Abstract
Amblyopia is the most common cause of preventable visual impairment in children and occurs as a result of unilateral or bilateral impairment in best-corrected visual acuity. Early diagnosis and proper treatment are crucial to prevent poor visual outcomes in adulthood. Advances in technology have provided more objective diagnostic tools, which can now be used by a wide range of healthcare providers. Here, we highlight tools that have gained popularity in the past two decades and compare clinically relevant parameters to guide primary care providers seeking to incorporate instrumental vision screening in pediatric patient care.
Keywords: Amblyopia, Amblyopia Risk Factors, Vision Screening, Instrumental Vision Screening, Preventive Ophthalmology
Introduction
Amblyopia occurs when a decrease in visual stimulation results in suboptimal development of the visual pathways in the brain. Studies have shown that the prevalence of amblyopia is between 2-6% in the general pediatric population [1-3] and up to 20% in certain populations at risk of developing this condition [4,5]. Risk factors for developing amblyopia include refractive errors (myopia, astigmatism, hyperopia), anisometropia (unequal refractive error leading to better vision in one eye compared to the other), strabismus (crossed eyes), and media opacities. Screening ensures early identification of children who are at risk so that they may be treated while there is significant plasticity in the developing visual pathways – typically until age 7 [6]. Although traditional screening tools such as letter and symbol charts are available, they can be time-consuming, erroneous, and challenging in younger children or those with disabilities.
The advent of instrumental vision screening has provided primary and eye care providers with more objective tools to detect amblyopia risk factors, especially in preverbal children as young as 6 months [7]. These instruments are also useful for pediatric ophthalmologists in screening children with disabilities, for which the standard cycloplegic retinoscopy examination might be difficult. In this article, we highlight the most common vision screening instruments in 2020 and compare statistical measures of utility and clinically relevant parameters to guide providers seeking to incorporate these instruments into their practices.
Methods
Amblyopia risk factors (ARFs) were obtained from the guidelines drafted by the American Association for Pediatric Ophthalmology and Strabismus (AAPOS) and the American Academy of Ophthalmology (AAO) in 2003 and 2013 [7,8]. We subsequently conducted a web search for “vision screening instruments” and included portable pediatric instruments that screened for amblyopia risk factors (ARFs). Once the instruments were selected, we obtained cost, conditions screened, as well as several clinically useful parameters for each instrument. The cost of equipment was obtained from online vendors (Active Forever, Alibaba.com, CME Corp, Jaken Medical, Medex Supply, Medical Device Depot, Serfinity Medical, and Tiger Medical) and/or company representatives. The clinically useful parameters we obtained include the presence of an EMR interface, battery life (after being fully charged), the transmission of protected health information (PHI), remote analysis of data obtained from vision screening, and weight of the instrument. We excluded instruments without publications testing their utility in screening for amblyopia risk factors in clinical settings. Lastly, we conducted searches on MEDLINE and OVID to extract literature published on each instrument and report sensitivities (SN), specificities (SP), positive predictive values (PPVs), and negative predictive values (NPVs) of each instrument (ranges) based on different referral criteria.
