Abstract
Objective
Determine whether hematological and transfusion patterns following, the onset of NEC can identify infants likely to develop fulminant, fatal necrotizing enterocolitis (NEC).
Design
Determine hematological predictors of fulminant NEC.
Results
Of 336 neonates with NEC, 35 (10%) who developed fulminant NEC were born with higher birth weights and more frequently developed radiologically evident pneumoperitoneumand/or portal venous gas. Following the diagnosis of NEC, these infants were more likely to rapidly develop thrombocytopenia, lymphopenia, neutropenia, and lower total white blood cell counts compared to medical/surgical non-fulminant type. They were also more likely to have received a red blood cell (RBC) transfusion (76.7% vs. 53.1%, p = 0.001) within 48 h after disease onset and platelet transfusion (24.2% vs. 11.7%; p = 0.03) before the onset of NEC.
Conclusion
Neonates with fulminant NEC frequently developed thrombocytopenia, lymphopenia, neutropenia, and leukopenia, received RBC transfusions after or platelet transfusions before the onset of NEC developed the fulminant disease.
Subject terms: Paediatrics, Risk factors
Introduction
Necrotizing enterocolitis (NEC) is the most common acute gastrointestinal illness during the neonatal period, affecting about 5–10% of premature neonates with a birth weight ≤1500 g [1, 2]. Despite advances in neonatal intensive care, NEC remains a leading cause of surgical intervention, postoperative morbidity, and death in premature neonates [3–7]. A diagnosis of surgical NEC is associated with increased resource utilization and cost of care due to continued hospitalization that can last several months [8, 9]. In these neonates, the disease is frequently marked by extensive bowel necrosis and inflammation [5, 10–13]. Some neonates develop what has been termed fulminant NEC and die within 48 h of diagnosis.
In the premature intestine, developmental limitations in both the innate and adaptive arms of the mucosal immune system increase the risk of inflammatory injury and NEC. Systemic inflammation during NEC has been associated with several hematological abnormalities [14] with altered counts of platelets, leukocytes including monocytes, neutrophils, and lymphocytes; and coagulopathy, and these changes may convey important diagnostic and prognostic information [15–18]. Platelet counts provide important predictive information for the outcome in these patients [19]. Neutropenia can be seen in severe NEC and is associated with adverse outcomes [14, 15, 17, 18]. In another study, Lambert et al. reported that neonates who died of fulminant NEC within 48 h of onset had low lymphocyte counts [20].
Neonates with NEC are frequently treated with blood product transfusions. Both clinical and animal model studies show anemia to change the intestinal inflammatory milieu and subsequent RBC transfusions to activate these newly recruited leukocytes in the intestinal wall and precipitate NEC [21–24]. Neonates with surgical NEC commonly receive platelet transfusions to treat severe thrombocytopenia [25], which can also accentuate the mucosal inflammation and may increase mortality [26–28].
Fulminant NEC is a severe subtype, categorized by pan-intestinal necrosis and death within 48 h of diagnosis. No studies have reported nadir hematological counts and recovery timeline after disease onset in neonates with medical/surgical NEC. Thus, it is difficult to forecast disease progression and outcome in the first 24 h after the NEC onset. In this context, it may be useful to understand whether the chronological evolution that we frequently see in routine laboratory results, such as hematological counts, has any prognostic implications. With the widespread availability of automated hematological analyzers, these data have now become relatively inexpensive and readily available and are comparable between centers. To address this knowledge gap, we aimed to determine the hematological and clinical predictors of mortality in infants with fulminant NEC. We also evaluated whether red blood cell transfusion and platelet transfusions before and/or during the 48 h following NEC onset were associated with mortality.
Methods
This retrospective study was conducted at the University of Mississippi Medical Center (UMMC) at Jackson, Mississippi, after approval by the Institutional Review Board (2017-0127). The protocol was considered compliant with the Health Insurance Portability and Accountability Act of 1996. The UMMC houses a level 4 neonatal intensive care unit, which is a regional referral center for neonates with surgical NEC in the entire state. A detailed review of the electronic medical records identified 336 patients with medical and surgical NEC (NEC Bell stage 2 and above) who underwent management of NEC in the period between January 2000 and December 2018. Infants who did not show obvious pneumatosis on radiology were classified as ≥stage 2 NEC only if they displayed highly suggestive definite clinical (intestinal) signs with loss of bowel sounds, tenderness, and abdominal distension; systemic instability with increased needs for ventilatory and hemodynamic support; and radiological signs such as intestinal dilatation, ascites, and/or portal venous gas.
Definitions
We defined fulminant NEC as a severe subtype, with death occurring within 48 h of onset, and with pan-intestinal necrosis shown at necropsy. Records of patients qualifying for fulminant NEC were compared with cases who developed surgical non-fulminant NEC (SNF) and with cases who developed medical non-fulminant NEC (MNF). Both of the latter group were alive 48 h after NEC onset. The infants with MNF either had confirmed pneumatosis on radiology exam or had all the clinical signs of NEC and were treated for at least 5–7 days of antibiotics.
Clinical information
We recorded demographic characteristics including birth weight, gestational age, gender, race (African-American, Caucasian, or Latino), and mode of delivery (C-section/vaginal delivery), APGAR scores at 5 min, outborn status, and small for gestational age status. We noted the NEC features such as the age of onset, pneumatosis, presence of X-ray, and clinical presentation (abdominal distension, feeding intolerance, and bloody stools). We collected information regarding maternal factors, including pregnancy-induced hypertension, chorioamnionitis, and antenatal steroids. We collected information on clinical variables such as patent ductus arteriosus, PDA treatment medically or surgically, C-reactive protein at 24 and 48 h after NEC onset cholestasis (direct bilirubin >2 mg/dL), blood cultures drawn at the time of NEC onset, use of inotropes at 24 h, assisted ventilation, and antibiotic duration after NEC onset. We recorded information on the length of stay and mortality. The length of stay was defined as the number of days of hospitalization at the referring hospital and at UMMC. Mortality was defined as death due to any prior to hospital discharge.
Hematological/transfusion information and time frame assessment
We recorded complete blood cell count results from the electronic chart before the NEC onset (last available CBC inpatient record before NEC onset), on the day of NEC onset, 24 h, and 48 h after onset. The pre-NEC CBC was mostly available 3–7 days before the NEC onset. We also recorded and compared complete blood count (CBC) results on days 4 and 7 for neonates with non-fulminant medical/surgical NEC. We collected data on relative (presented as percentages) as well as on the absolute values. If we had multiple CBC on the same day, we recorded data from what we judged to be the most abnormal one.
We also collected data on platelet and RBC transfusion before and after the NEC onset. In our hospital, decisions to order RBC or platelet transfusions are made by the on-call neonatologist. In our unit, blood transfusions are considered if the hematocrit is ≤30% (hemoglobin ≤ 10 gm/dL) for neonates requiring moderate or significant mechanical (invasive/non-invasive) ventilation, defined as mean airway pressure >8-cm H2O and FiO2 > 0.40 on a conventional ventilator. In our practice, all neonates are considered for transfusion of platelet count, which is <30 × 103/cmm. Platelet transfusion is considered at counts <50 × 103/cmm if the neonate is <1000 g (<7 days old) and has h/o previous intraventricular hemorrhage and pulmonary bleed. At our blood bank, regarding platelet transfusions of neonates, AB positive is the preferred type, but if unavailable, type-specific platelets are provided. Platelets expire within 5 days. Standard platelet preservation is ACD-A, stored constantly agitated at 20–24 °C. Platelet donations are collected from single apheresis donors. Neither platelets nor RBCs are washed, but both are irradiated in-house before transfusion for all neonates <4 months old.
