Abstract
Purpose:
This study aimed to test the hypothesis that the elevation in internal body temperature during exercise in a hot environment is influenced by the combination of exercise intensity and BSA burned.
Methods:
Ten healthy participants (8 males, 2 females; 32 ± 9 yr; 75.3 ± 11.7 kg) completed eight exercise trials on a cycle ergometer, each with different combinations of metabolic heat productions (low, 4 W·kg−1; moderate, 6 W·kg−1) and simulated BSA burn in a hot environmental chamber (39.9°C ± 0.3°C, 20.1% ± 1.5% RH). Burns were simulated by covering 0%, 20%, 40%, or 60% of participants’ BSA with a highly absorbent, vapor-impermeable material. Gastrointestinal temperature (TGI) was recorded, with the primary analysis being the increase in TGI after 60 min of exercise.
Results:
We identified an interaction effect for the increase in TGI (P < 0.01), suggesting TGI was influenced by both intensity and simulated burn BSA. Regardless of the percentage BSA burn simulated, the increase in TGI was similar across low-intensity trials (0.70°C ± 0.26°C, P > 0.11 for all). However, during moderate-intensity exercise, the increase in TGI was greater for the 60% (1.78°C ± 0.38°C, P < 0.01) and 40% BSA coverage trials (1.33°C ± 0.44°C, P = 0.04), relative to 0% (0.82°C ± 0.36°C). There were no differences in TGI responses between 0% and 20% trials.
Conclusion:
These data suggest that exercise intensity influences the relationship between burn injury size and thermoregulatory responses in a hot environment.
Keywords: BURN SURVIVOR, HEAT STRESS, CORE TEMPERATURE
Survival from severe burns has increased dramatically in the past few decades, with a Lethal Dose 50 (the burn size that results in 50% lethality) improving to approximately 90% body surface area (BSA) burned (1). As a result, there are a large number of severely burned individuals requiring clinical considerations for their resumption of activities of daily living (2). Burn survivors with grafted skin demonstrate a decreased heat dissipation capacity due to impaired sweating and cutaneous vasodilation (3,4), even after long-term recovery (5). Indeed, burn survivors report problems with hot environmental temperatures persisting for years after their recovery (2). Multiple factors, including the severity/size of the burn injury and grafting techniques, may also influence the heat dissipating capacity of burn survivors. Such responses culminate in burn survivors, exhibiting exaggerated internal body temperature responses during exercise (6–9). This impairment is especially apparent in burn survivors whose size of injured skin exceeds the compensatory heat dissipation capacity of their remaining uninjured skin (10).
In the U.S. military, severe burn incidence is 2.7 times greater than the civilian population (11). There are policies in place that determine the permissibility of a burn survivor to begin or continue military service. The U.S. Department of Defense’s Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DoDI 6130.03, Section 5.21, w) states, “Prior burn injury involving 18 percent or more body surface area (including graft sites), or resulting in functional impairment to such a degree, due to scarring, as to interfere with satisfactorily performing military duty due to pain or decreased range of motion, strength, temperature regulation, or agility” disqualifies an individual from military service (12). This guideline supersedes previous U.S. Army standards (AR 40-501), which disqualified an individual with greater than 40% BSA burn injury (13). However, these policies are based on previous studies that used small sample sizes or methodologies that limit the application of the results (6–9).
Individuals with greater than 40% BSA burned exhibit higher internal body temperatures during moderate-intensity exercise in the heat when compared with both nonburned controls and groups with ~20% BSA burn (6,8,9). However, during lower-intensity exercise in a very small cohort (n = 3 per group), individuals with >60% BSA burned exhibit similar body temperature responses to those ~35% BSA burned (14). Despite this observation, no previous study has systematically examined the role of exercise intensity on body temperature responses in burn survivors. That is, most studies have omitted considerations for the influence of metabolic heat production on heat balance in burn survivors, which is particularly concerning because metabolic heat production accounts for a large portion of the interindividual variability in internal body temperature responses (15). This omission reverberates in military policies that only consider the total burned BSA, thereby omitting consideration of the metabolic heat generation associated with an assigned task. By contrast, the combination of burned BSA and exercise intensity may dictate the circumstances that determine uncompensable heat stress. As a result, potential decrements to safety and performance during training exercises, where most exertional heat illnesses occur (16), may differ for burn survivors when compared with nonburned soldiers.
Overall, in both military and civilian burn survivors, the role of exercise intensity on thermoregulatory dysfunction remains unknown. Therefore, the purpose of this study was to test the hypothesis that the magnitude of the elevation in internal body temperature during exercise in a hot environment is influenced by the combination of exercise intensity and simulated percentage BSA burned. Specifically, we sought to determine whether lower exercise intensity, and as a result lower metabolic heat production, attenuates the detrimental effects of burn surface area on thermoregulation.
METHODS
Ethical approval.
The Institutional Review Boards of the University of Texas Southwestern Medical Center, Texas Health Presbyterian Hospital Dallas, and the Human Research Protections Office of the Defense Health Agency approved this study protocol and associated informed consent. Before participation, all participants were informed of the study procedures and potential risks and provided written consent. All procedures conformed to the standards set forth in the Declaration of Helsinki.
Participants.
We completed sample size calculations a priori, concluding that 10 participants would be needed to achieve a statistical power of >0.8, using an α = 0.05 and an estimated Cohen’s f of 0.6 for the elevation in core body temperature based on previous work in our laboratory (repeated-measures ANOVA, within–between interaction; G*Power 3.1). Participants were required to be between 18 and 55 yr old, not take any medication, and not have any reported cardiovascular, respiratory, metabolic, or neurologic disease. We recruited 13 healthy individuals (2 withdrew voluntarily and 1 was excluded due to low fitness) to participate in this study, resulting in 10 participants (8 males and 2 females) who completed all study procedures. Participant characteristics are reported in Table 1. Of the two female participants, one was assessed during the early follicular phase of her menstrual cycle. For the other female, her menstrual cycle was inconsistent, perhaps because of the use of an intrauterine device. For this reason, we were unable to obtain data from all eight trials during the same phase of the menstrual cycle for that participant. However, because the primary variable of interest is the increase in internal body temperature to the exercise bouts, which is not influenced by the menstrual cycle (17), we do not view this approach as a limitation.