Results
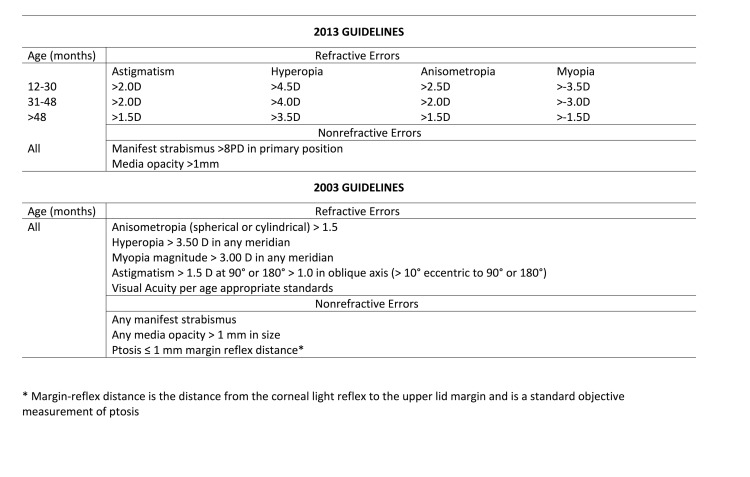
The risk factors for the development of amblyopia as outlined by AAPOS in the years 2003 and 2013 are reported in Figure 1 [7,8]. After excluding instruments without published testing or utility in clinical settings, seven portable vision-screening instruments were left. These include the Spot Vision Screener (Welch Allyn, Skaneateles Falls, NY), 2WIN (Adaptica, Padova, Italy), the S12C/R Mobile Vision Screener autorefractor (Plusoptix, Nuremberg, Germany), the iScreen 3000 photo screener (iScreen Vision Inc, Cordova, TN), the OPTEC 5500 vision screener (Stereo Optical Co., Inc., Chicago, IL), the GoCheck Kids smartphone photo screening application (Goquity Mobile Health, Scottsdale, AZ) on the iPhone (Apple Inc, Cupertino, CA), and the Pediatric Vision Scanner or “blinq” (Rebion, Boston, MA).
Figure 1.
American Association of Pediatric Ophthalmology and Strabismus (AAPOS) Amblyopia Risk Factors (ARFs) Guidelines.
The cost of each instrument, conditions screened, and clinically relevant information is reported in Table 1. Although all the instruments screen for refractive errors and strabismus, only the Plusoptix and iScreen instruments detect cataracts. The Optec instrument measures visual acuity (VA) and is suitable for children that can cooperate with VA testing. The Goquity and Plusoptix (S12 C) instruments are currently the only members of this group that can interface with the electrical medical record (EMR), although some other companies are working on interfaces in subsequent models. All but the iScreen and Goquity instruments automatically analyze tests. With the iScreen and Goquity, trained personnel conduct remote analyses of tests.
Table 1. Comparison of seven portable vision screening instruments outlining the cost, amblyopia risk factor screened, and clinically useful characteristics.
| Company | Welch Allyn | Adaptica | Plusoptix | iScreen | Optec | Goquity | Rebion | |
| Instrument | Spot Vision Screener | 2WIN | S12C/R | 3000 | 5500P | GoCheck Kids# | Blinq | |
| Place of Manufacture | Skaneateles Falls, NY, USA | Padova, Italy | Nuremberg, Germany | Cordova, TN, USA | Chicago, IL, USA | Scottsdale, AZ, USA | Boston, MA, USA | |
| Cost (US$) | 6750-8436 | 5000-6850 | 5495-8898 | 5000 | 3545-4716 | 169* | 7495-8995 | |
| Amblyopia Risk Factors (ARFs) | Refractive Errors | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Strabismus | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Cataracts (media opacity) | No | No | Yes | Yes | No | No | No | |
| EMR friendly interface§ | No | No | Yes | No | No | Yes | No~ | |
| Battery Life (minutes)§ | 240 | 240 | 180/variable** | 480 | - | 360-480 | 360 | |
| PHI Transmission§ | No | Yes | No | Yes | No | Yes | Yes | |
| Remote analysis§ | No | No | No | Yes | No | Yes | No | |
| Weight (pounds)§ | 2.55 | 1.88 | 1.76-2.20 | 4.35 | 15.50 | 0.30-0.46 | 4.00 |
# Currently only on the iPhone, * only monthly finance available, § Clinically useful characteristics, ~ EMR (electronic medical record) interface is currently being developed, ** Based on the rechargeable AA battery used.
Spot Vision Screener (Welch Allyn, Skaneateles Falls, NY), 2WIN (Adaptica, Padova, Italy), the S12C/R Mobile Vision Screener autorefractor (Plusoptix, Nuremberg, Germany), the iScreen 3000 photo screener (iScreen Vision Inc, Cordova, TN), the OPTEC 5500 vision screener (Stereo Optical Co., Inc., Chicago, IL), the GoCheck Kids smartphone photo screening application (Goquity Mobile Health, Scottsdale, AZ) on the iPhone (Apple Inc, Cupertino, CA), and the Pediatric Vision Scanner or “blinq” (Rebion, Boston, MA).
