Abstract
The adverse side effects and toxicity caused by the non-targeted delivery of doxorubicin has emphasized the demand of emerging a targeted delivery system. The goal of this study is to enhance the delivery of doxorubicin by formulating an aptamer-labeled liposomal nanoparticle delivery system that will carry and deliver doxorubicin specifically into Her-2+ breast cancer cells. Twelve liposomal batches were prepared using different saturated (HSPC and DPPC) and unsaturated (POPC and DOPC) lipids by thin film hydration. The liposomes were characterized for their particle size, zeta potential, and drug encapsulation efficiency. The particles were also assessed for in vitro toxicity and DOX delivery into the breast cancer cells. The formulations, F1 through F12, had a small particle size of less than 200 nm and a high entrapment efficiency of about 88 ± 5%. The best formulation, F5, had a particle size of 101 ± 14nm, zeta potential of + 5.63 ± 0.46 mV, and entrapment efficiency of ≈ 93%. The cytotoxicity studies show that the DOX-loaded liposomal formulations are more effective in killing cancer cells than the free DOX in both MCF-7 and SKBR-3 cells. The uptake studies show a significant increase in the uptake of the aptamer-labeled liposomes (i.e., F5) by more than 60% into Her-2+ MCF-7 and SKBR-3 breast cancer cells compare to non-aptamer-labeled nanoparticles. F5 also shows ≈ 1.79-fold increase in uptake of DOX in the Her-2+ cells compared to the Her-2- cells. This preliminary study indicates that aptamer-labeled F5 nanoparticles among several batches showed the highest uptake as well as the targeted delivery of doxorubicin into Her-2+ breast cancer cells. Thus, aptamer targeted approach results in substantial reduction in the dose of DOX and improves the therapeutic benefits by promoting the target specificity.
Keywords: liposome, doxorubicin hydrochloride, aptamer A6, doxorubicin uptake, Her-2+ breast cancer
INTRODUCTION
Breast cancer is one of the most frequent malignant tumors in women (1). The USA has more than 1 million new breast cancer cases every year, with an increased incidence of the disease by 0.3% each year. In the year 2020, estimated 325,010 women will be diagnosed with both invasive and non-invasive breast cancer in the USA and about 42,170 women are estimated to die of the disease (2). Chemotherapy is the primary choice of treatment for early stages of cancer and is also used in conjugation with surgery and radiation for the late stages of cancer. However, chemotherapy is associated with the unavoidable side effects, leading to toxicity to patients and development of drug resistance (3). The development of advanced drug delivery systems has shown great potential to yield enhanced therapeutic efficacy and comparatively higher accumulation of drug in tumor cells along with minimal exposure to normal tissues (4). But, the development of multidrug resistance (MDR) in cancer cells is of grave concern, limiting the efficacy of anticancer agents and, hence, the failure of therapy (5).
Doxorubicin is a very potent cytotoxic anticancer drug and was first isolated from Streptomyces peucetius. It directly inhibits topoisomerase II and nucleic acid synthesis. As a result of the inhibition, the proliferation of cancer cells is terminated. Drugs with anthracyclines, as the active ingredient, have been widely used for treating cancer (6). Clinical research and application revealed that in spite of its potential anticancer effects, doxorubicin is highly toxic, and its long-term application may cause dose-dependent irreversible cardiomyopathy, severe cardiac toxicity, or liver damage, thereby limiting its application in clinical practice (7). Fortunately, clinical application of liposomes could resolve most of these limitations and help to reduce the side effects of the drug (8).
Liposomes are bi-layered phospholipid vesicles with an aqueous core that can sheath both hydrophilic and hydrophobic drugs. In fact, liposomes can retain the drugs until being disrupted, indicating that they can promote sustained release formulation of drugs. Besides, they accumulate in malignant tumors, thereby enhancing the selectivity of the anticancer drugs which leads to reduced toxicity (9). The enhanced retention and permeability (EPR) effect plays a huge role of passively accumulating the nanoparticles into the tumor tissue. The EPR effect is caused by angiogenesis which gives rise to leaky and defective blood vessels near the tumor site (10). Nanoparticles have more benefits over traditional medication alongside their passive targeting. Nanoparticles have lesser side effects and are promising in overcoming multidrug resistance in cancer cells, which is also a massive challenge in recent tumor therapy (11).
There are several liposomal anticancer drugs authorized by the United State Food and Drug Administration including doxorubicin. A long-acting form of doxorubicin encapsulated in liposomes has been marketed since the mid-1990s for the treatment of various malignancies. It is also known as Doxil in the USA or Caelyx in Europe (12). This liposomal formulation contains polyethylene glycol (PEG) coated-liposomal doxorubicin, which is capable of transferring doxorubicin to tumor sites. At present, liposomal doxorubicin is a therapeutic option in the treatment of AIDS-related Kaposi’s sarcoma, metastatic breast cancer, advanced ovarian cancer, and relapsed/refractory multiple myelomas (13).
Cationic amphiphiles are commonly used as transfer agents for genes. DNA added to cationic liposomes are used for transfecting cultured cells. This involves the formation of liposomes-DNA complexes which occurs due to the interaction of excess positive charges on the liposomes that binds with the negative charge on the DNA. Thus the complexes can enter into the cells by endocytosis where the complexes are destabilized by the endosomal membrane and release the contents into the cell (14). There is very little information present on how the composition of cationic formulations can affect cellular membranes. On the other hand, fusion of liposomes has been extensively studied. Liposomal fusion occurs when the charged lipid bodies are neutralized due to changes in pH or presence of neutralizing multivalent ions. Fusion can be improved by using lipids with unsaturated fatty acyl chains or small uncharged headgroups. Thus, charge neutralization on the lipid surface leads to dehydration of the lipid bilayers leading to membrane fusion, which in turn results in non-bilayer lipid intermediates. Thus, understanding the lipid properties may open new doors to better lipid mediated transfection and that can be very useful in understanding the variation in transfection within different cell lines (15).
Furthermore, if the drug is not targeted specifically to the cancer cells, it might still have a significant contribution in treating cancer, however, because of non-targeted delivery, the drug can cause a significant adverse side effects and toxicity to non-cancerous normal cells and tissues. To prevent this drug-generated toxicity to normal cells and tissues, and more targeting the drug to cancer cells, a targeted delivery system has been developed by labeling the particles with Apt-A6 that can recognize and bind Her-2 receptors on Her-2+ breast cancer cells (16). Aptamers are short oligonucleotides of DNA or RNA that have been tested for targeted drug delivery (17). The properties such as smaller size, target specificity, lack of immunogenicity, and penetrability make them excellent nanoparticle carrier systems for drugs, nucleic acids etc. into the targeted cells.
In this paper, for the first time, we did a comparative study of liposomes with both saturated and unsaturated lipids. A thin film hydration method has been proposed to produce a stable DOX loaded PEGylated liposomes (DOXLP). We have also examined whether the surface labeling of the DOXLP with Apt-A6 helps in increasing the delivery of doxorubicin into Her-2+ breast cancer cells compare to non-targeted DOX liposomes and also with Her-2- breast cancer cells.
MATERIALS AND METHODS
Materials
L-α-phosphatidylcholine, hydrogenated (Soy) (HSPC); 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC); 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC); 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC); 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt) (DSPE-PEG-2000); 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (ammonium salt) (Mal-PEG-2000); Cholesterol (CHO); 1,2-dioleoyl-3-trimethylammonium-propane (chloride salt) (DOTAP); and dimethyldidodecylammonium bromide (DDAB) were purchased from Avanti Polar- lipids Inc. (Birmingham, AL, USA). DOX-NP (liposomal encapsulated doxorubicin) was purchased from Avanti Polar- lipids Inc. (Birmingham, AL, USA). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from VWR International (Texas, USA). Penicillin-streptomycin-neomycin antibiotics and fetal bovine serum albumin (BSA) were purchased from Gibco, Invitrogen Corp. (Carlsbad, CA, USA). Aptamers-A6 and GFP-aptamers were purchased from Life Technologies (Carlsbad, CA). SKBR3, MCF7 and MDA-MB-231 cells were bought from the American Type Culture Collection (ATCC, Manassas, VA). All other reagents were of analytical grade and were supplied by Sigma Chemical Co. (St. Louis, MO, USA).
