Abstract
Sweet cherry production faces new challenges that necessitate the exploitation of genetic resources such as varietal collections and landraces in breeding programs. A harmonized approach to characterization is key for an optimal utilization of germplasm in breeding. This study reports the genotyping of 63 sweet cherry accessions using a harmonized set of 11 simple sequence repeat (SSR) markers optimized in two multiplexed PCR reactions. Thirty-eight distinct allelic profiles were identified. The set of SSR markers chosen proved highly informative in these germplasm; an average of 6.3 alleles per locus, a PIC value of 0.59 and above-average expected and observed heterozygosity levels were detected. Additionally, 223 amplified fragment length polymorphism (AFLP) markers derived from eight selective primer combinations were employed to further differentiate 17 closely related accessions, confirming the SSR analysis. Genetic relationships between internationally known old cultivars were revealed: SSR fingerprints of “Schneiders Späte Knorpelkirsche” and “Germersdorfer” were found to be identical to those of the standard cultivar “Noire de Meched”, among others, whereas four accessions known as “Hedelfinger Riesenkirsche” and four known as “Große Schwarze Knorpelkirsche” showed allelic differences at various loci. The genetic diversity of locally-grown cultivars worldwide might be currently underestimated. Several autochthonous Austrian sweet cherry germplasm accessions were genotyped for the first time and their genetic relationships analyzed and discussed. Interestingly, seven Austrian sweet cherry landraces were shown to be clearly genetically separated from international and modern varieties, indicating that Austrian germplasm could include valuable genetic resources for future breeding efforts.
Keywords: sweet cherry, Prunus avium, breeding, Austrian germplasm, autochthonous varieties, genetic diversity, simple sequence repeat (SSR), amplified fragment length polymorphism (AFLP)
1. Introduction
Autochthonous varieties and their wild relatives are valuable genetic resources for the breeding and development of any domesticated crop [1,2,3,4]. Global climate change is a key driver for some of the challenges that sweet cherry production must address, such as double fruits [5] or fruit cracking [6] stem. The availability of well characterized genetic resources from different climatic zones and growing regions will continue to gain importance, especially since r modern sweet cherry breeding programs have focused on few selected genotypes as parents causing a severe genetic bottleneck [2]. Future breeding programs aiming to support sustainable sweet cherry cultivation will need adequate and diverse germplasm to answer to these challenges. The need for a harmonized approach to genetic characterization of national and international germplasm pools to maximize their usefulness as resources for breeding and research has been highlighted in various European-wide initiatives e.g. the European Cooperative Programme for Plant Genetic Resources (ECPGR) [7] and the European Cooperation in Science and Technology (COST) action FA1104 (http://www.cost.eu/COST_Actions/fa/FA1104).
Despite a long-standing history of sweet cherry growing [8], the economic importance of this crop in Austria has decreased drastically over the last decades: in 1995, 28.7 kt of sweet cherry were produced in extensive production systems—compared to only 4.1 kt in 2017, of which only around 1.4 kt were harvested from intensive commercial plantings in 2017 [9]. Only a handful of modern cultivars are used commercially mainly due to a strong emphasis on large fruit size by retailers. Little is known about the occurrence, identity and diversity of old and locally developed Austrian landraces still grown in the mostly abandoned traditional extensive production systems [8,10].
Recent surveys of sweet cherry landraces in the provinces Burgenland [11,12] and Upper Austria [13] provide detailed morphologic descriptions of 71 sweet cherry varieties, 19 of which could be identified based on pomological descriptions, whereas 52 distinct phenotypes could not be identified due to the lack of references in the literature. These were presumed to be autochthonous Austrian landraces.
Environmental and phytosanitary factors can lead to morphological variation in plants [14], potentially calling into question the accuracy of the varietal identification on phenotypes alone [15,16,17]. This limitation is particularly severe when working with clonally propagated perennial fruit tree species. Comparing specimens of diverse age, health and unclear phytosanitary status of rare varieties, as in the case of the above-mentioned germplasm surveys, can prove extremely challenging.
The deployment of genetic markers such as simple sequence repeats (SSRs), or single nucleotide polymorphisms (SNPs) to reveal a characteristic genetic fingerprint, considerably accelerates and simplifies the process of cultivar grouping and identification [15,18,19,20]. The analysis of the alleles at the self-incompatibility locus (S-alleles) has also been used in genetic studies in sweet cherry; it provides valuable genotypic information that is very relevant for fruit growing and production [21,22,23]. SSRs have been the marker of choice to analyze diversity and genetic structure in sweet cherry [15,16,19,24,25] over the last twenty years, and the ECPGR still recommends a standard set of 16 microsatellite markers and the inclusion of eight reference accessions in diversity studies to harmonize future fingerprints of sweet cherry collections with already existing datasets. This harmonization should enable the detection of synonyms and duplicates in and across collections as well as the verification of the phenotype-based identification of accessions. A reliable confirmation of “trueness-to-type” is key to help rationalize collections and to obtain comparable data for different sweet cherry germplasm sources [7].
SSR markers inherently cover a very limited part of the target genome. They are not universally polymorphic. A defined set of markers could reveal genetic polymorphism within one population but be less informative for a second group of genotypes, e.g., closely related landraces from another gene pool [25,26,27]. Amplified fragment length polymorphism (AFLP) markers have been identified as a powerful tool for differentiation between closely related individuals at the population level [18,28] and have been successfully used in sweet cherry [19,29,30]. A genome-wide 6K SNP array was developed in 2012 for genetic studies and breeding in sweet and sour cherry, but only a third of the SNPs were found to be informative [20]. This SNP array has only very recently been improved [31] and promises to become a powerful tool in sweet cherry research. For this study, SNP arrays were not applied.
This is the first study reporting genetic fingerprints of autochthonous Austrian landraces and germplasm accessions of sweet cherry. The research questions we wanted to address were the following: (1) Are the SSR marker set and the multiplex method we chose effective? Do they provide fast and reliable results that can be harmonized? (2) Can Austrian sweet cherry landraces be successfully differentiated by the selected set of SSR markers? (3) Have the selected accessions and landraces been correctly identified?
2. Materials and Methods
2.1. Plant Material
Plant material for SSR-analysis was collected at two locations. (1) Sample Set STB: Stoob, Burgenland, AT (n = 29) representing old landraces and probably regional selections of known cultivars in the traditional high-stem meadow orchard growing system; and (2) Sample Set BOK: University of Natural Resources and Life Sciences, Vienna (BOKU) germplasm collection, Vienna, AT (n = 23) including Austrian landraces and some modern varieties. Additionally, ECPGR reference genotypes from East Malling Research, United Kingdom (GB) (n = 5) and United States of America (US) reference genotypes (n = 6) were included in Sample Set IS. These reference genotypes were used to standardize the SSR data as described by [7]. The individual samples were given a code (A01–A63) and named according to their phenotype-based identification of a known variety or cultivar, respectively, or using the accession name in the germplasm collection. Varieties that could not be reliably identified were referred to by their tree numbers (TN). In this study, the term “accession” is used to address the individual plant in the collection or sample set, respectively. Tree numbers identify the individual tree in the collection (Table 1).
Table 1.
List of accessions for simple sequence repeat (SSR) analysis, listed by their variety name or tree number (TN). Variety names in double quotes are internal working names for unidentified varieties in the germplasm collection. Code: Identifies the individual sample in the study; TN in Collection: Identifies the specific tree in the respective collection; Sample Origin: Country-Code where the plant material was collected; Variety Origin: Country-Code of the Country where this variety originated; Sample Set: Accessions belong to one of three sample sets: STB: Stoob, BOK: BOKU, Vienna germplasm collection, IS: International Standards; Interesting traits of Austrian varieties: Some traits of Austrian landraces are listed, that could be interesting for breeders are listed.
