Abstract
Different oral hygiene practices are used to overcome endemic diseases such as dental caries and oral infections. In Mali (Africa), natural plant-based toothbrushes are used for eliminating bacterial biofilm. The repertoire of microorganisms associated with natural toothbrushes is unknown. The aim of our study is to study microbial flora in particular the methanogenic archaea associated with natural toothbrushes recently recognized as responsible for periodontitis and peri-implantitis. We investigated the methanogens and bacteria associated with 15 different natural plant toothbrushes collected in Bamako local market (Mali). Microbiological investigations consisted in culturing the bacteria on agar plates and searching archaea using molecular techniques. No archaea were demonstrated by molecular biology but 50 bacterial species, including 33 aero-anaerobic and 17 aerobic species, were isolated from natural toothbrushes. We isolated Pseudomonas sp., Staphylococcus sp. and Klebsiella pneumoniae, which are acknowledged as opportunistic human pathogens. This study has highlighted the likely impact of the use of natural toothbrushes in the spread of potentially pathogenic bacteria in the human oral cavity.
Keywords: Bacteria, Mali, methanogen, miswak, natural toothbrushes, oral cavity
Introduction
Different oral hygiene methods have been used to overcome widely endemic diseases such as dental caries and oral infections [1]. Archaeological excavations have revealed the use of many different methods and tools for cleaning teeth throughout history: toothpicks in wood, feather, thorn, porcupine hair and fibrous wood (stems of lentisque wood with frayed ends) found in Egyptian tombs dating from 3000 bc [2].
The use of natural toothbrushes or chewing sticks became popular in the Muslim world, including several African countries including Mali. These natural toothbrushes, also called Miswak, are prepared from the root, stem, twigs, or bark of trees and are used to clean the oral cavity [3]. Miswak is an Arabic word meaning tooth cleaning stick, becoming a common designation for Salvadora persica [4]. Salvadora persica is the most used plant for oral hygiene [5]. Today, the use of natural toothbrushes is very common for reasons of low cost, ease of availability and tradition.
There are around 173 different types of trees that can be used as chewing sticks, belonging to the families Salvadoraceae, Acacia, Fabaceae, Terminalia, Combretaceae, Lasianthera, Icacinaceae, Gouania and Rhamnaceae [[6], [7], [8]]. Studies have shown that these plants confer an essential antibacterial role, especially against cariogenic bacteria and periodontal pathogens [6]. A recent study has shown that the aqueous extract of the roots of Salvadora persica contains thermostable components with significant antiprotozoal activity against Blastocystis sp. subtypes [9]. As the correct decontamination of natural toothbrushes before use is not always carried out, this could constitute a pathway for the diffusion of environmental bacteria and archaea into the oral microbiota in humans. It is known that the main route of exposure of humans to resistant pathogens comes from other people, either in clinics or in community settings. Environmental dissemination routes for resistant bacteria have also been pointed out as potentially important for the spread of antibiotic resistance in humans [10]. Plants harbour a wide variety of microbiomes that are specific to each plant genotype, but also specific to each plant organ, for example roots, leaves and spheres [11]. Recent next-generation sequencing studies of the environmental microbiome revealed archaeal signatures in substantial amounts in diverse microbiomes associated with plants [12]. The microbial community associated with the use of natural toothbrushes has not been studied; hence the influence on the oral microbiota is unknown. In this preliminary study conducted in Mali, we examined for the first time the microbial flora that contaminates natural toothbrushes used for oral hygiene.
Materials and methods
Collection and identification of samples
Unused natural toothbrushes were collected in March 2018 from the local market in Bamako, Mali. Identification of the scientific names of plants was based on the literature by correspondence with local names [8,13,14]. The collection consisted of 15 natural toothbrushes from different plants, including Fagara xanthoxyloides, Balanites aegyptiaca, Salvadora persica, Guiera senegalensis, Prosopis africana, Burkea africana, Diospyros mespiliformis, Ziziphus mucronata, Ficus exasperata, Amona senegalensis, Cassia sieberiana, Ficus capensis, Grewia bicolor, Detarium microscarpum and Loeseneriella africana (Table 1). The samples were analysed at the IHU Mediterranée Infection laboratory (Marseille, France) for microbiological investigations.