TABLE 1.
Participant characteristics.
| Age (yr) | 32 ± 9 (20–47) |
| V̇O2peak (L·min−1) | 3.38 ± 0.59 (2.44–4.48) |
| V̇O2peak (mL·kg−1·min−1) | 46.5 ± 12.2 (34.0–75.1) |
| Maximum heart rate at V̇O2peak (bpm) | 180 ± 12 (165–200) |
| Body mass (kg) | 75.3 ± 11.7 (54.0–99.8) |
| Height (m) | 1.76 ± 0.07 (1.65–1.85) |
| BMI (kg·m−2) | 24.1 ± 2.9 (19.8–29.5) |
| BSA (m2) | 1.91 ± 0.17 (1.58–2.22) |
Data represent mean ± SD (range) for 10 participants, 8 males and 2 females.
V̇O2peak, peak oxygen consumption; BMI, body mass index.
Experimental protocol.
Participants completed nine study visits (one preliminary and eight experimental trials), each separated by at least 48 h. In a randomized, crossover fashion, participants completed experimental trials that were combinations of two exercise intensities (low, ~4 W·kg−1; moderate, ~6 W·kg−1) and four simulated burn areas (0%, 20%, 40%, and 60% BSA). For six participants, their moderate-intensity trials originated from a previous investigation with identical conditions (18). Before all study visits, participants abstained from allergy medicine, anti-inflammatory drugs, and aspirin for 36 h; exercise and alcohol for 24 h; and caffeine for 12 h. Study visits were performed at similar times of the day for a given participant to minimize the effects of the circadian rhythm on the primary variables of interest. Upon arrival to the laboratory, participants provided a urine sample that was analyzed for urine specific gravity (Atago Inc., Bellevue, WA), with a value >1.025 prohibiting testing on that day (19).
The preliminary trial consisted of screening procedures, including a 12-lead electrocardiogram and medical history, and a graded exercise test to determine peak oxygen consumption (V̇O2peak). Participants conducted V̇O2peak testing in a thermoneutral environment on a cycle ergometer (Lode Corival, Groningen, Netherlands). Participants first completed a warm-up of three 4-min stages of progressively increasing intensity cycling, followed by a 10-min rest. They then completed a graded exercise test that began at a work rate of 1 W·kg−1 of total body mass and increased by 20 or 25 W·min−1 until volitional exhaustion. Throughout testing, participants’ expired gases were collected and analyzed using indirect calorimetry (TrueOne 2400; Parvo Medics, Sandy, UT). The highest 30-s V̇O2 determined the participant’s V̇O2speak.
Participants wore similar clothing across experimental trials, consisting of shorts, socks, running shoes, and sports bra (females only). The decreased evaporative cooling observed in burn survivors was simulated by affixing absorbent pads with a vapor-impermeable exterior to the skin of the participants (18,20). BSA was calculated based on participants’ height (Detecto stadiometer, Webb City, MO) and mass (Mettler Toledo PBD655-BC120, Toledo, OH) (21). Based on the calculated BSA for each experimental day, we covered the skin of the torso, arms, and legs with a scaled amount of covering (20%, 40%, or 60%); one-half of the coverage for each condition was affixed to the anterior and posterior torso, with the remainder divided equally across the arms and legs. Pads were attached using surgical tape (3M Transpore, London, ON) and tubular net bandages (Owens & Minor MediChoice, Mechanicsville, VA). Sweat secreted in an area covered by the absorbent material is sequestered in that material, thereby preventing evaporative heat loss and thus simulating a similarly-sized burn injury.
Gastrointestinal temperature (TGI) was measured using a telemetric pill, with the responses sampled at 0.1 Hz, and that signal was sent to the data acquisition system (Biopac MP150, Santa Barbara, CA). The pill was ingested ~2 h before exercise (HQ Inc., Palmetto, FL), which is an acceptable duration post-ingestion being that TGI responses to exercise are not different when the pill was ingested 40 min or 24 h before the exercise bout (22). Heart rate was recorded from an electrocardiogram (GE Medical Systems, Madison, WI) and routed into the data acquisition system. After instrumentation, participants entered an environmental chamber with ambient conditions of 39.9°C ± 0.3°C and 20.1% ± 1.5% relative humidity. After 30 min of seated equilibration, participants exercised for 60 min on a cycle ergometer. Participants cycled at a fixed metabolic heat production, either ~4 or ~6 W·kg−1, which was measured from expired gases during minutes 0–10, 25–35, and 50–60, and accounting for external work on the cycle ergometer. Thus, the calculation of metabolic heat production accounted for oxygen consumption (V̇O2), RER, and external work (Wk):
where ec is the caloric equivalent for the oxidation of carbohydrates (21.12 kJ·L−1 O2) and ef is the caloric equivalent for the oxidation of fat (19.61 kJ·L−1 O2).
Throughout the protocol, participants were permitted to drink water ad libitum that was maintained at each participant’s internal body temperature via a water bottle immersed in a circulating water bath adjusted to body temperature (E100, Lauda, Germany). Participants were prevented from drinking ~5 min before critical time points (e.g., 0, 15, 30, 45, or 60 min) to minimize any influence of drinking on TGI. During exercise, a fan was directed at the participants, circulating air at 0.36 ± 0.38 m·s−1.
Statistical analyses.
TGI and heart rate values were analyzed using 2-min averages preceding 0-, 15-, 30-, 45-, and 60-min time points of exercise. In the moderate-intensity exercise condition, one participant with a 40% simulated burn and three participants with 60% simulated burn were unable to complete the full 60 min of exercise, either due to volitional fatigue or reaching the ethical upper limit of TGI, 39.5°C. For these four (out of 80) trials, the last exercising TGI and heart rate values were analyzed as the 60-min data point. This approach was selected because had the participants continued to exercise for the full 60 min; those 60-min TGI and heart rate values would have been equal to or greater than the values when they stopped exercising.
