Abstract
The discovery that new neurons are produced in some regions of the adult mammalian brain is a paradigm-shift in neuroscience research. These new-born cells are produced from neuroprogenitors mainly in the subventricular zone at the margin of the lateral ventricle, subgranular zone in the hippocampal dentate gyrus and in the striatum, a component of the basal ganglia, even in humans. In the human hippocampus, neuroblasts are produced even in elderlies. The regulation of adult neurogenesis is a complex phenomenon involving a multitude of molecules, neurotransmitters and soluble factors released by different sources including glial cells. Microglia, the resident macrophages of the central nervous system, are considered to play an important role on the regulation of adult neurogenesis both in physiological and pathological conditions. Following stroke and other acute neural disorders, there is an increase in the numbers of neuroblast production in the neurogenic niches. Microglial activation is believed to display both beneficial and detrimental role on adult neurogenesis after stroke, depending on the activation level and brain location. In this article, we review the scientific evidence addressing the role of microglial activation on adult neurogenesis after ischemia. A comprehensive understanding of the microglial role after stroke and other neural disorders it is an important step for development of future therapies based on manipulation of adult neurogenesis.
Keywords: adult neurogenesis, hippocampus, ischemia, microglia, neuroinflammation, neuroprotection, stroke, subventricular zone, therapy
Adult Neurogenesis: Historical Background
There was a long-lasting belief that the central nervous system (CNS) of adult mammals was unable to generate new neurons. Nevertheless, the pioneering studies performed by Joseph Altman (Altman and Das, 1965), Michael Kaplan (Kaplan and Hinds, 1977) and Fernando Nottebohm (Nottebohm et al., 1986), respectively, in the 1960s, 1970s and 1980s were the basis for in vitro studies performed in the 1990s (Reynolds and Weiss, 1992) that definitively confirmed that the adult brain possesses the capacity of producing new neurons (Lima and Gomes-leal W, 2019). An important contribution to the field was the demonstration that new granule neurons are produced in the dentate gyrus (DG) of adult rodents (Cameron, 1994; Gould et al., 1997). Therefore, the neural circuits of adult mammalian brains are not fixed and immutable as previously thought. Experimental findings suggest that the brain modulates and maintains preexisting neural circuits by adding newborn cells (Vicidomini et al., 2020). In addition, this phenomenon might be used to replace neurons lost after CNS diseases (Zhao et al., 2008).
Nowadays, is fully confirmed the existence of two main neurogenic niches in the mammalian brain: subventricular zone (SVZ) at the wall of lateral ventricles (Doetsch et al., 1997) and subgranular zone in the hippocampus, even in humans (Boldrini et al., 2018; Moreno-Jiménez et al., 2019). In the SVZ, astrocyte-like adult neural stem cells, termed B1 in mice, generate C cells, which in turn generate neuroblasts (A cells). These immature neurons migrate to the olfactory bulb (OB) to be constantly integrated into preexisting neural circuits, which seems to be very important for maintenance and repair of OB circuitry (Imayoshi et al., 2008). In the hippocampus, progenitor cells originate immature neurons, which migrate from subgranular zone to the granular layer of hippocampal dentate gyrus becoming granule cells, which are functionally integrated into hippocampal circuits (Li et al., 2009). A search was performed in the US National Library of Medline (PubMed). The search terms were microglia, review, adult neurogenesis, stroke.
Function of Adult Neurogenesis
The function of adult neurogenesis is not well established. Genetic labeling studies in mice suggest that adult neurogenesis is important for replacement of the majority of granule neurons in the OB and considerable addition of granule neurons into the hippocampal circuitry (Imayoshi et al., 2008). Genetic ablation of newly formed neurons in both OB and hippocampus results in conspicuous decrease in the population of granule cells with subsequent functional deficits (Imayoshi et al., 2008). In the hippocampus, there was impairment in some kinds of contextual and a spatial memory, which depends on the hippocampus integrity (Imayoshi et al., 2008). According to Imayoshi results, continuous neurogenesis is necessary for maintenance and reorganization of OB circuitry and refinement and modulation of adult hippocampal circuits, which are necessary for specific types of hippocampal-dependent memory.