Statistical methods
Patient demographics, and clinical and hematological information, were compared between the three NEC groups; MNF, SNF, and fulminant. Differences in continuous data were compared using the Kruskal–Wallis (Mann–Whitney U) test and summarized with medians and interquartile ranges (Q3–Q1). Categorical variables were compared using Chi-squared tests and reported with frequencies and percentages. To estimate the associations between NEC status and hematological characteristics, multivariate logistic regression was used. Hematological characteristics that showed significant differences between NEC groups within 24 h after NEC onset were used in the logistic model with patient demographic information as covariates. Odds ratios and confidence intervals (CIs) (OR [95% CI]) were reported to compare fulminant neonates to non-fulminant patients (reference group). All the statistical analyses were performed in R statistical software (version 3.6.3; The R Foundation for Statistical Computing). A p value of <0.05 was considered significant.
Results
Characteristics of the entire NEC cohort
Three hundred and thirty-six neonates with NEC were studied in three cohorts: 35 (10.5%) with fulminant NEC (death within 48 h of disease onset, and extensive bowel necrosis), 163 (48.5%) with SNF, and 138 (41%) with MNF NEC. In our entire cohort, the gestational age was mean ± standard deviation 27.9 ± 4 weeks, and the birth weight was 1087 ± 745 g. The mean age of NEC onset was 21.2 days (SD ± 18.8 days). Eighty-five (26.5%) died. The data have been summarized in Table 1.
Table 1.
Demographic and clinical data.
| Sample size | Combined | Medical non-fulminant NEC | Surgical non-fulminant NEC | Fulminant NEC | P1 value Ful vs. SNF | P2 Ful vs. MNF | P3 MNF vs. SNF | |
|---|---|---|---|---|---|---|---|---|
| N = 336 | N = 138 | N = 163 | N = 35 | |||||
| Maternal information | ||||||||
| Pregnancy-induced hypertension, n (%) | 319 | 62 (19.4) | 20 (15.3) | 36 (23.4) | 6 (17.6) | 0.21 | ||
| Chorioamnionitis, n (%) | 308 | 24 (7.7) | 8 (6.0) | 13 (9.0) | 3 (9.0) | 0.59 | ||
| Antenatal steroids, n (%) | 315 | 175 (55.6) | 57 (44.5) | 101 (65.2) | 17 (53.1) | 0.002 | <0.001 | |
| Demographic data | ||||||||
| Gestational age (weeks; mean ± SD) | 336 | 27.9 (4.0) | 28.3 (25; 30.6) | 26.5 (24.5; 28.4) | 26.5 (25.4; 29.5) | 0.359 | 0.288 | 0.001 |
| Birth weight (g; mean ± SD) | 336 | 1087 (745) | 1035 (710; 1635) | 740 (622; 990) | 917 (708; 1158) | 0.036 | 0.227 | <0.001 |
| Mode of delivery | 335 | 0.85 | ||||||
| C-section, n (%) | 224 (66.9) | 94 (68.6) | 107 (65.6) | 23 (65.7) | ||||
| Vaginal, n (%) | 111 (33.1) | 43 (31.4) | 56 (34.4) | 12 (34.3) | ||||
| Gender—Male, n (%) | 336 | 190 (56.5) | 71 (51.4) | 101 (62.0) | 18 (51.4) | 0.15 | ||
| Race | 331 | 0.12 | ||||||
| African-American, n (%) | 242 (73.1) | 92 (68.1) | 124 (77.0) | 26 (74.3) | ||||
| Caucasian, n (%) | 70 (21.1) | 33 (24.4) | 32 (19.9) | 5 (14.3) | ||||
| Other, n (%) | 19 (5.74) | 10 (7.41) | 5 (3.1) | 4 (11.4) | ||||
| AGA (appropriate for gestational age) | 325 | 0.29 | ||||||
| No, n (%) | 230 (70.8) | 99 (75.6) | 109 (67.7) | 22 (66.7) | ||||
| Yes, n (%) | 95 (29.2) | 32 (24.4) | 52 (32.3) | 11 (33.3) | ||||
| Apgar score <6 at 5 mins, n (%) | 288 | 62 (21.5) | 21 (20.8) | 34 (21.5) | 7 (24.1) | 0.92 | ||
| Outborn, n (%) | 286 | 163 (57.0) | 52 (51.5) | 94 (59.9) | 17 (60.7) | 0.37 | ||
| NEC features | ||||||||
| Clinical presentation | 336 | <0.001 | 0.013 | <0.001 | ||||
| Abdominal distension, n (%) | 262 (78.0) | 83 (60.1) | 149 (91.4) | 30 (85.7) | ||||
| Bloody stools, n (%) | 59 (17.6) | 45 (32.6) | 9 (5.52) | 5 (14.3) | ||||
| Feeding intolerance, n (%) | 15 (4.4) | 10 (7.2) | 5 (3.0) | 0 (0.0) | ||||
| NEC age onset (days), n (%) | 333 | 21.2 (18.8) | 23.2 (17.1) | 20.3 (20.9) | 17.5 (14.6) | 0.193 | 0.05 | |
| Pneumatosis, n (%) | 246 | 104 (42.3) | 43 (42.2) | 50 (40.7) | 11 (52.4) | 0.603 | ||
| Portal venous gas, n (%) | 294 | 26 (8.8) | 2 (1.82) | 18 (11.8) | 6 (18.8) | 0.002 | 0.005 | |
| Pneumoperitoneum, n (%) | 294 | 93 (31.6) | 5 (4.55) | 78 (51.3) | 10 (31.2) | 0.06 | <0.001 | <0.001 |
| Clinical information | ||||||||
| PDA before NEC onset, n (%) | 293 | 147 (50.2) | 45 (43.3) | 94 (58.8) | 8 (27.6) | 0.004 | 0.02 | |
| Cyclooxygenase inhibitors, n (%) | 265 | 45 (17.0) | 15 (15.3) | 28 (20.0) | 2 (7.4) | 0.27 | ||
| PDA surgical ligation, n (%) | 266 | 13 (4.8) | 4 (3.8) | 9 (6.6) | 0 (0.0) | 0.39 | ||
| Inotrope use at 24 h after NEC onset, n (%) | 277 | 147 (53.1) | 20 (20.4) | 103 (67.8) | 24 (88.9) | 0.04 | <0.001 | <0.001 |
| CRP at NEC onset, median (IQR) | 207 | 2.6 [0.8; 7.4] | 1.2 [0.5; 3.5] | 4.9 [1.4; 8.5] | 3.3 [1.3; 7.0] | 0.009 | <0.001 | |
| CRP at 24 h after NEC onset, median (IQR) | 175 | 6.2 [1.4; 15.1] | 1.8 [0.6; 6.7] | 12.6 [2.9; 19.8] | 6.4 [3.9; 18.9] | 0.011 | <0.001 | |
| CRP at 48 h after NEC onset, median (IQR) | 150 | 6.6 [1.4; 18.0] | 1.7 [0.7; 6.5] | 15.4 [4.5; 21.9] | 8.8 [3.3; 12.5] | 0.032 | <0.001 | |
| Assisted ventilation at the NEC onset intubated, n (%) | 197 (74.3) | 38 (41.8) | 136 (91.3) | 23 (92.0) | <0.001 | <0.001 | ||
| Hematocrit before NEC onset, median (IQR) | 224 | 34.2 [29.8; 41.6] | 36.4 [28.8; 42.7] | 33.9 [30.2; 39.8] | 35.0 [29.6; 45.3] | 0.77 | ||
| Positive blood culture at NEC onset, n (%) | 276 | 69 (25.0) | 20 (20.2) | 44 (28.4) | 5 (22.7) | 0.32 | ||
| Antibiotics duration, median (IQR) | 213 | 7.00 [5.00; 10.0] | 7.00 [5.00; 8.00] | 10.0 [7.00; 12.0] | 2.50 [1.75; 3.00] | <0.001 | <0.001 | <0.001 |
| Cholestasis at NEC onset, n (%) | 226 | 100 (44.2) | 19 (19.6) | 78 (68.4) | 3 (20.0) | 0.001 | <0.001 | |
| Feeding before NEC onset | ||||||||
| Breast milk, n (%) | 336 | 85 (25.3) | 1 (0.72) | 73 (44.8) | 11 (31.4) | <0.001 | <0.001 | |
| Donor milk, n (%) | 336 | 38 (11.3) | 3 (2.17) | 30 (18.4) | 5 (14.3) | 0.009 | <0.001 | |
| Formula feed, n (%) | 336 | 32 (9.5) | 15 (10.9) | 13 (7.9) | 4 (11.4) | 0.61 | ||
| Mixed feeding, n (%) | 336 | 25 (7.4) | 9 (6.5) | 16 (9.8) | 0 (0.0) | 0.11 | ||
| Length of hospitalization median (IQR) | 275 | 102 [48.0; 152] | 76.0 [40.0; 111] | 124 [83.0; 179] | 17.5 [3.00; 29.2] | <0.001 | <0.001 | |
| Death | 321 | <0.001 | <0.001 | <0.001 | ||||
| Death, n (%) | 85 (26.5) | 10 (8.06) | 40 (24.7) | 35 (100) | ||||
| Discharged, n (%) | 236 (73.5) | 114 (91.9) | 122 (75.3) | 0 (0.00) | ||||
Data are represented as n (%), mean (SD), or median (1st quartile, 3rd quartile). Differences in continuous measures were tested using a t-test or Mann–Whitney U test, ANOVA or Kruskal–Wallis test; differences in categorical measures were tested using a chi-square test or Fisher’s exact test; P1 = fulminant vs. surgical non-fulminant (SNF), P2 = fulminant (ful) vs. medical non-fulminant (MNF), P3 = MNF vs. SNF.
