Abstract
Aim:
Development and characterization of a novel injury-free preclinical model of migraine-like pain allowing mechanistic assessment of both acute and preventive treatments.
Methods:
A “two-hit” hyperalgesic priming strategy was used to induce vulnerability to a normally subthreshold challenge with umbellulone (UMB), a TRPA1 activator, in uninjured female and male C57BL/6 mice. Priming (i.e., the first hit) was induced by three consecutive daily episodes of restraint stress (RS); repeated UMB was also evaluated for potential priming effects. Sixteen days after the first RS, mice received inhalational UMB (i.e., the second hit) to elicit migraine-like pain. Medications currently used for acute or preventive migraine therapy including propranolol (a beta blocker) and sumatriptan (5HT1B/D agonist), as well as olcegepant, an experimental CGRP receptor antagonist and nor-BNI, an experimental long-acting KOR antagonist, were investigated for their efficacy to block priming and prevent or reverse UMB-induced allodynia in primed animals. To assess migraine-like pain, cutaneous allodynia (CA) was determined by responses to periorbital or hindpaw probing with von Frey filaments.
Results:
Repeated RS, but not UMB exposure, produced transient CA that resolved within 16 days. RS produced long-lasting priming that persisted beyond 16 days as demonstrated by reinstatement of CA following inhalational UMB challenge. Pretreatment with propranolol or nor-BNI prior to RS prevented both transient CA and priming demonstrated by a lack of UMB-induced CA. Following establishment of RS priming, olcegepant, but not propranolol or nor-BNI, prevented UMB-induced CA. When administered 1 hour after UMB, sumatriptan, but not olcegepant, reversed UMB-induced CA in RS primed rats.
Conclusion:
We have developed a novel injury-free model with translational relevance that can be used to study mechanisms relevant to migraine-like pain and to evaluate novel acute or preventive treatments. RS priming induced a state of vulnerability to a subthreshold stimulus that has been referred to as “latent sensitization” (LS). The development of LS could be prevented by blockade of stress-pathways with propranolol or with a KOR antagonist. Following establishment of LS, subthreshold stimulation with UMB reinstated CA likely from activation of meningeal TRPA1-expressing nociceptors. Accordingly, in RS-primed animals, sumatriptan reversed UMB-induced CA supporting peripheral sites of action, while propranolol or nor-BNI were not effective. Surprisingly, olcegepant was effective in mice with LS when given prior to, but not after, UMB challenge suggesting time-dependent contributions of CGRP receptor signaling in promoting migraine-like pain in this model. Activation of the CGRP receptor participates in initiating, but has a more limited role in maintaining, pain responses supporting the efficacy of small molecule CGRP antagonists as preventive medications. Additionally, the effectiveness of sumatriptan in reversal of established pain thus suggests modulation of additional, non-CGRP receptor-mediated nociceptive mechanisms. KOR antagonists may represent a novel preventive therapy for stress-related migraine.
Keywords: Migraine, stress, priming, umbellulone, KOR antagonist, olcegepant
Introduction
Migraine is one of the most common and disabling neurological disorders worldwide (1–4). Understanding underlying pathophysiology is difficult due to genetic underpinnings as well as disease complexity that includes multiple phases with a broad spectrum of symptoms reflecting the involvement of different neural networks (1–3, 5). In addition, migraine is a functional pain disorder commonly characterized by intermittent attacks of pain that occur in the absence of injury (6–9). These factors can limit the translatable potential of preclinical models (2). Recently, migraine had been described as a sensory threshold disease in which people with migraine generally exhibit lower sensory thresholds and increased vulnerability to pain episodes when compared to healthy controls (5). The mechanisms that underlie the alteration of pain thresholds and vulnerability to attacks is not completely understood.
One approach for studying mechanisms of migraine in humans is a provocation strategy involving administration of substances that promote headache in healthy volunteers (10–12). Similar approaches have been used in naïve animals where headache-like behaviors have been observed (13). However, provocation studies in healthy volunteers, rather than in individuals with the disease, neglect the role of pre-existing vulnerability, preventing the deeper investigation of underlying migraine pathophysiology (2, 5). A more realistic approach may be achieved by provocation studies in individuals susceptible to migraine. For example, while challenge with glyceryl trinitrate (GTN) produces headache in all subjects, a delayed migraine headache is produced only people with migraine (14). Similarly, other provocative agents such as environmental irritants can promote migraine attacks only in vulnerable individuals (15–17).
Environmental irritants include many substances that can activate the TRPA1 channel expressed on meningeal nociceptors providing a basis for generating migraine pain. The California bay leaf Umbellularia californica is commonly known as the “headache tree” and inhalational exposure to volatile extracts of the plant containing the major monoterpene ketone umbellulone (UMB) has been reported anecdotally to trigger cluster headache (18, 19). Preclinical mechanistic investigations have shown that the effect of UMB in inducing headache likely occurs through activation of TRPA1 channels in the trigeminal system and consequent increase of circulating CGRP peptide with presumably direct and indirect activation of trigeminovascular pathways (19–21). Notably, UMB has not been reported to elicit headache in humans who do not suffer from pre-existing headache disorders, again suggesting the need to establish a state of vulnerability to subthreshold stimuli, including TRPA1 activation.
As animals do not have the complex polygenetic or physiological underpinnings of people with migraine, medications that target these mechanisms cannot be investigated directly. However, increased translational relevance of preclinical mechanistic studies might be achieved by experimentally lowering sensory thresholds to provocative stimuli, mimicking to some extent, the state of vulnerability observed in people with migraine (5). Stress is the most frequently cited trigger associated with migraine attacks, and the frequency of migraine attacks is a risk factor in promoting the transition from episodic to chronic migraine states (22–24). These observations suggest that repeated stress may promote “sensitization” of peripheral and central neural circuits increasing the probability (i.e., potentially decreasing thresholds for nociceptor activation) of future attacks (22). Therefore, stress might represent one of the main factors in inducing vulnerability in people with migraine. While precise mechanisms remain to be determined, it is well established in preclinical studies that stress increases the actions of dynorphin, an endogenous opioid peptide that acts at the kappa opioid receptor (KOR). We have previously reported that blockade of KOR prevents stress-induced allodynia in a rodent model of medication overuse headache (MOH)(25).
In the present study, we have developed and characterized a novel injury-free preclinical model of migraine-like pain by using a “two-hit” priming strategy, whereby repeated restraint stress (RS) is used to induce latent sensitization (LS), a state of vulnerability to a normally subthreshold stimulus revealed by CA to inhalational UMB. We determined whether in this model, propranolol, a commonly used preventive treatment, or blockade of the stress response with the KOR antagonist nor-BNI could prevent the development of LS and/or block UMB-induced allodynia in RS-primed mice. Additionally, we investigated if acute migraine medications including a triptan (sumatriptan, serotonin 5HT1B/D receptor agonist) and a gepant (olcegepant, small molecule calcitonin gene-related peptide receptor antagonist) could block and/or reverse UMB-induced allodynia in RS-primed mice.
Materials and methods
Animals
Unless otherwise noted, all experiments were conducted using 6-weeks-old female C57BL/6J mice from Jackson-Labs (Sacramento, CA, USA). Key experiments were replicated in 6-weeks-old male C57BL/6J mice (see Supplementary figures). Housing conditions consisted of 12hr light/dark cycle (lights on 7am-7pm) in a climate- and humidity-controlled environment with food and water provided ad libitum in the University of Arizona animal facility. A total of 265 animals were used in these studies with 6 – 12 animals per group size for evaluation of behavior. Estrous cycle was not monitored for these studies. All experimental procedures were performed in accordance with the ARRIVE guidelines, the ethical guidelines of the International Association for the Study of Pain regulations on animal welfare and the National Institutes of Health guidelines for the care and use of laboratory animals. The experimental procedures have been previously approved by the Institutional Animal Care and Use Committee of the University of Arizona. Experimenters were blinded for the conditions and/or treatments. Mice were randomly divided into control and experimental groups. Every effort was made to minimize the number of animals and their suffering.
Drugs
Umbellulone (UMB; AdipoGen, San Diego, CA, USA) was prepared as a stock solution of 0.1M in 100% DMSO and freshly diluted to 0.01M with phosphate-Buffered Saline (PBS). Olcegepant (Tocris, Minneapolis, MN, USA) was dissolved using 20% DMSO in saline. Propranolol (Sigma, St. Louis, MO, USA) was diluted in DiH2O. Nor-BNI (Tocris, Minneapolis, MN, USA) was diluted in saline. UMB at 0.01M was delivered by inhalation (see below). Propranolol, olcegepant and sumatriptan were administered intraperitoneally (i.p.) at 20, 1 and 0.6 mg/kg, respectively, while nor-BNI was given subcutaneously (s.c.) at 10 mg/kg. Controls received the respective vehicles at 10 mL/kg.
