Abstract
Currently, ischemic stroke is the most prevalent form of stroke compared to hemorrhagic and there is a high incidence in older adults. Nutrition is a modifiable risk factor for stroke. B-vitamins are part of a metabolic network that integrates nutritional signals with biosynthesis, redox homeostasis, and epigenetics. These vitamins play an essential role in the regulation of cell proliferation, stress resistance, and embryo development. A deficiency in vitamin B12 is common in older adults and has been reported to be implicated in ischemic stroke. The aim of this review was to investigate whether vitamin B12 deficiencies impact the risk and outcome of ischemic stroke. Clinical data from our literature review strongly suggest that a deficiency in vitamin B12 is a risk factor for ischemic stroke and possible outcome. Our survey of the literature has identified that there is a gap in the understanding of the mechanisms through which a vitamin B12 deficiency leads to an increased risk of stroke and outcome. A vitamin B12 deficiency can increase homocysteine levels, which are a well-established risk factor for ischemic stroke. Another potential mechanism through which vitamin B12 deficient may impact neurological function and increase risk of stroke, is changes in myelination, however this link requires further investigation. Further studies are required in model systems to understand how a vitamin B12 deficiency changes the brain.
Keywords: B-vitamins, ischemic stroke, one-carbon, vitamin B12
Introduction
Vitamin B12, also known as cobalamin (Cbl), was first isolated in 1948 when it was used to treat megaloblastic anemia, a condition that results in unusually large, structurally abnormal and immature red blood cells, resulting in the first Nobel Prize pertaining to vitamin B12 being awarded (Reynolds, 2006; Scott and Molloy, 2012). The second Nobel Prize for vitamin B12 was awarded when researchers discovered the mechanism in which vitamin B12 is absorbed in the ileum (Scott and Molloy, 2012). Vitamin B12 is a component of one-carbon metabolism, which is a key metabolic network that integrates nutritional signaling with biosynthesis, redox homeostasis, and epigenetics (Murray et al., 2017; Tang et al., 2017).
Metabolism of Vitamin B12
Vitamin B12, along with other B-vitamins, acts as co-factors for specific enzymes to carry out metabolic functions in the body (Weir and Scott, 1999). Humans obtain vitamin B12 from animal source foods, such as meat, dairy, eggs, and fish. There are certain bacteria (e.g. Escherichia coli) in the gut that can synthesize vitamin B12, but the amount is insufficient in terms of demands (Watanabe, 2007; Fang et al., 2017). Chaperones, transport proteins, and receptors protect vitamin B12 and facilitate its gastrointestinal absorption and renal reabsorption for its retention and distribution (Zhang et al., 2016). Intrinsic factor, a glycoprotein secreted in the stomach, facilitates the absorption of vitamin B12 (Johansson et al., 2010).
In the cell, vitamin B12 is a co-factor involved in the methylation of homocysteine to methionine using the main circulating form of folate, 5-methyltetrahydrofolate (5-methylTHF) (Figure 1). Increased levels of plasma homocysteine (> 10 µM) or methylmalonic acid (> 210 nM) can be helpful in determining whether a patient has a deficiency in vitamin B12 (Selhub et al., 2010).
Figure 1.
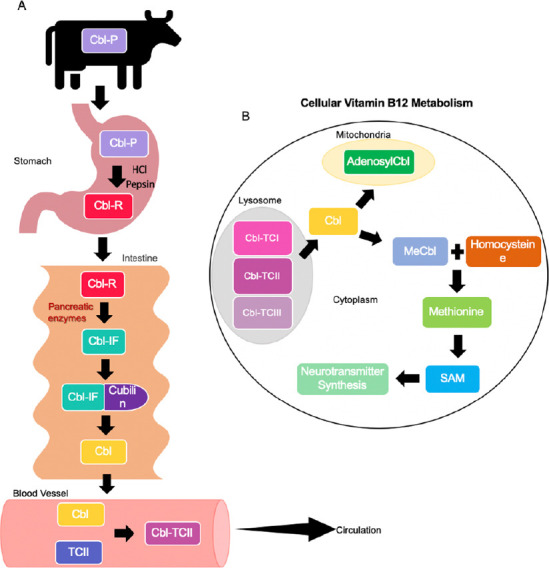
Vitamin B12 metabolism.
