Neuroinflammation in the brain is thought related to the emergence of various psychoses, although the identifying regional significance, the involvement of immune-cells and lymphocytic activity, and ways for the therapeutic recovery are under the effort of researchers. We recently revealed that the cerebellar acute inflammation causes the symptoms manifested in mood disorders or developmental disorders, which were associated with hyperexcitability due to immune-triggered plasticity and the overconnectivity between the inflamed cerebellum and prefrontal cortex (Yamamoto et al., 2019). Here, we give a perspective regarding the emergence of psychoses focusing on the aberrant immunity, the relevance of dysfunctions in brain regions including the cerebellum, and potential therapeutic ways via metabolism.
Psychosis is a complex brain disorder that typically appears in late adolescence or early adulthood (Tang et al., 2014). For instance, while 22q11.2 deletion is one of the most famous genetic loci of psychosis-spectrum disorders, the symptoms would be polygenic and related to the brain volume, gender, race, and history of viral infection during the developmental stage. In human 22q11.2 deletion syndrome (DiGeorge syndrome), the hypoplasia of thymus, cell-mediated immune deficiency, congenital dysfunction of the cardiovascular system, and facies palatina are observed. Due to the underdevelopment and dysfunction of the thymus, patients show a deficiency in the immune system and reduced innate immune responses. In the 22q11.2 deletion syndrome, it is also characteristic in terms of mild intellectual and learning disabilities. Patients also show attention-deficit/hyperactivity disorder (ADHD) with higher anxiety and developmental neuropsychiatric symptoms such as autism spectrum disorder (ASD), which affect communication and social interaction (Tang et al., 2014). Unfortunately, researchers in the field of mental illnesses tend to be unable to find the link between functional connectivity in the brain regions correlated with their cellular neurophysiology, impairments in the immune system, and gene profiling, while extensive efforts are devoted.
The cerebellum is the center for motor coordination and its learning. It is one of the distinct brain regions whose neural circuits and functions are well understood. The cerebellum converges the error between the inflowing information and the internal models by the plasticity of its neurophysiological function. For that reason, it has become known to be involved in complex intellectual abilities such as prediction, thinking, judgment, and language understanding. The anomalies of the cerebellum in synaptic and non-synaptic plasticity are observed in mental disease models and have been discussed in several studies (Schmahmann, 2004; Hansel, 2019; Ohtsuki et al., 2020). Clinical and basic research have indicated the significance of cerebellar impairments in cognitive dysfunction and psychiatric disorders (Schmahmann, 2004). These symptoms are considered as the dysmetria of thought, represented by ASD, depressive behaviors (mood disorders), ADHD, and schizophrenia (SCZ). From the viewpoint of animal models, modulation of neurons belonging to a subpopulation or a distinct circuit may induce a certain behavioral anomaly. In this perspective, we exemplify the cerebellum-related psychosis as ASD, SCZ, depression, and ADHD.
