Abstract
Background:
The majority of sudden cardiac deaths (SCD) occur in low risk populations often as the first manifestation of cardiovascular disease (CVD). Biomarkers are screening tools that may identify subclinical CVD and those at elevated risk for SCD. We sought to determine whether the total to high-density lipoprotein cholesterol ratio (TC:HDL), cardiac troponin I (hsTnI), B-type natriuretic peptide (NT-proBNP), or C-reactive protein (hsCRP) individually or in combination could identify individuals at higher SCD risk in large, free-living populations with and without CVD.
Methods:
We performed a nested case-control study within 6 prospective cohort studies utilizing 565 SCD cases matched to 1090 controls (1:2) by age, sex, ethnicity, smoking status, and presence of CVD.
Results:
The median study follow-up time until SCD was 11.3 years. When examined as quartiles or continuous variables in conditional logistic regression models, each of the biomarkers was significantly and independently associated with SCD risk after mutually controlling for cardiac risk factors and other biomarkers. The mutually adjusted odds ratios (95% CI) for the top compared to the bottom quartile were 1.90 (1.30–2.76) for TC:HDL, 2.59 (1.76–3.83) for hsTnI, 1.65 (1.12 −2.44) for NT-proBNP, and 1.65 (1. 13 −2.41) for hsCRP. A biomarker score that awarded one point when the concentration of any of those four biomarkers was in the top quartile (score range, 0–4) was strongly associated with SCD, with an adjusted OR (95% CI) of 1.56 (1.37–1.77) per 1 unit increase in the score.
Conclusions:
Widely available measures of lipids, subclinical myocardial injury, myocardial strain, and vascular inflammation show significant independent associations with SCD risk in apparently low risk populations. In combination these measures may have utility to identify individuals at risk for SCD.
Keywords: troponin, inflammation, NT-proBNP, sudden death, lipids, cholesterol
Introduction
Sudden cardiac death (SCD) is a common cause of death in the United States. While the relative risk of SCD increases with the presence of cardiovascular disease (CVD), the vast majority of SCDs occur in low-risk populations without known or established CVD at the time of death. As a result, identifying individuals at risk for SCD before a fatal event is difficult because of the need to screen large numbers of individuals who are each at seemingly low risk for the event.
In order to be feasible and gain widespread clinical acceptance, SCD risk stratification in such a broad population will require pragmatic screening tools that can be easily applied and integrated into routine care at relatively low cost. Accumulating data from population-based prospective cohorts support a potential prognostic role of several commercially available biomarkers on SCD risk. In particular, measures of ongoing myocardial injury (cardiac troponin) myocardial stress (B-type natriuretic peptides), and inflammation (hsCRP) have been associated with SCD in individual prospective studies.1–3 However, replication is limited and the relatively small numbers of SCD in individual prospective studies has precluded an adequate assessment of the independence and additive value of these biomarker SCD associations.
To address these issues, we measured cardiac troponin I (hsTnI), the amino-terminal fragment of the prohormone of B-type natriuretic peptide (NT-proBNP), and high-sensitivity C-reactive protein (hsCRP) in a prospective nested case-control study of 1655 subjects, which included 565 sudden cardiac death events and 1090 controls. In order to increase the number of cases, without decreasing specificity, SCD cases were pooled from six NIH-funded prospective cohorts utilizing identical rigorous adjudication methods and analyses that simultaneously controlled for cardiac risk factors and biomarkers were performed.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure, because insufficient sample remains to replicate results in all patients, and per our current IRB protocol, access to individual patient level data for analyses is limited to qualified affiliated study investigators as approved by Brigham and Women’s Hospital IRB.
Study Population
We designed a nested case control study, with subjects included from Physicians Health Study I and II, the Nurses’ Health Study, the Health Professionals Follow-Up Study, the Women’s Health Study, and the Women’s Antioxidant Cardiovascular Study. All 6 prospective cohorts employed similar methods to document the timing and mechanism of cardiovascular deaths as previously described 4 – including ascertainment and review of medical records (inclusive of emergency medical services, emergency room, hospital, and autopsy reports as available) and standardized interviews with next-of-kin or witnesses to obtain detailed descriptions of the circumstances surrounding the death. In total, 565 cases and 1090 controls with total and high-density lipoprotein (HDL) cholesterol, hsTnI, NT-proBNP, and hsCRP measurements were identified with risk set sampling. Control subjects were matched roughly 2:1 within each cohort on age (± 1–3 years), sex, race, smoking status (current, never, past), time and date of blood sampling, follow up time, and the presence or absence of cardiovascular disease (CVD; defined as myocardial infarction, coronary artery bypass graft surgery, angina, or stroke) either at the time of blood sampling or during study follow up. The study protocol was approved by the Brigham and Women’s Hospital/Partners Healthcare Institutional Review Board.
Endpoint Definitions
Sudden cardiac death (SCD) was defined as a sudden and/or arrhythmic death. Definite SCD was defined as a death or a fatal cardiac arrest that occurred within 1 hour of symptom onset, according to next of kin or medical record report (n=380) or with autopsy findings consistent with SCD (n=33). Deaths that occurred during sleep or deaths that were unwitnessed where the participant was documented to be symptom free within the preceding 24 hours were considered probable SCD and were included in the SCD endpoint (n=116).
Deaths were also classified as presumed arrhythmic or non-arrhythmic using the definition of Hinkle and Thaler.5 An abrupt spontaneous loss of pulse was considered an arrhythmic death provided there was no evidence of preceding circulatory impairment, such as with congestive heart failure or shock, or evidence neurological dysfunction. When the pulse gradually disappeared before death, the death was defined as non-arrhythmic and was excluded from the study primary endpoint of SCD. Deaths that fulfilled the criteria for arrhythmic death but were preceded by >1 hour of symptoms were included in the combined SCD endpoint (n=36).
Biomarker measurements
High sensitivity Troponin I (hsTnI) was measured using a high-sensitivity assay provided by Siemens for use on a Dimension Vista platform. The assay was investigational at the time of use for this study, but it has since been approved for commercial use by the U.S. Food and Drug Administration.6 The commercial assay has a limit of detection of 2.0 ng/L. Since the assay was investigational when we performed the analyses, the laboratory reported all values with a signal above the lowest calibrator value (which is set nominally at 0), and these lower values were included in the analysis. Of the 1655 study participants, 345 (21%) had an hsTnI concentration below the commercial LOD of 2 ng/L. The coefficient of variation at 4.4 ng/L was reported to be 8.5%.6, 7 The 99th percentile is reported as 59 ng/L in a population of men and women.8 NT-proBNP was also measured using an commercially available assay provided by Siemens Healthcare. All blood specimens were assayed centrally for total cholesterol, high-density cholesterol, and high-sensitivity C-reactive protein (hsCRP) using commercially available assays 2.
