MH. 1995. The relation between the color of the illuminant and the color of the illuminated object. Color Res Appl. 20(1):70–76. [Google Scholar]
- Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal. 9(2): 378–400. [Google Scholar]
- Campbell BC, Gerald MS. 2004. Body composition, age and fertility among free-ranging female rhesus macaques (Macaca mulatta). J Med Primatol. 33:70–77. [DOI] [PubMed] [Google Scholar]
- Carpenter CR. 1942. Sexual behavior of free ranging rhesus monkeys (Macaca mulatta). I. Specimens, procedures and behavioral characteristics of estrus. J Comp Psychol. 33(1):113. [Google Scholar]
- Changizi MA, Zhang Q, Shimojo S. 2006. Bare skin, blood and the evolution of primate colour vision. Biol Lett. 2:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T. 2009. Sexual selection in females. Anim Behav. 77(1):3–11. [Google Scholar]
- Clutton-Brock T, McAuliffe K. 2009. Female mate choice in mammals. Q Rev Biol. 84:3–27. [DOI] [PubMed] [Google Scholar]
- Coffin D. 2008. DCRAW: decoding raw digital photos in linux. [Google Scholar]
- Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M. 1993. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 14:303–347. [DOI] [PubMed] [Google Scholar]
- Darwin C. 1872. The expression of emotions in animals and man. London (UK): John Murray. [Google Scholar]
- Deschner T, Heistermann M, Hodges K, Boesch C. 2004. Female sexual swelling size, timing of ovulation, and male behavior in wild West African chimpanzees. Horm Behav. 46:204–215. [DOI] [PubMed] [Google Scholar]
- Dixson AF. 1983. Observations on the evolution and behavioral significance of “sexual skin” in female primates. In: Rosenblatt JS, Hinde RA, Beer C, Busnel M-C, editors. Advances in the Study of Behavior. Vol. 13. New York (NY): Academic Press. p. 63–106. [Google Scholar]
- Dixson AF. 2012. Primate sexuality: comparative studies of the prosimians, monkeys, apes, and humans. Oxford (England): Oxford University Press. [Google Scholar]
- Domb LG, Pagel M. 2001. Sexual swellings advertise female quality in wild baboons. Nature. 410:204–206. [DOI] [PubMed] [Google Scholar]
- Dubuc C, Allen WL, Cascio J, Lee DS, Maestripieri D, Petersdorf M, Winters S, Higham JP. 2016. Who cares? Experimental attention biases provide new insights into a mammalian sexual signal. Behav Ecol. 27:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C, Allen WL, Maestripieri D, Higham JP. 2014. Is male rhesus macaque red color ornamentation attractive to females? Behav Ecol Sociobiol. 68:1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C, Brent LJN, Accamando AK, Gerald MS, MacLarnon A, Semple S, Heistermann M, Engelhardt A. 2009. Sexual skin color contains information about the timing of the fertile phase in free-ranging Macaca mulatta. Int J Primatol. 30(6):777–789. [Google Scholar]
- Dubuc C, Coyne SP, Maestripieri D. 2013. Effect of mating activity and dominance rank on male masturbation among free-ranging male rhesus macaques. Ethology. 119(11):1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C, Hughes KD, Cascio J, Santos LR. 2012. Social tolerance in a despotic primate: co-feeding between consortship partners in rhesus macaques. Am J Phys Anthropol. 148:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C, Muniz L, Heistermann M, Engelhardt A, Widdig A. 2011. Testing the priority-of-access model in a seasonally breeding primate species. Behav Ecol Sociobiol. 65:1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C, Muniz L, Heistermann M, Widdig A, Engelhardt A. 2012. Do males time their mate-guarding effort with the fertile phase in order to secure fertilisation in Cayo Santiago rhesus macaques? Horm Behav. 61:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C, Ruiz-Lambides A, Widdig A. 2014. Variance in male lifetime reproductive success and estimation of the degree of polygyny in a primate. Behav Ecol Sociobiol. 25(4):878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C, Winters S, Allen WL, Brent LJ, Cascio J, Maestripieri D, Ruiz-Lambides AV, Widdig A, Higham JP. 2014. Sexually selected skin colour is heritable and related to fecundity in a non-human primate. Proc R Soc Lond B Biol Sci. 281(1794):20141602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edward DA, Chapman T. 2011. The evolution and significance of male mate choice. Trends Ecol Evol. 26:647–654. [DOI] [PubMed] [Google Scholar]