Preparation of Liposomes
A schematic representation of the preparation of doxorubicin-loaded liposome has been shown in Fig. 1. Non-targeted liposomes were prepared with various phosphatidylcholines, cholesterol, and mPEG-DSPE. The phosphatidylcholines were HSPC, POPC, DOPC, and DPPC as shown in Table I. Briefly, 100 mg phosphatidylcholines, 20 mg DSPE-PEG 2000, 0.25 mg Mal-PEG, 40 mg DOPE, 40 mg DOTAP/DDAB, and 30 mg CHO were added in 10 mL of HPLC-grade chloroform in a 100-mL round bottom flask. The flask was rotated at 100 rpm while dipping in water bath maintaining a temperature of 55°C. Once the film was formed, the flask was placed in a vacuum desiccator overnight to remove any organic solvent residue. The resulting film of the lipid polymer mixture was hydrated in 120 mM ammonium sulfate. All resulting liposomes were sonicated for 4min using probe sonicator (Branson Probe Sonicator, USA) maintaining a temperature of 55°C. Doxorubicin was loaded into liposomes using the ammonium sulfate loading method as shown in Fig. 1 (18).
Fig. 1.
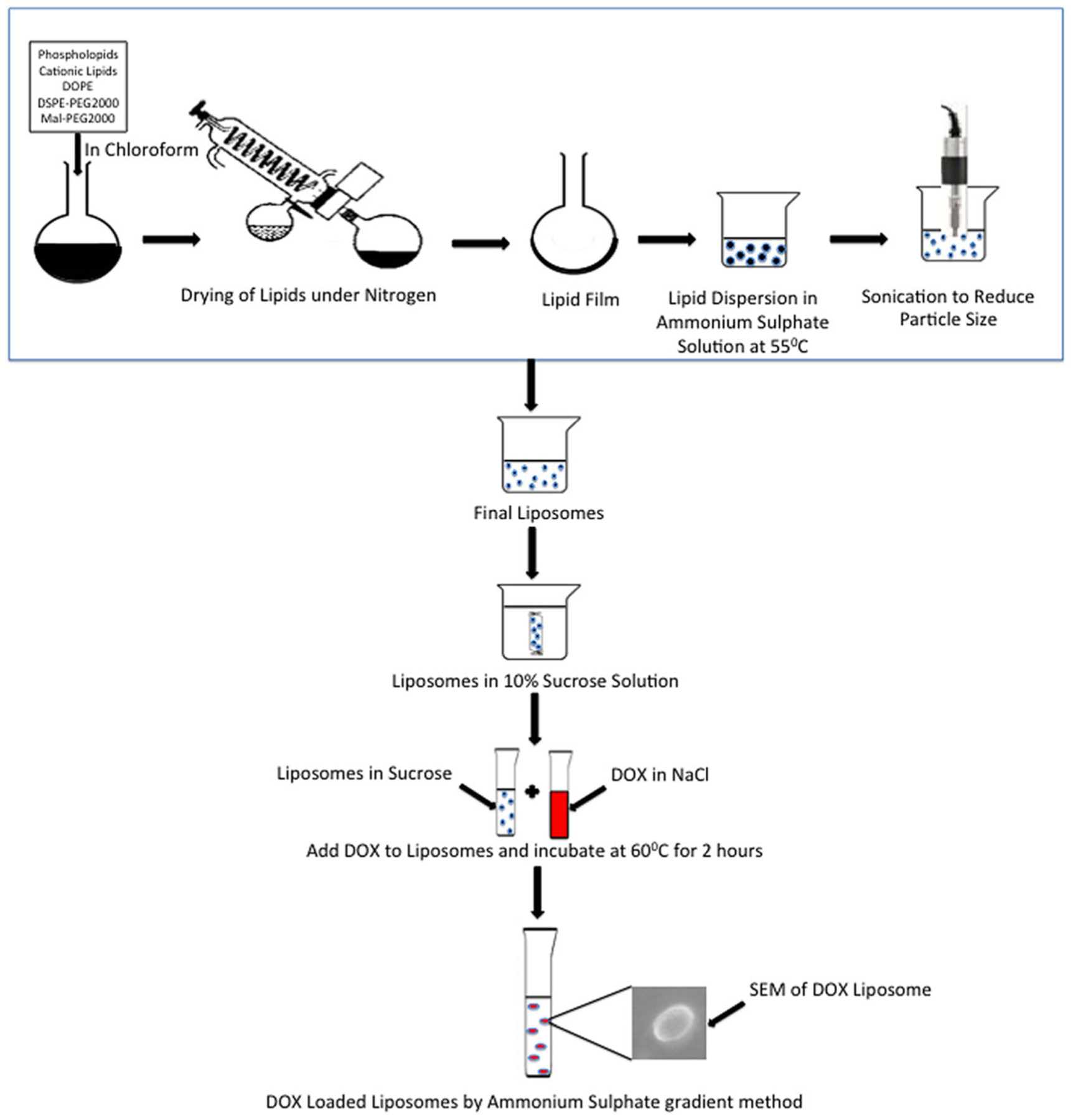
Schematic representation of preparation of doxorubicin-loaded liposomes
Table I.
Chemical Structure of Lipids
| Phospholipids | Chemical Structure |
|---|---|
| L-α-phosphatidylcholine, hydrogenated (Soy); HSPC | 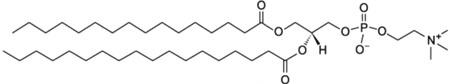 |
| 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine; POPC | 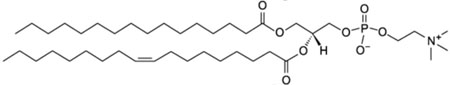 |
| 1,2-dioleoyl-sn-glycero-3-phosphocholine; DOPC | 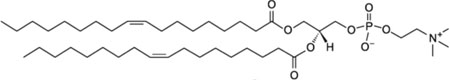 |
| 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; DPPC | 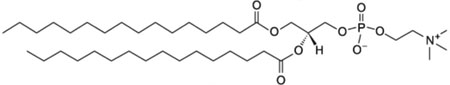 |
| 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt); DSPE-PEG-2000 | 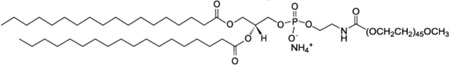 |
| 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (ammonium salt); Mal-PEG-2000 | 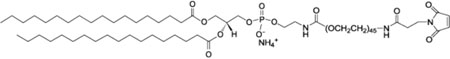 |
| Cholesterol; CHO | 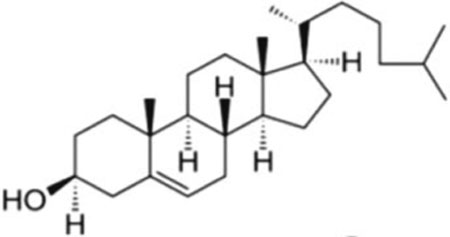 |
| 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOPE | 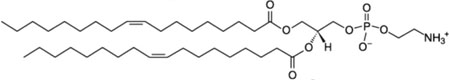 |
| 1,2-dioleoyl-3-trimethylammonium-propane (chloride salt); DOTAP | 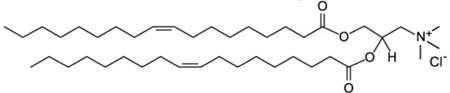 |
| Dimethyldidodecylammonium bromide; DDAB | 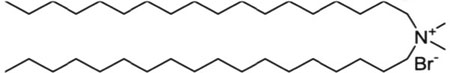 |
Preparation of Aptamer-Labeled Nanoparticles
The aptamer-labeled liposomes were prepared by following the protocol from Powell et al. (16). Briefly, the liposomes were prepared with the Mal-PEG on their surface for binding to the aptamer. A stock concentration of 10 μM aptamer A6 (NH2-Apt-6) was used to give a final concentration of 0.1 μM in the 6-well plate along with 0.1 μM DOX. The aptamer with/without DOX liposomes were incubated for 2 h at room temperature before treatment.
Physicochemical Characterization of Liposome
Particle Size Distribution, Polydispersity Index, and Zeta Potential Measurement
Particle size and polydispersity of the liposomes were determined by photon correlation spectroscopy (PCS) using Zeta sizer (PSS Systems, USA). Zeta potential of liposomes was determined by electrophoretic mobility determination using Zeta sizer (PSS systems, USA). The liposome samples were analyzed after appropriate dilution with deionized water.
Entrapment Efficiency
For the entrapment efficiency, the amount of DOX encapsulated in the liposomes was determined by HPLC. A reverse- phase HPLC column (Agilent Eclipse XDB-C18, 4.6 × 250 mm, 5 μm) was used. The mixture was centrifuged (Universal 32) for 60 min at 14000 rpm, the supernatant containing DOX was collected. The mobile phases was composed of 0.5% (v/v) glacial acetic acid (A) and acetonitrile (B) filtered through a membrane filter (0.2 μm) at a gradient program (A/B) of 80: 20 (t = 8 min) for 15 min at a flow rate of 0.7 mL/min. The column temperature was maintained at 30°C. The excitation and emission wavelengths were 470 nm and 590 nm, respectively. The peak area was also monitored at a wavelength of 253 nm. The injection volume was 10 μL. The calibration curve was linear in the range of 10–10,000 ng/mL with a regression coefficient R2 = 0.997. The drug encapsulation efficiency was defined as the percentage of the amount of DOX encapsulated in the liposomes to total amount of drug.