| Code | Variety Name or TN | TN in Collection | Sample Origin | Variety Origin | No of Trees of this Variety Name | Sample Set | Interesting Traits of Austrian Varieties | Reference |
|---|---|---|---|---|---|---|---|---|
| A60 | “Bigarreau Burlat” | 26113BSch | AT | FR | 2 | BOK | ||
| A59 | “Burlat VG“ | 26112BVG | AT | FR | 1 | BOK | ||
| A05 | “Butterkirsche” | TN34 | AT | AT | 5 | STB | taste, blushed type, early ripening | [12] |
| A06 | “Butterkirsche” | TN35 | AT | AT | 5 | STB | taste, blushed type, early ripening | [12] |
| A10 | “Butterkirsche” | TN44 | AT | AT | 5 | STB | taste, blushed type, early ripening | [12] |
| A13 | “Butterkirsche” | TN58 | AT | AT | 5 | STB | taste, blushed type, early ripening | [12] |
| A31 | “Butterkirsche” | TN165 | AT | AT | 5 | STB | taste, blushed type, early ripening | [12] |
| A63 | “Chelan” | US | CA | 1 | IS | |||
| A47 | “Donnerskircher Blaukirsche” | 2472Do | AT | AT | 1 | BOK | rich juice color, processed products | |
| A25 | “Early Burlat” | US | FR | 1 | IS | |||
| A01 | “F12/1” | GB | GB | 1 | IS | |||
| A62 | “Fruehe Kirsche Ubl” | 26325FKU | AT | AT | 1 | BOK | ||
| A52 | “Frueheste der Mark” | 10816FdM | AT | FR | 1 | BOK | early ripening | [8] |
| A39 | “Germersdorfer” | 2428Ge | AT | DE | 2 | BOK | ||
| A54 | “Germersdorfer” | 2426T1 | AT | DE | 2 | BOK | fruit size, fruit firmness | |
| A17 | “Goodnestone Black” | GB | GB | 1 | IS | |||
| A02 | “Grosse Schwarze Knorpelkirsche” | TN09 | AT | DE | 5 | STB | ||
| A03 | “Grosse Schwarze Knorpelkirsche” | TN29 | AT | DE | 5 | STB | ||
| A04 | “Grosse Schwarze Knorpelkirsche” | TN30 | AT | DE | 5 | STB | ||
| A15 | “Grosse Schwarze Knorpelkirsche” | TN87 | AT | DE | 5 | STB | ||
| A40 | “Grosse Schwarze Knorpelkirsche” | 2424GSK | AT | DE | 5 | BOK | ||
| A14 | “Hedelfinger Riesenkirsche” | TN60 | AT | DE | 5 | STB | ||
| A22 | “Hedelfinger Riesenkirsche” | TN128 | AT | DE | 5 | STB | ||
| A27 | “Hedelfinger Riesenkirsche” | TN146 | AT | DE | 5 | STB | ||
| A38 | “Hedelfinger Riesenkirsche” | 2444HR | AT | DE | 5 | BOK | ||
| A55 | “Hedelfinger Riesenkirsche” | 2437T2 | AT | DE | 5 | BOK | ||
| A23 | “Horitschoner Herzkirsche” | TN133 | AT | AT | 1 | STB | fruit size, fruit firmness | |
| A37 | “Horitschoner Herzkirsche” | 2452HH | AT | AT | 1 | BOK | fruit size, fruit firmness | |
| A51 | “Hybrid 222” | 10812Hy | AT | FR | 1 | BOK | ||
| A19 | “Jaboulay” | TN109 | AT | FR | 2 | STB | early ripening | |
| A43 | “Jaboulay” | 26120Sch | AT | FR | 2 | BOK | early ripening | |
| A44 | “Kritzendorfer Einsiedekirsche” Type I | 26225KrD1 | AT | AT | 1 | BOK | rich juice color, processed products | |
| A45 | “Kritzendorfer Einsiedekirsche” Type II | 2641KrD2 | AT | AT | 1 | BOK | rich juice color, processed products | |
| A46 | “Lambert” | 2456La | AT | US | 1 | BOK | ||
| A09 | “Lapins” | US | CA | 1 | IS | |||
| A16 | “Marzer Kirsche” | TN95 | AT | AT | 2 | STB | high fruit acidity, high ratio fruit weight/stone weight, early ripening | [8] |
| A36 | “Marzer Kirsche” | 2468Ma | AT | AT | 2 | BOK | high fruit acidity, high ratio fruit weight/stone weight, early ripening | [8] |
| A42 | “Melker Riesenkirsche” | 2431MRK | AT | AT | 1 | BOK | fruit size, fruit firmness | [8] |
| A33 | “Napoleon” | GB | DE | 1 | IS | |||
| A58 | “Noble” | GB | GB | 1 | IS | |||
| A49 | “Noire de Meched” | GB | IR | 1 | IS | |||
| A41 | “NY54” | US | US | 1 | IS | |||
| A26 | “Saemling von Sauerbrunn” | TN143 | AT | AT | 1 | STB | tolerance to fruit cracking | [8] |
| A61 | “Saemling von Sauerbrunn” | 26122LaSt | AT | AT | 2 | BOK | tolerance to fruit cracking | [8] |
| A48 | “Sarga Dragan” | 1071SD | AT | HU | 1 | BOK | ||
| A56 | “Schneiders Spaete Knorpelkirsche” | US | DE | 2 | IS | |||
| A53 | “Schneiders Spaete Knorpelkirsche” | 2422SSK | AT | DE | 2 | BOK | ||
| A57 | “Stella Spur” | 2453StP | AT | CA | 1 | BOK | ||
| A50 | “Tavriczskai” | 1082Ta | AT | HU | 1 | BOK | ||
| A20 | TN110 | TN110 | AT | AT | 1 | STB | early ripening, mechanical harvest | [12] |
| A21 | TN120 | TN120 | AT | AT | 1 | STB | early ripening | [12] |
| A24 | TN142 | TN142 | AT | AT | 1 | STB | early ripening, mechanical harvest | [12] |
| A28 | TN155 | TN155 | AT | AT | 1 | STB | ||
| A29 | TN157 | TN157 | AT | AT | 1 | STB | ||
| A30 | TN163 | TN163 | AT | AT | 1 | STB | mechanical harvest | [12] |
| A32 | TN175 | TN175 | AT | AT | 1 | STB | fruit size, fruit firmness | |
| A34 | TN177 | TN177 | AT | AT | 1 | STB | ||
| A07 | TN36 | TN36 | AT | AT | 1 | STB | ||
| A08 | TN39 | TN39 | AT | AT | 1 | STB | ||
| A11 | TN46 | TN46 | AT | AT | 1 | STB | mechanical harvest | [12] |
| A12 | TN52 | TN52 | AT | AT | 1 | STB | early ripening | [12] |
| A18 | TN96 | TN96 | AT | AT | 1 | STB | taste, early ripening | [12] |
| A35 | “Ulster” | US | US | 1 | IS |
AFLP analysis was conducted to further investigate the genetic identity and relationships of certain accessions showing identical SSR fingerprints. With AFLP analysis it is possible to detect polymorphisms between samples, covering the whole genome without prior knowledge of the DNA sequence [32]. Therefore, it is considered a suitable technique to differentiate between very closely related individuals of the same species [18,28]. We tested a subset of the BOKU germplasm accessions against the ECPGR standards “Noire de Meched” and “Noble” as well as landraces from two Austrian sweet cherry growing regions.
Plant material for the AFLP-analysis was collected at three locations: (1) Leithaberg, Burgenland (n = 4) (2) Scharten, Upper-Austria (n = 5) and (3) BOKU germplasm collection (n = 8), Vienna. ECPGR reference genotypes “Noire de Meched” and “Noble” were included in the analysis (Table 2). Samples from Stoob were not included in this analysis. For simplification of the graphs, samples are coded by their TN instead of variety names.
Table 2.
List of accessions for AFLP-analysis listed by their variety name. Code, TN: identifies the individual tree in the respective sample set for AFLP-analysis. Sample Origin: Country-Code where the plant material was collected, Variety Origin: Country-Code where this variety originated; Sample Set: Accessions for AFLP-analysis belong to one of four sample sets: Leitha: Leithaberg, Scharten, BOK: BOKU, Vienna germplasm collection, IS: International Standards; The column "SSR-analysis (Table 1)” indicates if this tree has also been included in the SSR-analysis presented in this study.
| Code, TN | Variety Name or TN | Sample Origin | Variety Origin | Sample Set | SSR-Analysis (Table 1) |
|---|---|---|---|---|---|
| 2451F12_1 | “F12/1” | GB | GB | IS | Yes |
| 2425T1 | “Germersdorfer” | AT | DE | BOK | No |
| 2428Ge | “Germersdorfer” | AT | DE | BOK | Yes |
| Ge_alt_VG | “Germersdorfer” | AT | DE | BOK | No |
| B04 | “Germersdorfer” | AT | DE | Leitha | No |
| P10 | “Germersdorfer” | AT | DE | Leitha | No |
| S20 | “Germersdorfer” | AT | DE | Scharten | No |
| S26 | “Germersdorfer” | AT | DE | Scharten | No |
| 2452HH | “Horitschoner Herzkirsche” | AT | AT | BOK | Yes |
| D10 | “Horitschoner Herzkirsche” | AT | AT | Leitha | No |
| P12 | “Horitschoner Herzkirsche” | AT | AT | Leitha | No |
| 2431MRK | “Melker Riesenkirsche” | AT | AT | BOK | Yes |
| P09 | “Melker Riesenkirsche” | AT | AT | Leitha | No |
| Noble 2013, Noble 2015 |
“Noble” | GB | GB | IS | Yes |
| N.d.M. 2013, N.d.M.2015 |
“Noire de Meched” | GB | IR | IS | Yes |
| K02 | “Rainkirsche” | AT | AT | Scharten | No |
| 2422SSK | “Schneiders Spaete Knorpelkirsche” | AT | DE | BOK | Yes |
| SSK_alt_VG | “Schneiders Spaete Knorpelkirsche” | AT | DE | BOK | No |
| SSKB | “Schneiders Spaete Knorpelkirsche” | AT | DE | Scharten | No |
2.2. DNA Extraction
For SSR analysis, genomic DNA was extracted from fresh leaves or winter buds with the DNeasy Plant Mini Kit (QIAGEN, Chatsworth, CA, USA) and diluted to a concentration of 2.5–4 ng/µL. For the reference accessions, lyophilized leaf-samples were extracted and diluted accordingly. The genomic DNA for the US reference accessions was kindly provided by Dr Amy Iezzoni’s team in Michigan State University.
For AFLP analysis, DNA was extracted from lyophilized leaf-samples according to [33] with minor modifications.