With regards to referral criteria, the Spot Vision Screener has unique referral criteria that are updated as newer models of the instrument are released, with the option for the user to enter a different set of criteria (eg, AAPOS 2013), if desired. Similarly, in addition to the option of entering user-specific criteria, Plusoptix instruments have five different referral criteria. These criteria were implemented based on studies [9-16] and are different combinations of SN-SP values, allowing the provider to choose the desired sensitivity or specificity. Several instruments have adopted the 2013 AAPOS criteria including the Adaptica with a slight revision to children > 48mo, as well as the iScreen and Goquity instruments. These are guidelines stating limits of hyperopia, myopia, astigmatism, anisometropia, strabismus, or media opacity above which the patients are referred for a criterion standard exam since these patients are at risk for developing amblyopia (Figure 1). The Optec and Rebion instruments are slightly different from the rest. Optec 5500P differs from other instruments as its measurements of visual acuity and phoria require output from the child while the others objectively assess refraction, alignment, fixation, and media clarity based on a child’s ability to fixate on a target. The Rebion instrument is designed to detect amblyopia and strabismus through disrupted bi-foveation, and signals to refer if abnormalities are found. In Table 2, we include the manufacturer and AAPOS referral criteria. Unless otherwise stated, the studies compare instrument referral criteria to criterion standard confirmatory examinations by ophthalmologists.
Table 2. Statistical Measures of Vision screening instruments stratified by referral criteria.
| Company | Instrument | Referral Criteria | Sensitivity, % (Median) | Specificity, % (Median) | PPV, % (Median) | NPV, % Median |
| Welch Allyn | Spot Vision Screener | Manufacturer (v. 1.03) [22] | 80 | 74 | 88 | 61 |
| Manufacturer (v.1.151) [19] | 88.1 | 71.9 | 79.3 | 83.1 | ||
| Manufacturer (v.2.0.16) [19] | 87.7 | 75.9 | 81.7 | 83.4 | ||
| 2003 AAPOS [23] | 77 | 87 | ||||
| 2013 AAPOS [19,20,22,24-26] | 60-92.6 (87) | 70.4-93 (84.5) | 58.1-86 (77.1) | 75-98.9 (89.3) | ||
| Adaptica | 2WIN | Manufacturer [27] | 71 | 67 | ||
| 2003 AAPOS* [28,29] | 68-91 (79.5) | 68-84 (76) | 84 | |||
| 2013 AAPOS [30] | 67.4 | 83.7 | 87.9 | 59.4 | ||
| Plusoptix | S12C/R | Manufacturer ROC [27] | 85 | 73 | ||
| Manufacturer ROC3 [31] | 86 | 84 | ||||
| Manufacturer ROC5 [32] | 69-83 (76) | |||||
| 2013 AAPOS [25,33,34] | 64-100 (90.2) | 61-93 (88) | 65-76 (65) | 87-100 (98.5) | ||
| iScreen | 3000 | Manufacturer [35] | 77.2 | 94.1 | ||
| 2003 AAPOS [23,36-38] | 66.2-90.7 (78) | 42.9-92 (81.8) | 81.8 | 75.5 | ||
| Optec | 5500 | 2013 AAPOS [39] | 77.4-81 (79.2) | 87-100 (93.5) | 91.9-100 (96.0) | 50-71.4 (60.7) |
| Goquity | Gocheck kids | 2003 AAPOS [40] | 81 | 91 | 92 | |
| 2013 AAPOS [41-45] | 65-90.5 (81) | 67.2-85 (68.1) | 50-76 (56.9) | 80-94 (88.5) | ||
| Rebion | blinq. (pediatric vision scanner) | Manufacturer [25,28,46-50] | 41-98 (96.5) | 75-96 (87) | 38-82 (47) | 78-100 (89) |
| 2003 AAPOS [28] | 75 | 68 | 81 |
* slightly modified version as outlined in Arnold, 2020 [28].
