Multiple sclerosis (MS) is a chronic autoimmune disorder of the central nervous system (CNS) that has both inflammatory and neurodegenerative components. Both genetic and environmental factors confer risk for developing the disease. Additionally, gut microbiota appears to play an important role in the risk for MS and potentially also in affecting disease severity, though our understanding of these interactions are still incomplete (Bhargava and Mowry, 2014). The circulating metabolome is at the intersection of these various factors – genome, gut microbiome and exposome and provides unique insights into the pathophysiology of the disease (Bhargava and Anthony, 2020).
Metabolic dysfunction in MS: Multiple studies have now established that the circulating metabolome, as well as the cerebrospinal fluid metabolome in MS, display multiple alterations compared to healthy controls (Bhargava and Anthony, 2020). The metabolic pathways involved are primarily linked to energy metabolism (glucose metabolism, ketone bodies, amino acid metabolism), oxidative stress (glutathione metabolism), lipid metabolism (short chain fatty acid, sphingolipid and phospholipid metabolism) and xenobiotic (xanthine and benzoate) metabolism. While some studies have attempted to link specific metabolic pathways (such as tryptophan metabolism) to disease risk or severity (Lim et al., 2017; Nourbakhsh et al., 2018), there is a paucity of large longitudinal studies that examine the utility of metabolic dysfunction as a diagnostic or prognostic biomarker. Additionally, there is a need for mechanistic studies that examine the potential pathophysiological consequences of observed metabolic alterations in MS.
Sources of metabolic dysfunction in MS: Currently, the precise mechanism underlying metabolic dysfunction in MS remains unclear. The potential sources of these alterations are genetic alterations in endogenous metabolism, alterations in peripheral immune cell metabolism, diet-related alterations, altered gut microbial metabolism or other environmental factors/exposures. There is extensive evidence that both genetic variation and the gut microbiota influence the level of circulating metabolites (Wikoff et al., 2009; Shin et al., 2014). There may also be an interaction between these two factors with genetic variation affecting metabolite levels through alterations in the gut microbiota (Wang et al., 2016). There is evidence that metabolic abnormalities exist in neurons in MS especially involving mitochondrial function (Mahad et al., 2015). Additionally, metabolic profiles appear to differ between pro- and anti-inflammatory subsets in both innate and adaptive immune cell types. Thus, explanations for alterations in energy metabolism related molecules would include changes in immune cell metabolism (Miyajima et al., 2017). The underlying cause of the various altered metabolic pathways noted in MS are likely to vary and future studies utilizing multi-omics approaches combining genomics, epigenomics, metagenomics and metabolomics will provide further insights into this important outstanding question. This information will be crucial when designing appropriate interventions to target these aberrant metabolic pathways in MS.
Altered bile acid metabolism in MS: Bile acids are cholesterol metabolites that play an important role in lipid absorption in the gut. Besides this function, these molecules can also be absorbed into the circulation and have effects on cells throughout the body. In our recent study, we noted that the levels of multiple bile acids were reduced in the circulation of both adults and children with MS from two academic MS Centers (Bhargava et al., 2020). These findings were initially noted on untargeted metabolomics assays and were then confirmed in an additional cohort using targeted metabolomics analyses. In the adult MS population, alterations were most pronounced in those with progressive disease. Interestingly, recent studies have also demonstrated alterations in bile acid metabolism in Alzheimer’s disease (Nho et al., 2018), noting an imbalance between certain circulating primary and secondary bile acid concentrations. We noted similar changes in the ratios of certain primary and secondary bile acid metabolites in MS, but additionally noted reductions in multiple other circulating bile acid concentrations compared to controls.
To further understand the relevance of these findings, we assessed the expression of bile acid receptors in the brain–using autopsy tissue from MS patients and controls. We noted the presence of both nuclear [Farnesoid X receptor (FXR)] and surface [G-protein coupled bile acid recetor-1 (GPBAR1)] bile acid receptors in MS lesions and the expression of these receptors was higher than in control tissue or in normal-appearing white matter. These receptors were present on both immune [Farnesoid X receptor and GPBAR1 on CD68 positive cells (macrophages/microglia)] and glial cells [GPBAR1 on glial fibrillary acidic portein positive cells (astrocytes)], demonstrating that bile acids could influence cells present in MS lesions. We then demonstrated that treatment of astrocytes and microglia in vitro with an endogenous bile acid-tauroursodeoxycholic acid (TUDCA) led to amelioration of inflammatory polarization of both these cell types. Since both these cell types play an important role in MS disease pathophysiology, especially in the progressive phase of the disease, reduced signaling through these receptors could predispose to greater neuroinflammation.