2.3. SSR Analysis
Twelve primer pairs recommended by the ECPGR [7] and suitable for multiplexing were combined in two multiplex (MP) PCRs depending on the fragment sizes of amplified PCR products: MP1: large (173–261 bp) and small (98–187 bp), MP2: medium (120–208 bp). Primers were labeled with fluorescent dyes (Table 3).
Table 3.
Primers used for SSR-analysis in multiplex PCR reactions.
| Multiplex | Primer | nmol in 13 µL PCR Reaction | Dye | Linkage Group | Reference |
|---|---|---|---|---|---|
| MP1 | EMPa002 | 2.50 | PET | LG1 | [34] |
| EMPaS12 | 1.75 | FAM | LG6 | [35] | |
| EMPaS02 | 3.75 | NED | LG3 | [35] | |
| UDP98-412 | 1.25 | VIC | LG6 | [36] | |
| CPPCT006 | 2.50 | PET | LG8 | [37] | |
| EMPaS01 | 5.00 | FAM | LG6 | [35] | |
| EMPa017 | 5.00 | NED | LG2 | [34] | |
| CPPCT022 | 2.50 | VIC | LG7 | [37] | |
| MP2 | CPSCT038 | 2.50 | NED | LG2 | [38] |
| BPPCT034 | 3.00 | PET | LG2 | [39] | |
| BPPCT037 | 1.75 | FAM | LG5 | [39] | |
| EMPaS10 | 1.75 | VIC | LG4 | [35] |
Primer concentrations in PCR reactions were optimized to produce similar intensity of chromatogram peaks to facilitate scoring. The final volume of PCR reactions was 13 µL containing 5–8 ng genomic DNA, 2× Type-it Multiplex PCR Master Mix (QIAGEN, Hilden, Germany), 1.25–5 nmol of primer, the exact quantity depending on each primer (Table 1).
PCR was started with a denaturing step at 95 °C for 5 min, followed by 10 circles of touchdown-PCR: 95 °C for 30 s, 60 °C for 1.5 min (−1 °C per cycle) and 72 °C for 30 s, followed by 18 cycles: 95 °C for 30 s, 50 °C for 1.5 min and 72 °C for 30 s, with a final elongation step of 60 °C for 30 min.
The amplicon sizes were measured against a LIZ 500 standard with an ABI 3130 Genetic Analyzer (Applied Biosystems, Waltham, MA, USA). Subsequent scoring was done with GENESCAN® and GENOTYPER® (Applied Biosystems, Waltham, MA, USA). Scored fragment sizes were harmonized with those of the reference genotypes using the allele sizes published by [7].
2.4. AFLP-Analysis
AFLP analysis was performed following the protocol by Vos et al. with the following modifications: Genomic DNA (0.3 µg) was incubated with 3.6 U Tru1I and 45 U EcoRI in 25 µL Tango-Buffer (Fermentas, St. Leon-Rot, Germany) for 1 hour at 37 °C, followed by two hours at 65 °C and 15 min at 85 °C. 5 µL of the adapter-ligation-solution as described by Vos et al. [40] were added and incubated overnight at 20 °C followed by a 1min-step at 65 °C to inactivate the T4 Ligase. For preamplification the template was diluted 1:5 and 2.25 µL were incubated in a 15 µL-PCR reaction with Taq DNA polymerase (5 U/µL) and 10× Taq buffer (Fermentas, St. Leon-Rot, Germany), 1.5 pM of each AFLP-primer without selective extensions, 3 mM MgCl2, 0.2 mM of dNTPs. The pre-amplified template was diluted 1:10 and 4 µL were incubated in a 10µL-PCR reaction with 0.5 pM of each of the selective primers. For this final selective amplification, 18 different primer combinations were tested on nine varieties. The eight most promising combinations were selected for the analysis: ATC/ATC, ACC/ATC, ACC/CAG, AGG/AGT, AGG/CAG, ATA/ATC, ATA/AGT, ATA/CAG. AFLP-fragments were run on a LI-COR (NEN Model 4300) analyzer (LI-COR Inc., Lincoln, NE, USA) and scored for presence (1) or absence (0) by hand with SagaTM (Version 3.0) (LI-COR inc., Lincoln, Nebraska USA). For all samples, except two, biological replicates (separate leaves, DNA extractions, digest, ligation, preamplification and selective amplification) for estimation of clonal variation were done and run side by side. Additionally, a technical control was run for four samples (same DNA extraction, separate digest, etc.). Three types of negative controls, one from the start and one for each of the PCRs were included. Only clearly visible bands between 90 and 400 bp of length were scored.
2.5. Data Analysis and Statistics:
For SSR data and allele frequencies, the identification of unique alleles was done in GenAlEx version 6.5 [41,42]. polymorphism information content (PIC)-values were subsequently calculated in Excel according to 43 [43].
The calculation of the genotype association curve (Figure 1), frequency based diversity estimators, allelic richness, observed heterozygosity, expected heterozygosity, the distance matrix, the dendrogram with Nei’s distance as well as the genetic diversity indices for the clusters resulting from Discriminant Analysis of Principal Components (DAPC) were done in the statistics environment R version 4.0.3 [44] using packages poppr version 2.3.0 [45,46] and adegenet version 2.1.3 [47,48].
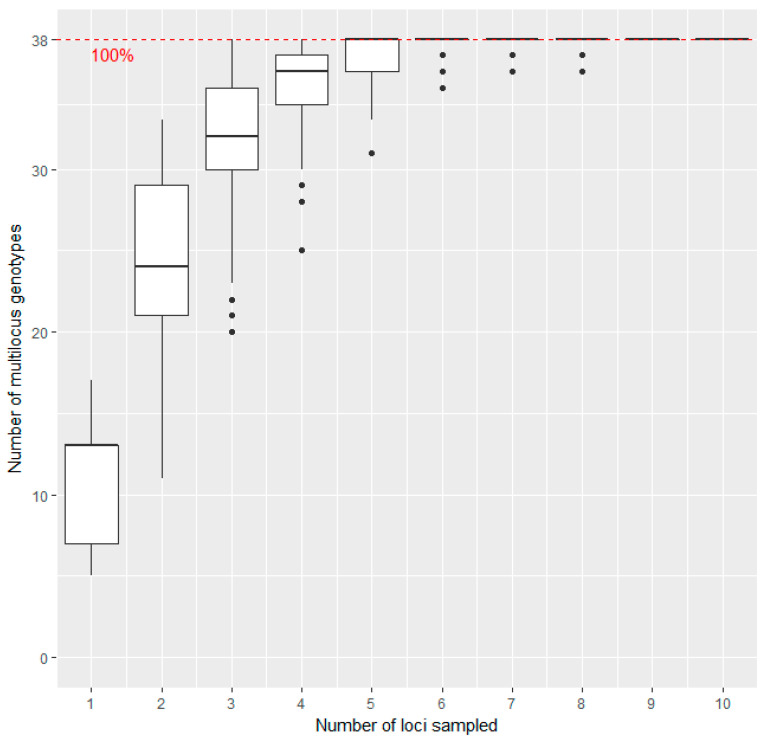
Figure 1.
Genotype association curve for SSR analysis of sweet cherry accessions: The horizontal axis represents the number of SSR loci randomly sampled from the dataset, while the vertical axis shows the number of Multilocus Genotypes (MLGs) observed. The red dashed line represents 100% of the total MLGs observed in the data set. The plateau is reached with five loci sampled, and variance decreases to a minimum with six loci.
The genotype association curve (Figure 1) reveals that with five loci sampled already 100% of the Multilocus Genotypes (MLGs) across the sampling set tested could be detected. We conclude that the method using 11 SSR markers has adequate power to discriminate between the unique individuals in our dataset and adding more markers would not reveal many additional genotypes.
The DAPC was done in R, package adegenet version 2.1.3 [47] for SSR -data. In contrast to the Principal Component Analysis (PCA) or Principal Coordinate Analysis (PCoA), this analysis doesn’t focus on the global genetic variation resp. diversity of the dataset, but instead optimizes the discriminant functions which show differences between groups, minimizing variation within clusters [49].
For AFLP-scores the PCoA was done in GenAlEx [41,42]. This analysis displays the overall genetic diversity present in the dataset.
3. Results
3.1. Method Evaluation of SSR-Analysis
Eleven of twelve SSR markers gave unambiguous results in the multiplex approach, which could be standardized. Scores of BPPCT034 [39] could not be standardized, because the allele lengths of the reference accessions were found to be ambiguous. Data for this marker were excluded prior to analysis. The remaining 11 markers were amplified in two multiplexed reactions, resulting in a fast and easy-to-use fingerprinting system. The calculated genotype association curve (Figure 1) showed that the number of loci obtained with these markers was sufficient to cover the genetic diversity present in the sample set. Other studies used similar numbers of makers for genetic fingerprints in Prunus avium, obtaining reliable results [7,19].
The number of alleles per locus ranged from 3 to 9, with an average of 6.3; PIC values ranged between 0.22 (EMPa017) and 0.78 (BPPCT037), with an average of 0.59. The observed and expected heterozygosity ranged between 0.06 resp. 0.23 (EMPA017) and 0.89 resp. 0.81 (BPPCT037) depending on the SSR marker (Table 4).
Table 4.