Discussion
Portable vision screening instruments are gaining popularity in the United States and the world, especially in developing countries where access to sophisticated and expensive vision screening instruments is limited [17,18]. The portability, affordability, and user-friendly nature of these instruments enable primary care providers and trained personnel to conduct vision screening, effectively expanding access to eye care. In May 2020, at least 6,500 US pediatricians had incorporated the new GoCheck Kids app into their practice [17]. Owing to decades of prospective studies showing the efficacy of instruments like the Spot Vision Screener, Plusoptix, 2WIN, and iScreen (Table 2), these instruments have since been adopted as part of routine eye screening by many primary care practices. These instruments could significantly expand access to vision screening on a global scale, providing frontline providers with an objective way of identifying children that need to be triaged to obtain a criterion standard ophthalmologic exam and amblyopia treatment. Thus, primary care providers and health administrators should be equipped with information to ensure that they select instruments that best serve their patient populations.
Sensitivity and specificity are traditionally used to determine the utility of a screening test but in pediatric vision screening, these values depend on a predetermined set of referral criteria. Referral criteria are thresholds beyond which an instrument recommends that a child be triaged for a criterion standard uniform exam by a specialist or treatment. Instrument manufacturers can recommend referral criteria that optimize SN and SP values either as detailed in the user manual or as a pre-programmed mode on the device. When available, receiver operating characteristic (ROC) curve analyses are also helpful as the area under those curves (AUC) provides a summary of the general performance of the instrument and allows for comparison of different referral criteria.
AAPOS and the American Academy of Ophthalmology (AAO) established guidelines in 2003 [7], which were later revised in 2013 [8] (Figure 1) to present a standard for comparing screening instruments, and to recognize that an instrument may detect amblyopia directly, instead of relying on ARFs. Despite the presence of guidelines, AAPOS recommends that with instrumental screening, providers can rely on the manufacturer-determined criteria if those criteria yield a more accurate test result for the instrument of interest. These manufacturer-specific criteria are available either on their respective websites or in the instrument manuals. A 2014 study comparing SN and SP values of different versions (v.1.1.51 and 2.0.16) of the Spot Vision Screener, each with unique manufacturer-recommended criteria reported higher values for sensitivity and specificity for the manufacturer’s criteria compared to the 2013 AAPOS criteria [19]. The manufacturer’s criteria had less stringent cutoffs for anisometropia, myopia, and hyperopia and also screened children from 6 to 12 months (AAPOS recommends screening from 12 months due to limited evidence supporting the benefit of screening before that age [8]).
SN and SP values depend on referral criteria as well as the age group being studied. While testing the Spot Vision Screener for ARFs using the 2013 AAPOS referral criteria, Forcina et al. found that children aged 6 to 11 months had a sensitivity of 100% (95% CI; 29.2-100) compared to those aged 12 to 23 months with a sensitivity of 82.4% (95% CI; 56.6-96.2) [20]. On the other hand, PPV was highest in children between 24 and 35 months at 64.3 (95% CI; 50.4-76.6) and lowest in the 6 to 11-month age group at 30 (95% CI; 6.7-65.3). A test with a higher PPV in conjunction with high sensitivity is preferred as it can identify children at risk of developing amblyopia while reducing over-referrals. For pre-school age children, a test with high specificity may reduce over-referrals, and also lead to finding those children before amblyopia is entrenched [8]. Thus, providers need to be aware of the ramifications of differences in SN, PPV, and SP in vision screening instruments for children of different ages.
Interestingly, we obtained a wide range of SN and SP values for instruments with several studies published. This could be due to the presence of user-specific differences in screening, highlighting the need for understanding the operating principles of the selected instrument as well as the proper training of screening personnel. Each manufacturer provides a unique set of instructions that are available either online or included upon purchase of the instrument. Sometimes, provisions are made for representatives to provide guidance and/or troubleshoot issues that may arise. Providers should make use of these resources when needed to achieve suitable SN and SPs values in their respective clinics.