Statistical Analyses
To account for the correlation within matched sets, baseline characteristics were compared using generalized estimated equations with PROC GENMOD of SAS for categorical variables and using linear models with an unstructured covariance matrix with PROC MIXED of SAS for continuous variables. We tested for Spearman correlations between biomarkers in the control population. We utilized conditional logistic regression to test for associations between baseline concentrations of total to high-density lipoprotein cholesterol ratio (TC:HDL), hsTnI, NT-proBNP, and hsCRP and the risk of SCD using conditional logistic regression.9 After demonstrating no significant heterogeneity across cohorts in individual study results in a random-effects meta-analysis, a pooled analysis was performed. Biomarkers were analyzed in quartiles based on the overall distribution in the controls (Table I in the Supplement) and using established clinical thresholds.
Biomarkers were also analyzed as continuous variables after natural logarithm transformations were performed to improve normality. For ease of comparison, we calculated the odds ratios per 1 standard deviation (SD) change in the biomarker. The SD for the log of each biomarker was calculated using measures from all available 1655 subjects (Table II in the Supplement). For NT-proBNP, a quadratic term (set equal to 0 at the mean NT-proBNP concentration) was added to these models to test for nonlinearity as suggested in the quartile results.
The above analyses were adjusted for possible confounders in multivariable models. Model 1 included further adjustment for imperfectly matched variables (age and smoking status). Model 2 adjusted for the covariables included in Model 1, as well as hypertension, family history of MI, history of hypercholesterolemia, body mass index, physical activity, alcohol intake, aspirin use, and fasting status. Sensitivity analyses controlling for hemoglobin A1c (n=429 cases and 820 controls) and estimated glomerular filtration rate (eGFR; n=551 cases and 1077 controls) were also performed in the subset of patients with these measures available. Stratified analyses and interaction terms were used to test for effect modification by sex and presence or absence of known cardiovascular disease
To evaluate the independence of biomarker associations, biomarkers were entered simultaneously into multivariable adjusted models. To explore the additive value of the biomarker measurements, we constructed a simple integer biomarker score that assigned 1 point for each biomarker concentration in the upper quartile of the biomarker distribution in the control population, and then examined the association of the score with SCD in separate multivariable conditional logistic regression models.
Results
Baseline Characteristics, Traditional Risk Factors, and Biomarkers
In total, 565 cases of SCD occurred during a median (IQR) follow up of 11.3 (8.8–21.9) years. These 565 cases were paired with 1090 matched controls. The baseline characteristics collected closest to the time of blood draw for the cases and controls are outlined in Table 1. SCD cases were more likely than controls to have a history of hypertension, diabetes, higher BMI, and were less likely to engage in regular physical activity. They also had a significantly lower high-density lipoprotein cholesterol (HDL-C), and higher TC:HDL-C ratio, hemoglobin A1c, hsTnI, NT-proBNP, and hsCRP concentrations at baseline. Aside from the known correlations between total cholesterol, HDL-C, and their ratio, only hsTnI and NT-proBNP showed moderate correlation (rho 0.26, P<0.0001; Table III in the Supplement).
Table 1.
Baseline characteristics of the study population according to case or control status. Continuous characteristics are presented as median and interquartile range and categorical variables are presented as n (%).
| Case (n=565) | Control (n=1090) | P value | |
|---|---|---|---|
| Age, years, median (Q1-Q3) | 64 (57–69) | 63 (57–69) | Matching Factor |
| Female, n (%) | 230 (41) | 440 (40) | Matching Factor |
| White, n (%) | 547 (97) | 1061 (97) | Matching Factor |
| Current Smoker, n (%) | 90 (16) | 163 (15) | Matching Factor |
| History of Prior CVD, n (%) | 198 (35) | 368 (34) | Matching Factor |
| Family History of MI <60 y of age, n (%) | 104 (18) | 166 (15) | 0.09 |
| History of Hypertension, n (%) | 316 (56) | 439 (40) | <0.0001 |
| History of High Cholesterol, n (%) | 226 (40) | 441 (40) | 0.86 |
| Body mass index, median (Q1-Q3), kg/m2 | 25.8 (23.7–28.6) | 24.8 (23.0–27.8) | 0.0002 |
| Diabetes, n (%) | 81 (14) | 78 (7) | <0.0001 |
| Hemoglobin A1C, median (Q1-Q3) | 5.8 (5.5–6.2) | 5.7 (5.5–6.0) | <0.0001 |
| Aspirin Use, n (%) | 229 (41) | 421 (39) | 0.43 |
| Alcohol Intake, gm/d, median (Q1-Q3) | 2.4 (0–13.0) | 3.5 (0–15.0) | 0.02 |
| Weekly Exercise, n (%) | 324 (57) | 684 (63) | 0.03 |
| Fasting, n (%) | 320 (57) | 604 (55) | 0.40 |
| Total cholesterol, mg/dL, median (Q1-Q3) | 205 (177–234) | 203 (179–232) | 0.25 |
| High-density lipoprotein cholesterol, mg/dL, median (Q1-Q3) | 46 (37–59) | 49 (40–60) | 0.0003 |
| Total to high-density lipoprotein cholesterol ratio (Q1-Q3) | 4.4 (3.5–5.4) | 4.1 (3.3–5.1) | <0.0001 |
| eGFR, ml/min/1.73m2 | 65.2 (52.7–86.9) | 63.5 (53.3–82.8) | 0.78 |
| High-sensitivity cardiac troponin I, ng/L, median (Q1-Q3) | 3.4 (2.4–6.0) | 2.7 (2.0–4.0) | <0.0001 |
| NT-proBNP, ng/L, median (Q1-Q3) | 87 (45–236) | 73 (38–130) | <0.0001 |
| High-sensitivity C-reactive protein, mg/L, median (Q1-Q3) | 2.0 (0.9–4.7) | 1.5 (0.7–3.7) | <0.0001 |
Abbreviations: CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction
Biomarkers and SCD Risk
Each of the four biomarkers was significantly associated with SCD both in minimally adjusted models (Model 1, Table IV in the Supplement) and in more fully adjusted estimates (Model 2, Figure 1). As compared to those in the lowest quartile of each biomarker, individuals in the highest quartile were at significantly elevated risk for SCD, with an adjusted OR (aOR; 95% CI) of 1.75 (1.22–2.49) for TC:HDL, 2.91 (2.02–4.19) for hsTnI, 2.05 (1.43–2.92) for NT-proBNP, and 1.75 (1.22–2.52) for hsCRP. The odds of SCD increased in a linear fashion across quartiles of the TC:HDL ratio, hsTnI and hsCRP. By contrast, the risk of SCD did not increase in a linear fashion across quartiles of NT-proBNP, raising the possibility of a non-linear association or threshold effect for the highest quartile, which was >130 ng/L in the pooled control subjects
Figure 1. Increasing Concentrations of Cardiovascular Biomarkers and the Risk of Sudden Cardiac Death.