However, how immunity in the cerebellum is associated with the various forms of plasticity (i.e., synaptic and non-synaptic plasticity), the resultant hyperexcitability in the cerebellum and even cognitive dysfunction had not been addressed; therefore, researchers begin to pay attention to that relevance in these days (Ohtsuki et al., 2020). Recently, one study suggested that the inflammatory cytokines are secreted from microglia, the resident immune cells of the central nervous system, during acute inflammation in the cerebellum, and these mediators induced various forms of neurophysiological modulation of cerebellar Purkinje cells (Yamamoto et al., 2019). The animals with acute inflammation in the cerebellum showed psychosis-like behaviors (Yamamoto et al., 2019). Cerebellar malformation and malfunctions may also be related to symptoms in mental illness. Classically, cerebellar pathology and atrophy of the vermis had been pointed out in the schizophrenic patients. According to a recent study from over 2300 participants of SCZ (Moberget et al., 2018), the effect size (Cohen’s d) of total cerebellar grey matter volume was significantly reduced in SCZ patients, compared to healthy controls. Their results suggested that the frontoparietal node was the most reduced across the age range (16 to 66-year-old). The cerebellar volume and cerebral cortical thickness in frontotemporal regions were correlated with each other (Moberget et al., 2018). Because the cerebral cortex returns projections to the pons and the cerebellar cortex, we cannot say that the cerebellum is the cardinal brain region in SCZ. Please note that we do not stress that the cerebellum is the master region of those psychiatric disease-like symptoms, but the cerebellum can be involved in the symptoms due to its efferent and afferent projections (e.g., via cerebello-frontal loop). Therefore, it would be proposed that the alteration of functional connectivity stemming from the cerebellum could be a marker of psychosis. Roalf et al. (2019) also suggested that lower fractional anisotropy of whole-brain white matter and higher radial diffusivity (i.e., disruption of white matter) were present in individuals with risk symptoms from a longitudinal diffusion-weighted imaging of their “psychosis spectrum” screening (including participants with positive, negative and disorganized subclinical-symptoms). The authors discussed the contribution of oligodendrocytes and myelin formation as a possible mechanism of neuronal dysfunction in psychosis (Roalf et al., 2019). Additionally, microglia also are known to refine the neuronal circuit. When considering the brain function is organized by glial cells and is affected by brain immunity, such psychiatric symptoms may emerge due to anomaly in the non-neuronal cell activity which modulates functional connectivity among brain regions, as well as due to the neurodevelopmental process.
Neurophysiological modulation during the cerebellar inflammation: Then, how does aberrant immune responses in the cerebellum induce anomaly of neuronal activity and dysfunction across the brains? As mentioned above, our recent study suggested that cerebellar microglia upregulated the Purkinje-cell excitability, mediated by inflammatory cytokines, tumor necrosis factor-α (TNF-α), and ATP (Yamamoto et al., 2019; Figure 1). Exposure to bacterial endotoxin lipopolysaccharide or heat-killed Gram-negative bacteria induced a potentiation of the intrinsic excitability of Purkinje neurons, which was prevented by suppression of microglial activity. TNF-α released from microglia via toll-like receptor 4 induced both intrinsic plasticity (a long-lasting increase in the action potential firing frequency), excitability increase in the Purkinje-cell dendrites, and increases in the presynaptic transmitter release. These signalings involve the activation of intra-neuronal phosphatases. Our ATP-imaging showed an increase in ATP concentration following endotoxin exposure, which were involved in the presynaptic plasticity. And, the immune-triggered hyperexcitability modulated animal behaviors. Region-specific inflammation in the cerebellum in vivo showed distinct behavioral modulations: animals with inflammation in the cerebellar anterior lobes showed depression-like behavior, while inflammation in the right hemisphere caused autistic-like behaviors. Furthermore, both TNF-α-inhibition and microglia-depletion reverted those behavioral abnormalities. Results of resting-state functional MRI suggested an overconnectivity between the inflamed cerebellum and prefrontal neocortical regions. This study suggested that aberrant immune activity in the cerebellum induces neuronal hyperexcitability and disruption of psychomotor behaviors (Yamamoto et al., 2019). Therefore, inflammation in the cerebellum and changes of the output activity in the cerebellar cortex and nuclei could influence cerebral activity, including the prefrontal cortex, via cerebello-frontal connectivity (Yamamoto et al., 2019; Ohtsuki et al., 2020; Figure 1). Of note, in the diagnosis of human ASD, the TNF-α expression level in macrophages is high in the blood samples. The efferent connections from the cerebellum to thalamic nuclei, red nuclei, and ventral tegmental area were characterized by their behavioral correlations in recent studies of rodent models (Ohtsuki et al., 2020; Figure 1). Notably, the output regions of the cerebellar nuclei are composed of four regions: fastigial, interpositus, dentate, and vestibular nuclei, which have distinct projections and modulate behavior in different ways. The difference in the behavioral phenotype (i.e., depressive- or autistic-like) may come from the inflamed region in the cerebellum. This gives us a lesson: the psychiatric effects of brain inflammation are not uniform across the brain because of the distinct circuitry, even in the cerebellum. Cerebellar inflammation may also alter dendritic integration of electrical current (i.e., electroconductivity) of Purkinje cells and clustering of synaptic input, via the downregulation of Ca2+-activated K+-channels (SK2 channels) (Ohtsuki, 2020; Figure 1). Therefore, aberrant inflammation in the cerebellum and resultant neurophysiological modulation cause disruption of distinct regions of the brain via connections.