Periorbital and hindpaw frequency of response evaluation
Periorbital and hindpaw frequency of response to tactile stimulation were performed prior and after RS, UMB or BLS exposure with minor adaptation (26). Mice were placed in clear Plexiglas chambers (4’’L × 4”W × 8’’H), on top of a wire mesh stand (0.635 cm² grid), for 2 hours. After habituation, 0.4 g and 1 g von Frey filaments (Stoelting, Wood Dale, IL, USA) were applied 10 times to periorbital and hindpaw region, respectively. The filament was gently applied until the filament slightly arched. Positive responses for the periorbital region were scored after each filament application as characterized by facial grooming, head shaking, and/or turning away after filament application. Positive responses for hindpaw region were characterized by sharp withdrawal of the paw, shaking, and/or licking the paw. Further, any such behaviors displayed before filament application were not considered positive responses. Frequency response was calculated by [(number of positive responses × 100)/10]. Only animals with an average baseline of ~30% frequency of response were used to complete the experiments.
Umbellulone inhalational exposure
Inhalational exposure to UMB was completely performed inside a biological safety cabinet and a N95 respirator mask worn by the experimenter to minimize vapor exposure. A multi-station isoflurane anesthesia board (Parkland Scientific, Coral Springs, FL, USA) was used with medical grade oxygen. A half square gauze (1”×1”) was placed in each nose cone of the anesthesia board and 500 µL of UMB at 0.01M or vehicle was pipetted onto each gauze. Animals were individually placed at each nose cone under a lightly anesthesia with 1.5 – 2% isoflurane, i.e. 1.5 – 2 L/min rate. Mice received inhalational exposure to UMB or vehicle under anesthesia for 30 minutes. During the exposure, heating pads were placed under the animals to avoid changes in body temperature. Animals were observed continuously during the 30 minutes of exposure with adjustments of air and isoflurane performed if needed. To evaluate a possible priming effect produced by repetitive inhalational exposure to UMB, animals were exposed to UMB for 30 minutes each day on days 0, 2, and 4. Periorbital and hindpaw measurements were made on day 6 after the first UMB exposure and animals were then exposed to a subsequent environmental stressor, i.e. bright light stress (BLS) followed by measurements of frequency of response hourly for an additional 5 hours.
Restraint stress
Animals were placed in plastic restrainers (Plas-labs INC, 551-BSRR, Lansing, MI, USA) to induce restraint stress (RS). The tail was pulled through the restrainers’ stoppers, which were pushed tightly enough against the animals to limit their movements without inhibiting respiration. Animals were observed continuously during the stress exposure. For repeated RS priming, mice underwent RS for 2 hours each day consecutively for 3 days. Control animals were kept in their home cages without food and water during the 2 hours. To evaluate the possible effect of RS in eliciting allodynia before or after challenge with UMB, periorbital and hindpaw response frequency was measured at baseline in both female and male mice followed by 3 daily episodes of RS. Allodynic frequency was measured on days 3, 5, 7, 9, 11, 14 and 16 after the first RS. On days 16, 18 and 20 or 16, 17 and 18 after the first RS, baseline measurement to tactile stimulation was collected and animals were challenged with inhalational exposure to UMB under isoflurane anesthesia or only isoflurane. After the exposure, frequency of response to tactile stimulation was performed at 30 minutes and hourly up to 5 – 6 hours and again at 24 hours after the exposure. Hindpaw data were collected for all treatments with the exception of studies with nor-BNI.
Environmental bright light stress challenge
Mice were kept in their home cages and received one episode of BLS on day 6 after the first inhalational exposure to UMB with similar conditions as previously described (27). BLS was induced by two LED lights placed on both sides of the cages to deliver approximately 1000 lux for 15 minutes.
General experimental design overview
We used a “two-hit” priming strategy to induce latent sensitization (LS) whereby normally subthreshold stimuli such as UMB or stress can produce allodynia. LS was induced with 3 consecutive episodes of RS in uninjured mice and was demonstrated by periorbital and hindpaw cutaneous allodynia (CA) following inhalational exposure to UMB. To evaluate the possibility that UMB might produce priming, we exposed mice to three days of inhalational UMB and then used BLS in this experiment only as the second hit stimulus with CA as the outcome measure. The potential translational relevance of the model was then addressed by evaluation of drugs currently approved as migraine therapeutics for acute or preventive treatment. The possible activity of an experimental KOR antagonist was also assessed. Propranolol, a migraine preventive medication, was given 1 hour prior to each RS or 1 hour prior to the UMB exposure. Nor-BNI, a long acting KOR antagonist, was given as a single injection 2 hours prior to the first RS episode or 24 hours prior to UMB exposure on day 16, in mice previously primed with RS. Sumatriptan, a 5HT1B/D agonist currently used for acute treatment of migraine and olcegepant, an experimental CGRP antagonist that has been shown to be clinically effective in treatment of migraine (28), were administered on day 16 after the last RS, either 30 minutes before or 1 hour after the UMB exposure. Evaluation of periorbital and hindpaw allodynia data showed that these endpoints developed in parallel. For this reason, periorbital CA was chosen as the outcome for these experiments.
Statistical analysis
Sample size was determined using the GPower 3.1 software, with an established significance level of p < 0.05. Statistical analyses were calculated using GraphPad Prism 7 (GraphPad Software, La Jolla, CA). Two-way analysis of variance (ANOVA) followed by Sidak or Tukey test were used for analysis of the time course experiments for sensory thresholds for two or more groups comparisons, respectively. The statistical analysis, numbers of animals used (n), p values and interaction F ratios are reported in Table 1. All data are presented as mean ± SEM and statistical significance was set at p < 0.05.
Table 1.
Summary of statistical analyses.
| Figure | Analysis | Interaction p | Interaction F | n |
|---|---|---|---|---|
| 1(a) | Two-way ANOVA Sidak | p = 0.8088 | F (26, 494) = 0.7516 | 6 – 12 |
| 1(b) | Two-way ANOVA Sidak | p < 0.0001 | F (27, 378) = 5.290 | 8 |
| 2(a) | Two-way ANOVA Sidak | p < 0.0001 | F (13, 221) = 4.549 | 9 – 10 |
| 2(b) | Two-way ANOVA Tukey | p > 0.05 | F (13, 130) = 2.156 | 6 |
| 3(a) | Two-way ANOVA Tukey | p < 0.0001 | F (48, 320) = 2.858 | 6 |
| 3(b) | Two-way ANOVA Tukey | p = 0.1757 | F (6, 90) = 1.535 | 7 – 8 |
| 3(c) | Two-way ANOVA Tukey | p = 0.0016 | F (39, 312) = 1.893 | 5 – 10 |
| 4(a) | Two-way ANOVA Tukey | p < 0.0001 | F (42, 280) = 2.754 | 5 – 7 |
| 4(b) | Two-way ANOVA Tukey | p = 0.9502 | F (28, 210) = 0.5918 | 5 – 7 |
| S1(a) | Two-way ANOVA Sidak | p = 0.4214 | F (26, 494) = 1.033 | 6 – 12 |
| S1(b) | Two-way ANOVA Sidak | p < 0.0001 | F (27, 378) = 3.356 | 8 |
| S2(a) | Two-way ANOVA Sidak | p < 0.0001 | F (27, 405) = 4.318 | 8 – 9 |
| S2(b) | Two-way ANOVA Sidak | p < 0.0001 | F (27, 405) = 2.791 | 8 – 9 |
| S3(a) | Two-way ANOVA Sidak | p = 0.6421 | F (6, 126) = 0.71 | 9 – 14 |
| S3(b) | Two-way ANOVA Sidak | p = 0.1061 | F (6, 126) = 1.791 | 9 – 14 |
| S4 | Two-way ANOVA Sidak | p < 0.0001 | F (13, 182) = 11.21 | 8 |
p values, interaction F ratios and n for statistical analyses used in Figures 1–4 and supplemental material, Figures S1 and S4.
Results
Characterization of inhalational UMB in naïve or RS primed mice
In naïve female mice, inhalational exposures to UMB for 30 minutes did not produce periorbital or hindpaw CA (Figure 1(a) and Supplementary Figure S1(a)). Mice did not develop CA after subsequent UMB exposures on days 2 and 4. In these mice, repeated UMB did not induce priming as CA was not observed on day 6 following a second hit challenge with BLS. There was no significant difference when UMB-primed animals were compared to the vehicle exposed control group (Figure 1(a) and Figure S1(a)).
Figure 1.