The absorbance was measured and the entrapment efficiency was calculated by using following formula:
where, EE is the concentration of entrapped drug (ng/mL), CT is the initial concentration of drug used in formulating the liposomes (ng/mL), CS is the concentration of drug in the supernatant (ng/mL), and EE (%) is the percentage of the drug’s entrapment.
Formation and Morphology
The scanning electron microscopy (SEM) pictures of liposomes were taken on a FEI Nova NanoSEM 400 machine fitted with a tungsten (W) filament. The liposome samples were dried in vacuum and mounted on a metal stub using double sided carbon tape followed by sputter-coating with gold at a plasma current of 30 mA for 120 s. Finally, the pictures were taken at an accelerating voltage of 10 kV and observed using 1-μm scale bars.
Cytotoxicity Assay
Her-2+ SKBR3 and MCF7 cells were bought from American Type Culture Collection (ATCC, Manassas, VA). In our previous study, we have reported the expression of Her-2 on the cells surface of those cells (16). Briefly, MCF7 and SKBR3 were plated at 10,000 cells/ well in 96-well plates. Cells were treated either with DOX solution or DOXLP at different concentrations for 48 h to check the effect of the liposomes. Cells were washed with phosphate buffered saline (PBS; 1X, pH 7.4) and 100 μL of 0.5% (v/v) glutaraldehyde was added for fixing the plate and incubated for 30 min. Next, 0.5% (w/v) crystal violet dye was added and the plate was incubated for another 20 min. The plate was washed with tap water and dried before adding 1% (w/v) sodium lauryl sulfate (SLS) and read at 570 nm.
Cellular Uptake
Microscopy
To observe the cellular uptake, two breast cancer cell lines, MCF-7 and SKBR3 cells cultured in DMEM media with 10% fetal bovine serum (FBS) were seeded in 6-well plates with a density of 1.5 × 105 cells/well and incubated in 37°C with 5% CO2 for 24 h. Apt-A6-FITC complexed with DOXLP was added into each well and incubated for 2 h, and then the cells were washed thrice with PBS, respectively. Afterward, image analysis of cells was performed with fluorescence microscopy (Olympus, Japan).
Flow Cytometry
For flow cytometry, cells were seeded in 6-well plates and treated with the liposomes when the cells were approximately 70% confluent. The Apt-A6-FITC and blank liposomes or DOXLP were incubated for 2 h at room temperature for forming the complex. The complex was added to each of the wells and further incubated for 2 h. The media was removed, the wells were washed with PBS twice, and cells were harvested by trypsination. Cell-associated fluorescence was detected using a flow cytometer (BD AccuriTM C6 Plus, Becton Dickinson, San Jose, CA). Minimum of 10,000 cells was analyzed after exclusion of the cellular debris from the analysis. The results are reported as the median of the distribution of cell fluorescence intensity obtained by analyzing 10,000 cells in the gate.
Authentication of Key Biological and Chemical Resources and Adherence to the Standard Biosecurity and Institutional Safety Procedures
Cell Line Used.
The cell lines used in this study were properly maintained and occasionally checked the expression profile of the known markers to determine the validity of those cell lines.
Chemicals Used.
All the chemicals used in this study were stored and handled as suggested by the representative manufacturer.
Adherence to the Standard Biosecurity and Institutional Safety Procedures.
The study was conducted following the standard biosecurity and institutional safety procedures. The disposal of biohazard materials was also conducted following the university mandated biohazard disposal guidelines.
Statistical Analysis
All of the results were remarked with the mean ± SD, and the one-way analysis of variance (ANOVA) was employed for statistical analysis of the data. All statistical analyses were performed using GraphPad prism V3.05 (GraphPad Software, La Jolla, California) and Microsoft excel.
RESULTS
Preparation of DTX Liposomes
DOX liposome formulation was prepared by thin film hydration method by using different ratios of phospholipid mixtures. The different compositions have been shown in Table II. A schematic representation of the preparation of doxorubicin-loaded liposomes has been shown in Fig. 1. Several batches of DOX liposomes were prepared which showed the particle size of DOX liposomes ranging from 98.7 to 181.2 nm and the entrapment efficiency of DTX ranged from 74.9 to 94.1%. Batches F1, F2, and F3 were formulated with HSPC; F4, F5, and F6 were formulated with POPC; F7, F8, and F9 were formulated with DOPC; and F10, F11, and F12 were formulated with DPPC. The batches were prepared with either DOTAP or DDAB as their cationic lipid or they had CHO. The batches prepared with DOTAP (F2, F5, F8, and F11) had the smallest particle size and the batches with CHO (F1, F4, F7, and F11) had the highest particle size. Batches F1, F4, F7, and F11 were standards for each of the four sets of formulation. Mal-PEG was added in the batches where cationic lipids were present. Mal-PEG was added so that it could bind to the aptamer and allow it to target the Her-2 receptors on the surface of the cancer cells. Optimized DOX liposome batches were made based on lowest particle size and highest entrapment efficiency and the composition of two of the best batches were 10 mg DOX, 150 mg POPC, 40 mg DOTAP, 40 mg DOPE, 20 mg DSPE-mPEG2000, and 0.25 mg Mal-PEG for batch F5; and 10 mg DOX, 150 mg POPC, 40 mg DOTAP, 40 mg DOPE, 20 mg DSPE-mPEG2000, and 0.25 mg Mal-PEG for batch F8. The formulations F5 and F8 had particle size of 101.70 ± 14.04 nm and 98.7 ± 13.25 nm, zeta potential of + 5.63 ± 0.46 mV and + 7.94 ± 0.32 mV, and entrapment efficiency of 92.8% and 94.1%, respectively. The optimized lipid composition was further used for complexation with Aptamer-A6. Particle size distribution, zeta potential, and entrapment efficiency data of different liposomal formulations are shown in Table II.
Table II.
Composition of the Different Liposomal Batches
| Formulation No. | Composition | |||||
|---|---|---|---|---|---|---|
| F1 | HSPC | DSPE-PEG-2000 | CHO | |||
| F2 | HSPC | DSPE-PEG-2000 | Mal-PEG-2000 | DOPE | DOTAP | |
| F3 | HSPC | DSPE-PEG-2000 | Mal-PEG-2000 | DOPE | DDAB | |
| F4 | POPC | DSPE-PEG-2000 | CHO | |||
| F5 | POPC | DSPE-PEG-2000 | Mal-PEG-2000 | DOPE | DOTAP | |
| F6 | POPC | DSPE-PEG-2000 | Mal-PEG-2000 | DOPE | DDAB | |
| F7 | DOPC | DSPE-PEG-2000 | CHO | |||
| F8 | DOPC | DSPE-PEG-2000 | Mal-PEG-2000 | DOPE | DOTAP | |
| F9 | DOPC | DSPE-PEG-2000 | Mal-PEG-2000 | DOPE | DDAB | |
| F10 | DPPC | DSPE-PEG-2000 | CHO | |||
| F11 | DPPC | DSPE-PEG-2000 | Mal-PEG-2000 | DOPE | DOTAP | |
| F12 | DPPC | DSPE-PEG-2000 | Mal-PEG-2000 | DOPE | DDAB |
HSPC, L-α-phosphatidylcholine, hydrogenated (Soy); POPC, 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine; DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; DSPE-PEG-2000, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt); Mal-PEG-2000, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (ammonium salt); CHO, cholesterol; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane (chloride salt); DDAB, dimethyldidodecylammonium bromide
Scanning Electron Microscopy Analysis
The scanning electron microscopy (SEM) images of the liposomes are shown in Fig. 2. The liposomes look like vesicles, which are spherical in shape and have a diameter of about 100–200 nm (S1). Agglomeration occurred due to the drying process before the SEM analysis. Also, the SEM pictures show that the particle size of the liposome has slightly increased, after the liposomes were loaded with the drug.
Fig. 2.
