Abstract
Background:
The transfer of passive immunity with convalescent plasma is a promising strategy for treatment and prevention of COVID-19, but donors with a history of nonsevere disease are serologically heterogenous. The relationship between SARS-Cov-2 antigen-binding activity and neutralization activity in this population of donors has not been defined.
Study Design and Methods:
Convalescent plasma units from 47 individuals with a history of nonsevere COVID-19 were assessed for antigen-binding activity of using three clinical diagnostic serology assays (Beckman, DiaSorin, and Roche) with different SARS-CoV-2 targets. These results were compared with functional neutralization activity using a fluorescent reporter strain of SARS-CoV-2 in a microwell assay.
Results:
Positive correlations of varying strength (Spearman r = 0.37–0.52) between antigen binding and viral neutralization were identified. Donors age 48 to 75 years had the highest neutralization activity. Units in the highest tertile of binding activity for each assay were enriched (75%–82%) for those with the highest levels of neutralization.
Conclusion:
The strength of the relationship between antigen-binding activity and neutralization varies depending on the clinical assay used. Units in the highest tertile of binding activity for each assay are predominantly comprised of those with the greatest neutralization activity.
The transfer of passive immunity with convalescent plasma is a promising strategy for treatment and prevention of COVID-19.1 Challenges include the heterogeneity of SARS-CoV-2 antibody responses2 and the variety of assay techniques to measure antigen-binding activities.3 Although individuals who experience more severe COVID-19 disease tend to show greater SARS-CoV-2 antibody titers,4 the majority of individuals diagnosed with COVID-19 (and potential convalescent plasma donors) have nonsevere disease.5 It is therefore especially important to assess the variability in antigen-binding activity of convalescent plasma from donors with less severe disease and to determine how readily available serologic measurements correlate with functional neutralization activity in this population.
Viral neutralization titers measure the ability of antibodies to prevent viral infection of a eukaryotic cell line in vitro. Both live and pseudotyped virus assays exist for SARS-CoV-26 as well as surrogate assays that measure blockage of the Spike-ACE2 protein interaction.8 Pseudotyped and recombinant protein assays require less restrictive biosafety procedures and facilities; however, results may differ from live viral assays since pseudotyped viruses typically express only a single viral entry protein. Neutralizing antibodies that impact other viral targets and processes may therefore not be detected by pseudotyped viral assays.9 Readouts of neutralization assays also vary, but standard viral plaque reduction readouts are labor-intensive. Fluorescence-based assays use an engineered viral particle with a fluorescent protein gene expressed upon viral infection of eukaryotic cells.10 One recently developed fluorescence microwell assay correlated well with plaque reduction but was faster and could be readily automated.7 In contrast, live SARS-CoV-2 neutralization assays remain available only in specialized laboratories and are not widely used to screen convalescent plasma due to complexity of implementation, high cost, low throughput, and biosafety concerns.
In addition, clinical assays that measure the binding of antibodies against SARS-CoV-2 to specific viral antigens have been rapidly developed. These assays were primarily designed to diagnose past COVID-19 exposure and none of the currently available assays measure the ability of antibodies to neutralize the virus or prevent viral entry into cells. However, many of these clinical assays can be run on high-throughput, automated instruments. As a result, these assays can be performed at low cost, low safety risk to laboratory staff, and high throughput and in almost any clinical laboratory.
Initial reports have suggested some correlation between binding antibody activity and viral neutralization titers.11 Data from other coronaviruses suggests that plasma with detectable antibodies at a 1-in-160 or 1-in-320 dilution using a clinical binding assay should have high neutralizing titers as well.11 However, these assumptions have not been rigorously tested and the dilution of samples requires specific assay validation and an extra step in the testing process. Even without dilution, many clinical serology assays provide a quantitative serologic score of antibody reactivity.
Despite these uncertainties, there is currently high demand for COVID-19 convalescent plasma for use in clinical trials and under the recent FDA emergency use authorization. Therefore, we sought to determine in a real-world cohort of donors with a history of nonsevere COVID-19, the relationship between antigen-binding activity measured by several FDA-approved clinical diagnostic assays under emergency authorization, and neutralization activity against live SARS-CoV-2 recombinantly engineered to express fluorescent mNeonGreen protein in infected Vero E6 cells.7,10
1 |. MATERIALS AND METHODS
1.1 |. Convalescent plasma donor recruitment
The NorthShore University HealthSystem COVID-19 convalescent plasma collection program was established in April 2020. This program and associated human subject research, performed in in accordance with the ethical standards of the Helsinki Declaration, were approved by the NorthShore University HealthSystem Institutional Review Board. All potential donors provided written consent for the study and provided information about their COVID-19 disease history and demographics. Disease history was reported in free-text format and the absence of a reported symptom was assumed to indicate that the symptom was not present. For the donors included in this study, reported symptoms included fatigue (49%), myalgia (47%), cough (47%), anosmia (43%), headache (40%), and other symptoms 20% each (Table S1). None of the donors had a history of hospitalization for COVID-19.