Adjusted odds ratios and 95% confidence intervals for sudden cardiac death according to increasing quartiles of natural logarithm transformed total to high-density lipoprotein cholesterol ratio (Ln-TC:HDL), high-sensitivity cardiac troponin I (hsTnI), NT-proBNP (NTproBNP), and high-sensitivity C-reactive protein (hsCRP). Odds ratios and 95% CI are adjusted for matching factors (age, sex, smoking status, white race and cardiovascular disease status), and further adjusted for age, aspirin use, overweight or obese, weekly exercise, alcohol use, hypertension, parental history of myocardial infarction, history of high cholesterol, and diabetes (Multivariable Model 2).
The common clinical thresholds used for each of the novel biomarkers and their association with SCD are displayed in Figure 2. As can be seen, the clinical thresholds for total cholesterol to HDL-C ratio (≥ 4 and ≥ 5), hsTnI ≥ 6 ng/L (vs. < 6 ng/L) and ≥ 10 ng/L (vs. <10 ng/L), NT-proBNP ≥ 125 ng/L (vs. < 125 ng/L) and ≥300 ng/L (vs. <300 ng/L) and hsCRP ≥2 mg/L and ≥3 mg/L (vs. <2 mg/L and <3 mg/L, respectively) are all associated with an increased risk, although the association with hsCRP was not statistically significant.
Figure 2. Common Clinical Thresholds for Cardiovascular Biomarkers and the Risk of Sudden Cardiac Death.
Adjusted odds ratios and 95% confidence intervals for sudden cardiac death for various clinical cutpoints of total to high-density cholesterol ratio (TC:HDL), high-sensitivity cardiac troponin I (hsTnI, in ng/L), NT-proBNP, in ng/L, and high-sensitivity C-reactive protein (hsCRP) in mg/L. Odds ratios are adjusted for matching factors (age, sex, smoking status, white race, and cardiovascular disease status) and further adjusted for age, aspirin use, overweight or obese, weekly exercise, alcohol use, hypertension, parental history of myocardial infarction, history of high cholesterol, and diabetes (Multivariable Model 2).
We then tested for evidence of a linear association between baseline concentrations of natural logarithm-transformed concentrations of TC:HDL-C ratio, hsCRP, hsTnI, and NT-proBNP, each normalized to per 1-SD unit increase (Table 2). Because of the shape of the relationship between NT-proBNP and SCD noted in Figure 1, we added a squared term for NT-proBNP to the multivariable model assessing the association between NT-proBNP and SCD. Each biomarker was significantly associated with SCD after adjustment for age, aspirin use, overweight or obesity, exercise, alcohol use, hypertension, parental history of MI, history of high cholesterol, and diabetes, with odds ratios (OR) (95% CI) per 1-SD change of 1.22 (1.07–1.38) for Ln(TC:HDL), 1.50 (1.33–1.71) for Ln(hsTnI), 1.33 (1.15–1.53) for Ln(NT-proBNP), and 1.23 (1.08–1.40) for Ln(hsCRP) (Table 2, Model 2). While there was a significant curvilinear relation of NT-proBNP to SCD, the linear term for NT-proBNP was also associated with SCD when we did not include the squared term in the model [OR=1.45 (1.27–1.66)].
Table 2.
Associations of lipid, cardiac, and inflammatory biomarkers with sudden cardiac death.
| Biomarker | Model 1* Adjusted Odds Ratio (95% CI) | Model 1 P-value | Model 2† Adjusted Odds Ratio (95% CI) | Model 2 P-value | Model 3‡ Adjusted Odds Ratio (95% CI) | Model 3 P-value |
|---|---|---|---|---|---|---|
| Ln(TC:HDL ratio)/SD | 1.26 (1.13–1.41) | <0.0001 | 1.22 (1.07–1.38) | 0.002 | 1.26 (1.10–1.44) | 0.0008 |
| Ln(hsTnI)/SD | 1.55 (1.37–1.74) | <0.0001 | 1.50 (1.33–1.71) | <0.0001 | 1.38 (1.20–1.58) | <0.0001 |
| Ln(NT-proBNP)/SD§ | 1.37 (1.20–1.57) | <0.0001 | 1.33 (1.15–1.53) | 0.0001 | 1.23 (1.05–1.43) | 0.01 |
| Ln(hsCRP)/SD | 1.32 (1.17–1.48) | <0.0001 | 1.23 (1.08–1.40) | 0.002 | 1.18 (1.03–1.35) | 0.02 |
Model 1 adjusted for matching factors (age, sex, current smoking, white race and cardiovascular disease status), and further adjusted for age.
Model 2 adjusted for matching factors and further adjusted for age, aspirin use, overweight or obese, weekly exercise, alcohol use, hypertension, parental history of myocardial infarction, history of high cholesterol, and diabetes.
Model 3 adjusted for matching factors, the covariates in Model 2, and mutually adjusted for Ln(TC:HDL-C ratio), Ln(hsTnI), Ln(NT-proBNP), and Ln(hsCRP).
A centered quadratic term for Ln(NT-proBNP) was added to Models 1, 2 and 3 and was statistically significant, with ORs (95% CI) of 1.13 (1.05–1.22; P=0.001) in Model 1, OR (95% CI) 1.13 (1.05–1.23; P=0.002) in Model 2, and OR 1.11 (1.03–1.20; P=0.01) in model 3. The P-values reported in the table are those for the linear NT-proBNP term after adjusting for the quadratic term.