Data are reported as mean ± SD. Heat balance parameters (effective BSA, external work, and Hprod) across exercise intensities and simulated burn areas were compared using two-way repeated-measures ANOVA (intensity × burn BSA). Changes in TGI and heart rate across simulated burn areas, within each exercise intensity, were analyzed using a two-way repeated-measures ANOVA (time × burn BSA, with Bonferroni-corrected multiple comparisons referenced to 0% simulated burn). To better elucidate the interaction of intensity and simulated burn area, which was the primary variable of interest, two-way repeated-measures ANOVA (intensity × burn BSA) of end-exercise TGI and heart rate were calculated. We performed Bonferroni-corrected multiple comparisons for any variable with a significant interactive effect. Statistical analyses were performed using Prism 8.3 (GraphPad, La Jolla, CA). Significance was set a priori at P < 0.05.
RESULTS
Table 2 displays heat balance parameters by exercise intensity and simulated burn area. Within an exercise intensity, there were no differences across simulated burn areas for external work (main effect of simulated burn, P = 0.90). As designed, there were no differences in metabolic heat production (main effect of simulated burn, P = 0.34), but there were main effects of exercise intensity on external work (P < 0.01), absolute heat production (P < 0.01), and mass-specific heat production (P < 0.01).
TABLE 2.
Heat balance parameters at low- and moderate-intensity exercise and graduated levels of simulated burn.
| 0% | 20% | 40% | 60% | |
|---|---|---|---|---|
| Low | ||||
| Effective BSA (m2) | 1.91 ± 0.17**,*** | 1.53 ± 0.14*,*** | 1.15 ± 0.11*,** | 0.76 ± 0.06*,**,*** |
| External work (W) | 62 ± 9 | 64 ± 10 | 64 ± 10 | 63 ± 9 |
| Hprod (W) | 305 ± 45 | 295 ± 41 | 296 ± 39 | 299 ± 39 |
| Hprod (W·kg−1) | 4.07 ± 0.49 | 3.96 ± 0.50 | 3.95 ± 0.46 | 4.00 ± 0.57 |
| Moderate | ||||
| Effective BSA (m2) | 1.90 ± 0.18**,*** | 1.53 ± 0.14*,*** | 1.14 ± 0.11*,** | 0.76 ± 0.07*,**,*** |
| External work (W) | 104 ± 16 | 101 ± 13 | 102 ± 17 | 102 ± 16 |
| Hprod (W) | 433 ± 68 | 425 ± 68 | 436 ± 69 | 435 ± 63 |
| Hprod (W·kg−1) | 5.85 ± 0.77 | 5.69 ± 0.61 | 5.70 ± 0.33 | 5.81 ± 0.73 |
Data are presented as mean ± SD.
Different from 0% (P < 0.05).
Different from 20% (P < 0.05).
Different from 40% (P < 0.05).
Hprod, metabolic heat production.
At the beginning of exercise, there were no differences in heart rate or TGI across simulated burn areas (heart rate, P = 0.85; TGI, P = 0.61) or exercise intensity (heart rate, P = 0.98; TGI, P = 0.63). The changes in TGI, relative to the beginning of exercise, for a given simulated burn area, are shown in Figure 1A (low-intensity exercise) and Figure 1B (moderate-intensity exercise). Overall, there was an interactive effect of time and simulated burn area for both low (P < 0.01) and moderate intensities (P < 0.01). However, unlike moderate-intensity exercise, for the low-intensity trial, there were no significant paired differences at any time point relative to the 0% coverage trial upon post hoc analyses (see Fig. 1A and 1B). Figure 2 expands this analysis by comparing the change in TGI at 60 min of exercise across exercise intensities and simulated burn area, with an interaction between simulated burn area and exercise intensity (P < 0.01). Further analyses (via Bonferroni multiple comparison test) revealed an absence of a difference in the magnitude of the elevation in TGI at 60 min of exercise across any paired comparisons for the low-intensity trial (P > 0.05). By contrast, at the end of moderate-intensity exercise, 60% simulated burn resulted in greater increases in TGI relative to both 20% and 40% simulated burn trials (P < 0.05), but there was no difference between TGI responses at the end of exercise between 20% and 40% simulated burn trials (P = 0.33).