Genetic parameters detected for SSR markers used to study autochthonous Austrian sweet cherry accessions and international varieties.
| Locus | N | Ho | He | PIC | PA | PA-MLGs |
|---|---|---|---|---|---|---|
| BPPCT037 | 8 | 0.89 | 0.81 | 0.78 | 2 | 2 |
| CPPCT006 | 8 | 0.89 | 0.77 | 0.73 | 2 | 2 |
| CPPCT022 | 8 | 0.51 | 0.59 | 0.56 | 4 | 6 |
| CPSCT038 | 3 | 0.58 | 0.61 | 0.54 | ||
| EMPa002 | 3 | 0.75 | 0.54 | 0.45 | ||
| EMPa017 | 5 | 0.06 | 0.23 | 0.22 | 1 | 1 |
| EMPaS01 | 5 | 0.73 | 0.70 | 0.66 | ||
| EMPaS02 | 7 | 0.84 | 0.72 | 0.68 | 1 | 1 |
| EMPaS10 | 8 | 0.49 | 0.60 | 0.53 | 4 | 5 |
| EMPaS12 | 5 | 0.83 | 0.75 | 0.71 | ||
| UDP98-412 | 9 | 0.71 | 0.70 | 0.67 | 1 | 1 |
| Total | 69 | |||||
| Average | 6.3 | 0.66 | 0.64 | 0.59 |
N, number of alleles per locus; Ho, observed heterozygosity; He, expected heterozygosity; PIC, polymorphic information content; PA, no. of private alleles per locus; PA-MLGs, Number of Multi locus genotypes with private alleles per locus.
Eight different genotypes (four genotypes from Stoob, two from the BOKU collection and two international standards) show unique alleles in up to three markers. The best markers in terms of detecting unique alleles in different genotypes were CPPCT022 and EMPaS10, with unique alleles amplified in six and five genotypes, respectively.
3.2. Variety Identification and Verification of Trueness to Type
The genotypes obtained by SSR fingerprinting allowed us to confirm or reject the morphological identification of landraces and germplasm accessions and revealed the occurrence of homonyms and synonyms in otherwise well-known varieties. Some phenotyping, grafting or labelling errors were also detected (e.g. “Burlat VG” which is not identical with the cultivar “Bigarreau Burlat”; “Lambert” and “Stella Spur” which surprisingly showed the same fingerprint). Four trees in Stoob were phenotyped as cultivar “Große Schwarze Knorpelkirsche”; only two showed the same genotype and all of them differed compared to the reference accession in the BOKU germplasm collection. Two of three trees phenotyped as “Hedelfinger Riesenkirsche” and collected in Stoob had the same fingerprint, but all of them differed from the two accessions of the same name in the BOKU germplasm collection, which also turned out to show two distinct genetic profiles. Some clonal variants were also identified such as the samples phenotyped as landrace “Butterkirsche” and the two types of “Kritzendorfer Einsiedekirsche”. Six of the sampled Austrian landraces showed to be identical to several germplasm accessions and standard cultivars from different origins. This group of seemingly identical genotypes includes the accessions “Germersdorfer”, “Schneiders Späte Knorpelkirsche”, the ECPGR reference cultivar “Noire de Meched” and the US-reference “Schneiders”, as well as traditional Austrian cultivars like “Melker Riesenkirsche” and “Horitschoner Herzkirsche”. For simplification purposes this group of accessions will be mentioned as the “Schneiders-Group” throughout this study.
An AFLP-analysis including further samples from two other sampling sites was conducted to confirm if these cultivars are true clones i.e., synonyms (see below).
3.3. Genetic Diversity
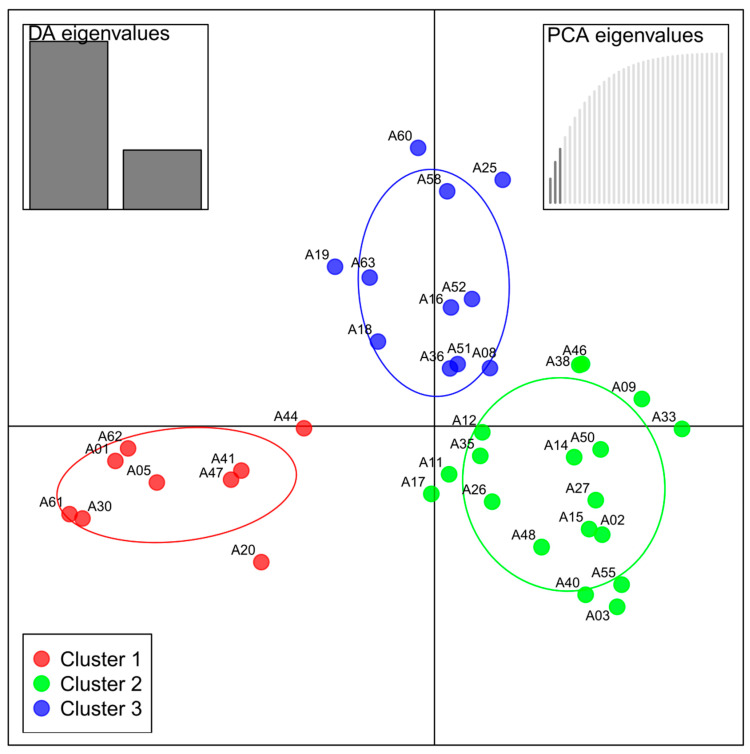
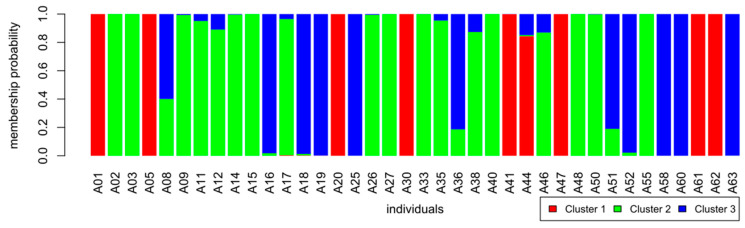
The Discriminant Analysis of Principal Components (DAPC, R, adegenet) was used to identify groups of genetically related individuals in all three sample sets. This analysis has been developed and is suitable for clonal or partly clonal populations, since it does not rely on Hardy–Weinberg or linkage disequilibria [50]. The varieties group into three defined separate clusters (Figure 2); each cluster contains members of the three sample sets: BOK = BOKU germplasm collection, IS = International Standard, STB = Stoob. Part of the samples showed to be admixed according to the estimated probability of group membership, which is depicted in the membership probability plot (Figure 3).
Figure 2.
Scatter plot of discriminant analysis of principal components (DAPC) for SSR-data, three PCs and three DAs retained. Color code and group-ellipses according to the three calculated clusters. The box on the upper left shows the retained DA eigenvalues, while the box on the upper right shows that three PCA eigenvalues have been retained.
Figure 3.
Membership probability of samples to the three identified clusters. Depiction of admixture. X-axis: individual samples, y-axis: membership probability to one of three clusters: 1: red, 2: green, 3: blue.
Cluster 1 consists of nine multi-locus-genotypes (MLGs) and contains landraces from Burgenland (“Butterkirsche”, “Sämling von Sauerbrunn” and “Donnerskircher Blaukirsche”) as well as the rootstocks “NY54” and “F12/1” (Table 1 and Table 5). Only one of these samples (“Kritzendorfer Einsiedekirsche”) shows to be admixed with cluster 3. The other two clusters share more admixed genotypes (Figure 3). In cluster 2, 18 samples or MLGs are found; some of the modern cultivars, all samples of the two widely distributed cultivars “Große Schwarze Knorpelkirsche” and “Hedelfinger Riesenkirsche” and some landraces denominated by tree numbers (TN). Cluster 3 comprises of the rest of the modern cultivars and the “Schneiders-Group” which was included in the analyses represented by one sample of this genotype: TN39 (Table 5).
Table 5.
Composition of clusters, membership of Multilocus Genotypes according to DAPC based on SSR data. Column 4 shows the code of the accessions with the same genotype where applicable. STB = Stoob, IS = International Standard, BOK = BOKU, Vienna, TN = Tree number. Variety names in double quotes are internal working names for unidentified varieties in the germplasm collection.
