Abstract
Spinal cord injury can lead to severe motor, sensory and autonomic nervous dysfunctions. However, there is currently no effective treatment for spinal cord injury. Neural stem cells and progenitor cells, bone marrow mesenchymal stem cells, olfactory ensheathing cells, umbilical cord blood stem cells, adipose stem cells, hematopoietic stem cells, oligodendrocyte precursor cells, macrophages and Schwann cells have been studied as potential treatments for spinal cord injury. These treatments were mainly performed in animals. However, subtle changes in sensory function, nerve root movement and pain cannot be fully investigated with animal studies. Although these cell types have shown excellent safety and effectiveness in various animal models, sufficient evidence of efficacy for clinical translation is still lacking. Cell transplantation should be combined with tissue engineering scaffolds, local drug delivery systems, postoperative adjuvant therapy and physical rehabilitation training as part of a comprehensive treatment plan to provide the possibility for patients with SCI to return to normal life. This review summarizes and analyzes the clinical trials of cell transplantation therapy in spinal cord injury, with the aim of providing a rational foundation for the development of clinical treatments for spinal cord injury.
Keywords: central nervous system, clinical trials, injury, plasticity, protection, regeneration, repair, spinal cord, stem cells
Introduction
Spinal cord injury (SCI) is a severe type of trauma to the central nervous system (CNS), and is associated with a high rate of disability. SCI can cause total or partial loss of sensory, motor and sphincter function below the injured segment (Anderson et al., 2018). The main clinical manifestations of patients with SCI are paraplegia, and urination and defecation dysfunctions. These symptoms hinder social reintegration, and place a heavy economic burden on society. According to current statistics, more than 600,000 people lose sensory and motor function in their limbs because of SCI caused by trauma every year (Tran and Silver, 2015). Therefore, nerve repair is an urgent unmet medical need for SCI patients (Tran and Silver, 2015). Research on SCI is gradually garnering the attention of researchers worldwide, and many studies with breakthrough findings have been published. These studies have mainly focused on the epidemiology, treatment and pathophysiology of SCI, among which the latter is the most well-studied. The pathophysiology of SCI can be classified into two stages—primary injury and secondary injury (Ahuja et al., 2017a). Primary injury is caused by direct damage to neural tissue by the primary mechanical insult. Secondary injury is caused by a series of secondary events, such as hemorrhage, edema, demyelination, and axonal and neuronal necrosis caused by the cascade of inflammatory reactions. The damage caused by the secondary injury may be much greater than the damage caused by the primary injury (Ahuja et al., 2017b). This not only gives us insight into the occurrence and development of SCI, but also provides a basis for its treatment.
The use of autologous or allogeneic cell transplantation to repair tissue defects at the site of SCI is a potential treatment (Figure 1), and researchers hope that these cells can promote tissue and functional reconstruction after SCI through different mechanisms, including neuroprotection, immune regulation, axonal regeneration, neuronal circuit formation and myelin regeneration. Currently, stem cell transplantation is the main cell therapy for SCI, but some differentiated mature cells have also been demonstrated to have a good therapeutic effect (Table 1). However, at present, the majority of studies on SCI are based on cell and animal models, including cats (Bamford et al., 2017), dogs (Wu et al., 2018), monkeys (Capogrosso et al., 2016) and rodents (Kjell and Olson, 2016), rather than patients with SCI. In particular, subtle changes in sensory function, nerve root movement and pain cannot be fully inves-tigated with animal studies. Because of a lack of data on mechanisms and safety, it is difficult to translate research results into clinical application (Cyranoski, 2019). Therefore, clinical randomized controlled trials are urgently needed for the treatment of SCI. After surveying current studies, we found that cell transplantation therapy, exercise therapy, electrophysiological stimulation therapy and a number of supplementary therapies have gradually entered the stage of clinical randomized controlled trials (Cyranoski, 2019). Here, we systematically review and analyze the results of clinical experiments related to SCI to provide constructive suggestions for the formulation and improvement of clinical treatments for SCI.
Figure 1.
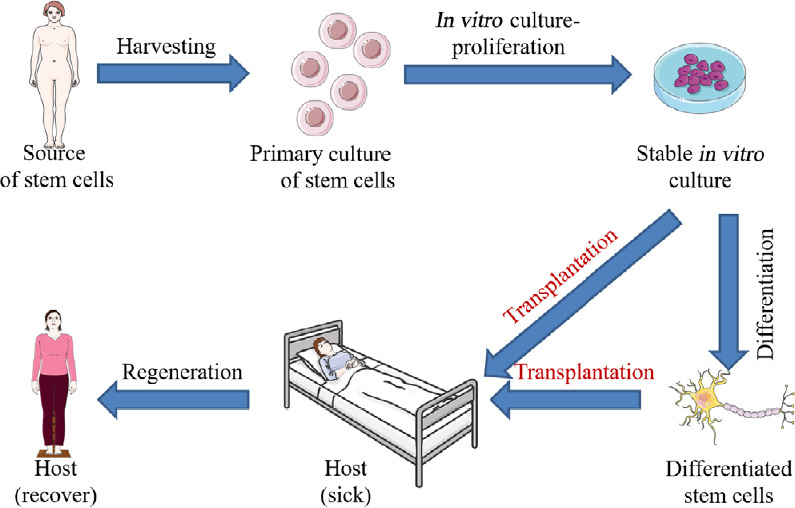
Basic procedure of cell transplantation in the treatment of spinal cord injury.
The basic process of cell transplantation in the treatment of spinal cord injury mainly includes three important steps: isolation and extraction of cells, purification and screening in vitro and stable culture, and transplantation of cells into patients. However, in some studies, the purified cells were induced to differentiate in vitro, which is not a routine, but increasingly common, procedure.
Table 1.
Cells with therapeutic potential for SCI
| Cells | Abbreviation | Stem cell | Nervous system | Source | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|---|
| Neural stem cells/neural precursor cells | NSCs/NPCs | √ | √ | Central nervous system | High security | Few sources of cells; Difficult to extract; Ethical disputes | Shin et al., 2015; Curtis et al., 2018; Levi et al., 2018 |
| Bone marrow mesenchymal stem cells | BMSCs | √ | × | Bone marrow | No problems of ethics and cell origin | Ectopic migration; Potential tumorigenicity | Attar et al., 2011; Karamouzian et al., 2012; Saito et al., 2012; Dai et al., 2013; El-Kheir et al., 2014; Mendonça et al., 2014; Jarocha et al., 2015; Bansal et al., 2016; Chhabra et al., 2016; Satti et al., 2016; Vaquero et al., 2016, 2017, 2018; Xiao et al., 2018 |
| Olfactory ensheathing cells | OECs | × | × | Olfactory mucosa | High security; no problems of ethics and cell origin | Low survival rate | Huang et al., 2006; Hummel et al., 2007; Mackay-Sim et al., 2008; Wu et al., 2012; Rao et al., 2013; Tabakow et al., 2013 |
| Umbilical cord mesenchymal stem cells | UCMSCs | √ | × | Umbilical cord | No ethical disputes; high safety and effectiveness | No enough evidence | Cheng et al., 2014 |
| Adipose mesenchymal stem cells | ASCs | √ | × | Fat | No enough evidence | No enough evidence | Hur et al., 2016 |
| Hematopoietic stem cells | HSCs | √ | × | Peripheral blood | No enough evidence | No enough evidence | Cristante et al., 2009; Frolov and Bryukhovetskiy, 2012 |
| Human embryonic stem cell-derived oligodendrocyte progenitor cells | AST-OPC1 | √ | √ | Embryonic | No obvious side effects; no pain; no risk of teratoma or tumor formation | Not found | Keirstead et al., 2005; Alsanie et al., 2013; Manley et al., 2017 |
| Macrophages | × | × | Peripheral blood | No enough evidence | No enough evidence | Knoller et al., 2005; Lammertse et al., 2012 | |
| Schwann cells | SCs | × | √ | Nervous system | No enough evidence | No enough evidence | Chen et al., 2014; Anderson et al., 2017 |
At present, most of the cells used for SCI cell transplantation are stem cells, of which BMSCs are the most widely used. NSCs/NPCs have also been used frequently by clinical researchers. UCMSCs, ASCs, HSCs and AST-OPC1 cells have also been investigated for the treatment of SCI. In addition to stem cells, OECs, which have the potential to regenerate, have received considerable attention. BMSCs, SCs and macrophages have also been used in the treatment of SCI. SCI: spinal cord injury.