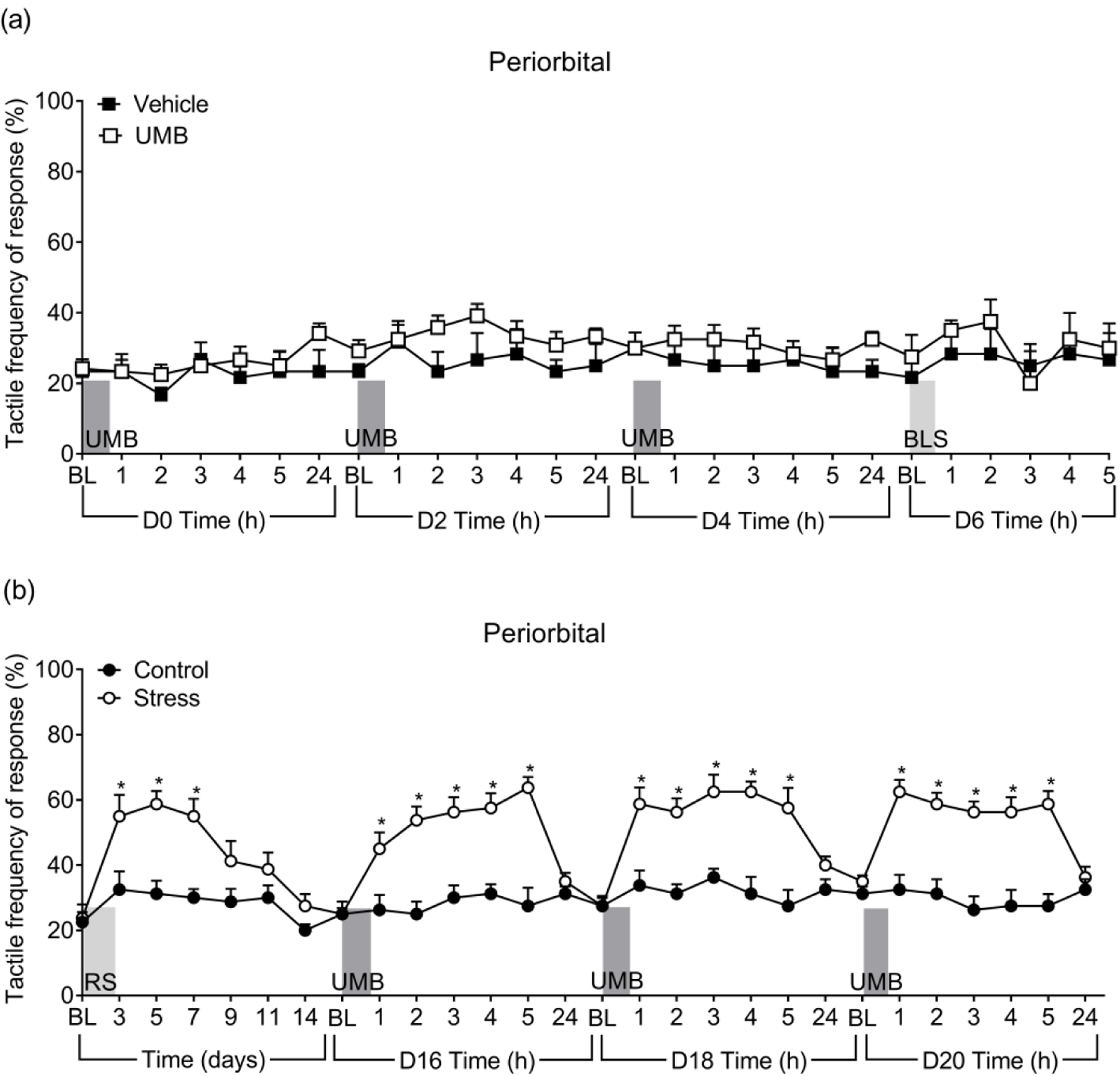
UMB induced periorbital CA only in female mice primed with RS. (a) Tactile frequency of response was assessed in naïve female mice at baseline (BL) and hourly after each of the 3 UMB inhalational exposures (0.01N/500 µL, each, under ~2% isoflurane anesthesia) and again after BLS exposure. (b) Tactile frequency of response at baseline (BL) was collected, followed by 3 consecutive days of RS. CA was assessed on indicated days after the first RS over a time course of 16 days. On days 16, 18 and 20, baseline (BL) response was collected, followed by inhalational exposure of UMB with measurements collected hourly for 5 hours. Data are presented as mean ± S.E.M. and analyzed using two-way ANOVA followed by Sidak’s multiple comparison test with * representing p < 0.05 in comparison to the Control group (n = 6 – 12).
In contrast, repeated episodes of RS on three consecutive days produced significant periorbital and hindpaw CA in naïve female mice (Figures 1(b) and S1(b)). Tactile thresholds began to return to baseline levels between days 7 – 11 and there was no significant CA by day 14 after RS. Similarly, repeated RS elicited periorbital and hindpaw CA in naïve male mice with similar time course observed in both sexes (Figure S2(a) and S2(b)).
Following resolution of CA, RS-primed and control mice were exposed to inhalational UMB on days 16, 18 and 20 to determine if RS sensitized the animals to UMB. Each UMB exposure elicited periorbital and hindpaw CA only in RS primed female mice that lasted for 4 – 5 hours (Figures 1(b) and S1(b)). Similarly, inhalational UMB on days 16, 17 and 18 also reinstated CA in RS primed male mice (Figure S2) evidenced by significantly increased withdrawal response frequencies compared to the control group. These findings reflect the development of latent sensitization (LS) induced by repeated episodes of stress in both male and female mice.
As isoflurane has also been reported to have TRPA1 agonist properties (29), separate cohorts of RS primed and control female mice received inhalational isoflurane without UMB on day 16 after RS. Inhalational isoflurane did not induce CA (Figure S3(a) and S3(b)) in either RS primed or control animals. These data confirm that the CA observed after inhalational exposure was due to the effects of UMB.
Propranolol prevents the development of RS-induced CA and LS, but does not block CA elicited by UMB in previously primed animals
Propranolol or vehicle were given prior to each RS in female mice. While vehicle-treated animals developed transient periorbital CA following RS priming, the propranolol-treated group shows no increases in withdrawal thresholds over baselines (Figure 2(a)). UMB exposure on day 16 elicited CA in vehicle-but not propranolol-treated mice. Withdrawal responses were significantly lower in the propranolol group, suggesting that propranolol administered prior to each RS abolished the development of CA as well as the induction of LS in female mice by preventing the consequences of stress. In contrast, in previously RS-primed female mice, a single treatment with propranolol given 1 hour before inhalational exposure to UMB on day 16 after RS failed to block periorbital CA (Figure 2(b)). These data confirmed the preventive effect of propranolol therapy in this model but indicate that propranolol is not effective in preventing TRPA1-induced headache in already sensitized state.
Figure 2.
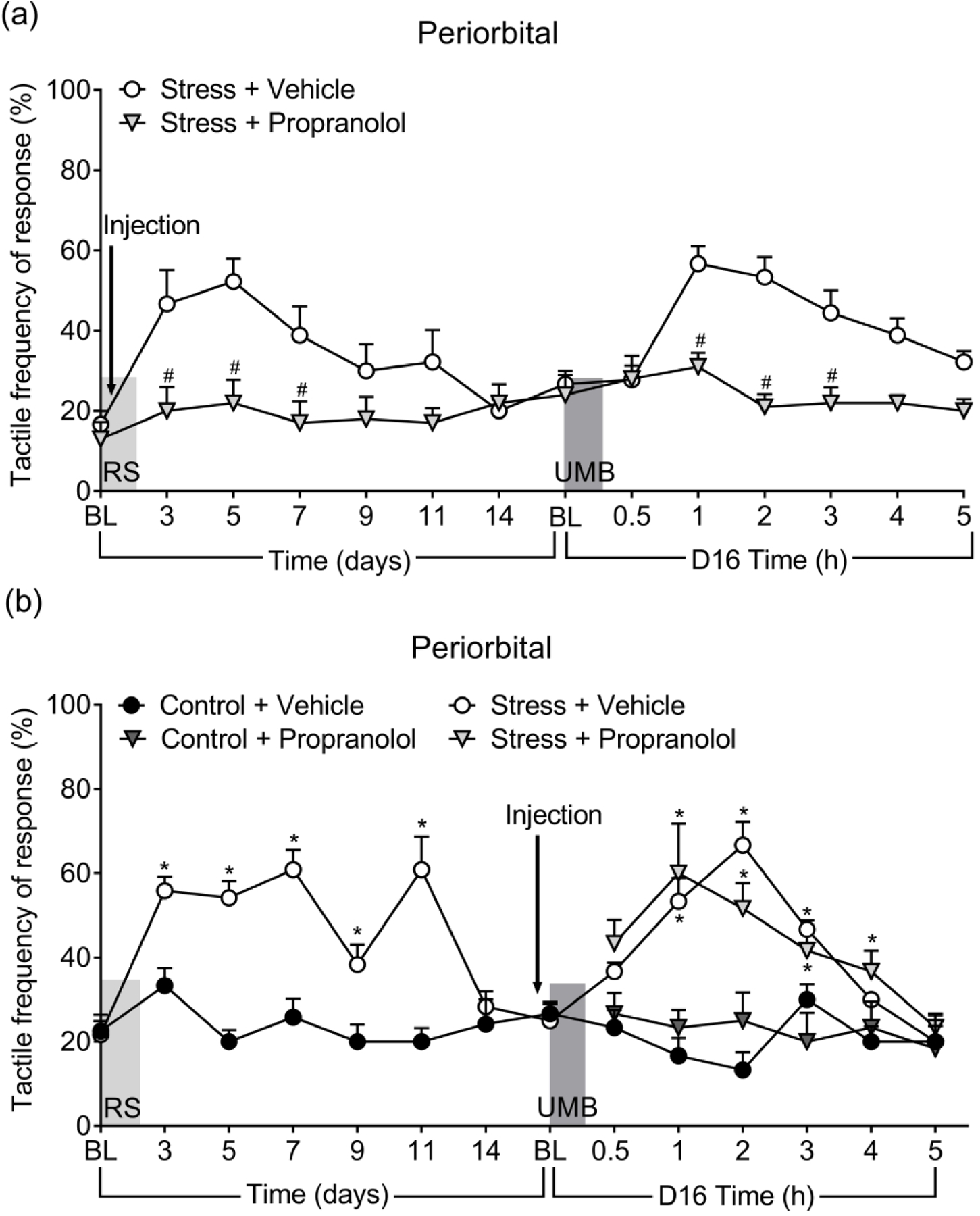
Effects of propranolol in prevention of RS-induced and UMB-induced CA. (a) Propranolol (20 mg/kg, i.p.) was given 1 hour prior each RS and CA was assessed over a time course of 16 days. On day 16, the mice were exposed to UMB and CA assessed over a 5-hour time course. (b) Propranolol was administered 1 hour prior to UMB inhalational exposure and CA assessed. Data are presented as mean ± S.E.M. and analyzed using two-way ANOVA followed by Sidak’s (a) or Tukey’s (b) multiple comparison test with # representing p < 0.05 in comparison to no Stress + Vehicle group, and * representing p < 0.05 in comparison to Control + Vehicle group (n = 6 – 10).