FIGURE 1—
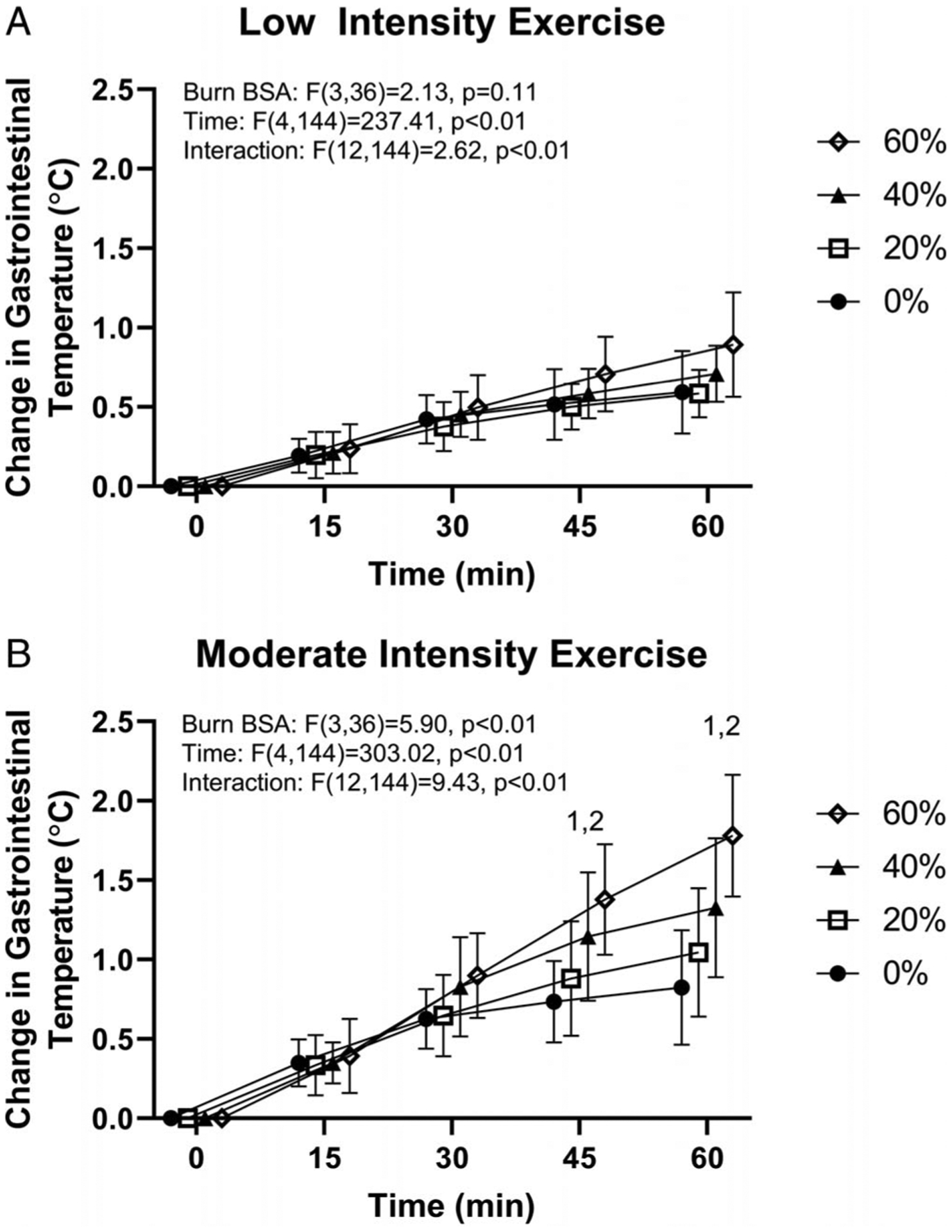
Change in TGI during 60 min of exercise at low (A, ~4 W·kg−1) and moderate (B, ~6 W·kg−1) exercise intensity with 0%, 20%, 40%, and 60% simulated BSA burn. 140% simulated burn different from 0% at the indicated time points within exercise intensity (P < 0.05). 260% simulated burn different from 0% at the indicated time points within exercise intensity (P < 0.01). For the moderate-intensity trial, one 40% and three 60% simulated burn values for the 60-min time point are end of exercise values due to these participants ending exercise prematurely (see text for details). For the moderate-intensity trial, at 30 min, the 60% simulated burn trial approached significance (P = 0.0508) relative to the 0% trial.
FIGURE 2—
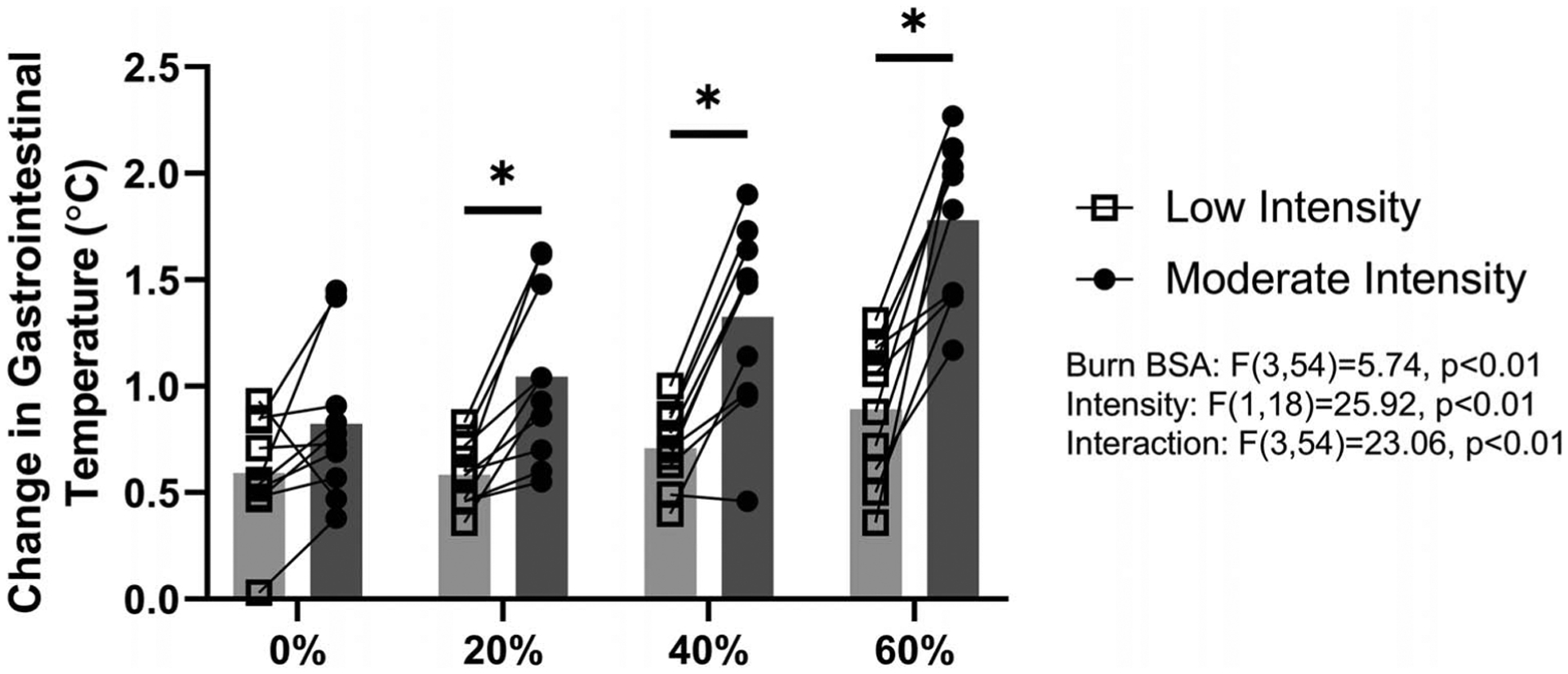
Change in TGI after 60 min of exercise at low (~4 W·kg−1) and moderate (~6 W·kg−1) exercise intensity with 0%, 20%, 40%, or 60% simulated BSA burn. *Different from corresponding moderate-intensity value (20%, P = 0.02; 40%, P < 0.01; 60%, P < 0.01). For the moderate-intensity trial, one 40% and three 60% simulated burn values for the 60-min time point are end of exercise values due to these participants ending exercise prematurely (see text for details).