Search Strategy
Related articles were searched in the Medline database with the English search term “spinal cord injury, clinical trial” from January 2014 to February 2020, and a total of 615 related articles were retrieved. Inclusion criteria: articles related to the treatment of SCI-related clinical research and the corresponding previous basic research; similar research ideas to select the latest articles published in authoritative journals. Exclusion criteria: repeat or retrospective studies. Through title and abstract reading, literature not related to the treatment of SCI and high-similarity studies were excluded. Overall, 70 articles were included in the reference catalogue.
Neural Stem Cells/Neural Precursor Cells
Neural stem cells (NSCs) have the potential to differentiate into neurons, astrocytes and oligodendrocytes, and produce a large number of brain cells through proliferation and differentiation (Shi et al., 2020). NSCs can be proliferated, cryopreserved and stored, while retaining key biological properties, including self-renewal, capacity for implantation, paracrine secretion of factors to enhance neural plasticity, migration ability and triple differentiation ability (neurons, oligodendrocytes and astrocytes). Because of these biological characteristics, NSC transplantation is one of the most promising strategies for the treatment of nervous system diseases (Zhu et al., 2018).
Curtis et al. (2018) conducted a phase I clinical trial of NSC transplantation in four patients with thoracic (T2–12) American Spinal Injury Association (ASIA) Impairment Scale grade A SCI. These patients received intraspinal injection of human spinal cord-derived NSCs (NSI-566 cells) at 12–24 months after injury. During the follow-up of 27 months, three of the four subjects showed improvements in motor and sensory function and electrophysiological examination, and no significant adverse reactions were found in any patient. This demonstrates that allogeneic NSCs can be used in cell transplantation therapy after SCI. Although the results were not statistically significant because of small sample size, the study suggests that further clinical trials of NSC transplantation are warranted, and provides a basis for future research on transplantation dose.
The safety and neurological effects of NPC transplantation for traumatic SCI were evaluated in another phase I/IIa open label nonrandomized controlled clinical trial conducted by Shin et al. (2015). In their study, a total of 19 patients with cervical SCI (17 patients with complete sensorimotor injury and two patients with complete motor injury) were enrolled. Fetal telencephalon-derived NSCs/NPCs were cultured into neurospheres and then transplanted into the spinal cord. One year later, patients who received cell transplantation had no adverse reactions such as syringomyelia, tumor formation, neurodeterioration, or aggravating neuropathic pain or spasm. At the same time, the ASIA impairment scale grade changed from A to C in five cases, from B to D in two cases, and from A to B in one case, while only one case in the control group showed symptom improvement from grade A to B.
In another clinical trial, Levi et al. (2018) injected NSCs into 12 patients with chronic traumatic thoracic SCI and 17 patients with chronic traumatic SCI in the neck. In the first year after injection, four of the 12 thoracic cases had four serious adverse events, and nine of the 17 patients with cervical spondylosis had 15 serious adverse events. Cervical magnetic resonance images showed slight increases in T2 signals in eight of the 17 transplant subjects, with no hypokinesia or neuropathic pain, and all T2 signal changes disappeared 6–12 months after transplantation.
Although these two clinical trials (Shin et al., 2015; Levi et al., 2018) demonstrate that the application of NSCs in the treatment of SCI is safe and effective, these two studies are limited by small sample size or low quality. Therefore, these studies are insufficient to support the use of NSC transplantation in the treatment of SCI. However, as phase I clinical trials, they lay the foundation for the next stage of clinical trials in this field. It is important to note that the dose of cells used in this type of cell transplantation therapy is relatively high, and therefore we believe that the most important factor hindering the development of this field is obtaining a sufficient number of cells through legal means for clinical transplantation (Shin et al., 2015; Curtis et al., 2018; Levi et al., 2018).
Bone Marrow Mesenchymal Stem Cells
Bone marrow mesenchymal stem cells (BMSCs) are adult stem cells with low immunogenicity (García et al., 2019). They are widely distributed in bone marrow and have the potential to self-renew and differentiate into various cell types (such as osteoblasts, chondrocytes and adipocytes) (He et al., 2018). In addition, BMSCs are easily isolated and expanded in vitro. Because of their paracrine and immunomodulatory functions, BMSCs can migrate to damaged tissue and can be induced to differentiate into specific cell types to reconstruct the local tissue microenvironment (Boido et al., 2014; He et al., 2018). By enhancing the function of endogenous cells and regulating the immune response, BMSCs participate in tissue repair, which makes them ideal seed cells for transplantation (Karaoz et al., 2012; Boido et al., 2014). BMSCs promote tissue repair following SCI by affecting inflammation, apoptosis, axonal regeneration, angiogenesis, tissue protection, astrocyte scar formation and motor recovery (Karaoz et al., 2012; Nakajima et al., 2012; Boido et al., 2014). Furthermore, the supply of BMSCs is relatively abundant, and there are no ethical issues, making them ideal candidates for SCI cell transplantation (Ning et al., 2019).
Bansal et al. (2016) successfully extracted BMSCs from patients and injected them into the L1/L2 level within a period of 12 weeks. In this clinical trial of 10 patients, improvement in ASIA grade was observed in six patients, virtual time to contact and walking were restored in eight patients, bladder control was improved in three patients, sexual function was improved in five patients, and spasm was relieved in eight patients. Jarocha et al. (2015) conducted a 2-year intensive treatment experiment of BMSC transplantation in a patient with SCI at the T2–3 level. In this study, the patient received a total of 1.54 × 108 BMSCs (intravenous and intrathecal injection of autologous bone marrow mononuclear cells 10 weeks after SCI, and BMSCs were injected five times every 4 months by lumbar puncture). Following treatment, the patient’s ASIA score increased from 112 to 231 (from grade A to C/D), the sensation level expanded from T1 to L3–4, and control of the body and urine were restored. In the MRI examination, hemorrhagic necrosis at the T2–3 level was low, and new tissue appeared. In a phase II clinical trial, Vaquero et al. (2018) administered three intrathecal injections of BMSCs (1 × 108) into chronic SCI patients, and performed follow-up for a period of 10 months from the first injection. Motor function, sensation, limb control ability, and urine and stool control ability were improved to varying degrees. In the clinical trial conducted by Dai et al. (2013), 20 patients with complete and chronic cervical SCI were treated with local injection of BMSCs. ASIA grade, ASIA score, residual urine volume, electromyography and paraspinal somatosensory evoked potential all showed that the BMSCs effectively improved neurological dysfunction, with an effectiveness rate of 50%. There were no adverse reactions such as wound infection, cerebrospinal fluid leakage, intracranial infection or aggravation of symptoms after transplantation. However, some patients showed tumors 6 months after injection. Thus, while this treatment is safe in the short term, its long-term safety needs to be evaluated further. Satti et al. (2016) conducted a clinical trial of BMSC transplantation in six patients with chronic SCI and three patients with subacute SCI. In this trial, the researchers isolated BMSCs by density gradient separation, and then transplanted them into the subarachnoid space by lumbar puncture. During a median follow-up of 644 days, no adverse events were reported. Furthermore, no intraspinal abnormal mass was found in follow-up MRI. This shows that BMSC transplantation is safe in patients with SCI. Saito et al. (2012) performed BMSC injection in five patients with cervical SCI and complete quadriplegia, and then followed up for 4 years. BMSC transplantation had a rapid and significant effect in patients with ASIA grades B and C, while effectiveness was slow and limited in patients with ASIA grade A. The effectiveness of BMSCs in the treatment of SCI is not limited to the chronic stage of SCI. Karamouzian et al. (2012) reported that BMSC transplantation is also effective in the treatment of patients with subacute SCI.