Sumatriptan reversed UMB-induced CA elicited in RS-primed animals
Sumatriptan, a 5HT1B/D agonist, or vehicle were administered systemically to female mice on day 16 after priming, 1 hour after inhalational UMB exposure, when significant periorbital allodynia compared to pre-UMB baseline is already observed. Sumatriptan fully reversed UMB-induced periorbital CA to similar levels as in control animals (Figure 3(a)). Control animals treated with sumatriptan did not show any changes in sensitivity to von Frey stimulation throughout the experiment (p < 0.05). These data confirmed the efficacy of triptans for acute migraine therapy in this model.
Figure 3.
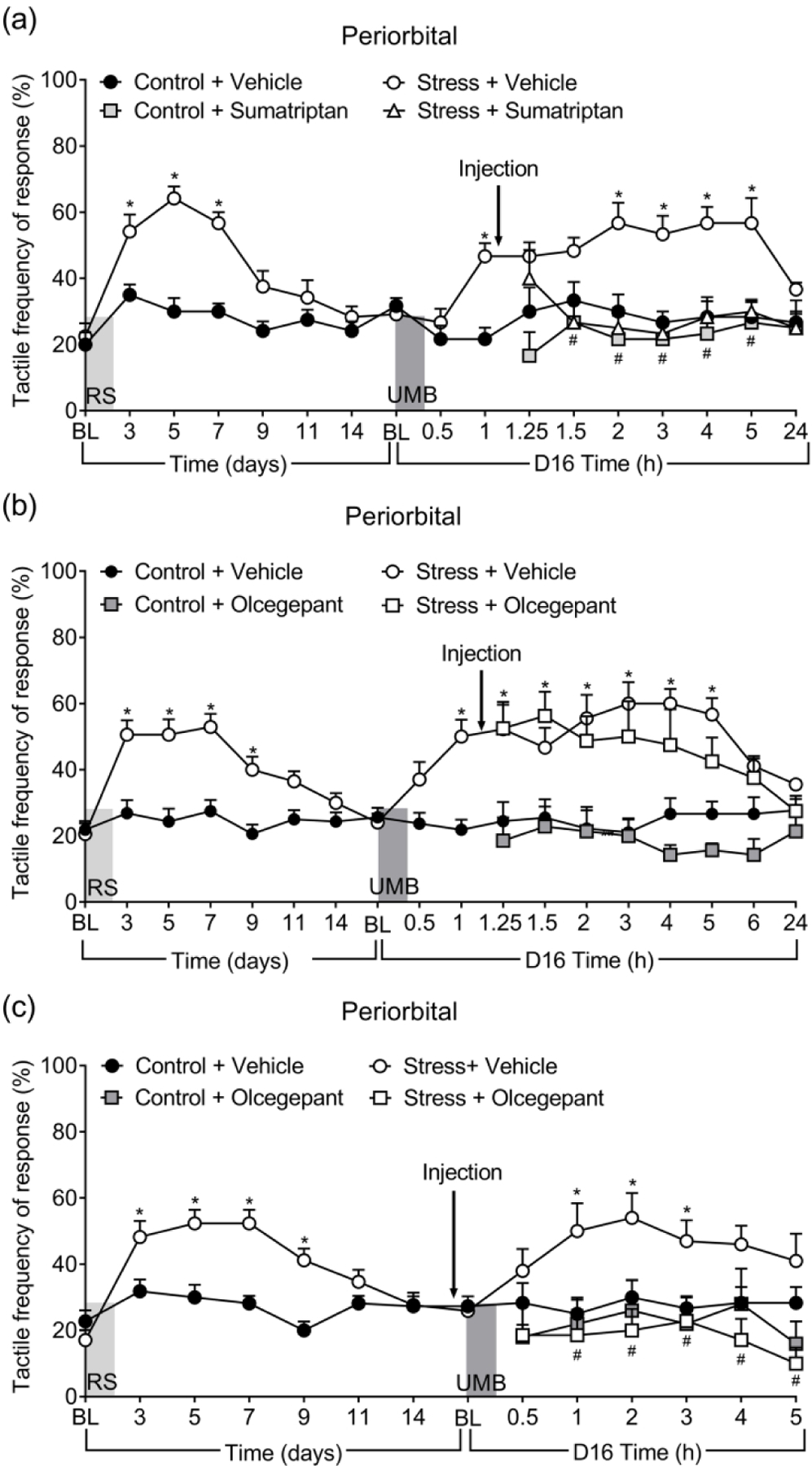
Effects of sumatriptan and olcegepant in reversal and prevention of UMB-induced CA. Sumatriptan (0.6 mg/kg, i.p.) (a) and olcegepant (1 mg/kg, i.p.) (b) was given 1 hour after UMB challenge in mice with RS for reversal of established CA. Olcegepant was also given 30 minutes prior to UMB in order to block CA (c). Data are presented as mean ± S.E.M. and analyzed using two-way ANOVA followed by Tukey’s multiple comparison test with * representing p < 0.05 in comparison to Control + Vehicle group, and # representing p < 0.05 in comparison to no Stress + Vehicle group (n = 7 – 9).
Olcegepant prevented UMB-induced CA elicited in RS-primed animals but was not effective in reversing already enduring CA
We next tested the efficacy of olcegepant in reversing UMB-induced allodynia in RS-primed mice, as in the sumatriptan experiment. In contrast to sumatriptan, however, olcegepant failed to reduce UMB-induced periorbital CA as there was no significant difference from tactile responses in RS-primed mice treated with vehicle (Figure 3(b)). We therefore tested if olcegepant would be effective when administered 30 minutes before the UMB challenge. Olcegepant fully blocked the development of UMB-induced CA in RS-primed mice (Figure 3(c)). Olcegepant had no effect in control animals (p < 0.05). These data suggest that small molecule CGRP antagonists may be effective when given before the onset of migraine, but may be less effective, or ineffective when taken during the headache phase.
Evaluation of a kappa opioid receptor (KOR) antagonist as a potential migraine therapy
A single dose of the long-acting KOR antagonist, nor-BNI, was administered prior to the first of three RS episodes. Pretreatment with nor-BNI prevented periorbital CA in female (Figure 4(a)) as well as male (Figure S4) mice as shown by significant reduction in tactile responses in comparison to the vehicle treated groups. In nor-BNI pretreated mice, UMB no longer elicited CA, suggesting that nor-BNI prevented the development of LS. Pretreatment with nor-BNI had no effect in control mice. In contrast, a single treatment with nor-BNI given before inhalational exposure to UMB in RS-primed female mice failed to block UMB-induced periorbital CA (Figure 4(b)). These data demonstrated the effect of the blockade of KOR as a possible preventive anti-migraine therapy.
Figure 4.