| Cluster | Variety | Sample Set | Accessions with Same Genotype | Code |
|---|---|---|---|---|
| 1 | “F12/1” | IS | A01 | |
| 1 | “Butterkirsche” | STB | A06, A07, A10, A13, A31 | A05 |
| 1 | TN110 | STB | A20 | |
| 1 | TN163 | STB | A30 | |
| 1 | “NY54” | IS | A41 | |
| 1 | “Kritzendorfer Einsiedekirsche” “Type I” | BOK | A45 | A44 |
| 1 | “Donnerskircher Blaukirsche” | BOK | A47 | |
| 1 | “Sämling von Sauerbrunn” | BOK | A61 | |
| 1 | “Frühe Kirsche Ubl” | BOK | A62 | |
| 2 | “Große Schwarze Knorpelkirsche” | STB | A02 | |
| 2 | “Große Schwarze Knorpelkirsche” | STB | A04 | A03 |
| 2 | “Lapins” | IS | A09 | |
| 2 | TN46 | STB | A11 | |
| 2 | TN52 | STB | A12 | |
| 2 | “Hedelfinger Riesenkirsche” | STB | A22 | A14 |
| 2 | “Große Schwarze Knorpelkirsche” | STB | A15 | |
| 2 | “Goodnestone Black” | IS | A17 | |
| 2 | “Sämling von Sauerbrunn” | STB | A26 | |
| 2 | “Hedelfinger Riesenkirsche” | STB | A27 | |
| 2 | “Napoleon” | IS | A33 | |
| 2 | “Ulster” | IS | A35 | |
| 2 | “Hedelfinger Riesenkirsche” | BOK | A38 | |
| 2 | “Große Schwarze Knorpelkirsche” | BOK | A40 | |
| 2 | “Lambert” | BOK | A57 | A46 |
| 2 | “Sarga Dragan” | BOK | A48 | |
| 2 | “Tavriczskai” | BOK | A50 | |
| 2 | “Tscholl 2” | BOK | A55 | |
| 3 | TN39 | STB | A23, A28, A29, A32, A34, A37, A39, A42, A49, A53, A54, A56 | A08 |
| 3 | “Marzer Kirsche” | STB | A16 | |
| 3 | TN96 | STB | A21, A24 | A18 |
| 3 | “Jaboulay” | STB | A43 | A19 |
| 3 | “Early Burlat” | IS | A25 | |
| 3 | “Marzer Kirsche” | BOK | A36 | |
| 3 | “Hybrid 222” | BOK | A59 | A51 |
| 3 | “Früheste der Mark” | BOK | A52 | |
| 3 | “Noble” | IS | A58 | |
| 3 | “Bigarreau Burlat” | BOK | A60 | |
| 3 | “Chelan” | IS | A63 |
According to Simpson’s diversity index (lambda, Table 6), DAPC-cluster 2, which includes many accessions of the germplasm-collection, is the most diverse, with 0.944, followed by cluster 3, with 0.90. Cluster 1 is the least diverse, with lambda 0.889; this cluster mostly consists of the landraces from Burgenland, which, unsurprisingly, were shown to be very closely related to each other.
Table 6.
Diversity indices for SSR data grouped according to DAPC clusters.
| Pop | N | MLG | eMLG | SE | lambda | Hexp | Ia | rbarD |
|---|---|---|---|---|---|---|---|---|
| 1 | 9 | 9 | 9 | 0 | 0.889 | 0.630 | 1.035 | 0.1075 |
| 2 | 18 | 18 | 10 | 5.43 × 10−7 | 0.944 | 0.595 | 0.316 | 0.0331 |
| 3 | 11 | 11 | 10 | 0 | 0.909 | 0.614 | 0.634 | 0.0654 |
| Total | 38 | 38 | 10 | 1.72 × 10−6 | 0.974 | 0.665 | 0.653 | 0.0662 |
Pop, population from DAPC analysis; N: no. of individuals; MLG: multi-locus-genotypes; eMLG, expected MLGs based on rarefaction; SE, standard error from rarefaction; lambda, Simpson’s index; Hexp, Neis expected heterozygosity; Ia, Index of association; rbarD, standardized index of association.
The expected heterozygosity (Hexp) of all three clusters were very similar, with values ranging from 0.595 (cluster 2) to 0.63 (cluster 1).
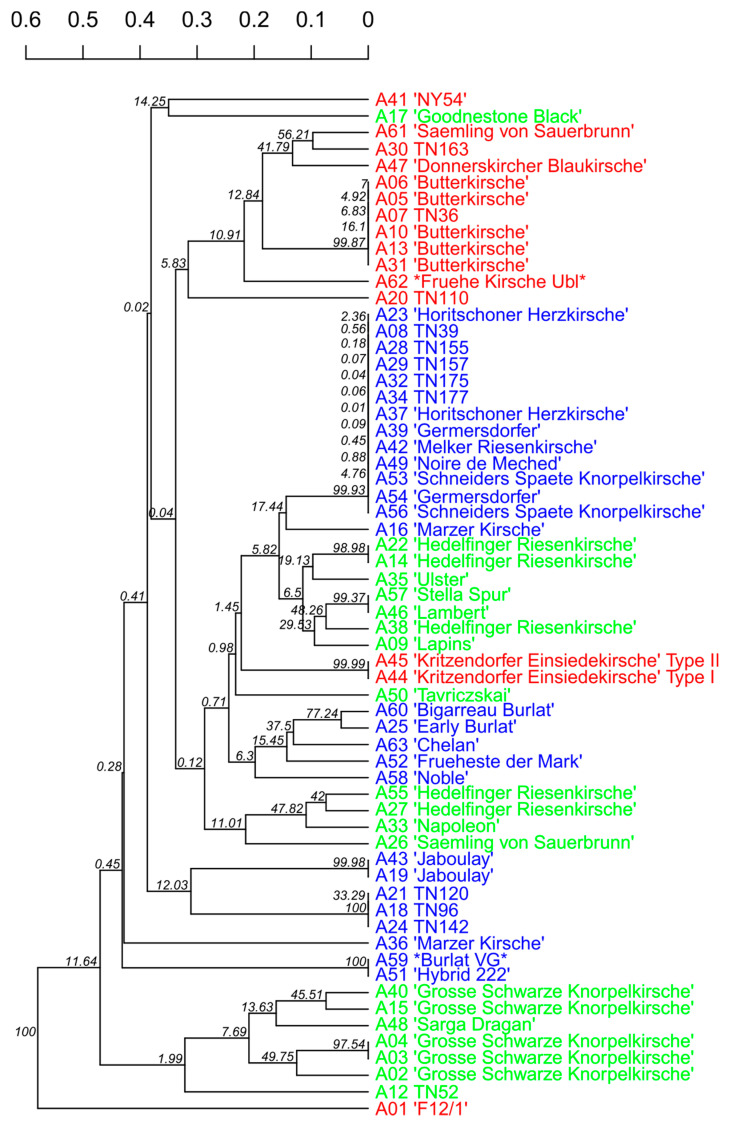
The dendrogram (Figure 4) was calculated based on Nei’s distance, bootstrapping (10,000) and UPGMA (unweighted pair group with arithmetic means). Variety A11 TN46 had to be excluded for this calculation, because the algorithm based on Nei’s distance cannot process missing data, and the SSR marker CPSCT038 did not amplify any PCR products for this genotype (see also Supplement-File S1). The dendrogram shows four main clusters of sweet cherry varieties. Though based on the same data, the clusters are composed differently compared to the DAPC (Figure 2, Table 3), as they are calculated using a different algorithm.
Figure 4.
Dendrogram based on SSR data, Nei’s distance, bootstrapping (10,000), unweighted pair group with arithmetic means (UPGMA); color code according to DAPC clusters: 1: red, 2: green, 3: blue.
DAPC-cluster 1 (red) is represented on the upper end missing “Kritzenorfer Einsiedekirsche”, which groups with the varieties of the cluster below. NY45 groups together with Goodnestone Black (uppermost end), and F12/1 appears separated from all other varieties on the bottom end of the dendrogram. The other two DAPC clusters appear to be admixed, whereas notably all genotypes of “Hedelfinger Riesenkirsche” appear in one cluster and all genotypes of “Große Schwarze Knorpelkirsche” in another. A separate cluster consists of “Hybrid 222”, i.e., “Burlat VG”. Unlike DAPC analysis, the dendrogram shows the rootstock “F12/1” as more genetically distant from the rest of the varieties (Figure 4). This might be a result of the clustering algorithm UPGMA assuming the occurrence of a hierarchical structure between the individuals and rooting this structure in one sample, which might not be an accurate assumption for our data set. The low bootstrap-values on the left-side nodes indicate that the separation of the main clusters as shown in the dendrogram is not very well supported by the data. This is probably due to the limited amount of processed data, combined with the fact that the sweet cherry varieties in this study are generally very closely related.
3.4. Results from AFLP-Analysis
For AFLP-analysis samples of “Germersdorfer” (n = 7), “Horitschoner Herzkirsche” (n = 3), “Melker Riesenkirsche” (n = 2), “Schneiders Späte Knorpelkirsche” (n = 3) of three origins and the ECPGR standard “Noire de Meched” were tested. We also included the clearly distinct phenotypes “Noble” (which also shows a different SSR-fingerprint), rootstock “F12/1” as well as the Austrian landrace “Rainkirsche” (K02) (Table 2). Four runs had to be excluded due to a high proportion of failed bands.
An AFLP analysis with eight selective primer combinations and 17 samples of four different origins resulted in 223 markers, of which 64 (28.7%) were polymorphic.