Although the puncture technique seems to be a safe and effective method for the treatment of SCI with BMSC transplantation, this relatively blind method may have large variability that reduces therapeutic effectiveness. However, assistance with imaging and biomarkers can greatly reduce the incidence of puncture errors, but not all medical institutions have the ability to implement these technologies. Therefore, cell transplantation using traditional surgery is a direct way to solve this problem. Attar et al. (2011) recruited two male and two female patients with traumatic SCI (ASIA grade A) and performed the following operations on them: anterior decompression, stabilization and fusion of vertebral trauma, and posterior implantation of BMSCs. After a 1-year follow-up, there were two cases of ASIA grade C, one case of ASIA grade B, one case without neurological change, and no obvious adverse reactions in any of the three patients. Mendonça et al. (2014) recruited 14 patients with thoracic or lumbar SCI (all of whom had undergone spinal cord decompression and stabilization surgery). After laminectomy and spinal canal decompression, the researchers opened the dura mater to expose the injured segment under the microscope, and injected a fixed number of cells (5 × 106/cm3) into each lesion under direct vision. The study showed the potential benefit of BMSC transplantation in varying degrees of motor and sensory improvement, clinical analgesia and urodynamic parameters. This study showed that BMSCs can effectively promote neural repair after SCI, and seven subjects (58.3%) changed from complete injury to incomplete injury (from grade A to C). Although one patient had cerebrospinal fluid leakage in this study, this is a common postoperative complication of spinal surgery and is not directly related to cell transplantation, and therefore, BMSC transplantation can be considered safe. In a clinical trial of 21 patients with ASIA grade A SCI, Chhabra et al. (2016) used both puncture injection transplantation and surgical incision site transplantation of BMSCs in different groups of patients. Unfortunately, although this study demonstrated the effectiveness and safety of BMSC transplantation to an extent, it did not make a detailed analysis of the difference between the two transplantation methods. This is unfortunate. If this study had made a comprehensive and detailed analysis of the data, no matter which transplantation method had a better therapeutic effect, or even if the results were not statistically significant, it would have provided a very valuable theoretical basis for further research on BMSC transplantation therapy.
BMSCs need a conducive local microenvironment to maintain their ability to proliferate and differentiate after transplantation. Thus, giving cells a special liquid environment during transplantation is a good strategy. Vaquero et al. (2017) performed BMSC transplantation (30 × 106) into the subarachnoid space of 10 patients with incomplete SCI on the 1st, 4th, 7th and 10th months of a clinical study. What distinguishes this study from the team’s previous study (Vaquero et al., 2017) is that the BMSCs in this study were transplanted with the support of the patients’ autologous plasma. The clinical parameters (motor, sensory, sphincter and limb control, neuropathic pain, spasm, sexual function) of all patients showed gradual improvement, but did not reach a steady state at the end of the follow-up period. Fortunately, however, the investigators found that spinal cord tissue repair began after the first transplantation.
In another study, Vaquero et al. (2016) conducted a clinical trial of 12 patients with complete and chronic paraplegia, consisting of early intravenous injection and late subarachnoid injection of autologous plasma-supported BMSCs, in which the dose of BMSCs was 100–230 × 106 and 30 × 106, respectively. The patients were followed up clinically, with urodynamics, neurophysiology and neuroimaging at 3, 6, 9 and 12 months after injection. All patients showed improvement, in terms of sensitivity and sphincter control. Motor function was also improved in some patients.
Although many studies have shown that BMSC transplantation in SCI is safe and relatively effective, its therapeutic effectiveness is still unclear. Therefore, BMSC transplantation combined with other treatment methods may help enhance recovery following SCI. In the controlled single-blind phase I/II clinical trial conducted by El-Kheir et al. (2014), 70 patients with chronic cervical and thoracic SCI with injury duration of at least 12 months were treated with intrathecal injection of autologous adherent bone marrow cells combined with physiotherapy or physiotherapy alone. ASIA, electrophysiological somatosensory evoked potentials, MRI and functional independence measures were used for clinical and neurological assessment. Chronic cervical and thoracic SCI patients treated with autologous adherent bone marrow cells combined with physiotherapy (15 ASIA grade A patients and 35 ASIA grade B patients) showed improved function compared with the control group given physiotherapy alone (10 ASIA grade A patients and 10 ASIA grade B patients), and there were no side effects associated with long-term cell therapy. At 18 months after treatment, of the 50 patients who received cell therapy, 23 (46%) showed continuous functional improvement. Compared with cervical spine injury, the duration of thoracic injury is shorter, myelopathy is less severe, and the rate of functional improvement is higher. However, this study did not compare the efficacy and safety of combined therapy with that of cell therapy alone, and therefore it did not demonstrate that physiotherapy can potentiate the efficacy of BMSC transplantation.
The biggest hidden danger of the application of BMSC transplantation in SCI is that the cells cannot effectively attach to the lesion area after entering the patient, but migrate to other parts with the circulation of cerebrospinal fluid. This phenomenon of ectopic migration not only greatly reduces the efficacy of BMSC transplantation, but also may cause damage to other tissues (Dai et al., 2013). Therefore, how to accurately target the BMSCs to the injured site and prevent them from migrating to other locations is an important and difficult problem that needs to be addressed. Nerve tissue engineered scaffolds are the most direct and effective way to solve this problem. Xiao et al. (2018) successfully prepared a NeuroRegen scaffold composed of ordered collagen fibers from bovine tendon sheath. This scaffold exhibited low antigenicity, good biocompatibility and suitable biodegradability, and is therefore a biomaterial suitable for clinical use. Then they implanted BMSCs and NeuroRegen stents into two patients with acute complete SCI. In the follow-up the following year, sensory and motor functions were improved, suggesting that the application of NeuroRegen stents combined with BMSCs is a safe and effective treatment for SCI (Xiao et al., 2018).
Current research suggests that BMSCs may have more application potential than NSCs/NPCs (El-Kheir et al., 2014; Vaquero et al., 2016, 2017; Xiao et al., 2018), and their use is not limited by ethical problems or source. However, the application of BMSCs in SCI cannot yet be clinically implemented. Ectopic migration and potential tumorigenicity remain great hurdles to the clinical application of BMSCs. Therefore, the combination of BMSC transplantation and other treatments will bring great progress to the field. First, nerve tissue engineered scaffolds can provide a local environment that supports BMSC adhesion and colonization. These scaffolds can also be loaded with bioactive factors or drugs to promote BMSC proliferation and differentiation. Second, the multidirectional differentiation ability of BMSCs means that they cannot differentiate exclusively into neurons after entering the human body. Therefore, BMSCs need to be induced to differentiate into neurons or other CNS cells in vitro before transplantation. Thus, BMSC transplantation has great application potential in the treatment of SCI, but further improvements in technology and concept are required.
Olfactory Ensheathing Cells
Olfactory ensheathing cells (OECs) are a type of glial cell present in both the peripheral and central nervous systems (Lindsay et al., 2010). They establish synaptic connections in the olfactory bulb to ensure the accurate transmission of the sense of smell by wrapping the bundle of olfactory nerve fibers between the nasal mucosa and the olfactory bulb (Barnett, 2004). At the same time, they nourish nerve fibers and promote synapse formation by secreting a variety of cytokines. From the point of view of nerve regeneration, the key ability of OECs is their migration from the peripheral to the CNS (Gómez et al., 2018). OECs have been shown to accurately migrate to the injured site and promote the recon-struction of injured axons (Anna et al., 2017; Yao et al., 2018). Because OECs exhibit continuous regeneration and the ability to stimulate axonal growth, numerous studies have transplanted OECs into the injured spinal cord for potential therapeutic use (Anna et al., 2017; Yao et al., 2018). OECs have been demonstrated effective and safe in animal models of SCI, and therefore clinical trials of OECs in the treatment of SCI have been carried out in many countries (Gomes et al., 2016; Thornton et al., 2018; Zhang et al., 2019). Huang et al. (2006) implanted fetal OECs into the injured spinal cord in 300 patients with complete SCI (ASIA grade A) and incomplete SCI (ASIA grade D). Wu et al. (2012) also used OECs from aborted fetus in clinical trials in patients with SCI. In their study, they tested safety in six patients with thoracic injury, and effectiveness in five patients with cervical injury. Although OECs showed good safety, therapeutic effectiveness was very limited. Although these cells are extracted and derived from the olfactory bulb of 12 to 16-week-old human fetuses, some investigators have ques-tioned whether these cells should be termed “OECs” (Hummel et al., 2007). Thus, it is controversial whether these studies can be defined as clinical trials of OECs in SCI.