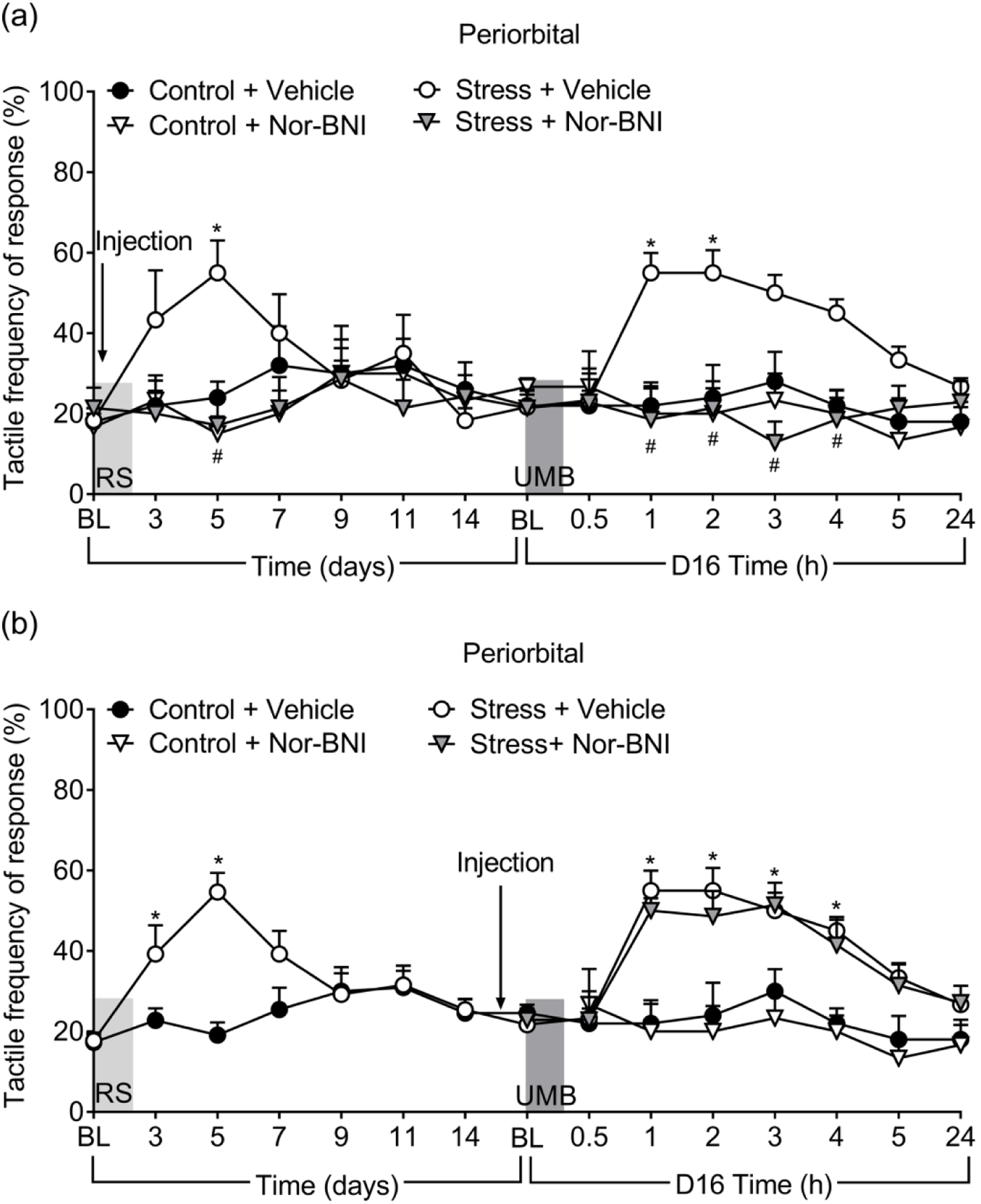
Effects of nor-BNI in prevention of RS-induced and UMB-induced CA in female mice. Nor-BNI (10 mg/kg, s.c.) was given either 2 hours before the first RS (a) or 24 hours prior to UMB exposure (b) and CA was measured at indicated times. Data are presented as mean ± S.E.M. and analyzed using two-way ANOVA followed by Tukey’s multiple comparison test with * representing p < 0.05 in comparison to Control + Vehicle group, and # representing p < 0.05 in comparison to no Stress + Vehicle group (n = 5 – 7).
Discussion
Several findings of our study are notable. First, our approach was to use an injury-free priming strategy to establish vulnerability to provocation-induced migraine-like pain, more closely resembling the human experience and potentially increasing translational relevance. Second, we found that the effects of the second hit with UMB promoted migraine-like pain only following induction of LS, consistent with the observation that TRPA1 activators induce migraine only in people with an underlying primary headache disorder (18). Third, olcegepant, prevented but did not reverse, migraine-like pain in animals with LS. In contrast, sumatriptan was effective in reversing migraine-like pain in animals with LS. These findings suggest a time-dependent role for the CGRP receptor with participation in the initiation of migraine-like pain, but with a more limited role in its maintenance. Additionally, the effectiveness of sumatriptan in reversal of established pain suggests the modulation of additional non-CGRP receptor-mediated nociceptive mechanisms (2, 30–32). Fourth, propranolol inhibited the induction of LS from repeated episodes of RS so that UMB, the second hit stimulus, no longer induced pain-like behaviors. A single administration of propranolol prior to UMB in mice with LS, however, was not effective, consistent with its activity as a preventive and not an acute medication (33). Finally, consistent with our previous findings in a model of medication overuse headache (MOH) with stress-induced allodynia, we demonstrated that a KOR antagonist given before RS priming could prevent pain from an UMB challenge, supporting the possible use of KOR antagonists as preventive therapies (25).
Migraine has been conceptualized as a threshold disorder where normally subthreshold stimuli can provoke attacks of headache pain in vulnerable individuals (5). Changes in thresholds could occur as a result of either peripheral and/or central sensitization (5). Repeated migraine pain episodes are predictive of increased risk of transformation of episodic to chronic migraine (2), suggesting a priming phenomenon that induces vulnerability to subsequent stimuli through central and possibly peripheral sensitization. Stress is one of the major self-identified factors associated with triggering migraine attacks (22, 34, 35). Repeated stress may therefore be relevant in promoting central sensitization and increased vulnerability to subthreshold stimuli that can activate trigeminal nociceptive afferents.
Exposure to UMB has been shown to induce pain-like behavior in animals (19, 20, 36). Administration of UMB intranasally or directly onto the dura mater of rats induced migraine-like pain behavior and mediated CGRP release from trigeminal neurons through activation of TRPA1 channels (19, 20). TRPA1 receptors are expressed throughout the trigeminal system including the sensory innervation of the nasal mucosa and on meningeal nociceptors (19, 37). Many substances classified as environmental irritants activate the TRPA1 channel on meningeal nociceptors and that are thought to promote the pain phase of migraine (17, 38–40). It should be noted, however, that previous studies with TRPA1 agonists including UMB, acrolein and mustard oil demonstrated pain-like behaviors in naïve animals without inducing vulnerability (19, 20, 41). These mechanistic observations differ from human reports. UMB is a known headache trigger in humans based on an anecdotal report of cluster headache in a susceptible patient who suffered from cluster headache during his youth (18). The effects of UMB are consistent with clinical observation that environmental irritants including cigarette smoke, acrolein, perfumes and other substances are known to provoke migraine attacks in people with migraine, but not in those without migraine (17, 42, 43). Collectively, these findings suggest that subthreshold activation with TRPA1 agonists could trigger migraine in those with the disorder.
We therefore adapted the “two-hit” hyperalgesic priming model thought to promote central sensitization, an amplification mechanism that is likely important in promoting transformation of episodic to chronic migraine (44). In this approach, repeated RS acts as the “first hit” to induce latent sensitization (LS), a primed state whereby a subthreshold stimulus including for example an environmental irritant can act as the “second hit” to promote pain. We found that repeated inhalation of UMB did not produce pain-like behaviors in mice without LS and that isoflurane, a drug reported to have TRPA1 properties, (29) did not produce pain-like behaviors with our experimental conditions. Differences in the outcomes of our studies and previous preclinical reports (19, 20, 30, 36) may relate to the UMB dose that was used, timing of the exposure or other factors. In our protocol, the migraine-like pain induced by inhalational exposure likely reflects the amplification of effects of a subthreshold UMB exposure that stimulate TRPA1 receptors on trigeminal afferents (19, 42). Our results are consistent with a study by Durham and colleagues who showed that inhalation of an oil extracted from California bay leaf, which contained UMB, did not produce pain behaviors in animals that had not previously received an inflammatory stimulus in the neck muscles, suggesting a requirement for sensitization of the trigeminal system to lower response thresholds to induce pain (36). While our studies are conceptually similar, differences are also notable. Inflammation is an injury that recruits generalized immune responses occurring over several days, biological processes that likely differ from the injury-free priming induced by RS in our model. Additionally, priming with RS also allows for the determination of possible preventive and acute interventions, as the animals were allowed to recover from the transient CA promoted by stress before exposure to UMB. Nevertheless, our data, and these previous findings (36), are consistent with the view that plasticity in the nervous system is required to increase vulnerability to migraine triggers (1, 22, 35). Notably, this vulnerable state may change in different phases of migraine, where reduced thresholds are associated with the headache phase, recovering during the postdrome phase, and gradually reducing again until the next attack (5). Sensory thresholds in people with migraine have been shown to be lower than in controls even in the interictal period (45, 46). The molecular and circuit mechanisms that underlie these oscillating rhythms remain to be clarified (5).