Statistically significant p < 0.05 values are in bold.
CRP C-reactive protein, PDA patent ductus arteriosus.
Comparison between fulminant and SNF
Neonates with fulminant NEC had higher mean birth weight [917 g (IQR 708; 1158) vs.740 g (IQR 622; 990), p = 0.036]. Those with fulminant NEC received significantly less exposure to antenatal steroids (53.1% vs.SNF 65.2%; p = 0.002) and more likely to need ionotropic support at 24 h after NEC onset (88.9% vs. SNF 67.8%; p = 0.04) compared to neonates with SNF NEC. Fewer had evidence of PDA on echocardiography (27.6% vs. SNF 58.8%; p = 0.004) and cholestasis (20% vs. SNF 68.4%, p < 0.001) neonates.
Fulminant vs. MNF
Neonates with fulminant NEC were more likely to have pneumatosis (52.4% vs. 42.2%), portal venous gas (18.8% vs. 1.8%, p = 0.001), and pneumoperitoneum (31.2% vs. MNF 4.5%; p < 0.001) on abdominal radiographs. A larger proportion of those with fulminant NEC received inotropes (88.9 % vs. 20.4% in MNF, p ≤ 0.001) and assisted ventilation (92% vs. MNF 41.8%, p < 0.001). C-reactive protein levels were significantly higher at NEC onset, and 2 days, later in those with fulminant NEC (p ≤ 0.001 vs. MNF).
In the fulminant NEC group, 20/35 infants were alive at 24 h after NEC onset, and only eight infants were alive at 36 h of NEC onset. The Kaplan–Meier curve depicting the trend of mortality in neonates with fulminant NEC has been summarized in Fig. 1. All neonates with fulminant NEC died while 40 (24.7%) with SNF and 10 (8.0%) in the MNF group died (<0.001), all beyond 48 h (Table 1).
Fig. 1. Kaplan–Meier curve (survival curve) showing mortality in neonates with fulminant necrotizing enterocolitis.
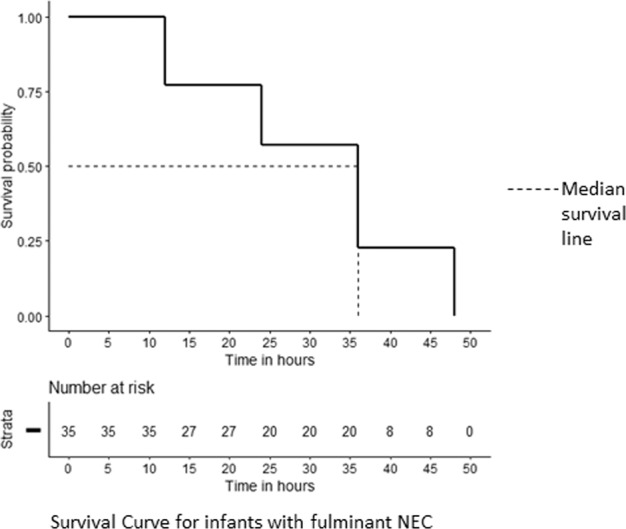
The dotted line represents the median survival line. X-axis represents the time in h. The numerical 0 on the X-axis represents the time of NEC onset. Y-axis represents the survival probablity.
Blood transfusions
One hundred and forty-one (47%) infants had received RBC transfusion from birth until the NEC onset. The three groups did not differ in the number of RBC transfusions received before the onset of NEC. Infants who developed fulminant NEC (77.4%) and SNF NEC (77.4%) were more likely to have received RBC transfusions in the 48-h period following the onset of NEC than those with MNF (30.1%; p < 0.001). These data are shown in Table 2.
Table 2.
Transfusion frequency in three subtypes of necrotizing enterocolitis.
| Combined cohort (N = 336) | Medical non-fulminant NEC (N = 138) | Surgical non-fulminant NEC (N = 163) | Fulminant NEC (N = 35) | P1 Ful vs. SNF | P2 Ful vs. MNF | P3 SNF vs. MNF | |
|---|---|---|---|---|---|---|---|
| Platelet transfusion before NEC | n = 226 | n = 56 | n = 152 | n = 18 | |||
| Number of platelet transfusion, n (%) | 0.319 | 0.337 | 0.892 | ||||
| 0 | 192 (85.0%) | 48 (85.7%) | 130 (85.5%) | 14 (77.8%) | |||
| 1 | 17 (7.52%) | 5 (8.93%) | 11 (7.24%) | 1 (5.56%) | |||
| 2 | 8 (3.54%) | 1 (1.79%) | 6 (3.95%) | 1 (5.56%) | |||
| 3+ | 9 (3.98%) | 2 (3.57%) | 5 (3.29%) | 2 (11.1%) | |||
| Platelet transfusion after 48 h | n = 226 | n = 53 | n = 154 | n = 19 | |||
| Number of platelet transfusion, n (%) | 0.373 | 0.002 | <0.001 | ||||
| 0 | 149 (65.9%) | 48 (90.6%) | 90 (58.4%) | 11 (57.9%) | |||
| 1 | 46 (20.4%) | 3 (5.66%) | 36 (23.4%) | 7 (36.8%) | |||
| 2 | 17 (7.52%) | 1 (1.89%) | 16 (10.4%) | 0 (0.00%) | |||
| 3+ | 14 (6.19%) | 1 (1.89%) | 12 (7.79%) | 1 (5.26%) | |||
| RBC transfusion before NEC | n = 296 | n = 122 | n = 143 | n = 31 | |||
| Number of RBC transfusion, n (%) | 0.392 | 0.335 | 0.246 | ||||
| 0 | 155 (52.4%) | 67 (54.9%) | 71 (49.7%) | 17 (54.8%) | |||
| 1 | 29 (9.80%) | 12 (9.84%) | 12 (8.39%) | 5 (16.1%) | |||
| 2 | 35 (11.8%) | 9 (7.38%) | 22 (15.4%) | 4 (12.9%) | |||
| 3+ | 77 (26.0%) | 34 (27.9%) | 38 (26.6%) | 5 (16.1%) | |||
| RBC transfusion after 48 h NEC | n = 281 | n = 113 | n = 137 | n = 31 | |||
| Number of RBC transfusion, n (%) | 0.574 | <0.001 | <0.001 | ||||
| 0 | 117 (41.6%) | 79 (69.9%) | 31 (22.6%) | 7 (22.6%) | |||
| 1 | 91 (32.4%) | 29 (25.7%) | 48 (35.0%) | 14 (45.2%) | |||
| 2 | 40 (14.2%) | 4 (3.54%) | 32 (23.4%) | 4 (12.9%) | |||
| 3+ | 33 (11.7%) | 1 (0.88%) | 26 (19.0%) | 6 (19.4%) |
Data represented as n (%); differences in categorical measures tested using the Chi-squared test or Fisher’s exact test; P1 = Fulminant vs. surgical non-fulminant (SNF), P2 = fulminant (ful) vs. medical non-fulminant (MNF), P3 = MNF vs. SNF, p < 0.05 significant.