Mackay-Sim et al. (2008) conducted a clinical trial of OEC transplantation in six patients with ASIA grade A SCI (three patients with cervical SCI and three patients with thoracic SCI). In their study, the researchers performed the following procedures: laminectomy, and after a dural incision, cells were injected into the spinal cord in multiple parts of the entire injured spinal cord, and into the proximal and distal ends of the intact spinal cord as well. During a follow-up of up to 3 years, they followed patients with medical, psychosocial, radiological and neurological tests, as well as specialized tests for neurological and functional defects. These assessments showed that OEC transplantation was safe for SCI. Tabakow et al. (2013) recruited six patients with ASIA grade A SCI for a phase I clinical trial, in which three patients received OEC transplantation and the other three patients received non-transplant therapy. Patients in the transplantation group received 1.5–10 × 104 OECs within 15 seconds, while patients in the non-transplantation group did not receive cell transplantation. During the 1-year follow-up after transplantation, there were no complications such as neurological deterioration, neuropathic pain, nerve infection, cerebrospinal fluid leakage or local infection at the surgical site. In terms of therapeutic effect, patient 1 changed from ASIA grade A to C and patient 2 from ASIA grade A to B. Although patient 3 was still ASIA A, an improvement in sensory and motor function was nonetheless observed. This demonstrates that OEC transplantation is effective. Rao et al. (2013) recruited eight patients with SCI, and injected autologous OECs into the area around the injury site under the guidance of MRI, twice a week for 4 weeks. The ASIA impairment scale, ASIA sensory and motor score and functional independence measure score were evaluated before treatment and at follow-up at 3, 6, 12 and 24 months after treatment. At 3 and 6 months after treatment, the average ASIA score and functional independence measure scores were significantly improved compared with those before treatment, but there was no further improvement in ASIA score 1 year after treatment. Sensory and motor functions of various muscles below the injury level were examined in three patients during the follow-up period. In addition, the bladder function of two patients recovered. Additionally, over the entire follow-up period, no adverse reactions were found.
OECs have unique advantages, and have accordingly garnered as much attention as BMSCs for the treatment of SCI. Compared with NSCs/NPCs, OECs have no ethical problems, and compared with BMSCs, OECs are safer. Therefore, the use of OECs for cell transplantation in the treatment of SCI has great application potential. However, it is important to note that although many studies have reported that OECs can effectively improve the neuroanatomy and function of the injured spinal cord, it has also been found that OECs transplanted in the injured nerve tissue have great limitations in terms of cell survival and function. In addition, the outcomes of these clinical trials have not been satisfactory, and therefore whether OECs can effectively promote nerve repair after SCI is still unknown. Thus, to further promote OEC transplantation for SCI, the first problem to solve is that of cell purification, and the solution to this problem must be based on the search for molecular markers of OECs. In view of the current situation, researchers should focus on OECs themselves, identify molecular markers that specifically detect OECs, and optimize the process of cell extraction and purification, to provide a solid foundation for future clinical trials.
Umbilical Cord Mesenchymal Stem Cells
Umbilical cord mesenchymal stem cells (UCMSCs) are typical adult stem cells (Bartolucci et al., 2017). Distinct from bone marrow stem cells, UCMSCs have the advantages of painless collection and faster self-renewal (Liu et al., 2020). Hematopoietic stem cells (HSCs) are also not fraught by ethical source considerations. They can differentiate into the three germ layers, promote tissue repair, and regulate the immune response. They also possess antitumor properties. Therefore, they are attractive autologous or allogeneic cells for the treatment of malignant and non-malignant solid and soft tissue cancers. Human UCMSCs can also be used as a feeder layer for embryonic stem cells or other pluripotent stem cells (Ding et al., 2015). Under specific induction conditions, UCMSCs can be induced to differentiate into neurons, glial cells and vascular endothelial cells. UCMSCs also produce cytokines and neurotrophic factors, including vascular endothelial growth factor, glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor, which support nerve regeneration, promote axonal growth and stimulate damaged neurons. In addition, UCMSCs can inhibit the formation of the glial scar and activate endogenous NSCs (Zhang et al., 2009; Malgieri et al., 2010; Zhilai et al., 2012; Badner et al., 2016).
Cheng et al. (2014) randomly divided 34 patients with thoracolumbar SCI into three groups. The stem cell transplantation group received UCMSC transplantation twice under the guidance of CT. The rehabilitation group received rehabilitation treatment. The control group did not receive any treatment. Outcome was evaluated by ASIA grading, ASIA scoring, muscle strength and muscle tension scale, the Barthel index and urodynamic examination. During the follow-up, the motor, selfcare ability and muscle tone of seven of the 10 patients in the UCMSC group showed a significant and stable improvement, and five of the 14 patients in the rehabilitation group also showed some improvement in these indexes. Urodynamic examination showed that maximum urinary flow rate and maximum bladder volume increased, and residual urine volume and maximum detrusor pressure decreased in the UCMSC group. The maximum bladder capacity decreased in the rehabilitation group, but there was no significant change in maximum urinary flow rate, residual urine volume or maximum detrusor pressure. These results indicate that UCMSC transplantation can safely and effectively promote the recovery of neurological function after SCI (Cheng et al., 2014).
From the point of view of ethics, safety and effectiveness, UCMSCs are one of the best choices for cell transplantation in the treatment of SCI. However, at present, there is only one clinical trial of UCMSCs in SCI. Although the design of this clinical trial is very rigorous, it still cannot be used as evidence for the clinical use of UCMSCs. Therefore, more clinical trials should be carried out to further explore the safety and efficacy of UCMSC transplantation in SCI.
Adipose Mesenchymal Stem Cells
Adipose mesenchymal stem cells (ASCs) first discovered in the early 21st century, are mesenchymal stem cells with self-renewal ability and multilineage differentiation potential. Compared with BMSCs, ASCs have the advantages of faster culture, easier expansion, and maintain a stem cell phenotype and pluripotency for a greater number of passages. Compared with NPCs/NSCs, ACSs can be extracted from subcutaneous adipose tissue by a very simple separation and purification procedure, and this process can be repeated many times (Damia et al., 2018; Liu et al., 2020).
Hur et al. (2016) recruited 14 patients with SCI, including 12 cases of ASIA grade A, one case of B, and one case of D, and the duration of injury was 28 months. These patients included six cases of cervical SCI, one case of combined neck and chest injury, six cases of thoracic SCI and one case of lumbar SCI. The researchers isolated and purified ASCs from subcutaneous adipose tissue and transplanted the purified cells (9 × 107) by lumbar puncture. The follow-up results showed that although the patients had varying degrees of improvement in motor, sensory and sphincter control, three patients had adverse reactions, including urinary tract infection, headache, nausea and vomiting. Through an analysis of the results, we posit that the occurrence of headache, nausea and vomiting in this study may be related to the lumbar puncture procedure. Patients had to undergo a 5-minute lumbar puncture in the study, which may directly cause intraspinal homeostasis problems and CNS irritation. The occurrence of urinary tract infection may be related to inadequate nursing management and poor personal hygiene habits of patients, and not to the cell transplantation therapy per se.
Hematopoietic Stem Cells
HSCs are an adult stem cell present in the blood system, and have strong self-renewal ability and multi-directional differentiation ability (Xiong et al., 2017). HSC have a long history of research, and have optimized methods for extraction, separation and purification. Therefore, the use of HSC transplantation in the treatment of SCI has been studied by an increasing number of researchers (Koda et al., 2005).
Frolov and Bryukhovetskiy (2012) recruited 20 patients (15 male and five female, mean age 32.41 years) with chronic complete and incomplete traumatic SCI in the C4–8 segments, and 10 matched patients with SCI as controls. After receiving recombinant human granulocyte colony-stimulating factor at a dose of 2.5–6.8 μg/kg (average 4.3 μg/kg) twice a day for 4 days, on the 5th day, the researchers extracted HSCs using a blood cell separator and transplanted them into the subarachnoid space by lumbar puncture. During the follow-up, somatosensory evoked potentials returned to normal in three patients, inter-peak amplitude of somatosensory evoked potential N20P23 increased in four patients with median nerve stimulation, somatosensory evoked potential P38 latency shortened in two patients with tibial nerve stimulation, and motor evoked potential recovery occurred in three patients. In this study, the location and degree of injury were important factors affecting the efficacy of HSC transplantation. Cristante et al. (2009) conducted a clinical trial of HSCs in 39 patients with complete cervicothoracic SCI without cortical response for at least 2 years in a public tertiary hospital in Sao Paulo, Brazil. Similar to Frolov and Bryukhovetskiy’s scheme (Frolov and Bryukhovetskiy, 2012), the researchers extracted HSCs from peripheral blood and implanted them into the body. However, unlike Frolov and Bryukhovetskiy’s study (Frolov and Bryukhovetskiy, 2012), they chose arteriography. HSC transplantation also showed robust therapeutic effectiveness in this study, with 26 patients (67%) showing recovery in somatosensory evoked responses to peripheral stimuli during the 2.5-year follow-up (Cristante et al., 2009).
Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cells
AST-OPC1 is a cell therapy product derived from GRNOPC1, which is mainly composed of oligodendrocyte precursor cells (OPCs) derived from H7 human embryonic stem cell line 6 (Nistor et al., 2005). The results of early clinical trials show that AST-OPC1 can be used in preclinical trials and clinical development. AST-OPC1 expresses early markers of OPCs, including nestin, neuronal/glial antigen 2 and platelet-derived growth factor receptor α (Hu et al., 2009). In in vitro and animal models, AST-OPC1 exhibits a variety of activities related to CNS repair, including secretion of neurotrophic factors, stimulation of axonal growth, inhibition of parenchymal cavitation, maturation of oligodendrocyte-produced myelin sheath, and promotion of motor behavioral recovery after SCI (Sharp et al., 2010; Priest et al., 2015).
Manley et al. (2017) investigated the efficacy and safety of AST-OPC1 in the nude mouse model of SCI. Their results showed that this approach effectively improves motor function after SCI, especially forelimb function. This is very important for patients with SCI, because recovery of the upper limbs not only means that they can care of themselves, but also that they can re-enter the labor force. Notably, AST-OPC1s were limited to the spinal cord and lower brain stem after transplantation, while cerebro-spinal fluid and blood leakage were minimal. Furthermore, AST-OPC1 transplant had no obvious side effects, no pain, and no risk of teratoma or tumor formation. Therefore, AST-OPC1s are a safe and effective cell transplantation therapy for SCI. AST-OPC1 is a cellular product originally developed by Geron Corporation and its name was later changed to AST-OPC1 after the acquisition of Geron’s hu-man embryonic stem cell technology by Asterias (Alsanie et al., 2013). AST-OPC1 has professional research and business teams to support its technology development and marketing, and has been approved by the U.S. Food and Drug Administration for clinical trials (Keirstead et al., 2005; Manley et al., 2017). Therefore, based on its scientific and commercial nature, we have reason to believe that the clinical trials and transformation of AST-OPC1 will be achieved more quickly than any other cell transplantation therapy.
Macrophages
Macrophages are heterogeneous cells with wide functional plasticity (Wang et al., 2015). Macrophages can be divided into two main types, termed M1 and M2 (Gordon, 2003; Mantovani et al., 2004). M1 macrophages produce pro-inflammatory cytokines, reactive oxygen species and nitric oxide, which lead to tissue inflammation and damage. In contrast, M2 macrophages have a reduced ability to produce anti-inflammatory cytokines and pro-inflammatory molecules, which contribute to wound healing and tissue remodeling. Macrophages have the ability to change from one phenotype to another by induction by factors in the inflammatory microenvironment after injury or infection (Mosser and Edwards, 2008; Wolfs et al., 2011). Most of the macrophages/microglia in the injured spinal cord are M1 cells, and only a few are M2. After SCI, M1 macrophages predominate, while M2 macrophages are fewer, resulting in chronic inflammatory reaction and secondary injury (David and Kroner, 2011). The characterization of macrophage phenotype and the post-SCI biochemical microenvironment should clarify how macro-phages participate in the pathogenesis of SCI and pave the way for new treatment strategies (Kigerl et al., 2009).
Knoller et al. (2005) evaluated the safety and efficacy of autologous macrophage transplantation in the treatment of acute SCI in a phase I clinical trial. In this experiment, the researchers first extracted mac-rophages from the peripheral blood of patients and cultured them in vitro. Macrophages were then injected into the patients’ caudal spinal cord within 14 days after SCI. Of the eight patients in this study, three recovered clinically significant neuromotor and sensory functions, and changed from ASIA grade A to C. However, during the study, a number of adverse events occurred, the most serious of which were two cases of pulmonary embolism and one case of osteomyelitis. Although these complications may not be related to the macrophage transplantation itself, but to the surgical procedure, the influence of the transplantation scheme itself cannot be ruled out. In another phase II clinical trial, Lammertse et al. (2012) performed autologous macrophage transplantation in 26 patients with acute SCI. Of the 43 participants (26 treated subjects and 17 controls), seven treated subjects and 10 control participants experienced ASIA A to B or better conversion, and two treated subjects and two control participants experienced ASIA grade A to C conversion, suggesting that macrophage transplantation did not have any beneficial effect in patients with SCI. In the statistical analysis of the follow-up results, neither the effectiveness index nor the safety index were statistically significant. Therefore, we believe that macrophage transplantation for the treatment of SCI is still worthy of further, more stringent, assessment.
Schwann cells
Schwann cells (SCs) are glial cells of the peripheral nervous system (Jessen et al., 2015). Damaged nerve fibers and cells can regenerate new axons in the presence of SCs. Numerous studies have found that SCs have neuroprotective effects, reducing cavitation, and promoting axonal regeneration and myelin formation. Therefore, SCs can be used for cell transplantation after SCI (Cerqueira et al., 2018). Anderson et al. (2017) conducted a phase I clinical trial in six patients with subacute SCI. They removed the sural nerve by surgery, and isolated and purified SCs, which were then surgically trans-planted into the injured spinal cord within 7 weeks after injury. The first group received 5 × 106 cells in 50 μL, the second group received 10 × 106 cells in 100 μL, and the third group received 15 × 106 cells in 150 μL. During follow-up, the researchers found no adverse events related to cell therapy, and there was no evidence of additional SCI, mass damage, or cavity formation. Therefore, the transplantation of SCs in SCI is safe. Chen et al. (2014) investigated the safety and efficacy of SCs combined with OEC transplantation for SCI, and compared the combined transplantation regimen with the single transplan-tation regimen. A total of 28 eligible patients with chronic SCI were recruited, including three cases of OEC intraspinal transplantation, one case of SC implantation and one case of combined therapy. All patients who received single or combined transplantation of OECs and SCs showed functional im-provement. Both single and combined transplantation of OECs and SCs were safe and had beneficial effects. However, these results are not sufficient to compare the advantages and disadvantages of the three transplantation strategies. Therefore, it is necessary to conduct further research on a larger cohort of patients to adequately assess the benefits and risks of the various intervention strategies (Anderson et al., 2017).
Conclusion and Future Prospects
Cell transplantation for the treatment of SCI is a promising strategy for the clinical treatment of SCI, particularly as current procedures are inadequate. Stem cells have the best prospects in the treatment of SCI, with their potential for nerve regeneration, neuroprotection and immune regulation, while macrophages and SCs, as the most important participants in the pathophysiological development of SCI, also have good developmental potential. However, irrespective of the cell type used in the treatment of SCI, there are differences in the evaluation of safety and efficacy in the various clinical trials. Differences are also found in the various steps in the implementation of cell transplantation therapy. We found that the main factors affecting the effectiveness and safety of cell transplantation therapy in SCI are injury severity, cell type, location of injury, cell delivery system, and the procedure for cell transplantation. However, the severity and location of the injury are variables beyond the control of clinicians and researchers, and the cell type cannot be changed for the specific cell transplantation therapy. Despite these constraints, future trials should nonetheless attempt to standardize the various steps in the experimental protocol as much as feasible.
For transplantation, obtaining the needed cells safely and efficiently is the first crucial step. However, differences in the techniques and instruments used by the surgeons cause variabilities in the cells obtained. Therefore, it is currently difficult to achieve a consistent cell extraction. The best way to resolve this problem is to identify and screen the cells before transplantation. However, this goal is based on the establishment of quality standards for different cell grafts, which must be recognized by most researchers, although the task is difficult.
Second, the investigator must choose between single cell type transplantation and multiple cell type (combined) transplantation. However, cotransplantation appears unfeasible in the short term. Because the efficacy and safety of the different cell types are not currently clear, the optimal cell combinations for transplantation are unknown. Therefore, we consider it logical to evaluate the efficacy and safety of each of the various cell types individually for the treatment of SCI through clinical trials, then optimize cell dosage, and then finally investigate the effects of combined transplantation.
Finally, the choice of transplantation method is a key issue worthy of discussion. Lumbar puncture is a simple and easy to tolerate method. The requirements for both patients and doctors are low, and can be carried out in most hospitals. However, it is unknown whether the cells will accurately localize to the site of injury after injection into the subarachnoid space. After injection into the subarachnoid space, the cells will disperse and travel in the circulating cerebrospinal fluid, and only a small number will likely colonize the site of trauma. This entails a significant reduction in efficacy, as well as risk. Therefore, a good option is to expose the injured part of the spinal cord by surgery, and manually transplant the cells into the site of tissue damage. However, this approach requires patients to have a good tolerance to the surgery, and also requires surgeons to have a high operating ability. Because this method cannot be repeated many times, if it fails, the patient will lose the opportunity to benefit from cell transplant treatment. Furthermore, for treatment with long-term repeated cell transplantation, surgical transplantation is not a suitable option. We propose that the use of imaging guidance to accurately im-plant cells into the injured site through puncture or minimally invasive surgery may be the best solution at present.