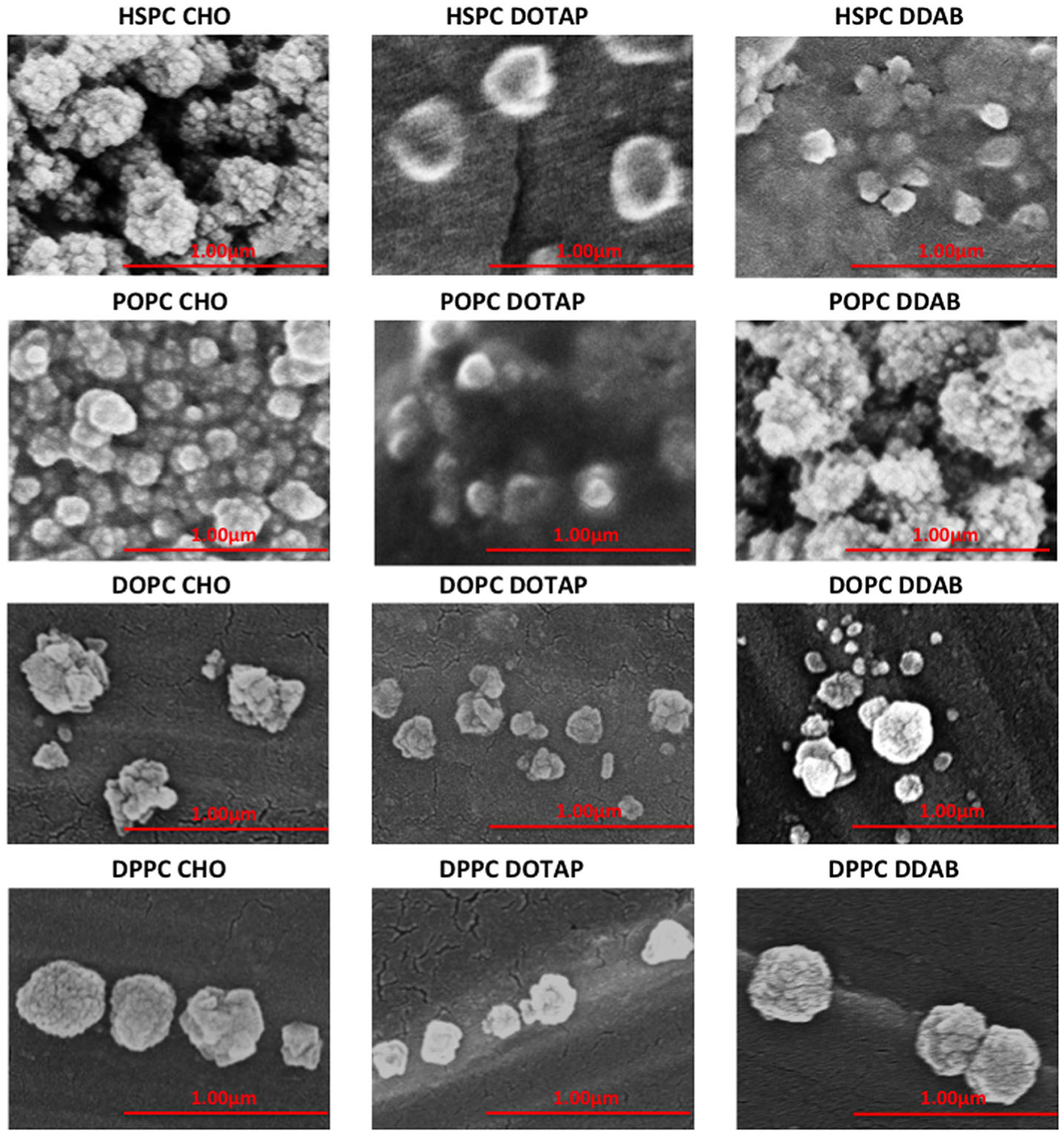
Scanning electron microscopy (SEM) images of DOX-loaded liposomal formulations
Cytotoxicity of DOX Liposomes on Different Breast Cancer Cells
As seen from Fig. 3, the IC50 of designed liposomal DOX and free DOX on different breast cancer cell lines were compared. The IC50 for DOX solution were 0.68 ± 0.03 μM and 0.67 ± 0.01 μM for MCF7 and SKBR3, respectively. The IC50 of liposomal DOX for MCF-7 for all the formulations were in the range from 0.34 ± 0.003 μM to 0.63 ± 0.01 μM and for SKBR3 were in the range from 0.41 ± 0.02 μM to 0.65 ± 0.03 μM. The DOTAP formulations showed lower IC50s when the unsaturated lipids, POPC and DOPC, were used, and the DDAB formulations showed lower IC50s when saturated lipids, HSPC and DPPC, were used. Overall, liposomal formulations were more effective in the breast cancer cells than the free DOX (S2) in both the cell lines.
Fig. 3.
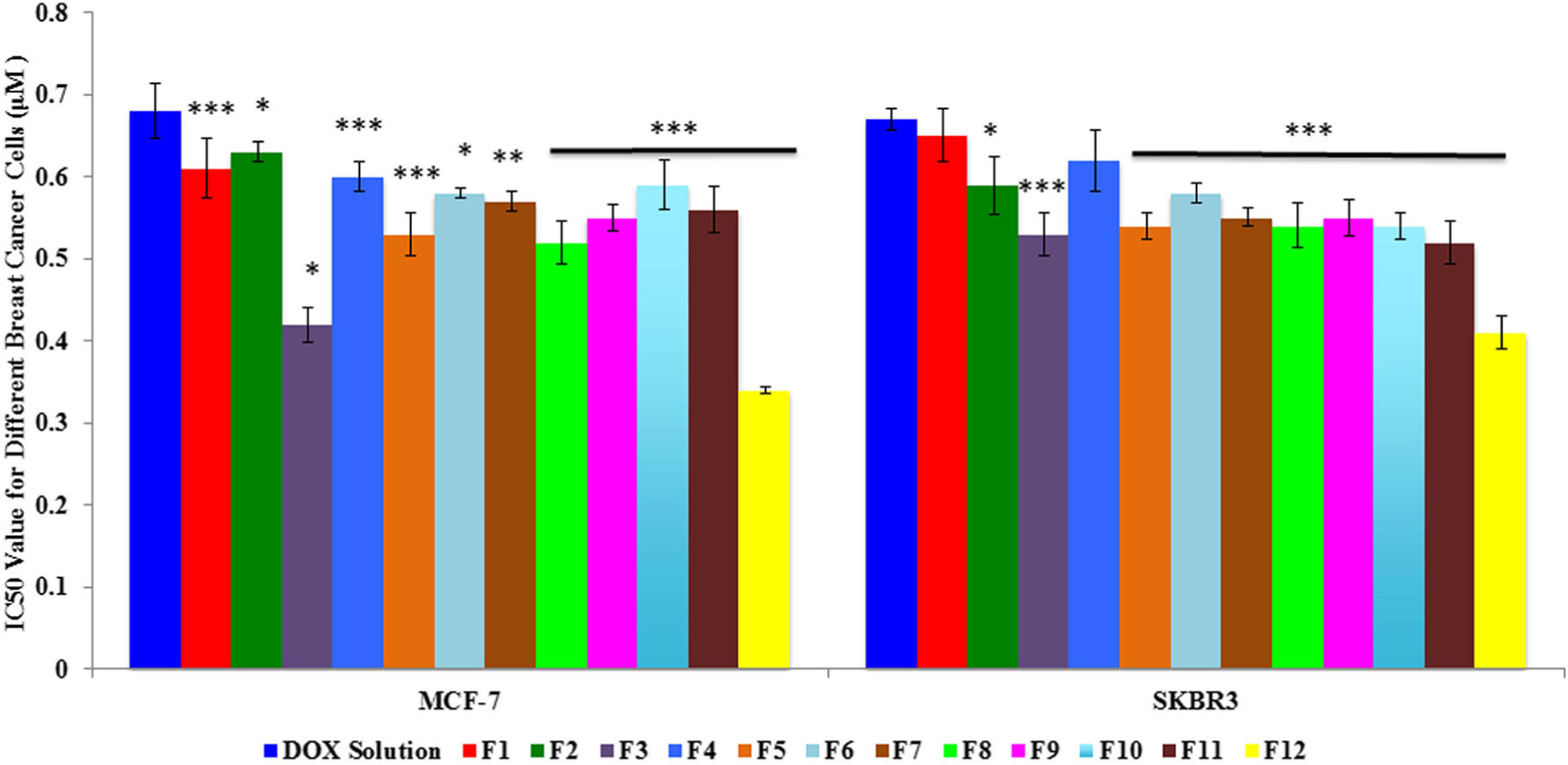
Inhibitory concentration 50 (IC50) for doxorubicin hydrochloride and different doxorubicin-loaded liposomal formulations (F1–F12) on MCF7 and SKBR3 breast cancer cell lines. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the doxorubicin solution
Measurement of Cellular Uptake of DOX Liposomes by Fluorescence Microscopy
The cellular fluorescence uptake of the aptamer-FITC liposomal complex is shown in Fig. 4. The cells were incubated with liposome-aptamer complexes of formulations F1, F5, F8, and F12 on individual wells for 2 h. Selective uptake of the liposomes by Her-2+ cells was initially investigated by fluorescence microscopy utilizing the green fluorescence of FITC. After incubation of cell lines with liposomes, the liposomes would bind to the Her-2 receptors on the surface of the breast cancer cells and enter into the cell membrane where they would appear green due to the presence of FITC attached to the aptamer. The lowest fluorescence is seen in F1 for both MCF7 and SKBR3 that suggests that F1 liposomes were not targeted, as it did not have mal-PEG labeled on the surface to bind to the Aptamer. Higher amount of fluorescence is detected for F5, F8, and F12, and F5 showed the highest fluorescence compared to F8 and F12. All these three formulations had Mal-PEG to bind to the aptamer and hence were more specific in targeting the Her-2+ breast cancer cells and F5 had the most uptake among all the four formulations.