Our studies showed that repeated stress induced maladaptive changes demonstrated by long-lasting increased vulnerability to subthreshold TRPA1 activation. Repeated stress likely involves central sensitization mechanisms that may be modulated by the beta-adrenergic blockade (22, 47). Administration of the beta-adrenergic blocker, propranolol, prior to each RS episode was able to prevent both transient CA, as well as the development of LS. Following LS, however, a single dose of propranolol was not effective in reducing the CA promoted by UMB exposure. These observations are consistent with the use of this drug as a preventive, rather than an acute, migraine treatment (33) and suggest a central site of action rather than direct inhibition of TRPA1 activation of peripheral trigeminal neurons. RS priming may therefore model to some extent the sensitization that occurs with repeated attacks of migraine. A recent report has shown that RS priming was CGRP-dependent (48). Consistent with this, our pilot experiments have detected increased CGRP blood levels after RS.
Additionally, stress mobilizes corticotropin releasing factor (CRF) to promote the release of dynorphin and subsequent activation of kappa opioid receptors (KOR) (25, 49–51). We found that like propranolol, a single administration of nor-BNI, a very long acting KOR antagonist (52), blocked both the transient stress-induced CA as well as the induction of LS. These findings support the role of central stress pathways in promoting increased vulnerability through dynorphin-KOR signaling and are in agreement with our previous reports that KOR antagonists can protect against stress-induced pain in a model of MOH (25). In that study, animals were primed with sumatriptan and then challenged with BLS. Blockade of the KOR was effective in preventing medication-induced priming and was also able to prevent BLS-triggered CA when given after priming supporting a central site of action in stress circuits. In the present study, however, a single treatment of nor-BNI following RS priming was not effective in reducing the CA promoted by UMB exposure that likely directly activates peripheral nociceptors. Recently, BTRX-335140, a selective KOR antagonist (53) has been advanced to Phase II clinical trials for the treatment of neuropsychiatric disorders suggesting a potential opportunity for evaluation as a migraine preventive medication.
Although female prevalence is a well-established feature of migraine (54), we did not observe sex differences in the development of stress-induced LS. The preventive effect induced by the blockade of KOR with nor-BNI treatment was also not noticeably different between sexes consistent with our previous reports of blockade of sensitization in a model of MOH in male rats (25). While experimental stress might act to elicit sensitization in our model, it should be noted that stress may impact men and women differently and may be only one factor that contributes to the female prevalence of this disorder.
We also explored the translational relevance of our approach with assessment of therapies for acute migraine intervention. Preclinical studies have demonstrated that UMB promotes the release of CGRP (19). Surprisingly, however, we did not observe reversal of UMB-induced CA with olcegepant (30–32) when administered 1 hour after UMB when CA is already present, while sumatriptan was fully effective in reversal of UMB-induced CA under the same conditions. Importantly, olcegepant given prior to UMB fully blocked the development of CA suggesting that in this model, the CGRP receptor participates in the initiation, but has a less prominent role in the maintenance, of migraine-like pain. A potential explanation is a time-dependent progression of migraine pain from a CGRP receptor dependent initiation phase where CGRP is one of multiple mediators that collectively promote activation of nociceptors to reach the threshold for nociceptor activation and pain perception and a subsequent maintenance phase where blockade of the CGRP receptor prevents only one of many potential mechanisms driving dural nociceptors and pain.
Whether such distinctions can be drawn clinically remains unclear. Clinical trials with ubrogepant, another member of the gepant family of small molecule CGRP receptor antagonists have demonstrated efficacy when used as an acute anti-migraine therapy (55, 56). These studies, however, report that ubrogepant is most effective when given early in the migraine attack and when headache pain is mild, rather than when pain is severe (55, 56). Similarly, rimegepant, another small molecule CGRP receptor antagonist, was reported to be effective for the acute treatment of migraine. However, the pain-free efficacy two hours after administration and magnitude of treatment effect of both ubrogepant and rimegepant is modest and inferior to triptans (57, 58). Interestingly, recent data show that administration of an optional second dose of ubrogepant significantly increases the two-hour pain-free response rate raising the question of whether sufficient target engagement is achieved with an initial dose of the antagonist (59). Early intervention, before mild pain becomes more severe, is also more responsive to triptans, agonists at the 5HT1B/D receptors (60). Nevertheless, sumatriptan remained effective in reversal of established pain in this model. Prevention of the actions of CGRP has been clinically validated by the efficacy of anti-CGRP peptide monoclonal antibodies (mAb) including framanezumab, galcanezumab and eptinezumab, as well as of erenumab, a mAb to the CGRP receptor (61–64). The activity of olcegepant in our preclinical model would therefore be consistent with a preventive effect. In spite of the remarkable efficacy of these antibodies in migraine prevention, a substantial number of people with migraine continue to experience migraine attacks even after antibody therapy supporting CGRP-independent mechanisms of migraine pain (61–63). Triptans have been widely suggested to act primarily via modulation of CGRP release (2). Our data suggest, however, that sumatriptan is likely to additionally modulate non-CGRP receptor-mediated nociceptive mechanisms. This conclusion is also consistent with the efficacy of triptans for breakthrough migraine attacks in patients treated with anti-CGRP mAbs (62, 65).
We note that a limitation of our studies was the evaluation of only single doses of therapeutic compounds in a single strain of mouse. The timepoints chosen, and the routes of administration of these compounds may also be additional limitations. It is also not clear if the subthreshold inhalational dose of UMB used in our studies reflect the concentration of irritants that may be achieved in humans. Further, we used a single type of stress that is effective in rodents but that may not reflect the types of stress that are experienced in humans. Another limitation is the use of only one trigger, a TRPA1 agonist. Finally, other migraine triggers including different chemical substances and physiological triggers such as stress, sleep deprivation, hormonal changes etc. may engage mechanisms that respond differently to acute migraine medications. Future research is required to address these limitations.
Nevertheless, there is a need to improve preclinical models that have translational relevance to the human condition and our studies may provide insights relevant across species. While the exact cause of migraine remains poorly understood, it seems likely that different patients could experience attacks initiated either from peripheral activation of nociceptors by subthreshold stimuli, as in the case of environmental irritants, or from dysfunction of central circuits in response to stress or cyclical hypothalamic activity. In this regard, classification of medicines currently in use as preventive agents include both mAbs that inhibit CGRP and its receptors, which do not readily cross the blood brain barrier, as well as drugs that act within the brain such as topiramate or propranolol. The present studies developed and characterized a preclinical model of migraine that promotes vulnerability without injury that may increase forward translation of both acute treatments or preventive medications with peripheral or central mechanisms. Importantly, our studies are consistent with the conclusion that there is a temporal role for the CGRP receptor in migraine-like pain. In these circumstances, antagonism of the CGRP receptor may be most effective for prevention or when receptor blockade can be achieved early in the attack.
Supplementary Material
Supplementary Figure 1. UMB induced hindpaw CA only in female mice primed with RS. (a) Tactile frequency of response was assessed in naïve female mice at baseline (BL) and hourly after each of the 3 UMB inhalational exposure (0.01N/500 µL, each) or after BLS exposure. (b) Baseline (BL) response was collected, followed by 3 consecutive days of RS. CA was assessed on indicated days after the first RS over a time course of 16 days. On days 16, 18 and 20, baseline (BL) was collected, followed by inhalational exposure of UMB, with CA measurements collected hourly for 5 hours. Data are presented as mean ± S.E.M. and analyzed using two-way followed by Sidak’s multiple comparison test with * representing p < 0.05 in comparison to Control group (n = 6 – 12).
Supplementary Figure 2. UMB induced CA only in male mice primed with RS. (a) Periorbital and (b) hindpaw tactile frequency of response was collected at baseline (BL), followed by 3 days of RS. CA was assessed on indicated days after the first RS over a time course of 16 days. On days 16, 17 and 18, baseline (BL) was collected, followed by inhalational exposure of UMB, with measurements collected hourly for 5 hours. Data are presented as mean ± S.E.M. and analyzed using two-way ANOVA followed by Sidak’s multiple comparison test with * representing p < 0.05 in comparison to Control group (n = 8 – 9).
Supplementary Figure 3. TRPA1 agonist, isoflurane, did not induce CA in female mice primed with RS. (a) Periorbital and (b) hindpaw baseline (BL) tactile frequency of response was collected, followed by 3 days of RS. CA was assessed on indicated days over a time course of 16 days. On day 16, baseline (BL) was collected, animals were exposed to inhalation of isoflurane and tactile responses collected hourly for 5 hours. Data are presented as mean ± S.E.M. and analyzed using two-way ANOVA followed by Sidak’s multiple comparison test with * representing p < 0.05 in comparison to Control + Isoflurane group (n = 9 – 14).
Supplementary Figure 4. Effects of nor-BNI in prevention of RS-induced and UMB-induced CA in male mice. Nor-BNI (10 mg/kg, s.c.) was given 2 hours prior the first RS in male mice and tactile responses assessed for 16 days and again in response to UMB on day 16. Data are presented as mean ± S.E.M. and analyzed using Two-way ANOVA followed by Tukey’s multiple comparison test with * representing p < 0.05 in comparison to Stress + Vehicle group, (n = 8/group).