For the application of cell combination therapy in SCI, the combination of different cell types, the interaction between them and the immune response that may be brought by combined transplantation are all important factors worthy of attention. The future of combined transplantation therapy in the treatment of SCI may involve the use of stents, nutritional factors and drugs. Only when cells, scaffolds, nutritional factors and drugs are systematically combined to form a microenvironment conducive to local regeneration of the injured spinal cord can the potential of combined transplantation be fully realized. However, it is essential that clinical trials of combined transplantation therapy in SCI be carried out with adequate knowledge of the safety and efficacy of cells, stents and drugs in the human body. This is because their combined use introduces additional potential risk factors. Therefore, clinical trials of combined transplantation therapy need a firmer empirical basis to ensure the safety of patients.
In conclusion, cell transplantation has great therapeutic potential in the treatment of SCI. However, the clinical application of cell transplantation in SCI requires further high-quality clinical trials (systematic and comprehensive randomized control trials and evidence-based medicine) (Santamaría et al., 2019). Cell transplantation alone (whether single-cell transplantation or multicell transplantation) does not seem to be the endpoint for the treatment of SCI. Cell transplantation should be combined with tissue engineering scaffolds, local drug delivery systems, postoperative adjuvant therapy and physical rehabilitation training as part of a comprehensive treatment plan to provide the possibility for patients with SCI to return to normal life.
Acknowledgments
We thank Hong-Wei Li, Min-Xuan Yang and Yong Yang from Lanzhou University Second Hospital, China for proofreading the entire manuscript.
Footnotes
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Patel D, Yu J, Song CP; T-Editor: Jia Y
Conflicts of interest: The authors declare that there is no conflict of interests.
Financial support: This work was supported by 2019 Scientific Research Project of Traditional Chinese Medicine in Gansu Province of China, No. GZK-2019-46 (to XWK and YBL).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by 2019 Scientific Research Project of Traditional Chinese Medicine in Gansu Province of China, No. GZK-2019-46 (to XWK and YBL).
References
- 1.Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017a;3:17018. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 2.Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D, Fehlings MG. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017b;80:S9–S22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 3.Alsanie WF, Niclis JC, Petratos S. Human embryonic stem cell-derived oligodendrocytes: protocols and perspectives. Stem Cells Dev. 2013;22:2459–2476. doi: 10.1089/scd.2012.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson KD, Guest JD, Dietrich WD, Bartlett Bunge M, Curiel R, Dididze M, Green BA, Khan A, Pearse DD, Saraf-Lavi E, Widerström-Noga E, Wood P, Levi AD. Safety of autologous human schwann cell transplantation in subacute thoracic spinal cord injury. J Neurotrauma. 2017;34:2950–2963. doi: 10.1089/neu.2016.4895. [DOI] [PubMed] [Google Scholar]
- 5.Anderson MA, O’Shea TM, Burda JE, Ao Y, Barlatey SL, Bernstein AM, Kim JH, James ND, Rogers A, Kato B, Wollenberg AL, Kawaguchi R, Coppola G, Wang C, Deming TJ, He Z, Courtine G, Sofroniew MV. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature. 2018;561:396–400. doi: 10.1038/s41586-018-0467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anna Z, Katarzyna JW, Joanna C, Barczewska M, Joanna W, Wojciech M. Therapeutic potential of olfactory ensheathing cells and mesenchymal stem cells in spinal cord injuries. Stem Cells Int. 2017;2017:3978595. doi: 10.1155/2017/3978595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attar A, Ayten M, Ozdemir M, Ozgencil E, Bozkurt M, Kaptanoglu E, Beksac M, Kanpolat Y. An attempt to treat patients who have injured spinal cords with intralesional implantation of concentrated autologous bone marrow cells. Cytotherapy. 2011;13:54–60. doi: 10.3109/14653249.2010.510506. [DOI] [PubMed] [Google Scholar]
- 8.Badner A, Vawda R, Laliberte A, Hong J, Mikhail M, Jose A, Dragas R, Fehlings M. Early intravenous delivery of human brain stromal cells modulates systemic inflammation and leads to vasoprotection in traumatic spinal cord injury. Stem Cells Transl Med. 2016;5:991–1003. doi: 10.5966/sctm.2015-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bamford JA, Marc Lebel R, Parseyan K, Mushahwar VK. The fabrication, implantation, and stability of intraspinal microwire arrays in the spinal cord of cat and rat. IEEE Trans Neural Syst Rehabil Eng. 2017;25:287–296. doi: 10.1109/TNSRE.2016.2555959. [DOI] [PubMed] [Google Scholar]
- 10.Bansal H, Verma P, Agrawal A, Leon J, Sundell IB, Koka PS. Autologous Bone Marrow-Derived Stem Cells in Spinal Cord Injury. J Stem Cells. 2016;11:51–61. [PubMed] [Google Scholar]
- 11.Barnett SC. Olfactory ensheathing cells: unique glial cell types. J Neurotrauma. 2004;21:375–382. doi: 10.1089/089771504323004520. [DOI] [PubMed] [Google Scholar]
- 12.Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, Rojo P, Palma I, Lamich R, Pedreros PA, Valdivia G, Lopez VM, Nazzal C, Alcayaga-Miranda F, Cuenca J, Brobeck MJ, Patel AN, Figueroa FE, Khoury M. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]) Circ Res. 2017;121:1192–1204. doi: 10.1161/CIRCRESAHA.117.310712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boido M, Garbossa D, Fontanella M, Ducati A, Vercelli A. Mesenchymal stem cell transplantation reduces glial cyst and improves functional outcome after spinal cord compression. World Neurosurg. 2014;81:183–190. doi: 10.1016/j.wneu.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud EM, Mignardot JB, Buse N, Gandar J, Barraud Q, Xing D, Rey E, Duis S, Jianzhong Y, Ko WK, Li Q, Detemple P, Denison T, Micera S, Bezard E, Bloch J, et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature. 2016;539:284–288. doi: 10.1038/nature20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerqueira SR, Lee YS, Cornelison RC, Mertz MW, Wachs RA, Schmidt CE, Bunge MB. Decellularized peripheral nerve supports Schwann cell transplants and axon growth following spinal cord injury. Biomaterials. 2018;177:176–185. doi: 10.1016/j.biomaterials.2018.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Huang H, Xi H, Zhang F, Liu Y, Chen D, Xiao J. A prospective randomized double-blind clinical trial using a combination of olfactory ensheathing cells and Schwann cells for the treatment of chronic complete spinal cord injuries. Cell Transplant. 2014;23(Suppl 1):S35–44. doi: 10.3727/096368914X685014. [DOI] [PubMed] [Google Scholar]
- 17.Cheng H, Liu X, Hua R, Dai G, Wang X, Gao J, An Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J Transl Med. 2014;12:253. doi: 10.1186/s12967-014-0253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chhabra HS, Sarda K, Arora M, Sharawat R, Singh V, Nanda A, Sangodimath GM, Tandon V. Autologous bone marrow cell transplantation in acute spinal cord injury--an Indian pilot study. Spinal Cord. 2016;54:57–64. doi: 10.1038/sc.2015.134. [DOI] [PubMed] [Google Scholar]
- 19.Cristante AF, Barros-Filho TE, Tatsui N, Mendrone A, Caldas JG, Camargo A, Alexandre A, Teixeira WG, Oliveira RP, Marcon RM. Stem cells in the treatment of chronic spinal cord injury: evaluation of somatosensitive evoked potentials in 39 patients. Spinal Cord. 2009;47:733–738. doi: 10.1038/sc.2009.24. [DOI] [PubMed] [Google Scholar]