Fig. 4.
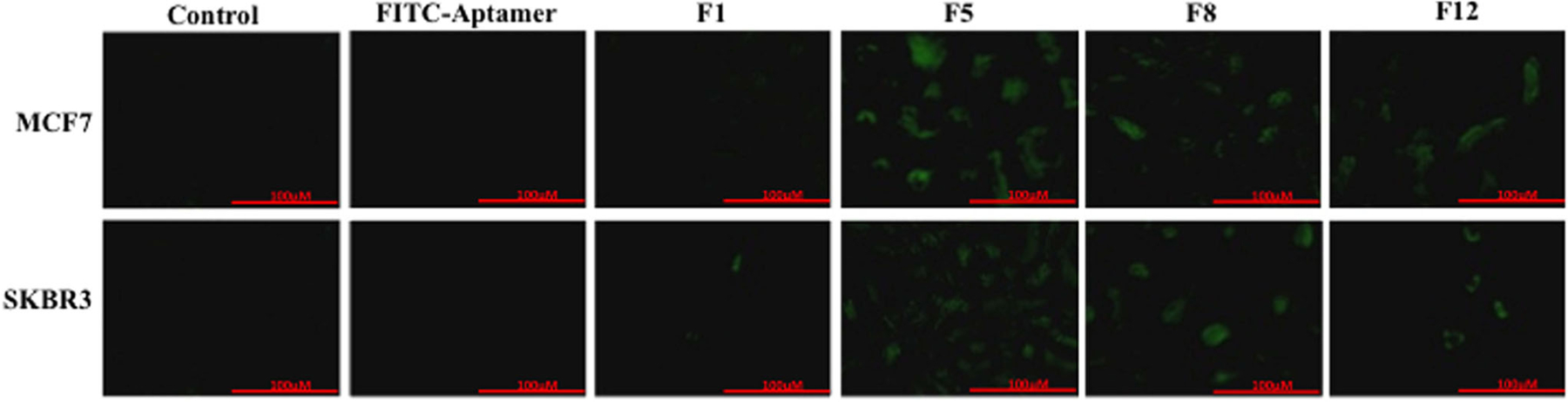
In vitro expression of FITC-Aptamer and liposomal complexes for formulations F1, F5, F8, and F12. Control indicates cells without any treatment. FITC-Aptamer indicates cells treated with only FITC-Aptamer in solution. F1, F5, F8, and F12 indicate FITC-Aptamer liposomal complexes. Scale bar is of 100 μm size
Cellular Uptake of DOX Liposomes by Flow Cytometry
The uptake of the FITC-Aptamer-A6 liposomal complex by the MCF7 and SKBR3 cells is shown in Fig. 4 and Fig. 5. The flow cytometry uptake study further confirms the improved uptake of the FITC-aptamer-A6 for both the MCF7 and the SKBR3 breast cancer cells. Similar to the uptake study done by fluorescence microscopy, the highest uptake was seen for formulation F5. F5 showed a 98.60% uptake in SKBR3 and 66.45% uptake in the MCF7 cells. Furthermore, we have also seen 1.79 fold increase in DOX uptake in the SKBR3 cells compared to the MDA-MB-231 cells (Fig. 6). This confirms the specific targeting of the aptamer-labeled particles on the Her-2+ breast cancer cells.
Fig. 5.
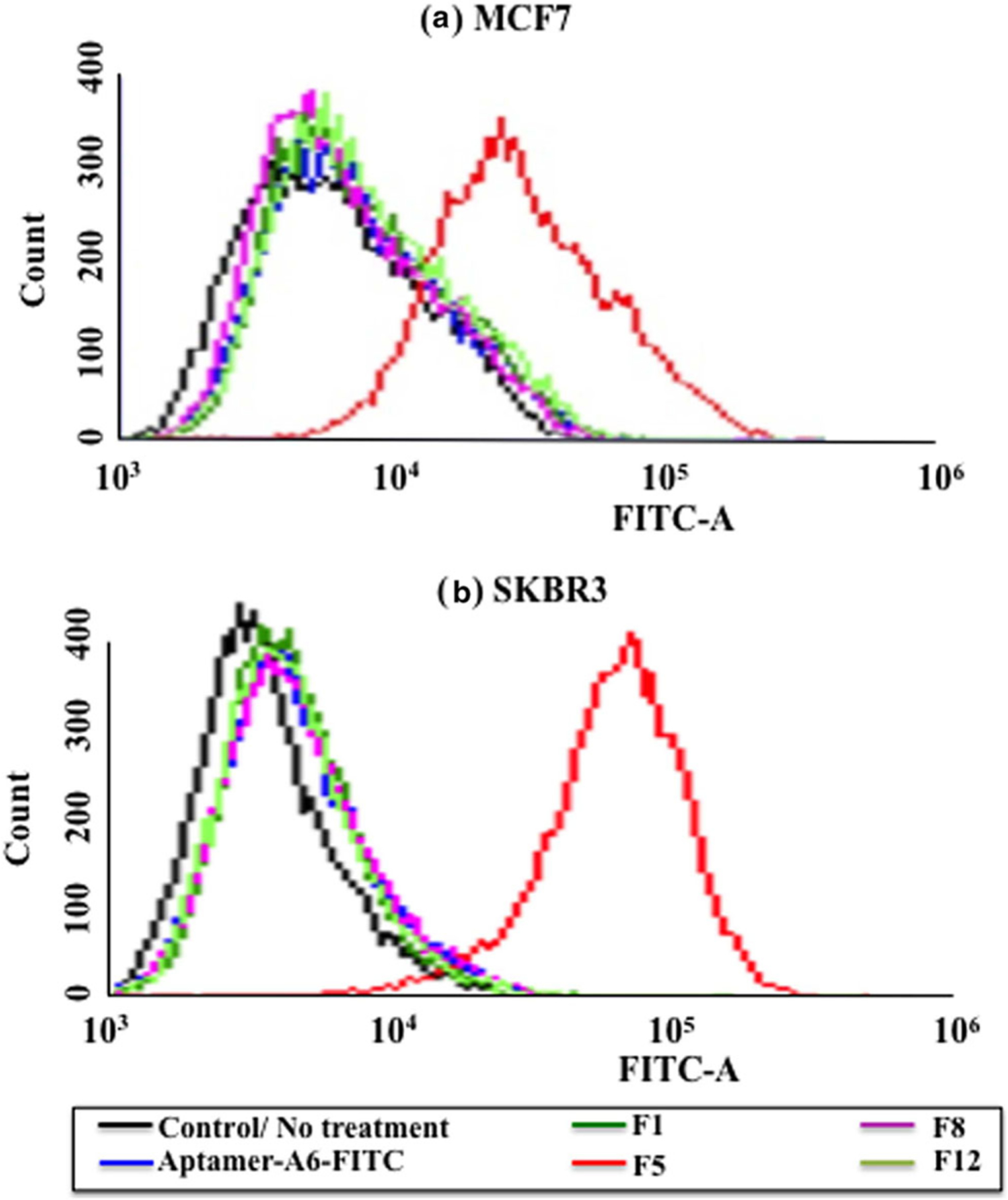
Flow cytometry histograms illustrating the uptake of Aptamer-FITC complex with different formulations (F1, F5, F8, and F12) on HER2+ cells a MCF7 and b SKBR3
Fig. 6.