1.2 |. Sample collection, storage, and transport
Samples were collected at the time of donation using serum separator tubes (BD, Franklin Lakes, NJ), centrifuged, aliquoted, and frozen at −80°C. For each sample included in this study, an aliquot was shipped to the University of Minnesota for viral neutralization titer measurement on dry ice.
1.3 |. Clinical serology testing
An aliquot was thawed and tested at NorthShore using the Elecsys anti-SARS-CoV-2 (Roche Diagnostics), Access SARS-CoV-2 IgG (Beckman Coulter), and LIAISON SARS-CoV-2 S1/S2 IgG (DiaSorin). Both the quantitative cutoff index and the qualitative results were recorded. Samples were divided into tertiles based on the Roche assay results, and then 47 were randomly selected to equally sample each tertile.
1.4 |. SARS-CoV-2 S1 RBD immunoglobulin enzyme-linked immunosorbent assay
The anti-SARS-CoV-2 S1 RBD total immunoglobulin assay employed a standard indirect enzyme-linked immunosorbent assay (ELISA), described in detail elsewhere, using a secondary antibody recognizing all human immunoglobulin isotypes (goat anti-human IgG H+L-HRP, Invitrogen/ThermoFisher).12,13 Threefold serum dilutions were tested: 1 in 50 to more than 1 in 12 150. ELISA titer was reported as the dilution at which absorbance of each sample tested exceed the 50% maximal absorbance signal for a positive control sample on the same ELISA plate.
1.5 |. Live SARS-CoV-2 virus neutralization assay
Vero E6 cells (2.5 × 104) were seeded in each well of a 96-well black/clear flat bottom TC-treated plate (Falcon) and incubated in DMEM overnight at 37°C with 5% CO2 before infection. Plasma samples were twofold serially diluted (from 1 in 20 to 1 in 5120) in DMEM and incubated with mNeonGreen SARS-CoV-2 at 37°C for 1 hour. Medium was removed from cells and the virus-plasma mixture was added to achieve a final multiplicity of infection of 0.1 plaque-forming units per cell. The cells were incubated at 37°C with the virus-plasma mixture for 24 to 26 hours. After incubation, cells were fixed in 4% paraformaldehyde at 4°C for 30 minutes. The paraformaldehyde-virus-plasma mixture was removed, cells were washed once with PBS, and 50 μL of PBS was added to each well. The fluorescence signal was determined by reading the plates on a hybrid multimode reader (Synergy H1, BioTek), using excitation/emission wavelengths of 488/517 nm. Percent maximal infection was determined for each dilution as the ratio of the fluorescent signal to the maximal signal for non-serum-treated controls in the same plate. A nonlinear regression method was used to determine the dilution that neutralized 50% of mNeonGreen fluorescence (NT50) by using Prism 8 (GraphPad). If a plasma titration failed to generate 50% inhibition within the range of concentrations tested, a titer value of one-half (10) of the highest serum concentration tested was ascribed to it. Each sample was tested in duplicate.
1.6 |. Statistical analysis
Assay results were compared using linear regression and Spearman correlation. Results from each assay were broken into equal tertiles for comparison; tertiles were compared using the Kruskal-Wallis test. Comparisons of NT50 between two groups used the Mann-Whitney U-test.
2 |. RESULTS
2.1 |. Fluorescent SARS-CoV-2 neutralization
We tested 47 units of convalescent plasma units from individuals with a history of nonsevere COVID-19 for viral neutralization activity. There was a broad range of neutralization against live SARS-CoV-2, with 6 (13%) units demonstrating high NT50 values of more than 500, and six (13%) with undetectable NT50 values of less than 20 (Figure S1). Neutralization assays results yielded a robust calculation of NT50 with R2 values of more than 0.9 for curve fitting (Figure 1A) in the majority of samples with detectable neutralization activity.
FIGURE 1.