- 20.Curtis E, Martin JR, Gabel B, Sidhu N, Rzesiewicz TK, Mandeville R, Van Gorp S, Leerink M, Tadokoro T, Marsala S, Jamieson C, Marsala M, Ciacci JD. A first-in-human, phase i study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell. 2018;22:941–950e6. doi: 10.1016/j.stem.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Cyranoski D. Japan’s approval of stem-cell treatment for spinal-cord injury concerns scientists. Nature. 2019;565:544–545. doi: 10.1038/d41586-019-00178-x. [DOI] [PubMed] [Google Scholar]
- 22.Dai G, Liu X, Zhang Z, Yang Z, Dai Y, Xu R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013;1533:73–79. doi: 10.1016/j.brainres.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Damia E, Chicharro D, Lopez S, Cuervo B, Rubio M, Sopena JJ, Vilar JM, Carrillo JM. Adipose-derived mesenchymal stem cells: are they a good therapeutic strategy for osteoarthritis. Int J Mol Sci. 2018;19:1926. doi: 10.3390/ijms19071926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 25.Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24:339–347. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- 26.El-Kheir WA, Gabr H, Awad MR, Ghannam O, Barakat Y, Farghali HA, El Maadawi ZM, Ewes I, Sabaawy HE. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014;23:729–745. doi: 10.3727/096368913X664540. [DOI] [PubMed] [Google Scholar]
- 27.Frolov AA, Bryukhovetskiy AS. Effects of hematopoietic autologous stem cell transplantation to the chronically injured human spinal cord evaluated by motor and somatosensory evoked potentials methods. Cell Transplant. 2012;21(Suppl 1):S49–55. doi: 10.3727/096368912x633761. [DOI] [PubMed] [Google Scholar]
- 28.García E, Rodríguez-Barrera R, Buzoianu-Anguiano V, Flores-Romero A, Malagón-Axotla E, Guerrero-Godinez M, De la Cruz-Castillo E, Castillo-Carvajal L, Rivas-Gonzalez M, Santiago-Tovar P, Morales I, Borlongan C, Ibarra A. Use of a combination satrategy to improve neuroprotection and neuroregeneration in a rat model of acute spinal cord injury. Neural Regen Res. 2019;14:1060–1068. doi: 10.4103/1673-5374.250627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomes ED, Mendes SS, Leite-Almeida H, Gimble JM, Tam RY, Shoichet MS, Sousa N, Silva NA, Salgado AJ. Combination of a peptide-modified gellan gum hydrogel with cell therapy in a lumbar spinal cord injury animal model. Biomaterials. 2016;105:38–51. doi: 10.1016/j.biomaterials.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Gómez RM, Sánchez MY, Portela-Lomba M, Ghotme K, Barreto GE, Sierra J, Moreno-Flores MT. Cell therapy for spinal cord injury with olfactory ensheathing glia cells (OECs) Glia. 2018;66:1267–1301. doi: 10.1002/glia.23282. [DOI] [PubMed] [Google Scholar]
- 31.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 32.He Y, Chen D, Yang L, Hou Q, Ma H, Xu X. The therapeutic potential of bone marrow mesenchymal stem cells in premature ovarian failure. Stem Cell Res Ther. 2018;9:263. doi: 10.1186/s13287-018-1008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu BY, Du ZW, Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc. 2009;4:1614–1622. doi: 10.1038/nprot.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang H, Wang H, Chen L, Gu Z, Zhang J, Zhang F, Song Y, Li Y, Tan K, Liu Y, Xi H. Influence factors for functional improvement after olfactory ensheathing cell transplantation for chronic spinal cord injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20:434–438. [PubMed] [Google Scholar]
- 35.Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264:237–243. doi: 10.1007/s00405-006-0173-0. [DOI] [PubMed] [Google Scholar]
- 36.Hur JW, Cho TH, Park DH, Lee JB, Park JY, Chung YG. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: A human trial. J Spinal Cord Med. 2016;39:655–664. doi: 10.1179/2045772315Y.0000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarocha D, Milczarek O, Wedrychowicz A, Kwiatkowski S, Majka M. Continuous improvement after multiple mesenchymal stem cell transplantations in a patient with complete spinal cord injury. Cell Transplant. 2015;24:661–672. doi: 10.3727/096368915X687796. [DOI] [PubMed] [Google Scholar]
- 38.Jessen KR, Mirsky R, Lloyd AC. Schwann cells: development and role in nerve repair. Cold Spring Harb Perspect Biol. 2015;7:a020487. doi: 10.1101/cshperspect.a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karamouzian S, Nematollahi-Mahani SN, Nakhaee N, Eskandary H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin Neurol Neurosurg. 2012;114:935–939. doi: 10.1016/j.clineuro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Karaoz E, Kabatas S, Duruksu G, Okcu A, Subasi C, Ay B, Musluman M, Civelek E. Reduction of lesion in injured rat spinal cord and partial functional recovery of motility after bone marrow derived mesenchymal stem cell transplantation. Turk Neurosurg. 2012;22:207–217. doi: 10.5137/1019-5149.JTN.5412-11.1. [DOI] [PubMed] [Google Scholar]
- 41.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kjell J, Olson L. Rat models of spinal cord injury: from pathology to potential therapies. Dis Model Mech. 2016;9:1125–1137. doi: 10.1242/dmm.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knoller N, Auerbach G, Fulga V, Zelig G, Attias J, Bakimer R, Marder JB, Yoles E, Belkin M, Schwartz M, Hadani M. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J Neurosurg Spine. 2005;3:173–181. doi: 10.3171/spi.2005.3.3.0173. [DOI] [PubMed] [Google Scholar]
- 45.Koda M, Okada S, Nakayama T, Koshizuka S, Kamada T, Nishio Y, Someya Y, Yoshinaga K, Okawa A, Moriya H, Yamazaki M. Hematopoietic stem cell and marrow stromal cell for spinal cord injury in mice. Neuroreport. 2005;16:1763–1767. doi: 10.1097/01.wnr.0000183329.05994.d7. [DOI] [PubMed] [Google Scholar]
- 46.Lammertse DP, Jones LA, Charlifue SB, Kirshblum SC, Apple DF, Ragnarsson KT, Falci SP, Heary RF, Choudhri TF, Jenkins AL, Betz RR, Poonian D, Cuthbert JP, Jha A, Snyder DA, Knoller N. Autologous incubated macrophage therapy in acute, complete spinal cord injury: results of the phase 2 randomized controlled multicenter trial. Spinal Cord. 2012;50:661–671. doi: 10.1038/sc.2012.39. [DOI] [PubMed] [Google Scholar]
- 47.Levi AD, Okonkwo DO, Park P, Jenkins AL, 3rd, Kurpad SN, Parr AM, Ganju A, Aarabi B, Kim D, Casha S, Fehlings MG, Harrop JS, Anderson KD, Gage A, Hsieh J, Huhn S, Curt A, Guzman R. Emerging safety of intramedullary transplantation of human neural stem cells in chronic cervical and thoracic spinal cord injury. Neurosurgery. 2018;82:562–575. doi: 10.1093/neuros/nyx250. [DOI] [PubMed] [Google Scholar]
- 48.Lindsay SL, Riddell JS, Barnett SC. Olfactory mucosa for transplant-mediated repair: a complex tissue for a complex injury. Glia. 2010;58:125–134. doi: 10.1002/glia.20917. [DOI] [PubMed] [Google Scholar]
- 49.Liu AM, Chen BL, Yu LT, Liu T, Shi LL, Yu PP, Qu YB, So KF, Zhou LB. Human adipose tissue- and umbilical cord-derived stem cells: which is a better alternative to treat spinal cord injury. Neural Regen Res. 2020;15:2306–2317. doi: 10.4103/1673-5374.284997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackay-Sim A, Féron F, Cochrane J, Bassingthwaighte L, Bayliss C, Davies W, Fronek P, Gray C, Kerr G, Licina P, Nowitzke A, Perry C, Silburn PA, Urquhart S, Geraghty T. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain. 2008;131:2376–2386. doi: 10.1093/brain/awn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3:248–269. [PMC free article] [PubMed] [Google Scholar]