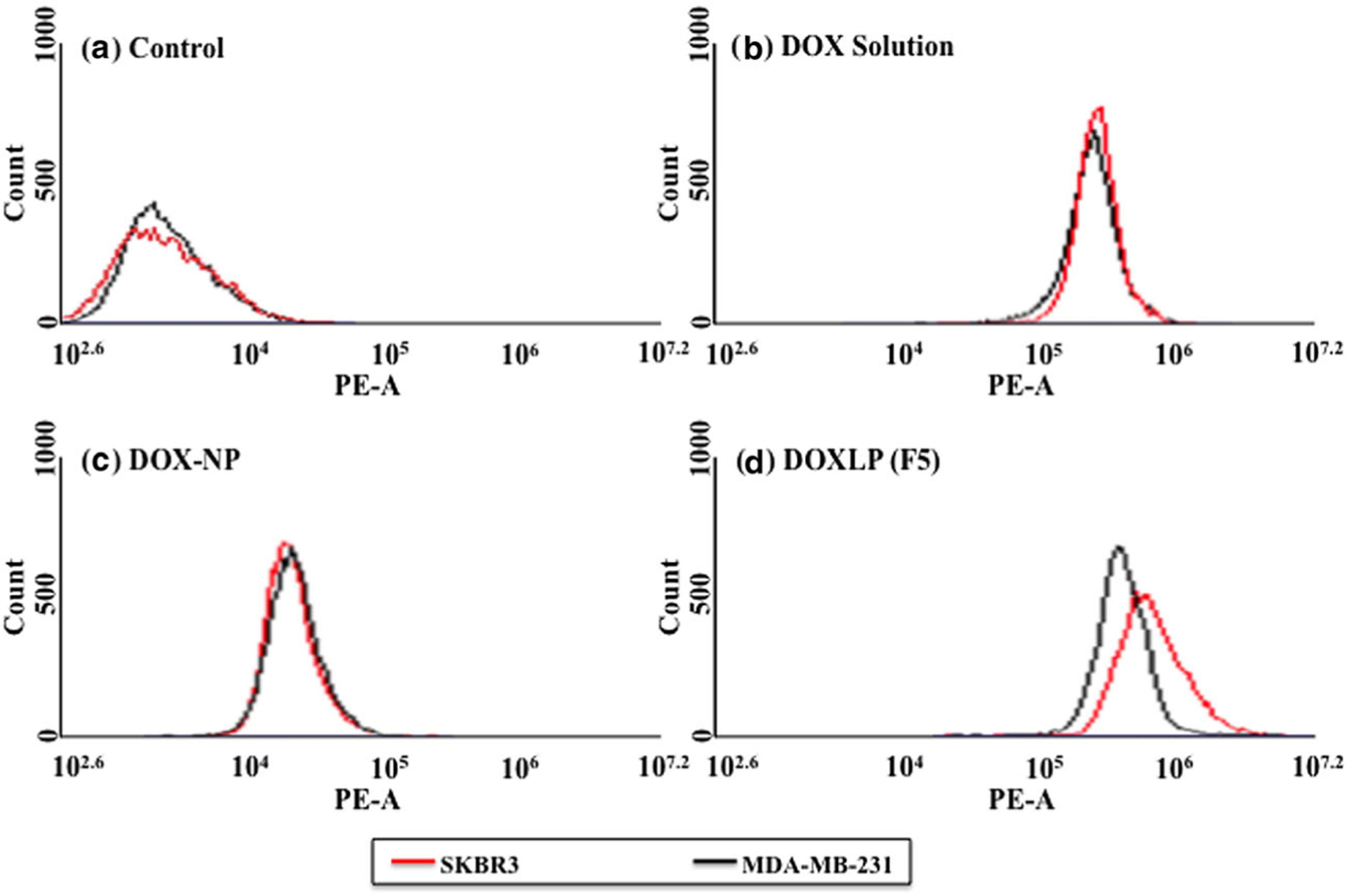
Flow cytometry histograms illustrating the uptake of doxorubicin in HER2+ (SKBR3) and HER2− (MDA-MB-231) breast cancer cells
DISCUSSION
With the advance in time, nano-sized liposome formulations have become the leading delivery systems for transporting cytotoxic drugs (19). Liposomal anticancer drugs were the first nano-based formulations approved for cancer therapy by the FDA (20). Liposomes can enhance drug solubility, lower the dose-limiting toxicities, and modify unwanted pharmacokinetics (21–23). Such formulations can be triggered to release the drug contents within the cell by changing the temperature or pH of the system. They can also be used in targeted delivery by binding ligands on the surface and making them specific towards certain diseases; hence, the ligand targeting attracts much attention compared to the other delivery systems (24). In this study, we developed a targeted DOX delivery system that is specific for the Her-2-overexpressing breast cancer cells.
We have developed several batches of liposomes by changing the composition of the lipids and modified them by adding targeting moieties and cationic lipids. We then studied their physicochemical characterization. The formulations have been composed of HSPC, POPC, DOPC, DPPC, DOPE, CHO, DOTAP, DDAB, and DSPE-PEG 2000, which are commonly used in preparation of liposome formulations. We have incorporated lipids, such as HSPC, POPC, and CHO that are clinically used for liposomal formulations. In our study, we have prepared four different sets of liposomes (1) liposomes of HSPC, (2) liposomes of POPC, (3) liposomes of DOPC, and (4) liposomes of DPPC. The particle size of the liposomes has been checked to determine the desired liposomal size range. The particle size of all the twelve formulations is shown in Table III. The formulations having DOTAP (F2, F5, F8, and F11) have the lowest particle size, followed by DDAB (F3, F6, F9, and F12) and finally, the ones having CHO in their composition (F1, F4, F7, and F10) have the highest particle sizes. This could be because cationic lipids, such as DOTAP, tend to form particles with smaller sizes due to the relatively small headgroup of these lipids that leads to conical shape that is more readily accommodated in the inner monolayer of a membrane (25,26). From all the formulations, formulations F5 and F8 have the smallest particle size 101.70 ± 14.04 nm and 98.7 ± 13.25 nm, respectively. Jensen et al. observed a similar trait, where he found the size of nanoparticles were decreasing on addition of DOTAP (27). We also observed that the polydispersity index for all the liposomes is below 0.3; this indicates a homogenous population of the liposomes (28). The size of the liposome is an important characteristic since it affects the interaction of the liposomes with the biological membranes. The small size of the liposome is also important for the EPR effect that was first described by Matsumura and Maeda in 1986 (29). The nanosize of the particles indicates that the developed liposomes would be able to provide tumor tissue penetration through the EPR effect in the breast tumors (22,30). Thus, for intravenous administration, the small size will be able to diffuse to the tumor site through the leaky vascular capillaries and accumulated in the tumors. The particle size of our liposomes was further confirmed from scanning electron microscopy where the liposomes had a size range of 100–200 nm.
Table III.
Physiochemical Characteristics of Different Liposomes
| Formulation No. | Before loading | After loading | ||||
|---|---|---|---|---|---|---|
| Particle size (nm) | Zeta potential (mV) | Polydispersity | Particle size (nm) | Zeta potential (mV) | Entrapment efficiency (%) | |
| F1 | 162.10 ± 7.59 | − 2.73 ± 0.94 | 0.237 | 176.83 ± 13.30 | − 8.99 ± 1.29 | 89.2 |
| F2 | 124.36 ± 17.73 | + 5.14 ± 0.43 | 0.270 | 154.65 ± 20.44 | + 6.51 ± 0.98 | 88.8 |
| F3 | 147.80 ± 5.26 | + 3.77 ± 0.62 | 0.237 | 164.70 ± 13.24 | + 14.27 ± 0.41 | 87.6 |
| F4 | 181.15 ± 73.88 | −5.54 ± 0.37 | 0.232 | 225.87 ± 143.32 | − 5.92 ± 0.20 | 88.4 |
| F5 | 101.70 ± 14.04 | + 5.63 ± 0.46 | 0.124 | 172.08 ± 29.14 | + 17.63 ± 4.45 | 92.8 |
| F6 | 117.70 ± 16.87 | + 9.04 ± 1.28 | 0.269 | 183.16 ± 75.87 | + 16.19 ± 0.75 | 85.1 |
| F7 | 110 ± 22.30 | −5.27 ± 1.12 | 0.235 | 131.37 ± 26.99 | − 7.99 ± 1.13 | 90.4 |
| F8 | 98.7 ± 13.25 | + 7.94 ± 0.32 | 0.205 | 107.6 ± 16.57 | + 8.3 ± 0.33 | 94.1 |
| F9 | 105.57 ± 9.49 | + 6.05 ± 0.18 | 0.255 | 127.15 ± 5.76 | + 12.07 ± 1.32 | 88.6 |
| F10 | 175.6 ± 24.32 | − 6.5 ± 0.16 | 0.296 | 190.45 ± 60.69 | − 26.49 ± 2.38 | 86.3 |
| F11 | 121.46 ± 6.62 | + 7.07 ± 0.766 | 0.166 | 131.42 ± 58.12 | + 10.85 ± 0.54 | 74.9 |
| F12 | 126.83 ± 5.05 | + 9.89 ± 0.63 | 0.179 | 172.52 ± 13.61 | + 16.63 ± 1.32 | 89.6 |
The results show mean of three independent batches (n = 3) with standard deviation (SD)
Different phospholipids were used to formulate the DOX loaded liposomes and the entrapment efficiency of DOX was compared in the formulations. Usually, liposomes with the same polar head groups have similar encapsulation efficiencies regardless of their different hydrocarbon chains length or the saturation degrees. This suggests that the %EE of the liposomes does not depend on the hydrophobic component of the phospholipid (31). Thus, we see very similar %EE for all our formulations, with an average of 87.98 ± 4.80% in all the formulations. Within all the prepared formulations F5 and F8 having slightly higher %EE of 92.8% and 94.1%, respectively. This data suggests that the presence of cationic lipids plays no role in the entrapment of DOX into the liposomes. Thus, no significant difference in the encapsulation efficiency was observed.