- Emery MA, Whitten PL. 2003. Size of sexual swellings reflects ovarian function in chimpanzees (Pan troglodytes). Behav Ecol Sociobiol. 54(4):340–351. [Google Scholar]
- Engelhardt A, Hodges JK, Niemitz C, Heistermann M. 2005. Female sexual behavior, but not sex skin swelling, reliably indicates the timing of the fertile phase in wild long-tailed macaques (Macaca fascicularis). Horm Behav. 47:195–204. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick CL, Altmann J, Alberts SC. 2014. Sources of variance in a female fertility signal: exaggerated estrous swellings in a natural population of baboons. Behav Ecol Sociobiol. 68:1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S. 2011. An R companion to applied regression. 2nd ed. Thousand Oaks: Sage. [Google Scholar]
- Fujita S. 2010. Interaction between male and female mating strategies and factors affecting reproductive outcome. In: Nakagawa N, Nakamichi M, Sugiura H, editors. The Japanese macaques. Tokyo (Japan): Springer Japan. (Primatology Monographs). p. 221–239. [Google Scholar]
- Girard-Buttoz C, Higham JP, Heistermann M, Wedegärtner S, Maestripieri D, Engelhardt A. 2011. Urinary C-peptide measurement as a marker of nutritional status in macaques. PLoS One. 6:e18042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogarten JF, Koenig A. 2013. Reproductive seasonality is a poor predictor of receptive synchrony and male reproductive skew among nonhuman primates. Behav Ecol Sociobiol. 67(1):123–134. [Google Scholar]
- Hartig F. 2020. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.3.3.0. https://cran.r-project.org/package=DHARMa [Google Scholar]
- Heistermann M, Ademmer C, Kaumanns W. 2004. Ovarian cycle and effect of social changes on adrenal and ovarian function in Pygathrix nemaeus. Int J Primatol. 25(3):689–708. [Google Scholar]
- Heistermann M, Finke M, Hodges JK. 1995. Assessment of female reproductive status in captive-housed Hanuman langurs (Presbytis entellus) by measurement of urinary and fecal steroid excretion patterns. Am J Primatol. 37:275–284. [DOI] [PubMed] [Google Scholar]
- Heistermann M, Palme R, Ganswindt A. 2006. Comparison of different enzyme-immunoassays for assessment of adrenocortical activity in primates based on fecal analysis. Am J Primatol. 68:257–273. [DOI] [PubMed] [Google Scholar]
- Hernández‐Pacheco R, Rawlins RG, Kessler MJ, Delgado DL, Ruiz‐Lambides AV, Sabat AM. 2016. Discovery of a secular trend in Cayo Santiago macaque reproduction. Am J Primatol. 78(2):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP. 2006. The reproductive ecology of female olive baboons (Papio hamadryas anubis) at Gashaka-Gumti National Park, Nigeria. Lond Roehampton Univ. [Google Scholar]
- Higham JP, Brent LJ, Dubuc C, Accamando AK, Engelhardt A, Gerald MS, Heistermann M, Stevens M. 2010. Color signal information content and the eye of the beholder: a case study in the rhesus macaque. Behav Ecol. 21:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham J, Dubuc C. 2015. The evolution of female fertility signals in macaques. In: eLS (Encyclopedia of Life Sciences). Hoboken (NJ): Wiley-Blackwell. [Google Scholar]