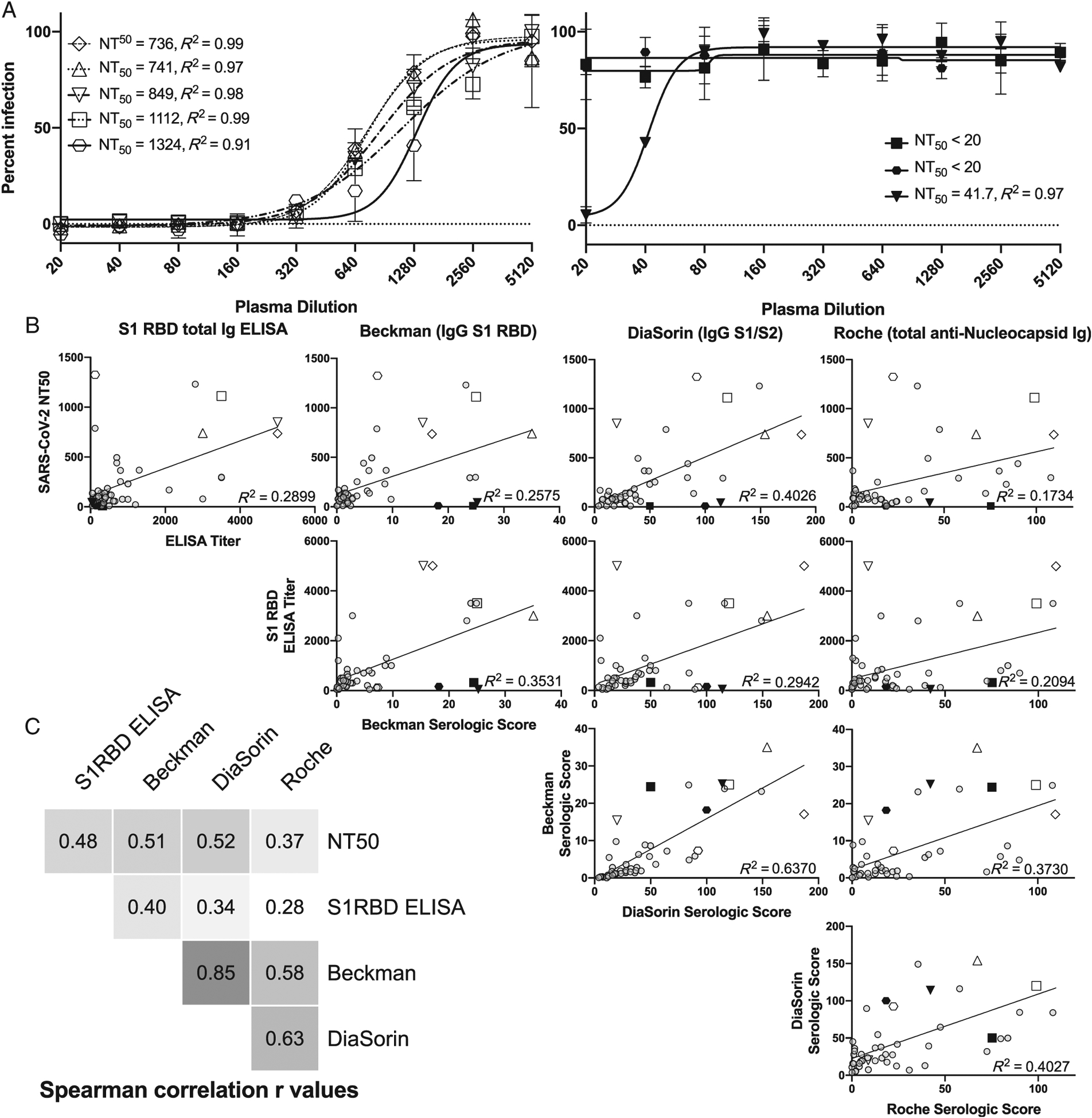
Comparison of nonsevere COVID-19 convalescent plasma SARS-CoV-2 neutralization activity and antigen-binding assays. A, Dilution curves with example of nonlinear curve fitting showing R2 and NT50 calculation for five highly neutralizing units (open symbols) and 3 units with low or undetectable neutralization activity (black, filled symbols). B, Correlation plots comparing all assays tested, with linear curve fitting R2 value shown. Several samples with either high or low neutralization activity, as represented by specific symbols in Figure 1A, are also represented by the same symbols in Figure 1B. C, Spearmanʼs correlation r values for pairwise comparisons of each assay tested
2.2 |. Binding and neutralization assay comparison
We compared the neutralization activity in these samples to binding activity as measured by an in-house ELISA for S1 RBD total immunoglobulin as well as three clinical diagnostic assays that use different viral antigenic targets: Beckman (IgG anti-S1 RBD), DiaSorin (IgG anti-S1/S2 protein), and Roche (total anti-Nucleocapsid immunoglobulin). The geometric mean, median, and quartiles (first, second, third, and fourth) for each assay were as follows: Beckman (2.75, 2.81, 1.2, 2.81, 8.75, 35.06), DiaSorin (31.41, 31.9, 16.85, 31.9, 59.6, 187), and Roche (10.84, 16.11, 4.38, 16.11, 41.70, 109.5). There was a universally positive relationship between NT50 and all four binding assays tested, with Spearman correlation r ranging from 0.37 to 0.52 and R2 values of 0.17 to 0.40 reflecting a weak linear relationship (Figure 1B,C). The strongest positive correlation between assays was the Beckman and DiaSorin assays (Spearman r = 0.85), which measure different aspects of anti-Spike protein-binding activity. The Roche total antinucleocapsid assay had the lowest overall correlation with NT50, but stronger positive correlation with the Beckman and DiaSorin assays (Figure 1C).