Pattern separation is a neural computational task performed by hippocampal DG, which allows separation of similar information in the memory system. Plenty of evidences suggest that adult hippocampal neurogenesis plays a pivotal role on pattern separation (Sahay et al., 2011a; Anacker and Hen, 2017). Experimental ablation of adult hippocampal neurogenesis using optogenetic impairs the ability to discriminate very similar contexts (Clelland et al., 2009) and genetic expansion of adult neurogenesis in DG by enhancing survival of adult-born granule cells increases highly similar context discrimination (Sahay et al., 2011b). In addition, adult born cells may also play different roles on DG functions including pattern integrators and contribution to cognitive flexibility (Anacker and Hen, 2017; Anacker et al., 2018). Decrease on pattern separation and cognitive flexibility may underlie the pathophysiology of mood disorders including anxiety, depression, post-traumatic stress disorders (Anacker and Hen, 2017; Anacker et al., 2018). This can be related to decrease in stress resilience in these pathological conditions (Anacker et al., 2018; Toda et al., 2019).
Microglia and Adult Neurogenesis
There is a complex regulation of adult neurogenesis by intrinsic and extrinsic factors comprising several growth factors, calcium and neurotransmitters (Song et al., 2016). It seems that glial cells play an important role on the regulation of adult neurogenesis in both physiological and pathological conditions (Song et al., 2016). It has been reported that microglia instruct neurogenesis in vitro (Aarum et al., 2003) and continuous loss of microglia in culture is detrimental for neurogenesis (Walton et al., 2006). In vivo, microglia seem to regulate neurogenesis in the hippocampus of adrenalectomized rats (Battista et al., 2006) and also the proliferation of progenitor cells by working in synergism with lymphocytes (Ziv et al., 2006). In DG, newborn cells dye by apoptosis between 1 to 4 days after their birth and are efficiently removed by microglial cells, which shape hippocampal adult neurogenesis through apoptosis-coupled phagocytosis (Sierra et al., 2010). It has been also shown that synaptic pruning by microglial is necessary for normal brain development (Paolicelli et al., 2011), while synaptic integration of adult hippocampal newborn cells is tightly controlled by astrocytes (Sultan et al., 2015).
Stroke and Adult Neurogenesis
Adult neurogenesis is influenced by stroke, trauma and other pathological conditions (Arvidsson et al., 2002; Gomes-Leal, 2012; Liang et al., 2019). There is conspicuous neuroblast migration to striatum (Arvidsson et al., 2002) and cortex (Liang et al., 2019) after experimental stroke. Arvidsson et al. (2002) showed for the first time that neuroblasts migrate to ischemic striatum and partially replace neurons that are lost after stroke. Similar results were reported by a other group a short time later (Parent, 2003). Since then, other studies have shown migration of neuroblasts to other brain areas, including cerebral cortex (Liang et al., 2019).
It has been recently shown that cortical ischemia influences proliferation of neuroblasts in the SVZ (Liang et al., 2019). SVZ neuroblasts migrate to peri-infarct cortical area contributing to spontaneous functional recovery in this experimental paradigm (Liang et al., 2019). It has been also shown that immature adult-born GABAergic migrate from SVZ and integrate into the peri-infarct area after cortical ischemia, although their contribution to functional recovery was not determined (Kannangara et al., 2018). In addition, neuroblasts migrate toward ischemic cortex contributing to increase in the number of total cortical neurons at 65 days post-injury (Palma-Tortosa et al., 2017). Finally, it has been reported that an endogenous population of cortical progenitors may originate adult-born GABAergic neurons in layer 1 of neocortex, which migrate to other cortical layers following damage (Ohira et al., 2010).
Microglia are considerably activated after stroke with beneficial and detrimental actions for adult neurogenesis (Thored et al., 2009; Gomes-Leal, 2012; Lopes et al., 2016). These glial cells together with astrocytes seem to contribute to neuroblast migration toward striatum after stroke by releasing factors including monocyte chemoattractant protein-1 (Yan et al., 2006) and stromal cell-derived factor1 α (Thored et al., 2006). We have previously shown that long-lasting microglia activation occurs in both SVZ and striatum following middle cerebral artery occlusion (MCAO) (Thored et al., 2009). In this study, we have reported that SVZ microglia display a more ramified morphological phenotype in both acute and chronic survival times after MCAO presenting a proneurogenic phenotype (Figure 1) by expressing and releasing insulin-like growth factor (Thored et al., 2009).
Figure 1.
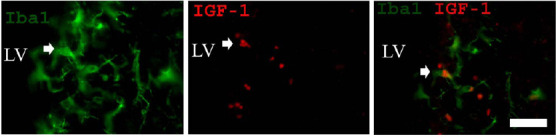
Expression of insulin-like growth factor 1 (IGF-1) by microglia in the subventricular zone (SVZ).