The zeta potential of all the formulations ranges from − 8.99 ± 1.29 mV to + 17.63 ± 4.45 mV. This shows that the particles are stable in the liposomal system. Furthermore, it was observed that for each group of liposome formulation, the ones having DOTAP had slightly higher zeta potential than the ones with DDAB. The batches prepared with cholesterol were negatively charge. Cholesterol is a non-polar molecule but the presence of negatively charged hydroxyl group allows it to associate with the water molecules inside and outside of the liposomes. The negative zeta potential of the batches prepared with cholesterol is presumably due to the presence of hydroxyl group on the cholesterol (32). The charge on the liposomes is a crucial criterion when working with liposomal complexes. Marty et al. observed that DNA forms more stable complexes with cationic lipids than with neutral lipids, with DOTAP having the highest stability, cholesterol having the lowest stability and DDAB having better stability than cholesterol (33). This is similar to our observation as shown in Table IV, where we saw F5 having DOTAP was stable up to 10 weeks and F1 having cholesterol precipitated in 2 weeks, and F12 having DDAB precipitated in 3 weeks. The overall formulation composition plays a role in the stability of the liposomes. Among several factors reported, it was reported that the inclusion of cationic lipids into the liposome formulations not only supports to reduce the particle size but also helps to improve the stability of the particles. For example, Campbell et al. has shown that increasing the concentration of DOTAP significantly increases the physical stability of paclitaxel-containing liposomes (34). Hence, F1 having no cationic lipid incorporated showed the least stability among all the formulations tested. The stability is also assumably provided by the presence of PEG-2000 and Mal-PEG into the liposomal formulations that prevents cross-linking and stops aggregation of the liposomes (35).
Table IV.
Physical Stability of the Liposomes F1, F5, F8, and F12
| Formulations | Phospholipids | Physical stability at 4°C |
|---|---|---|
| F1 | HSPC | Stable for 1–2 weeks, then precipitates |
| F5 | POPC | Stable for 8–10 weeks |
| F8 | DOPC | Stable for 3–4 weeks, then precipitates |
| F12 | DPPC | Stable for 2–3 weeks, then forms gel |
Furthermore, most of our formulations were prepared with Mal-PEG and PEG-2000. PEG chains are hydrophilic and orient themselves towards external aqueous phase of the liposomal particles, thus improve the overall solubility and decrease aggregation, which results in further decrease of the particle size. Among 4 batches (F1, F5, F8, and F12) tested for the physical stability, only F1 is lacking a cationic lipid and Mal-PEG. In general, the PEG-2000 (incorporated into all 12 batches) has been used to improve the circulation time and get more stable particles, whereas, the Mal-PEG-2000 is used to provide a binding site for the Aptamer-A6. So considerably, the higher amount of PEG moiety into F5, F8, and F12 compare to F1 is assumed to offer more stability to those particles.
The most commonly used functional groups for modifying PEG are maleimide, carboxylic acids, hydrazine, thiol, avidin, ether, and ester. In our study, we have used Mal-PEG-2000 to provide a binding site for the Aptamer-A6. The labeling of Mal-PEG-2000 on the liposome surface allows it to binds to the amino-terminal of Aptamer-A6 and facilitates target binding of the particles to the overexpressed Her-2 receptors on the surface of breast cancer cells as Aptamer-A6 is specific to Her-2 receptors. Salvati et al. formulated amyloid-β-targeting liposomes in presence of Mal-PEG2000 to bind to the transferrin receptors in the brain (36). The liposomes showed significant uptake in the blood-brain barrier model and proved to be useful in targeting amyloid-β and other defective proteins in Alzheimer’s disease. The PEG2000 in our liposome will reduce the reticuloendothelial uptake by creating a hydrophilic surface on the liposomes and improves circulation time. PEG 2000 is commonly used in marketed preparations such as Doxil which is a pegylated doxorubicin HCl liposome. Doxil shows better stability, reduced leakage, lower toxicity, and increased accumulation in the solid tumors by 20-folds due to the presence of DSPE-PEG-2000 (37).
MCF7 and SKBR3 breast cancer cells overexpress Her-2 receptors. The cytotoxicity studies show that the DOX-loaded liposomal formulations are more effective in killing cancer cells than the free DOX in both MCF7 and SKBR3 cells. This is because liposomes are quickly internalized by endocytosis leading to lower degradation of sensitive drugs, whereas the drug solutions enter the cells by passive diffusion only (38). Furthermore, DOTAP formulations had a lower IC50 when the unsaturated lipids were used whereas, the DDAB formulations had a lower IC50 when saturated lipids were used. POPC and DOPC are unsaturated lipids and HSPC and DPPC are saturated lipids. Increased saturated lipids are usually found in aggressive breast cancer that reduces the membrane fluidity and helps to advance the disease (39).
The fluorescent microscopy and flow cytometry is widely used to study the internalization of fluorescent dyes in cells (40). From the fluorescent microscopy studies, we found that free FITC-Aptamer solution had almost no fluorescence as the aptamer had no binding site and would not be taken up by the cells. F1 also did not have any fluorescence as it did not have Mal-PEG in its’ composition to bind to the FITC-aptamer to form the complex. Formulations F5, F8, and F12 had significant fluorescence as they could complex with the aptamer-FITC due to the presence of Mal-PEG undergoing receptor mediated uptake. Upon quantification of the uptake through flow cytometry, we have seen that F5 had the highest fluorescence intensity of 98.60% and 66.45% on the SKBR3 and MCF7 cells, respectively. Hence, F5 was significantly internalized by the cells due to the interaction of the Mal-PEG with the Her-2 receptors on the surface of MCF7 and SKBR3 breast cancer cells. Furthermore, upon incubation of F5 in SKBR3 and MDA-MB-231, we saw 1.79-fold increase in the DOX uptake in the SKBR3 Her-2+ cells compared to the MDA-MB-231 triple negative breast cancer cells. We also observed that there was no significant difference in the uptake of DOX-NP or DOX solution in the two cell lines. This indicates the target specificity of the aptamer-A6 towards the Her-2+ breast cancer cells only.
CONCLUSION
In conclusion, we have observed an improved uptake of DOX delivered by the aptamer-labeled liposomes in the Her-2+ cell lines and a reduced IC50 value. The formulations, F1 through F12, had a small particle size of less than 200 nm and a high entrapment efficiency of mostly above 85%. The best formulation, F5, had a particle size of 101 ± 14 nm, zeta potential of + 5.63 ± 0.46 mV, and entrapment efficiency of ≈ 93%. F5 also showed a significant improvement in uptake of the aptamer in both MCF7 and SKBR3 cells by more than 60%, and an improved uptake in the Her-2 positive cells than the Her-2 negative cells. These factors can be critically important in reducing the dose-dependent side effects of DOX and enhancing the tumor specific toxicity of DOX. Thus, the aptamer targeted approach can be very promising for adjuvant and neoadjuvant chemotherapy for the treatment of various cancers.
Supplementary Material
ACKNOWLEDGMENTS
The authors would want to extend their gratitude to Dr. Syed Muniruzzaman of the Department of Biology, Xavier University of Louisiana, New Orleans, LA, for his enormous guidance and contribution to this project.
FUNDING INFORMATION
This research was supported with funding from NIGMS grant number P20GM12345, P20GM103424-17 and Louisiana Board of Regents grant LEQSF(2015-20)-INBRE-Match, LCRC, NIMHD grant number TL4GM118968 and T34GM007716, NIGMS grant number UL1GM118967 and R25GM060926, grant no. LS-LAMP: NSF, HRD-1503226 and CUR from Xavier University of Louisiana.
