Abstract
Oral health reflects the general health, and it is fundamental to well-being and quality of life. An infection in the oral cavity can be associated with serious complications in human health. Local therapy of these infections offers many advantages over systemic drug administration, targeting directly to the diseased area while minimizing systemic side effects. Specialized drug delivery systems into the oral cavity have to be designed in such a fashion that they resist to the aqueous environment that is constantly bathed in saliva and subject to mechanical forces. Additionally, a prolonged release of drug should also be provided, which would enhance the efficacy and also decrease the repeated dosing. This review is aimed to summarize the current most relevant findings related to local drug delivery of various drug groups for prevention and treatment of infections (viral, bacterial, fungal) and infection-related manifestations in the oral cavity. Current therapeutic challenges in regard to effective local drug delivery systems will be discussed, and the recent approaches to overcome these obstacles will be reviewed. Finally, future prospects will be overviewed to promote novel strategies that can be implemented in clinical management for prevention and treatment of oral infections.
Graphical abstract
Keywords : Oral infections, Topical drug delivery, Nanoparticulate systems, Hydrogels, Fibers, Strips, New therapeutic agents
Introduction
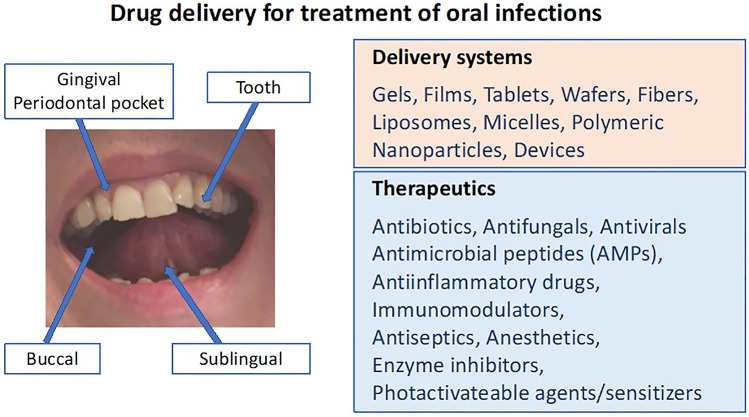
The oral cavity, which is the main entrance for two systems vital to human function and physiology, the gastrointestinal and respiratory systems, consists of the teeth, the buccal, sublingual and gingival mucosa, soft and hard palate, and tongue (Fig. 1). It has a very large and diverse microbiota, harboring numerous microorganisms which include bacteria, fungi, viruses, and protozoa. There is a homeostasis between this microbiota and the host, which forms an environment with specific dynamics, and plays a crucial role in maintaining the oral health as well as the systemic health. Disruption of this balance by various factors will result in dysbiosis, allowing for the survival and establishment of a more virulent polymicrobial community impairing the efficient immune responses. Subsequently, these events can clinically manifest as oral infectious diseases [1]. For the pathogenesis of the oral infections, not only the microbiological aspects but also the immunological host response needs to be considered as crucial elements.
Fig. 1.
Drug groups and delivery systems for treatment of oral infections
Oral diseases pose a major health burden for many countries and affect people throughout their lifetime, causing pain, discomfort, disfigurement and even death. These diseases share common risk factors with other major noncommunicable diseases. It is estimated by the World Health Organisation (WHO) that oral diseases affect nearly 3.5 billion people [2]. Oral health is considered as a key indicator of overall health, well-being and quality of life. In 2019, oral health has been included in the Political Declaration on Universal Health Coverage of United Nations Political Declaration [3]. Several systemic diseases manifest in the oral cavity. Vice versa, specific conditions in the oral cavity may create foci of infection that can affect many other vital systems, such as the cardiovascular and renal systems.
Virus-related oral infections
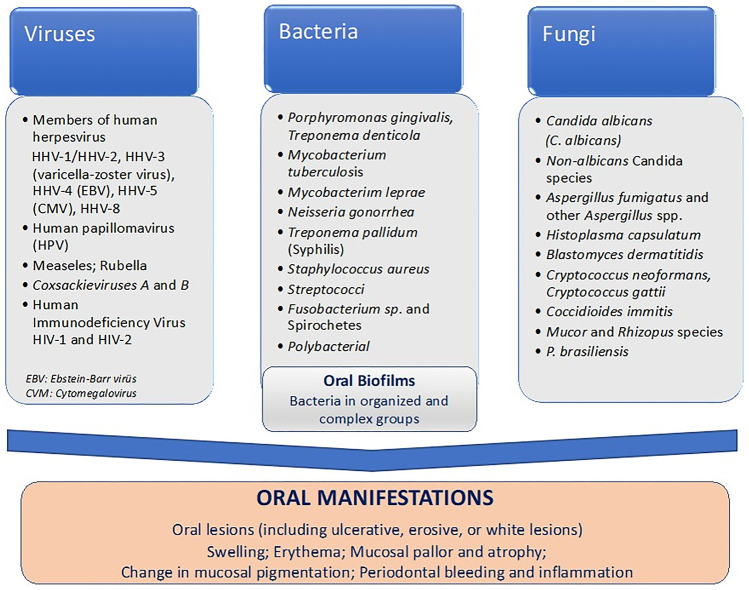
Oral infections, based on their microbial etiology, can be reviewed in three essential groups, which are viral, fungal, and bacterial. Although frequency of oral viral infections is not very high as the bacterial infections and exerts diagnostic challenges, it may be linked to some severe results in the oral cavity [4–6].
Many dormant viruses that are present in the human oral cavity can be activated and produce a variety of pathological changes in the oral mucosa in individuals immunocompromised by age, illness, or as a side effect of therapy (e.g., anticancer therapy) [7]. Oral manifestations of general viral infections may be presented as a primary sign of disease, co-symptom of the disease, or the only sign observed in such viral disease. Among the major types of viruses that are responsible for oral infections are Paramyxoviridae, Coxsackieviruses (a subgroup of the RNA enteroviruses), oral papillomas (human Papillomavirus of the Papovavirus family), and human herpesvirus family (Fig. 2). HPV infections have received particular attention in recent years, as persistent strains might increase the risk of experiencing malignant transformation in the oral mucosa. Many other viral infections can affect the oral cavity in humans, either as localized or systemic infections [8, 9]. Diagnosis and early management of viral infections is very critical, as certain viral infections result in serious conditions [4]. Recurrent Herpes Labialis (RHL) is a commonly occurring condition in herpes simplex virus (HSV) infection, characterized as a lesion located on the lips and occasionally the attached gingiva (known as a fever blister or cold sore). Secondary infections from oral bacteria can prolong the healing process. Recurrent intraoral herpes (RIH), which is observed more often in immunocompromised patients, may be difficult to distinguish clinically from other oral mucosal disorders, such as aphthous stomatitis [10]. Topical therapies for oral HSV infections can be categorized as palliative, preventive, and antiviral. Among the palliative topical agents are anesthetics such as benzocaine and lidocaine which are helpful in reducing pain associated with an oral HSV infection. Recurrent herpetic lesions are usually treated with topical antiviral medications such as acyclovir and penciclovir [11]. In immunocompetent patients, oral or parenteral administration has been shown to be more effective [4]. Currently, available antiviral medications or treatments are limited and their efficacy is inadequate.
Fig. 2.
Pathogens causing oral infections and resulting symptoms/manifestations
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a highly transmissible and pathogenic coronavirus, has emerged in late 2019, and it causes acute respiratory disease, named “coronavirus disease 2019” (COVID-19), which threatens human health and public safety. On March 2020, WHO has defined COVID-19 as pandemic [12]. At the time this paper was submitted (January 2020), more than 90 million cases and more than 1.9 million deaths have been reported, with an enormously rapid day-by-day increase in these numbers due to mutations in the virus. SARS-CoV-2 is transmitted from human-to-human by either direct transmission such as cough, sneeze, and droplet inhalation or contact transmission like saliva, contact through mucous membranes of the mouth, nose, and eyes. SARS-CoV-2 uses ACE2 as the receptor and human proteases as entry activators; subsequently, it fuses the viral membrane with the cell membrane and achieves invasion [13, 14]. ACE-2 expression highly occurs in the oral mucosa epithelium, and the expression is more in the dorsum of the tongue [15]. Wang et al. [16] have reported that SARS-CoV-2 might induce acute sialadenitis and associated symptoms, such as pain, discomfort, inflammation, and secretory dysfunction in salivary glands by fusing with them, replicating and lysing the cells. As oral mucosa is the first area infected with SARS- CoV-2 through droplets [17], oral mucosal lesions, painful ulcers, or blisters could be signs of COVID-19, and they should be examined thoroughly [18]. Currently, there is a low certainty of evidence regarding cause–effect relationship between coronavirus infection and the appearance of oral lesions; however, similar to that of HIV infection, COVID-19 patients were reported to develop oral lesions related to immunosuppression. Taste alteration is the most prevalent reported oral manifestation. The multiple clinical aspects suggest coinfections, immunity impairment, and adverse reactions rather than a genuine oral mucosa infection primarily caused by SARS-CoV-2 [19]. Lack of oral hygiene, opportunistic infections, stress, immunosuppression, vasculitis, and hyper-inflammatory response secondary to COVID-19 have been reported as the most important predisposing factors for onset of oral lesions in COVID-19 patients. Among the most common oral manifestations of COVID-19 disease, oral dryness, vesiculobullous lesions, aphthous-like lesions, herpetiform lesions, candidiasis, and oral lesions of Kawasaki-like disease have been reported [18, 20–23]. Currently, there are no specific medications for COVID-19-related oral manifestations, but progress in investigations is indeed going faster than usual, because it is considered as a “Once-in-a-Century Pandemic”; hence, there would be new effective treatments available for clinical applications in coming months.
Fungi-related oral infections
Candidal and non-candidal fungal infections in oral mucosa occur generally as a result of defects in the immune system. Candidiasis (candidosis) is the most common fungal infection of the oral cavity, whilst the incidence of noncandidal oral fungal infections such as aspergillosis, cryptococcosis, histoplasmosis, blastomycosis, paracoccidioidomycosis, zygomycosis (mucormycosis), oral geotrichosis, Rhodotorula infection, and fusariosis is rather low [24]. The most commonly seen fungal oral infections are summarized in Fig. 1. Fungal infections can be superficial, may cause serious lesions in the oral cavity, or can be indicative of a more serious systemic illness that may even result in mortality [25].
Various systemic and topical agents are used in treatment of candidiasis. Topical delivery has been preferred predominantly in uncomplicated cases, whilst systemic delivery is indicated when topical agents are ineffective or not tolerated in cases such as immunocompromised HIV or patients with cancer. Nystatin, amphotericin B, miconazole, ketoconazole, and clotrimazole are the most commonly used topical antifungal drugs. Other systemic (oral or parenteral) treatment alternatives such as itraconazole, voriconazole, posaconazole, anidulafungin, caspofungin, and isavuconazole have also found applications in treatment [26–28].
Bacteria-related oral infections
The oral cavity harbors more than 700 different bacterial species [29]. Oral bacterial infections can occur with intense clinical symptoms, chronic or without apparent symptoms, or clinical findings without impairing the host defenses like disrupting the mucosal barriers. Oral infections commonly originate from an odontogenic (tooth) source in adults and from tonsil and lymphatic sources in children. Odontogenic infections arise from advanced dental caries or periodontal disease. Nonodontogenic oral infections are related to salivary gland infection, lymph node abscess, postoperative infection, chemical, thermal, or trauma injury and may be associated with almost any microorganism. Sexually transmitted pathogens such as herpes simplex, Neisseria gonorrhea, and Treponema pallidum may be considered [30]. These nonodontogenic infections can be potentially life threatening.
The oral mucosal infections can be widespread (stomatitis/mucositis, glossitis, gingivitis) or localized (white lesions, red lesions, ulcers) [31]. The clinical manifestations and bacteria responsible for oral infections are summarized in Fig. 2. Complex biofilms of varying compositions of bacteria can colonize the surfaces of the oral cavity. Dental plaque is the term commonly used for the biofilm formed on teeth; however, this term has now been extended to include biofilms on all oral surfaces. These biofilms consist of complex microbial communities embedded in a matrix of polymers of bacterial and salivary origin, and they are recognized as a virulence factor in many oral infectious diseases, including dental caries, periodontitis, and endodontic infections [32, 33]. Periodontitis, which is a chronic inflammatory disease, may result in progressive destruction of the periodontal ligament and alveolar bone with periodontal pocket formation, gingival recession, or both. Periodontitis can also affect many other vital systems, such as the cardiovascular and renal [34].
On the other hand, severe periodontal inflammation or bleeding may require careful investigation of conditions such as diabetes mellitus, human immunodeficiency virus infection, thrombocytopenia, and leukemia [35]. It is important to diagnose correctly the underlying local or systemic condition of the oral diseases for the right treatment. Examination of the oral cavity should include evaluation for mucosal changes, periodontal inflammation and bleeding, and general condition of the teeth. Oral manifestations of specific systemic conditions are oral lesions (including ulcerative, erosive, or white lesions), swelling, and erythema, mucosal pallor and atrophy, change in mucosal pigmentation, periodontal bleeding, and inflammation [35, 36].
Antibacterial agents such as chlorhexidine, metronidazole, and tetracycline are used topically in the management of these infections; hence, higher concentration of the antibiotic can be available in the affected area and a much lower concentration throughout the rest of the body. By this means, the systemic side effects, as well as the risk of bacterial resistance, are decreased. Furthermore, antibacterial agents have been shown to be effective in the disruption/inhibition of oral biofilm. Nevertheless, current antimicrobial treatments have been reported to treat the problem only provisionally and are not effective at complete elimination of the infections, and the challenge of precisely and continuously eliminating the specific pathogens without disturbing the microbial ecology still exists. Alternate strategies against biofilms such as biofilm-inhibition agents, to prevent the early stages of biofilm formation, or biofilm-dispersal agents to disrupt the biofilm cell community have not been sufficiently efficient in direct treatment and eradication of the established biofilms [37]. Hence, investigation of alternative agents to antibiotics as well as new delivery systems play a key role to improve the efficacy of the antibacterial therapy against oral infections, without necessarily inducing microbial dysbiosis of the oral cavity. In following sections, such approaches will be reviewed with current examples.
Topical drug delivery for treatment of oral infections
Topical drug delivery plays an important role in the management of oral infections. Topical drugs have been extensively used as the first line of therapy in many conditions related to viral, bacterial, and fungal infections. A large number of clinical studies have established the clinical efficacy of topical antimicrobials and antivirals which provides targeted drug delivery options for the treatment of local oral lesions (see Tables 1, 2, and 3) [38]. In general, when compared to systemic delivery, topical drug delivery has a number of advantages such as the ability to deliver drug more selectively to a specific site at higher concentrations, lowering risk of systemic adverse events, and avoiding fluctuations in drug levels, inter- and intra-patient variations, and suitability for self-medication, hence improved patient compliance. The other advantages of topical delivery will be mentioned where appropriate. Nonetheless, there are also some obstacles faced with topical drug delivery into oral cavity such as taste alterations, limited surface area, poor tissue penetration, and rapid removal due to continuous saliva flow and tongue movement and accidental swallowing. The total surface area of the oral mucosa is relatively small (~ 200 cm2). The teeth, keratinized epithelium, and non-keratinized epithelium occupy about 20%, 50%, and 30% of the total surface area, respectively. The average volumes of saliva present in the mouth before and after swallowing were estimated to be 0.77 and 1.07 mL, respectively, and the average thickness of the salivary film in the mouth was calculated as 70 and 100 µm [39]. The permeability to the topical drugs differs significantly in different oral regions, depending on the pattern of epithelial differentiation, such as thickness and the extent of keratinization. Buccal and sublingual regions in the oral cavity are lined by non-keratinized, stratified squamous epithelium, which is 100–200 μm and 8–12 cells thick in the sublingual region, and 500–800 μm and 40–50 cells thick in the buccal region. The loss of the permeability barrier in the oral mucosa, which can be encountered due to oral manifestations, may lead to rapid diffusion of the drug into tissues when compared to the intact mucosa. The oral epithelium is covered by a complex mucus layer with an average thickness of 70–100 μm, which has an impact on the mobility of delivery systems and drug molecules. Mucus forms a protective coating on epithelial surfaces and plays a key role in host defense. Mucins are the primary structural components of mucus that create its viscoelastic properties as well as its protecting functions in oral diseases such as HIV/AIDS, oral candidiasis, and dental caries [40].
Table 1.