Statistically significant p < 0.05 values are in bold.
NEC necrotizing enterocolitis, RBC red blood cell.
Thirty-four (15%) patients had received platelet transfusions from birth until the onset of NEC. The three groups did not differ in the number of platelet transfusions prior to the onset of NEC. Seventy-seven (34%) of these neonates had received platelet transfusions in the 48 h following the onset of NEC. Those with fulminant (42.1%) and SNF NEC (41.6%) were more likely to have received platelet transfusions within 48 h after developing NEC than those with MNF NEC (9.4%; p < 0.001).
First 48 h after NEC
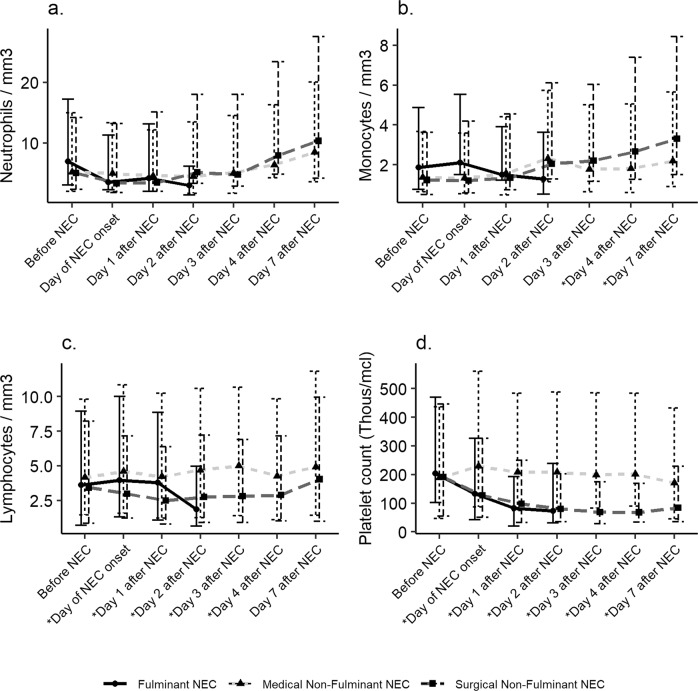
Patients with fulminant NEC on the day of NEC onset were more likely to have had lower absolute lymphocyte count [3.96 (IQR 2, 64; 6.04) fulminant NEC vs. 4.59 (IQR 1.00; 5.25) SNF; p = 0.017], lower median platelet count [134 (IQR 91.8; 193) in fulminant group vs. 230 (IQR 143; 331) in MNF; p ≤ 0.001]. At 24 h after NEC onset, neonates with fulminant NEC had significantly lower lymphocyte count [3.78 (IQR 2.70; 5.08) fulminant NEC vs. 4.23 (IQR 3.00; 6.00) SNF; p = 0.021], lower platelet count [82 (IQR 61.8; 110) in fulminant group vs. 208 (IQR 133; 276) in MNF; p ≤ 0.001] as compared to the surgical and MNF group. At 48 h after NEC onset, neonates with fulminant NEC had significantly lower median platelet count [73 (IQR 41; 166) in fulminant group vs. 208 (IQR 134; 280) in MNF; p = 0.015], lower total white blood cell count [9.3 (IQR 4.4; 10.3) vs. 11.4 (IQR 9.15; 18.4) MNF, p = 0.04), lower absolute neutrophil count [2.96 (IQR 1.52; 3.16) fulminant NEC vs. 4.48 (IQR 2.82; 8.96) MNF; p = 0.039] and significantly lower absolute lymphocyte count [1.86 [IQR 1.21; 3.12] fulminant NEC group vs. 4.70 [IQR 3.44; 5.88] in MNF; p = 0.001] as compared to neonates with MNF group. Blood count profiles are shown in detail in Supplementary Table 1 and Fig. 2.
Fig. 2. Heamatological changes before and seven days after NEC onset.
Absolute blood values of neutrophils (a), monocytes (b), lymphocytes (c), platelet count (d) in three different subtypes of NEC. The circle, triangle, and sqaure symbol represent neonates with fulminant, medical non-fulminant, and surgical non-fulminant NEC. The data have been depicted as a median (IQR). The asterisk * on the x-axis represents p < 0.05.
Relative blood values
On the day of NEC onset, those neonates with fulminant NEC had lower neutrophil percentages (26% vs. MNF 42%, p < 0.009; vs. SNF 41%, p = 0.016), and, however, the monocyte percentages were significantly higher in neonates with SNF NEC group [14.0 (IQR 10.0; 21.0) fulminant group vs. 11.0 (IQR 7.00; 16.8) in MNF; p = 0.03] as compared to the MNF group on the day of NEC onset. The data are shown in Supplementary Table 1 and Figs. 1, 2.
Blood profile, SNF, and MNF, days 3–7
Those with SNF NEC had lower platelet and lymphocyte counts than did those with MNF NEC, from the day of onset until day 7 (p < 0.001). In both groups, the fall in platelets continued until day 4 (p < 0.001) with recovery by day 7. Those with SNF NEC had higher monocyte percentages and absolute monocyte counts on days 3 (p = 0.02), 4 (p = 0.03), and 7 (p = 0.04). The eosinophil counts did not differ between the two groups, except on day 7 (p = 0.04).
Using unadjusted modeling, a difference in absolute neutrophil count (p = 0.04) and platelet count (p = 0.01) between pre-NEC values and those found at NEC onset was associated with increased odds of fulminant NEC (or death). Also, the platelet count at 24 h after NEC onset was associated with increased odds of fulminant NEC (p = 0.03). The absolute neutrophil count and WBC count at 48 h were associated with increased odds of fulminant NEC (or death). We found similar trends at different time assessment points after adjusting the predictive model for gestational age and birth weight. That data are summarized in Table 3. On predictive modeling, for a 5% increase in neutrophils on the day of NEC diagnosis relative to pre-NEC neutrophil percentages, the odds of fulminant NEC decreased by 16% [OR 0.84 (95% CI; 0.74, 0.94); p = 0.002] after adjusting for birth weight, gestational age, and clinical presentation of abdominal distention. The model did not reveal other relationships with blood values. That data are summarized in Table 4. We also analyzed the interplay of different blood component counts at different time points after NEC onset with each other by interaction predictive modeling, as shown in Supplementary Table 2. The interactive model did not find any significant interaction except for absolute lymphocyte count and platelet count on the day of NEC onset (p = 0.006).