Table 1.
Bacterial diversity of natural toothbrushes
| Vernacular name (Bambara) | Scientific name | Plant parts used | Bacteria |
|---|---|---|---|
| Wo | Fagara xanthoxyloides | Root and twig | Enterococcus faecium; Pediococcus acidilactici; Enterococcus casseliflavus; Pantoea septica; Cronobacter sakazakii; Pantoea massiliensis; Staphylococcus epidermidis |
| Zéguéné ou Séréné | Balanites aegyptiaca | Rod | Enterobacter cloacae; Enterococcus faecium; Bacillus marisflavi; Bacillus megaterium; Bacillus pumilus |
| Souraka gèsè | Salvadore persica | Branch | Pantoea intestinalis; Klebsiella pneumoniae; Pantoea septica; Pantoea massiliensis; Pseudomonas balearica; Cronobacter sakazakii; Enterococcus faecium; Pantoea dispersa; Enterobacter cloacae |
| Kounjè | Guiera senegalensis | Root | Enterobacter tabaci; Enterobacter cloacae; Enterobacter asburiae; Klebsiella pneumoniae; Enterobacter asburiae; Bacillus pumilus; Pantoea massiliensis; Pantoea septica; Cronobacter sakazaki; Escherichia coli; Kosakonia cowanii; Bacillus megaterium; Kocuria marina |
| Gwele | Prosopis africana | Rod | Pantoea septica; Klebsiella pneumoniae; Pantoea massiliensis; Bacillus firmus; Bacillus soli; Paenibacillus yunnanensis; Pantoea dispersa; Pantoea eucrina; Enterococcus faecium; Enterobacter cloacae; Escherichia coli; Cronobacter sakazaki; Pantoea massiliensis; Cronobacter helveticus; Raoultella ornithinolytida |
| Siri | Burkea africana | Branch | Klebsiella pneumoniae; Kosakonia radicincitans; Staphylococcus pasteuri; Staphylococcus epidermidis; Enterobacter cloacae; Pantoea septica; Cronobacter sakazaki; Pantoea intestinalis |
| Sunsun gèsè | Diospyros mespiliformis | Branch | Klebsiella pneumoniae; Enterobacter cloacae; Pantoea calida; Pantoea massiliensis; Cronobacter sakazakii |
| Surukou ntomono | Ziziphus mucronata | Branch | Raoultella ornithinolytica; Escherichia coli; Pantoea septica; Klebsiella oxytoca; Salmonella sp.; Pantoea calida; Pantoea massiliensis; Kosakonia radicincitans |
| Suruku nièniè | Ficus exasperate | Branch | Bacillus megaterium; Bacillus pumilus; Pantoea septica; Staphylococcus epidermidis |
| Mandé sunsun | Amona senegalensis | Branch | Pseudomonas aeruginosa; Klebsiella pneumoniae; Enterobacter cloacae; Cronobacter sakazakii; Stenotrophomonas maltophila; Enterococcus casseliflavus; Enterococcus faecium; Enterococcus gallinarum; Acinetobacter baumannii |
| Sinjan | Cassia sieberiana | Roots | Enterobacter cloacae; Klebsiella pneumoniae; Enterobacter tabaci; Enterobacter asburiae; Acinetobacter variabilis; Pantoea massiliensis; Pantoea septica; Pantoea calida |
| Seretoro | Ficus capensis | Branch | Enterococcus casseliflavus; Enterobacter cloacae; Klebsiella pneumoniae; Acinetobacter calcoaceticus; Bacillus circulans; Bacillus flexus |
| Nogo nogo | Grewia bicolor | Branch | Pantoea septica; Pantoea massiliensis; Enterococcus faecium; Enterobacter hormachei; Enterococcus casseliflavus; Pantoea calida; Bacillus megaterium; Cronobacter helveticus; Candida fabianii; Bacillus idriensis; Pantoea intestinalis |