- Higham JP, Girard-Buttoz C, Engelhardt A, Heistermann M. 2011. Urinary C-peptide of insulin as a non-invasive marker of nutritional status: some practicalities. PLoS One. 6:e22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Heistermann M, Maestripieri D. 2011. The energetics of male–male endurance rivalry in free-ranging rhesus macaques, Macaca mulatta. Anim Behav. 81(5):1001–1007. [Google Scholar]
- Higham JP, Heistermann M, Saggau C, Agil M, Perwitasari-Farajallah D, Engelhardt A. 2012. Sexual signalling in female crested macaques and the evolution of primate fertility signals. BMC Evol Biol. 12:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Hughes KD, Brent LJ, Dubuc C, Engelhardt A, Heistermann M, Maestriperi D, Santos LR, Stevens M. 2011. Familiarity affects the assessment of female facial signals of fertility by free-ranging male rhesus macaques. Proc R Soc Lond B Biol Sci. 278(1723):3452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Kimock CM, Mandalaywala TM, Heistermann M, Cascio J, Petersdorf M, Winters S, Allen WL, Dubuc C. 2020. Female ornaments: is red skin color attractive to males and related to condition in rhesus macaques? Behav Ecol. doi: 10.5061/dryad.6m905qfz3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, MacLarnon AM, Ross C, Heistermann M, Semple S. 2008. Baboon sexual swellings: information content of size and color. Horm Behav. 53:452–462. [DOI] [PubMed] [Google Scholar]
- Higham JP, Maestripieri D. 2014. The costs of reproductive success in male rhesus macaques (Macaca mulatta) on Cayo Santiago. Int J Primatol. 35(3–4):661–676. [Google Scholar]
- Hill GE. 1993. Male mate choice and the evolution of female plumage coloration in the house finch. Evolution. 47:1515–1525. [DOI] [PubMed] [Google Scholar]
- Hoffman CL, Higham JP, Heistermann M, Coe CL, Prendergast BJ, Maestripieri D. 2011. Immune function and HPA axis activity in free-ranging rhesus macaques. Physiol Behav. 104:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchard E, Courtiol A, Benavides JA, Knapp LA, Raymond M, Cowlishaw G. 2009. Can fertility signals lead to quality signals? Insights from the evolution of primate sexual swellings. Proc R Soc B Biol Sci. 276(1663):1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives HE. 1912. Studies in the photometry of lights of different colors. I. Spectral luminosity curves obtained by the equality of brightness photometer and flicker photometer under similar conditions. Philos Mag. 24:149–188. [Google Scholar]
- Kraaijeveld K, Kraaijeveld-Smit FJL, Komdeur J. 2007. The evolution of mutual ornamentation. Anim Behav. 74(4):657–677. [Google Scholar]
- Kutsukake N, Nunn CL. 2006. Comparative tests of reproductive skew in male primates: the roles of demographic factors and incomplete control. Behav Ecol Sociobiol. 60(5):695–706. [Google Scholar]
- Lee PC. 1987. Nutrition, fertility and maternal investment in primates. J Zool. 213(3):409–422. [Google Scholar]
- Ma WJ, Higham JP. 2018. The role of familiarity in signaller-receiver interactions. J R Soc Interface. 15:20180568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandalaywala TM, Higham JP, Heistermann M, Parker KJ, Maestripieri D. 2014. Physiological and behavioural responses to weaning conflict in free-ranging primate infants. Anim Behav. 97:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JH. 1995. Do female rhesus macaques choose novel males? Am J Primatol. 37:285–296. [DOI] [PubMed] [Google Scholar]
- Michael RP, Zumpe D. 1970. Sexual initiating behaviour by female rhesus monkeys (Macaca mulatta) under laboratory conditions. Behaviour. 36(3):168–186. [Google Scholar]
- Møller AP, Alatalo RV. 1999. Good-genes effects in sexual selection. Proc R Soc Lond B Biol Sci. 266(1414):85–91. [Google Scholar]
- Nunn CL. 1999. The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim Behav. 58:229–246. [DOI] [PubMed] [Google Scholar]