Table 3.
Association between fulminant NEC and hematological measures using multinomial logistic regression.
| Unadjusted models | Adjusted models | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Difference | β | OR | p value | 95% CI | β | OR | p value | 95% CI | ||
| Δ Absolute neutrophil count | ||||||||||
| Δ ANC before and day of NEC | 0.07 | 1.07 | 0.04 | 1.003 | 1.14 | 0.07 | 1.07 | 0.04 | 1.003 | 1.14 |
| Δ ANC before and 24 h NEC | 0.05 | 1.05 | 0.29 | 0.96 | 1.15 | 0.05 | 1.05 | 0.27 | 0.96 | 1.15 |
| Δ ANC before and 48 h NEC | 0.08 | 1.09 | 0.09 | 0.99 | 1.20 | 0.10 | 1.10 | 0.07 | 0.99 | 1.22 |
| Absolute lymphocyte count | ||||||||||
| Δ ALC before and day of NEC | −0.03 | 0.97 | 0.73 | 0.81 | 1.16 | −0.04 | 0.96 | 0.68 | 0.80 | 1.16 |
| Δ ALC before and 24 h NEC | 0.14 | 1.15 | 0.29 | 0.89 | 1.47 | 0.14 | 1.15 | 0.29 | 0.89 | 1.48 |
| Δ ANC before and 48 h NEC | 0.24 | 1.27 | 0.19 | 0.89 | 1.82 | 0.25 | 1.29 | 0.18 | 0.89 | 1.86 |
| Platelet count | ||||||||||
| Δ Platelet before and day of NEC | 0.01 | 1.01 | 0.01 | 1.001 | 1.01 | 0.004 | 1.00 | 0.02 | 1.001 | 1.01 |
| Δ Platelet before and 24 h NEC | 0.01 | 1.01 | 0.03 | 1.001 | 1.01 | 0.01 | 1.01 | 0.05 | 1.000 | 1.01 |
| Δ Platelet before and 48 h NEC | 0.01 | 1.01 | 0.11 | 1.00 | 1.01 | 0.01 | 1.01 | 0.14 | 0.99 | 1.01 |
| Absolute monocytes count | ||||||||||
| Δ AMC before and day of NEC | 0.17 | 1.18 | 0.2 | 0.92 | 1.52 | 0.18 | 1.19 | 0.17 | 0.93 | 1.53 |
| Δ AMC before and 24 h NEC | 0.12 | 1.12 | 0.46 | 0.82 | 1.53 | 0.13 | 1.14 | 0.43 | 0.83 | 1.57 |
| Δ AMC before and 48 h NEC | 0.24 | 1.27 | 0.22 | 0.87 | 1.84 | 0.26 | 1.29 | 0.20 | 0.87 | 1.91 |
| WBC count | ||||||||||
| Δ WBC before and day of NEC | 0.03 | 1.03 | 0.13 | 0.99 | 1.08 | 0.03 | 1.03 | 0.14 | 0.99 | 1.08 |
| Δ WBC before and 24 h NEC | 0.05 | 1.05 | 0.12 | 0.99 | 1.11 | 0.05 | 1.05 | 0.09 | 0.99 | 1.12 |
| Δ WBC before and 48 h NEC | 0.06 | 1.06 | 0.04 | 1.003 | 1.12 | 0.06 | 1.07 | 0.03 | 1.01 | 1.13 |
| Eosinophil count | ||||||||||
| Δ AEC before and day of NEC | −0.002 | 1.00 | 0.98 | 0.86 | 1.16 | −0.02 | 0.99 | 0.85 | 0.84 | 1.16 |
| Δ AEC before and 24 h NEC | −0.02 | 0.98 | 0.77 | 0.84 | 1.14 | −0.03 | 0.98 | 0.74 | 0.84 | 1.13 |
| Δ AEC before and 48 h NEC | −0.08 | 0.93 | 0.35 | 0.79 | 1.08 | −0.07 | 0.93 | 0.40 | 0.80 | 1.10 |
| Monocytes (%) | ||||||||||
| Δ % Monocyte before and day of NEC | 0.02 | 1.02 | 0.48 | 0.96 | 1.08 | 0.02 | 1.02 | 0.48 | 0.96 | 1.08 |
| Δ % Monocyte before and 24 h NEC | −0.01 | 0.99 | 0.69 | 0.93 | 1.05 | −0.01 | 0.99 | 0.71 | 0.93 | 1.05 |
| Δ % Monocyte before and 48 h NEC | −0.04 | 0.96 | 0.27 | 0.89 | 1.03 | −0.05 | 0.96 | 0.25 | 0.88 | 1.03 |
| Neutrophil (%) | ||||||||||
| Δ % Neutrophil before and day of NEC | 0.03 | 1.03 | 0.02 | 1.004 | 1.05 | 0.03 | 1.03 | 0.02 | 1.003 | 1.05 |
| Δ % Neutrophil before and 24 h NEC | 0.02 | 1.02 | 0.17 | 0.99 | 1.05 | 0.02 | 1.02 | 0.18 | 0.99 | 1.05 |
| Δ % Neutrophil before and 48 h NEC | 0.04 | 1.04 | 0.04 | 1.002 | 1.08 | 0.04 | 1.04 | 0.05 | 1.00 | 1.08 |
| Lymphocyte (%) | ||||||||||
| Δ % Lymphocyte before and day of NEC | −0.02 | 0.98 | 0.20 | 0.96 | 1.008 | −0.02 | 0.98 | 0.20 | 0.96 | 1.01 |
| Δ % Lymphocyte before and 24 h NEC | 0.001 | 1.001 | 0.95 | 0.97 | 1.04 | 0.001 | 1.00 | 0.99 | 0.97 | 1.04 |
| Δ % Lymphocyte before and 48 h NEC | 0.002 | 1.002 | 0.94 | 0.95 | 1.05 | 0.003 | 1.00 | 0.90 | 0.96 | 1.05 |
Adjusted models: models are adjusted for gestational age and birth weight; all values reported as OR (95% CI); ref: medical non-fulminant NEC; p value compares fulminant NEC and medical non-fulminant group. 48-h time point represents the data from alive infants between 24 and 48 h.
Statistically significant p < 0.05 values are in bold.
OR odds ratio, CI confidence interval.
Table 4.
Associations between fulminant NEC and blood profile.
| Model | OR (95% CI) | p value |
|---|---|---|
| Day of NEC | ||
| White blood cells (%)a | 0.94 (0.81, 1.09) | 0.42 |
| Neutrophils (%)a | 0.84 (0.74, 0.94) | 0.002 |
| Absolute lymphocytes count | 0.98 (0.85, 1.14) | 0.8 |
| Lymphocytes (%)a | 1.01 (0.98, 1.03) | 0.39 |
| Mean platelet volume | 0.75 (0.54, 1.04) | 0.09 |
| Platelet count | 0.99 (0.989, 0.99) | 0.02 |
| 24 h after NEC | ||
| Neutrophils (%)a | 0.96 (0.84, 1.08) | 0.48 |
| Absolute lymphocytes | 0.96 (0.82, 1.13) | 0.64 |
| Lymphocytes (%)a | 0.93 (0.80, 1.08) | 0.32 |
| Platelet count | 0.98 (0.98, 0.99) | <0.001 |
All values reported as OR (95% CI). Each model was adjusted for birth weight, gestational age.
Statistically significant p < 0.05 values are in bold.
OR odds ratio, CI confidence interval.
a5% change.