These findings indicate that there are a variety of vision screening instruments that are effective for screening children with amblyopia. Most are based on identifying ARFs, although the Pediatric Vision Scanner identifies patients with amblyopia and strabismus directly. Moreover, these instruments are compact, user-friendly, require minimal participation by children, and are reimbursable by several private insurers and Medicaid in some states in the US (CPT codes 99177 and 99174). Given that the traditional letter and symbol charts have varying efficacy [21], these instruments provide an objective method for early detection and subsequent treatment of amblyopia in this population. Pediatricians can use these findings to determine which instrument they find suitable to incorporate into their practice.
Our report has several limitations. First, different studies sometimes used different models of instruments, so we decided to group instruments by referral criteria or version, when available. Secondly, we report ranges of reported sensitivities and specificities by instrument based on studies identified in a literature search, some of which are wide. Due to the wide range reported, we also include the median values in Table 2. We have attached a supplement that contains all of the studies included in this report with sensitivities and specificities broken down for individual review (Appendix A).
Glossary
- ARFs
Amblyopia Risk Factors
- NPV
Negative Predictive Value
- PPV
Positive Predictive Value
- SN
Sensitivity
- SP
Specificity
Appendix A.
Author Contributions
AS: Conception and design; Data Analysis; Writing; Review and Editing. BKY: Conception and design. MAH: Conception and design; Data Analysis; Critical Review and Editing.
References
- Preslan MW, Novak A. Baltimore Vision Screening Project. Ophthalmology. 1996. January;103(1):105–9. 10.1016/S0161-6420(96)30753-7 [DOI] [PubMed] [Google Scholar]
- Simons K. Preschool vision screening: rationale, methodology and outcome. Surv Ophthalmol. 1996. Jul-Aug;41(1):3–30. 10.1016/S0039-6257(97)81990-X [DOI] [PubMed] [Google Scholar]
- Pai AS, Wang JJ, Samarawickrama C, Burlutsky G, Rose KA, Varma R, et al. Prevalence and risk factors for visual impairment in preschool children the sydney paediatric eye disease study. Ophthalmology. 2011. August;118(8):1495–500. 10.1016/j.ophtha.2011.01.027 [DOI] [PubMed] [Google Scholar]
- Fozailoff A, Tarczy-Hornoch K, Cotter S, Wen G, Lin J, Borchert M, et al. Writing Committee for the MEPEDS Study Group. Prevalence of astigmatism in 6- to 72-month-old African American and Hispanic children: the Multi-ethnic Pediatric Eye Disease Study. Ophthalmology. 2011. February;118(2):284–93. 10.1016/j.ophtha.2010.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson V, Harvey EM, Miller JM, Clifford-Donaldson CE. Anisometropia prevalence in a highly astigmatic school-aged population. Optom Vis Sci. 2008. July;85(7):512–9. 10.1097/OPX.0b013e31817c930b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcido AA, Bradley J, Donahue SP. Predictive value of photoscreening and traditional screening of preschool children. J AAPOS. 2005. April;9(2):114–20. 10.1016/j.jaapos.2003.10.011 [DOI] [PubMed] [Google Scholar]
- Donahue SP, Arnold RW, Ruben JB, AAPOS Vision Screening Committee. Preschool vision screening: what should we be detecting and how should we report it? Uniform guidelines for reporting results of preschool vision screening studies. J AAPOS. 2003. October;7(5):314–6. 10.1016/S1091-8531(03)00182-4 [DOI] [PubMed] [Google Scholar]