Drug delivery systems for treatment of bacterial infection-related conditions in the oral cavity
| Drug | Target | Delivery system | Ingredients | In vitro studies | In vivo studies | Results | Reference |
|---|---|---|---|---|---|---|---|
| Antimicrobials | |||||||
| Ampicillin and metronidazole | Oral mucosa | Fiber | Polylactide |
- Antibacterial activity agar diffusion assay - Cytocompatibility human gingival fibroblasts |
- |
- Antibacterial effect against A. actinomycetemcomitans, F. nucleatum, P. gingivalis and E. Faecalis - No cytotoxic effect |
[183] |
| Cefuroxime axetil | Oral mucosa | Mono and bilayered film and wafer | Chitosan and HPMC |
- Drug release Franz diffusion cells - Antimicrobial activity agar disk diffusion method |
- |
- Prolonged release adhesive chitosan backing layer and HPMC based drug loaded layer with suitable mucoadhesion - Increased antimicrobial activity against E. coli and S. aureus |
[182] |
| Chlorhexidine digluconate |
Oral mucosa Periodontal pocket |
Film, gel | Chitosan, TPP, glycerin, lactic acid |
- Mucoadhesion Texture analyzer (porcine buccal mucosa) - Antimicrobial activity blood agar plates |
- |
- Suitable mucoadhesion - Enhanced antimicrobial activity against Porphyromonas gingivalis in presence of chitosan |
[87] |
| Chlorhexidine | Oral cavity | Mouthwash | Chitosan | - |
Healthy volunteers - Plaque index, gingival index Quickley–Hein plaque index (QPI), probing depth -Antimicrobial activity on dental plaques Agar diffusion |
- Significant reduction in clinical parameters in presence of chitosan - Enhanced antimicrobial effect against S. mutans or enterococci in presence of chitosan - |
[184] |
| Chlorhexidine | Tooth surface | Varnish | - Ethyl cellulose and poly ethylene glycol in ethanol | - |
Orthodontic patients (10 -16 year-old) - Antimicrobial activity in sputum samples of orthodontic patients |
- A significant decrease in S mutans levels for 3 weeks - No significant change in Actinomyces viscosus levels |
[185] |
| Chlorhexidine/thymol | Tooth root surface | Varnish |
Vinyl acetate co-polymer and acrylate co-polymer Ethanol or ethyl acetate as solvents |
- Antibacterial activity (agar difussion assay) | - Patients (35 and 55 year-old) with one tooth with buccal gingival recession of 1–2 mm and initial root caries (between) |
- Significant reduction in Streptococci and Lactobacilli in supragingival plaque - Stronger antibacterial activity against A.actinomycetemcomitans with ethyl acetate when compared to ethanol as a solvent - Highest activity against strains: P. gingivalis and Fusobacterium Nucleatum |
[186] |
|
Chlorhexidine and diclofenac sodium Chlorhexidine and Betamethasone Chlorhexidine and Lidocaine |
Buccal mucosa | Film | HPMC, PEG 400 and Carbopol 917 |
- Anti-inflammatory activity prostaglandin E2 levels - Antibacterial activity - Cytotoxicity test HaCaT keratinocyte cell line |
- |
- Anti-inflammatory activity by reducing prostaglandin E2 levels - Antibacterial activity against planktonic and biofilm bacteria - No cytotoxic effect |
[176] |
| Doxycycline | Periodontal pocket |
Nanoparticle loaded gel |
Nanoparticles: chitosan Gel: PVA, PVP, glycerol and PEG 400 |
- |
- Patients with moderate chronic periodontitis - IL-6 and TNF-a levels in gingival crevicular fluid |
- Reduced probing pocket depth - Decreased levels of IL6 and TNF-a |
[156] |
| Doxycycline | Subgingival placement | Strip | Methylcellulose | - |
- Patients with inflammatory periodontal disease - Gingival index, probing depth, attachment loss, and gingival shrinkage - Microbiological evaluation in subgingival fluid |
- Significant decrease in clinical parameters at week 8 - Marked decrease in anaerobic count by week 10 |
[167] |
| Metronidazole | Periodontal pocket | Microcapsuleloaded hydrogel | Chitosan, PVA |
- Drug release Dialysis diffusion method - Bacteriostasis activity |
Ligation induced periodontitis in Wistar rats |
- Prolonged drug release - Prolonged in vitro antibacterial activity - Enhanced in vivo antibacterial activity - Reduced probing depth of the periodontal pocket |
[104] |
| Metronidazole | Periodontal pocket | Fiber | Polylactide |
- Drug release (immersing fiber in liquid medium) - Antibacterial activity agar diffusion method - Cytotoxicity human gingival fibroblasts |
- |
- Prolonged drug release after day 3 - Antibacterial activity against F. nucleatum, A. actinomycetemcomitans and P. gingivalis - No cytotoxic effect |
[187] |
| Metronidazole | Periodontal pocket | Gel | Chitosan, lactic acid | - |
Patients with moderate to severe chronic periodontitis - Gingival recession, plaque index, gingival index, and gingival bleeding time |
- Significant decrease in clinical parameters and similar to that of a commercial gel | [86] |
| Metronidazole and levofloxacin | Periodontal pocket | Film | Chitosan |
- Drug release Placing films in vial containing McIlvaine buffer, pH 6.6) - Antibacterial activity disc diffusion method |
Patients with chronic periodontitis - Gingival index, plaque index and pocket depth |
- Prolonged drug release - Significant decrease in clinical parameters - Antibacterial activity against S. aureus and E. coli |
[188] |
| Minocycline | Periodontal pocket | In-situ forming cubic liquid crystal | Phytantriol /propylene glycol |
- Drug release Dialysis membrane diffusion method |
Ligation induced periodontitis in SPF rats |
- Sustained release for four days - Reduction in gingival index, probing depth and alveolar bone loss |
[91] |
| Minocycline | Periodontal pocket | Liposome | Hydrogenated soy phosphatidylcholine and cholesterol | - Cell proliferation rate MTT assay murine macrophages (ANA-1) | - |
- Inhibition of the proliferation of macrophages - Stronger anti-inflammatory effects by suppression of TNF-α mRNA expression |
[68] |
| Minocycline | Periodontal pocket | Strip | Polycaprolactone | - |
Patients with chronic periodontitis - Subgingival plaque bacterial counts on day 3 (strips inserted in periodontal pocket) |
Significant reduction in the proportions of C. gracillis, P. melaninogenica, and F. necrogenes by day 6 | [189] |
| Moxifloxacin | Periodontal pocket |
Nanoparticle loaded in situ gel |
Nanoparticles: PLGA, PVA Gel: Poloxamer 407 |
- Drug release Dialysis diffusion method |
- Ligation induced periodontitis in Sprague-Dawley rats - γ-scintigraphy analysis in rabbits |
- Extended drug release and enhanced retention of the system - Higher efficacy with once-a-week application compared to that of twice-a-day application of a commercial gel - Almost complete recovery in 3 weeks |
[156] |
| Moxifloxacin | Periodontal pocket | In situ gel | Poloxamer 407, Gellan gum, Carbopol 934P |
- Drug release Franz diffusion cell - The antibacterial activity using agar cup method |
- |
- Prolonged drug release (9h) - Antimicrobial activity against S. aureus and E. coli in gel |
[190] |
| Moxifloxacin | Oral cavity | Gel | Chitosan, Carbopol 940, HPMC |
-Drug release Franz diffusion cells - Mucoadhesion Texture analyzer -Antimicrobial activity disk diffusion method |
- |
- Prolonged drug release - Enhanced antimicrobial activity against S. aureus and S. mutans in presence of chitosan |
[96] |
| Tetracycline | Periodontal pocket | Nanofiber | PLGA and gum tragacanth |
- Drug release (immersing membrane in PBS, pH 7.4) - Biocompatibility using Human dermal fibroblast cells - The antibacterial activity using agar plate method |
- |
- Sustained release for 75 days - Biocompatible - Antibacterial activity against S. aureus and P. aeruginosa |
[191] |
| Tetracycline | Oral mucosa | Nanofiber | Chitosan and PVA |
- Drug release Vial method - The antibacterial activity (using samples collected from human periodontal subgingival pocket of patients with chronic periodontitis) - Cytotoxicity analysis MTT assay (neonatal human dermal fibroblast cells) |
- |
- Sustained release (14 h) days - Antibacterial activity against F. nucleatum, P. micra, P. nigrescens, P. intermedia, E. nodatum, C. gracilis, C. rectus and C. showae, T. denticola, T. forsythia and P. gingivalis - No cytotoxic effect |
[174] |
| Tetracycline | Implant surface | Nanofiber | PLA, PCL, and gelatin |
- Antimicrobial activity agar diffusion assay - Murine derived osteoprecursor cell (MC3T3-E1) response |
- |
- Antimicrobial activity against A. actinomycetemcomitans, F. nucleatum, P. gingivalis, and P. intermedia - Significant increase in alkaline phosphatase levels indicating an osteogenic differentiation |
[192] |
| Antiinflammatory agents | |||||||
| Aspirin and erythropoietin | Submucoperiosteous tissue | Hydrogel | Chitosan, β-sodium glycerophosphate, gelatin |
- Drug release (adding PBS to hydrogel containing plates) - Cytotoxicity MTT assay (rat bone marrow stromal cells) |
Ligature-induced periodontitis in nude mice and Wistar rats |
- Sustained release for 21 days - Anti-inflammatory activity and significant periodontium regeneration - No cytotoxicity |
[193] |
| Tenoxicam | Buccal mucosa | Film | Chitosan | - Drug release study (immersing films in artificial saliva) |
Healthy volunteers - Mucoadhesion |
- Controlled release for 6 h - Mucoadhesion time: 1.25 ± 0.17 h |
[194] |
| Atorvastatin | Periodontal pocket | Gel | Base and water soluble chitosan | - |
Ligature induced periodontitis in Wistar rats - Antiinflammatory and osteoclastic activity |
- Enhanced anti-inflammatory effect in presence of chitosan - Bone and tissue healing after week 3 - No difference between water soluble and base chitosan |
[85] |
| Atorvastatin and atorvastatin solid dispersions | Periodontal pocket | Gel | Base and water soluble chitosan |
-Drug release Franz diffusion cells - Mucoadhesion and syringability Texture analyzer - Anti-inflammatory activity human gingival fibroblast induced cells |
- |
- Prolonged drug release - Suitable mucoadhesion and syringability - Decreased release of pro-inflammatory cytokines (IL-1β, IL-6, IL-8) and anti-inflammatory cytokines (IL-10, TGF-β1, TGF-β2 and TGF-β3), enhanced in presence of chitosan - No difference between atorvastatin and soluble atorvastatin solid dispersions |
[195] |
| Natural products | |||||||
| Ziziphus jujuba extract | Buccal mucosa | Nanofibrous membrane | - Carbopol, polyacrylonitrile |
- Drug release (immersing membrane in artificial saliva, pH 6.9) - Mucoadhesion using Universal Testing Machine -Antimicrobial activity against using the disk diffusion susceptibility test - Anti-inflammatory activity permm Permeability assay (Human umbilical vein endothelial cells-HUVEC) |
- |
- 80% drug release in 1h - Suitable mucoadhesion - Improved antimicrobial activity against P. gingivalis and F. nucleatum - Improved anti-inflammatory function on HUVEC |
[196] |
| Scutellaria baicalensis and chlorhexidine | Buccal mucosa | Nanoparticle | Water-ethanol | -Antibacterial activity broth microdilution assay | - | - Inhibition of biofilms of S. mutans, S. sobrinus, F. nucleatum, and A. actinomycetemcomitans | [132] |
|
Eucalyptol, menthol, thymol Sodium fluoride, eucalyptol, menthol, thymol |
Oropharynx | Mouthwash | Alcohol, benzoic acid, methyl salicylate, poloxamer 407 | - Antimicrobial activity agar plate test | - Male patients with pharyngeal gonorrhoea |
- Significant reduction of total N. gonorrhoeae counts in vitro after 1-min exposure - Significantly reduced count of N. gonorrhoeae on the pharyngeal surface |
[197] |
| Propolis | Periodontal pocket | Magnetic nanoparticle in liquid crystalline |
Nanoparticle: Iron oxide Liquid crystal: Isopropyl myristate, polyoxyethylene oleyl ether |
- Drug release Periodontal pocket simulator apparatus with a flow system - Antifungal activity broth macrodilution test - Cytotoxicity fibroblasts cell line (ATCC CCL-1.3) |
- |
- Prolonged drug release - Fungicide activity against Candida spp. - Very low cytotoxicity |
[198] |
| Green tea Catechin | Periodontal pocket | Strip | Hydroxypropylcellulose | - |
- Patients with advanced periodontitis - Antimicrobial study Gingival crevicular fluid (GCF) - The pocket depths (PD) measured using a standard periodontal probe |
- Reduced pocket depth - Decrease in proportion of Prevotella spp. and P. gingivalis |
[199] |
| Curcumin | Periodontal pocket | Sponge | Collagen | - |
Patients with chronic periodontitis - Plaque index, gingival index, probing pocket depth and clinical attachment levels - microbiology N-benzoyl-DL-arginine-β-naphthylamide (BANA) test and microbial colony count |
-Significant reduction in clinical and microbiological parameters, yet, lower efficacy when compared to chlorhexidine chip | [200] |
| Resveratrol | Periodontal pocket | Nanofiber | Polycaprolactone |
- Drug release using USP Apparatus II - Morphology |
- | - Rapid release in the first 4 h, followed by a prolonged release up to 12h | [201] |
| Royal Jelly(bee product) | Oral mucosa | Film | Chitosan and sodium alginate | - Drug release modified JP XIV dissolution apparatus |
5-fluorouracil and mild abrasion induced oral mucositis in seven-week-old Golden Syrian hamsters -Myeloperoxidase activity (MPO) - Microscobic and macroscopic evaluations -Antiinflammatory activity Pro-inflammatory cytokines (TNF-α, interleukin-1β) |
- Drug release for 4 h - Decrease in MPO activity - Improved recovery, on day 8 -Induction of pro-inflammatory cytokines |
[202] |
| Miscellaneous | |||||||
| Metformin | Periodontal pocket | Film | Chitosan |
- Drug release Vial method - Antibacterial activity disc diffusion method |
Ligature induced + LPS injected periodontitis in Wistar rats |
- Sustained drug release (11 days) - Antibacterial activity against P. gingivalis and T. forsythia - Effectively reduced alveolar bone destruction |
[203] |
| Lactobacillus fermentum | Oral cavity | Film | Carboxymethylcellulose | - Probiotic bacteria release study (in simulated salivary fluid) | - |
- Complete bacterial release in 4 min - Maintenance of probiotic viability and antioxidant activity |
[204] |
| Bismuth subsalicylate | Oral mucosa | Nanoparticle |
- Antibacterial activity agar diffusion - Cytotoxicity using human gingival fibroblast (HGF-1) cell line |
- |
- High antibacterial activity against A. actinomycetemcomitans, C. gingivalis, and P. Gingivalis - Low cytotoxicity |
[136] | |
| PolymP-n Active nanoparticles with silver and doxycycline | Coating hydroxyapatite discs | Nanoparticles | - |
- Anti-biofilm activity - Antibacterial activity agar diffusion |
- |
- Destruction of biofilm formation - Antibacterial activity against S. oralis, A. naeslundii, V. parvula, F. nucleatum, P. gingivalis and A. actinomycetemcomitans |
[205] |
| Fe3O4 | Dentinal tubule | Liposome | PEG |
- Ex-vivo evaluation in extracted human teeth |
- | - Diffusion into dentinal tubules | [206] |
| Indocyanine green | Oral cavity | Nanosphere | PLGA, chitosan |
- Antibacterial activity activity Blood agar plates |
- | - Antimicrobial effect on P. gingivalis with indocyanine green ‐Nano/c with low‐level diode laser (0.5 W; 805 nm) irradiation | [150] |
| Pac-525(antimicrobial peptide) | Oral mucosa | Nanofiber |
Composite membrane: Gelatin/Chitosan Hydroxyapatite nanoparticles Microspheres: PLGA |
- Drug release study (immersing membrane in PBS, pH 7.4) - Osteogenic activity using rat bone marrow mesenchymal stem cells (rBMSCs) - The antibacterial activity using agar diffusion method |
- |
- A rapid release in the first 24 h, then a second burst release at around 4 days followed by a long-term sustained release - Promoted osteogenic differentiation - A good antibacterial activity against S. aureus and E. coli up to one month |
[207] |
PVA polyvinyl alcohol, PLGA poly(lactic-co-glycolic acid), PLA polylactic acid, PCL polycaprolactone, PVP polyvinylpyrrolidone, PEG polyethylene glycol, HPMC hydroxypropyl methylcellulose, TPP tripolyphosphate pentasodium, TNF tumor necrosis factor, IL interleukin, MTT dimethylthiazol-diphenyltetrazolium bromide
Table 2.