Figure 1.
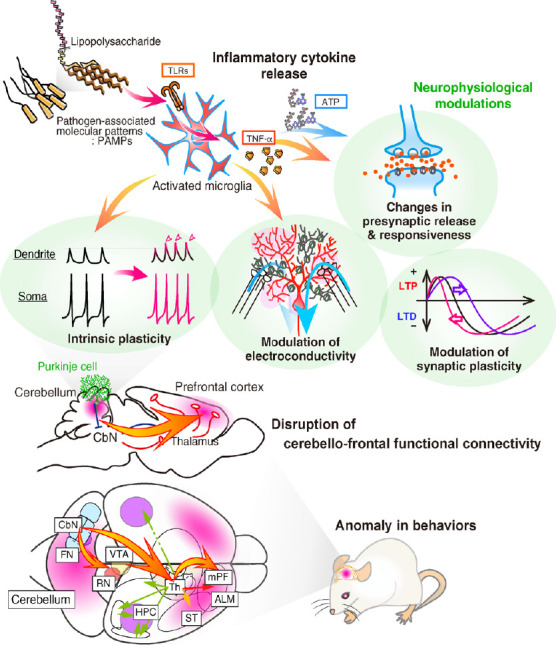
Functional physiology of the aberrant inflammation in the cerebellum.
PAMPs, including bacterial lipopolysaccharide, activate microglia, and promote the secretion of inflammatory cytokines via TLRs. Released TNF-α and ATP modulate neurophysiological properties: intrinsic excitability of neuronal soma and dendrites, electroconductivity of synaptic currents on dendrites, threshold of the synaptic plasticity induction, and synaptic transmission. Those modulations result in the hyperexcitability of Purkinje cells in the cerebellum. Through the efferent pathways from the cerebellum to higher-cognitive regions in the brain regions, the activity of inflamed cerebellum enhances the activity of target regions and causes anomaly in behaviors. ALM: Anterior lateral motor cortex; CbN: cerebellar nuclei; FN: fastigial nuclei (medial cerebellar nuclei); HPC: hippocampus; LTD: long-term depression; LTP: long-term potentiation; mPF: medial prefrontal cortex; PAMPs: pathogen-associated molecular patterns; RN: red nuclei; ST: striatum; Th: thalamus; TLRs: toll-like receptors; TNF-α: tumor necrosis factor-α; VTA: ventral tegmental area.
Psychosis symptoms following aberrant immunity: How does the aberrant immunity occur in the brain? And, what is the chronic effect? Brains in neonatal and developing stages are very vulnerable. It would be true that the phenotype is dependent on the genome-environment interplay. A review study by Brown et al. (2010) had emphasized that the prenatal and early-life infection with influenza virus, herpes simplex virus type 2 (HSV2), and Toxoplasma gondii (a parasitic protists) is a risk factor for the SCZ among offspring and those affected, as referred in many epidemiologic and translational studies. Infectious RNA viruses; influenza virus, measles virus, Japanese encephalitis virus, human immunodeficiency virus, Rotavirus, West Nile virus, and SARS-Coronavirus-2, and DNA viruses; cytomegalovirus (herpesvirus), and Adenovirus, are known to cause the encephalitis. Many microbial and viral infections and resultant encephalitis can cause high cytokine conditions in the brain and in the uterus, and serious inflammation symptoms associated with cognitive dysfunctions. However, the mechanism of these after-effects were not well understood.