Figure 3 shows the change in heart rate over time within an exercise intensity across simulated burn areas. For both exercise intensities, there was an interaction of time and simulated burn area (low, P < 0.01; moderate, P < 0.01). For the change in heart rate at the end of exercise, main effects of simulated burn area (P < 0.01) and exercise intensity were identified (P < 0.01); however, an interactive effect was not identified (P = 0.26). Comparing within levels of simulated burn, in all instances, moderate-intensity exercise trials exhibited higher heart rates (P < 0.01).
FIGURE 3—
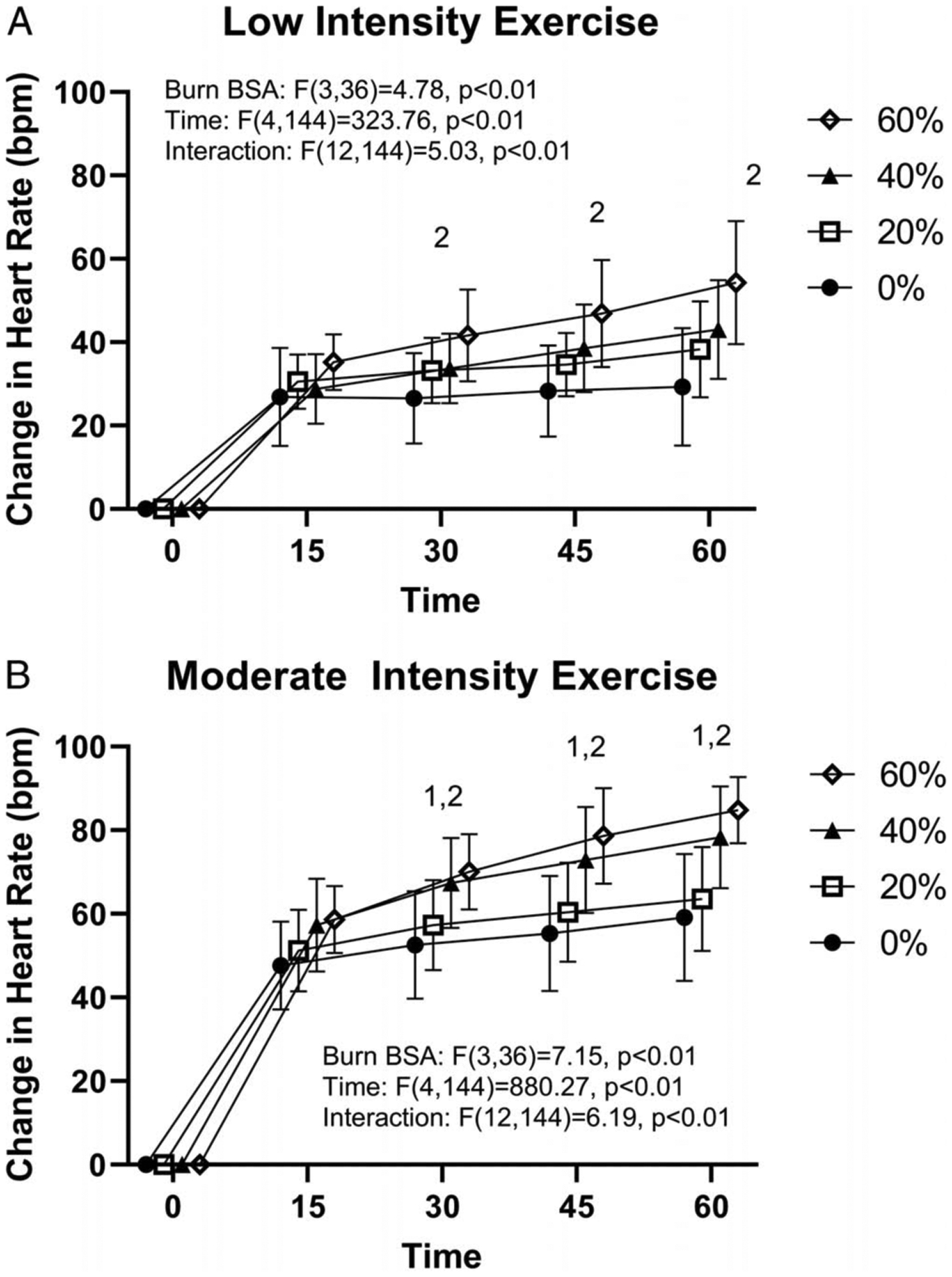
Change in heart rate during 60 min of exercise at low (A, ~4 W·kg−1) and moderate (B, ~6 W·kg−1) exercise intensity with 0%, 20%, 40%, or 60% simulated BSA burn. 140% simulated burn different from 0% at the indicated time points within exercise intensity (P < 0.05). 260% simulated burn different from 0% at the indicated time points within exercise intensity (P < 0.05). For the moderate-intensity trial, one 40% and three 60% simulated burn values for the 60-min time point are end of exercise values due to these participants ending exercise prematurely (see text for details).