Drug delivery systems for treatment of viral infection–related conditions in the oral cavity
| Virus | Drug | Target | Delivery system | Ingredients | In vitro studies | In vivo studies | Results | Reference |
|---|---|---|---|---|---|---|---|---|
| HSV | Acyclovir | Oral mucosa | In situ gel | Poloxamer 407, Carbopol 934, and HPMC |
-Drug release -Ex-vitro (porcine oral mucosa) drug permeation -Mucoadhesion (porcine oral mucosa) using modified physical balance |
- |
-Drug release up to 6 h -Suitable mucoadhesion |
[208] |
| HSV-1/2 | Acyclovir | Buccal mucosa | Films impregnated with nanospheres |
Nanosphere: PLGA, PVA Film: HPMC K15, Eudragit RL 100, Carbopol 974P, PEG 200, Ethyl cellulose |
-Drug release paddle over disc method using USP II apparatus -Drug permeation Franz diffusion cells -Mucoadhesion Texture analyzer using rabbit buccal mucosa |
White male rabbits |
- High permeation and controlled release of drug over an extended period of time - Enhanced bioavailability by ∼ 8 fold |
[209] |
| HSV-1 | Acyclovir | Buccal mucosa | Tablet (Sitavig®) | Hypromellose, milk protein concentrate, sodium lauryl sulfate, magnesium stearate, MCC, povidone, colloidal silicon dioxide | - | Patients, with at least four herpes episodes in the previous year |
- Prolonged plasma drug levels - Reduction of duration of the herpes episode - Less primary vesicular lesions |
[210] |
| HSV-1 |
Penciclovir Acyclovir |
- | Cream | - | - | Patients with herpes simplex facialis/labialis (five times daily for 7 days) | - 1% penciclovir and 3% acyclovir equally effective | [211] |
| HHV-4 (EBV) |
Podophyllin resin Penciclovir Acyclovir |
Tongue | Cream | - | - | Patients with HIV infection and oral hairy leukoplakia (related to EBV) |
- Effective clinical healing within 7–8 weeks - Faster clinical healing with podophyllin resin + acyclovir |
[212] |
| HHV-5 (CMV) | Plasmid DNA (CMV-β-gal) and β-galactosidase | Buccal mucosa | Bilayer film | Polycarbophil and Eudragit S-100 |
- Drug release vial method - Mucoadhesion time using a glass model |
Female New Zealand White rabbits | - IgG titers comparable to that of subcutaneous administration | [213] |
CMV cytomegalovirus, EBV Epstein-Barr virus, HSV Herpes simplex virus, HHV human herpes virus, HPMC hydroxypropyl methylcellulose, NaCMC sodium carboxymethyl cellulose, MCC microcrystalline cellulose, PVP polyvinylpyrrolidone, PVA polyvinyl alcohol, PLGA poly(lactic-co-glycolic acid), PEG polyethylene glycol
Table 3.
Drug delivery systems for treatment of fungal infection-related conditions in the oral cavity
| Fungus | Drug | Target | Delivery system | Ingredients | In vitro studies | In vivo studies | Results | Reference |
|---|---|---|---|---|---|---|---|---|
| Candida albicans | Chlorhexidine digluconate | Oral cavity | Gel, film | Chitosan, glycerin, TPP, lactic acid |
- Drug release Franz diffusion cells - Antifungal activity |
- |
- Prolonged drug release - Enhanced antifungal activity obtained in presence of chitosan |
[94] |
| Candida albicans | Nystatin | Buccal mucosa | Gel, film | Chitosan, glycerin, TPP, lactic acid, glacial acetic acid, aspartame |
- Drug release Franz diffusion cells |
- Young male golden Syrian hamsters (5-fluorouracil induced mucositis) - Healthy volunteers |
- Prolonged release - Increased reduction of granulation tissue and formation of scar tissue in presence of chitosan - Drug concentrations above MIC value for maintained for 90 min at the application site |
[95] |
| Candida albicans | Ciclopirox olamine | Buccal mucosa | Bilayer film | Polyethylene oxide, Eudragit, glycerol |
- Drug release USP paddle apparatus - Drug permeation (porcine buccal mucosa) using modified Franz cells |
White SPF European rabbits intraorally infected with Candida albicans (ATCC90028) |
- Drug release for 12 h - Accumulation of drug in porcine buccal mucosa in ex vivo studies. - Prolonged plasma levels - Progressive healing in stomatitis without organ pathologies |
[214] |
| Candida albicans | Clotrimazole | Buccal mucosa |
pH triggered in situ gel Ion triggered in situ gel |
Carbopol - HPMC Gellan gum-HPMC |
- Drug release using flow through device cell - Antifungal activity agar diffusion method |
- |
- Prolonged release (6 h) in presence of gellan gum - Comparable antifungal activity to that of a commercial product |
[215] |
| Candida albicans | Fluconazole | Buccal mucosa | Oral strip | Eudragit RS 100, Eudragit RL 100, HPMC E50, HPMC K100M, PEG 400 |
- Drug release dialysis bag - Ex vivo drug permeation (bovine buccal mucosa) using Franz diffusion cells - Antifungal activity (agar well diffusion method) - Mucoadhesion texture analyzer - Cytotoxicity MTT assay with Chinese hamster ovary (CHO) cells |
- |
- Fast disintegration (5-30 s), prolonged release - Enhanced antifungal activity - Suitable mucoadhesion with Eudragit and HPMC combination -No drug permeation across bovine buccal mucosa - No cytotoxic effect |
[216] |
| Candida albicans | Miconazole | Sublingual and buccal mucosa | Nanostructured lipid carrier (NLC) based hydrogel |
- Hydrogel: Carbopol 2001 (PFC®) and triethanolamine - NLC: Gelucire® 43/01, Miglyol® 812, Tween® 80 |
- Drug release dialysis bag - Antifungal activity (agar-well diffusion method) |
- |
- Controlled drug release (16% and 22% in 48 h) - Antifungal activity with lowered dose |
[217] |
| Candida albicans |
Miconazole Clotrimazole |
Buccal mucosa Oral mucosa |
Tablet Troche |
- Croscarmellose sodium, magnesium stearate, MCC, povidone, dextrates | - |
- HIV positive patients with oropharyngeal candidiasis ( ≥18 years of age) - Buccal tablet adhesion time - Local inflammation (gingival index) - Once daily buccal tablets of miconazole and 5 times daily clotrimazole troches for 14 days |
- Effective, safe, and well-tolerated treatment with once-daily dose buccal miconazole - Similar efficacy between once-daily buccal tablet and 5 times daily troche |
[218] |
| Candida albicans | Natamycin | Buccal mucosa | Bilayered tablet | Carbopol 974, HPMC |
- Drug release studies USP rotating paddle method - Adhesion (membrane) using Texture Analyzer - Antifungal activity (broth microdilution method) |
- Ten females and two males (22-29 year-old) with no history of dry mouth conditions and oral lesions - Tablets placed on buccal mucosa - Saliva samples collected from different regions in the oral cavity |
- Unidirectional drug release obtained in prolonged fashion - Drug concentration maintained above the MIC value -Highest drug levels on application side, lowest drug levels in sublingual region |
[219] |
| Candida albicans | Nystatin | Buccal mucosa | Nanoparticles incorporated in toothpaste, oral gel and oral films |
Nanoparticles: PLA, PLGA and alginate Toothpaste: xanthan gum, glycerol, sorbitol, citric acid buffer, NaF, CaCO3, Microcrystalline, cellulose, sodium, lauryl sulfate Gel: Sodium hydroxide, Carbopol 940 Film: HPMC, glycerol |
- Mucoadhesion Texture Analyzer and retention studies with mucus-secreting HT29-MTX cells | - |
- Enhanced mucoadhesion in order of: film with the PLGA nanoparticles > gel with PLA nanoparticles > toothpaste with alginate nanoparticles |
[220] |
| Candida albicans | Nystatin | Buccal mucosa | Microspheres | Alginate,chitosan, calcium carbonate, calcium chloride, acetic acid, soya oil, and Span® 80 |
- Drug release Franz diffusion cell - Antifungal activity (Sabouraud Dextrose medium) |
Female crossbred (Landrace × Large White) pigs - Drug plasma levels - Histopathology after sacrification |
- High fungal activity - No nystatin in systemic circulation, assuring the safety of the treatment - Nystatin retained in the tissue without any tissue damage |
[221] |
| Candida albicans, Candida glabrata, Candida parapsilosis | Clotrimazole | Buccal and sublingual mucosa | Nanoemulsion | Capry-locaproyl macrogol-8 glycerides, medium-chain triglycerides, propylene glycol monocaprylate, propylenglycol |
- Drug release and permeation Franz difussion cells (porcine buccal and sublingual mucosa) - Antifungal activity (broth microdilution method) |
- |
- Prolonged drug release (48 h) - Drug permeation similar to that of a commercial product - Significant antifungal activity against Candida ssp. |
[222] |
| Candida albicans, Candida parapsilosis, Candida. krusei | Posaconazole | Buccal mucosa | Film | Alginate oligosaccharides, sodium alginate, glycerol |
- Mucoadhesion using (bovine buccal mucosa) - Antifungal activity (broth microdilution method) |
- Human volunteers Mucoadhesion of placebo films |
- Prolonged release (5 h) and suitable mucoadhesive property - Improved antifungal activity against |
[223] |
| Candida albicans, Candida parapsilosis, Candida krusei | Amphotericin B | Oropharyngeal cavity | Film | HPMC acetate succinate, maltodextrin, sorbitol, dextran, microcrystalline cellulose, sodium carboxymethylcellulose, HPC |
- Disintegration test - Antifungal activity (agar diffusion assay) |
- |
- Fast disintegration (60 s) - High antifungal activity |
[224] |
| Cryptococcus neoformans, Candida albicans, Sporothrix schenckii | Miconazole nitrate | Buccal mucosa | Gel | - HPMC, carbopol 940, methyl paraben, propyl paraben, PEG 400, propylene glycol, hydroxyethyl cellulose, NaCMC, Tween 20, Tween 80, triethanolamine |
- Ex vivo permeation study (goat buccal mucosa) using modified USP II type dissolution apparatus - Strength and mucoadhesion studies using Texture Analyzer - Antifungal activity (agar diffusion method) |
- |
- Efficient permeation - High adhesion and strength - Broader zone of growth inhibition compared to marketed formulation |
[225] |
PLA polylactic acid, PLGA polylactic-co-glycolic acid, TPP tripolyphosphate pentasodium, HPMC hydroxypropyl methylcellulose, MIC minimum inhibitory concentration, MCC microcrystalline cellulose, PEG polyethylene glycol, MTT dimethylthiazol-diphenyltetrazolium bromide
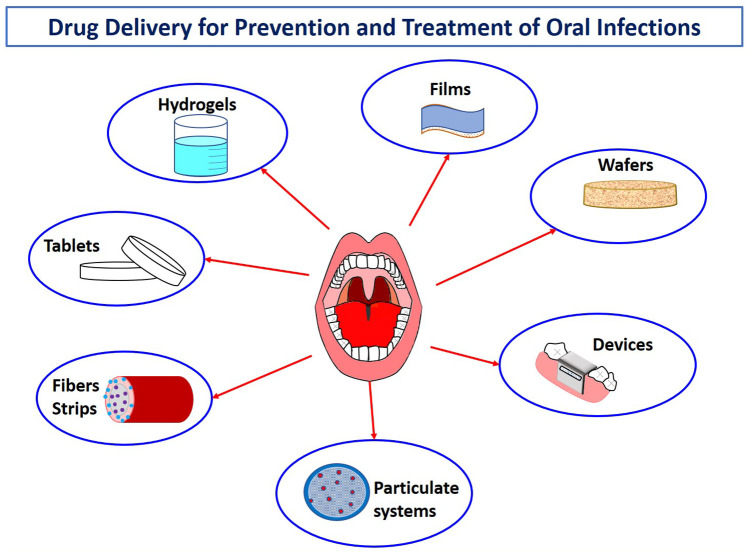
Topical drug delivery systems are traditionally formulated as solid dosage forms (e.g., tablets, wafers, films, fibers, and patches), liquid dosage forms (e.g., sprays and drops), and semi-solid dosage forms (e.g., gels, ointments) [41–45]. Conventional topical dosage forms are commonly affected by physiological factors, which can reduce the contact of the formulation with the mucosa and lead to reduced efficacy. Hence, numerous strategies have been proposed in order to overcome these difficulties and improve the retention and permeation of drugs in the oral cavity [32, 46]. In 1950s, antimicrobials were incorporated into dental cements and resins in order to provide local drug release of antimicrobials [47]. Recognition of local antimicrobial delivery systems in the management of bacterial infections in the oral cavity resulted in a shift in treatment modalities of dental diseases [48]. Chlorhexidine chip [49], metronidazole oral gel [50], and minocycline dental gel [51] are amongst the first formulations brought to the market.
For a successful local drug delivery, mucoadhesive delivery systems have been widely utilized to avoid the rapid removal of the system from the side of application due to physiological conditions in the oral cavity [52, 53]. Interaction between mucin and mucoadhesive polymer enables the system to remain attached on the application site and also provides prolonged release of drug. Penetration enhancers are also incorporated into delivery systems to generate improved efficacy for both local and systemic drug delivery. In designing a local delivery system, it is also important to take into consideration the condition of the disease, as each condition may require distinct penetration and drug retention/distribution profiles for an optimized efficacy. In most conditions, the drug is required to penetrate to deeper layers of the epithelium. Consequently, regarding all the requirements mentioned above, various delivery systems (e.g., liposomes, polymeric nanoparticles, lipid nanoparticles, hydrogels, fibers, films) other than conventional formulations have been investigated for an improved treatment of the oral infections [54].
Liposomes
In dentistry, liposomes have been used topically to control the oral biofilm (preventing caries and gingivitis), to treat oral lesions and periodontitis, and in photodynamic therapy. Liposomes are synthetic nano-sized vesicles consisting of one or more phospholipid bilayers, able to accommodate hydrophilic and lipophilic molecules. Liposomes may be formulated with a range of characteristics including different size, charge, and drug retention, which can be customized for a given drug and target site [55, 56]. In early 80 s, Mezei and Gulasekharam [57, 58] have shown the applicability of liposomes as drug carriers for the topical administration using triamcinolone as a model drug. Later, the potential of liposomes as drug carriers to the ulcerated oral mucosa was investigated in vivo in hamsters using radioactive triamcinolone acetonide palmitate [59]. Liposomes were shown to increase local and decrease systemic drug concentration. In addition, the authors suggested that liposomes decrease drug diffusion into neighboring tissues and localize the drug in the area of inflammation. Proteoliposomes with surface-bound succinylated concanavalin A were prepared to deliver triclosan for elimination of Streptococcus sanguis biofilms [60]. It was shown that triclosan delivered in liposome was a more effective growth inhibitor than free triclosan. Further, reactive liposomes were prepared encapsulating the enzymes, glucose oxidase (GO) and GO in combination with horse radish peroxidase (HRP) to eliminate the biofilms of the oral bacterium Streptococcus gordonii [61]. Increased bacterial inhibition was observed with the reactive liposomes. Antibacterial activity in the presence of saliva was also observed with the reactive liposomes.
Immunoliposomes were developed to increase the specificity and affinity of bactericide delivery to a specific model bacterium [62]. Antibacterial immunoliposomes were prepared using covalently bound antibody, extended to the cell surface of the bacterium Streptococcus oralis, and chlorhexidine and triclosan were incorporated as the bactericides. For short exposure times to the biofilms, several times enhanced growth inhibition of S. oralis was obtained with immunoliposomes when compared to free bactericides.
Variable results have been reported in regard to relation between the surface charge of the liposomes and their effect on biofilms, most likely due to the differences between test methods used in the studies. Nguyen et al. [63] have reported that negatively charged liposomes, specifically targeting for the teeth, appeared to be the most suitable for use in the oral cavity because these liposomes were found to be the least reactive with the components of parotid saliva. On the other side, Sugano et al. [64] have investigated the behavior of cationic liposomes on S. mutans in planktonic cells and biofilms and they reported that cationic liposomes have higher affinity not only to oral bacterial cells but also biofilms than conventional liposomes. It was demonstrated microscopically that cationic liposomes interacted with the negative charge on the bacterial surface and penetrated the deep layers of biofilms.
Lectin-conjugated liposomes were prepared using wheat germ agglutinin (WGA) to serve as bioadhesive drug carrier that can rapidly bind to oral epithelial cells within minutes, and stay on the cells to provide sustained, localized drug release for the management of oral ulcerative lesions and other related complications [65]. A significant reduction in oral cell damage was obtained when the bacterially infected cells were treated with amoxicillin-loaded WGA liposomes compared to the untreated controls.
Erjavec et al. [66] have investigated liposome formulations of varying composition and size to identify a suitable carrier for drug delivery to oral mucosal lesions by assessing the effects of a hyperaemic drug on the oral mucosa using in vivo EPR (electron paramagnetic resonance)-oximetry. They have reported that multi-lamellar liposomes made from hydrogenated soy lecithin appeared to be the most appropriate for local drug delivery to oral mucosa.
Liposome formulations have been widely investigated for treatment of periodontitis. It was shown in vivo that local delivery of liposome-encapsulated superoxide dismutase and catalase suppressed periodontal inflammation in experimentally induced periodontitis beagle dogs [67]. As an adjunctive treatment for chronic periodontitis, liposome formulation for an antimicrobial drug, minocycline was developed and investigated in vitro on murine macrophages (ANA-1) [68]. Liposomes were shown to have stronger and longer inhibition effect on LPS-stimulated TNF-α secretion of macrophages cell when compared to that of solution of the drug. This result was attributed to the specificity of liposomes for macrophages and also controlled delivery provided by the liposomes.
pH-responsive quaternary ammonium chitosan (TMC)-liposome formulations loaded with doxycycline were developed for periodontal treatment [69]. The periodontitis healing capacity of the developed formulations was evaluated in rats. The formulations showed antimicrobial activity against P. gingivalis and Prevotella intermedia, strong inhibition on biofilm formation and prevented alveolar bone absorption in vivo.