Abbreviations: HDL, high-density lipoprotein cholesterol; hsTnI, high sensitivity cardiac troponin I; hsCRP, high-sensitivity C-reactive protein; NT-proBNP, amino-terminal prohormone B-type natriuretic peptide; SD, standard deviation; TC, total cholesterol
In a sensitivity analysis which adjusted for hemoglobin A1c (429 cases and 820 controls) in addition to the covariates in Model 2, the results were essentially unchanged, although the relationship between hsCRP and SCD was modestly attenuated after control for hemoglobin A1c [OR (95% CI) per 1SD change in Ln(hsCRP) 1.15 (0.98–1.34, P=0.08)]. A second sensitivity analysis which added eGFR (551 cases and 1077 controls) to the covariables in Model 2 revealed no substantial change in the results observed associations (data not shown). Results were also similar in sensitivity analyses restricted to “definite” SCD cases (407 cases matched with 777 controls) as well (data not shown).
Stratified Analyses:
In stratified analyses (Table 3), we observed statistically significant evidence that sex modified the association between the TC:HDL-C ratio and the risk of SCD. Specifically, the association with SCD was higher among men (OR 2.45, 95% CI 1.51–3.96) than it was among women (OR 1.11, 95% CI 0.60–2.05; P-interaction=0.04). Sex did not appear to modify the association of the other biomarkers with SCD. In analyses stratified according to whether clinically detected CVD was present before SCD, the magnitude of the association with SCD per 1-SD change in Ln(hsTnI) was significantly greater in those without known CVD (OR 1.87, 95% CI 1.52–2.31) than in those with CVD (OR 1.36, 95% CI 1.08–7.71; P-interaction=0.04). There was no evidence for effect modification by CVD status for the TC: HDL-C ratio, NTproBNP, or hsCRP.
Table 3.
Associations of novel biomarkers with sudden cardiac death, stratified by sex or the presence or absence of established cardiovascular disease at baseline.
| Biomarker | Model 2* Adjusted Odds Ratio (95% CI) | Model 2 P-value |
|---|---|---|
| Sex-stratified analysis | ||
| Ln(TC:HDL)/SD | ||
| Male | 2.45 (1.51–3.96) | 0.0003 |
| Female | 1.11 (0.60–2.05) | 0.73 |
| Test for interaction† | 0.04 | |
| Ln(hsTnI)/SD | ||
| Male | 1.57 (1.31–1.89) | <0.0001 |
| Female | 1.77 (1.35–2.31) | <0.0001 |
| Test for interaction† | 0.48 | |
| Ln(NT-proBNP)/SD | ||
| Male | 1.45 (1.25–1.68) | <0.0001 |
| Female | 1.28 (1.06–1.55) | 0.01 |
| Test for interaction† | 0.34 | |
| Ln(hsCRP)/SD | ||
| Male | 1.26 (1.09–1.47) | 0.002 |
| Female | 1.12 (0.94–1.33) | 0.22 |
| Test for interaction† | 0.28 | |
| Established CVD-stratified analysis | ||
| Ln(TC:HDL)/SD | ||
| Without CVD | 2.16 (1.32–3.54) | 0.002 |
| With CVD | 1.45 (0.82–2.56) | 0.21 |
| Test for interaction† | 0.28 | |
| Ln(hsTnI)/SD | ||
| Without CVD | 1.87 (1.52–2.31) | <0.0001 |
| With CVD | 1.36 (1.08–1.71) | 0.0009 |
| Test for interaction† | 0.04 | |
| Ln(NT-proBNP)/SD | ||
| Without CVD | 1.37 (1.18–1.59) | <0.0001 |
| With CVD | 1.41 (1.16–1.70) | 0.0004 |
| Test for interaction† | 0.82 | |
| Ln(hsCRP)/SD | ||
| Without CVD | 1.22 (1.07–1.41) | 0.004 |
| With CVD | 1.15 (0.95–1.40) | 0.15 |
| Test for interaction† | 0.61 |
Model 2 adjusted for matching factors (age, sex, current smoking, white race) and further adjusted for age, aspirin use, overweight or obese, weekly exercise, alcohol use, hypertension, parental history of myocardial infarction, history of high cholesterol, and diabetes.
Evidence of effect modification tested with a sex*biomarker or CVD*biomarker interaction term in a model containing terms for the main effects of CVD or sex and the main effects of the biomarker.
Abbreviations: HDL, high-density lipoprotein cholesterol; hsTnI, high sensitivity cardiac troponin I; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein cholesterol; NT-proBNP, amino-terminal prohormone B-type natriuretic peptide; TC, total cholesterol
Independence and Additive Biomarker-SCD Associations
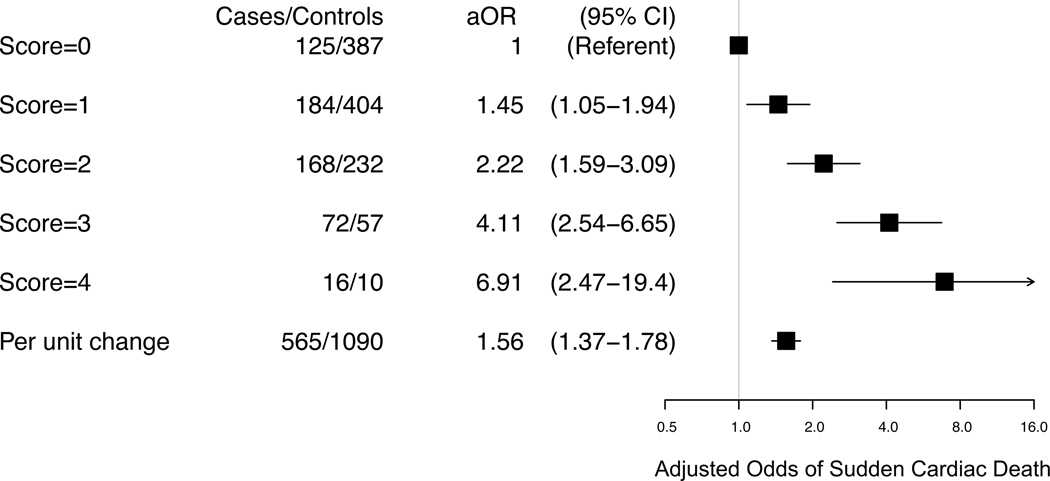
In multivariable models that mutually adjusted for all the biomarkers (Model 3, Table 2 and Table 4), the continuous and quartile associations described above for each of the biomarkers retained statistical significance. The associations per 1-SD change in natural logarithm transformed biomarker concentrations of hsTnI (OR 1.38, 95% CI 1.20–1.58), NT-proBNP (OR 1.23, 95% CI 1.05–1.43), and hsCRP (OR 1.18, 95% CI 1.03–1.35) were modestly attenuated, whereas the association for TC:HDL-C was not (OR 1.26, 95% CI 1.10–1.44) (Model 3, Table 2). The additive association of elevated biomarker measurements are demonstrated in Figure 3. Those with biomarker concentrations in the top quartile for all 4 biomarkers (Score =4), had 6.9 times higher odds of SCD (95%CI, 2.5–19.4) than those without any biomarkers in the top quartile (Score=0). Each one unit increase in the score was association with a 56% increase in the odds of SCD (P<0.0001). Because the top risk group (Score=4) has relatively few subjects (16 cases and 10 controls) we conducted a sensitivity analysis in which the top two scores (Score = 3 and Score = 4) were combined into one group. For this combined group of 88 cases and 67 controls, the odds of SCD compared to those with a score=0 was 4.38 (95% CI 2.75–6.98).