Transfusion number and mortality
The neonates who died were more likely to have received a platelet transfusion before NEC onset (24.2% vs. 11.7%; p = 0.03). However, platelet transfusions during the 48 h after NEC onset did not discriminate between non-survivors vs. survivors (p = 0.98). RBC transfusions were not more likely before NEC onset among non-survivors than survivors (52.4% vs. 46.1%; p = 0.48). However, RBC transfusion in the 48 h after NEC onset was more likely in non-survivors (76.7% vs. 53.1%, p = 0.001). This data is given in Table 5.
Table 5.
Transfusion and mortality.
| ALL | Combined group | Discharged | ||||||
|---|---|---|---|---|---|---|---|---|
| Death | Discharged | p value | ALL | MNF NEC | SNF NEC | p value | ||
| N = 321 | N = 85 | N = 236 | N = 236 | N = 114 | N = 122 | |||
| Platelet tx before NEC | n = 225 | n = 62 | n = 163 | n = 163 | n = 51 | n = 112 | ||
| Platelet tx before NEC | 0.09 | 0.832 | ||||||
| 0 | 191 (84.9%) | 47 (75.8%) | 144 (88.3%) | 144 (88.3%) | 44 (86.3%) | 100 (89.3%) | ||
| 1 | 17 (7.56%) | 7 (11.3%) | 10 (6.13%) | 10 (6.13%) | 4 (7.84%) | 6 (5.36%) | ||
| 2 | 8 (3.56%) | 4 (6.45%) | 4 (2.45%) | 4 (2.45%) | 1 (1.96%) | 3 (2.68%) | ||
| 3+ | 9 (4.00%) | 4 (6.45%) | 5 (3.07%) | 5 (3.07%) | 2 (3.92%) | 3 (2.68%) | ||
| Total yes | 34 (15.1%) | 15 (24.2%) | 19 (11.7%) | 19 (11.7%) | 7 (13.7%) | 12 (10.7%) | ||
| Platelet tx 48 h after NEC | n = 225 | n = 63 | n = 162 | n = 162 | n = 47 | n = 115 | ||
| Platelet tx 48 h after NEC | 0.99 | <0.001 | ||||||
| 0 | 148 (65.8%) | 42 (66.7%) | 106 (65.4%) | 106 (65.4%) | 43 (91.5%) | 63 (54.8%) | ||
| 1 | 46 (20.4%) | 13 (20.6%) | 33 (20.4%) | 33 (20.4%) | 3 (6.38%) | 30 (26.1%) | ||
| 2 | 17 (7.56%) | 4 (6.35%) | 13 (8.02%) | 13 (8.02%) | 1 (2.13%) | 12 (10.4%) | ||
| 3+ | 14 (6.22%) | 4 (6.35%) | 10 (6.17%) | 10 (6.17%) | 0 (0.00%) | 10 (8.70%) | ||
| Total yes | 77 (34.2%) | 21 (33.3%) | 56 (34.6%) | 56 (34.6%) | 4 (8.51%) | 52 (45.2%) | ||
| Blood tx before NEC | n = 282 | n = 73 | n = 209 | n = 209 | n = 100 | n = 109 | ||
| Blood tx before NEC | 0.823 | 0.036 | ||||||
| 0 | 147 (52.1%) | 35 (47.9%) | 112 (53.6%) | 112 (53.6%) | 56 (56.0%) | 56 (51.4%) | ||
| 1 | 27 (9.57%) | 8 (11.0%) | 19 (9.09%) | 19 (9.09%) | 9 (9.00%) | 10 (9.17%) | ||
| 2 | 32 (11.3%) | 8 (11.0%) | 24 (11.5%) | 24 (11.5%) | 5 (5.00%) | 19 (17.4%) | ||
| 3+ | 76 (27.0%) | 22 (30.1%) | 54 (25.8%) | 54 (25.8%) | 30 (30.0%) | 24 (22.0%) | ||
| Total yes | 135 (47.9%) | 38 (52.1%) | 97 (46.4%) | 97 (46.4%) | 44 (44.0%) | 53 (48.6%) | ||
| Blood tx before NEC | n = 267 | n = 73 | n = 194 | n = 194 | n = 91 | n = 103 | ||
| Blood tx 48 h after NEC | 0.003 | <0.001 | ||||||
| 0 | 108 (40.4%) | 17 (23.3%) | 91 (46.9%) | 91 (46.9%) | 66 (72.5%) | 25 (24.3%) | ||
| 1 | 89 (33.3%) | 28 (38.4%) | 61 (31.4%) | 61 (31.4%) | 22 (24.2%) | 39 (37.9%) | ||
| 2 | 37 (13.9%) | 16 (21.9%) | 21 (10.8%) | 21 (10.8%) | 2 (2.20%) | 19 (18.4%) | ||
| 3+ | 33 (12.4%) | 12 (16.4%) | 21 (10.8%) | 21 (10.8%) | 1 (1.10%) | 20 (19.4%) | ||
| Total yes | 159 (59.6%) | 56 (76.7%) | 103 (53.1%) | 103 (53.1%) | 25 (27.5%) | 78 (75.7%) | ||
Cells are represented as n (%), differences in categorical measures were tested using a chi-square test or Fisher’s exact test.
MNF medical non-fulminant NEC, SNF surgical non-fulminant NEC.
Discussion
Our data demonstrate that neonates who develop fulminant NEC have a different hematological profile than those with non-fulminant medical or surgical disease. In the 48 h following diagnosis, infants with fulminant NEC had lower total leukocyte counts, and the absolute counts of neutrophils, lymphocytes, and eosinophils, but higher monocyte counts. The platelet counts were lower. Clinically, the fulminant disease occurred at an earlier postnatal age. As anticipated, the fulminant disease course was associated with more extensive clinical disease seen with prominent radiological signs such as portal venous gas prior to surgery and greater needs for assisted ventilation and inotropes before and after surgery. In infants with the non-fulminant disease, the clinical features were closer to our anticipations. Compared to MNF NEC, infants with surgical but non-fulminant NEC had lower gestation age and birth weights and had a higher severity of illness related to prematurity with a higher frequency of PDA and need for assisted ventilation. As anticipated, those who needed surgery had higher CRP levels and more frequently documented pneumoperitoneum.
The incidence of fulminant NEC in our study (10%) was lower than in the study by Lin et al. [29], who reported 352 neonates, 112 of whom (31.8%) met the fulminant NEC criteria. They had, on average, a higher gestational age (37.0 weeks) and birth weight (2610 g) than ours (mean GA 27.9 ± 4.0 weeks and mean birth weight 1087 ± 745) and showed different hematological findings. They had lower leukocyte and neutrophil counts but no differences in platelet count, compared with non-fulminant cases. In another study, Lambert et al. reported fulminant NEC in 6.7% of 523 cases, which was less than in our study [20]. The gestational age and birth weight of neonates with fulminant NEC resembled our cohort, at 27.5 ± 2.4 weeks and 1089 ± 545 g. They reported a higher rate of portal venous gas in fulminant cases (N = 20/35, 57%).
Several previous studies [14, 15, 17, 18] have reported thrombocytopenia in surgical NEC. However, only a few commented on their nadir and none on the recovery timeline (trend toward pre-NEC levels). We found significant thrombocytopenia in fulminant and non-fulminant NEC (73.0 [41.8; 166] and 68.0 [33.8; 101] SNF) lasting 48 and 96 h, respectively, and noticed recovery generally after 4 days in the survivors. Our patients with fulminant NEC (all of whom died within 48 h, by definition) had the lowest platelet counts at 73 TH/cmm [41.8; 166] than 80 TH/cmm [45.0; 123] SNF and 208 TH/cmm [134; 280] MNF, which resembled the observations in other studies [15, 19]. The exact mechanism causing thrombocytopenia in these NEC is still uncertain, but consumptive disorders are likely and include the formation of microthrombi in the diseased intestine [30] and platelet activation from bacterial products leading to aggregation in the microvasculature [31].