Periodontal therapy usually requires also local anesthesia. A liposomal lidocaine/prilocaine, thermosetting anesthetic gel formulation delivered into periodontal pocket was investigated for pain control during scaling and root planing (anti-infective periodontal therapy) in 40 volunteers with moderate to severe chronic periodontitis [70]. It was reported that the intra-pocket anesthetic gel would be a good option for anxious patients, or those who have a fear of needles.
Micelles
Micelles are self-assembling colloidal systems obtained by the aggregation of block or graft amphiphilic copolymers [71]. Micelles have found applications in dentistry for a targeted-delivery of antimicrobials to the tooth surface against biofilm formation. Chen et al. [72], have used alendronate terminated Pluronic copolymers to prepare triclosan-loaded tooth-binding micelles and demonstrated that micelles were able to inhibit initial biofilm growth of S. mutans. The use of alendronate as a binding moiety, however, has raised concerns on the safety of these tooth-binding micelles; therefore, the same group has replaced alendronate with diphosphoserine and conjugated it to the chain termini of Pluronic P123 and combined it with another biodegradable tooth-binding moiety, pyrophosphate (PPi) [73]. Tooth-binding potential and binding stability as well as anti-biofilm activity against S. mutans of the developed micelles were found to be significant. Recently, a multifunctional matrix for the treatment of periodontitis and enhancement of regeneration of the periodontal tissue was prepared from vitamin E containing hydrogel made of alginate and gelatin, and doxycycline HCl containing methoxy poly(ethylene glycol)-block-polycaprolactone micelles [74]. A sustained drug release and enhanced antimicrobial activity were observed against E. coli and S. aureus.
Nanoemulsions
Nanoemulsions have been recognized to have unique properties that make them more versatile than other emulsion systems. Nanoemulsions consist of droplets 100 nm or smaller in diameter of immiscible liquids stabilized by surface-active materials [75]. They are distinguished from microemulsions by their stability. Microemulsions are indefinitely stable thermodynamic phases, whereas nanoemulsions are transient, kinetically stable structures. Nanoemulsions can be fabricated with great flexibility to deliver different drug moieties with different characteristics. Several studies have shown the efficacy of nanoemulsions in prevention and treatment of diseases of the oral cavity [76]. Nanoemulsions are reported to provide improved penetration of the drug into deeper layers of the oral mucosa due to their size, thereby resulting in better and complete cure of the disease. Nanoemulsions with high surfactant content have also been shown to exert antimicrobial activity. Karthikeyan et al. [77] have prepared oil-in-water nanoemulsion composed of soybean oil, cetylpyridiniumchloride, and Triton X-100 and demonstrated its antimicrobial activity against cariogenic Streptococcus mutans, suggesting this formulation for prevention of dental caries.
Hydrogels
Hydrogels have a three-dimensional porous and interconnected structures composed of hydrophilic, cross-linked macromolecules that absorb water, aqueous solutions, or physiological fluids, but remain insoluble due to their network structure [78, 79]. They provide a biocompatible microenvironment for cell attachment and proliferation and possess many unique advantages on the targeted delivery systems for hydrophilic and hydrophobic agents and other biomolecules. Localized application is possible with hydrogels, and they can be tailored to release the drug for a long time by controlling the hydrogel architectures, network pores, and gelation mechanisms (physical and chemical gelation). Synthetic (poly(hydroxyethyl methacrylate) (polyHEMA, PHEMA) polyethylene glycol and derivatives, poly(vinyl alcohol), polyvinylpyrrolidone, polyimide, polyacrylate, polyurethane [80], and natural (chitosan, alginate, collagen, gelatin etc.) [81] polymers have been used for preparation of hydrogels. Most of these polymers also exert adhesive properties which enables a longer retention of the system on application site. Hydrogels have found applications in dentistry for regenerative therapies to provide recovery of the function of tissues lost due to oral and dental pathologies of infection as well as traumatic and neoplastic origin [82–84]. Furthermore, various hydrogel formulations have been used for treatment of oral lesions and also for delivery of antimicrobials, anesthetics, and antiinflammatory drugs [85–91]. Our group has investigated gel formulations based on chitosan, which is a material widely investigated in dental field both for its bioactive properties such as wound healing, tissue regeneration, antimicrobial, and as a biocompatible, bioadhesive biopolymer for delivery of drugs, especially the anti-inflammatory and antimicrobial molecules [84, 92]. Chitosan gel itself has been shown to exhibit antimicrobial activity against various dental patogens [93]. Antimicrobial activity was found to depend on the properties of the chitosan used (source—animal or non-animal, molecular weight, solubility, degree of deacetylation etc.) as well as the type of the strains tested. Furthermore, when incorporated with various antimicrobial drugs such as chlorhexidine [87, 94], nystatin [95], moxifloxacin [96], metronidazole [86], and anti-inflammatory drug such as atorvastatin [85, 97], the effect of the drug was found to be enhanced in presence of chitosan, besides the improved retention time and prolonged drug release. Chitosan gel itself has also been shown in vivo in human to be promising for periodontal tissue regeneration [98].
Hydrogels for the anesthetic drug lidocaine hydrochloride were prepared for buccal application using chitosan glutamate, or its binary mixture with glycerin. The anesthetic activity of mucoadhesive hydrogels was assessed in healthy volunteers in comparison to commercial semisolid formulations. Prolonged release of drug, which resulted in local anesthetic activity lasting for 20 to 30 min upon application, was obtained. The developed hydrogels were suggested as potential delivery system reducing the pain symptoms that characterize aphthosis and other mouth diseases [99].
Hydrogels exert appropriate syringeability properties which make it suitable for administration into periodontal pocket [100]. The injectable thermosensitive hydrogels have gained more attention, especially for unapproachable periodontal pockets. An injectable thermogel system for the treatment of oral mucosa-related ulcers was developed by Luo et al. [101]. These thermogels were formed from a series of chitosan-based conjugates, composed of a chitosan backbone and synthetic side chains of thermosensitive poly(N-isopropylacrylamide) (PNIPAAM). Ulcer healing was investigated in vivo in rats and the antibacterial activity against Staphylococcus aureus as well as proliferation promotion; hemostasis effect of the developed formulation was demonstrated. Ji et al. [102] have developed a thermosensitive hydrogel based on chitosan, quaternized chitosan, and β-glycerophosphate loaded with 0.1% w/w chlorhexidine. Higher antimicrobial activity against P. gingivalis and Prevotella intermedia was obtained with gels prepared using quaternized chitosan when compared to that with chitosan.
A thermo-reversible poly-isocyanopeptide (PIC), which is a water-soluble polymer forming a gel at very low polymer concentrations with good injectability properties, and has a sol–gel transition temperature of 15–18 °C [103], was investigated as a hydrogel for delivery of doxycycline and/or lipoxin A 4 for antimicrobial and anti-inflammatory treatment [100]. The PIC hydrogel facilitated the drug for around 4 days in vitro. When applied in dogs, local or systemic adverse effects were observed. The subgingival bacterial load and pro-inflammatory interleukin-8 level were shown to reduce with the hydrogel formulations. Gingival clinical attachment was improved when compared to mechanical debridement.
Dong et al. [104] have incorporated metronidazole loaded microcapsules into a poly(vinyl alcohol) injectable hydrogel by dynamic covalent bonding and ionic interaction through a 4-carboxyphenylboronic acid bridge. The developed formulation exhibited desirable antibacterial activity against P. gingivalis and Fusobacterium nucleatum for 1-week period on the rats.
Hydrogels have been used also to deliver antimicrobial peptides (AMPs), which are one of the most well-studied classes of biofilm eradication agents [105, 106]. AMPs are a diverse group of host-defense molecules that include defensins, cathelicidins, histatins, neuropeptides, peptide hormones, and many other proven and putative peptides. In the oral cavity, the AMPs are produced by the salivary glands and the oral epithelium [107]. AMPs are effective defensive weapons and have been shown to modify cellular functions such as chemotaxis, apoptosis, gene transcription, and cytokine production. Further, they play role in stimulation of wound healing and angiogenesis. Due to their antibacterial, anti-inflammatory, and/or immune modulatory actions, they are used to control oral infections [108–114]. Sani et al. [89] have developed a hydrogel based on a visible-light-activated naturally derived polymer (gelatin) and an antimicrobial peptide (AMP) for treatment of peri-implant diseases. An enhanced antimicrobial activity against P. gingivalis was obtained with the gels.
In oral mucosal conditions related to immunological pathogenesis, clinical studies have shown that topical immunomodulators such as cyclosporine, tacrolimus, and pimecrolimus are also effective when compared to the steroids which are the conventionally used drugs [115–120]. In order to enhance their activities, these immunomodulators were incorporated into bioadhesive gels. For the treatment of oral lichen planus, clobetasol and cyclosporin adhesive gels based on hydroxyethyl cellulose were applied twice a day on dried lesions for 2 months and significant healing was observed with the gels [121].
Currently, there are commercially available products based on hydrogels. A two syringe mixing system (Atridox) is a subgingival controlled-release product composed of the syringe A: Atrigel® Delivery System, which is a bioabsorbable, flowable polymeric formulation composed of 36.7% poly(DLlactide) (PLA) dissolved in 63.3% N-methyl-2-pyrrolidone (NMP) and syringe B containing doxycycline hyclate [122]. Upon contact with the crevicular fluid, the liquid product solidifies and then allows for controlled release of drug for a period of 7 days. In addition, numerous gel formulations of metronidazole are also available on the market.
Nanomaterials and polymeric nanoparticles
Materials in nano-size and drug-incorporated nanoparticles as well as their combination have found wide applications in dentistry for prevention, diagnosis, therapeutic, restoration, and tissue regeneration purposes [54, 123–125].
Metallic nanoparticles such as silver, gold, and zinc oxide due to their broad-spectrum antibacterial activity have been used to eliminate the biofilms in the oral cavity [126–131]. The large surface area and high charge density of these nanoparticles enable them to interact with the negatively charged surface of bacterial cells to a greater extent resulting in enhanced antimicrobial activity. In order to enhance the antimicrobial activity, these metals have been combined with other antimicrobial agents such as chlorhexidine [132]. Recently, the antimicrobial efficacy of silver and gold nanoparticles with diode laser was investigated against S. mutans in teeth sample, and the greatest reduction in colony-forming units (CFU) was observed with the combination of silver nanoparticles with diode laser group [133].
Metallic nanoparticles combined with polymers or coated onto biomaterial surfaces have been shown to exhibit superior antimicrobial properties in the oral cavity [132, 134, 135]. Besides silver, gold, and zinc oxide, bismuth subsalicylate nanoparticles have also been shown to inhibit the growth of several periodontal pathogens including A. actinomycetemcomitans, C. gingivalis, and P. gingivalis [136].
Further, mesoporous silica nanoparticles, which have a porous structure with large surface area, have been investigated as anti-biofilm agents [137, 138]. When combined with another antimicrobial such as chlorhexidine, antibacterial activity against S. mutans, F. nucleatum, A. actinomycetemcomitans, and P. gingivalis was shown to be enhanced [139].
Recently, graphene family nanomaterials, due to their superior mechanical, chemical, and biological properties, have gained great attention in dentistry. Graphene oxide (GO), as the derivative of graphene, was investigated for its antimicrobial property against various dental pathogens including S. mutans, Fusobacterium nucleatum, and P. gingivalis, and GO nanosheets were reported to be highly effective in inhibiting the growth of dental pathogens [140]. It was also shown by transmission electron microscopy that the cell wall and membrane of bacteria lost their integrity and the intracellular contents leaked out after they were treated by GO. Furthermore, graphene oxide (GO) has been widely investigated as a nanodelivery system for variety of drugs [141, 142], which makes it a promising material for treatment of infections in the oral cavity.
In the past decade, the application of antimicrobial photodynamic therapy (aPDT) on oral infectious diseases has attracted great interest. The bacteria can be killed when induced with light in the presence of a sensitizing agent, by means of generation of cytotoxic, reactive oxygen species (ROS) [143]. There are a number of sensitizers that interact with bacterial cell and generate ROS such as methylene blue, erythrosine, indocyanine green, eosin-Y, psoralen, and toluidine blue ortho [105]. Erythrosine has been applied with white light (500–650 nm) which successfully killed S. mutans and inhibited biofilm formation [144]. A dental light with haematoporphyrin sensitizer was investigated against S. mutans, A. actinomycetemcomitans, and E. faecalis, and it was found that the sensitizer can penetrate gram-positive bacteria cell, whilst by A. actinomycetemcomitans, the sensitizer is taken up in presence of 10% EDTA [145]. Furthermore, dental LEDs with blue light absorbing photosensitizer were demonstrated to disrupt E. faecalis biofilm depending on the concentration of sensitizer [146]. However, due to the hydrophobic characteristics of the photosensitizers, aPDT was not very effective on the viability of biofilms; hence, nanomaterials (metal and metal oxide nanoparticles) or polymeric nanoparticles have been used in order to enhance the antimicrobial performance of aPDT [147, 148].
The photosensitizer indocyanine green (ICG) was incorporated into chitosan nanoparticles, and A. actinomycetemcomitans ATCC 33384 strain was treated with these nanoparticles, which was excited with a diode laser [149]. The expression of rcpA gene which is involved in biofilm formation of A. actinomycetemcomitans was found to be significantly downregulated upon using nanoparticles for aPDT, indicating a promising approach for control of periodontal pathogens. Similarly, indocyanine green was incorporated into PLGA nanoparticles coated with chitosan for aPDT [150]. A significantly higher antibacterial activity against P. gingivalis was observed. De Freitas et al. [151] have investigated the effect of aPDT on human dental plaque bacteria using methylene blue (MB)-loaded poly(lactic-co-glycolic) nanoparticles in a clinical pilot study with 10 adult human subjects with chronic periodontitis. Patients were treated either with ultrasonic scaling and scaling and root planing (US + SRP) or ultrasonic scaling + SRP + aPDT with MB-nanoparticles. The clinical study demonstrated the safety of aPDT. At month three, more profound effect (28.82%) on gingival bleeding index was observed in ultrasonic SRP + aPDT group when compared to ultrasonic SRP.
In literature, there are numerous studies on polymeric nanoparticles used to deliver drugs into oral cavity for treatment of oral infections [152–156]. Due to their versatile characteristics such as surface charge, dimension, and hydrophobicity, it has been possible to prepare tailor-made polymeric nanoparticles for an enhanced local treatment of oral infections (see Tables 1, 2, and 3).
Microparticles
Polymer-based microparticles have also been investigated to maintain therapeutic drug concentrations for longer periods of time for treatment of dental and mucosal infections in the oral cavity [157, 158]. Numerous synthetic (e.g., PLGA) and natural polymers (e.g., chitosan) have been successfully used in preparation of microparticles for drug delivery [159]. Due to its own antimicrobial activity, chitosan alone in microparticle form has also been investigated. Kawatika et al. [160] have compared the effect of chitosan aqueous dispersion and microparticles on mature biofilms of S. mutans and demonstarted that chitosan in microparticle form reduced the bacterial viability and acidogenicity more effectively than the dispersions, thereby was more effective to control the growth of mature biofilms. Moura et al. [161] have investigated the release of locally delivered doxycycline-loaded PLGA microspheres in the periodontal pocket of patients with chronic periodontitis. The microspheres were demonstrated to provide sustained release after local administration, as an adjunct to non-surgical periodontal therapy.
Currently, there is a commercially available subgingival sustained-release product (Arestin®), which consists of minocycline hydrochloride incorporated microspheres prepared using bioresorbable polymer, poly (glycolide-co-dl-lactide). It is used in combination with scaling and root planing procedures to treat patients with adult periodontitis [162].
Strips/fibers
Strips and fibers composed of polymeric matrix have been used to deliver antimicrobials as an adjunct to mechanical treatment of periodontal disease [163]. They can be designed in appropriate dimension which allows practical insertion in periodontal pocket resulting in desirable clinical outcomes [164]. Various polymers and their combinations have been used to prepare strips and fiber for delivery of antimicrobials such as chlorhexidine, doxycycline, tetracycline, minocycline, and metronidazole [165–167]. In early years, acrylic polymers have been widely used providing significant improvements in various clinical conditions by effective microbial eradication from the pockets [164]. However, due to several disadvantages such as being non-absorbable, removal required after therapy, which may impair the regenerating tissue at the site, other polymers such as cellulose derivatives (hydroxypropyl cellulose, hydroxypropyl methylcellulose, ethyl cellulose), polycaprolactone, polyhydroxybutyric acid, poly- methylmethacrylate, and PLGA have been preferred over acrylic polymers.