Double immunofluorescence for Iba1 (green, microglial marker) and IGF-1 (red) in the SVZ. Iba-1 positive cells are also positive for IGF-1. LV: Lateral ventricle. Laboratory of Experimental Neuroprotection and Neuroregeneration image data bank. See Thored et al. (2009) for details on the protocol for IGF-1 immunohistochemistry. Scale bar: 30 µm.
In a follow up study, we have described clusters of ramified microglia spatially correlated with clusters of neuroblasts in the SVZ suggesting a beneficial role of microglia for striatal neurogenesis (Gomes-Leal, 2012). Surprisingly, in the same study, we also described hyper activated microglia in regions of absence of neuroblast migration in the dorsal striatum (Gomes-Leal, 2012), while in the same animal activated but more ramified microglia were present together with migrating neuroblasts in the ventral striatum (Gomes-Leal, 2012). Based on these results, we hypothesized that after MCAO beneficial and detrimental microglia are present in different anatomical niches in the damaged striatum (Gomes-Leal, 2012).
Microglia are also detrimental for adult neurogenesis. Treatment with the nonsteroidal anti-inflammatory drug indomethacin reduced microglia activation and increased survival and numbers of neuroblasts in the striatum following transient focal ischemia (Hoehn et al., 2005). Chronic treatment with minocycline also reduced microglia activation and increased number of neuroblasts in the hippocampal DG after MCAO (Liu et al., 2007). Recently, we provided further evidence that inhibition of microglia with indomethacin increases numbers of neuroblasts in both SVZ and striatum mainly at 14 days after endothelin-1-induced striatal stroke even in the absence of infarct area reduction (Lopes et al., 2016). In addition, indomethacin treatment enhances adult neurogenesis in the hippocampal DG giving further support that this anti-inflammatory drug may modulate microglia activation contributing to increased neurogenesis (Hain et al., 2018).
The pathological environment is non-permissive to survival of migrating neuroblasts (Arvidsson et al., 2002). 80% of neuroblasts migrating to ischemic striatum dye in the first 2 weeks after MCAO (Arvidsson et al., 2002), although a continuous neuroblast migration has been reported (Thored et al., 2006). Excessive inflammation certainly contributes to neuroblast death after stroke and a proper modulation of microglia activation is suitable approach to increase adult neurogenesis as previously discussed. It follows that minocycline and indomethacin may modulate microglial phenotypes after stroke maximizing expression of M2 microglia, which release growth factors and contribute to repair after stroke (Hu et al., 2012). This seems to be valid for stem cell survival as we have also shown that modulation of microglial activation with minocycline improves therapeutic actions of bone marrow mononuclear cells transplanted into the acute phase of both cortical (Franco et al., 2012) and striatal stroke (Cardoso et al., 2013).
Recent studies have addressed the mechanisms underlying the influence of microglia on adult neurogenesis suggesting both beneficial (Chapman et al., 2015; Sellner et al., 2016) and detrimental roles (Jin et al., 2014; Ortega et al., 2014; Moraga et al., 2015), as previously reported. It has been shown that inflammation, even in the absence of neuronal damage, is a major inducer of neuroblast migration to striatum (Chapman et al., 2015). Blockage of K(ATP) -channel in microglia using glibenclamide enhances striatal neurogenesis following stroke (Ortega et al., 2013, 2014), suggesting that very reactive microglia may impair adult neurogenesis. Nevertheless, a proper modulation of microglial activation seems to contribute to neurogenesis as glibenclamide may increase monocyte chemoattractant protein-1 release by microglia contributing to neuroblast migration (Ortega et al., 2013, 2014). In addition, increased microglial proliferation has been demonstrated with aging with a subsequent impairment of adult neurogenesis and neuroblast migration to striatum following stroke in aged animals (Moraga et al., 2015).
The “Janus face” of microglia seems to be related to their immune functions (Gomes-Leal, 2019). Different microglial phenotypes (M1, M2, etc) are likely a product of soluble factors released by different sources in the pathological environment. Recently, we have introduced the “friend fire hypothesis” to explain the dual role of microglia after CNS diseases (Gomes-Leal, 2019). According to this hypothesis, microglial can be beneficial or detrimental depending on the kind of pattern recognition receptors that are activated by “danger signals” released by dying cells in the pathological environment. We proposed that “danger signals” might activate some receptors, like toll-like receptors, activated by pathogens during infection even in the absence of a real infection (Gomes-Leal, 2019). Under these circumstances, microglia would use the same biochemical weapons normally used to eliminate pathogens during infectious diseases killing neurons during stroke, trauma and other neural disorders (Gomes-Leal, 2019). These mechanisms might underlie the dual role of microglia on adult neurogenesis, as previously discussed.