| Tanba kunba | Detarium microscarpum | Branch | Klebsiella pneumoniae; Cronobacter sakazakii; Enterobacter cloacae; Enterobacter aerogenes; Pseudomonas putida; Leclercia adecarboxylata; Enterococcus casseliflavus; Erwina sp.; Kosakonia radicincitans; Salmonella sp.; Escherichia vulneris |
| Mankana | Loeseneriella africana | Branch | Klebsiella pneumoniae; Pantoea septica; Pantoea calida; Pantoea massiliensis; Bacillus pumilus; Cronobacter sakazaki; Raoultella ornithinolytica |
Culture of the samples
The different samples were rehydrated in Falcon tubes containing sterile phosphate-buffered saline for 24 hours at room temperature. The suspension was used to inoculate the agars at different dilutions. Culture of bacteria was performed at 37°C on 5% sheep-blood-enriched Columbia agar and PolyViteX agar (bioMérieux, Marcy l’Etoile, France) under aerobic and anaerobic atmospheres, for 48 hours.
Identification by MALDI-TOF MS
All microbial colonies that grew on agar plates were identified by matrix-assisted laser desorption–ionization time-of-flight mass spectrometry (MALDI-TOF MS) using a Microflex spectrometer (Bruker Daltonics, Bremen, Germany) [15,16].
PCR sequencing
Methanogens were searched for by PCR sequencing. A 0.3-g quantity of acid-washed beads (B106 mm; Sigma, Saint-Quentin Fallavier, France) was added to each tube containing 250 μL of toothbrush sample, the suspension was shaken to achieve a mechanical lysis in a FastPrep BIO 101 apparatus (Qbiogene, Strasbourg, France) at level 6.5 for 2 min. Then 200 μL of buffer TL and OB Protease Solution (1.5 mL) from the E.Z.N.A. Tissue DNA Kit (OMEGA, bio-tek, Norcross, GA, USA) were added. The mixture was incubated overnight at 56°C. After a second cycle of mechanical lysis, the mixture was incubated for 60 min at 70°C. Extracted DNA was eluted with 100 μL of elution buffer and the DNA was stored at –20°C.
Amplification of the archaea 16S rRNA gene (primers used: SDArch0333aS15, 5′-TCCAGGCCCTACGGG-3′ and SDArch0958aA19, 5′-YCCGGCGTTGAMTCCAATT-3′) and the methyl-coenzyme M (mcrA) gene (primers used: mcrAFor, 5′-GCTCTACGACCAGATMTGGCTTGG-3′ and mcrARev, 5′-CCGTAGTACGTGAAGTCATCCAGCA-3′) was performed as previously described [17]. Sequencing reactions were carried-out using the Big-Dye Terminator, version 1.1, cycle sequencing kit DNA according to the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA). Nucleotide sequences were assembled using Chromas Pro software, version 1.7 (Technelysium Pty Ltd., Tewantin, QLD, Australia) and compared with the GenBank database by similarity search using the BLASTN program (http://www.ncbi.nlm.nih.gov/blast/).
Results and discussion
In this study, we were interested in the different types of natural toothbrushes used in Mali. We obtained a collection of 15 natural toothbrushes from different plants that are frequently used in Mali (Table 1). The natural toothbrushes that presented the most bacteria were: Prosopis africana and Guiera senegalensis with respectively 15 and 13 bacterial species (Table 1).
We isolated 50 bacterial species from natural toothbrushes, including 33 aero-anaerobic and 17 aerobic species.