DISCUSSION
The purpose of this study was to examine the interaction of exercise intensity and percentage BSA burned using a simulated burn injury model on TGI responses. Our main finding was that exercise intensity modulates the detrimental effect of a simulated burn on internal body temperature responses. During low-intensity exercise in a hot environment, at each time point, the increase in TGI was similar across all levels of simulated burn when compared with the 0% coverage condition (Fig. 1A). However, during moderate-intensity exercise, the 40% and 60% simulated burn trials exhibited greater increases in TGI than the 0% trials at minutes 45 and 60 of exercise (Fig. 1B). These findings indicate that clinical guidelines outlining the potential influence of metabolic heat production on temperature regulation in burn survivors should reflect both the external workload performed and the extent of grafting. Such observations also have important implications for burn survivors to achieve a fully rehabilitated state, given that exercise is a primary mode of rehabilitation.
This study is unique as it is the first to systematically investigate the interactive effect of burn size and exercise intensity on thermoregulation, using a model to simulate thermoeffector dysfunction in burn survivors. Previous investigations identified a 40% BSA burn injury as a functional limitation for appropriate thermoregulation during low-intensity exercise (7–9). However, Austin et al. (14) found similar internal body temperature responses in individuals with greater than 60% BSA burned relative to those with less than 35% BSA burned, despite a more intense exercise protocol. However, they did not account for the effect of morphological differences on internal body temperature across burn survivors and the participant numbers were very low (e.g., n = 3 in both groups) (14). In the present study, wherein we controlled for metabolic heat production across participants, we observed no differences in TGI between simulated burn of BSA up to 60% during low-intensity exercise.
During moderate-intensity exercise, 40% and 60% BSA simulated burns resulted in greater increases in TGI at minutes 45 and 60 compared with the 0% BSA simulated burn trial. Interestingly, because TGI differences were not apparent until minute 45, these data would suggest that survivors with 40%–60% BSA burned could sustain moderate-intensity exercise for up to 30 min in the heat without adverse thermoregulatory consequences. These data support the observations from McEntire et al. (23), who found that burned children (>40% BSA) could sustain submaximal exercise (75% of peak aerobic power) for 20 min without excessive elevations in TGI.
We observed differences in heart rate between 0% and 60% simulated burn during low-intensity exercise after 30 min of exercise, indicating that although impaired heat dissipation may not affect internal body temperature, it may increase cardiovascular strain at this exercise intensity. During moderate-intensity exercise, heart rate responses in the 40% and 60% simulated burn trials generally reflected the TGI responses. When comparing heart rate responses between exercise intensities, moderate-intensity exercise resulted in an expected greater physiological strain (i.e., higher TGI and heart rate) than low-intensity when participants had a simulated burn. However, TGI was similar between the two exercise intensities during 0% simulated burn trials.
The U.S. Department of Defense’s Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DoDI 6130.03, Section 5.21, w) excludes any individual with a burn injury greater than 18% BSA (12). From a thermoregulatory standpoint, our results do not support these guidelines. That is, individuals with simulated burns up to 20% BSA had similar increases in TGI responses relative to the 0% BSA burn trial during moderate-intensity exercise. Moreover, there were no differences in TGI responses regardless of BSA simulated burn at any time point for the low-intensity trial. Based on this observation, thermoregulatory impairments associated with burn injuries should be considered in the context of the workload demands of military activities, inclusive of training (i.e., boot camp).
According to U.S. Army training guidelines, in the present study’s environmental conditions (~28.1°C WBGT) (24), easy work (metabolic rates of 250 W) can be conducted without a duration limit, moderate work (metabolic rates of 425 W) can be conducted with an interval of 50 min work to 10 min for rest, and hard work (metabolic rates of 600 W) can be conducted at an interval of 30 min work to 30 min of rest (25). In the context of our findings (metabolic rate: low-intensity exercise, 362 ± 46 W; moderate-intensity exercise, 531 ± 76 W), it would appear that percent BSA burned has little effect on thermoregulation during relatively light work (e.g., rifle fire in a prone position, walking on a hard surface at 1.56 m·s−1, lift and carry 45-kg artillery shell at 3 min−1) (26). Conversely, individuals with 40% or more BSA burns would be at a greater risk for excessive hyperthermia compared with their “nonburned” counterparts during moderate to hard work activities (e.g., emplacement digging, walking on a hard surface at 2 m·s−1, field assaults) while in the assessed environmental conditions (26). We note that these guidelines consider soldiers who are average sized, heat acclimatized, and wearing battle dress uniform, whereas the assessed participants were not heat acclimatized and were seminude. The latter point is particularly important given our recent findings that the severity of a burn injury on thermoregulatory responses during exercise is minimized if the individual is wearing a battle dress uniform (27). Never-theless, not all military activities are performed in battle dress uniforms, with many heat-related injuries occurring during training, many of which could occur while the individuals are wearing just shorts and a T-shirt.
Outside of the training environment, where activities can be modified based on environmental conditions, the obtained results can also be interpreted based on data from actual field operations. For example, soldiers performing rifle squad operations in Afghanistan exhibit an average metabolic rate between 297 and 544 W (28). Our data would support that individuals with up to 20% BSA burn would be able to perform these activities without risk of excessive hyperthermia (i.e., remain in a compensable heat stress state) when compared with their nonburned counterparts during these activities in a 40°C climate with low humidity.
The obtained findings also have important implications for burn survivors within the civilian sector. For such individuals, exercise is an important mode to achieve full rehabilitation and to curtail the associated morbidity risks associated with a sedentary lifestyle (29). The present data suggest that, regardless of the severity of the burn injury (up to 60% BSA burned), burn survivors can perform 60 min of low-intensity exercise in the heat without the risk of excessive hyperthermia. These data also suggest that burn survivors with less severe burn injuries (i.e., 20% BSA burned) could perform 60 min of moderate-intensity exercise in the heat without a risk of excessive hyperthermia. Finally, these data demonstrate that all burn survivors, with injuries covering up to 60% BSA, could perform moderate-intensity exercise in the heat for up to 30 min without the risk of excessive hyperthermia. By informing the burn survivor communities, and associated clinicians, of these capabilities and limitations, burn survivors should be less apprehensive in performing physical activities that are beneficial for cardiovascular and metabolic health, even in hot environmental conditions.