A recent mouse study reported thrombin-mediated platelet activation [32, 33]. Few human studies have reported decreased immature platelet fraction and increased mean platelet volume in neonates with NEC [34, 35]. In our study, we did not find significant differences in mean platelet volume in three groups.
Our findings suggest that neonates with surgical fulminant and the non-fulminant group had significantly lower absolute neutrophil counts from the day of NEC until 48 h after onset, and recovery was noticed on day 3 in those with surgical NEC. Other investigators reported neutropenia in neonates with fulminant NEC; however, those studies did not report serial timing of neutropenia in relation to NEC onset [15, 29]. Defining the timeline might give an opportunity for early diagnostic and therapeutic inventions. Hutter et al. studied the bone marrow of neonates who died due to NEC and reported normal granulocyte precursors [15]. Our predictive model suggests that improvement in the neutrophil count by every 5% is associated with decreased risk of fulminant NEC.
Lambert et al. reported lower lymphocyte counts [(<4000/μl) (p = 0.018)] in neonates with fulminant NEC [20]. Our findings also suggest significant lymphopenia in neonates with fulminant NEC from the day of NEC onset until day 2 and until day 7 in neonates with SNF NEC. The data indicate a longer-lasting duration of lymphopenia/or delayed recovery as compared to neutropenia in neonates with SNF NEC. We also observed significantly lower eosinophil count in neonates with fulminant and non-fulminant NEC. This needs further investigation to understand the role of eosinophils in fulminant NEC. We observed a variable pattern of monocytes. The neonates with SNF NEC had lower absolute monocyte counts as compared to those with medical NEC until 48 h after NEC onset, and the significant opposite pattern was [36] seen on days 4 and 7. This may be explained due to tissue/intestinal infiltration with monocytes in the NEC condition, as shown in animal models [37, 38]. The increase in monocyte counts noted from day 4 onwards most probably reflects mobilization, an attempt at recovery.
In our study, the non-survivors received significantly more platelet transfusions before NEC onset. A recently published randomized trial by Carley et al. demonstrated that neonates who receive platelet transfusions at a platelet-count threshold of 50,000 per cubic millimeter had a significantly higher rate of death or major bleeding within 28 days after randomization than those who received platelet transfusions at a platelet-count threshold of 25,000 per cubic millimeter [28]. A previously published study by Kenton et al. did not find an effect of platelet transfusion on mortality. However, they reported an increased frequency of morbidities, such as short bowel syndrome and cholestasis [27]. In a recent report [26], which studied 598 very-low-birth-weight neonates, 44 developed NEC. In unadjusted analysis, platelet transfusion rate was 30.3 (95% CI, 11.5–80.1) per 100 infant-days among neonates who died, compared to 6.0 (95% CI, 3.2–11.2) among survivors (incidence rate ratio, 5.1; 95% CI, 1.6–16.2; p = 0.006). The contribution of platelet transfusions to mortality may be explained due to increased levels of pro-inflammatory mediators such as neuropeptide Y in the stored platelets [26].
In our study, the rate of blood transfusion from birth until the NEC onset did not affect mortality. A recent study by Wang et al. reported associations between the number of RBC transfusions and in-hospital mortality and morbidity. The number of RBC transfusions within 7 days of birth was not different among those who went on to develop NEC (3.0 ± 2.6) vs. those who did not develop NEC (2.8 ± 1.7, p = 0.521) [39]. Similarly, in our previous meta-analysis, we did not find an association between RBC transfusion and NEC onset [23]. In the present study, the non-survivors were more likely to receive an RBC transfusion within 48 h after NEC onset.
Our study’s strengths include our evaluation of nadir and recovery of different hematological variables, perhaps providing insights about disease pathophysiology and direction for further research. Our data validate the observation that platelet transfusion before NEC onset was associated with adverse outcomes. We acknowledge study limitations, including the single-center retrospective design and the relatively small sample size of infants with fulminant NEC.
In conclusion, neonates with medical, surgical, and fulminant NEC have somewhat different clinical and hematological patterns after the NEC onset. The fulminant NEC neonates were more likely to be associated with lower platelet count and lower total white blood cell count, lower neutrophil count, and lower lymphocyte count after the NEC onset. In addition, the higher frequency of platelet transfusions before NEC onset was associated with an increased risk of fulminant NEC. In the future, larger, prospective multicenter studies that include additional clinical detail (e.g., mesenteric perfusion using NIRS) and laboratory predictors such as hematological and stool biomarkers may inform earlier recognition of or risk factors for fulminant NEC.
Supplementary information
Acknowledgements
We would like to thank Dr Robert Christensen (Professor and Division Chief of Neonatology at Intermountain Medical Center, Utah), Dr Akhil Maheshwari (Professor, Department of Pediatrics/Neonatology at John Hopkins Hospital, Baltimore), and Dr Martha C. Sola-Visner (Department of Neonatology, Boston Children Hospital, and Boston, Massachusetts) for critically reviewing the manuscript and providing for all the feedback and guidance.
Author contributions
PMG designed the study. PMG, VO, AC, BV, HH, HM, JLP, MAYA, CDJ collected and analyzed the data. PMG, MAYA wrote the manuscript. All the authors contributed to and approved the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41372-021-01044-3.
References
- 1.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–64. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sankaran K, Puckett B, Lee DS, Seshia M, Boulton J, Qiu Z, et al. Variations in incidence of necrotizing enterocolitis in Canadian neonatal intensive care units. J Pediatr Gastroenterol Nutr. 2004;39:366–72. doi: 10.1097/00005176-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Sjoberg Bexelius T, Ahle M, Elfvin A, Bjorling O, Ludvigsson JF, Andersson RE. Intestinal failure after necrotising enterocolitis: incidence and risk factors in a Swedish population-based longitudinal study. BMJ Paediatrics Open. 2018;2:e000316. doi: 10.1136/bmjpo-2018-000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allin BSR, Long AM, Gupta A, Lakhoo K, Knight M. One-year outcomes following surgery for necrotising enterocolitis: a UK-wide cohort study. Arch Dis Child Fetal Neonatal Ed. 2018;103:F461–6. doi: 10.1136/archdischild-2017-313113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knell J, Han SM, Jaksic T, Modi BP. Current status of necrotizing enterocolitis. Curr Probl Surg. 2019;56:11–38. doi: 10.1067/j.cpsurg.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santulli TV, Schullinger JN, Heird WC, Gongaware RD, Wigger J, Barlow B, et al. Acute necrotizing enterocolitis in infancy: a review of 64 cases. Pediatrics. 1975;55:376–87. [PubMed] [Google Scholar]