(A) Ingestion of dietary cobalamin bound to animal protein (P) via animal foods such as meat, eggs and poultry which naturally contain cobalamin (Cbl). The cobalamin-animal protein complex (Cbl-P) enters the stomach where cobalamin is cleaved from the animal protein via digestive enzymes such as Pepsin and HCl. Free cobalamin (Cbl) then binds to R-protein (R), a protein secreted from salivary and parietal cells, to form a cobalamin-R-protein complex (Cbl-R) and travels to the intestine. It should be noted that intrinsic factor (IF), a glycoprotein produced by parietal cells of the stomach, is present in the stomach, however, it does not bind to cobalamin until it reaches the intestine due to the acidic environment of the stomach. In the intestine, pancreatic enzymes cleave the Cbl-R complex, resulting in free cobalamin. Cobalamin can now bind to intrinsic factor to create the cobalamin-intrinsic factor complex (Cbl-IF). The complex will then bind to its receptor cubilin, located on the mucosal cells, and is endocytosed into the mucosal cell. Immediately after entering the mucosal cell, the complex is cleaved, resulting in free cobalamin. Free cobalamin now enters the circulation where it binds to transcobalamin I, II or III (TCI, TCII, TCIII), creating a cobalamin-transcobalamin complex, and travels to its target. Cobalamin-transcobalamin II (Cbl-TCII) is diagramed here to highlight its unique ability to transport cobalamin to all parts of the body, unlike TCI and TCIII. It should also be noted that cobalamin individually binds to all three transcobalamin while in circulation. (B) The Cbl-TCII complex is endocytosed into target cells and immediately cleaved, resulting in free cobalamin. Free cobalamin is then converted into its two active forms in the human body, methyl cobalamin (MeCbl) and adenosyl cobalamin (AdenosylCbl). MeCbl remains in the cytoplasm while adenosyl cobalamin travels to the mitochondria. Homocysteine can be methylated to methionine using methyl cobalamin and the enzyme, methionine synthase. Methionine is then converted to S-adenosyl methionine (SAM) which in the brain is involved in neurotransmitter synthesis.
Vitamin B12 Metabolism in Older Adults
As we age, our metabolism and absorption of nutrients, including vitamins, also change (Bottiglieri et al., 2000; Shlisky et al., 2017; Moore et al., 2018). Greater than 20%, of older adults (> 60 years old) have a vitamin B12 deficiency (Andrès et al., 2004; Lachner et al., 2012). A deficiency in vitamin B12 is defined as plasma levels of Cbl that are less than 150 pM. A measure of holotranscobalamin levels or metabolites such as homocysteine and methylmalonic acid will adequately assess if a patient is vitamin B12 deficient (Andrès et al., 2004; Selhub et al., 2010; Spence and Stampfer, 2011; Lachner et al., 2012). Pernicious anemia is a type of megaloblastic anemia that results in the reduction of red blood cells and is secondary to a vitamin B12 deficiency caused by low levels of intrinsic factor. The most frequently affected by a vitamin deficiency or pernicious anemia are the elderly (Andrès et al., 2004).
A vitamin B12 deficiency can be caused by a reduced dietary intake, frequently in the case of a vegetarian diet, and or changes in absorption. The Framingham Heart Study reported that 24% of 60 to 69-year-old patients have atrophic gastritis, with increasing prevalence (37%) in 80-year-old people (Krasinski et al., 1986). The high prevalence of a vitamin B12 deficiency in older adults is mostly due to malabsorption or increased atrophic gastritis, which leads to changes in gastric emptying and decreased secretion of intrinsic factor. However, only in severe cases of gastric atrophy does intrinsic factor secretion become a rate-limiting factor for vitamin B12 absorption. Atrophic gastritis has been reported to limit bioavailability of vitamin B12 from food proteins and peptides due to impaired acid secretion and reduced digestion by pepsin (Selhub et al., 2010). Other consequences of atrophic gastritis include bacterial overgrowth, which leads to bacteria consuming vitamin B12, and increased pH in the stomach and proximal small intestine resulting in reduced folic acid absorption (Selhub et al., 2000).
Individuals with high plasma levels of homocysteine may be encouraged to increase intake of food high in folates or take folic acid supplements, to help reduce plasma levels of homocysteine. However, if the cause of the elevated homocysteine is a vitamin B12 deficiency, and dietary levels of folic acid are increased, this will result in the exacerbation of biochemical and clinical symptoms associated with a vitamin B12 deficiency. This is referred to as the methyl trap (Reynolds, 2006; Selhub et al., 2010). Treatment of a vitamin B12 deficiency can include intramuscular injections, as well as oral or nasal administration of the vitamin (Andrès et al., 2004).