Article highlights.
A novel, injury free model of vulnerability for evaluation of migraine mechanisms and therapies
Use of translatable provocative stimuli to induce pain
Temporal contributions of the CGRP receptor in initiation and maintenance of migraine-like pain
Additional, non-CGRP receptor dependent modulation of nociception by sumatriptan
Identification of kappa opioid receptor antagonists as a novel preventive strategy for stress-related migraine
Acknowledgments
Funding
This research was funded by an unrestricted grant from AstraZeneca to FP.
Footnotes
Declaration of conflicting interests
IC is employee of AstraZeneca. CK received postdoctoral support from AstraZeneca. FP received laboratory support for this study from AstraZeneca. DWD reports the following conflicts: Personal fees: Amgen, AEON, Association of Translational Medicine, University Health Network, Daniel Edelman Inc., Autonomic Technologies, Axsome, Allergan, Alder BioPharmaceuticals, Biohaven, Charleston Laboratories, Clexio, Dr Reddy’s Laboratories/Promius, Electrocore LLC, Eli Lilly, eNeura, Neurolief, Novartis, Ipsen, Impel, Satsuma, Supernus, Sun Pharma (India), Theranica, Teva, Vedanta, WL Gore, Nocira, PSL Group Services, XoC, Zosano, ZP Opco, Foresite Capital, Oppenheimer; Upjohn (Division of Pfizer), Pieris, Revance, Equinox, Salvia, Amzak Health. Speaking fees: Eli Lilly, Novartis Canada, Amgen, Lundbeck. Speakers Bureaus: None. CME fees or royalty payments: HealthLogix, Medicom Worldwide, MedLogix Communications, Mednet, Miller Medical, PeerView, WebMD Health/Medscape, Chameleon, Academy for Continued Healthcare Learning, Universal Meeting Management, Haymarket, Global Scientific Communications, Global Life Sciences, Global Access Meetings, Catamount, UpToDate (Elsevier), Oxford University Press, Cambridge University Press, Wolters Kluwer Health; Stock options: Precon Health, Aural Analytics, Healint, Theranica, Second Opinion/Mobile Health, Epien, Nocira, Matterhorn, Ontologics, King-Devick Technologies; Consulting without fee: Aural Analytics, Healint, Second Opinion/Mobile Health, Epien; Board of Directors: Paranet North America, Precon Health, Epien, Matterhorn, Ontologics, King-Devick Technologies. Patent: 17189376.1–1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis without fee; Research funding: American Migraine Foundation, US Department of Defense, PCORI, Henry Jackson Foundation; Professional society fees or reimbursement for travel: American Academy of Neurology, American Brain Foundation, American Headache Society, American Migraine Foundation, International Headache Society, Canadian Headache Society.
References
- 1.Brennan KC, Pietrobon D. A Systems Neuroscience Approach to Migraine. Neuron 2018;97(5):1004–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dodick DW. Migraine. Lancet 2018;391(10127):1315–30. [DOI] [PubMed] [Google Scholar]
- 3.Dodick DW. A Phase-by-Phase Review of Migraine Pathophysiology. Headache 2018;58 Suppl 1:4–16. [DOI] [PubMed] [Google Scholar]
- 4.Goadsby PJ, Holland PR. An Update: Pathophysiology of Migraine. Neurol Clin 2019;37(4):651–71. [DOI] [PubMed] [Google Scholar]
- 5.Peng KP, May A. Migraine understood as a sensory threshold disease. Pain 2019;160(7):1494–501. [DOI] [PubMed] [Google Scholar]
- 6.Bourke JH, Langford RM, White PD. The common link between functional somatic syndromes may be central sensitisation. J Psychosom Res 2015;78(3):228–36. [DOI] [PubMed] [Google Scholar]
- 7.Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain 2012;13(10):936–44. [DOI] [PubMed] [Google Scholar]
- 8.Nahman-Averbuch H, Granovsky Y, Coghill RC, Yarnitsky D, Sprecher E, Weissman-Fogel I. Waning of “conditioned pain modulation”: a novel expression of subtle pronociception in migraine. Headache 2013;53(7):1104–15. [DOI] [PubMed] [Google Scholar]
- 9.Teepker M, Kunz M, Peters M, Kundermann B, Schepelmann K, Lautenbacher S. Endogenous pain inhibition during menstrual cycle in migraine. Eur J Pain 2014;18(7):989–98. [DOI] [PubMed] [Google Scholar]
- 10.Amin FM, Asghar MS, Guo S, Hougaard A, Hansen AE, Schytz HW, et al. Headache and prolonged dilatation of the middle meningeal artery by PACAP38 in healthy volunteers. Cephalalgia 2012;32(2):140–9. [DOI] [PubMed] [Google Scholar]
- 11.Birk S, Kruuse C, Petersen KA, Tfelt-Hansen P, Olesen J. The headache-inducing effect of cilostazol in human volunteers. Cephalalgia 2006;26(11):1304–9. [DOI] [PubMed] [Google Scholar]
- 12.Tvedskov JF, Iversen HK, Olesen J, Tfelt-Hansen P. Nitroglycerin provocation in normal subjects is not a useful human migraine model? Cephalalgia 2010;30(8):928–32. [DOI] [PubMed] [Google Scholar]
- 13.Harriott AM, Strother LC, Vila-Pueyo M, Holland PR. Animal models of migraine and experimental techniques used to examine trigeminal sensory processing. J Headache Pain 2019;20(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomsen LL, Kruuse C, Iversen HK, Olesen J. A nitric oxide donor (nitroglycerin) triggers genuine migraine attacks. Eur J Neurol 1994;1(1):73–80. [DOI] [PubMed] [Google Scholar]
- 15.Irlbacher K, Meyer BU. Nasally triggered headache. Neurology 2002;58(2):294. [DOI] [PubMed] [Google Scholar]
- 16.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007;27(5):394–402. [DOI] [PubMed] [Google Scholar]
- 17.Kunkler PE, Ballard CJ, Oxford GS, Hurley JH. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain 2011;152(1):38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benemei S, Appendino G, Geppetti P. Pleasant natural scent with unpleasant effects: cluster headache-like attacks triggered by Umbellularia californica. Cephalalgia 2010;30(6):744–6. [DOI] [PubMed] [Google Scholar]
- 19.Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, De Siena G, et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain 2012;135(Pt 2):376–90. [DOI] [PubMed] [Google Scholar]
- 20.Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, Materazzi S, et al. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain 2012;153(9):1949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong J, Minassi A, Prenen J, Taglialatela-Scafati O, Appendino G, Nilius B. Umbellulone modulates TRP channels. Pflugers Arch 2011;462(6):861–70. [DOI] [PubMed] [Google Scholar]
- 22.Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron 2012;73(2):219–34. [DOI] [PubMed] [Google Scholar]
- 23.Haque B, Rahman KM, Hoque A, Hasan AT, Chowdhury RN, Khan SU, et al. Precipitating and relieving factors of migraine versus tension type headache. BMC Neurol 2012;12:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robbins L. Precipitating factors in migraine: a retrospective review of 494 patients. Headache 1994;34(4):214–6. [DOI] [PubMed] [Google Scholar]
- 25.Xie JY, De Felice M, Kopruszinski CM, Eyde N, LaVigne J, Remeniuk B, et al. Kappa opioid receptor antagonists: A possible new class of therapeutics for migraine prevention. Cephalalgia 2017;37(8):780–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luiz AP, Schroeder SD, Rae GA, Calixto JB, Chichorro JG. Contribution and interaction of kinin receptors and dynorphin A in a model of trigeminal neuropathic pain in mice. Neuroscience 2015;300:189–200. [DOI] [PubMed] [Google Scholar]
- 27.Navratilova E, Rau J, Oyarzo J, Tien J, Mackenzie K, Stratton J, et al. CGRP-dependent and independent mechanisms of acute and persistent post-traumatic headache following mild traumatic brain injury in mice. Cephalalgia 2019;39(14):1762–75. [DOI] [PubMed] [Google Scholar]
- 28.Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, et al. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol 2000;129(3):420–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ton HT, Phan TX, Abramyan AM, Shi L, Ahern GP. Identification of a putative binding site critical for general anesthetic activation of TRPA1. Proc Natl Acad Sci U S A 2017;114(14):3762–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moldovan Loomis C, Dutzar B, Ojala EW, Hendrix L, Karasek C, Scalley-Kim M, et al. Pharmacologic Characterization of ALD1910, a Potent Humanized Monoclonal Antibody against the Pituitary Adenylate Cyclase-Activating Peptide. J Pharmacol Exp Ther 2019;369(1):26–36. [DOI] [PubMed] [Google Scholar]
- 31.Petersen KA, Lassen LH, Birk S, Lesko L, Olesen J. BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin Pharmacol Ther 2005;77(3):202–13. [DOI] [PubMed] [Google Scholar]