We identified several individual donors with discordant binding and neutralization activity, including some with high neutralization and low binding activities in individual assays (Figure 1A,B, open hexagon and open inverted triangle). We also identified individual units characterized by low neutralization despite relatively high binding activity in individual assays (Figure 1A,B, black square and black inverted triangle).
2.3 |. Tertile analysis to enrich for highly neutralizing convalescent plasma units
Because the optimal NT50 that corresponds to functional immune protection is not known, we assigned each convalescent plasma unit to a neutralization tertile and sought to determine how different donor and binding characteristics enrich for units found in the top two tertiles of neutralization activity. These included units with NT50 values of either 1 in 78 to 180 or more than 1 in 180. There was a significant difference in NT50 depending on donor age. Donors in the oldest tertile (age 48–75 years) had the highest enrichment for the top two neutralization tertiles (P = .04). However, no other donor demographic including sex, fever, symptom duration, or reported COVID-19 symptoms correlated with NT50 (Figure 2A and S2). We observed no association between neutralization or binding and the time from either symptom onset or symptom end to sample collection (Figure S2). Finally, there was no statistically significant correlation between the number of symptoms reported and NT50 (Pearson correlation = 0.10, P = .48).
FIGURE 2.

Enrichment for highly neutralizing nonsevere COVID-19 convalescent plasma by donor characteristics and serologic tertile. A, Dot plots demonstrating NT50 values for all units tested as categorized by key donor characteristics. Dotted horizontal lines represent transition points between neutralization tertiles. B, Comparison of NT50 values between tertiles of binding activity for each serologic assay tested. C, Heatmaps indicating the percentage of each binding tertile that is made up of units from each neutralization tertile. Significance was assessed by Mann-Whitney U test for comparisons of two donor categories. Kruskal-Wallis test was used to compare groups of three categories
Most highly neutralizing antibodies were in the top tertile of binding activity for each assay. A total of 69% of the units in the top tertile of binding activity for the S1 RBD ELISA assay were in the highest tertile of neutralization activity and 13% in the middle neutralization tertile (82% were highly neutralizing;, see Figure 2B,C). Clinical serologic assays were similar in this regard, 75% to 82% of the units in the highest binding tertile were highly neutralizing. Only the S1 RBD ELISA contained no units with undetectable neutralization activity in the highest binding tertile (Figure 2B), whereas each clinical assay contained at least one nonneutralizing sample in the highest binding tertile.
2.4 |. Using multiple assays to predict neutralization titer
Results from the three emergency use authorization-approved assays were combined by summing the assay-specific tertile rank for each sample (range 3–9; with 9 indicating the highest reactivity tertiles across all three assays). For samples with a score of 7 or higher, 15.8% were in the lowest NT50 tertile, 10.5% were in the middle NT50 tertile, and 63.1% were in the highest NT50 tertile.
3 |. DISCUSSION
These findings illustrate the difficulty in deriving information about functional antibody responses using anti-SARS-CoV-2–binding assays. Although a correlation exists, the relatively high discordance rate in donors with a history of nonsevere COVID-19 may adversely affect the interpretation of convalescent plasma clinical trial data. Furthermore, the discordance observed implies that SARS-CoV-2 patients develop a broad antibody repertoire against multiple proteins and epitopes, each of which may only partially contribute to the overall neutralization of the virus.