Strips containing tetracycline hydrochloride or metronidazole 25% in polyhydroxybutyric acid as a biodegradable polymer matrix were evaluated in patients suffering from advanced periodontal disease [168]. The greatest response to therapy was observed with tetracycline hydrochloride strips inserted into periodontal pockets at 4-day intervals for 16 days, compared with an untreated control group. Metronidazole strips or root-planing tended not to be as effective. The clinical improvement produced by each treatment was not maintained when treatment was terminated. A commercially available periodontal fiber (Actisite®) for periodontal pocket placement has been developed in 90 s, which consists of a 23-cm monofilament of ethylene/vinyl acetate copolymer, 0.5 mm in diameter, impregnated with 25% tetracycline hydrochloride, providing continuous release of tetracycline for 10 days. It was demonstrated that the local delivery system was more effective than scaling and root planing (SRP) with respect to decreasing probing depth, increasing attachment level, and decreasing bleeding on probing (BOP) [169]. Later, the tetracycline strips were prepared with the identical polymer system to that of the fiber except for its physical shape and method of placement and investigated in patients following administration singly or in multiples in conjunction with root planing, versus root planing alone, or to an untreated control [166]. It was concluded that multiple strips which fill the periodontal pocket were superior to a single strip in reducing BOP and that use of locally delivered tetracycline was superior to SRP alone in decreasing probing depth.
The commercially available biodegradable local delivery system (PerioChip), which contains chlorhexidine gluconate in a biodegradable matrix of hydrolyzed gelatin (cross-linked with glutaraldehyde) developed by Steinberg et al. [170], can also be considered as a strip with rectangular shape. Drug concentration was shown to remain above the minimum inhibitory concentration for more than 99% of periodontal pocket flora for up to nine days.
In recent years, nanostructured polymeric fibers have been developed in order to enhance the efficacy of the drugs at the application site [171]. The advantages of nanofibers involve large surface area, high porosity, and high mechanical strength which make it a potential candidate for the application into the periodontal pocket [172]. Electrospinning technique is the mostly used method for the production of nanofibers, which allows adjustment of fiber size, drug loading, and mechanical properties can be adjusted [173]. In a recent study, the antibacterial activity of chitosan nanofiber, cross-linked with tetracycline comprising polyvinyl alcohol (PVA), was evaluated and enhanced antibacterial activity against a wide range of periodontal pathogens was demonstrated [174]. Mahmoud et al. [175] have developed polymeric electrospun fibers using PLGA, poly(L-lactic acid) (PLLA), and polycaprolactone (PCL) alone or blended with polyethylene oxide (PEO), incorporated with an antimicrobial peptide (BAR), and evaluated their safety and functionality against P. gingivalis/S. gordonii biofilms. The most promising formulation was found to be PLGA:PEO providing a sustained drug release and a dose-dependent inhibition of biofilm formation.
Films and wafers
Films containing antimicrobials and anti-inflammatory drugs have been designed for treatment of oral infections [176, 177]. Film formulations can be applied on the oral mucosa as well as into the periodontal pockets. They are thin and flexible, and particularly, mucoadhesive films can resist to physiological conditions in the oral cavity [178]. Numerous synthetic or natural polymers have been investigated to develop mucoadhesive films with one or more layers, or films based on stimuli responsive hydrogel. Chitosan is one of the most investigated polymers for preparation of films. Due to its bioadhesive properties, it can retain on the application site in prolonged periods of time and also exerts synergetic effect due to it antimicrobial activity [94, 179]. Recently, a two layer-polymeric film was prepared using a polymeric gel-like blend (including chitosan, HPMC, methocel at various ratios), with the basal layer (with lidocaine hydrochloride for a faster release than the apical layer with benzydamine HCl and N-acetyl-cysteine [180]. The single patient study showed that the association of polymers with the addition of analgesics, anti-inflammatories, and mucolytics promoted the reduction of inflammation, tumefaction, and an erythematous halo with significant mucosal regeneration in 30 days.
Exerting similar properties to that of films, wafers have been also investigated for local delivery to the oral cavity. The advantage of wafers of films is reported to be low residual moisture and increased drug loading [181]. Recently, our group has developed monolayer and bilayered mucoadhesive film and wafer formulations as local drug delivery platforms for treatment of oral infections, using chitosan and hydroxypropyl methylcellulose (HPMC) [182]. Cefuroxime axetil (CA) was used as the model drug. Antimicrobial activity was evaluated against E. coli and S. aureus. HPMC-based formulations were found to disintegrate within less than 30 min, whereas chitosan-based formulations remained intact up to 6 h. Significantly higher drug release was obtained with wafer formulations. Antimicrobial activity was found to increase in presence of chitosan, and HPMC was also observed to contribute to antimicrobial activity. Bilayered wafer formulation with adhesive chitosan backing layer and HPMC-based drug-loaded layer is suggested as a promising local delivery system for treatment of the infections in the oral cavity.
Conclusion and future perspectives
The mouth is said to be the gateway to one’s overall health, because the oral cavity may exhibit manifestations of underlying systemic infectious or autoimmune, hematologic, endocrine, and neoplastic-related diseases and serve as an indicator of overall health. On the other hand, oral cavity can act also as the site of origin for dissemination of pathogenic organisms to distant body sites, especially in immunocompromised hosts such as patients suffering from diabetes or rheumatoid arthritis or receiving immunosuppressive treatment. The oral microbiome encompasses a highly diverse microbiota, consisting of over 700 microorganisms, including bacteria, fungi, and viruses. In order to maintain the oral health, it is important to diagnose precisely the underlying local or systemic condition of the oral diseases in order to take the right actions for prevention and treatment. Appropriate drugs and delivery systems are crucial to reduce /eliminate the local as well as the systemic complications related to oral infections. Currently, there are numerous drugs available for preventive and palliative therapies, which reduce the infection incidence but cannot adequately eliminate the infection. Topical use of antivirals and antimicrobials has been successfully used for treatment of oral infections; however, there are still some obstacles to be addressed related to the drugs used as well as the delivery systems. Antimicrobial resistance has emerged as a huge challenge to the effective treatment of infectious diseases with antibiotics. Owing to the challenges faced with discovery of a new drug and the limited number of new classes of antibiotics, researchers have focused more on non-conventional approaches that could serve as alternatives to antibiotics. The alternative approaches to antibiotics include immunomodulators, competitive exclusion of pathogenic bacteria via probiotics and their combination, antimicrobial peptides, and photoactivateable agents. Another widely investigated approach against antimicrobial resistance is use of nanomaterials either as the active agent (e.g., silver or gold nanoparticles, nanographene, zinc oxide) or for drug delivery (antibiotics or above-mentioned alternate compounds). Nano delivery systems have the potential to deliver the antibiotic payload into the infected cells; therefore, enhancing penetration and release of antibiotics inside the infected cells. By this means, they can reduce the antibiotic overuse and help prevent antimicrobial resistance. Furthermore, nano systems have the potential to treat biofilm-forming pathogen infections by acting as a protective coat, shielding against interactions of drug by biofilm compartments and resident enzymes. Nevertheless, oral cavity is a complex environment for local drug delivery due to its distinct characteristics such as different epithelial structure (keratinized or non-keratinized, thickness etc.) in different regions, continuous secretion of saliva, movement of the tongue, and swallowing. Specialized delivery systems are required to deliver the drug in the desired period of time, resisting the physiological conditions of the oral cavity and avoiding the removal of the drug from the application site. Furthermore, for delivery to certain regions in the oral cavity such as periodontal pocket and tooth, systems with certain shape and dimension are required.
Currently, there are numerous products available on the market, which are mostly based on conventional dosage forms and aimed for inhibition of bacterial growth and biofilm formation, for reducing inflammation or for tissue regeneration. On the other side, many promising results have been reported for prevention and treatment of oral infections with the newly developed advanced systems, particularly, with those that apply nanotechnology and/or use novel compounds alternative to those currently used; however, up to date, very few have reached to the market. More studies in human are needed to prove the safety and efficacy of these systems, so that they can be available for routine clinical applications. In regard to manufacturing of these systems, commercial scale-up may pose some problems due to their complexities; however, the delivery market opportunities are plentiful. There are indeed some exciting developments within topical delivery into the oral cavity. For future, the most lacking research area appears to be the development of vaccines for prevention of oral infections. This is indeed an important aspect which the researchers should focus more.
Author contribution
Authors have contributed equally in this manuscript.
Declarations
Consent for publication
All authors have the consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Oral health. In: Newsroom. 2020. https://www.who.int/news-room/fact-sheets/detail/oral-health. Accessed 4 Jan 2021.
- 3.Moving together to build a healthier world. In: Political Declaration of the High-level Meeting on Universal Health Coverage. 2019. https://www.un.org/pga/73/wp-content/uploads/sites/53/2019/07/FINAL-draft-UHC-Political-Declaration.pdf. Accessed 4 Jan 2021.
- 4.Santosh ABR, Muddana K. Viral infections of oral cavity. J Family Med Prim Care. 2020;9(1):36. doi: 10.4103/jfmpc.jfmpc_807_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gondivkar S, Gadbail A, Sarode GS, Sarode SC, Patil S, Awan KH. Infectious diseases of oral cavity. Dis Mon. 2019;65(6):164. doi: 10.1016/j.disamonth.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Donà MG, Spriano G, Pichi B, Rollo F, Laquintana V, Covello R, et al. Human papillomavirus infection and p16 overexpression in oropharyngeal squamous cell carcinoma: a case series from 2010 to 2014. Future Microbiol. 2015;10(8):1283–1291. doi: 10.2217/FMB.15.55. [DOI] [PubMed] [Google Scholar]
- 7.Asai D, Nakashima H. Pathogenic viruses commonly present in the oral cavity and relevant antiviral compounds derived from natural products. Medicines. 2018;5(4):120. doi: 10.3390/medicines5040120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott DA, Coulter WA, Biagioni PA, O'Neill HO, Lamey PJ. Detection of herpes simplex virus type 1 shedding in the oral cavity by polymerase chain reaction and enzyme-linked immunosorbent assay at the prodromal stage of recrudescent herpes labialis. J Oral Pathol Med. 1997;26(7):305–309. doi: 10.1111/j.1600-0714.1997.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 9.Rautava J, Syrjänen S. Human papillomavirus infections in the oral mucosa. J Am Dent Assoc. 2011;142(8):905–14. 10.14219/jada.archive.2011.0297 [DOI] [PubMed]
- 10.Stoopler ET, Balasubramanlam R. Topical and systemic therapies for oral and perioral herpes simplex virus infections. J Calif Dent Assoc. 2013;41(4):259. [PubMed] [Google Scholar]
- 11.Hammer KD, Dietz J, Lo TS, Johnson EM. A systematic review on the efficacy of topical acyclovir, penciclovir, and docosanol for the treatment of herpes simplex labialis. Dermatology. 2018.
- 12.WHO. Coronavirus disease 2019 (COVID-19) Situation Report-51, https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf. Accessed 7 Jan 2021.
- 13.de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu B, Guo H, Zhou P, Shi Z-L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2020:1–14. 10.1038/s41579-020-00459-7 [DOI] [PMC free article] [PubMed]
- 15.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C, Wu H, Ding X, Ji H, Jiao P, Song H, et al. Does infection of novel coronavirus cause acute and/or chronic sialadenitis? Med Hypotheses. 2019;2020:109789. doi: 10.1016/j.mehy.2020.109789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng L, Hua F, Bian Z. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J Dent Res. 2020;99(5):481–487. doi: 10.1177/0022034520914246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin Carreras-Presas C, Amaro Sanchez J, Lopez-Sanchez AF, Jane-Salas E, Somacarrera Perez ML. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. 2020 doi: 10.1111/odi.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amorim dos Santos J, Normando A, Carvalho da Silva R, Acevedo A, De Luca Canto G, Sugaya N et al. Oral manifestations in patients with COVID-19: a living systematic review. J Dent Res. 2020:0022034520957289. 10.1177/0022034520957289 [DOI] [PubMed]
- 20.Iranmanesh B, Amiri R, Zartab H, Aflatoonian M. Oral manifestations of COVID-19 disease: a review article. Dermatol Ther. 2020 doi: 10.1111/dth.14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J, Li Y, Gan F, Du Y, Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J Dent Res. 2020:0022034520918518. [DOI] [PubMed]
- 22.Xu R, Cui B, Duan X, Zhang P, Zhou X, Yuan Q. Saliva: potential diagnostic value and transmission of 2019-nCoV. Int J Oral Sci. 2020;12(1):1–6. doi: 10.1038/s41368-020-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Díaz Rodríguez M, Jimenez Romera A, Villarroel M. Oral manifestations associated with COVID-19. Oral Dis. 2020 doi: 10.1111/odi.13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnan PA. Fungal infections of the oral mucosa. Indian J Dent Res. 2012;23(5):650. doi: 10.4103/0970-9290.107384. [DOI] [PubMed] [Google Scholar]
- 25.Telles DR, Karki N, Marshall MW. Oral fungal infections: diagnosis and management. Dent Clin. 2017;61(2):319–349. doi: 10.1016/j.cden.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Cuesta C, Sarrion-Pérez M-G, Bagán JV. Current treatment of oral candidiasis: a literature review. J Clin Exp Dent. 2014;6(5):e576. doi: 10.4317/jced.51798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quindós G, Gil-Alonso S, Marcos-Arias C, Sevillano E, Mateo E, Jauregizar N et al. Therapeutic tools for oral candidiasis: current and new antifungal drugs. Med Oral Patol Oral Cir Bucal. 2019;24(2):e172. 10.4317/medoral.22978 [DOI] [PMC free article] [PubMed]
- 28.Nowak A, Christensen JR, Mabry TR, Townsend JA, Wells MH. Pediatric Dentistry-E-Book: infancy through adolescence. Elsevier Health Sci; 2018.
- 29.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levi ME, Eusterman VD. Oral infections and antibiotic therapy. Otolaryngol Clin North Am. 2011;44(1):57–78. doi: 10.1016/j.otc.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Dahlen G. Bacterial infections of the oral mucosa. Periodontol. 2000;2009(49):13–38. doi: 10.1111/j.1600-0757.2008.00295.x. [DOI] [PubMed] [Google Scholar]
- 32.Jiao Y, Tay FR, Niu L-n, Chen J-h. Advancing antimicrobial strategies for managing oral biofilm infections. Int J Oral Sci. 2019;11(3):1–11. 10.1038/s41368-019-0062-1 [DOI] [PMC free article] [PubMed]
- 33.Spratt D, Pratten J. Biofilms and the oral cavity. Rev Environ Sci Biotechnol. 2003;2(2–4):109–120. doi: 10.1023/B:RESB.0000040466.82937.df. [DOI] [Google Scholar]
- 34.Newman MG, Takei HH, Klokkevold PR. Carranza’s clinical periodontology.12th ed. St Louis: Elsevier; 2015.
- 35.Chi AC, Neville BW, Krayer JW, Gonsalves WC. Oral manifestations of systemic disease. Am Fam Physician. 2010;82(11):1381–1388. [PubMed] [Google Scholar]
- 36.Rivera‐Hidalgo F, Stanford TW. Oral mucosal lesions caused by infective microorganisms I. Viruses and bacteria. Periodontol 2000. 1999;21(1):106–24. 10.1111/j.1600-0757.1999.tb00171.x [DOI] [PubMed]
- 37.Verderosa AD, Totsika M, Fairfull-Smith KE. Bacterial biofilm eradication agents: a current review. Front Chem. 2019;7:824. doi: 10.3389/fchem.2019.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shastry SP, Sanjay C, Kaul R, Mahima V, Doggalli N. Topical drug delivery: an essential aid in the management of oral diseases. J Adv Clin Res Insights. 2015;2(6):269–75. 10.15713/ins.jcri.92
- 39.Collins L, Dawes C. The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. J Dent Res. 1987;66(8):1300–1302. doi: 10.1177/00220345870660080201. [DOI] [PubMed] [Google Scholar]
- 40.Frenkel ES, Ribbeck K. Salivary mucins in host defense and disease prevention. J Oral Microbiol. 2015;7(1):29759. doi: 10.3402/jom.v7.29759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fenton OS, Olafson KN, Pillai PS, Mitchell MJ, Langer R. Advances in biomaterials for drug delivery. Adv Mater. 2018;30(29):1705328. doi: 10.1002/adma.201705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.George A, Shah PA, Shrivastav PS. Natural biodegradable polymers based nano-formulations for drug delivery: a review. Int J Pharm. 2019;561:244–264. doi: 10.1016/j.ijpharm.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Bruschi ML, de Freitas O. Oral bioadhesive drug delivery systems. Drug Dev Ind Pharm. 2005;31(3):293–310. doi: 10.1081/DDC-52073. [DOI] [PubMed] [Google Scholar]
- 44.Zięba M, Chaber P, Duale K, Martinka Maksymiak M, Basczok M, Kowalczuk M, et al. Polymeric carriers for delivery systems in the treatment of chronic periodontal disease. Polymers. 2020;12(7):1574. doi: 10.3390/polym12071574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parhi R. Drug delivery applications of chitin and chitosan: a review. J Environmental Chemistry Letters. 2020:1–18. 10.1007/s10311-020-00963-5
- 46.Hua S. Advances in nanoparticulate drug delivery approaches for sublingual and buccal administration. Front Pharmacol. 2019;10. 10.3389/fphar.2019.01328 [DOI] [PMC free article] [PubMed]
- 47.Colton MB, Ehrlich E. Bactericidal effect obtained by addition of antibiotics to dental cements and direct filling resins. J Am Dent Assoc. 1953;47(5):524–31. 10.14219/jada.archive.1953.0206 [DOI] [PubMed]
- 48.Greenstein G, Polson A. The role of local drug delivery in the management of periodontal diseases: a comprehensive review. J Periodontol. 1998;69(5):507–520. doi: 10.1902/jop.1998.69.5.507. [DOI] [PubMed] [Google Scholar]
- 49.Soskolne W, Heasman P, Stabholz A, Smart G, Palmer M, Flashner M, et al. Sustained local delivery of chlorhexidine in the treatment of periodontitis: a multi-center study. J Periodontol. 1997;68(1):32–38. doi: 10.1902/jop.1997.68.1.32. [DOI] [PubMed] [Google Scholar]
- 50.Sander L, Frandsen EVG, Arnbjerg D, Warrer K, Karring T. Effect of local metronidazole application on periodontal healing following guided tissue regeneration. Clinical findings J Periodontol. 1994;65(10):914–920. doi: 10.1902/jop.1994.65.10.914. [DOI] [PubMed] [Google Scholar]
- 51.Graca M, Watts T, Wilson R, Palmer R. A randomized controlled trial of a 2% minocycline gel as an adjunct to non-surgical periodontal treatment, using a design with multiple matching criteria. J Clin Periodontol. 1997;24(4):249–253. doi: 10.1111/j.1600-051X.1997.tb01838.x. [DOI] [PubMed] [Google Scholar]
- 52.Senel S, Hıncal AA. Drug permeation enhancement via buccal route: possibilities and limitations. J Control Release. 2001;72(1–3):133–144. doi: 10.1016/S0168-3659(01)00269-3. [DOI] [PubMed] [Google Scholar]
- 53.Senel S. Potential applications of chitosan in oral mucosal delivery. J Drug Deliv Sci Tec. 2010;20(1):23–32. doi: 10.1016/S1773-2247(10)50003-0. [DOI] [Google Scholar]
- 54.Fabio Oliveira de Sousa F, Ferraz C, de Santiago Nojosa J, Yamauti M. Nanotechnology in dentistry: drug delivery systems for the control of biofilm-dependent oral diseases. Curr Drug Deliv. 2014;11(6):719–28. [DOI] [PubMed]
- 55.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 56.Gregoriadis G, Perrie Y. Liposomes. Encyclopedia of life sciences (ELS). JohnWiley & Sons, Ltd: Chicheste. 2010.