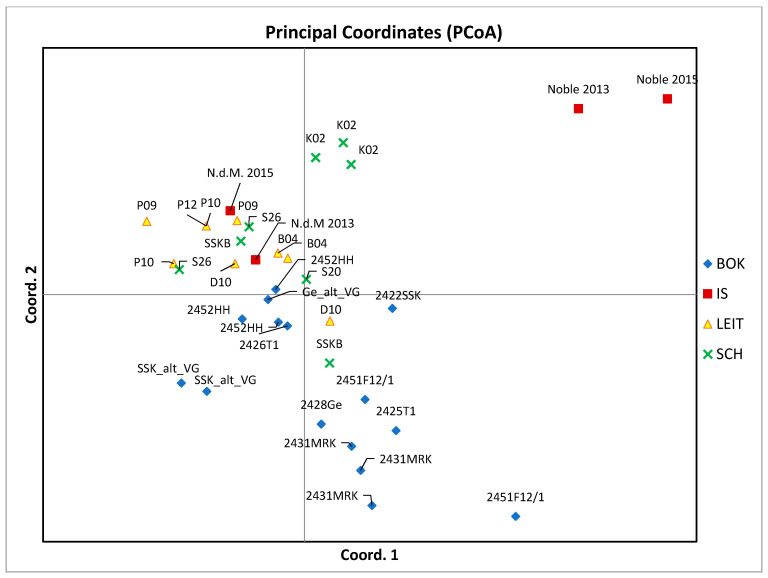
The error-rate was moderate, with 1.36–1.79% of difference in band-occurrence between technical replicates. Biological replicates differed up to 6.73%. The samples of “Noble” could clearly be separated from the other samples with PCoA (Figure 5). The percentage of variance explained by the first two axes was 32.7% and 44.17% by the three main coordinates. This low power of explanation of variance is probably due to the low number of samples and the generally low variation between the tested genotypes. Therefore, these results should be interpreted with caution.
Figure 5.
Principal Coordinates 1 and 2 (% of var. explained: 32.7%) of AFLP-data of sweet cherry accessions. Blue: BOK = BOKU, red: IS = International Standard, yellow: LEIT = Leithaberg, green: SCH = Scharten.
4. Discussion
The optimized multiplex-PCR approach to SSR genotyping was broadly successful; 11 of the 12 SSR-markers were easily scored and standardized against published data for five reference accessions (data not shown). This gives us confidence in the quality of the data generated which should prove straightforward to compare with similarly standardized data for other germplasm collections in the future The number of markers was comparable to that of similar studies [3,4,23,27,51] and showed to be sufficient to reveal the genetic diversity of MLGs expected to be present in the samples analyzed. Furthermore, with an average of 6.3 alleles per locus and PIC-value of 0.59 as well as the above-average expected and observed heterozygosity-levels, it is clear that the set of SSR-markers chosen was highly informative in the sweet cherry varieties in this study. The method of characterization proved effective and provided solid results that can be reliably harmonized with future studies.
The chosen primer combination was found to be effective for differentiating between most of the Austrian sweet cherry landraces tested. Regarding the accurate identification of duplicates (i.e. synonyms) and homonyms (i.e. genetically heterogenous groups phenotyped as the same cultivar), both could be detected by SSR-analysis, providing essential information for the optimal management of the germplasm collection and future breeding approaches.
In DAPC cluster 2 (Figure 2), there are two heterogenous groups. In five samples, all phenotyped as “Große Schwarze Knorpelkirsche”, four distinct genotypes could be detected. This is understandable; this cultivar dates back to the 16th century and has been one of the most important in central Europe [52].
Four of five samples phenotyped as “Hedelfinger Riesenkirsche” showed differences in allele sizes. The cultivar “Hedelfinger Riesenkirsche” originates from Hedelfingen in Germany where it was selected from seedlings and taken to Hohenheim (Germany) around 1850. Thereafter, this cultivar has been widely distributed by tree nurseries [8] and is found in many places around the world nowadays often referred to as “Hedelfingen”.
“Große Schwarze Knorpelkirsche” as well as “Hedelfinger Riesenkirsche” were assigned to the same DAPC cluster; both have been referred to as “population cultivar”, indicating that the cultivar consists of several phenotypes [53]. This could be due to mixed vegetative and sexual reproduction combined with selection for a certain fruit morphology—which makes these cultivars relatively easy to phenotype—over the past decades. Another possibility is clonal variation, i.e., the vegetative propagation of sport mutants.
On the other hand, three not previously identified genotypes of distinct phenotypes found in Stoob showed the same fingerprint (TN96, TN120, TN142; Figure 4). In that case, it has to be considered that the discriminating power of the limited set of SSR markers might not be adequate for these genotypes.
The identification of synonyms or duplicates helps to reduce the number of accessions and therefore the running costs in conservation efforts such as germplasm collections [54]. On the other hand, mutations and thus clonal variation occur, especially in long-lived tree species [55,56]. Certain clones potentially harbor desirable superior traits such as yield [27], climatic adaptation or tolerance to pests and diseases, and thus the identification of true clonal variation within germplasm collections is essential.
A group of accessions showing the same genetic SSR fingerprint includes presumably different late-ripening, heart-shaped, dark-red, firm cultivars with considerable fruit size. Surprisingly, very well-known, Europe-wide distributed cultivars like “Germersdorfer” and “Schneiders Späte Knorpelkirsche” as well as typical Austrian cultivars like “Melker Riesenkirsche” and “Horitschoner Herzkirsche” (named after Melk and Horitschon—two towns in the eastern part of Austria) are found in this group, along with the ECPGR reference cultivar “Noire de Meched” and the US-standard “Schneiders”. Although these findings could be due to limited SSR-marker resolution, suspicions that some of the cultivars could be clones and their names therefore synonyms have been raised before. Braun-Lüllemann and Bannier [52] found that in Germany the cultivar denominated as “Germersdorfer“ in the past decades is morphologically identical to the cultivar known as “Schneiders Späte Knorpelkirsche“.
This finding is especially intriguing since this latter cultivar was found to be comparable in fruit size to widely distributed modern cultivars like “Regina” or “Kordia”, and thus represents a potential candidate for breeding. Considering that maximizing fruit size still is one of the most important objectives in sweet cherry breeding [57], it is essential to find out whether the studied varieties are in fact clones. The verification of this hypothesis would probably reduce the number of suitable high-fruit-sized parent-candidates for future breeding programs. Differences in the phenotype due to clonal or epigenetic variation would have to be studied in appropriate trials, to select for the best clones. Moreover, for a cost-effective rationalization of germplasm collections, it is important to know if those Austrian cultivars are in fact all duplicates.
To our knowledge, until now there has been no such study. To gather additional evidence on the correct genetic identity of the above-mentioned varieties which have identical SSR profiles, an AFLP analysis was conducted.
We compared the ECPGR standard “Noire de Meched”, accessions of the Austrian germplasm collection “Germersdorfer”, “Schneiders Späte Knorpelkirsche”, “Melker Riesenkirsche” and “Horitschoner Herzkirsche”, as well as samples from two different sites consisting of several landraces identified as one of the just mentioned cultivars. Three genetically and phenotypically different accessions were included for comparison.
Based on the results of the AFLP analysis, the tested varieties in general show low genetic variation; only about 29% of markers were shown to be polymorphic. This is in agreement with reported polymorphic rates of 21% for AFLP markers in sweet cherry [19]. Technical error rates are as high as 1.79%. For biological replicates (same tree, different branch), differences of up to 6.73% were recorded. These differences could be explained by clonal variation resp. sport mutation, and therefore it is also probable that morphologically identical or very similar varieties are clones of one and the same widespread cultivar. As expected, samples of the cultivar “Noble” could clearly be separated from the rest by PCoA (Figure 5). Nevertheless, samples of the other two very different phenotypes “F12/1” and “Rainkirsche” appear close to or inside the cluster of “Schneiders-Group” cultivars. This could be due to the proportion of technical errors combined with the comparatively small genetic distance between Prunus avium varieties, which puts more weight on such technical errors. To sum up: based on the results shown in this study genetic differences among the tested varieties exist. These genotypes might represent valuable resources for future breeding efforts, if they have superior traits, e.g., disease resistance or superior fruit size.
The genetic diversity of the Austrian landraces evaluated was subsequently compared to that of international standard cultivars based on the results of the DAPC and calculated diversity indices. The DAPC sorted the samples into three clusters. In cluster 2, all samples of “Hedelfinger Riesenkirsche” and “Große Schwarze Knorpelkirsche” group together with “Goodnestone Black”, “Napoleon”, “Stella Spur”, “Lambert”, “Lapins”, “Ulster”, “Tavriczskai” and “Sarga Dragan”. “Chelan” groups with “Noble”, “Early Burlat”, “Burlat”, “Jaboulay” and “Früheste der Mark” in DAPC cluster 3, whereas DAPC cluster 1 comprised only varieties from Burgenland, i.e., landraces not mentioned in the available literature. These autochthonous varieties seemed clearly distinct from the other groups and may therefore constitute a valuable germplasm for breeding as members of a regional gene pool. Details on valuable phenotypic and physicochemical characteristics of these varieties such as unique taste, low susceptibility to rain-induced cracking or high content of polyphenols in the fruit have been recorded in prior studies [11,12,58]. The landraces are probably admixed with the wild cherry population of this specific region. Interestingly, rootstocks “NY45” and “F12/1” are also assigned to this cluster.
5. Conclusions
A successful method of fast and easy-to-use multiplex SSR analysis for international harmonization of sweet cherry accessions was presented. The investigated collection of autochthonous Austrian sweet cherry landraces is highly diverse and could constitute a valuable germplasm for future breeding programs, since Austrian landraces were shown to represent a regional gene pool. They exhibit interesting traits that might be valuable for breeders (Table 1) and are most probably adapted to the local climate and environmental factors, since they comprise ecotypes that have been cultivated in the same region for decades. Furthermore, they might harbor certain traits like tolerance to fruit cracking or tolerances to diseases and pests, which has to be evaluated in further studies.
Concerning the various genotypes of “Große Schwarze Knorpelkirsche” and 2Hedelfinger Riesenkirsche”, marker assisted selection (MAS), field trials and cultivar evaluations should be conducted to identify the most valuable of the clones for breeding purposes.