Vitamin B12 in the Brain
The brain has a minute to minute dependence on nutrient supply which can be impacted by dietary intake. In older adults, low levels of vitamin B12 have been linked to reduced cognitive function and Alzheimer’s disease (Bottiglieri, 1996; Selhub et al., 2010; Lachner et al., 2012). The Rotterdam Scan Study found that poor vitamin B12 status was significantly associated with greater severity of white-matter lesions in the brain, which may be a result of reduced myelin integrity (de Lau et al., 2009). Furthermore, peripheral neuropathy is more common during vitamin B12 deficiency compared to other B-vitamin deficiencies (Weir and Scott, 1999; Reynolds, 2006). Neurological diseases due to a vitamin B12 deficiency can occur in both males and females between the age of 40 and 90, and peak at 60–70 years of age (Reynolds, 2006). In community-dwelling volunteers aged 61 to 87 years old, decreased brain volume was reported and it was even greater among those with low vitamin B12 levels (Vogiatzoglou et al., 2008). With aging, the transport mechanisms of getting vitamin B12 across the blood-brain barrier via receptor-mediated endocytic pathways become damaged (Selhub et al., 2010). The global population is aging (Benjamin et al., 2018), and diseases of aging, such as ischemic stroke, are predicted to increase (Feigin et al., 2015; Katan and Luft, 2018). Ischemic stroke is the result of blockage in blood flow within the brain that results in reduced oxygen and glucose, which leads to cell death and functional impairments (Kumar et al., 2016). Nutrition, especially reduced levels of B vitamins, is a modifiable risk factor for stroke possibly through their role in metabolism of homocysteine (Hankey, 2012).
Role of B vitamins in stroke
Adequate nutrition reduces the risk of ischemic stroke (Mozaffarian et al., 2016; Spence, 2019). Elevated levels of homocysteine increase the risk for vascular diseases, such as stroke (Graham et al., 1997; Nygård et al., 1997; Castro et al., 2006; Clarke et al., 2010). There is also a decline in the risk of stroke when homocysteine levels are reduced (Huang et al., 2017). At the cellular level, increased levels of homocysteine lead to reduced levels of nitric oxide bioavailability, leading to changes in endothelial mediated dilation causing vascular damage due to the production of free radicals, as well as lipid peroxidation (Lai and Kan, 2015; Tsybikov et al., 2016; McCully, 2018). The presence of atrial fibrillation, vitamin B12 deficiency, and resultant elevated levels of homocysteine increases with age are a risk factor for stroke (Spence, 2007; Spence and Stampfer, 2011). In the Framingham study, atrial fibrillation was an attributable risk for stroke in 23% patients aged 80–89 years old (Wolf et al., 1991).
B-vitamins metabolize homocysteine (Murray et al., 2017), therefore increasing levels of B-vitamins can be beneficial for patients with elevated levels of homocysteine in terms of reducing stroke risk. Since 2015, this has been tested in approximately 68,131 patients worldwide (Spence, 2007; Huo et al., 2015). Past clinical trials have demonstrated that the impact of supplementing with B-vitamins is complicated, when it comes to reducing the risk of stroke with the main factor being the form of vitamin B12 administered to patients and the health status of patient (e.g., renal failure or diabetic) (Toole et al., 2004; Lonn et al., 2006; Spence, 2007; Saposnik et al., 2009). The Vitamin Intervention for Stroke Prevention trial was conducted in the US and included 3680 participants that were administered 25 mg of folic acid, 25 mg pyridoxine (B6), and 400 µg cyanocobalamin (B12). The trial showed no reduction on recurrent stroke or in secondary outcomes, including coronary heart disease and cardiovascular disease (Toole et al., 2004). These outcomes might be a result of caveats, due to the fact that the study did not include a placebo group, there was a coinciding folic acid fortification of grain supply in the US, and patients with low vitamin B12 at the start of the study received B12 injections. A subgroup analysis which excluded patients with a vitamin B12 deficiency and renal impairment from the Vitamin Intervention for Stroke Prevention trial, showed that there was a 38% reduction in stroke and myocardial infarction risk (Spence et al., 2005). Another subgroup analysis of the Vitamin Intervention for Stroke Prevention trial reported that patients with the GG phenotype of transcobalamin 2, a transport protein for vitamin B12, were responsive to the B-vitamin supplementation (Hsu et al., 2011). These two subgroup analyses demonstrate some positive impact of B-vitamin supplementation on stroke risk for specific populations.
In another trial, the Heart Outcomes Prevention Evaluation clinical trial, 5522 patients aged 55 years or older who had vascular disease or diabetes, received a combination of folic acid (2.5 mg), vitamin B6 (50 mg) and vitamin B12 (1 mg) or a placebo for an average of 5 years. The primary outcome was death from cardiovascular disease (Lonn et al., 2006). The Heart Outcomes Prevention Evaluation trial observed a reduction in homocysteine levels in the supplemented group versus the placebo group, although there was no benefit of B vitamin supplementation on cardiovascular disease risk. However, when homocysteine-lowering therapy and stroke risk were assessed, there was a reduction on the incidence of stroke and risk of nonfatal stroke, and the severity of the stroke or disability was not affected by supplementation (Saposnik et al., 2009).