- Osorio D, Vorobyev M. 2005. Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc R Soc B Biol Sci. 272(1574):1745–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostner J, Nunn CL, Schülke O. 2008. Female reproductive synchrony predicts skewed paternity across primates. Behav Ecol. 19:1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel M. 1994. The evolution of conspicuous oestrous advertisement in Old World monkeys. Anim Behav. 47(6):1333–1341. [Google Scholar]
- Paul A. 2002. Sexual selection and mate choice. Int J Primatol. 23(4):877–904. [Google Scholar]
- Petersdorf M, Weyher AH, Kamilar JM, Dubuc C, Higham JP. 2019. Sexual selection in the Kinda baboon. J Hum Evol. 135:102635. [DOI] [PubMed] [Google Scholar]
- Plavcan JM. 2004. Sexual selection, measures of sexual selection, and sexual dimorphism in primates. In: Kappeler PM, Van Schaik CP, editors. Sexual selection in primates: new and comparative perspectives. New York (NY): Cambridge University Press. p. 230–252. [Google Scholar]
- R Development Core Team . 2020. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. [Google Scholar]
- Rhodes L, Argersinger ME, Gantert LT, Friscino BH, Hom G, Pikounis B, Hess DL, Rhodes WL. 1997. Effects of administration of testosterone, dihydrotestosterone, oestrogen and fadrozole, an aromatase inhibitor, on sex skin colour in intact male rhesus macaques. J Reprod Fertil. 111:51–57. [DOI] [PubMed] [Google Scholar]
- Rigaill L, Higham JP, Winters S, Garcia C. 2019. The redder the better? Information content of red skin coloration in female Japanese macaques. Behav Ecol Sociobiol. 73(8):103. [Google Scholar]
- Rigaill L, MacIntosh AJ, Higham JP, Winters S, Shimizu K, Mouri K, Furuichi T, Garcia C. 2015. Multimodal advertisement of pregnancy in free-ranging female Japanese macaques (Macaca fuscata). PLoS One. 10:e0135127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell JM, Charpentier M, Wickings EJ. 2005. Mate guarding and paternity in mandrills: factors influencing alpha male monopoly. Anim Behav. 70(5):1105–1120. [Google Scholar]
- Southwick CH, Siddiqi MF. 2011. India’s rhesus populations: protectionism versus conservation management. In: Gumert MD, Jones-Engel L, Fuentes A, editors. Monkeys on the edge: ecology and management of long-tailed macaques and their interface with humans. New York (NY): Cambridge University Press. p. 275–292. [Google Scholar]
- Stephen ID, Coetzee V, Law Smith M, Perrett DI. 2009. Skin blood perfusion and oxygenation colour affect perceived human health. PLoS One. 4:e5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Párraga CA, Cuthill IC, Partridge JC, Troscianko TS. 2007. Using digital photography to study animal coloration. Biol J Linn Soc. 90(2):211–237. [Google Scholar]
- Stevens M, Stoddard MC, Higham JP. 2009. Studying primate color: towards visual system-dependent methods. Int J Primatol. 30(6):893–917. [Google Scholar]
- Torres R, Velando A. 2005. Male preference for female foot colour in the socially monogamous blue-footed booby, Sula nebouxii. Anim Behav. 69(1):59–65. [Google Scholar]
- Trivers R. 1972. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man 1871-1971. New York (NY): Aldine. p. 136-179. . [Google Scholar]
- Waitt C, Gerald MS, Little AC, Kraiselburd E. 2006. Selective attention toward female secondary sexual color in male rhesus macaques. Am J Primatol. 68:738–744. [DOI] [PubMed] [Google Scholar]
- Wallen K, Winston LA, Gaventa S, Davis-DaSilva M, Collins DC. 1984. Periovulatory changes in female sexual behavior and patterns of ovarian steroid secretion in group-living rhesus monkeys. Horm Behav. 18:431–450. [DOI] [PubMed] [Google Scholar]
- Watkins GG. 1997. Inter-sexual signalling and the functions of female coloration in the tropidurid lizard Microlophus occipitalis. Anim Behav. 53(4):843–852. [Google Scholar]
- Zinner D, Alberts SC, Nunn CL, Altmann J. 2002. Evolutionary biology: significance of primate sexual swellings. Nature. 420(6912):142–143. [DOI] [PubMed] [Google Scholar]