It would be interesting to compare the genetic diversity of Austrian landraces with those from the French collection described by Mariette et al. [2]. Does it comprise a different gene pool? What is the influence of the wild cherry population in Austria and how is this gene pool different compared to the French wild cherries?
Phenotype-based surveys on sweet cherry diversity have been conducted for some Austrian regions [10,11,12,13], and Austrian landraces were shown to bear valuable characteristics such as a high content of polyphenols in the fruit [58]. While important first steps have recently been taken to preserve and protect these landraces in the future, considerable gaps of knowledge still need to be filled to effectively preserve the Austrian sweet cherry diversity.
Part of these gaps could effectively be filled by genetic evaluation, as has been shown in this study. Homonyms, synonyms and labeling errors were detected. The genetic data help to evaluate the genetic diversity, identity and trueness to type of Austrian sweet cherry accessions and thus serves as an important and valuable tool for the management of Austrian germplasm collections.
Acknowledgments
We would like to thank Sebastian Arming and Manuel Curto for software support and suggestions on earlier versions. Open access funding provided by BOKU Vienna Open Access Publishing Fund.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/12/3/322/s1, Table S1: SSR-data, Table S2: AFLP-data.
Author Contributions
Conceptualization, all authors; methodology, E.S., F.F.F. and U.A.-B.; formal analysis, investigation and validation, E.S., F.F.F., L.A. and U.A-B.; data curation, E.S., writing—original draft preparation, E.S.; writing—review and editing, all authors; visualization, E.S.; supervision, F.F.F., U.A.-B., A.S., A.F., project administration, E.S.; funding acquisition, E.S. and F.F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the funding of a Short Term Scientific Mission through COST Action 1104 and East Malling Research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data from SSR- and AFLP-analysis is provided as supplemental files (Supplement S1, Supplement S2) to this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gecer M.K., Kan T., Gundogdu M., Ercisli S., Ilhan G., Sagbas H.I. Physicochemical characteristics of wild and cultivated apricots (Prunus armeniaca L.) from Aras valley in Turkey. Genet. Resour. Crop Evol. 2020;67:935–945. doi: 10.1007/s10722-020-00893-9. [DOI] [Google Scholar]
- 2.Mariette S., Tavaud M., Arunyawat U., Capdeville G., Millan M., Salin F. Population structure and genetic bottleneck in sweet cherry estimated with SSRs and the gametophytic self-incompatibility locus. Genetics. 2010;11:1471–2156. doi: 10.1186/1471-2156-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kocsisné G.M., Bolla D., Anhalt-Brüderl U.C.M., Forneck A., Taller J., Kocsis L. Genetic diversity and similarity of pear (Pyrus communis L.) cultivars in Central Europe revealed by SSR markers. Genet. Resour. Crop Evol. 2020;67:1755–1763. doi: 10.1007/s10722-020-00937-0. [DOI] [Google Scholar]
- 4.Mihaljevic M.Z., Anhalt U.C.M., Rühl E., Mugosa M.T., Maras V., Forneck A., Zdunic G., Preiner D., Pejic I. Cultivar Identity, Intravarietal Variation, and Health Status of Native Grapevine Varieties in Croatia and Montenegro. Am. J. Enol. Vitic. 2015;66:531–541. doi: 10.5344/ajev.2015.15023. [DOI] [Google Scholar]
- 5.Roversi A., Monteforte A., Panelli D., Folini L., Fajt N. Observations on the occurrence of sweet cherry double-fruits in Italy and Slovenia. Acta Hortic. ISHS. 2008:849–854. doi: 10.17660/ActaHortic.2008.795.137. [DOI] [Google Scholar]
- 6.Sekse L. Fruit Cracking in Sweet Cherries—Some Recent Advances. Acta Hortic. 2008:615–624. doi: 10.17660/ActaHortic.2008.795.96. [DOI] [Google Scholar]
- 7.Clarke J.B., Tobutt K.R. A Standard Set of Accessions, Microsatellites and Genotypes for Harmonising the Fingerprinting of Cherry Collections for the ECPGR. Acta Hortic. ISHS. 2009;814:615–618. doi: 10.17660/ActaHortic.2009.814.104. [DOI] [Google Scholar]
- 8.Bodo F. Burgenlands Kirschensorten. Victor Horáth; Neusiedl am See, Austria: 1936. [Google Scholar]
- 9.BMNT Grüner Bericht 2018. Bericht Über die Situation der Österreichischen Land- und Forstwirtschaft im Jahr 2017. [(accessed on 23 February 2021)];2018 Available online: www.gruenerbericht.at.
- 10.Leifer H. Kartierung und Beschreibung von Kirschbäumen und alten Kirschensorten in Pöttsching (Burgenland). Diplomar-Beit. University of Natural Resources and Life Sciences; Vienna, Austria: 2002. [Google Scholar]
- 11.Spörr T., Schüller E., Keppel H., Spornberger A. Mapping of regionally typical old cherry cultivars in the gourmet region ‘Genussregion Leithaberger Edelkirsche’. Mitteilungen Klosterneuburg. 2014;64:82–95. [Google Scholar]
- 12.Schüller E., Pilz V., Holler C., Keppel H., Spornberger A. Mapping and description of old cherry trees and regional cherry cultivars in Stoob, Mittelburgenland. Mitteilungen Klosterneuburg. 2016;66:113–126. [Google Scholar]
- 13.Putz S. Master’s Thesis. University of Natural Resources and Life Sciences; Vienna, Austria: 2014. Survey and Identification of Old Local Sweet Cherry (Prunus avium) cultivars in Scharten, Upper Austria. [Google Scholar]
- 14.Schoedl K., Denk A., Hummelbrunner S., Modl P., Forneck A. No improvement in fruit quality through chemical flower thinning in sweet cherry (Prunus avium L.) J. Sci. Food Agric. 2009;89:1236–1240. doi: 10.1002/jsfa.3581. [DOI] [Google Scholar]
- 15.Wünsch A., Hormaza J.I. Cultivar identification and genetic fingerprinting of temperate fruit tree species using DNA markers. Euphytica. 2002;125:59–67. doi: 10.1023/A:1015723805293. [DOI] [Google Scholar]
- 16.Turkoglu Z., Bilgener S., Ercisli S., Bakir M., Koc A., Akbulut M., Gercekcioglu R., Gunes M., Esitken A. Simple sequence repeat-based assessment of genetic relationships among Prunus rootstocks. Genet. Mol. Res. 2010;9:2156–2165. doi: 10.4238/vol9-4gmr957. [DOI] [PubMed] [Google Scholar]
- 17.Anhalt U.C., Martini K., Ruehl E.-H., Forneck A. Tracing Heterozygosity in the Vvlexp1 Locus in Grapevine by Sequenc-ing and High-resolution Melt Analysis. J. Am. Soc. Hort. Sci. 2013;138:120–124. doi: 10.21273/JASHS.138.2.120. [DOI] [Google Scholar]
- 18.Powell W., Machray G.C., Provan J. Polymorphism revealed by simple sequence repeats. Trends Plant Sci. 1996;1:215–221. doi: 10.1016/S1360-1385(96)86898-0. [DOI] [Google Scholar]
- 19.Struss D., Ahmad R., Southwick S.M., Boritzki M. Analysis of sweet cherry (Prunus avium L.) cultivars using SSR and AFLP markers. J. Am. Soc. Hortic. Sci. 2003;128:904–909. doi: 10.21273/JASHS.128.6.0904. [DOI] [Google Scholar]
- 20.Peace C., Bassil N., Main D., Ficklin S., Rosyara U.R., Stegmeir T., Sebolt A., Gilmore B., Lawley C., Mockler T.C., et al. Development and evaluation of a genome-wide 6K SNP array for diploid sweet cherry and tetraploid sour cherry. PLoS ONE. 2012;7:e48305. doi: 10.1371/journal.pone.0048305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaughan S.P., Bošković R.I., Gisbert-Climent A., Russell K., Tobutt K.R. Characterisation of novel S-alleles from cherry (Prunus avium L.) Tree Genet. Genomes. 2008;4:531–541. doi: 10.1007/s11295-007-0129-6. [DOI] [Google Scholar]
- 22.Marchese A., Giovannini D., Leone A., Mafrica R., Palasciano M., Cantini C., Di Vaio C., de Salvador F.R., Giacalone G., Caruso T., et al. S-genotype identification, genetic diversity and structure analysis of Italian sweet cherry germplasm. Tree Genet. Genomes. 2017;13:1–20. doi: 10.1007/s11295-017-1176-2. [DOI] [Google Scholar]
- 23.Ercisli S., Agar G., Yildirim N., Karlidag H., Duralija B., Vokurka A. Genetic diversity in wild sweet cherries (Prunus avium) in Turkey revealed by SSR markers. Genet. Mol. Res. 2011;10:1211–1219. doi: 10.4238/vol10-2gmr1196. [DOI] [PubMed] [Google Scholar]
- 24.Xuan H., Wang R., Büchele M., Möller O., Hartmann W. Microsatellite Markers (SSR) as a Tool to Assist in Identification of Sweet (Prunus avium) and Sour Cherry (Prunus cerasus) Acta Hortic. 2009 doi: 10.17660/ActaHortic.2009.839.69. [DOI] [Google Scholar]