- 57.Mezei M, Gulasekharam V. Liposomes a selective drug delivery system for the topical route of administration. Life Sci. 1980;26:1473–1477. doi: 10.1111/j.2042-7158.1982.tb04767.x. [DOI] [PubMed] [Google Scholar]
- 58.Mezei M. Liposomes a selective drug delivery system for the topical route of administration: gel dosage. J Pharm Pharmacol. 1982;34:473–474. doi: 10.1016/0024-3205(80)90268-4. [DOI] [PubMed] [Google Scholar]
- 59.Harsanyi B, Hilchie J, Mezei M. Liposomes as drug carriers for oral ulcers. J Dent Res. 1986;65(9):1133–1141. doi: 10.1177/00220345860650090501. [DOI] [PubMed] [Google Scholar]
- 60.Jones MN, Francis SE, Hutchinson FJ, Handley PS, Lyle IG. Targeting and delivery of bactericide to adsorbed oral bacteria by use of proteoliposomes. Biochim Biophys Acta Biomembr. 1993;1147(2):251–261. doi: 10.1016/0005-2736(93)90010-W. [DOI] [PubMed] [Google Scholar]
- 61.Hill KJ, Kaszuba M, Creeth JE, Jones MN. Reactive liposomes encapsulating a glucose oxidase-peroxidase system with antibacterial activity. Biochim Biophys Acta Biomembr. 1997;1326(1):37–46. doi: 10.1016/S0005-2736(97)00007-2. [DOI] [PubMed] [Google Scholar]
- 62.Robinson AM, Creeth JE, Jones MN. The use of immunoliposomes for specific delivery of antimicrobial agents to oral bacteria immobilized on polystyrene. J Biomater Sci Polym Ed. 2000;11(12):1381–1393. doi: 10.1163/156856200744408. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen S, Hiorth M, Rykke M, Smistad G. The potential of liposomes as dental drug delivery systems. Eur J Pharm Biopharm. 2011;77(1):75–83. doi: 10.1016/j.ejpb.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 64.Sugano M, Morisaki H, Negishi Y, Endo-Takahashi Y, Kuwata H, Miyazaki T, et al. Potential effect of cationic liposomes on interactions with oral bacterial cells and biofilms. J Liposome Res. 2016;26(2):156–162. doi: 10.3109/08982104.2015.1063648. [DOI] [PubMed] [Google Scholar]
- 65.Wijetunge SS, Wen J, Yeh C-K, Sun Y. Lectin-conjugated liposomes as biocompatible, bioadhesive drug carriers for the management of oral ulcerative lesions. ACS Appl Bio Mater. 2018;1(5):1487–1495. doi: 10.1021/acsabm.8b00425. [DOI] [PubMed] [Google Scholar]
- 66.Erjavec V, Pavlica Z, Sentjurc M, Petelin M. In vivo study of liposomes as drug carriers to oral mucosa using EPR oximetry. Int J Pharm. 2006;307(1):1–8. doi: 10.1016/j.ijpharm.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 67.Petelin M, Pavlica Z, Ivanuša T, Šentjurc M, Skalerič U. Local delivery of liposome-encapsulated superoxide dismutase and catalase suppress periodontal inflammation in beagles. J Clin Periodontol. 2000;27(12):918–925. doi: 10.1034/j.1600-051x.2000.027012918.x. [DOI] [PubMed] [Google Scholar]
- 68.Liu D, Yang P. Minocycline hydrochloride nanoliposomes inhibit the production of TNF-α in LPS-stimulated macrophages. Int J Nanomedicine. 2012;7:4769. doi: 10.2147/IJN.S34036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu F, Zhou Z, Xu Q, Fan C, Wang L, Ren H, et al. A novel pH-responsive quaternary ammonium chitosan-liposome nanoparticles for periodontal treatment. Int J Biol Macromol. 2019;129:1113–1119. doi: 10.1016/j.ijbiomac.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 70.Moraes GS, Santos IBD, Pinto SCS, Pochapski MT, Farago PV, Pilatti GL et al. Liposomal anesthetic gel for pain control during periodontal therapy in adults: a placebo-controlled RCT. J Appl Oral Sci. 2020;28. 10.1590/1678-7757-2019-0025 [DOI] [PMC free article] [PubMed]
- 71.Capretto L, Mazzitelli S, Colombo G, Piva R, Penolazzi L, Vecchiatini R, et al. Production of polymeric micelles by microfluidic technology for combined drug delivery: application to osteogenic differentiation of human periodontal ligament mesenchymal stem cells (hPDLSCs) Int J Pharmaceut. 2013;440(2):195–206. doi: 10.1016/j.ijpharm.2012.07.057. [DOI] [PubMed] [Google Scholar]
- 72.Chen F, Rice KC, Liu X-M, Reinhardt RA, Bayles KW, Wang D. Triclosan-loaded tooth-binding micelles for prevention and treatment of dental biofilm. Pharm Res. 2010;27(11):2356–2364. doi: 10.1007/s11095-010-0119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen F, Jia Z, Rice KC, Reinhardt RA, Bayles KW, Wang D. The development of dentotropic micelles with biodegradable tooth-binding moieties. Pharm Res. 2013;30(11):2808–2817. doi: 10.1007/s11095-013-1105-5. [DOI] [PubMed] [Google Scholar]
- 74.Isik G, Hasirci N, Tezcaner A, Kiziltay A. Multifunctional periodontal membrane for treatment and regeneration purposes. J Bioact Compat Polym. 2020;35(2):117–138. doi: 10.1177/0883911520911659. [DOI] [Google Scholar]
- 75.Sheth T, Seshadri S, Prileszky T, Helgeson ME. Multiple nanoemulsions. Nat Rev Mater. 2020;5(3):214–228. doi: 10.1038/s41578-019-0161-9. [DOI] [Google Scholar]
- 76.Narang JK, Narang RS. Emerging role of nanoemulsions in oral health management. Int J Pharm Investig. 2017;7(1):1. doi: 10.4103/jphi.JPHI_32_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karthikeyan R, Amaechi BT, Rawls HR, Lee VA. Antimicrobial activity of nanoemulsion on cariogenic Streptococcus mutans. Arch Oral Biol. 2011;56(5):437–445. doi: 10.1016/j.archoralbio.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoffman A. Hydrogel biomedical articles. Adv Drug Deliver Rev. 2002;54:3–12. doi: 10.1016/S0169-409X(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 79.Peppas N, Slaughter B, Kanzelberger M. Hydrogels. In Polymer science: a comprehensive reference. Amsterdam: Elsevier; 2012. pp. 385–395. 10.1016/B978-0-444-53349-4.00226-0
- 80.Chai Q, Jiao Y, Yu X. Hydrogels for biomedical applications: their characteristics and the mechanisms behind them. Gels. 2017;3(1):6. doi: 10.3390/gels3010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Catoira MC, Fusaro L, Di Francesco D, Ramella M, Boccafoschi F. Overview of natural hydrogels for regenerative medicine applications. J Mater Sci Mater Med. 2019;30(10):115. doi: 10.1007/s10856-019-6318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yen A-H, Yelick P. Dental tissue regeneration—a mini-review. Gerontology. 2011;57(1):85–94. doi: 10.1159/000314530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alexandre Chisini L, Cristian Muniz Conde M, Grazioli G, Schmidt San Martin A, Fernando Demarco F. Bone, Periodontal and dental pulp regeneration in dentistry: a systematic scoping review. Braz Dent J. 2019;30:77–95. 10.1590/0103-6440201902053 [DOI] [PubMed]
- 84.Şenel S, Aksoy EA, Akca G. Application of chitosan based scaffolds for drug delivery and tissue engineering in dentistry. Marine-Derived Biomaterials for Tissue Engineering Applications. Springer; 2019. p. 157–78
- 85.Ozdogan AI, Ilarslan YD, Kosemehmetoglu K, Akca G, Kutlu HB, Comerdov E, et al. In vivo evaluation of chitosan based local delivery systems for atorvastatin in treatment of periodontitis. Int J Pharm. 2018;550(1–2):470–476. doi: 10.1016/j.ijpharm.2018.08.058. [DOI] [PubMed] [Google Scholar]
- 86.Akıncıbay H, Şenel S, Yetkin AZ. Application of chitosan gel in the treatment of chronic periodontitis. J Biomed Mater Res B Appl Biomater. 2007;80(2):290–296. doi: 10.1002/jbm.b.30596. [DOI] [PubMed] [Google Scholar]
- 87.Ikinci G, Senel S, Akincibay H, Kas S, Ercis S, Wilson CG, et al. Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis. Int J Pharm. 2002;235(1–2):121–127. doi: 10.1016/S0378-5173(01)00974-7. [DOI] [PubMed] [Google Scholar]
- 88.Cicciù M, Fiorillo L, Cervino G. Chitosan use in dentistry: a systematic review of recent clinical studies. Mar Drugs. 2019;17(7):417. doi: 10.3390/md17070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sani ES, Lara RP, Aldawood Z, Bassir SH, Nguyen D, Kantarci A, et al. An antimicrobial dental light curable bioadhesive hydrogel for treatment of peri-implant diseases. Matter. 2019;1(4):926–944. doi: 10.1016/j.matt.2019.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hamed R, AbuRezeq Aa, Tarawneh O. Development of hydrogels, oleogels, and bigels as local drug delivery systems for periodontitis. Drug Dev Ind Pharm. 2018;44(9):1488–97. 10.1080/03639045.2018.1464021 [DOI] [PubMed]
- 91.Yang Z, Liang X, Jiang X, Guo J, Tao Y, Wang S, et al. Development and evaluation of minocycline hydrochloride-loaded in situ cubic liquid crystal for intra-periodontal pocket administration. Molecules. 2018;23(9):2275. doi: 10.3390/molecules23092275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Şenel S. Current status and future of chitosan in drug and vaccine delivery. React Funct Polym. 2019:104452. 10.1016/j.reactfunctpolym.2019.104452
- 93.Akca G, Ozdemir A, Oner ZG, Senel S. Comparison of different types and sources of chitosan for the treatment of infections in the oral cavity. Res Chem Intermediat. 2018;44(8):4811–4825. doi: 10.1007/s11164-018-3338-8. [DOI] [Google Scholar]
- 94.Senel S, Ikinci G, Kas S, Yousefi-Rad A, Sargon MF, Hincal AA. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int J Pharm. 2000;193(2):197–203. doi: 10.1016/S0378-5173(99)00334-8. [DOI] [PubMed] [Google Scholar]
- 95.Aksungur P, Sungur A, Unal S, Iskit AB, Squier CA, Senel S. Chitosan delivery systems for the treatment of oral mucositis: in vitro and in vivo studies. J Control Release. 2004;98(2):269–279. doi: 10.1016/j.jconrel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 96.Azeran N, Zazali N, Timur S, Özdogan I, Ekizoglu M, Sheshala R, et al. Moxifloxacin loaded chitosan gel formulations for the treatment of periodontal diseases. J Polym Mater. 2017;34:157–170. [Google Scholar]
- 97.Ozdogan AI, Akca G, Senel S. Development and in vitro evaluation of chitosan based system for local delivery of atorvastatin for treatment of periodontitis. Eur J Pharm Sci. 2018;124:208–216. doi: 10.1016/j.ejps.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 98.Boynuegri D, Ozcan G, Senel S, Uc D, Uraz A, Ogus E et al. Clinical and radiographic evaluations of chitosan gel in periodontal intraosseous defects: a pilot study. J Biomed Mater Res B. 2009;90b(1):461–6. 10.1002/jbm.b.31307 [DOI] [PubMed]
- 99.Pignatello R, Basile L, Puglisi G. Chitosan glutamate hydrogels with local anesthetic activity for buccal application. Drug deliv. 2009;16(3):176–181. doi: 10.1080/10717540902861267. [DOI] [PubMed] [Google Scholar]
- 100.Wang B, Booij-Vrieling HE, Bronkhorst EM, Shao J, Kouwer PH, Jansen JA, et al. Antimicrobial and anti-inflammatory thermo-reversible hydrogel for periodontal delivery. Acta Biomater. 2020;116:259–267. doi: 10.1016/j.actbio.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 101.Luo Z, Xue K, Zhang X, Lim JY, Lai X, Young DJ, et al. Thermogelling chitosan-based polymers for the treatment of oral mucosa ulcers. Biomater Sci. 2020;8(5):1364–1379. doi: 10.1039/C9BM01754B. [DOI] [PubMed] [Google Scholar]
- 102.Ji QX, Chen XG, Zhao QS, Liu CS, Cheng XJ, Wang LC. Injectable thermosensitive hydrogel based on chitosan and quaternized chitosan and the biomedical properties. J Mater Sci Mater Med. 2009;20(8):1603–1610. doi: 10.1007/s10856-009-3729-x. [DOI] [PubMed] [Google Scholar]
- 103.Zimoch J, Padial JS, Klar AS, Vallmajo-Martin Q, Meuli M, Biedermann T, et al. Polyisocyanopeptide hydrogels: a novel thermo-responsive hydrogel supporting pre-vascularization and the development of organotypic structures. Acta biomater. 2018;70:129–139. doi: 10.1016/j.actbio.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 104.Dong Z, Sun Y, Chen Y, Liu Y, Tang C, Qu X. Injectable adhesive hydrogel through a microcapsule cross-link for periodontitis treatment. ACS Appl Bio Mater. 2019;2(12):5985–5994. doi: 10.1021/acsabm.9b00912. [DOI] [PubMed] [Google Scholar]
- 105.Allaker RP, Ian DC. Non-conventional therapeutics for oral infections. Virulence. 2015;6(3):196–207. doi: 10.4161/21505594.2014.983783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Borro BC, Nordström R, Malmsten M. Microgels and hydrogels as delivery systems for antimicrobial peptides. Colloids Surf B. 2020:110835. 10.1016/j.colsurfb.2020.110835 [DOI] [PubMed]
- 107.Zasloff M. Antimicrobial peptides in health and disease. N Engl J Med. 2002;347(15):1199-. 10.1038/415389a [DOI] [PubMed]
- 108.Niu JY, Yin IX, Wu WKK, Li Q-L, Mei ML, Hung CC. Antimicrobial peptides for the prevention and treatment of dental caries: a concise review. Arch Oral Biol. 2020:105022. 10.1016/j.archoralbio.2020.105022 [DOI] [PubMed]
- 109.Wattanarat O, Makeudom A, Sastraruji T, Piwat S, Tianviwat S, Teanpaisan R, et al. Enhancement of salivary human neutrophil peptide 1–3 levels by probiotic supplementation. BMC Oral Health. 2015;15(1):19. doi: 10.1186/s12903-015-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee J-K, Chang SW, Perinpanayagam H, Lim S-M, Park Y-J, Han SH, et al. Antibacterial efficacy of a human β-defensin-3 peptide on multispecies biofilms. J Endod. 2013;39(12):1625–1629. doi: 10.1016/j.joen.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 111.Zhou L, Wong HM, Zhang YY, Li QL. Constructing an antibiofouling and mineralizing bioactive tooth surface to protect against decay and promote self-healing. ACS Appl Mater Interfaces. 2019;12(2):3021–3031. doi: 10.1021/acsami.9b19745. [DOI] [PubMed] [Google Scholar]
- 112.Krzyściak W, Jurczak A, Piątkowski J, Kościelniak D, Gregorczyk-Maga I, Kołodziej I et al. Effect of histatin-5 and lysozyme on the ability of Streptococcus mutans to form biofilms in vitro conditions. Postepy Hig Med Dosw. 2015;69. [PubMed]
- 113.He J, Yarbrough DK, Kreth J, Anderson MH, Shi W, Eckert R. Systematic approach to optimizing specifically targeted antimicrobial peptides against Streptococcus mutans. Antimicrob Agents Chemother. 2010;54(5):2143–2151. doi: 10.1128/AAC.01391-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lundy FT, O’Hare MM, McKibben BM, Fulton CR, Briggs JE, Linden GJ. Radioimmunoassay quantification of adrenomedullin in human gingival crevicular fluid. Arch Oral Biol. 2006;51(4):334–338. doi: 10.1016/j.archoralbio.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 115.Elad S, Epstein JB, von Bültzingslöwen I, Drucker S, Tzach R, Yarom N. Topical immunomodulators for management of oral mucosal conditions, a systematic review; Part II: miscellaneous agents. Expert Opin Emerg Drugs. 2011;16(1):183–202. doi: 10.1517/14728214.2011.528390. [DOI] [PubMed] [Google Scholar]
- 116.Jungell P, Malmström M. Cyclosporin A mouthwash in the treatment of oral lichen planus. Int J Oral Maxillofac Surg. 1996;25(1):60–62. doi: 10.1016/S0901-5027(96)80014-2. [DOI] [PubMed] [Google Scholar]
- 117.Eisen D, Ellis CN. Topical cyclosporine for oral mucosal disorders. J Am Acad Dermatol. 1990;23(6):1259–1264. doi: 10.1016/0190-9622(90)70352-I. [DOI] [PubMed] [Google Scholar]
- 118.Voûte AB, Schulten EA, Langendijk PN, Nieboer C, van der Waal I. Cyclosporin A in an adhesive base for treatment of recalcitrant oral lichen planus: an open trial. Oral Surg Oral Med Oral Pathol. 1994;78(4):437–441. doi: 10.1016/0030-4220(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 119.Thongprasom K, Chaimusig M, Korkij W, Sererat T, Luangjarmekorn L, Rojwattanasirivej S. A randomized-controlled trial to compare topical cyclosporin with triamcinolone acetonide for the treatment of oral lichen planus. J Oral Pathol Med. 2007;36(3):142–146. doi: 10.1111/j.1600-0714.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 120.Elad S, Epstein JB, Yarom N, Drucker S, Tzach R, Bültzingslöwen Iv. Topical immunomodulators for management of oral mucosal conditions, a systematic review; part I: calcineurin inhibitors. Expert Opin Emerg Drugs. 2010;15(4):713–26. 10.1517/14728214.2010.528389 [DOI] [PubMed]
- 121.Conrotto D, Carbone M, Carrozzo M, Arduino P, Broccoletti R, Pentenero M et al. Ciclosporin vs. clobetasol in the topical management of atrophic and erosive oral lichen planus: a double‐blind, randomized controlled trial. Br J Dermatol. 2006;154(1):139–45. 10.1111/j.1365-2133.2005.06920.x [DOI] [PubMed]
- 122.Atridox® - Den-Mat Holdings, LLC. DailyMed. 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=2550dbc0-8bdd-4bca-81d5-eec77ee6fbd5&type=display. Accessed 4 Jan 2021.