- Donahue SP, Arthur B, Neely DE, Arnold RW, Silbert D, Ruben JB, POS Vision Screening Committee. Guidelines for automated preschool vision screening: a 10-year, evidence-based update. J AAPOS. 2013. February;17(1):4–8. 10.1016/j.jaapos.2012.09.012 [DOI] [PubMed] [Google Scholar]
- Joost AK, Kirchhoff S, Ehrt O. Screening for amblyogenic refractive errors with the VisionScreener in a paediatricians’ population. Munich, Germany: European Strabismological Society; 2008. [Google Scholar]
- Matta NS, Singman EL, Silbert DI. Performance of the Plusoptix vision screener for the detection of amblyopia risk factors in children. J AAPOS. 2008. October;12(5):490–2. 10.1016/j.jaapos.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Matta NS, Singman EL, Silbert DI. Performance of the plusoptiX S04 photoscreener for the detection of amblyopia risk factors in children aged 3 to 5. J AAPOS. 2010. April;14(2):147–9. 10.1016/j.jaapos.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Moghaddam AA, Kargozar A, Zarei-Ghanavati M, Najjaran M, Nozari V, Shakeri MT. Screening for amblyopia risk factors in pre-verbal children using the Plusoptix photoscreener: a cross-sectional population-based study. Br J Ophthalmol. 2012. January;96(1):83–6. 10.1136/bjo.2010.190405 [DOI] [PubMed] [Google Scholar]
- Arthur BW, Riyaz R, Rodriguez S, Wong J. Field testing of the plusoptiX S04 photoscreener. J AAPOS. 2009. February;13(1):51–7. 10.1016/j.jaapos.2008.08.016 [DOI] [PubMed] [Google Scholar]
- Nathan NR, Donahue SP. Modification of Plusoptix referral criteria to enhance sensitivity and specificity during pediatric vision screening. J AAPOS. 2011. December;15(6):551–5. 10.1016/j.jaapos.2011.08.008 [DOI] [PubMed] [Google Scholar]
- Bloomberg JD, Suh DW. The accuracy of the plusoptiX A08 photoscreener in detecting risk factors for amblyopia in central Iowa. J AAPOS. 2013. June;17(3):301–4. 10.1016/j.jaapos.2013.03.014 [DOI] [PubMed] [Google Scholar]
- Referral Criteria for PlusOptix S09: Alaska Blind Child Discovery (ABCD); [cited 2020 May 11].
- News Releases GoCheck Kids [cited 2021 February 7]. Available from: https://www.gocheckkids.com/press/
- Newsroom: Adaptica; [cited 2021 February 7]. Available from: https://www.adaptica.com/testimonials/
- Peterseim MM, Papa CE, Wilson ME, Davidson JD, Shtessel M, Husain M, et al. The effectiveness of the Spot Vision Screener in detecting amblyopia risk factors. J AAPOS. 2014. December;18(6):539–42. 10.1016/j.jaapos.2014.07.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcina BD, Peterseim MM, Wilson ME, Cheeseman EW, Feldman S, Marzolf AL, et al. Performance of the Spot Vision Screener in Children Younger Than 3 Years of Age. Am J Ophthalmol. 2017. June;178:79–83. 10.1016/j.ajo.2017.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi H, Yekta A, Jafarzadehpur E, Ostadimoghaddam H, Asharlous A, Nabovati P, et al. Sensitivity and Specificity of Preschool Vision Screening in Iran. Iran J Public Health. 2017. February;46(2):207–15. [PMC free article] [PubMed] [Google Scholar]
- Silbert DI, Matta NS. Performance of the Spot vision screener for the detection of amblyopia risk factors in children. J AAPOS. 2014. April;18(2):169–72. 10.1016/j.jaapos.2013.11.019 [DOI] [PubMed] [Google Scholar]
- Arnold RW, Armitage MD. iCheckKids, SPOT, iScreen, and Plusoptix performance in a high-risk, young pediatric eye practice. J AAPOS. 2013;17(1):e2. 10.1016/j.jaapos.2012.12.008 [DOI] [Google Scholar]