Table 4.
Mutually adjusted associations of quartiles of total cholesterol to high-density lipoprotein cholesterol ratio, high-sensitivity cardiac troponin I, NT-proBNP, and high-sensitivity C-reactive protein, with sudden cardiac death.
| Model 2 Adjusted* Odds Ratio (95% CI) | |||||
|---|---|---|---|---|---|
| Biomarker | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trend |
| TC:HDL | 1.0 (referent) | 1.11 (0.78–1.59) | 1.35 (0.94–1.93) | 1.90 (1.30–2.76) | 0.0005 |
| hsTnI | 1.0 (referent) | 1.24 (0.85–1.79) | 2.09 (1.43–3.06) | 2.59 (1.76–3.83) | <0.0001 |
| NT-proBNP | 1.0 (referent) | 1.17 (0.83–1.64) | 0.76 (0.51–1.12) | 1.65 (1.12–2.44) | 0.02 |
| hsCRP | 1.0 (referent) | 1.38 (0.97–1.96) | 1.53 (1.06–2.21) | 1.65 (1.13–2.41) | 0.01 |
Adjusted for matching factors (age, sex, white race, current smoking and cardiovascular disease status) and further adjusted for age, aspirin use, overweight or obese, weekly exercise, alcohol use, hypertension, parental history of myocardial infarction, history of high cholesterol, and diabetes.
Abbreviations: HDL, high-density lipoprotein cholesterol; hsTnI, high sensitivity cardiac troponin I; hsCRP, high-sensitivity C-reactive protein; NT-proBNP, amino-terminal prohormone B-type natriuretic peptide; TC, total cholesterol
Figure 3. Biomarker Score and the Risk of Sudden Cardiac Death.
Adjusted odds ratios and 95% confidence intervals for sudden cardiac death according to increasing values for a biomarker risk score constructed from the concentrations of total to high-density cholesterol ratio, high-sensitivity cardiac troponin I, NT-proBNP, and high-sensitivity C-reactive protein at baseline. Each participant is given one point for each biomarker level that falls in the top quartile of the distribution in the control population. Odds ratios are adjusted for matching factors (age, sex, smoking status, white race, and cardiovascular disease status) and further adjusted for age, aspirin use, overweight or obese, weekly exercise, alcohol use, hypertension, parental history of myocardial infarction, history of high cholesterol, and diabetes (Multivariable Model 2).
Discussion
In this nested case-control study of 565 SCD cases drawn from 6 prospective cohorts of men and women, we found that baseline concentrations of TC:HDL-C ratio, hsCRP, hsTnI, and NT-proBNP were independently associated with risk for future SCD. Each of these biomarkers was consistently associated with SCD even among those without established CVD, raising the possibility that they may be useful in identifying otherwise asymptomatic men and women at risk for SCD as their first manifestation of CVD. Each of the four biomarkers, marking distinct biological pathways of dyslipidemia (TC:HDL-C ratio), myocardial injury (hsTnI), myocardial stretch or strain (NT-proBNP), and subclinical vascular inflammation (hsCRP) were independent and additive of one another in their association with future SCD.
To our knowledge, our study comprises the largest collection of SCD cases with prospectively collected blood samples and is the first to report on biomarker-SCD associations after controlling for other biomarkers. Our results are generally consistent with prior studies reporting the association of individual biomarkers and SCD, some of which are included in this larger sample 1–3, 10, 11. However, our results differ from a more recent report from the Cardiovascular Health Study (CHS) involving 171 SCD cases, where significant associations for each of these three biomarkers were attenuated and became non-significant after adjusting for established cardiovascular risk factors 12. These later results were contradictory to prior published results from the CHS utilizing a larger number of SCD cases 1, 3, 11 controlling for a larger number of factors, and presumably the discrepancy is due to sample size or variable control for confounders.
We believe these results are of clinical importance for several reasons. First, SCD is often the first manifestation of CVD in otherwise asymptomatic individuals. Even among those with known CVD, the majority of SCDs do not occur in patients deemed to be at high risk.13, 14 Cardiac arrest usually does not have antecedent symptoms, and is often fatal.15, 16 Thus, there is a clinical need for tools to help identify individuals at elevated risk of SCD in broad populations of seemingly healthy individuals. Here, we have demonstrated that measures of TC:HDL, hsTnI, NTproBNP, and hsCRP were all associated with SCD independent of one another and of other established risk factors for CVD. We also demonstrate that these biomarker SCD associations were additive, such that participants with measurements in the top quartile of each biomarker from the four distinct biological pathways were at a marked ~7-fold higher SCD risk than those who were not in the top quartile for any biomarker.