Limitations.
Due primarily to an IRB-imposed safety limit of body temperature <39.5°C, in 4 of the 80 conducted trials, a participant was unable to complete the entirety of the study protocol during moderate-intensity exercise with 40% BSA simulated burn (n = 1) and 60% BSA simulated burn (n = 3). Such missing data are unfortunate as these individuals’ responses to the exercise heat stress were the most pronounced. To address this limitation, in these four trials, the final TGI and heart rate values during exercise were analyzed as the 60-min data point. We contest this is appropriate given that if participants were allowed to continue, their TGI and heart rate would have, at a minimum, be equivalent to these early termination values. In light of the relatively small sample size (n = 10), it is possible that the observed TGI differences in the moderate-intensity trial are the result of a type I error. Although this sample size was supported by a priori power calculations, we recognize that to fully evaluate the interactive nature of exercise intensity across a spectrum of sizes of BSA burned, studies with larger sample sizes may be necessary. Although metabolic heat production accounts for a large amount of the variability in internal body temperature responses during exercise (15,30,31), environmental conditions can also affect the compensability of an environment (32,33). Cramer et al. (18) demonstrated that a simulated burn, regardless of BSA “injured,” did not affect TGI responses at 25°C environmental temperature. However, at 40°C, both a 40% and a 60% simulated BSA burn exhibited greater TGI responses after 60 min of exercise than the 0% BSA trial. In contrast to that study, the present study considered only one environmental condition, 39.95°C ± 0.31°C and 20.1% ± 1.5% relative humidity, but at two exercise intensities. In 0% BSA simulated burn trials, this environment should have nearly maximized evaporative capacity; however, the introduction of simulated burn reduced evaporative heat capacity in proportion to the “injury.” We expect that a burn injury would modify the relationship between environmental conditions and exercise intensity and create a unique set of circumstances that delineates uncompensable and compensable heat stress in burn survivors. We also expect that clothing and equipment worn during field operations would influence the thermoregulatory responses of burn survivors. In fact, we recently described that the detrimental effect of a burn injury on thermoregulatory responses during exercise in the heat is minimized if the participants were wearing a battle dress uniform (27).
Healthy, noninjured participants, rather than burn survivors, were assessed in this study. The recruitment of burn survivors to systematically address this question would be extremely challenging, primarily given the variability in burn size between patients. We further acknowledge that our model of simulated burn does not entirely reflect the physiological responses of burn survivors to exercise in the heat, particularly as it pertains to whole-body sweat loss and cardiovascular responses. Although the absorbent pads with a vapor-impermeable exterior successfully mimic impaired evaporative heat loss of the covered area, grafted skin in a burn survivor is also deficient in cutaneous vasodilation (5,10,34). Thus, we recognize that the disturbance to cardiovascular homeostasis caused by profound cutaneous vasodilation under the simulated burn potentially exaggerates the reported heart rate results. Although we anticipate these effects are minimal, future studies should specifically evaluate the cardiovascular responses in actual burn survivors to different exercise intensities and different environmental conditions to further delineate the effects of impaired cutaneous vasodilation on such cardiovascular responses.
CONCLUSION
The present investigation evaluated the interactive influence of exercise intensity and simulated burn size on thermoregulatory responses in a hot environment. A larger simulated burn (i.e., 40% + BSA burned) resulted in greater increases in TGI during more intense exercise in hot and dry environmental conditions. Specifically, during low-intensity exercise, TGI responses were similar across all levels of simulated burn when compared with the nonburned condition, suggesting burn survivors with injuries ≥20% of total BSA are capable of safely performing prolonged, low-intensity exercise in a hot environment despite current Department of Defense policy, which excludes burn survivors with injuries >18% BSA from military service. However, during moderate-intensity exercise, 40% and 60% simulated burn BSA trials exhibited higher TGI after 45 min of exercise. These findings support the notion that clinical guidance and military policy for burn survivors should consider the intensity of exercise as well as the extent of burn injury when determining exercise limitations and exclusion criteria. Specifically, low-intensity exercise may negate the need for restrictions on burn survivors participating in activities in hot environments.
Acknowledgments
The authors sincerely appreciate the study participants for their time and effort. They also thank Ollie Jay for his contributions toward the experimental design. This work was supported by an award from the Department of Defense (W81XWH-15-1-0647 to C. G. C.), the NIH (R01GM068865 to C. G. C.), and a Natural Sciences and Engineering Research Council of Canada Postdoctoral Fellowship (to M. N. C.).