Several larger studies that included hospitalized patients with more severe disease have shown higher COVID-19 antibody-binding reactivity in individuals who had been hospitalized or received treatment for COVID-19. Interestingly, COVID-19 antibody levels, as measured by binding assays, are high in hospitalized patients during their hospitalization, and formation of these antibodies is not related to a decline in viral load.2 Future studies are needed to understand the significance of these observations. It is possible that high-titer-binding antibodies with low neutralization potential lead to antibody-dependent enhancement, suggesting that selecting convalescent plasma units based on high binding activity alone may cause harm.14 Similarly, future studies are needed to determine if developing high-titer-binding antibodies with low neutralization potential is a poor prognostic sign.
It is possible that combining binding titer results from multiple assays may produce a better prediction of neutralization titer than any single assay alone. In this study of limited sample size and three different emergency use authorization-approved assays, there did not appear to be an increased predictive power by combining results. However, additional studies are warranted, since any inconsistency in convalescent plasma potency could obscure a signal of potential benefit in the setting of a clinical trial.
In this study, increasing age correlated with NT50, consistent with other reports,15 perhaps due to relatively more severe disease processes and greater viral exposure in this age group, although the same association was not seen with symptom duration or fever. However, given the heterogeneity of COVID-19 disease symptoms, it is unclear how well symptom history reflects the degree of immunologic stimulation.
Although the neutralizing antibody dose needed for clinical benefit is unknown, units in our top two neutralization tertiles had activity consistent with current FDA recommendations (NT50 > 160 or > 80). Other factors such as the number of units transfused, the viral load at the time of transfusion, the degree of irreversible end-organ damage at the time of transfusion, and the extra-cellular fluid volume of the recipient may be critical factors in determining whether a unit with a certain concentration of neutralizing antibody shows clinical benefit.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Christine Ronayne for administrative and logistical laboratory support and members of the UMN BSL3 program.
Funding information
This work was supported by UMN Department of Medicine funding (T.D.B.), NIH T32AI055433 (J.M.T.), T32007741 (W.E.M.), 1R01AI153602 (V.D.M.), and by private donations to the NorthShore Foundation for the NorthShore COVID-19 convalescent plasma program.
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Investig. 2020;130:2757–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020; 581:465–469. [DOI] [PubMed] [Google Scholar]
- 3.Krammer F, Simon V. Serology assays to manage COVID-19. Science. 2020;368:1060–1061. [DOI] [PubMed] [Google Scholar]
- 4.Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. [DOI] [PubMed] [Google Scholar]
- 5.Tenforde MW, Billig Rose E, Lindsell CJ, et al. Characteristics of adult outpatients and inpatients with COVID-19 – 11 Academic Medical Centers, United States, March-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(26):841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford KHD, Eguia R, Dingens AS, et al. Protocol and reagents for pseudotyping lentiviral particles with SARS-CoV-2 spike protein for neutralization assays. Viruses. 2020;12(5):513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muruato AE, Fontes-Garfias CR, Ren P, et al. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nature Communications. 2020;11 (1). 10.1038/s41467-020-17892-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein-protein interaction. Nat Biotechnol. 2020; 38:1073–1078. [DOI] [PubMed] [Google Scholar]
- 9.Du P Comparisons of VLP-based ELISA, neutralization assays with native HPV, and neutralization assays with PsV in detecting HPV antibody responses in HIV-infected women. J AIDS Clin Res. 2015;6(3):433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie X, Muruato A, Lokugamage KG, et al. An infectious cDNA clone of SARS-CoV-2. Cell Host Microbe. 2020;27: 841–848.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salazar E, Kuchipudi SV, Christensen PA, et al. Convalescent plasma anti-SARS-CoV-2 spike protein ectodomain and receptor binding domain IgG correlate with virus neutralization. J Clin Investig. 2020; 10.1172/jci141206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas SN, Altawallbeh G, Zaun C, et al. Initial determination of COVID-19 seroprevalence among outpatients and healthcare workers in Minnesota using a novel SARS-CoV-2 total antibody ELISA. SSRN Electron J. 2020; [Preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stadlbauer D, Amanat F, Chromikova V, et al. A detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol. 2020;57:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coish JM, MacNeil AJ. Out of the frying pan and into the fire? Due diligence warranted for ADE in COVID-19. Microbes Infect. 2020; 10.1016/j.micinf.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Guo X, Xin Q, et al. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis. 2020; 10.1093/cid/ciaa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