- 25.Fernández i Martí A., Athanson B., Koepke T., Font I Forcada C., Dhingra A., Oraguzie N. Genetic diversity and relat-edness of sweet cherry (Prunus avium L.) cultivars based on single nucleotide polymorphic markers. Front. Plant Sci. 2012;3:116. doi: 10.3389/fpls.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wünsch A., Hormaza J.I. Molecular characterisation of sweet cherry (Prunus avium L.) genotypes using peach (Prunus per-sica (L.) Batsch) SSR sequences. Heredity. 2002;89:56–63. doi: 10.1038/sj.hdy.6800101. [DOI] [PubMed] [Google Scholar]
- 27.Clausen S.K., Andersen S.B., Henriksen K., Toldam-Andersen T.B., Grout B. Assessment of genetic diversity within sour cherry clones. Sci. Hortic. 2013;164:556–562. doi: 10.1016/j.scienta.2013.10.012. [DOI] [Google Scholar]
- 28.Janes J.K., Steane D.A., Vaillancourt R.E. What does population structure analysis reveal about the Pterostylis longifolia complex (Orchidaceae)? Ecol. Evol. 2012;2:2631–2644. doi: 10.1002/ece3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Struss D., Boritzki M., Glozer K., Southwick S.M. Detection of genetic diversity among populations of sweet cherry (Prunus avium L.) by AFLPs. J. Hortic. Sci. Biotechnol. 2001;76:362–367. doi: 10.1080/14620316.2001.11511378. [DOI] [Google Scholar]
- 30.Zhou L., Kappel F., Hampson C., Wiersma P.A., Bakkeren G. Genetic Analysis and Discrimination of Sweet Cherry Cu-litvars and Selections Using Amplified Fragment Length Polymorphism Fingerprints. J. Am. Soc. Horti Cult. Sci. 2002;127:786–793. doi: 10.21273/JASHS.127.5.786. [DOI] [Google Scholar]
- 31.Vanderzande S., Zheng P., Cai L., Barac G., Gasic K., Main D., Iezzoni A., Peace C. The cherry 6+9K SNP array: A cost-effective improvement to the cherry 6K SNP array for genetic studies. Sci. Rep. 2020;10:7613. doi: 10.1038/s41598-020-64438-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powell W., Morgante M., Andre C., Hanafey M., Vogel J., Tingey S., Rafalski A. The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol. Breed. 1996;2:225–238. doi: 10.1007/BF00564200. [DOI] [Google Scholar]
- 33.Xu Q., Wen X., Deng X. A simple protocol for isolating genomic DNA from chestnut rose (Rosa roxburghii tratt) for RFLP and PCR analyses. Plant Mol. Biol. Rep. 2004;22:301–302. doi: 10.1007/BF02773140. [DOI] [Google Scholar]
- 34.Clarke J.B., Tobutt K.R. Development and characterization of polymorphic microsatellites from Prunus avium ‘Napoleon’. Mol. Ecol. Notes. 2003;3:578–580. doi: 10.1046/j.1471-8286.2003.00517.x. [DOI] [Google Scholar]
- 35.Vaughan S.P., Russell K. Characterization of novel microsatellites and development of multiplex PCR for large-scale popu-lation studies in wild cherry, Prunus avium. Mol. Ecol. Notes. 2004;4:429–431. doi: 10.1111/j.1471-8286.2004.00673.x. [DOI] [Google Scholar]
- 36.Testolin R., Marrazzo T., Cipriani G., Quarta R., Verde I., Dettori M.T., Pancaldi M., Sansavini S. Microsatellite DNA in peach [Prunus persica (L.) Batsch] and its use in fingerprinting and testing the genetic origin of cultivars. Genome. 2000;43:512–520. doi: 10.1139/g00-010. [DOI] [PubMed] [Google Scholar]
- 37.Aranzana M.J., Garcia-Mas J., Carbo J., Arus P. Development and variability of microsatellite markers in peach. Plant Breed. 2002;121:87–92. doi: 10.1046/j.1439-0523.2002.00656.x. [DOI] [Google Scholar]
- 38.Mnejja M., Garcia-Mas J., Howad W., Badenes M.L., Arus P. Simple-sequence repeat (SSR) markers of Japanese plum (Prunus salicina Lindl.) are highly polymorphic and transferable to peach and almond. Mol. Ecol. Notes. 2004;4:163–166. doi: 10.1111/j.1471-8286.2004.00603.x. [DOI] [Google Scholar]
- 39.Dirlewanger E., Cosson P., Tavaud M., Aranzana M.J., Poizat C., Zanetto A.e.a. Development of microsatellite markers in peach [Prunus persica (L.) Batsch] and their use in genetic diversity analysis in peach and sweet cherry (Prunus avium L.) Theor. Appl. Genet. 2002;105:127–138. doi: 10.1007/s00122-002-0867-7. [DOI] [PubMed] [Google Scholar]
- 40.Vos P., Hogers R., Bleeker M., Reijans M., van de Lee T., Hornes M., Frijters A., Pot J., Peleman J., Kuiper M., et al. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peakall R., Smouse P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mo-Lecular Ecol. Notes. 2006;6:288–295. doi: 10.1111/j.1471-8286.2005.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peakall R., Smouse P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics. 2012;28:2537–2539. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hildebrand C.E., Torney D.C., Wagner R.P. Informativeness of Polymorphic DNA Markers. Los Alamos Sci. 1992;20:100–102. [Google Scholar]
- 44.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2020. [(accessed on 23 February 2021)]. Available online: https://r-project.org. [Google Scholar]
- 45.Kamvar Z.N., Tabima J.F., Grünwald N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ. 2014;2:e281. doi: 10.7717/peerj.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamvar Z.N., Brooks J.C., Grünwald N.J. Novel R tools for analysis of genome-wide population genetic data with empha-sis on clonality. Front. Genet. 2015;6:208. doi: 10.3389/fgene.2015.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jombart T. adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics. 2008;24:1403–1405. doi: 10.1093/bioinformatics/btn129. [DOI] [PubMed] [Google Scholar]
- 48.Jombart T., Ahmed I. adegenet 1.3-1: New tools for the analysis of genome-wide SNP data. Bioinformatics. 2011;27:3070–3071. doi: 10.1093/bioinformatics/btr521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jombart T., Collins C. A Tutorial for Discriminant Analysis of Principal Components (DAPC) Using Adegenet 2.0. 0. Imperial College London, MRC Centre for Outbreak Analysis and Modelling; London, UK: 2015. pp. 1–43. [Google Scholar]
- 50.Jombart T., Devillard S., Balloux F. Discriminant analysis of principal components: A new method for the analysis of genet-ically structured populations. BMC Genet. 2010;11:94. doi: 10.1186/1471-2156-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wünsch A., Hormaza J.I. Molecular evaluation of genetic diversity and S-allele composition of local Spanish sweet cherry (Prunus avium L.) cultivars. Genet. Resour. Crop Evol. 2004;51:635–641. [Google Scholar]
- 52.Braun-Lüllemann A., Bannier H.-J. Obstsortenwerk Alte Süßkirschsorten. Erstellt im Rahmen eines Modell- und Demonstrationsvorhabens im Bereich der Biologischen Vielfalt, Gefördert Durch die BLE. 2010.
- 53.Modl P., Spornberger A. Kirschen für den Naturnahen Hausgarten. Österreichischer Agrarverlag; Wien, Austria: 2009. [Google Scholar]
- 54.Barth S., Forneck A., Verzeletti F., Blaich R., Schumann F. Genotypes and phenotypes of an ex situ Vitis vinifera ssp. syl-vestris (Gmel.) Beger germplasm collection from the Upper Rhine Valley. Genet. Resour. Crop Evol. 2009;56:1171–1181. doi: 10.1007/s10722-009-9443-1. [DOI] [Google Scholar]
- 55.McKey D., Elias M., Pujol B., Duputie A. The evolutionary ecology of clonally propagated domesticated plants. New Phytol. 2010;186:318–332. doi: 10.1111/j.1469-8137.2010.03210.x. [DOI] [PubMed] [Google Scholar]
- 56.Forneck A. Plant Breeding: Clonality—A Concept for Stability and Variability During Vegetative Propagation. In: Esser K., Lüttge U., Beyschlag W., Murata J., editors. Progress in Botany. Springer; Berlin/Heidelberg, Germany: 2005. pp. 164–183. [Google Scholar]
- 57.Zhang G., Sebolt A.M., Sooriyapathirana S.S., Wang D., Bink M.C., Olmstead J.W., Iezzoni A.F. Fruit size QTL analysis of an F1 population derived from a cross between a domesticated sweet cherry cultivar and a wild forest sweet cherry. Tree Genet. Genomes. 2010;6:25–36. doi: 10.1007/s11295-009-0225-x. [DOI] [Google Scholar]
- 58.Schüller E., Halbwirth H., Mikulic-Petkovsek M., Slatnar A., Veberic R., Forneck A., Stich K., Spornberger A. High con-centrations of anthocyanins in genuine cherry-juice of old local Austrian Prunus avium varieties. Food Chem. 2015;173:935–942. doi: 10.1016/j.foodchem.2014.10.113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from SSR- and AFLP-analysis is provided as supplemental files (Supplement S1, Supplement S2) to this manuscript.