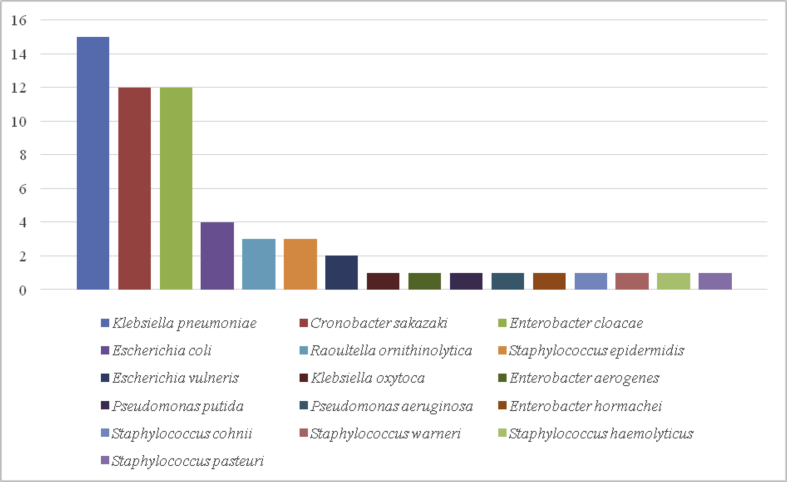
Among them, a variety of potential pathogenic bacteria [[16], [17], [18]] of the oral cavity were found. Among all bacteria isolated by culture, 70% can cause pathologies in humans, including seven bacterial genera that could potentially be responsible for respiratory infection (Fig. 1). Enterobacteria were the most represented in our study. Among these bacteria, 15 Klebsiella pneumoniae strains were isolated followed by 12 Cronobacter sakazaki, 12 Enterobacter cloacae, four Escherichia coli and three Raoultella ornithinolytica (Fig. 1). Klebsiella pneumoniae is an opportunistic bacterial pathogen known for its high frequency and diversity of antimicrobial resistance genes [19]. Natural toothbrushes can contribute to the spread of multidrug-resistant strains in humans from the environment [19].
Fig. 1.
Potential opportunistic pathogens isolated on natural toothbrushes collected in Bamako, Mali. The x-axis indicates the different bacterial species potentially pathogenic for humans, the y-axis indicates the number of bacteria isolated for each species.
Raoultella ornithinolytica is a bacterium often found in aquatic environments, soil, insects, fish, ticks, and termites [20]. This bacterium is involved in several pathologies in humans, including in the oral cavity [20,21].
In this study, we did not detect the presence of methanogens and archaea in general on the toothbrushes using molecular biology, suggesting that natural toothbrushes are not a source for methanogens commonly found in the oral cavity, chiefly Methanobrevibacter oralis [[22], [23], [24]], Methanobrevibacter smithii and Methanobrevibacter massiliensis [18].
We isolated nine different bacterial species from the natural toothbrush Salvadora persica despite its previously reported antimicrobial activity against both aerobic and anaerobic bacteria [4,6,25]. However, previous studies had shown some disadvantages of using natural toothbrushes. A study by Eid et al. [26] showed that natural toothbrush users had significantly more sites with gingival recession than industrial toothbrush users, and this may influence the periodontal health. The gingival recession may have different origins, such as the absence or low thickness of keratinized tissue, the traction of a frenum or even a thin external table of bone, or traumatic or excessive brushing [27]. Another study showed that the antimicrobial substances contained in natural toothbrushes or chewing sticks provided no additional benefits to those produced by the antimicrobial activity of commercially available toothpastes [28]. The mechanical effect exerted by natural toothbrushes during dental hygiene could lead to micro-wounds through which methanogens and bacteria that are present in the oral flora could enter the body and cause systemic diseases [29]. These practices may pose a risk to the elderly, immunosuppressed individuals and those following chemotherapy or radiotherapy, who are more likely to develop both oral cavity and systemic diseases.