- 32.Yao G, Yu T, Han X, Mao X, Li B. Therapeutic effects and safety of olcegepant and telcagepant for migraine: A meta-analysis. Neural Regen Res 2013;8(10):938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprenger T, Viana M, Tassorelli C. Current Prophylactic Medications for Migraine and Their Potential Mechanisms of Action. Neurotherapeutics 2018;15(2):313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipton RB, Buse DC, Hall CB, Tennen H, Defreitas TA, Borkowski TM, et al. Reduction in perceived stress as a migraine trigger: testing the “let-down headache” hypothesis. Neurology 2014;82(16):1395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon HJ, Seo JG, Park SP. Perceived stress in patients with migraine: a case-control study. J Headache Pain 2017;18(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawkins JL, Cornelison LE, Blankenship BA, Durham PL. Vagus nerve stimulation inhibits trigeminal nociception in a rodent model of episodic migraine. Pain Rep 2017;2(6):e628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shankland WE 2nd. The trigeminal nerve. Part III: The maxillary division. Cranio 2001;19(2):78–83. [DOI] [PubMed] [Google Scholar]
- 38.Benemei S, De Cesaris F, Fusi C, Rossi E, Lupi C, Geppetti P. TRPA1 and other TRP channels in migraine. J Headache Pain 2013;14:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benemei S, Dussor G. TRP Channels and Migraine: Recent Developments and New Therapeutic Opportunities. Pharmaceuticals (Basel) 2019;12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goadsby PJ. Herbalism helps headache. Brain 2012;135(Pt 2):318–9. [DOI] [PubMed] [Google Scholar]
- 41.Kunkler PE, Zhang L, Johnson PL, Oxford GS, Hurley JH. Induction of chronic migraine phenotypes in a rat model after environmental irritant exposure. Pain 2018;159(3):540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finger TE, Bottger B. Peripheral peptidergic fibers of the trigeminal nerve in the olfactory bulb of the rat. J Comp Neurol 1993;334(1):117–24. [DOI] [PubMed] [Google Scholar]
- 43.Tietjen GE, Khubchandani J, Ghosh S, Bhattacharjee S, Kleinfelder J. Headache symptoms and indoor environmental parameters: Results from the EPA BASE study. Ann Indian Acad Neurol 2012;15(Suppl 1):S95–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci 2009;32(12):611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russo A, Coppola G, Pierelli F, Parisi V, Silvestro M, Tessitore A, et al. Pain Perception and Migraine. Front Neurol 2018;9:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwedt TJ. Multisensory integration in migraine. Curr Opin Neurol 2013;26(3):248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci 2004;1032:1–7. [DOI] [PubMed] [Google Scholar]
- 48.Avona A, Mason BN, Lackovic J, Wajahat N, Motina M, Quigley L, et al. Repetitive stress in mice causes migraine-like behaviors and calcitonin gene-related peptide-dependent hyperalgesic priming to a migraine trigger. Pain 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 2010;1314:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crowley NA, Kash TL. Kappa opioid receptor signaling in the brain: Circuitry and implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry 2015;62:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry 1999;46(9):1167–80. [DOI] [PubMed] [Google Scholar]
- 52.Kishioka S, Kiguchi N, Kobayashi Y, Yamamoto C, Saika F, Wakida N, et al. Pharmacokinetic evidence for the long-lasting effect of nor-binaltorphimine, a potent kappa opioid receptor antagonist, in mice. Neurosci Lett 2013;552:98–102. [DOI] [PubMed] [Google Scholar]
- 53.Guerrero M, Urbano M, Kim EK, Gamo AM, Riley S, Abgaryan L, et al. Design and Synthesis of a Novel and Selective Kappa Opioid Receptor (KOR) Antagonist (BTRX-335140). J Med Chem 2019;62(4):1761–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 2017;16(1):76–87. [DOI] [PubMed] [Google Scholar]
- 55.Dodick DW, Lipton RB, Ailani J, Halker Singh RB, Shewale AR, Zhao S, et al. Ubrogepant, an Acute Treatment for Migraine, Improved Patient-Reported Functional Disability and Satisfaction in 2 Single-Attack Phase 3 Randomized Trials, ACHIEVE I and II. Headache 2020;60(4):686–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dodick DW, Lipton RB, Ailani J, Lu K, Finnegan M, Trugman JM, et al. Ubrogepant for the Treatment of Migraine. N Engl J Med 2019;381(23):2230–41. [DOI] [PubMed] [Google Scholar]
- 57.Lipton RB, Croop R, Stock EG, Stock DA, Morris BA, Frost M, et al. Rimegepant, an Oral Calcitonin Gene-Related Peptide Receptor Antagonist, for Migraine. N Engl J Med 2019;381(2):142–9. [DOI] [PubMed] [Google Scholar]
- 58.Tfelt-Hansen P, Loder E. The Emperor’s New Gepants: Are the Effects of the New Oral CGRP Antagonists Clinically Meaningful? Headache 2019;59(1):113–7. [DOI] [PubMed] [Google Scholar]
- 59.Ailani J, Blumenfeld A, Klein B, Finnegan M, Severt L, Liu C, et al. An Optional Second Dose of Ubrogepant is Effective in Achieving 2-Hour Pain Freedom in the Acute Treatment of Migraine (166). Neurology 2020;94(15 Supplement):166. [Google Scholar]
- 60.Goadsby PJ, Zanchin G, Geraud G, de Klippel N, Diaz-Insa S, Gobel H, et al. Early vs. non-early intervention in acute migraine-’Act when Mild (AwM)’. A double-blind, placebo-controlled trial of almotriptan. Cephalalgia 2008;28(4):383–91. [DOI] [PubMed] [Google Scholar]
- 61.Ceriani CEJ, Wilhour DA, Silberstein SD. Novel Medications for the Treatment of Migraine. Headache 2019;59(9):1597–608. [DOI] [PubMed] [Google Scholar]
- 62.Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: a new era in migraine therapy. Lancet 2019;394(10210):1765–74. [DOI] [PubMed] [Google Scholar]
- 63.Huang IH, Wu PC, Lin EY, Chen CY, Kang YN. Effects of Anti-Calcitonin Gene-Related Peptide for Migraines: A Systematic Review with Meta-Analysis of Randomized Clinical Trials. Int J Mol Sci 2019;20(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dodick DW, Lipton RB, Silberstein S, Goadsby PJ, Biondi D, Hirman J, et al. Eptinezumab for prevention of chronic migraine: A randomized phase 2b clinical trial. Cephalalgia 2019;39(9):1075–85. [DOI] [PubMed] [Google Scholar]
- 65.Dubowchik GM, Conway CM, Xin AW. Blocking the CGRP Pathway for Acute and Preventive Treatment of Migraine: The Evolution of Success. J Med Chem 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. UMB induced hindpaw CA only in female mice primed with RS. (a) Tactile frequency of response was assessed in naïve female mice at baseline (BL) and hourly after each of the 3 UMB inhalational exposure (0.01N/500 µL, each) or after BLS exposure. (b) Baseline (BL) response was collected, followed by 3 consecutive days of RS. CA was assessed on indicated days after the first RS over a time course of 16 days. On days 16, 18 and 20, baseline (BL) was collected, followed by inhalational exposure of UMB, with CA measurements collected hourly for 5 hours. Data are presented as mean ± S.E.M. and analyzed using two-way followed by Sidak’s multiple comparison test with * representing p < 0.05 in comparison to Control group (n = 6 – 12).
Supplementary Figure 2. UMB induced CA only in male mice primed with RS. (a) Periorbital and (b) hindpaw tactile frequency of response was collected at baseline (BL), followed by 3 days of RS. CA was assessed on indicated days after the first RS over a time course of 16 days. On days 16, 17 and 18, baseline (BL) was collected, followed by inhalational exposure of UMB, with measurements collected hourly for 5 hours. Data are presented as mean ± S.E.M. and analyzed using two-way ANOVA followed by Sidak’s multiple comparison test with * representing p < 0.05 in comparison to Control group (n = 8 – 9).
Supplementary Figure 3. TRPA1 agonist, isoflurane, did not induce CA in female mice primed with RS. (a) Periorbital and (b) hindpaw baseline (BL) tactile frequency of response was collected, followed by 3 days of RS. CA was assessed on indicated days over a time course of 16 days. On day 16, baseline (BL) was collected, animals were exposed to inhalation of isoflurane and tactile responses collected hourly for 5 hours. Data are presented as mean ± S.E.M. and analyzed using two-way ANOVA followed by Sidak’s multiple comparison test with * representing p < 0.05 in comparison to Control + Isoflurane group (n = 9 – 14).
Supplementary Figure 4. Effects of nor-BNI in prevention of RS-induced and UMB-induced CA in male mice. Nor-BNI (10 mg/kg, s.c.) was given 2 hours prior the first RS in male mice and tactile responses assessed for 16 days and again in response to UMB on day 16. Data are presented as mean ± S.E.M. and analyzed using Two-way ANOVA followed by Tukey’s multiple comparison test with * representing p < 0.05 in comparison to Stress + Vehicle group, (n = 8/group).