Second, the association between TC:HDL-C ratio and SCD in our study suggests that atherosclerotic CVD remains a potentially preventable cause of SCD in the population 17, 18, particularly among men. Consistent with these findings, a recent study of this population found variants in the low-density lipoprotein receptor in SCD cases but not in controls 19. Of the biomarkers tested in this study, only lipids – here represented by the total to HDL cholesterol ratio - are recommended as a screening test to help target preventive therapy for CVD 20. Statin therapy has been demonstrated to reduce the risk of CVD mortality in patients at a broad range of baseline risk21, 22 and has been associated with a modest reduction in SCD risk in a meta-analysis of randomized trials23. Although the majority of autopsy studies, performed primarily in populations of Caucasian ethnicity, have suggested that atherothrombotic coronary lesions and/or pathologic signs of chronic ischemic heart disease are present in a majority of individuals who die suddenly 24–28, a recent multiethnic coroner-based study in San Francisco found underlying ischemic heart disease in only 32% of SCD cases 29. This later study, and several others, have also found that ischemic heart disease underlies a smaller proportion of SCD in women as compared to men.13, 27, 29–31. Consistent with these findings, we observed statistically significant evidence that sex modifies the association between the TC:HDL-C ratio and SCD, such that TC:HDL-C ratio was associated with markedly elevated risks for SCD in men but not in women. Thus, third, these data in aggregate suggest that sex-specific SCD prevention strategies may be needed.
These data also provide important clues regarding the pathophysiologic mechanisms underlying SCD. In our data, high-sensitivity cardiac troponin was associated with an increased risk of SCD, independent of the association observed for TC:HDL-C ratio. In addition to their association with atherosclerotic cardiovascular disease 32–35, high-sensitivity assays for cardiac troponin are also closely associated with measures of cardiac structure and function, including left ventricular hypertrophy and heart failure 32. Our results combined with these known associations suggest that hsTnI may associate with SCD through mechanisms beyond atherosclerosis alone. In our subgroup analyses, we found evidence that increasing concentrations of hsTnI have a stronger association with SCD among those who were not known to have clinically evident cardiovascular disease prior to SCD. While these results may be due to chance, these also serve to emphasize the potential importance of undiagnosed myocardial injury and/or structural heart disease as a cause of SCD in apparently healthy individuals. Interestingly, the observation that hsTnI has a stronger association with SCD in patients without clinically evident CVD is parallel to our observations for HbA1c and SCD in these cohorts36 raising the possibility that both glycemia and ongoing myocardial injury play a more important role in identifying patients at increased risk for SCD among those without CVD as compared to those with established CVD.36 Subclinical myocardial injury and glycemia are also known to be linked, so these two observations may be related to a common pathophysiologic process.37, 38
NT-proBNP, which is known to be a powerful marker of heart failure risk, has also emerged as one of the most powerful markers of future atherosclerotic cardiovascular disease risk.39–41 Our results suggest that even mild, subclinical volume or pressure overload is related to an increase risk of SCD. The strength of the association between NT-proBNP and SCD may be due to the biomarker’s ability to integrate the risk of heart failure and atherosclerotic-related arrhythmic events.39 Indeed, the association between NT-proBNP and SCD was consistent regardless of CVD status. The subclinical LV dysfunction and associated volume and/or pressure overload that triggers natriuretic peptide release may be related to SCD risk, even before patients develop clinical symptoms and signs consistent with heart failure.
Associations for hsCRP, a biomarker released in the setting of inflammation, and SCD were more modest, but did remain statistically significant after controlling for the other biomarkers suggesting a potentially independent contribution of inflammation to SCD risk. Prior studies examining associations between markers of inflammation with SCD or ventricular arrhythmic events have reported stronger associations for interleukin-6 than hsCRP,11, 42, 43 and potentially a stronger association might have been found with an alternative inflammatory biomarker.
Preventing SCD is challenging. While it remains a common cause of death, SCD often occurs in men and women without known CVD, and thus the target population is large and the individual absolute risk is low. Due to our case-control study design, we are unable to extrapolate these relative risk elevations to estimates of absolute SCD risk. However, we believe these data support the hypothesis that using these four biomarkers to identify individuals with otherwise unrecognized coronary or structural heart disease could help prevent SCD. These markers are often used to identify patients at risk of non-fatal manifestations of atherosclerosis. However, we would anticipate that standard preventive therapy taken to prevent non-fatal first manifestations of atherosclerosis would also prevent SCD as the initial manifestation of the disease. Abnormalities in these readily available biomarkers might prompt further diagnostic evaluation, with a history, physical exam, electrocardiogram, echocardiogram, and depending on the results, initiation of preventive therapy for newly diagnosed CHD and/or left ventricular dysfunction such as statins, anti-platelet therapy, beta blockers, afterload reduction and/or even an ICD if appropriate. This approach will require testing and validation in other community-based cohorts to determine if it might be effective in predicting and preventing SCD, as well as other CVD endpoints. The cost implications of implementing such wide screening in general populations will also need to be carefully considered.
The strengths and limitations of our study need to be considered. The strengths include the nested case-control, prospective design, with 565 cases of strictly defined SCD from 6 cohorts of men and women. While this represents a large number of cases, the potential for misclassification of non-sudden deaths as sudden remains. However, our sensitivity analysis that used only definite SCD cases produced similar results. One key limitation is that we did not have information on baseline history of HF in all the cohorts, nor did we routinely collect electrocardiographic or imaging information (e.g., left ventricular ejection fraction). We note, however, that the majority of SCDs in the community do not occur among those with reduced left ventricular ejection fraction.14, 44 Our study design is not optimal for assessing the discriminative properties of these biomarkers or determining absolute risk elevations, which represent crucial next steps in the evaluation of the clinical utility of these biomarkers for SCD risk prediction. In addition, we were unable to adjust for changes in the use of preventive medications that may have occurred during study follow up. However, If these therapies are effective at CV event reduction, we would expect a bias towards the null. For example, patients with the highest TC:HDL ratio are also the most likely to start statin therapy, which is then likely to prevent or delay a major adverse cardiovascular event, attenuating any association between elevations in TC:HDL ratio at baseline and the outcome of interest (SCD).
In conclusion, we report robust evidence that biomarkers of TC:HDL-C ratio, hsTnI, NT-proBNP, and hsCRP all have robust, statistically significant and independent associations with future SCD. Use of these widely available and inexpensive biomarkers for risk stratification for patients at risk for SCD warrants further consideration.
Supplementary Material
Clinical Perspective.
It must address the following 2 questions: 1) What is new? (no more than 100 words, formatted as 2–3 bullets) and 2) What are the clinical implications? (no more than 100 words, formatted as 2–3 bullets).
1). What is new?
Sudden cardiac death is the most common cause of death in the United States, but identifying individuals at increased risk before they suffer a fatal event is challenging.
This study reports that four common, readily available blood biomarkers – total cholesterol to HDL cholesterol ratio, cardiac troponin I, NT-proBNP, and high-sensitivity C-reactive protein - are all independently and additively associated with sudden death in a large case-control study of men and women.