- 123.Chi M, Qi M, Wang P, Weir MD, Melo MA, Sun X, et al. Novel Bioactive and therapeutic dental polymeric materials to inhibit periodontal pathogens and biofilms. Int J Mol Sci. 2019;20(2):278. doi: 10.3390/ijms20020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lboutounne H. Dental medicine nanosystems: nanoparticles and their use in dentistry and oral health care. Int J Dent Oral Health. 2017;3(10):145–57. 10.25141/2471-657X-2017-10.0150
- 125.Benoit DS, Sims KR, Jr, Fraser D. Nanoparticles for oral biofilm treatments. ACS Nano. 2019;13(5):4869–4875. doi: 10.1021/acsnano.9b02816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Noronha VT, Paula AJ, Duran G, Galembeck A, Cogo-Mueller K, Franz-Montan M, et al. Silver nanoparticles in dentistry. Dent Mater. 2017;33(10):1110–1126. doi: 10.1016/j.dental.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 127.Bapat RA, Chaubal TV, Joshi CP, Bapat PR, Choudhury H, Pandey M, et al. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater Sci Eng C. 2018;91:881–898. doi: 10.1016/j.msec.2018.05.069. [DOI] [PubMed] [Google Scholar]
- 128.Nguyen S, Hiorth M. Advanced drug delivery systems for local treatment of the oral cavity. Ther delivery. 2015;6(5):595–608. doi: 10.4155/tde.15.5. [DOI] [PubMed] [Google Scholar]
- 129.Pokrowiecki R, Wojnarowicz J, Zareba T, Koltsov I, Lojkowski W, Tyski S, et al. Nanoparticles and human saliva: a step towards drug delivery systems for dental and craniofacial biomaterials. Int J Nanomedicine. 2019;14:9235. doi: 10.2147/IJN.S221608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Corrêa JM, Mori M, Sanches HL, Cruz ADd, Poiate E, Poiate IAVP. Silver nanoparticles in dental biomaterials. Int J Biomater. 2015;2015. 10.1155/2015/485275 [DOI] [PMC free article] [PubMed]
- 131.Schmalz G, Hickel R, Van Landuyt KL, Reichl FX. Scientific update on nanoparticles in dentistry. Int Dent J. 2018;68(5):299–305. doi: 10.1111/idj.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leung KC-F, Seneviratne CJ, Li X, Leung PC, Lau CBS, Wong C-H et al. Synergistic antibacterial effects of nanoparticles encapsulated with Scutellaria baicalensis and pure chlorhexidine on oral bacterial biofilms. Nanomaterials. 2016;6(4):61. 10.3390/nano6040061 [DOI] [PMC free article] [PubMed]
- 133.Sadony DM, Abozaid HE-s. Antibacterial effect of metallic nanoparticles on Streptococcus mutans bacterial strain with or without diode laser (970 nm). Bull Natl Res Cent. 2020;44(1):2. 10.1186/s42269-019-0262-z
- 134.Targino AGR, Flores MAP, dos Santos Junior VE, Bezerra FdGB, de Luna Freire H, Galembeck A et al. An innovative approach to treating dental decay in children. A new anti-caries agent. J Mater Sci Mater Med. 2014;25(8):2041–7. 10.1007/s10856-014-5221-5 [DOI] [PubMed]
- 135.Song W, Ge S. Application of antimicrobial nanoparticles in dentistry. Molecules. 2019;24(6):1033. doi: 10.3390/molecules24061033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vega-Jiménez A, Almaguer-Flores A, Flores-Castañeda M, Camps E, Uribe-Ramírez M, Aztatzi-Aguilar O, et al. Bismuth subsalicylate nanoparticles with anaerobic antibacterial activity for dental applications. Nanotechnology. 2017;28(43):435101. doi: 10.1088/1361-6528/aa8838. [DOI] [PubMed] [Google Scholar]
- 137.Cousins B, Allison H, Doherty P, Edwards C, Garvey M, Martin D, et al. Effects of a nanoparticulate silica substrate on cell attachment of Candida albicans. J Appl Microbiol. 2007;102(3):757–765. doi: 10.1111/j.1365-2672.2006.03124.x. [DOI] [PubMed] [Google Scholar]
- 138.Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed Nanotechnol Biol Med. 2015;11(2):313–327. doi: 10.1016/j.nano.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 139.Seneviratne CJ, Leung KC-F, Wong C-H, Lee S-F, Li X, Leung PC et al. Nanoparticle-encapsulated chlorhexidine against oral bacterial biofilms. PLoS One. 2014;9(8):e103234. 10.1371/journal.pone.0103234 [DOI] [PMC free article] [PubMed]
- 140.He J, Zhu X, Qi Z, Wang C, Mao X, Zhu C, et al. Killing dental pathogens using antibacterial graphene oxide. ACS Appl Mater Interfaces. 2015;7(9):5605–5611. doi: 10.1021/acsami.5b01069. [DOI] [PubMed] [Google Scholar]
- 141.Xie H, Cao T, Rodríguez-Lozano FJ, Luong-Van EK, Rosa V. Graphene for the development of the next-generation of biocomposites for dental and medical applications. Dent Mater. 2017;33(7):765–774. doi: 10.1016/j.dental.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 142.Daniyal M, Liu B, Wang W. Comprehensive review on graphene oxide for use in drug delivery system. Curr Med Chem. 2020 doi: 10.2174/13816128256661902011296290. [DOI] [PubMed] [Google Scholar]
- 143.Nagata JY, Hioka N, Kimura E, Batistela VR, Terada RSS, Graciano AX, et al. Antibacterial photodynamic therapy for dental caries: evaluation of the photosensitizers used and light source properties. Photodiagn Photodyn Ther. 2012;9(2):122–131. doi: 10.1016/j.pdpdt.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 144.Wood S, Metcalf D, Devine D, Robinson C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother. 2006;57(4):680–684. doi: 10.1093/jac/dkl021. [DOI] [PubMed] [Google Scholar]
- 145.Maisch T, Wagner J, Papastamou V, Nerl HJ, Hiller KA, Szeimies RM, et al. Combination of 10% EDTA, photosan, and a blue light hand-held photopolymerizer to inactivate leading oral bacteria in dentistry in vitro. J Appl Microbiol. 2009;107(5):1569–1578. doi: 10.1111/j.1365-2672.2009.04342.x. [DOI] [PubMed] [Google Scholar]
- 146.Pileggi G, Wataha JC, Girard M, Grad I, Schrenzel J, Lange N, et al. Blue light-mediated inactivation of Enterococcus faecalis in vitro. Photodiagn Photodyn Ther. 2013;10(2):134–140. doi: 10.1016/j.pdpdt.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 147.Perni S, Piccirillo C, Pratten J, Prokopovich P, Chrzanowski W, Parkin IP, et al. The antimicrobial properties of light-activated polymers containing methylene blue and gold nanoparticles. Biomaterials. 2009;30(1):89–93. doi: 10.1016/j.biomaterials.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 148.Qi M, Chi M, Sun X, Xie X, Weir MD, Oates TW, et al. Novel nanomaterial-based antibacterial photodynamic therapies to combat oral bacterial biofilms and infectious diseases. Int J Nanomedicine. 2019;14:6937. doi: 10.2147/IJN.S212807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rad MR, Pourhajibagher M, Rokn AR, Barikani HR, Bahador A. Effect of antimicrobial photodynamic therapy using indocyanine green doped with chitosan nanoparticles on biofilm formation-related gene expression of Aggregatibacter actinomycetemcomitans. Front Dent. 2019;16(3):187. 10.18502/fid.v16i3.1590 [DOI] [PMC free article] [PubMed]
- 150.Nagahara A, Mitani A, Fukuda M, Yamamoto H, Tahara K, Morita I, et al. Antimicrobial photodynamic therapy using a diode laser with a potential new photosensitizer, indocyanine green-loaded nanospheres, may be effective for the clearance of P orphyromonas gingivalis. J Periodontal Res. 2013;48(5):591–599. doi: 10.1111/jre.12042. [DOI] [PubMed] [Google Scholar]
- 151.De Freitas LM, Calixto GMF, Chorilli M, Giusti JSM, Bagnato VS, Soukos NS, et al. Polymeric nanoparticle-based photodynamic therapy for chronic periodontitis in vivo. Int J Mol Sci. 2016;17(5):769. doi: 10.3390/ijms17050769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rizvi SA, Saleh AM. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm J. 2018;26(1):64–70. doi: 10.1016/j.jsps.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Costa EM, Silva S, Veiga M, Tavaria FK, Pintado MM. A review of chitosan’s effect on oral biofilms: perspectives from the tube to the mouth. J Oral Biosci. 2017;59(4):205–210. doi: 10.1016/j.job.2017.07.001. [DOI] [Google Scholar]
- 154.Aithal GC, Nayak UY, Mehta C, Narayan R, Gopalkrishna P, Pandiyan S, et al. Localized in situ nanoemulgel drug delivery system of quercetin for periodontitis: development and computational simulations. Molecules. 2018;23(6):1363. doi: 10.3390/molecules23061363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Madi M, Pavlic V, Samy W, Alagl A. The anti-inflammatory effect of locally delivered nano-doxycycline gel in therapy of chronic periodontitis. Acta Odontol Scand. 2018;76(1):71–76. doi: 10.1080/00016357.2017.1385096. [DOI] [PubMed] [Google Scholar]
- 156.Beg S, Dhiman S, Sharma T, Jain A, Sharma RK, Jain A, et al. Stimuli responsive in situ gelling systems loaded with PLGA nanoparticles of moxifloxacin hydrochloride for effective treatment of periodontitis. AAPS Pharm Sci Tech. 2020;21(3):76. doi: 10.1208/s12249-019-1613-7. [DOI] [PubMed] [Google Scholar]
- 157.Álvarez AL, Espinar FO, Méndez JB. The application of microencapsulation techniques in the treatment of endodontic and periodontal diseases. Pharmaceutics. 2011;3(3):538–571. doi: 10.3390/pharmaceutics3030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kmiec M, Pighinelli L, Tedesco M, Silva M, Reis V. Chitosan-properties and applications in dentistry. Adv Tissue Eng Regen Med. 2017;2(4):00035. 10.15406/atroa.2017.02.00035
- 159.Virlan MJR, Miricescu D, Totan A, Greabu M, Tanase C, Sabliov CM et al. Current uses of poly (lactic-co-glycolic acid) in the dental field: a comprehensive review. J Chem. 2015;2015. 10.1155/2015/525832
- 160.Kawakita ER, Ré ACS, Peixoto MPG, Ferreira MP, Ricomini-Filho AP, Freitas O, et al. Effect of chitosan dispersion and microparticles on older Streptococcus mutans biofilms. Molecules. 2019;24(9):1808. doi: 10.3390/molecules24091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Moura LA, Ribeiro FV, Aiello TB, Duek EADR, Sallum EA, Nociti Junior FH, et al. Characterization of the release profile of doxycycline by PLGA microspheres adjunct to non-surgical periodontal therapy. J Biomater Sci Polym Ed. 2015;26(10):573–584. doi: 10.1080/09205063.2015.1045249. [DOI] [PubMed] [Google Scholar]
- 162.Arestin® - OroPharma. 2020. https://www.arestin.com/. Accessed 4 Jan 2021.