Abbreviations:
- %EE
Entrapment efficiency (%)
- CHO
Cholesterol
- DDAB
Dimethyldidodecylammonium bromide
- DOPC
1,2-Dioleoyl-snglycero-3-phosphocholine
- DOTAP
1,2-Dioleoyl-3-trimethylammonium-propane(chloride salt)
- DOX
Doxorubicin hydrochloride
- DOXLP
Doxorubicin-loaded PEGylated liposomes
- DOX-NP
Liposomal encapsulated doxorubicin
- DPPC
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine
- DSPE-PEG-2000
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt)
- Her-2
Human epidermal growth factor receptor 2
- HSPC
L-α-phosphatidylcholine, hydrogenated (Soy)
- IC50
Inhibitory concentration 50
- Mal-PEG-2000
1, 2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (ammonium salt)
- PBS
Phosphate buffered saline
- POPC
1-Palmitoyl-2-oleoyl-glycero-3-phosphocholine
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1208/s12249-020-01743-8) contains supplementary material, which is available to authorized users.
REFERENCES
- 1.Chowdhury N, Vhora I, Patel K, Bagde A, Kutlehria S, Singh M. Development of hot melt extruded solid dispersion of Tamoxifen citrate and resveratrol for synergistic effects on breast cancer cells. AAPS PharmSciTech. 2018;19(7):3287–97. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Breast Cancer Statistics. https://www.breastcancerorg/symptoms/understand_bc/statistics. Updated Feb 13, 2019.
- 3.Huang C-Y, Ju D-T, Chang C-F, Muralidhar Reddy P, Velmurugan BK. A review on the effects of current chemotherapy drugs and natural agents in treating non-small cell lung cancer. Biomedicine (Taipei). 2017;7(4):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018;9(1):1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Seebacher N, Shi H, Kan Q, Duan Z. Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget. 2017;8(48):84559–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marinello J, Delcuratolo M, Capranico G. Anthracyclines as topoisomerase II poisons: from early studies to new perspectives. Int J Mol Sci. 2018;19(11):3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2010;115(2):155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamichhane N, Udayakumar TS, D’Souza WD, Simone CB 2nd, Raghavan SR, Polf J, Mahmood J. Liposomes: clinical applications and potential for image-guided drug delivery. Molecules. 2018;23(2):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwendener RA, Schott H. Liposome formulations of hydrophobic drugs. Methods Mol Biol (Clifton, NJ). 2010;605:129–38. [DOI] [PubMed] [Google Scholar]
- 10.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–51. [DOI] [PubMed] [Google Scholar]
- 11.Rizvi SAA, Saleh AM. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm J. 2018;26(1):64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waterhouse DN, Tardi PG, Mayer LD, Bally mB. A comparison of liposomal formulations of doxorubicin with drug administered in free form: changing toxicity profiles. Drug Saf. 2001;24(12):903–20. [DOI] [PubMed] [Google Scholar]
- 13.Duggan ST, Keating GM. Pegylated liposomal doxorubicin: a review of its use in metastatic breast cancer, ovarian cancer, multiple myeloma and AIDS-related Kaposi’s sarcoma. Drugs. 2011;71(18):2531–58. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X-X, McIntosh TJ, Grinstaff MW. Functional lipids and lipoplexes for improved gene delivery. Biochimie. 2012;94(1):42–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell D, Chandra S, Dodson K, Shaheen F, Wiltz K, Ireland S, et al. Aptamer-functionalized hybrid nanoparticle for the treatment of breast cancer. Eur J Pharm Biopharm. 2017;114:108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu FX, Karnik R, Wang AZ, Alexis F, Levy-Nissenbaum E, Hong S, et al. Targeted nanoparticles for cancer therapy. Nano Today. 2007;2(3):14–21. [Google Scholar]
- 18.Bolotin EM, Cohen R, Bar LK, Emanuel N, Ninio S, Barenholz Y, et al. Ammonium sulfate gradients for efficient and stable remote loading of amphipathic weak bases into liposomes and ligandoliposomes. J Liposome Res. 1994;4(1):455–79. [Google Scholar]
- 19.Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ventola CL. Progress in nanomedicine: approved and investigational nanodrugs. P T. 2017;42(12):742–55. [PMC free article] [PubMed] [Google Scholar]
- 21.Daeihamed M, Dadashzadeh S, Haeri A, Akhlaghi MF. Potential of liposomes for enhancement of oral drug absorption. Curr Drug Deliv. 2017;14(2):289–303. [DOI] [PubMed] [Google Scholar]
- 22.Chowdhury N, Vhora I, Patel K, Doddapaneni R, Mondal A, Singh M. Liposomes co-loaded with 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3 (PFKFB3) shRNA plasmid and Docetaxel for the treatment of non-small cell lung cancer. Pharm Res. 2017;34(11):2371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bitounis D, Fanciullino R, Iliadis A, Ciccolini J. Optimizing druggability through liposomal formulations: new approaches to an old concept. ISRN Pharm. 2012;2012:738432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshpande PP, Biswas S, Torchilin VP. Current trends in the use of liposomes for tumor targeting. Nanomedicine (Lond). 2013;8(9):1509–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulkarni JA, Darjuan MM, Mercer JE, Chen S, van der Meel R, Thewalt JL, et al. On the formation and morphology of lipid nanoparticles containing ionizable cationic lipids and siRNA. ACS Nano. 2018;12(5):4787–95. [DOI] [PubMed] [Google Scholar]
- 26.Kolasinac R, Kleusch C, Braun T, Merkel R, Csiszar A. Deciphering the functional composition of fusogenic liposomes. Int J Mol Sci. 2018;19(2):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen DK, Jensen LB, Koocheki S, Bengtson L, Cun D, Nielsen HM, et al. Design of an inhalable dry powder formulation of DOTAP-modified PLGA nanoparticles loaded with siRNA. J of control release. 2012;157(1):141–8. [DOI] [PubMed] [Google Scholar]
- 28.Danaei M, Dehghankhold M, Ataei S, Davarani FH, Javanmard R, Dokhani A, Khorasani S, Mozafari MR. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–92. [PubMed] [Google Scholar]
- 30.Golombek SK, May J-N, Theek B, Appold L, Drude N, Kiessling F, et al. Tumor targeting via EPR: strategies to enhance patient responses. Adv Drug Deliv Rev. 2018;130:17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colletier JP, Chaize B, Winterhalter M, Fournier D. Protein encapsulation in liposomes: efficiency depends on interactions between protein and phospholipid bilayer. BMC Biotechnol. 2002;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yandrapati RK. Effect of lipid composition on the physical properties of liposomes: a light scattering study. 2012: 6864. [Google Scholar]
- 33.Marty R, N’soukpoé-Kossi CN, Charbonneau DM, Kreplak L, Tajmir-Riahi H-A. Structural characterization of cationic lipid—tRNA complexes. Nucleic Acids Res. 2009;37(15):5197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell RB, Balasubramanian SV, Straubinger RM. Influence of cationic lipids on the stability and membrane properties of paclitaxel-containing liposomes. J Pharm Sci. 2001;90(8):1091–105. [DOI] [PubMed] [Google Scholar]
- 35.Meyer O, Kirpotin D, Hong K, Sternberg B, Park JW, Woodle MC, et al. Cationic liposomes coated with polyethylene glycol as carriers for oligonucleotides. J Biol Chem. 1998;273(25):15621–7. [DOI] [PubMed] [Google Scholar]
- 36.Salvati E, Re F, Sesana S, Cambianica I, Sancini G, Masserini M, et al. Liposomes functionalized to overcome the blood-brain barrier and to target amyloid-beta peptide: the chemical design affects the permeability across an in vitro model. Int J Nanomedicine. 2013;8:1749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soundararajan A, Bao A, Phillips WT, Perez R 3rd, Goins BA. [(186)Re]liposomal doxorubicin (Doxil): in vitro stability, pharmacokinetics, imaging and biodistribution in a head and neck squamous cell carcinoma xenograft model. Nucl Med Biol. 2009;36(5):515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J, Bahreman A, Daudey G, Bussmann J, Olsthoorn RCL, Kros A. Drug delivery via cell membrane fusion using lipopeptide modified liposomes. ACS Cent Sci. 2016;2(9):621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilvo M, Denkert C, Lehtinen L, Muller B, Brockmoller S, Seppanen-Laakso T, et al. Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res. 2011;71(9):3236–45. [DOI] [PubMed] [Google Scholar]
- 40.Illien F, Rodriguez N, Amoura M, Joliot A, Pallerla M, Cribier S, et al. Quantitative fluorescence spectroscopy and flow cytometry analyses of cell-penetrating peptides internalization pathways: optimization, pitfalls, comparison with mass spectrometry quantification. Sci Rep. 2016;6:36938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