In 2015, the China Stroke Primary Prevention Trial demonstrated supplementation with folic acid in combination with enalapril, an angiotensin converting enzyme inhibitor used to treat hypertension, reduced risk of stroke by 24% in hypertensive patients (Huo et al., 2015). Furthermore, the dietary levels of vitamin B12 that were above the median when combined with the benefits of supplementation was transferred to patients with a polymorphism in the gene for methylenetetrahydrofolate reductase (MTHFR), an enzyme involved in one carbon metabolism (Zhao et al., 2017). Further, subgroup analysis showed that younger male patients with lower folate levels and higher systolic blood pressure, total cholesterol, and blood glucose and the MTHFR C677T CT or TT genotype had the most lifelong benefit (Zhang et al., 2020). A follow-up study investigated the interaction between folate, vitamin B12 and enalapril on risk of ischemic stroke, the effect of folate and B12 are additive; serum B12 levels above the median (from diet) were associated with a 72% reduction of stroke with folic acid and enalapril on TT heterozygotes (Qin et al., 2020).
Additionally, clinical trials of B vitamin supplementation to reduce risk of stroke found that cyanocobalamin is harmful among patients with renal impairment (Spence et al., 2017). The form of vitamin B12 (cyanocobalamin vs. methylcobalamin) needs to be carefully considered when B-vitamin supplementation is prescribed to patients that are at a higher risk for stroke or stroke affected patients (Spence, 2007). While it is evident in prior research that there is a significant correlation between homocysteine and folate levels with the risk of stroke, the role of vitamin B12 is still unclear (He et al., 2004). Studies suggest that vitamin B12 plays an important role in reducing homocysteine levels, but research on the direct association between vitamin B12 and ischemic stroke is not well investigated (He et al., 2004). The aim of this review is to investigate how deficiencies in vitamin B12 impact stroke risk and outcome.
Search Strategy
To describe the relationship between vitamin B12 deficiency and risk of ischemic stroke, a variety of clinical studies were included in this review. Publications using medical subject headings (MeSH) keywords, vitamin B12 deficiency, and stroke, were retrieved. For each study we collected the following data: study design, sample size, the country the study was conducted in, folic acid fortification, measures used to determine vitamin B12, and major findings. All of the findings for each individual study are summarized in Table 1.
Table 1.
Summary of clinical studies investigating vitamin B12 in patients
| Reference | Study design | Sample size | Country | Fortification present | Measure used to determine vitamin B12 levels | Major findings |
|---|---|---|---|---|---|---|
| He et al. (2004) | Longitudinal Cohort study/large prospective follow-up study | 725 stroke cases (455 ischemic, 125 hemorrhagic, 145 unknown) *Men only | USA | Yes | Studied intake levels only. Dietary information was assessed every 4 years for 14 years using detailed and validated semiquantitative food frequency questionnaire. | Intake of vitamin B12 and folate, but not B6 was inversely related to risk of ischemic stroke. No statisticgroupy significant associations with hemorrhagic stroke risk. |
| van Guelpen et al. (2005) | Prospective, Nested Case-Refernt Study, population based | 396 (334 ischemic and 62 hemorrhagic stroke cases) from Northern Sweden Health and Disease Cohort | Sweden | No | Venous blood samples drawn with minimum of 4-hour fasting, Folate and B12 levels were analyzed by Quantaphase II radioassay in heparinized serum/plasma. | Neither plasma nor dietary vitamin B12 was statisticgroupy significantly associated with either stroke type. |
| Zacharia et al. (2017) | Unusual case | 1 (35-year-old male vegetarian) | USA | Yes | Tested serum B12 levels before and after supplementation over 18 months. | Two months after beginning vitamin B12 supplemental treatment, patient’s symptoms improved dramaticgroupy. This included resolution of aphasia and improvement of hemiparesis. Vitamin B12 increased from 206 to 1249 ng/L and homosystein levels decreased from 55.7 to 28.5 μM. |
| Ahmed et al. (2019) | Cohort study | 4055 patients | Canada | Yes | Serum B12 levels measured by immunoassay. | In stroke patients, 8.2% of patients were biochemicgroupy deficient in vitamin B12, 10.6% patients were deficient in metabolic B12, and 19.1% had high homocysteine levels. In patients aged 80 years or older, 18.1% of patients were deficient in metabolic B12 and 35% had high homocysteine levels. |
Vitamin B12 in clinical stroke studies
In a longitudinal cohort study of 725 stroke cases (455 ischemic, 125 hemorrhagic, and 145 unknown incidents), increased dietary intake of vitamin B12 was statistically significantly associated with a decreased risk of ischemic stroke in men aged 40–75 years (P < 0.05) when adjusted for body mass index and intake of vitamin E, fiber, and potassium (He et al., 2004). Comparing men in the highest quintile of vitamin B12 intake with those in the lowest, the multivariate risk ratio, when adjusted for body mass index was 0.73 (95%CI: 0.53 to 0.99; P < 0.04) and the relation remained with additional adjustment for fiber, potassium, and vitamin E intakes (P < 0.05). It should be noted that this relationship was no longer statistically significant (P > 0.05) when adjusted for factors of cigarette smoking and age (He et al., 2004). Beneficial effects of intakes of folate, vitamin B6, and B12 were expected due to their inverse relationship with blood homocysteine.