Conclusions and Future Perspective
Further studies are necessary to precisely establish the role of microglia on adult neurogenesis following stroke. Nevertheless, a critical analysis of the papers discussed above indicates that the final contribution that the final contribution of microglia to adult neurogenesis will depend on how the pathological environment determines the fate of microglial phenotypes. Anti-inflammatory drugs such indomethacin and minocycline may inhibit specific populations of M1 microglia maximizing the beneficial effects of M2 microglial phenotypes contributing to adult neurogenesis improvement. It seems clear that a complete inhibition of microglia is not a suitable approach to improve adult neurogenesis following stroke. Future studies are mandatory to establish the contribution of adult neurogenesis to functional recovery after stroke and this will depend on the functional integration of new born neurons into preexisting neural circuits.
Acknowledgments
We are grateful to Olle Lindvall and Zaal Kokaya for scientific support during post-doctoral training of WGL.
Footnotes
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was supported by the Brazilian National Council for Scientific Research.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the Brazilian National Council for Scientific Research.
References
- 1.Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 3.Anacker C, Hen R. Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat Rev Neurosci. 2017;18:335–346. doi: 10.1038/nrn.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B, Hen R. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature. 2018;559:98–102. doi: 10.1038/s41586-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 6.Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- 7.Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22:589–599. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron HA. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 9.Cardoso MM, Franco EC, de Souza CC, da Silva MC, Gouveia A, Gomes-Leal W. Minocycline treatment and bone marrow mononuclear cell transplantation after endothelin-1 induced striatal ischemia. Inflammation. 2013;36:197–205. doi: 10.1007/s10753-012-9535-5. [DOI] [PubMed] [Google Scholar]
- 10.Chapman KZ, Ge R, Monni E, Tatarishvili J, Ahlenius H, Arvidsson A, Ekdahl CT, Lindvall O, Kokaia Z. Inflammation without neuronal death triggers striatal neurogenesis comparable to stroke. Neurobiol Dis. 2015;83:1–15. doi: 10.1016/j.nbd.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Y, Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36:2718–2724. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- 14.Franco EC, Cardoso MM, Gouveia A, Pereira A, Gomes-Leal W. Modulation of microglial activation enhances neuroprotection and functional recovery derived from bone marrow mononuclear cell transplantation after cortical ischemia. Neurosci Res. 2012;73:122–132. doi: 10.1016/j.neures.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Gomes-Leal W. Microglial physiopathology: how to explain the dual role of microglia after acute neural disorders. Brain Behav. 2012;2:345–356. doi: 10.1002/brb3.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes-Leal W. Why microglia kill neurons after neural disorders. The friendly fire hypothesis. Neural Regen Res. 2019;14:1499–1502. doi: 10.4103/1673-5374.255359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gould E, McEwen B S, Tanapat P, Galea LAM, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hain EG, Sparenberg M, Rasińska J, Klein C, Akyüz L, Steiner B. (2018) Indomethacin promotes survival of new neurons in the adult murine hippocampus accompanied by anti-inflammatory effects following MPTP-induced dopamine depletion. J Neuroinflammation. 2018:162. doi: 10.1186/s12974-018-1179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 20.Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 21.Jin Q, Cheng J, Liu Y, Wu J, Wang X, Wei S, Zhou X, Qin Z, Jia J, Zhen X. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav Immun. 2014;40:131–142. doi: 10.1016/j.bbi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Kannangara TS, Carter A, Xue Y, Dhaliwal JS, Béïque JC, Lagace DC. Excitable adult-generated GABAergic neurons acquire functional innervation in the cortex after stroke. Stem Cell Reports. 2018;11:1327–1336. doi: 10.1016/j.stemcr.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Mu Y, Gage FH. Development of neural circuits in the adult hippocampus. Curr Top Dev Biol. 2009;87:149–174. doi: 10.1016/S0070-2153(09)01205-8. [DOI] [PubMed] [Google Scholar]