- Srinivasan G, Russo D, Taylor C, Guarino A, Tattersall P, Moore B. Validity of the Spot Vision Screener in detecting vision disorders in children 6 months to 36 months of age. J AAPOS. 2019;23(5):278.e1-.e6. https://doi.org/ 10.1016/j.jaapos.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Wong A, Cohen A, Thorpe K, Maurer D. Choosing appropriate tools and referral criteria for vision screening of children aged 4-5 years in Canada: a quantitative analysis. BMJ Open. 2019. September;9(9):e032138. 10.1136/bmjopen-2019-032138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arana Mendez M, Arguello L, Martinez J, Salas Vargas M, Alvarado Rodriguez AM, Papa CE, et al. Evaluation of the Spot Vision Screener in young children in Costa Rica. J AAPOS. 2015. October;19(5):441–4. 10.1016/j.jaapos.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Kirk S, Armitage MD, Dunn S, Arnold RW. Calibration and validation of the 2WIN photoscreener compared to the PlusoptiX S12 and the SPOT. J Pediatr Ophthalmol Strabismus. 2014. Sep-Oct;51(5):289–92. 10.3928/01913913-20140701-01 [DOI] [PubMed] [Google Scholar]
- Arnold RW. Comparative AAPOS Validation of the Birefringent Amblyopia Screener with Isolated Small-Angle Strabismus. Clin Ophthalmol. 2020. January;14:325–9. 10.2147/OPTH.S242335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SL, Arnold AW, Sprano JH, Arnold RW. Performance of the 2WIN Photoscreener With “CR” Strabismus Estimation in High-Risk Patients. Am J Ophthalmol. 2019. November;207:195–203. 10.1016/j.ajo.2019.04.016 [DOI] [PubMed] [Google Scholar]
- Racano E, Alessi S, Pertile R. Comparison of 2Win and plusoptiX A12R refractometers with Retinomax handheld autorefractor keratometer. J AAPOS. 2019;23(5):276.e1-.e5. https://doi.org/ 10.1016/j.jaapos.2019.05.017. [DOI] [PubMed] [Google Scholar]
- Kinori M, Molina I, Hernandez EO, Robbins SL, Granet DB, Coleman AL, et al. The PlusoptiX Photoscreener and the Retinomax Autorefractor as Community-based Screening Devices for Preschool Children. Curr Eye Res. 2018. May;43(5):654–8. 10.1080/02713683.2018.1437453 [DOI] [PubMed] [Google Scholar]
- Ugurbas SC, Kucuk N, Isik I, Alpay A, Buyukuysal C, Ugurbas SH. Objective vision screening using PlusoptiX for children aged 3-11 years in rural Turkey. BMC Ophthalmol. 2019. March;19(1):73. 10.1186/s12886-019-1080-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescioni M, Miller JM, Harvey EM. Accuracy of the Spot and Plusoptix photoscreeners for detection of astigmatism. J AAPOS. 2015. October;19(5):435–40. 10.1016/j.jaapos.2015.07.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang J, Li Y, Jiang B. Diagnostic test accuracy of Spot and Plusoptix photoscreeners in detecting amblyogenic risk factors in children: a systemic review and meta-analysis. Ophthalmic Physiol Opt. 2019. July;39(4):260–71. 10.1111/opo.12628 [DOI] [PubMed] [Google Scholar]
- Omran SS, Donahue SP. Modification of iScreen Vision Screener referral criteria to optimize sensitivity and specificity in detection of AAPOS amblyopia risk factors. J AAPOS. 2012;16(1):e26–7. 10.1016/j.jaapos.2011.12.102 [DOI] [Google Scholar]
- Silbert DI, Arnold RW, Matta NS. Comparison of the iScreen and the MTI photoscreeners for the detection of amblyopia risk factors in children. J AAPOS. 2013. February;17(1):34–7. 10.1016/j.jaapos.2012.09.015 [DOI] [PubMed] [Google Scholar]