The mechanical effect made by natural toothbrushes during dental hygiene, as mentioned above, could cause micro-wounds and promote the haematogenous spread of microorganisms in the body.
Natural toothbrushes contaminated with Pseudomonas sp., Staphylococcus sp. and Klebsiella pneumoniae may be a source for these opportunistic pathogens, posing a threat to oral as well as general health in natural toothbrush users.
This pilot study made it possible to show the microbial diversity contaminating natural toothbrushes. In this work, we have also shown that natural toothbrushes could lead to the spread of multidrug-resistant bacteria in humans.
Further studies are needed to compare the oral flora of individuals using natural toothbrushes and industrial toothbrushes. In order to test the hypotheses formulated in this study, it would also be interesting to test the sensitivity of environmental bacteria to antibiotics and to determine the resistance genes circulating for a better oral health perspective.
Conflict of interest
There is no conflict of interest.
References
- 1.Niazi F., Naseem M., Khurshid Z., Zafar M.S., Almas K. Role of Salvadora persica chewing stick (miswak): a natural toothbrush for holistic oral health. Eur J Dent. 2016;10:301–308. doi: 10.4103/1305-7456.178297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyson J.M. History of the toothbrush. J Hist Dent. 2003;51:73–80. [PubMed] [Google Scholar]
- 3.Wu C.D., Darout I.A., Skaug N. Chewing sticks: timeless natural toothbrushes for oral cleansing. J Periodontal Res. 2001;36:275–284. doi: 10.1034/j.1600-0765.2001.360502.x. [DOI] [PubMed] [Google Scholar]
- 4.Haque M.M., Alsareii S.A. A review of the therapeutic effects of using miswak (Salvadora persica) on oral health. Saudi Med J. 2015;36:530–543. doi: 10.15537/smj.2015.5.10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordin A., Bin Saim A., Ramli R., Abdul Hamid A., Mohd Nasri N.W., Bt Hj, Idrus R. Miswak and oral health: an evidence-based review. Saudi J Biol Sci. 2020;27:1801–1810. doi: 10.1016/j.sjbs.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sukkarwalla A., Ali S.M., Lundberg P., Tanwir F. Efficacy of Miswak on oral pathogens. Dent Res J. 2013;10:314–320. doi: 10.4103/1735-3327.115138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Njoroge G.N., Kaibui I.M., Njenga P.K., Odhiambo P.O. Utilisation of priority traditional medicinal plants and local people’s knowledge on their conservation status in arid lands of Kenya (Mwingi District) J Ethnobiol Ethnomedicine. 2010;6:22. doi: 10.1186/1746-4269-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moyo M., Aremu A.O., Van Staden J. Medicinal plants: an invaluable, dwindling resource in sub-Saharan Africa. J Ethnopharmacol. 2015;174:595–606. doi: 10.1016/j.jep.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 9.El-Bali M.A., Abdulhakim A., Mohamed R.T., El-Malky M.A., Bakri R.A., Al-Harthi S.A. Antiprotozoal potential of Salvadora persica against three virulent subtypes of Blastocystis sp. J Parasit Dis Off Organ Indian Soc Parasitol. 2020;44:694–701. doi: 10.1007/s12639-020-01247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bengtsson-Palme J., Kristiansson E., Larsson D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol Rev. 2018;42(1) doi: 10.1093/femsre/fux053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moissl-Eichinger C., Pausan M., Taffner J., Berg G., Bang C., Schmitz R.A. Archaea are interactive components of complex microbiomes. Trends Microbiol. 2018;26:70–85. doi: 10.1016/j.tim.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Buée M., De Boer W., Martin F., van Overbeek L., Jurkevitch E. The rhizosphere zoo: an overview of plant-associated communities of microorganisms, including phages, bacteria, archaea, and fungi, and of some of their structuring factors. Plant Soil. 2009;321:189–212. [Google Scholar]