Patients with a vitamin B12 deficiency during an ischemic stroke have been reported to have worse outcome post-stroke (Pieters et al., 2009; Zacharia et al., 2017). In patients with their first lacunar stroke, low vitamin B12 levels were associated with more periventricular white matter lesions (Pieters et al., 2009). The role of vitamin B12 was observed further in a case of recurrent stroke in a 35-year-old male patient. The patient suffered two ischemic stroke incidents in a 3-month span and was recorded as having relatively low levels of vitamin B12 at 206 ng/L (at the lower end of the normal range of 180–914 ng/L) as well as high homocysteine levels at 55.7 μM (with a normal range of 5–15 μM) (Zacharia et al., 2017). In addition to statins and aspirin, the patient was treated with vitamin B12 supplements at a dose of 1000 μg daily via intramuscular injections and then orally, as well as folic acid. After 2 months of B vitamin supplemental therapy, the patient’s symptoms of aphasia and right-sided hemiparesis showed drastic improvement (Zacharia et al., 2017). The patient’s vitamin B12 levels increased to 1249 ng/L and homocysteine levels decreased to 28.5 μM. However, the patient was also discovered to be homozygous for the MTHFR polymorphism at bp677TT (Zacharia et al., 2017). Additional studies are needed to further monitor vitamin B12 levels in stroke patients, as well as the benefits of adding supplementation vitamin B12 therapy to existing treatments regimes.
In another study, the dietary intake and blood plasma concentration of vitamin B12 were measured in 396 stroke cases by a food frequency questionnaire over a 14-year period. In this prospective population-based study, intake and plasma levels of vitamin B12 were not statistically significantly associated with risk of either ischemic or hemorrhagic stroke (Van Guelpen et al., 2005). The plasma concentration and dietary intake of vitamin B12 were not found to be statistically significantly associated reduced with stroke risk (P > 0.05). Interestingly vitamin B12 levels did not change with multivitamin use in this study, suggesting a decrease in absorption of vitamin B12 with age, as the average age for ischemic stroke group was 54.8 years. The main limitation of the study was the inability to control for use of aspirin or other nonsteroidal anti-inflammatory drugs (Van Guelpen et al., 2005).
In a cohort study, the prevention of secondary stroke with use of B vitamin supplementation was conducted using 4055 patient records from the Urgent Transient Ischemic Attack Clinic between 2002 and 2017 (Ahmed et al., 2019). The use of holotranscobalamin or other vitamin B12 metabolites, such as methylmalonic acid or total homocysteine as a more accurate measure of metabolic B12 levels is noted in the article; a common misconception amongst health professionals who frequently mistake a normal serum B12 for having healthy functional B12 levels. According to the study, 8.2% of patients were found to be deficient in biochemical levels of B12, but 10.6% of patients were found to be deficient in metabolic B12 when deficiency is measured as being < 156 pM and < 258 pM for biochemical and metabolic levels respectively (Ahmed et al., 2019). In patients aged 80 years or older, metabolic B12 deficiency increased to 18.1% (Ahmed et al., 2019). These deficiencies may be slightly underestimated due to the inability to control for B vitamin supplementation. The study also found that 19.1% of all patients had high homocysteine levels which increased to 35% for patients aged 80 years or older (Ahmed et al., 2019). Seeing as B12 deficiencies and high homocysteine levels are linked to transient ischemic attack and stroke, it is pertinent that health professionals test for metabolic B12 levels rather than serum B12 alone when working to prevent stroke incidents; this is especially true in older patients (Ahmed et al., 2019).
Conclusion
In our survey of the literature, we did not find specific studies investigating the mechanisms of how a vitamin B12 deficiency changes the brain to impact stroke outcome. Research using model systems is needed to understand the specific mechanisms through which a vitamin B12 deficiency changes the brain.
Adequate levels of vitamin B12 are needed for successful aging (Vogiatzoglou et al., 2008). Clinical data and the results from our literature review strongly suggest that low levels of vitamin B12 are a risk factor for ischemic stroke. In addition, our survey of the literature has identified that there is a gap in the understanding of the mechanisms in which a vitamin deficiency leads to increased risk of stroke. One idea is increased levels of homocysteine, which is a well-known risk factor for ischemic stroke. Another mechanism is the role of vitamin B12 on neurological function, specifically myelination, this is not as well known. Further studies are required in model systems to understand how a vitamin B12 deficiency changes the brain, making it more vulnerable to ischemic stroke.