We also tested the effect of supplementation of TUDCA on disease severity in an animal model of MS (experimental autoimmune encephalomyelitis). We noted reduced severity of the disease and this effect was lost in mice lacking the GPBAR1 receptor, suggesting that TUDCA ameliorates neuroinflammation through its actions at this receptor.
Overall, these results demonstrated that bile acid metabolism is altered in MS and that signaling through GPBAR1 in the CNS may help in ameliorating neuroinflammation (Figure 1).
Figure 1.
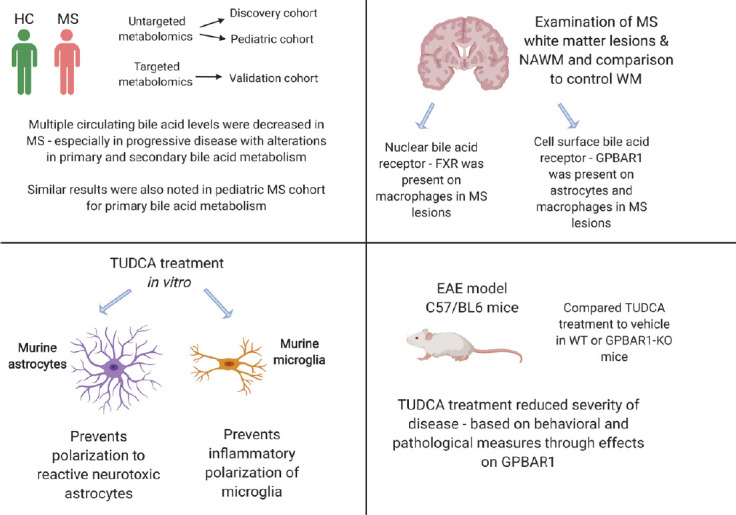
Schematic representation of results from recent study identifying altered bile acid metabolism in MS and the effects of bile acid supplementation on neuroinflammation.
GPBAR1: G protein-coupled bile acid-activated receptor 1; HC: healthy control; MS: multiple sclerosis; NAWM: normal-appearing white matter; TUDCA: tauroursodeoxycholic acid; WM: white matter; WT: wild type.
Targeting aberrant metabolic pathways in MS: As we unravel the pathophysiological relevance of metabolic abnormalities in MS, we will likely identify new therapeutic targets to impact the disease. Targeting metabolic dysfunction, will also likely require additional information regarding the origin of these abnormalities. Interventions could then include those targeted at the microbiota-probiotics, prebiotics or fecal microbiota transplantation versus supplementation with specific metabolites. Based on current knowledge it is likely that cells in both the immune system (innate and adaptive) as well as the CNS (glial and neuronal) are likely to be targets of interventions aimed at modulating aberrant metabolic pathways. For example, a short-chain fatty acid altered adaptive immune cell function (as noted below), while bile acid supplementation had effects at both innate immune and glial cells (as noted above).
A recent study, demonstrated that supplementation with propionate, as short chain fatty acid that is reduced in the circulation and stool of MS patients, leads to increased regulatory T cells and a reduction in pro-inflammatory T cells with an accompanying reduction in inflammatory MS disease activity (Duscha et al., 2020). This is an excellent example of how metabolic alterations in MS could be targeted to treat the disease, provided we have insight into the pathophysiological relevance of these changes.
Based on our study described above, we are currently in the process of testing the effect of supplementation of TUDCA in progressive MS patients to determine whether four months of treatment results in changes in the circulating metabolome, gut microbiome and peripheral immune profile (NCT03423121). If successful, this would lead to larger studies to determine whether we are able to alter disease progression in this patient population.
It would also be important to consider that given the heterogeneity in disease presentation and course in people with MS, alterations in specific metabolic pathways may exist in a subset of this population. Metabolomics analyses could also help in personalizing interventions aimed at a specific metabolic pathway to those individuals that demonstrate abnormality in the pathway of interest, thus enabling precision medicine by maximizing the likelihood of benefit and potentially minimizing adverse effects.
Conclusion: Metabolic alterations are present in people with MS and mechanistic studies examining the relevance of these metabolic pathways to MS pathophysiology are beginning to identify novel targets for therapeutic intervention. Future studies are likely to unravel pathophysiological roles for additional metabolic pathways and to provide additional treatment targets or interventions. Additionally, multi-omics studies will shed more light on the origins of specific metabolic alterations and will also help in personalizing interventions aimed at targeting these metabolic pathways.