- 163.Dixit R, Puthli S. Oral strip technology: overview and future potential. J Control Release. 2009;139(2):94–107. doi: 10.1016/j.jconrel.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 164.Joshi D, Garg T, Goyal AK, Rath G. Advanced drug delivery approaches against periodontitis. Drug Deliv. 2016;23(2):363–377. doi: 10.3109/10717544.2014.935531. [DOI] [PubMed] [Google Scholar]
- 165.Rajeshwari H, Dhamecha D, Jagwani S, Rao M, Jadhav K, Shaikh S, et al. Local drug delivery systems in the management of periodontitis: a scientific review. J Control Release. 2019;307:393–409. doi: 10.1016/j.jconrel.2019.06.038. [DOI] [PubMed] [Google Scholar]
- 166.Friesen LR, Williams KB, Krause LS, Killoy WJ. Controlled local delivery of tetracycline with polymer strips in the treatment of periodontitis. J Periodontol. 2002;73(1):13–19. doi: 10.1902/jop.2002.73.1.13. [DOI] [PubMed] [Google Scholar]
- 167.Mahajania M, Laddha R, Shelke A, Gadhiya N, Narkhede S, Shetty G. Effect of subgingival doxycycline placement on clinical and microbiological parameters in inflammatory periodontal disease: both in vivo and in vitro studies. J Contemp Dent Pract. 2018;19(10):1228–1234. doi: 10.5005/jp-journals-10024-2409. [DOI] [PubMed] [Google Scholar]
- 168.Deasy P, Collins AE, Maccarthy DJ, Russell R. Use of strips containing tetracycline hydrochloride or metronidazole for the treatment of advanced periodontal disease. J Pharm Pharmacol. 1989;41(10):694–699. doi: 10.1111/j.2042-7158.1989.tb06343.x. [DOI] [PubMed] [Google Scholar]
- 169.Goodson J, Cugini M, Kent R, Armitage G, Cobb C, Fine D, et al. Multicenter evaluation of tetracycline fiber therapy: II. Clinical response J Periodontal Res. 1991;26(4):371–379. doi: 10.1111/j.1600-0765.1991.tb02076.x. [DOI] [PubMed] [Google Scholar]
- 170.Steinberg D, Friedman M, Soskolne A, Sela M. A new degradable controlled release device for treatment of periodontal disease: in vitro release study. J Periodontol. 1990;61(7):393–398. doi: 10.1902/jop.1990.61.7.393. [DOI] [PubMed] [Google Scholar]
- 171.Meireles AB, Corrêa DK, da Silveira JV, Millás AL, Bittencourt E, de Brito-Melo GE, et al. Trends in polymeric electrospun fibers and their use as oral biomaterials. Exp Biol Med. 2018;243(8):665–676. doi: 10.1177/1535370218770404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Steinberg D, Friedman M. Sustained-release delivery of antimicrobial drugs for the treatment of periodontal diseases: Fantasy or already reality? Periodontol. 2020;84(1):176–187. doi: 10.1111/prd.12341. [DOI] [PubMed] [Google Scholar]
- 173.Chou S-F, Carson D, Woodrow KA. Current strategies for sustaining drug release from electrospun nanofibers. J Control Release. 2015;220:584–591. doi: 10.1016/j.jconrel.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.dos Santos DM, Chagas PA, Leite IS, Inada NM, de Annunzio SR, Fontana CR, et al. Core-sheath nanostructured chitosan-based nonwovens as a potential drug delivery system for periodontitis treatment. Int J Biol Macromol. 2020;142:521–534. doi: 10.1016/j.ijbiomac.2019.09.124. [DOI] [PubMed] [Google Scholar]
- 175.Mahmoud MY, Sapare S, Curry KC, Demuth DR, Steinbach-Rankins JM. Rapid release polymeric fibers for inhibition of Porphyromonas gingivalis adherence to Streptococcus gordonii. Front Chem. 2020;7:926. doi: 10.3389/fchem.2019.00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lim SY, Dafydd M, Ong J, Ord-McDermott LA, Board-Davies E, Sands K, et al. Mucoadhesive thin films for the simultaneous delivery of microbicide and anti-inflammatory drugs in the treatment of periodontal diseases. Int J Pharm. 2020;573:118860. doi: 10.1016/j.ijpharm.2019.118860. [DOI] [PubMed] [Google Scholar]
- 177.Barat R, Srinatha A, Pandit JK, Anupurba S, Mittal N. Chitosan inserts for periodontitis: influence of drug loading, plasticizer and crosslinking on in vitro metronidazole release. Acta Pharm. 2007;57(4):469–77.10.2478/v10007-007-0037-1 [DOI] [PubMed]
- 178.Karki S, Kim H, Na S-J, Shin D, Jo K, Lee J. Thin films as an emerging platform for drug delivery. Asian J Pharm Sci. 2016;11(5):559–574. doi: 10.1016/j.ajps.2016.05.004. [DOI] [Google Scholar]
- 179.Atac M, Senel S, Eren A. Application of chitosan films in sulcoplasty operations. Adv Chitin Sci. 2005;4:270–274. [Google Scholar]
- 180.Alves TF, Rios AC, da Silva PK, Portella DL, Aranha N, Severino P, et al. Bilayer mucoadhesive buccal film for mucosal ulcers treatment: development, characterization, and single study case. Pharmaceutics. 2020;12(7):657. doi: 10.3390/pharmaceutics12070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Costa JSR, de Oliveira CK, Oliveira-Nascimento L. A mini-review on drug delivery through wafer technology: formulation and manufacturing of buccal and oral lyophilizates. J Adv Res. 2019;20:33–41. doi: 10.1016/j.jare.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Timur SS, Yüksel S, Akca G, Şenel S. Localized drug delivery with mono and bilayered mucoadhesive films and wafers for oral mucosal infections. Int J Pharm. 2019;559:102–112. doi: 10.1016/j.ijpharm.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 183.Schkarpetkin D, Reise M, Wyrwa R, Völpel A, Berg A, Schweder M, et al. Development of novel electrospun dual-drug fiber mats loaded with a combination of ampicillin and metronidazole. Dent Mater. 2016;32(8):951–960. doi: 10.1016/j.dental.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 184.Uraz A, Boynueğri D, Özcan G, Karaduman B, Uc D, Şenel S, et al. Two percent chitosan mouthwash: a microbiological and clinical comparative study. J Dent Sci. 2012;7(4):342–349. doi: 10.1016/j.jds.2012.05.003. [DOI] [Google Scholar]
- 185.Beyth N, Redlich M, Harari D, Friedman M, Steinberg D. Effect of sustained-release chlorhexidine varnish on Streptococcus mutans and Actinomyces viscosus in orthodontic patients. Am J Orthod Dentofac Orthop. 2003;123(3):345–348. doi: 10.1067/mod.2003.19. [DOI] [PubMed] [Google Scholar]
- 186.Jentsch H, Eckert FR, Eschrich K, Stratul SI, Kneist S. Antibacterial action of chlorhexidine/thymol containing varnishes in vitro and in vivo. Int J Dent Hyg. 2014;12(3):168–173. doi: 10.1111/idh.12079. [DOI] [PubMed] [Google Scholar]
- 187.Reise M, Wyrwa R, Muller U, Zylinski M, Volpel A, Schnabelrauch M, et al. Release of metronidazole from electrospun poly(L-lactide-co-D/L-lactide) fibers for local periodontitis treatment. Dent Mater. 2012;28(2):179–188. doi: 10.1016/j.dental.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 188.Khan G, Yadav SK, Patel RR, Nath G, Bansal M, Mishra B. Development and evaluation of biodegradable chitosan films of metronidazole and levofloxacin for the management of periodontitis. AAPS PharmSciTech. 2016;17(6):1312–1325. doi: 10.1208/s12249-015-0466-y. [DOI] [PubMed] [Google Scholar]
- 189.Leung WK, Jin L, Yau JY, Sun Q, Corbet EF. Microflora cultivable from minocycline strips placed in persisting periodontal pockets. Arch Oral Biol. 2005;50(1):39–48. doi: 10.1016/j.archoralbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 190.Swain GP, Patel S, Gandhi J, Shah P. Development of Moxifloxacin Hydrochloride loaded in-situ gel for the treatment of periodontitis: In-vitro drug release study and antibacterial activity. J Oral Biol Craniofac Res. 2019;9(3):190–200. doi: 10.1016/j.jobcr.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ranjbar-Mohammadi M, Zamani M, Prabhakaran M, Bahrami SH, Ramakrishna S. Electrospinning of PLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mater Sci Eng C. 2016;58:521–531. doi: 10.1016/j.msec.2015.08.066. [DOI] [PubMed] [Google Scholar]
- 192.Bottino MC, Münchow EA, Albuquerque MT, Kamocki K, Shahi R, Gregory RL, et al. Tetracycline-incorporated polymer nanofibers as a potential dental implant surface modifier. J Biomed Mater Res B. 2017;105(7):2085–2092. doi: 10.1002/jbm.b.33743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xu X, Gu Z, Chen X, Shi C, Liu C, Liu M, et al. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta biomater. 2019;86:235–246. doi: 10.1016/j.actbio.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 194.Ashri LY, Amal El Sayeh F, Ibrahim MA, Alshora DH. Optimization and evaluation of chitosan buccal films containing tenoxicam for treating chronic periodontitis: In vitro and in vivo studies. Journal of Drug Delivery Science and Technology 2020:101720.
- 195.Özdoğan AI, Akca G, Şenel S. Development and in vitro evaluation of gel formulation of atorvastatin solid dispersions. J Drug Deliv Sci Tec. 2020:102199. 10.1016/j.jddst.2020.102199
- 196.Hosseinzadeh S, Hamedi S, Esmaeili E, Kabiri M, Babaie A, Soleimani M, et al. Mucoadhesive nanofibrous membrane with anti-inflammatory activity. Polym Bull. 2019;76(9):4827–4840. doi: 10.1007/s00289-018-2618-1. [DOI] [Google Scholar]
- 197.Chow EP, Howden BP, Walker S, Lee D, Bradshaw CS, Chen MY, et al. Antiseptic mouthwash against pharyngeal Neisseria gonorrhoeae: a randomised controlled trial and an in vitro study. Sex Transm Infect. 2017;93(2):88–93. doi: 10.1136/sextrans-2016-052753. [DOI] [PubMed] [Google Scholar]
- 198.de Toledo LdAS, Rosseto HC, Dos Santos RS, Spizzo F, Del Bianco L, Montanha MC et al. Thermal magnetic field activated propolis release from liquid crystalline system based on magnetic nanoparticles. AAPS PharmSciTech. 2018;19(7):3258–71. 10.1208/s12249-018-1163-4. [DOI] [PubMed]
- 199.Hirasawa M, Takada K, Makimura M, Otake S. Improvement of periodontal status by green tea catechin using a local delivery system: a clinical pilot study. J Periodontal Res. 2002;37(6):433–438. doi: 10.1034/j.1600-0765.2002.01640.x. [DOI] [PubMed] [Google Scholar]
- 200.Gottumukkala SN, Sudarshan S, Mantena SR. Comparative evaluation of the efficacy of two controlled release devices: chlorhexidine chips and indigenous curcumin based collagen as local drug delivery systems. Contemp Clin Dent. 2014;5(2):175. doi: 10.4103/0976-237X.132310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zupančič Š, Baumgartner S, Lavrič Z, Petelin M, Kristl J, Technology. Local delivery of resveratrol using polycaprolactone nanofibers for treatment of periodontal disease. J Drug Deliv Sci Tec. 2015;30:408–16. 10.1016/j.jddst.2015.07.009
- 202.Watanabe S, Suemaru K, Takechi K, Kaji H, Imai K, Araki H. Oral mucosal adhesive films containing royal jelly accelerate recovery from 5-fluorouracil–induced oral mucositis. J Pharmacol Sci. 2013:12181FP. 10.1254/jphs.12181FP [DOI] [PubMed]
- 203.Khajuria DK, Patil ON, Karasik D, Razdan R. Development and evaluation of novel biodegradable chitosan based metformin intrapocket dental film for the management of periodontitis and alveolar bone loss in a rat model. Arch Oral Biol. 2018;85:120–129. doi: 10.1016/j.archoralbio.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 204.Saha S, Tomaro-Duchesneau C, Daoud JT, Tabrizian M, Prakash S. Novel probiotic dissolvable carboxymethyl cellulose films as oral health biotherapeutics: in vitro preparation and characterization. Expert Opin Drug Deliv. 2013;10(11):1471–1482. doi: 10.1517/17425247.2013.799135. [DOI] [PubMed] [Google Scholar]
- 205.Sánchez M, Toledano-Osorio M, Bueno J, Figuero E, Toledano M, Medina-Castillo A et al. Antibacterial effects of polymeric PolymP-n Active nanoparticles. An in vitro biofilm study. Dent Mater. 2019;35(1):156–68. 10.1016/j.dental.2018.11.015 [DOI] [PubMed]
- 206.Di Turi G, Riggio C, Vittorio O, Marconcini S, Briguglio F, Funel N, et al. Sub-Micrometric liposomes as drug delivery systems in the treatment and periodontitis. Int J Immunopathol Pharmacol. 2012;25(3):657–670. doi: 10.1177/039463201202500312. [DOI] [PubMed] [Google Scholar]
- 207.He Y, Jin Y, Wang X, Yao S, Li Y, Wu Q, et al. An antimicrobial peptide-loaded gelatin/chitosan nanofibrous membrane fabricated by sequential layer-by-layer electrospinning and electrospraying techniques. Nanomaterials. 2018;8(5):327. doi: 10.3390/nano8050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chaudhary B, Verma S. Preparation and evaluation of novel in situ gels containing acyclovir for the treatment of oral herpes simplex virus infections. Sci World J. 2014;2014. 10.1155/2014/280928 [DOI] [PMC free article] [PubMed]
- 209.Al-Dhubiab BE, Nair AB, Kumria R, Attimarad M, Harsha S. Formulation and evaluation of nano based drug delivery system for the buccal delivery of acyclovir. Colloids Surf B Biointerfaces. 2015;136:878–884. doi: 10.1016/j.colsurfb.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 210.Downing C, Moayyad J, Tamirisa A, Tyring S. Acyclovir Lauriad®: a muco-adhesive buccal tablet for the treatment of recurrent herpes labialis. Expert Rev Anti Infect Ther. 2014;12(3):283–287. doi: 10.1586/14787210.2014.880337. [DOI] [PubMed] [Google Scholar]
- 211.Lin L, Chen X, Cui P, Wang J, Guo Z, Lu N, et al. Topical application of penciclovir cream for the treatment of herpes simplex facialis/labialis: a randomized, double-blind, multicentre, aciclovir-controlled trial. J Dermatol Treat. 2002;13(2):67–72. doi: 10.1080/095466302317584412. [DOI] [PubMed] [Google Scholar]
- 212.Moura MDG, Haddad JPA, Senna MIB, e Ferreira EF, Mesquita RA. A new topical treatment protocol for oral hairy leukoplakia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(5):611–7. 10.1016/j.tripleo.2010.05.015 [DOI] [PubMed]
- 213.Cui Z, Mumper RJ. Bilayer films for mucosal (genetic) immunization via the buccal route in rabbits. Pharm Res. 2002;19(7):947–953. doi: 10.1023/A:1016454003450. [DOI] [PubMed] [Google Scholar]
- 214.Gajdošová M, Vetchý D, Muselík J, Gajdziok J, Juřica J, Vetchá M et al. Bilayer mucoadhesive buccal films with prolonged release of ciclopirox olamine for the treatment of oral candidiasis: In vitro development, ex vivo permeation testing, pharmacokinetic and efficacy study in rabbits. Int J Pharm. 2020:120086. 10.1016/j.ijpharm.2020.120086 [DOI] [PubMed]
- 215.Harish N, Prabhu P, Charyulu R, Gulzar M, Subrahmanyam E. Formulation and evaluation of in situ gels containing clotrimazole for oral candidiasis. Indian J Pharm Sci. 2009;71(4):421. doi: 10.4103/0250-474X.57291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rençber S, Karavana SY, Yilmaz FF, Eraç B, Nenni M, Gurer-Orhan H et al. Formulation and evaluation of fluconazole loaded oral strips for local treatment of oral candidiasis. J Drug Deliv Sci Tec. 2019;49:615–21.10.1016/j.jddst.2018.12.035
- 217.Mendes A, Silva A, Catita J, Cerqueira F, Gabriel C, Lopes CM. Miconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: improving antifungal activity. Colloids Surf B Biointerfaces. 2013;111:755–763. doi: 10.1016/j.colsurfb.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 218.Vazquez JA, Patton LL, Epstein JB, Ramlachan P, Mitha I, Noveljic Z, et al. Randomized, comparative, double-blind, double-dummy, multicenter trial of miconazole buccal tablet and clotrimazole troches for the treatment of oropharyngeal candidiasis: study of miconazole Lauriad® efficacy and safety (SMiLES) HIV Clin Trials. 2010;11(4):186–196. doi: 10.1310/hct1104-186. [DOI] [PubMed] [Google Scholar]
- 219.Uzunoğlu B, Wilson CG, Sağıroğlu M, Yüksel S, Şenel S. Mucoadhesive bilayered buccal platform for antifungal drug delivery into the oral cavity. Drug Deliv Transl Res. 2020:1–10. 10.1007/s13346-020-00798-1 [DOI] [PubMed]
- 220.Roque L, Alopaeus J, Reis C, Rijo P, Molpeceres J, Hagesaether E, et al. Mucoadhesive assessment of different antifungal nanoformulations. Bioinspir Biomim. 2018;13(5):055001. doi: 10.1088/1748-3190/aad488. [DOI] [PubMed] [Google Scholar]
- 221.Martin MJ, Calpena AC, Fernandez F, Mallandrich M, Gálvez P, Clares B. Development of alginate microspheres as nystatin carriers for oral mucosa drug delivery. Carbohyd Polym. 2015;117:140–149. doi: 10.1016/j.carbpol.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 222.Soriano-Ruiz JL, Calpena-Capmany AC, Cañadas-Enrich C, Bozal-de Febrer N, Suñer-Carbó J, Souto EB, et al. Biopharmaceutical profile of a clotrimazole nanoemulsion: evaluation on skin and mucosae as anticandidal agent. Int J Pharm. 2019;554:105–115. doi: 10.1016/j.ijpharm.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 223.Szekalska M, Wróblewska M, Trofimiuk M, Basa A, Winnicka K. Alginate oligosaccharides affect mechanical properties and antifungal activity of alginate buccal films with posaconazole. Mar Drugs. 2019;17(12):692.10.3390/md17120692 [DOI] [PMC free article] [PubMed]
- 224.Serrano DR, Fernandez-Garcia R, Mele M, Healy AM, Lalatsa A. Designing fast-dissolving orodispersible films of amphotericin B for oropharyngeal candidiasis. Pharmaceutics. 2019;11(8):369. doi: 10.3390/pharmaceutics11080369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rai VK, Yadav NP, Sinha P, Mishra N, Luqman S, Dwivedi H, et al. Development of cellulosic polymer based gel of novel ternary mixture of miconazole nitrate for buccal delivery. Carbohyd Polym. 2014;103:126–133. doi: 10.1016/j.carbpol.2013.12.019. [DOI] [PubMed] [Google Scholar]