Footnotes
C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y Review
Conflicts of interest: None.
Financial support: This work was supported by Midwestern University Startup Funds (to NMJ) and American Heart Association, No. 20AIREA35050015 (to NMJ).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by Midwestern University Startup Funds (to NMJ) and American Heart Association, No. 20AIREA35050015 (to NMJ).
References
- 1.Ahmed S, Bogiatzi C, Hackam DG, Rutledge AC, Sposato LA, Khaw A, Mandzia J, Azarpazhoo MR, Hachinski V, Spence JD. Vitamin B 12 deficiency and hyperhomocysteinaemia in outpatients with stroke or transient ischaemic attack: a cohort study at an academic medical centre. BMJ Open. 2019;9:1–8. doi: 10.1136/bmjopen-2018-026564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrès E, Loukili NH, Noel E, Kaltenbach G, Ben Abdelgheni M, Perrin AE, Noblet-Dick M, Maloisel F, Schlienger JL, Blicklé JF. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ. 2004;171:251–259. doi: 10.1503/cmaj.1031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 4.Bottiglieri T. Folate, vitamin B12, and neuropsychiatric disorders. Nutr Rev. 1996;54:382–390. doi: 10.1111/j.1753-4887.1996.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 5.Bottiglieri T, Reynolds EH, Laundy M. Folate in CSF and age. J Neurol Neurosurg Psychiatry. 2000;69:562. doi: 10.1136/jnnp.69.4.562a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro R, Rivera I, Blom HJ, Jakobs C, Tavares de Almeida I. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: an overview. J Inherit Metab Dis. 2006;29:3–20. doi: 10.1007/s10545-006-0106-5. [DOI] [PubMed] [Google Scholar]
- 7.Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, Manson JE, Bonaa K, Spence JD, Nygard O, Jamison R, Gaziano JM, Guarino P, Bennett D, Mir F, Peto R, Collins R. B-Vitamin Treatment Trialists Collaboration (2010) Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific. Arch Intern Med. 170:1622. doi: 10.1001/archinternmed.2010.348. [DOI] [PubMed] [Google Scholar]
- 8.de Lau LML, Smith AD, Refsum H, Johnston C, Breteler MMB. Plasma vitamin B12 status and cerebral white-matter lesions. J Neurol Neurosurg Psychiatry. 2009;80:149–157. doi: 10.1136/jnnp.2008.149286. [DOI] [PubMed] [Google Scholar]
- 9.Fang H, Kang J, Zhang D. Microbial production of vitamin B12: a review and future perspectives. Microb Cell Fact. 2017;16:15. doi: 10.1186/s12934-017-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feigin VL, Mensah GA, Norrving B, Murray CJL, Roth GA. Atlas of the global burden of stroke (1990-2013): the GBD 2013 study. Neuroepidemiology. 2015;45:230–236. doi: 10.1159/000441106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham LM, Daly LE, Refsum HM, Robinson K, Brattström LE, Ueland PM, Palma-Reis RJ, Boers GH, Sheahan RG, Israelsson B, Uiterwaal CS, Meleady R, McMaster D, Verhoef P, Witteman J, Rubba P, Bellet H, Wautrecht JC, de Valk HW, Sales Lúis AC, et al. Plasma homocysteine as a risk factor for vascular disease: the European concerted action project. J Am Med Assoc. 1997;277:1775–1781. doi: 10.1001/jama.1997.03540460039030. [DOI] [PubMed] [Google Scholar]
- 12.Hankey GJ. Nutrition and the risk of stroke. Lancet Neurol. 2012;11:66–81. doi: 10.1016/S1474-4422(11)70265-4. [DOI] [PubMed] [Google Scholar]
- 13.He K, Merchant A, Rimm EB, Rosner BA, Stampfer MJ, Willett WC, Ascherio A. Folate, vitamin B6, and B12 intakes in relation to risk of stroke among men. Stroke. 2004;35:169–174. doi: 10.1161/01.STR.0000106762.55994.86. [DOI] [PubMed] [Google Scholar]
- 14.Hsu FC, Sides EG, Mychaleckyj JC, Worrall BB, Elias GA, Liu Y, Chen WM, Coull BM, Toole JF, Rich SS, Furie KL, Sale MM. Transcobalamin 2 variant associated with poststroke homocysteine modifies recurrent stroke risk. Neurology. 2011;77:1543–1550. doi: 10.1212/WNL.0b013e318233b1f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang X, Li Y, Li P, Li J, Bao H, Zhang Y, Wang B, Sun N, Wang J, He M, Yin D, Tang G, Chen Y, Cui Y, Huang Y, Hou FF, Qin X, Huo Y, Cheng X. Association between percent decline in serum total homocysteine and risk of first stroke. Neurology. 2017;89:2101–2107. doi: 10.1212/WNL.0000000000004648. [DOI] [PubMed] [Google Scholar]