Viral infection during pregnancy increases the risk of psychosis to the offspring, such as SCZ, ASD, bipolar disorders, and developmental disorders. In the mouse model, it is known that maternal immune activation during pregnancy yields offspring with autistic phenotypes and malformation in the cortical layers of a fetus through immune signaling like via interleukin (IL)-17A, released from T helper 17 cells (Th17), and its receptors (IL-17RA) (Choi et al., 2016). However, it is unclear how IL-17RA signaling induced dysplasia of the cortex area, what is the role of IL-17 for brain homeostasis, and whether IL-17A from peripheral Th17 close to brain parenchyma is important. On the other hand, elevated interferon-I/interferon-β signaling after maternal immune activation was also shown to affect brain pathology and behavior (Ben-Yehuda et al., 2020).
Brain inflammation via innate immunity is initially induced by activation of microglia. Recent view provided by the transcriptomic analysis gives us notions about microglia as follows: a developmental program of gene expression patterns and chromatin dynamics, regional heterogeneity of gene profile, different renewal rates, distinct morphology and density, and even a stress-induced mental state (Nie et al., 2018). These profiles inspire an idea of the variety in microglial subtypes. When considering the pathophysiological effects of brain immune cells, it is problematic if we suppose that the function of immune cells is homogenous. Rather, microglia per se have the heterogeneity across brain regions, developmental timing, and cytokine milieu in rodents and humans. In any case, in such psychosis-like symptoms of rodents, effectors are considered interleukins and interferons from helper T cells and microglia. Regulatory T cells would also contribute. And, those effects are probably without the brain region-dependency but are depending on the immune cells’ localization.
Vascular system and potential therapeutic ways through the metabolism: Although many mental illnesses are certainly thought to be potentially due to a genetic factor, immune and metabolic abnormalities may be phenotypically the cause of many symptoms. Therefore, it might be possible to seek therapeutic targets out of those processes. Present optical microscopy has been remarkably advanced for deep tissue imaging. For example, multiphoton imaging can visualize the regulation of capillary blood flow which delivers molecules or reagents into the central nervous system. By expressing fluorescent molecules in endothelial cells of the neurovascular unit in vivo, it would be possible to see relationships between the vascular activity and psychological phenotypes. In the brain, physiologically active eicosanoids, prostaglandins, are involved in functional hyperemia, vasodilation, stress responses, and fever (Furuyashiki and Narumiya, 2011). A prostaglandin can promote metabolism effectively. In addition, in circulatory systems, Da Mesquita et al. (2018) succeeded in rediscovering cerebrospinal meningeal lymphatic vessels and showed its significance to cognition in the neurological disease model. Their results suggested relationships between molecular transport through the vessels and memory decline with aging. By injecting fluorescent molecules (Visudyne) into the cerebrospinal fluid and expressing fluorescent proteins as markers in T cells (e.g., CD3e) and lymphatic endothelial cells (e.g., CD31), the transporting mechanism of substances between lymphatic vessels and lymphatic endothelial cells was visualized (Da Mesquita et al., 2018). These results succeeded in the improvement of the cognitive deficits by promoting meningeal lymphatic drainage and brain metabolism.
Conclusion: Here, we briefly reviewed potential immune-related psychosis, which is derived from the anomaly of the neuronal excitability and functional circuit among distinct brain regions. Following viral infection, chronic inflammation involving aberrant immune-cell activation (microglia, and Th) increased risk for emerging mental illnesses. This short perspective also addressed a possible link between the cerebellar dysfunction and psychosis, which underlie the cerebello-frontal pathways (Figure 1); so that, if the mechanisms including immunity and metabolism are revealed thoroughly, a therapeutic way could be lit up.
We would like to present our deep appreciation to Profs. Christian Hansel, Tomoo Hirano, and Shu Narumiya for supports on the research. We thank Nao Katsurada for invaluable comments on the manuscript.