- Arnold RW, Arnold AW, Armitage MD, Shen JM, Hepler TE, Woodard TL. Pediatric photoscreeners in high risk patients 2012: a comparison study of Plusoptix, Iscreen and SPOT. Binocul Vis Strabolog Q Simms Romano. 2013;28(1):20–8. [PubMed] [Google Scholar]
- Wang JG, Suh DW. Comparison between the Plusoptix and iScreen photoscreeners in detecting amblyopic risk factors in children. J AAPOS. 2012;16(1):e29. 10.1016/j.jaapos.2011.12.113 [DOI] [Google Scholar]
- Haschke M, Kinberg H, Morgan LA, Suh DW. Assessment of an Advanced Vision Screener in the Detection of Amblyopia in the Nebraska Pediatric Population. J Pediatr Ophthalmol Strabismus. 2018. May;55(3):189–93. 10.3928/01913913-20171205-01 [DOI] [PubMed] [Google Scholar]
- Arnold RW, Armitage MD. Performance of four new photoscreeners on pediatric patients with high risk amblyopia. J Pediatr Ophthalmol Strabismus. 2014. Jan-Feb;51(1):46–52. 10.3928/01913913-20131223-02 [DOI] [PubMed] [Google Scholar]
- Law MX, Pimentel MF, Oldenburg CE, de Alba Campomanes AG. Positive predictive value and screening performance of GoCheck Kids in a primary care university clinic. J AAPOS. 2020;24(1):17.e1-.e5. https://doi.org/ 10.1016/j.jaapos.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M, Duvall A, Daniels M, Doan M, Edmondson LE, Cheeseman EW, et al. Effectiveness of the iPhone GoCheck Kids smartphone vision screener in detecting amblyopia risk factors. J AAPOS. 2020;24(1):16.e1-.e5. https://doi.org/ 10.1016/j.jaapos.2019.10.007. [DOI] [PubMed] [Google Scholar]
- Peterseim MM, Rhodes RS, Patel RN, Wilson ME, Edmondson LE, Logan SA, et al. Effectiveness of the GoCheck Kids Vision Screener in Detecting Amblyopia Risk Factors. Am J Ophthalmol. 2018. March;187:87–91. 10.1016/j.ajo.2017.12.020 [DOI] [PubMed] [Google Scholar]
- Arnold RW, O’Neil JW, Cooper KL, Silbert DI, Donahue SP. Evaluation of a smartphone photoscreening app to detect refractive amblyopia risk factors in children aged 1-6 years. Clin Ophthalmol. 2018. August;12:1533–7. 10.2147/OPTH.S171935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold RW, Arnold AW, Hunt-Smith TT, Grendahl RL, Winkle RK. The Positive Predictive Value of Smartphone Photoscreening in Pediatric Practices. J Pediatr Ophthalmol Strabismus. 2018. November;55(6):393–6. 10.3928/01913913-20180710-01 [DOI] [PubMed] [Google Scholar]
- Yanni S, Jost R, Beauchamp C, Stager D, David S, Dao L, et al. Beyond Screening for Risk Factors…The Objective Detection of Strabismus and Amblyopia. Invest Ophthalmol Vis Sci. 2013;54(15):5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost RM, Yanni SE, Beauchamp CL, Stager DR Sr, Stager D Jr, Dao L, et al. Beyond screening for risk factors: objective detection of strabismus and amblyopia. JAMA Ophthalmol. 2014. July;132(7):814–20. 10.1001/jamaophthalmol.2014.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp CL, Stager DR Sr, Stager D Jr, Birch EE. High sensitivity and specificity of the Pediatric Vision Scanner in detecting strabismus and amblyopia in preschool children. J AAPOS. 2013;17(1):e18. 10.1016/j.jaapos.2012.12.065 [DOI] [Google Scholar]
- Kane J, Omran SS, Donahue SP. Detecting amblyopia and strabismus in children with the Pediatric Vision Scanner (PVS). J AAPOS. 2012;16(1):e20. 10.1016/j.jaapos.2011.12.078 [DOI] [Google Scholar]
- Loudon SE, Rook CA, Nassif DS, Piskun NV, Hunter DG. Rapid, high-accuracy detection of strabismus and amblyopia using the pediatric vision scanner. Invest Ophthalmol Vis Sci. 2011. July;52(8):5043–8. 10.1167/iovs.11-7503 [DOI] [PubMed] [Google Scholar]