- 16.Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, Tang G, Wang B, Chen D, He M, Fu J, Cai Y, Shi X, Zhang Y, Cui Y, Sun N, Li X, Cheng X, Wang J, Yang X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China the CSPPT randomized clinical trial. JAMA. 2015;313:1325–1335. doi: 10.1001/jama.2015.2274. [DOI] [PubMed] [Google Scholar]
- 17.Johansson I, Van Guelpen B, Hultdin J, Johansson M, Hallmans G, Stattin P. Validity of food frequency questionnaire estimated intakes of folate and other B vitamins in a region without folic acid fortification. Eur J Clin Nutr. 2010;64:905–913. doi: 10.1038/ejcn.2010.80. [DOI] [PubMed] [Google Scholar]
- 18.Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38:208–211. doi: 10.1055/s-0038-1649503. [DOI] [PubMed] [Google Scholar]
- 19.Krasinski SD, Russell RM, Samloff IM, Jacob RA, Dallal GE, McGandy RB, Hartz SC. Fundic atrophic gastritis in an elderly population. Effect on hemoglobin and several serum nutritional indicators. J Am Geriatr Soc. 1986;34:800–806. doi: 10.1111/j.1532-5415.1986.tb03985.x. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Aakriti, Gupta V. A review on animal models of stroke: an update. Brain Res Bull. 2016;122:35–44. doi: 10.1016/j.brainresbull.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Lachner C, Steinle NI, Regenold WT. The neuropsychiatry of vitamin B 12 deficiency in elderly patients. J Neuropsychiatry Clin Neurosci. 2012;24:5–15. doi: 10.1176/appi.neuropsych.11020052. [DOI] [PubMed] [Google Scholar]
- 22.Lai WKC, Kan MY. Homocysteine-induced endothelial dysfunction. Ann Nutr Metab. 2015;67:1–12. doi: 10.1159/000437098. [DOI] [PubMed] [Google Scholar]
- 23.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest J Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 24.McCully KS. Review: Chemical pathology of homocysteine VI, aging, cellular senescence, and mitochondrial dysfunction. Ann Clin Lab Sci. 2018;48:677–687. [PubMed] [Google Scholar]
- 25.Moore K, Hughes CF, Ward M, Hoey L, McNulty H. Diet, nutrition and the ageing brain: current evidence and new directions. Proc Nutr Soc. 2018;77:152–163. doi: 10.1017/S0029665117004177. [DOI] [PubMed] [Google Scholar]
- 26.Murray L, Emmerson J, Jadavji NM. The role of folates in neurological functions. In: Stephen M, Lee, editors. Folic acid: sources, health effects and role in disease. Hauppauge, NY: Nova Publishers Science Inc; pp. 81–104. [Google Scholar]
- 27.Nygård O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337:230–236. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 28.Pieters B, Staals J, Knottnerus I, Rouhl R, Menheere P, Kessels AF, Lodder J. Periventricular white matter lucencies relate to low vitamin b12 levels in patients with small vessel stroke. Stroke. 2009;40:1623–1626. doi: 10.1161/STROKEAHA.108.523431. [DOI] [PubMed] [Google Scholar]
- 29.Qin X, Spence JD, Li J, Zhang Y, Li Y, Sun N, Liang M, Song Y, Zhang Y, Wang B, Cheng X, Zhao L, Wang X, Xu X, Huo Y. Interaction of serum vitamin B12 and folate with MTHFR genotypes on risk of ischemic stroke. Neurology. 2020;94:e1126–1136. doi: 10.1212/WNL.0000000000008932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5:949–960. doi: 10.1016/S1474-4422(06)70598-1. [DOI] [PubMed] [Google Scholar]
- 31.Saposnik G, Ray JG, Sheridan P, McQueen M, Lonn E. Homocysteine-lowering therapy and stroke risk, severity and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365–1372. doi: 10.1161/STROKEAHA.108.529503. [DOI] [PubMed] [Google Scholar]
- 32.Scott JM, Molloy AM. The discovery of vitamin B12. Ann Nutr Metab. 2012;61:239–245. doi: 10.1159/000343114. [DOI] [PubMed] [Google Scholar]
- 33.Selhub J, Bagley LC, Miller J, Rosenberg IH. B vitamins, homocysteine, and neurocognitive function in the elderly. Am J Clin Nutr. 2000;71:614S–620S. doi: 10.1093/ajcn/71.2.614s. [DOI] [PubMed] [Google Scholar]
- 34.Selhub J, Troen A, Rosenberg IH. B vitamins and the aging brain. Nutr Rev. 2010;68(Suppl 2):S112–118. doi: 10.1111/j.1753-4887.2010.00346.x. [DOI] [PubMed] [Google Scholar]