A score that awarded 1 point when any of these four biomarkers was elevated was strongly associated with sudden death.
2). What are the clinical implications?
These biomarkers are readily available for clinical use, and their association with sudden death raises the possibility that they may be used to identify otherwise asymptomatic individuals who are at risk for sudden death as their first manifestation of cardiovascular disease.
Further prospective study is required to determine whether routine use of these biomarkers in at-risk populations might lead to strategies to prevent sudden death.
Acknowledgements:
The authors gratefully acknowledge the support of the Channing Division of Network Medicine at Brigham and Women’s Hospital.
Sources of Funding: The sudden cardiac death case-control cohort was supported by grants HL-03783, HL-26490, HL-34595, HL-34594, HL-35464, HL-043851, HL-46959, HL-099355 and HL-080467 from the National Heart, Lung, and Blood Institute and CA-167552, CA-186107, CA-49449CA-34944, CA-40360, CA-47988, CA-55075, CA-87969, and CA-97193 from the National Cancer Institute. The sudden death confirmation was supported by grants from the NIH (HL-068070) and Novo Nordisk Foundation, Copenhagen, Denmark. Biomarker measurements were performed by Siemens Diagnostics. The work was also supported by HL-091069.
Disclosures: Dr. Everett reports significant grants from NHLBI during the study, significant personal fees from Amgen, and modest personal fees from Amarin, the U.S. Food and Drug Administration, Merck, NIDDK, Roche Diagnostics, and Novartis outside the present work. Drs. Moorthy, Tikkanen, and Cook report no relevant disclosures. Dr. Albert reports significant investigator-initiated grants from NHLBI, Abbott, Roche, and St. Jude Medical and modest personal fees from Roche outside the present work.
Non-Standard Abbreviations and Acronyms
- CI
Confidence interval
- CVD
Cardiovascular disease
- eGFR
Estimated glomerular filtration rate
- HDL
High-density lipoprotein cholesterol
- hsCRP
High-sensitivity C-reactive protein
- hsTnI
High-sensitivity cardiac troponin I
- IQR
Interquartile range
- MI
Myocardial infarction
- NT-proBNP
Amino-terminal pro-hormone B-type natriuretic peptide
- OR
Odds ratio
- SCD
Sudden cardiac death
- SD
Standard deviation
- TC
Total cholesterol
Footnotes
Supplemental Materials:
Supplemental Tables I - IV
References
- 1.Patton KK, Sotoodehnia N, DeFilippi C, Siscovick DS, Gottdiener JS and Kronmal RA. N-terminal pro-B-type natriuretic peptide is associated with sudden cardiac death risk: the Cardiovascular Health Study. Heart Rhythm. 2011;8:228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korngold EC, Januzzi JL Jr., Gantzer ML, Moorthy MV, Cook NR and Albert CM. Amino-terminal pro-B-type natriuretic peptide and high-sensitivity C-reactive protein as predictors of sudden cardiac death among women. Circulation. 2009;119:2868–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussein AA, Gottdiener JS, Bartz TM, Sotoodehnia N, deFilippi C, Dickfeld T, Deo R, Siscovick D, Stein PK and Lloyd-Jones D. Cardiomyocyte injury assessed by a highly sensitive troponin assay and sudden cardiac death in the community: the Cardiovascular Health Study. J Am Coll Cardiol. 2013;62:2112–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newton-Cheh C, Cook NR, VanDenburgh M, Rimm EB, Ridker PM and Albert CM. A common variant at 9p21 is associated with sudden and arrhythmic cardiac death. Circulation. 2009;120:2062–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinkle LE Jr. and Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457–464. [DOI] [PubMed] [Google Scholar]
- 6.McKie PM, Heublein DM, Scott CG, Gantzer ML, Mehta RA, Rodeheffer RJ, Redfield MM, Burnett JC Jr. and Jaffe AS. Defining high-sensitivity cardiac troponin concentrations in the community. Clin Chem. 2013;59:1099–1107. [DOI] [PubMed] [Google Scholar]
- 7.Apple FS, Collinson PO and Biomarkers ITFoCAoC. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 2012;58:54–61. [DOI] [PubMed] [Google Scholar]
- 8.Seimens I High Sensitivity Troponin I Assay. Accessed March 19, 2020. Accessed at: https://static.healthcare.siemens.com/siemens_hwem-hwem_ssxa_websites-context-root/wcm/idc/groups/public/@global/@lab/documents/download/mda5/mzi2/~edisp/30-17-10376-01-76_dimension_vista_tnih_ss_ous-06781449.pdf.