- 52.Manley NC, Priest CA, Denham J, Wirth ED, 3rd, Lebkowski JS. Human embryonic stem cell-derived oligodendrocyte progenitor cells: preclinical efficacy and safety in cervical spinal cord injury. Stem Cells Transl Med. 2017;6:1917–1929. doi: 10.1002/sctm.17-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Mendonça MV, Larocca TF, de Freitas Souza BS, Villarreal CF, Silva LF, Matos AC, Novaes MA, Bahia CM, de Oliveira Melo Martinez AC, Kaneto CM, Furtado SB, Sampaio GP, Soares MB, dos Santos RR. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014;5:126. doi: 10.1186/scrt516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT, Johnson WE, Baba H. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614–1625. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ning GZ, Song WY, Xu H, Zhu RS, Wu QL, Wu Y, Zhu SB, Li JQ, Wang M, Qu ZG, Feng SQ. Bone marrow mesenchymal stem cells stimulated with low-intensity pulsed ultrasound: Better choice of transplantation treatment for spinal cord injury: Treatment for SCI by LIPUS-BMSCs transplantation. CNS Neurosci Ther. 2019;25:496–508. doi: 10.1111/cns.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 59.Priest CA, Manley NC, Denham J, Wirth ED, 3rd, Lebkowski JS. Preclinical safety of human embryonic stem cell-derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regen Med. 2015;10:939–958. doi: 10.2217/rme.15.57. [DOI] [PubMed] [Google Scholar]
- 60.Rao Y, Zhu W, Liu H, Jia C, Zhao Q, Wang Y. Clinical application of olfactory ensheathing cells in the treatment of spinal cord injury. J Int Med Res. 2013;41:473–481. doi: 10.1177/0300060513476426. [DOI] [PubMed] [Google Scholar]
- 61.Saito F, Nakatani T, Iwase M, Maeda Y, Murao Y, Suzuki Y, Fukushima M, Ide C. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: a pilot study. Restor Neurol Neurosci. 2012;30:127–136. doi: 10.3233/RNN-2011-0629. [DOI] [PubMed] [Google Scholar]
- 62.Santamaría AJ, Benavides FD, DiFede DL, Khan A, Pujol MV, Dietrich WD, Marttos A, Green BA, Hare JM, Guest JD. Clinical and neurophysiological changes after targeted intrathecal injections of bone marrow stem cells in a C3 tetraplegic subject. J Neurotrauma. 2019;36:500–516. doi: 10.1089/neu.2018.5716. [DOI] [PubMed] [Google Scholar]
- 63.Satti HS, Waheed A, Ahmed P, Ahmed K, Akram Z, Aziz T, Satti TM, Shahbaz N, Khan MA, Malik SA. Autologous mesenchymal stromal cell transplantation for spinal cord injury: A Phase I pilot study. Cytotherapy. 2016;18:518–522. doi: 10.1016/j.jcyt.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 64.Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28:152–163. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi X, Liu JS, Wan R, Wang YS. Research progress in mesenchymal stem cells for treating spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2020;24:4081–4087. [Google Scholar]
- 66.Shin JC, Kim KN, Yoo J, Kim IS, Yun S, Lee H, Jung K, Hwang K, Kim M, Lee IS, Shin JE, Park KI. Clinical trial of human fetal brain-derived neural stem/progenitor cell transplantation in patients with traumatic cervical spinal cord injury. Neural Plast. 2015;2015:630932. doi: 10.1155/2015/630932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tabakow P, Jarmundowicz W, Czapiga B, Fortuna W, Miedzybrodzki R, Czyz M, Huber J, Szarek D, Okurowski S, Szewczyk P, Gorski A, Raisman G. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. 2013;22:1591–1612. doi: 10.3727/096368912X663532. [DOI] [PubMed] [Google Scholar]
- 68.Thornton MA, Mehta MD, Morad TT, Ingraham KL, Khankan RR, Griffis KG, Yeung AK, Zhong H, Roy RR, Edgerton VR, Phelps PE. Evidence of axon connectivity across a spinal cord transection in rats treated with epidural stimulation and motor training combined with olfactory ensheathing cell transplantation. Exp Neurol. 2018;309:119–133. doi: 10.1016/j.expneurol.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tran AP, Silver J. Neuroscience, Systemically treating spinal cord injury. Science. 2015;348:285–286. doi: 10.1126/science.aab1615. [DOI] [PubMed] [Google Scholar]
- 70.Vaquero J, Zurita M, Rico MA, Bonilla C, Aguayo C, Montilla J, Bustamante S, Carballido J, Marin E, Martinez F, Parajon A, Fernandez C, Reina L. An approach to personalized cell therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II clinical trial. Cytotherapy. 2016;18:1025–1036. doi: 10.1016/j.jcyt.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 71.Vaquero J, Zurita M, Rico MA, Aguayo C, Bonilla C, Marin E, Tapiador N, Sevilla M, Vazquez D, Carballido J, Fernandez C, Rodriguez-Boto G, Ovejero M. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline. Cytotherapy. 2018;20:806–819. doi: 10.1016/j.jcyt.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 72.Vaquero J, Zurita M, Rico MA, Bonilla C, Aguayo C, Fernández C, Tapiador N, Sevilla M, Morejón C, Montilla J, Martínez F, Marín E, Bustamante S, Vázquez D, Carballido J, Rodríguez A, Martínez P, García C, Ovejero M, Fernández MV, et al. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy. 2017;19:349–359. doi: 10.1016/j.jcyt.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 73.Wang X, Cao K, Sun X, Chen Y, Duan Z, Sun L, Guo L, Bai P, Sun D, Fan J, He X, Young W, Ren Y. Macrophages in spinal cord injury: phenotypic and functional change from exposure to myelin debris. Glia. 2015;63:635–651. doi: 10.1002/glia.22774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolfs IM, Donners MM, de Winther MP. Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation. Thromb Haemost. 2011;106:763–771. doi: 10.1160/TH11-05-0320. [DOI] [PubMed] [Google Scholar]
- 75.Wu GH, Shi HJ, Che MT, Huang MY, Wei QS, Feng B, Ma YH, Wang LJ, Jiang B, Wang YQ, Han I, Ling EA, Zeng X, Zeng YS. Recovery of paralyzed limb motor function in canine with complete spinal cord injury following implantation of MSC-derived neural network tissue. Biomaterials. 2018;181:15–34. doi: 10.1016/j.biomaterials.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 76.Wu J, Sun T, Ye C, Yao J, Zhu B, He H. Clinical observation of fetal olfactory ensheathing glia transplantation (OEGT) in patients with complete chronic spinal cord injury. Cell Transplant. 2012;21(Suppl 1):S33–37. doi: 10.3727/096368912X633743. [DOI] [PubMed] [Google Scholar]
- 77.Xiao Z, Tang F, Zhao Y, Han G, Yin N, Li X, Chen B, Han S, Jiang X, Yun C, Zhao C, Cheng S, Zhang S, Dai J. Significant improvement of acute complete spinal cord injury patients diagnosed by a combined criteria implanted with NeuroRegen scaffolds and mesenchymal stem cells. Cell Transplant. 2018;27:907–915. doi: 10.1177/0963689718766279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiong LL, Liu F, Deng SK, Liu J, Dan QQ, Zhang P, Zou Y, Xia QJ, Wang TH. Transplantation of hematopoietic stem cells promotes functional improvement associated with NT-3-MEK-1 activation in spinal cord-transected rats. Front Cell Neurosci. 2017;11:213. doi: 10.3389/fncel.2017.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yao R, Murtaza M, Velasquez JT, Todorovic M, Rayfield A, Ekberg J, Barton M, St John J. Olfactory ensheathing cells for spinal cord injury: sniffing out the issues. Cell Transplant. 2018;27:879–889. doi: 10.1177/0963689718779353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang L, Zhuang X, Chen Y, Xia H. Intravenous transplantation of olfactory bulb ensheathing cells for a spinal cord hemisection injury rat model. Cell Transplant. 2019;28:1585–1602. doi: 10.1177/0963689719883842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang L, Zhang HT, Hong SQ, Ma X, Jiang XD, Xu RX. Cografted Wharton’s jelly cells-derived neurospheres and BDNF promote functional recovery after rat spinal cord transection. Neurochem Res. 2009;34:2030–2039. doi: 10.1007/s11064-009-9992-x. [DOI] [PubMed] [Google Scholar]
- 82.Zhilai Z, Hui Z, Anmin J, Shaoxiong M, Bo Y, Yinhai C. A combination of taxol infusion and human umbilical cord mesenchymal stem cells transplantation for the treatment of rat spinal cord injury. Brain Res. 2012;1481:79–89. doi: 10.1016/j.brainres.2012.08.051. [DOI] [PubMed] [Google Scholar]
- 83.Zhu Y, Uezono N, Yasui T, Nakashima K. Neural stem cell therapy aiming at better functional recovery after spinal cord injury. Dev Dyn. 2018;247:75–84. doi: 10.1002/dvdy.24558. [DOI] [PubMed] [Google Scholar]