- 35.Shlisky J, Bloom DE, Beaudreault AR, Tucker KL, Keller HH, Freund-Levi Y, Fielding RA, Cheng FW, Jensen GL, Wu D, Meydani SN. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv Nutr. 2017;8:17–26. doi: 10.3945/an.116.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spence J, Stampfer M. Understanding the complexity of homocysteine lowering with vitamins. JAMA. 2011;306:9–11. doi: 10.1001/jama.2011.1834. [DOI] [PubMed] [Google Scholar]
- 37.Spence JD. Homocysteine-lowering therapy: a role in stroke prevention. Lancet Neurol. 2007;6:830–838. doi: 10.1016/S1474-4422(07)70219-3. [DOI] [PubMed] [Google Scholar]
- 38.Spence JD. Nutrition and risk of stroke. Nutrients. 2019;11:647. doi: 10.3390/nu11030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spence JD, Bang H, Chambless LE, Stampfer MJ. Vitamin intervention for stroke prevention trial: an efficacy analysis. Stroke. 2005;36:2404–2409. doi: 10.1161/01.STR.0000185929.38534.f3. [DOI] [PubMed] [Google Scholar]
- 40.Spence JD, Yi Q, Hankey GJ. B vitamins in stroke prevention: time to reconsider. Lancet Neurol. 2017;16:750–760. doi: 10.1016/S1474-4422(17)30180-1. [DOI] [PubMed] [Google Scholar]
- 41.Tang S, Fang Y, Huang G, Xu X, Padilla-Banks E, Fan W, Xu Q, Sanderson SM, Foley JF, Dowdy S, McBurney MW, Fargo DC, Williams CJ, Locasale JW, Guan Z, Li X. Methionine metabolism is essential for SIRT1-regulated mouse embryonic stem cell maintenance and embryonic development. EMBO J. 2017;36:3175–3193. doi: 10.15252/embj.201796708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toole JF, Rene M, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, Sides EG, Wang C. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke. J Am Med Assoc. 2004;291:565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 43.Tsybikov NN, Fefelova EV, Tereshkov PP, Izmestyev SV. Endothelial dysfunction in experimental hyperhomocysteinemia. Patol Fiziol Eksp Ter. 2016;60:42–46. [PubMed] [Google Scholar]
- 44.Van Guelpen B, Hultdin J, Johansson I, Stegmayr B, Palmqvist R, Winkvist A, Guelpen V. Hemorrhagic stroke a prospective, nested case-referent study of plasma concentrations and dietary intake. Stroke. 2005;36:1426–1431. doi: 10.1161/01.STR.0000169934.96354.3a. [DOI] [PubMed] [Google Scholar]
- 45.Vogiatzoglou A, Refsum H, Johnston C, Smith SM, Bradley KM, de Jager C, Budge MM, Smith AD. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology. 2008;71:826–832. doi: 10.1212/01.wnl.0000325581.26991.f2. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe F. Vitamin B12 sources and bioavailability. Exp Biol Med. 2007;232:1266–1274. doi: 10.3181/0703-MR-67. [DOI] [PubMed] [Google Scholar]
- 47.Weir DG, Scott JM. Brain function in the elderly: Role of vitamin B12 and folate. Br Med Bull. 1999;55:669–682. doi: 10.1258/0007142991902547. [DOI] [PubMed] [Google Scholar]
- 48.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 49.Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti SD, JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM LJ, Lisabeth LD, Liu S, Mackey RH, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 50.Zacharia G, Shani D, Ortiz RA. Recurrent stroke in a patient with vitamin B 12 deficiency and MTHFR mutation. Neurol Clin Pract. 2017;7:e1–4. doi: 10.1212/CPJ.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang T, Lin T, Wang Y, Wang B, Qin X, Xie F, Cui Y, Huo Y, Wang X, Zhang Z, Jiang J. Estimated stroke-free survival of folic acid therapy for hypertensive adults: projection based on the CSPPT. Hypertension. 2020;75:339–346. doi: 10.1161/HYPERTENSIONAHA.119.14102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Hodgson NW, Trivedi MS, Abdolmaleky HM, Fournier M, Cuenod M, Do KQ, Deth RC. Decreased brain levels of vitamin B12 in aging, autism and schizophrenia. PLoS One. 2016;11:e0146797. doi: 10.1371/journal.pone.0146797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao M, Wang X, He M, Qin X, Tang G, Huo Y, Li J, Fu J, Huang X, Cheng X, Wang B, Hou FF, Sun N, Cai Y. Homocysteine and stroke risk: modifying effect of methylenetetrahydrofolate reductase C677T polymorphism and folic acid intervention. Stroke. 2017;48:1183–1190. doi: 10.1161/STROKEAHA.116.015324. [DOI] [PubMed] [Google Scholar]