This work was supported by grants from the Kowa Life Science Foundation, the Japanese Society for Promotion of Science (KAKENHI, Grant-in-Aid for Young Scientists (A) 26710002), the Brain Science Foundation, the Tokyo Biochemical Research Foundation, the Naito Foundation, and the Hakubi-project grant (Kyoto University) (all to GO).
Footnotes
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Ben-Yehuda H, Matcovitch-Natan O, Kertser A, Spinrad A, Prinz M, Amit I, Schwartz M. Maternal Type-I interferon signaling adversely affects the microglia and the behavior of the offspring accompanied by increased sensitivity to stress. Mol Psychiatry. 2020;25:1050–1067. doi: 10.1038/s41380-019-0604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furuyashiki T, Narumiya S. Stress responses: the contribution of prostaglandin E(2) and its receptors. Nat Rev Endocrinol. 2011;7:163–175. doi: 10.1038/nrendo.2010.194. [DOI] [PubMed] [Google Scholar]
- 5.Hansel C. Deregulation of synaptic plasticity in autism. Neurosci Lett. 2019;688:58–61. doi: 10.1016/j.neulet.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, Da Mesquita S, Frost EL, Gaultier A, Harris TH, Cao R, Hu S, Lukens JR, Smirnov I, Overall CC, Oliver G, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018;21:1380–1391. doi: 10.1038/s41593-018-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moberget T, Doan NT, Alnæs D, Kaufmann T, Córdova-Palomera A, Lagerberg TV, Diedrichsen J, Schwarz E, Zink M, Eisenacher S, Kirsch P, Jönsson EG, Fatouros-Bergman H, Flyckt L; KaSP, Pergola G, Quarto T, Bertolino A, Barch D, Meyer-Lindenberg A, et al. Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: a multisite mega-analysis of 983 patients and 1349 healthy controls. Mol Psychiatry. 2018;23:1512–1520. doi: 10.1038/mp.2017.106. [DOI] [PubMed] [Google Scholar]
- 8.Nie X, Kitaoka S, Tanaka K, Segi-Nishida E, Imoto Y, Ogawa A, Nakano F, Tomohiro A, Nakayama K, Taniguchi M, Mimori-Kiyosue Y, Kakizuka A, Narumiya S, Furuyashiki T. The innate immune receptors TLR2/4 mediate repeated social defeat stress-induced social avoidance through prefrontal microglial activation. Neuron. 2018;99:464–479. doi: 10.1016/j.neuron.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Ohtsuki G. Modification of synaptic-input clustering by intrinsic excitability plasticity on cerebellar Purkinje cell dendrites. J Neurosci. 2020;40:267–282. doi: 10.1523/JNEUROSCI.3211-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohtsuki G, Shishikura M, Ozaki A. Synergistic excitability plasticity in cerebellar functioning. FEBS J. 2020 doi: 10.1111/febs.15355. doi:101111/febs. [DOI] [PubMed] [Google Scholar]
- 11.Roalf DR, de la Garza AG, Rosen A, Calkins ME, Moore TM, Quarmley M, Ruparel K, Xia CH, Rupert PE, Satterthwaite TD, Shinohara RT, Elliott MA, Gur RC, Gur RE. Alterations in white matter microstructure in individuals at persistent risk for psychosis. Mol Psychiatry. 2019 doi: 10.1038/s41380-019-0360-1. doi:101038/s41380-019-0360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 13.Tang SX, Yi JJ, Calkins ME, Whinna DA, Kohler CG, Souders MC, McDonald-McGinn DM, Zackai EH, Emanuel BS, Gur RC, Gur RE. Psychiatric disorders in 22q11.2 deletion syndrome are prevalent but undertreated. Psychol Med. 2014;44:1267–1277. doi: 10.1017/S0033291713001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto M, Kim M, Imai H, Itakura Y, Ohtsuki G. Microglia-triggered plasticity of intrinsic excitability modulates psychomotor behaviors in acute cerebellar inflammation. Cell Rep. 2019;28:2923–2938. doi: 10.1016/j.celrep.2019.07.078. [DOI] [PubMed] [Google Scholar]