- 13.Portères R. Les baguettes végétales mâchées servant de frotte-dents (fin) J Agric Tradit Bot Appliquée. 1974;21:111–150. [Google Scholar]
- 14.Pageard R. Plantes à brûler chez les Bambaras. J Afr. 1967;37:87–130. [Google Scholar]
- 15.Seng P., Drancourt M., Gouriet F., La Scola B., Fournier P.-E., Rolain J.M. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis Off Publ Infect Dis Soc Am. 2009;49:543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 16.Seng P., Rolain J.-M., Fournier P.E., La Scola B., Drancourt M., Raoult D. MALDI-TOF-mass spectrometry applications in clinical microbiology. Future Microbiol. 2010;5:1733–1754. doi: 10.2217/fmb.10.127. [DOI] [PubMed] [Google Scholar]
- 17.Grine G., Boualam M.A., Drancourt M. Methanobrevibacter smithii, a methanogen consistently colonising the newborn stomach. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2017;36:2449–2455. doi: 10.1007/s10096-017-3084-7. [DOI] [PubMed] [Google Scholar]
- 18.Bansal M., Khatri M., Taneja V. Potential role of periodontal infection in respiratory diseases—a review. J Med Life. 2013;6:244–248. [PMC free article] [PubMed] [Google Scholar]
- 19.Wyres K.L., Holt K.E. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr Opin Microbiol. 2018;45:131–139. doi: 10.1016/j.mib.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Hajjar R., Ambaraghassi G., Sebajang H., Schwenter F., Su S.-H. Raoultella ornithinolytica: emergence and resistance. Infect Drug Resist. 2020;13:1091–1104. doi: 10.2147/IDR.S191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaya S., Bayramoğlu G., Sönmez M., Köksal İ. Raoultella ornithinolytica causing fatal sepsis. Braz J Infect Dis Off Publ Braz Soc Infect Dis. 2015;19:230–231. doi: 10.1016/j.bjid.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huynh H.T.T., Nkamga V.D., Drancourt M., Aboudharam G. Genetic variants of dental plaque Methanobrevibacter oralis. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:1097–1101. doi: 10.1007/s10096-015-2325-x. [DOI] [PubMed] [Google Scholar]
- 23.Ferrari A., Brusa T., Rutili A., Canzi E., Biavati B. Isolation and characterization of Methanobrevibacter oralis sp. nov. Curr Microbiol. 1994;29:7–12. [Google Scholar]
- 24.Huynh H.T.T., Pignoly M., Nkamga V.D., Drancourt M., Aboudharam G. The repertoire of archaea cultivated from severe periodontitis. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0121565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Ayed M.S.Z., Asaad A.M., Qureshi M.A., Attia H.G., AlMarrani A.H. Antibacterial activity of Salvadora persica L. (Miswak) extracts against multidrug resistant bacterial clinical isolates. Evidence-Based Compl Alt Med. 2016 doi: 10.1155/2016/7083964. Article ID 7083964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eid M.A., Selim H.A., al-Shammery A.R. The relationship between chewing sticks (Miswak) and periodontal health. 3. Relationship to gingival recession. Quintessence Int Berl Ger. 1985;22:61–64. 1991. [PubMed] [Google Scholar]
- 27.Jati A.S., Furquim L.Z., Consolaro A. Gingival recession: its causes and types, and the importance of orthodontic treatment. Dent Press J Orthod. 2016;21:18–29. doi: 10.1590/2177-6709.21.3.018-029.oin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norton M.R., Addy M. Chewing sticks versus toothbrushes in West Africa. A pilot study. Clin Prev Dent. 1989;11:11–13. [PubMed] [Google Scholar]
- 29.Gendron R., Grenier D., Maheu-Robert L.-F. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbe. Infect. 2000;2:897–906. doi: 10.1016/s1286-4579(00)00391-9. [DOI] [PubMed] [Google Scholar]