- 26.Liang H, Zhao H, Gleichman A, Machnicki M, Telang S, Tang S, Rshtouni M, Ruddell J, Carmichael ST. Region-specific and activity-dependent regulation of SVZ neurogenesis and recovery after stroke. Proc Natl Acad Sci U S A. 2019;116:13621–13630. doi: 10.1073/pnas.1811825116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lima SA, Gomes-Leal W. Neurogenesis in the hippocampus of adult humans: controversy “fixed” at last. Neural Regen Res. 2019;14:1917–1918. doi: 10.4103/1673-5374.259616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- 29.Lopes RS, Cardoso MM, Sampaio AO, Barbosa MS, Jr, Souza CC, MC DAS, Ferreira EM, Freire MA, Lima RR, Gomes-Leal W. Indomethacin treatment reduces microglia activation and increases numbers of neuroblasts in the subventricular zone and ischaemic striatum after focal ischaemia. J Biosci. 2016;41:381–394. doi: 10.1007/s12038-016-9621-1. [DOI] [PubMed] [Google Scholar]
- 30.Moraga A, Pradillo JM, Garcia-Culebras A, Palma-Tortosa S, Ballesteros I, Hernandez-Jimenez M, Moro MA, Lizasoain I. Aging increases microglial proliferation, delays cell migration, and decreases cortical neurogenesis after focal cerebral ischemia. J Neuroinflammation. 2015;12:87. doi: 10.1186/s12974-015-0314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno-Jiménez EP, Flor-Garcia M, Terreros-Roncal J, Rabano A, Cafini F, Pallas-Bazarra N, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- 32.Nottebohm F, Nottebohm ME, Crane L. Developmental and seasonal changes in canary song and their relation to changes in the anatomy of song-control nuclei. Behav Neural Biol. 1986;46:445–471. doi: 10.1016/s0163-1047(86)90485-1. [DOI] [PubMed] [Google Scholar]
- 33.Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, Funatsu N, Shimizu K, Oishi T, Hayashi M, Miyakawa T, Kaneko T, Nakamura S. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci. 2010;3:173–179. doi: 10.1038/nn.2473. [DOI] [PubMed] [Google Scholar]
- 34.Ortega FJ, Jolkkonen J, Mahy N, Rodriguez MJ. Glibenclamide enhances neurogenesis and improves long-term functional recovery after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2013;33:356–364. doi: 10.1038/jcbfm.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortega FJ, Vukovic J, Rodriguez MJ, Bartlett PF. Blockade of microglial KATP -channel abrogates suppression of inflammatory-mediated inhibition of neural precursor cells. Glia. 2014;62:247–258. doi: 10.1002/glia.22603. [DOI] [PubMed] [Google Scholar]
- 36.Palma-Tortosa S, García-Culebras A, Moraga A, Hurtado O, Perez-Ruiz A, Durán-Laforet V, Parra J, Cuartero MI, Pradillo JM, Moro MA, Lizasoain I. Specific features of SVZ neurogenesis after cortical ischemia: a longitudinal study. Sci Rep. 2017;27:16343. doi: 10.1038/s41598-017-16109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 39.Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011a;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011b;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sellner S, Paricio-Montesinos R, Spiess A, Masuch A, Erny D, Harsan LA, Elverfeldt DV, Schwabenland M, Biber K, Staszewski O, Lira S, Jung S, Prinz M, Blank T. Microglial CX3CR1 promotes adult neurogenesis by inhibiting Sirt 1/p65 signaling independent of CX3CL1. Acta Neuropathol Commun. 2016;4:102. doi: 10.1186/s40478-016-0374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song J, Olsen RH, Sun J, Ming GL, Song H. Neuronal Circuitry Mechanisms Regulating Adult Mammalian Neurogenesis. Cold Spring Harb Perspect Biol. 2016 doi: 10.1101/cshperspect.a018937. doi:101101/cshperspecta018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 44.Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, Nygren JM, Jacobsen SE, Ekdahl CT, Kokaia Z, Lindvall O. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- 45.Toda T, Parylak SL, Linker SB, Gage FH. The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry. 2019;24:67–87. doi: 10.1038/s41380-018-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vicidomini C, Guo N, Sahay A. Communication, cross talk, and signal integration in the adult hippocampal neurogenic niche. Neuron. 2020;105:220–235. doi: 10.1016/j.neuron.2019.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, Scheffler B, Steindler DA. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- 48.Yamashima T, Tonchev AB, Yukie M. Adult hippocampal neurogenesis in rodents and primates: endogenous, enhanced, and engrafted. Rev Neurosci. 2007;18:67–82. doi: 10.1515/revneuro.2007.18.1.67. [DOI] [PubMed] [Google Scholar]
- 49.Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;27:1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- 50.Zhao CS, Overstreet-Wadiche L. Integration of adult generated neurons during epileptogenesis. Epilepsia. 2008;49:3–12. doi: 10.1111/j.1528-1167.2008.01632.x. [DOI] [PubMed] [Google Scholar]
- 51.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]