This work was supported in part by a Career Transition Fellowship from the National MS Society.
Additional file: Open peer review report 1 (80.6KB, pdf) .
Footnotes
P-Reviewer: Kazanis I; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
Copyright license agreement: The Copyright License Agreement has been signed by the author before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Ilias Kazanis, University of Cambridge, UK.
References
- 1.Bhargava P, Anthony D. Metabolomics in multiple sclerosis disease course and progression. Mult Scler. 2020;26:591–598. doi: 10.1177/1352458519876020. [DOI] [PubMed] [Google Scholar]
- 2.Bhargava P, Mowry EM. Gut microbiome and multiple sclerosis. Curr Neurol Neurosci Rep. 2014;14:492. doi: 10.1007/s11910-014-0492-2. [DOI] [PubMed] [Google Scholar]
- 3.Bhargava P, Smith MD, Mische L, Harrington EP, Fitzgerald KC, Martin KA, Kim S, Reyes AAA, Gonzalez-Cardona J, Volsko C, Tripathi A, Singh S, Varanasi K, Lord HN, Meyers KR, Taylor M, Gharagozloo M, Sotirchos ES, Nourbakhsh B, Dutta R, et al. Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation. J Clin Invest. 2020 doi: 10.1172/JCI129401. doi:101172/JCI129401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duscha A, Gisevius B, Hirschberg S, Yissachar N, Stangl GI, Eilers E, Bader V, Haase S, Kaisler J, David C, Schneider R, Troisi R, Zent D, Hegelmaier T, Dokalis N, Gerstein S, Del Mare-Roumani S, Amidror S, Staszewski O, Poschmann G, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. 2020;180:1067–1080e16. doi: 10.1016/j.cell.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 5.Lim CK, Bilgin A, Lovejoy DB, Tan V, Bustamante S, Taylor B V, Bessede A, Brew BJ, Guillemin GJ. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci Rep. 2017;7:41473. doi: 10.1038/srep41473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahad DH, Trapp BD, Lassman H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–93. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 7.Miyajima M, Zhang B, Sugiura Y, Sonomura K, Guerrini MM, Tsutsui Y, Maruya M, Vogelzang A, Chamoto K, Honda K, Hikida T, Ito S, Qin H, Sanuki R, Suzuki K, Furukawa T, Ishihama Y, Matsuda F, Suematsu M, Honjo T, et al. Metabolic shift induced by systemic activation of T cells in PD-1-deficient mice perturbs brain monoamines and emotional behavior. Nat Immunol. 2017;18:1342–1352. doi: 10.1038/ni.3867. [DOI] [PubMed] [Google Scholar]
- 8.Nho K, Kueider-Paisley A, MahmoudianDehkordi S, Arnold M, Risacher SL, Louie G, Blach C, Baillie R, Han X, Kastenmüller G, Jia W, Xie G, Ahmad S, Hankemeier T, van Duijn CM, Trojanowski JQ, Shaw LM, Weiner MW, Doraiswamy PM, Saykin AJ, et al. (2018) Altered bile acid profile in mild cognitive impairment and Alzheimer’s disease: Relationship to neuroimaging and CSF biomarkers. Alzheimer’s Dement. 15:232–244. doi: 10.1016/j.jalz.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nourbakhsh B, Bhargava P, Tremlett H, Hart J, Graves J, Waubant E. Altered tryptophan metabolism is associated with pediatric multiple sclerosis risk and course. Ann Clin Transl Neurol. 2018;5:1211–1221. doi: 10.1002/acn3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, Arnold M, Erte I, Forgetta V, Yang TP, Walter K, Menni C, Chen L, Vasquez L, Valdes AM, Hyde CL, Wang V, Ziemek D, Roberts P, Xi L, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. 2014;46:543–550. doi: 10.1038/ng.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Thingholm LB, Skiecevičienė J, Rausch P, Kummen M, Hov JR, Degenhardt F, Heinsen FA, Rühlemann MC, Szymczak S, Holm K, Esko T, Sun J, Pricop-Jeckstadt M, Al-Dury S, Bohov P, Bethune J, Sommer F, Ellinghaus D, Berge RK, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. 2016;48:1396–1406. doi: 10.1038/ng.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