- 8.Mowitz ME, Dukhovny D, Zupancic JAF. The cost of necrotizing enterocolitis in premature infants. Semin fetal Neonatal Med. 2018;23:416–9. doi: 10.1016/j.siny.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Ganapathy V, Hay JW, Kim JH, Lee ML, Rechtman DJ. Long term healthcare costs of infants who survived neonatal necrotizing enterocolitis: a retrospective longitudinal study among infants enrolled in Texas Medicaid. BMC Pediatr. 2013;13:127. doi: 10.1186/1471-2431-13-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurscheid T, Holschneider AM. Necrotizing enterocolitis (NEC)-mortality and long-term results. Eur J Pediatr Surg. 1993;3:139–43. doi: 10.1055/s-2008-1063530. [DOI] [PubMed] [Google Scholar]
- 11.Thakkar HS, Lakhoo K. The surgical management of necrotising enterocolitis (NEC) Early Hum Dev. 2016;97:25–8. doi: 10.1016/j.earlhumdev.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Raval MV, Moss RL. Current concepts in the surgical approach to necrotizing enterocolitis. Pathophysiol: Off J Int Soc Pathophysiol. 2014;21:105–10. doi: 10.1016/j.pathophys.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Rees CM, Hall NJ, Eaton S, Pierro A. Surgical strategies for necrotising enterocolitis: a survey of practice in the United Kingdom. Arch Dis Child Fetal Neonatal Ed. 2005;90:F152–5. doi: 10.1136/adc.2004.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maheshwari A. Immunologic and hematological abnormalities in necrotizing enterocolitis. Clin Perinatol. 2015;42:567–85. doi: 10.1016/j.clp.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutter JJ, Jr., Hathaway WE, Wayne ER. Hematologic abnormalities in severe neonatal necrotizing enterocolitis. J Pediatr. 1976;88:1026–31. doi: 10.1016/S0022-3476(76)81069-4. [DOI] [PubMed] [Google Scholar]
- 16.Ragazzi S, Pierro A, Peters M, Fasoli L, Eaton S. Early full blood count and severity of disease in neonates with necrotizing enterocolitis. Pediatr Surg Int. 2003;19:376–9. doi: 10.1007/s00383-003-1014-5. [DOI] [PubMed] [Google Scholar]
- 17.Patel CC. Hematologic abnormalities in acute necrotizing enterocolitis. Pediatr Clin North Am. 1977;24:579–84. doi: 10.1016/S0031-3955(16)33466-6. [DOI] [PubMed] [Google Scholar]
- 18.Kenton AB, O’Donovan D, Cass DL, Helmrath MA, Smith EO, Fernandes CJ, et al. Severe thrombocytopenia predicts outcome in neonates with necrotizing enterocolitis. J Perinatol. 2005;25:14–20. doi: 10.1038/sj.jp.7211180. [DOI] [PubMed] [Google Scholar]
- 19.Ververidis M, Kiely EM, Spitz L, Drake DP, Eaton S, Pierro A. The clinical significance of thrombocytopenia in neonates with necrotizing enterocolitis. J Pediatr Surg. 2001;36:799–803. doi: 10.1053/jpsu.2001.22964. [DOI] [PubMed] [Google Scholar]
- 20.Lambert DK, Christensen RD, Baer VL, Henry E, Gordon PV, Besner GE, et al. Fulminant necrotizing enterocolitis in a multihospital healthcare system. J Perinatol. 2012;32:194–8. doi: 10.1038/jp.2011.61. [DOI] [PubMed] [Google Scholar]
- 21.MohanKumar K, Namachivayam K, Song T, Jake Cha B, Slate A, Hendrickson JE, et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat Commun. 2019;10:3494. doi: 10.1038/s41467-019-11199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA. 2016;315:889–97. doi: 10.1001/jama.2016.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg P, Pinotti R, Lal CV, Salas AA. Transfusion-associated necrotizing enterocolitis in preterm infants: an updated meta-analysis of observational data. J Perinat Med. 2018;46:677–85. doi: 10.1515/jpm-2017-0162. [DOI] [PubMed] [Google Scholar]
- 24.Garg PM, Ravisankar S, Bian H, Macgilvray S, Shekhawat PS. Relationship between packed red blood cell transfusion and severe form of necrotizing enterocolitis: a case control study. Indian Pediatr. 2015;52:1041–5. doi: 10.1007/s13312-015-0770-3. [DOI] [PubMed] [Google Scholar]
- 25.Cremer M, Sola-Visner M, Roll S, Josephson CD, Yilmaz Z, Bührer C, et al. Platelet transfusions in neonates: practices in the United States vary significantly from those in Austria, Germany, and Switzerland. Transfusion. 2011;51:2634–41. doi: 10.1111/j.1537-2995.2011.03208.x. [DOI] [PubMed] [Google Scholar]
- 26.Patel RM, Josephson CD, Shenvi N, Maheshwari A, Easley KA, Stowell S, et al. Platelet transfusions and mortality in necrotizing enterocolitis. Transfusion. 2019;59:981–8. doi: 10.1111/trf.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenton AB, Hegemier S, Smith EO, O’Donovan DJ, Brandt ML, Cass DL, et al. Platelet transfusions in infants with necrotizing enterocolitis do not lower mortality but may increase morbidity. J Perinatol. 2005;25:173–7. doi: 10.1038/sj.jp.7211237. [DOI] [PubMed] [Google Scholar]
- 28.Curley A, Stanworth SJ, Willoughby K, Fustolo-Gunnink SF, Venkatesh V, Hudson C, et al. Randomized trial of platelet-transfusion thresholds in neonates. N Engl J Med. 2019;380:242–51. doi: 10.1056/NEJMoa1807320. [DOI] [PubMed] [Google Scholar]
- 29.Lin L, Xia X, Liu W, Wang Y, Hua Z. Clinical characteristics of neonatal fulminant necrotizing enterocolitis in a tertiary Children’s hospital in the last 10 years. PLoS ONE. 2019;14:e0224880. doi: 10.1371/journal.pone.0224880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyman PE, Abrams CE, Zipser RD. Enhanced urinary immunoreactive thromboxane in neonatal necrotizing enterocolitis. A diagnostic indicator of thrombotic activity. Am J Dis Child. 1987;141:686–9. doi: 10.1001/archpedi.1987.04460060102046. [DOI] [PubMed] [Google Scholar]
- 31.Hsueh W, Caplan MS, Qu XW, Tan XD, De Plaen IG, Gonzalez-Crussi F. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol. 2003;6:6–23. doi: 10.1007/s10024-002-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Namachivayam K, MohanKumar K, Shores DR, Jain SK, Fundora J, Everett AD, et al. Targeted inhibition of thrombin attenuates murine neonatal necrotizing enterocolitis. Proc Natl Acad Sci U S A. 2020;117:10958–69. doi: 10.1073/pnas.1912357117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Namachivayam K, MohanKumar K, Garg L, Torres BA, Maheshwari A. Neonatal mice with necrotizing enterocolitis-like injury develop thrombocytopenia despite increased megakaryopoiesis. Pediatr Res. 2017;81:817–24. doi: 10.1038/pr.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cremer M, Weimann A, Szekessy D, Hammer H, Bührer C, Dame C. Low immature platelet fraction suggests decreased megakaryopoiesis in neonates with sepsis or necrotizing enterocolitis. J Perinatol. 2013;33:622–6. doi: 10.1038/jp.2013.21. [DOI] [PubMed] [Google Scholar]
- 35.Cekmez F, Tanju IA, Canpolat FE, Aydinoz S, Aydemir G, Karademir F, et al. Mean platelet volume in very preterm infants: a predictor of morbidities? Eur Rev Med Pharm Sci. 2013;17:134–7. [PubMed] [Google Scholar]
- 36.Desiraju S, Bensadoun J, Bateman D, Kashyap S. The role of absolute monocyte counts in predicting severity of necrotizing enterocolitis. J Perinatol. 2020;40:922–7. doi: 10.1038/s41372-020-0596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MohanKumar K, Kaza N, Jagadeeswaran R, Garzon SA, Bansal A, Kurundkar AR, et al. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am J Physiol Gastrointest Liver Physiol. 2012;303:G93–102. doi: 10.1152/ajpgi.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Remon J, Kampanatkosol R, Kaul RR, Muraskas JK, Christensen RD, Maheshwari A. Acute drop in blood monocyte count differentiates NEC from other causes of feeding intolerance. J Perinatol. 2014;34:549–54. doi: 10.1038/jp.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang YC, Chan OW, Chiang MC, Yang PH, Chu SM, Hsu JF, et al. Red blood cell transfusion and clinical outcomes in extremely low birth weight preterm infants. Pediatr Neonatol. 2017;58:216–22. doi: 10.1016/j.pedneo.2016.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.