Footnotes
The authors have no conflicts of interest to disclose. The results of the present study do not constitute an endorsement by the American College of Sports Medicine. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
REFERENCES
- 1.Klein MB, Goverman J, Hayden DL, et al. Benchmarking outcomes in the critically injured burn patient. Ann Surg. 2014;259(5): 833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holavanahalli RK, Helm PA, Kowalske KJ. Long-term outcomes in patients surviving large burns: the skin. J Burn Care Res. 2010;31(4): 631–9. [DOI] [PubMed] [Google Scholar]
- 3.Davis SL, Shibasaki M, Low DA, et al. Impaired cutaneous vasodilation and sweating in grafted skin during whole-body heating. J Burn Care Res. 2007;28(3):427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conway H. Sweating function of transplanted skin. Surg Gynec Obstet. 1939;69:756–61. [Google Scholar]
- 5.Davis SL, Shibasaki M, Low DA, et al. Sustained impairments in cutaneous vasodilation and sweating in grafted skin following long-term recovery. J Burn Care Res. 2009;30(4):675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGibbon B, Beaumont WV, Strand J, Paletta FX. Thermal regulation in patients after the healing of large deep burns. Plast Reconstr Surg. 1973;52(2):164–70. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Simchon C, Tsur H, Keren G, Epstein Y, Shapiro Y. Heat tolerance in patients with extensive healed burns. Plast Reconstr Surg. 1981;67(4):499–504. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro Y, Epstein Y, Ben-Simchon C, Tsur H. Thermoregulatory responses of patients with extensive healed burns. J Appl Physiol Respir Environ Exerc Physiol. 1982;53(4):1019–22. [DOI] [PubMed] [Google Scholar]
- 9.Roskind JL, Petrofsky J, Lind AR, Paletta FX. Quantitation of thermoregulatory impairment in patients with healed burns. Ann Plast Surg. 1978;1(2):172–6. [DOI] [PubMed] [Google Scholar]
- 10.Crandall CG, Davis SL. Cutaneous vascular and sudomotor responses in human skin grafts. J Appl Physiol. 2010;109(5): 1524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kauvar DS, Wade CE, Baer DG. Burn hazards of the deployed environment in wartime: epidemiology of noncombat burns from ongoing United States military operations. J Am Coll Surg. 2009; 209(4):453–60. [DOI] [PubMed] [Google Scholar]
- 12.United States Department of Defense. Medical Standards for Appointment, Enlistment, Or Induction into the Military Services. 2018. Available from: https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883.
- 13.United States Department of the Army. Standards of Medical Fitness. 2017. Available from: https://armypubs.army.mil/ProductMaps/PubForm/Details.aspx?PUB_ID=1004688.
- 14.Austin KG, Hansbrough JF, Dore C, Noordenbos J, Buono MJ. Thermoregulation in burn patients during exercise. J Burn Care Rehabil. 2003;24(1):9–14. [DOI] [PubMed] [Google Scholar]
- 15.Cramer MN, Jay O. Explained variance in the thermoregulatory responses to exercise: the independent roles of biophysical and fitness/fatness-related factors. J Appl Physiol. 2015;119(9):982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Update: Heat illness, active component, U.S. Armed forces, 2019. MSMR. 2020;27(4):4–9. [PubMed] [Google Scholar]
- 17.Lei TH, Cotter JD, Schlader ZJ, et al. On exercise thermoregulation in females: interaction of endogenous and exogenous ovarian hormones. J Physiol. 2019;597(1):71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cramer MN, Moralez G, Huang MU, Kouda K, Poh PYS, Crandall CG. Exercise thermoregulation with a simulated burn injury: impact of air temperature. Med Sci Sports Exerc. 2020;52(3):712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenefick RW, Cheuvront SN. Hydration for recreational sport and physical activity. Nutr Rev. 2012;70(2 Suppl):S137–42. [DOI] [PubMed] [Google Scholar]
- 20.Cramer MN, Moralez G, Huang MU, Kouda K, Poh PYS, Crandall CG. Exercise core temperature response with a simulated burn injury: effect of body size. Med Sci Sports Exerc. 2020;52(3):705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du Bois D, DuBois EF. Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;XVII(6_2). doi: 10.1001/archinte.1916.00080130010002. [DOI] [Google Scholar]
- 22.Domitrovich JW, Cuddy JS, Ruby BC. Core-temperature sensor ingestion timing and measurement variability. J Athl Train. 2010;45(6):594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McEntire SJ, Herndon DN, Sanford AP, Suman OE. Thermoregulation during exercise in severely burned children. Pediatr Rehabil. 2006;9(1):57–64. [DOI] [PubMed] [Google Scholar]
- 24.Liljegren JC, Carhart RA, Lawday P, Tschopp S, Sharp R. Modeling the wet bulb globe temperature using standard meteorological measurements. J Occup Environ Hyg. 2008;5(10):645–55. [DOI] [PubMed] [Google Scholar]
- 25.United States Department of the Army. Technical Bulletin Medical 507: Heat Stress Control and Heat Casualty Management. 2003. Available from: https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/tbmed507.pdf.
- 26.Sawka M, Pandolf KB. Physical exercise in hot climates: physiology, performance, and biomedical issues. In: Wenger CB, editor. Medical Aspects of Harsh Environments. Washington (DC): Office of The Surgeon General at TMM Publications; 2002. [Google Scholar]
- 27.Fischer M, Cramer MN, Huang M, et al. Burn injury does not exacerbate heat strain during exercise while wearing body armor. Med Sci Sports Exerc. 2020;52(10):2235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welles AP, Buller MJ, Margolis L, Economos D, Hoyt RW, Richter MW. Thermal-work strain during marine rifle squad operations in Afghanistan. Mil Med. 2013;178(10):1141–8. [DOI] [PubMed] [Google Scholar]
- 29.Ganio MS, Pearson J, Schlader ZJ, et al. Aerobic fitness is disproportionately low in adult burn survivors years after injury. J Burn Care Res. 2015;36(4):513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cramer MN, Jay O. Selecting the correct exercise intensity for unbiased comparisons of thermoregulatory responses between groups of different mass and surface area. J Appl Physiol. 2014;116(9):1123–32. [DOI] [PubMed] [Google Scholar]
- 31.Jay O, Cramer MN. A new approach for comparing thermoregulatory responses of subjects with different body sizes. Temperature (Austin). 2015;2(1):42–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung SS, McLellan TM, Tenaglia S. The thermophysiology of uncompensable heat stress. Physiological manipulations and individual characteristics. Sports Med. 2000;29(5):329–59. [DOI] [PubMed] [Google Scholar]
- 33.Lind AR. Human Tolerance to Hot Climates. In: Terjung R, editor. Comprehensive Physiology. 2011. [Google Scholar]
- 34.Pearson J, Ganio MS, Schlader ZJ, et al. Post junctional sudomotor and cutaneous vascular responses in noninjured skin following heat acclimation in burn survivors. J Burn Care Res. 2017;38(1): e284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