- 9.Prentice RL and Breslow NE. Retrospective studies and failure time models. Biometrika. 1978;65:153–158. [Google Scholar]
- 10.Albert CM, Ma J, Rifai N, Stampfer MJ and Ridker PM. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105:2595–2599. [DOI] [PubMed] [Google Scholar]
- 11.Hussein AA, Gottdiener JS, Bartz TM, Sotoodehnia N, DeFilippi C, See V, Deo R, Siscovick D, Stein PK and Lloyd-Jones D. Inflammation and sudden cardiac death in a community-based population of older adults: the Cardiovascular Health Study. Heart Rhythm. 2013;10:1425–1432. [DOI] [PubMed] [Google Scholar]
- 12.Deo R, Norby FL, Katz R, Sotoodehnia N, Adabag S, DeFilippi CR, Kestenbaum B, Chen LY, Heckbert SR, Folsom AR, et al. Development and Validation of a Sudden Cardiac Death Prediction Model for the General Population. Circulation. 2016;134:806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chugh SS, Uy-Evanado A, Teodorescu C, Reinier K, Mariani R, Gunson K and Jui J. Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: The Ore-SUDS (Oregon Sudden Unexpected Death Study). J Am Coll Cardiol. 2009;54:2006–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J and Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161–1166. [DOI] [PubMed] [Google Scholar]
- 15.Deo R and Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation. 2012;125:620–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta D, Curwin J, Gomes JA and Fuster V. Sudden death in coronary artery disease: acute ischemia versus myocardial substrate. Circulation. 1997;96:3215–3223. [DOI] [PubMed] [Google Scholar]
- 18.Epstein SE, Quyymi AA and Bonow RO. Sudden cardiac death without warning. Possible mechanisms and implications for screening asymptomatic populations. N Engl J Med. 1989;321:320–324. [DOI] [PubMed] [Google Scholar]
- 19.Khera AV, Mason-Suares H, Brockman D, Wang M, VanDenburgh MJ, Senol-Cosar O, Patterson C, Newton-Cheh C, Zekavat SM, Pester J, et al. Rare Genetic Variants Associated With Sudden Cardiac Death in Adults. J Am Coll Cardiol. 2019;74:2623–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
- 21.Cholesterol Treatment Trialists C, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cholesterol Treatment Trialists C, Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahimi K, Majoni W, Merhi A and Emberson J. Effect of statins on ventricular tachyarrhythmia, cardiac arrest, and sudden cardiac death: a meta-analysis of published and unpublished evidence from randomized trials. European heart journal. 2012;33:1571–1581. [DOI] [PubMed] [Google Scholar]
- 24.Davies MJ, Bland JM, Hangartner JR, Angelini A and Thomas AC. Factors influencing the presence or absence of acute coronary artery thrombi in sudden ischaemic death. Eur Heart J. 1989;10:203–208. [DOI] [PubMed] [Google Scholar]
- 25.el Fawal MA, Berg GA, Wheatley DJ and Harland WA. Sudden coronary death in Glasgow: nature and frequency of acute coronary lesions. Br Heart J. 1987;57:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman M, Manwaring JH, Rosenman RH, Donlon G, Ortega P and Grube SM. Instantaneous and sudden deaths. Clinical and pathological differentiation in coronary artery disease. JAMA. 1973;225:1319–1328. [DOI] [PubMed] [Google Scholar]
- 27.Haukilahti MAE, Holmstrom L, Vahatalo J, Kentta T, Tikkanen J, Pakanen L, Kortelainen ML, Perkiomaki J, Huikuri H, Myerburg RJ, et al. Sudden Cardiac Death in Women. Circulation. 2019;139:1012–1021. [DOI] [PubMed] [Google Scholar]
- 28.Vahatalo JH, Huikuri HV, Holmstrom LTA, Kentta TV, Haukilahti MAE, Pakanen L, Kaikkonen KS, Tikkanen J, Perkiomaki JS, Myerburg RJ, et al. Association of Silent Myocardial Infarction and Sudden Cardiac Death. JAMA Cardiol. 2019;4:796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tseng ZH, Olgin JE, Vittinghoff E, Ursell PC, Kim AS, Sporer K, Yeh C, Colburn B, Clark NM, Khan R, et al. Prospective Countywide Surveillance and Autopsy Characterization of Sudden Cardiac Death: POST SCD Study. Circulation. 2018;137:2689–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haukilahti MAE, Holmström L, Vähätalo J, Kenttä T, Tikkanen J, Pakanen L, Kortelainen M-L, Perkiömäki J, Huikuri H, Myerburg RJ, et al. Sudden Cardiac Death in Women. Circulation. 2019;139:1012–1021. [DOI] [PubMed] [Google Scholar]
- 31.Ashar FN, Mitchell RN, Albert CM, Newton-Cheh C, Brody JA, Muller-Nurasyid M, Moes A, Meitinger T, Mak A, Huikuri H, et al. A comprehensive evaluation of the genetic architecture of sudden cardiac arrest. Eur Heart J. 2018;39:3961–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Defilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M and Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation. 2011;123:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blankenberg S, Salomaa V, Makarova N, Ojeda F, Wild P, Lackner KJ, Jorgensen T, Thorand B, Peters A, Nauck M, et al. Troponin I and cardiovascular risk prediction in the general population: the BiomarCaRE consortium. Eur Heart J. 2016;37:2428–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel RB, Moorthy MV, Chiuve SE, Pradhan AD, Cook NR and Albert CM. Hemoglobin A1c levels and risk of sudden cardiac death: A nested case-control study. Heart Rhythm. 2017;14:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee AK, McEvoy JW, Hoogeveen RC, Ballantyne CM and Selvin E. Severe Hypoglycemia and Elevated High-Sensitivity Cardiac Troponin T in Older Adults With Diabetes: The ARIC Study. J Am Coll Cardiol. 2016;68:1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rezende PC, Everett BM, Brooks MM, Vlachos H, Orchard TJ, Frye RL, Bhatt DL and Hlatky MA. Hypoglycemia and Elevated Troponin in Patients With Diabetes and Coronary Artery Disease. J Am Coll Cardiol. 2018;72:1778–1786. [DOI] [PubMed] [Google Scholar]
- 39.Natriuretic Peptides Studies Collaboration, Willeit P, Kaptoge S, Welsh P, Butterworth AS, Chowdhury R, Spackman SA, Pennells L, Gao P, Burgess S, et al. Natriuretic peptides and integrated risk assessment for cardiovascular disease: an individual-participant-data meta-analysis. The lancet Diabetes & endocrinology. 2016;4:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Everett BM, Berger JS, Manson JE, Ridker PM and Cook NR. B-type Natriurietic Peptides Improve Cardiovascular Disease Risk Prediction in a Cohort of Women. J Am Coll Cardiol. 2014;64:1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Everett BM, Zeller T, Glynn RJ, Ridker PM and Blankenberg S. High-sensitivity cardiac troponin I and B-type natriuretic peptide as predictors of vascular events in primary prevention: impact of statin therapy. Circulation. 2015;131:1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng A, Zhang Y, Blasco-Colmenares E, Dalal D, Butcher B, Norgard S, Eldadah Z, Ellenbogen KA, Dickfeld T, Spragg DD, et al. Protein biomarkers identify patients unlikely to benefit from primary prevention implantable cardioverter defibrillators: findings from the Prospective Observational Study of Implantable Cardioverter Defibrillators (PROSE-ICD). Circ Arrhythm Electrophysiol. 2014;7:1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Empana JP, Jouven X, Canoui-Poitrine F, Luc G, Tafflet M, Haas B, Arveiler D, Ferrieres J, Ruidavets JB, Montaye M, et al. C-Reactive protein, interleukin 6, fibrinogen and risk of sudden death in European middle-aged men: the PRIME study. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:2047–2052. [DOI] [PubMed] [Google Scholar]
- 44.Narayanan K, Reinier K, Teodorescu C, Uy-Evanado A, Aleong R, Chugh H, Nichols GA, Gunson K, London B, Jui J, et al. Left ventricular diameter and risk stratification for sudden cardiac death. Journal of the American Heart Association. 2014;3:e001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.