Abstract
The global increase in lifespan noted not only in developed nations, but also in large developing countries parallels an observed increase in a significant number of non-communicable diseases, most notable neurodegenerative disorders. Neurodegenerative disorders present a number of challenges for treatment options that do not resolve disease progression. Furthermore, it is believed by the year 2030, the services required to treat cognitive disorders in the United States alone will exceed $2 trillion annually. Mammalian forkhead transcription factors, silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae), the mechanistic target of rapamycin, and the pathways of autophagy and apoptosis offer exciting avenues to address these challenges by focusing upon core cellular mechanisms that may significantly impact nervous system disease. These pathways are intimately linked such as through cell signaling pathways involving protein kinase B and can foster, sometimes in conjunction with trophic factors, enhanced neuronal survival, reduction in toxic intracellular accumulations, and mitochondrial stability. Feedback mechanisms among these pathways also exist that can oversee reparative processes in the nervous system. However, mammalian forkhead transcription factors, silent mating type information regulation 2 homolog 1, mechanistic target of rapamycin, and autophagy can lead to cellular demise under some scenarios that may be dependent upon the precise cellular environment, warranting future studies to effectively translate these core pathways into successful clinical treatment strategies for neurodegenerative disorders.
Keywords: Alzheimer's disease, apoptosis; autophagy, erythropoietin, forkhead, FoxO, mechanistic target of rapamycin, silent mating type information regulation 2 homolog 1
Life Expectancy and the Impact on Neurodegenerative Disorders
Although with some cyclic changes, life expectancy is increasing globally. Recently in the US, life expectancy was decreasing over a four-year decline, but with a recent reduction in deaths from opioid overdoses, life expectancy is increasing again in the US (National Center for Health Statistics, 2019). Currently, life expectancy has reached eighty years of age. From the years 2000 through 2011, the age-adjusted death rate has been marked by a one percent decrease. Over the prior 50 years, the number of people over the age of 65 has doubled. It is predicted that large countries such as China and India will see an increase in the elderly population from 5% to 10% over multiple decades. Yet, the ten leading causes of death that include cardiac disease, cancer, trauma, respiratory disease, stroke, Alzheimer’s disease (AD), diabetes, influenza and pneumonia, kidney disease, and suicide continue to remain the same (National Center for Health Statistics, 2019). Several reasons can account for the increased lifespan, but increased access to preventive medical care, improved public health guidelines and sanitation measures, and new treatments for multiple disease entities appear to have promoted an increased lifespan (Maiese, 2017; Stefanatos and Sanz, 2018; Mladenovic Djordjevic et al., 2020).
The observed life expectancy increase has corresponded to the rising prevalence of non-communicable diseases (NCDs) (Hu et al., 2020; Maiese, 2020; Schiano et al., 2020). Almost seventy percent of the annual deaths that occur each year are the result of NCDs and over forty million people die from NCDs each year (World Health Organization, 2017). Of the 40 million individuals, at least fifteen million are between the ages of thirty and sixty-nine, demonstrating that all age groups be affected by NCDs. In addition, NCDs affect at least ten percent of people that are less than sixty years of age in high-income countries. In low and middle-income countries, NCDs can affect a much larger proportion of people with at least one-third of the population impacted under 60 years of age.
One significant disease entity for NCDs is neurodegenerative disorders. Nervous system diseases comprise over 600 disorders that can progressively lead to death and disability (Maiese, 2019; Corti et al., 2020; Xu et al., 2020). Both acute and chronic diseases of the nervous system affect large numbers of individuals throughout the globe that can exceed more than one billion individuals. This represents approximately 15% the world’s population and at least 7 million die each year from neurodegenerative disorders (Maiese, 2020).
All countries bear a significant financial burden from neurodegenerative disorders. The cost of neurodegenerative disorders is greater than $700 billion in the US alone and this includes dementia, stroke, back pain, epilepsy, trauma to the nervous system, and Parkinson’s disease (PD). Caring and treating dementia is considered the most significant cost factor with more than $800 billion a year required for dementia care at the present time (World Health Organization, 2017). Currently, the estimated costs for dementia treatment approximates 2% of the Gross Domestic Product in the world. Required medical and behavior care expenses in the US are estimated to be 2 trillion dollars annually in the year 2030. These projections do not account for the additional expenses necessary to provide adult living care, social outreach programs, and companion care.
Neurodegenerative disorders are expected to increase in prevalence throughout the globe as a result of the progressive increase in lifespan. As an example of the growing prevalence of neurodegenerative disorders, at least sixteen million medical care workers are necessary to provide unpaid care for people with AD or other dementias in the US. Sporadic cases of AD are increasing in the world with dementia now ranked as the 7th leading cause of death (Maiese, 2019). Dementia occurs in all countries throughout the world at a significant financial burden (World Health Organization, 2017). Greater than 5 million people suffer with cognitive disorders in the US and most of these cases, approximately 60%, are from AD (Maiese, 2020). Currently, 50 million people in the world, or 5% of the global population, have dementia. By 2030, dementia will affect 82 million individuals, and by 2050, 152 million individuals will be impacted by dementia. These numbers are compounded by other factors. Over 60 million additional medical workers and behavioral health walkers will be necessary (World Health Organization, 2017). It is also believed that dementia is under diagnosed throughout the world (Maiese, 2019). Furthermore, assuming the diagnosis is correct, dementia can be difficult to treat especially if the disease has progressed to later stages necessitating multiple modalities of treatment for both physical health and behavioral health.
An electronic search of the MEDLINE database for literature describing neurodegeneration OR mammalian forkhead transcription factors OR mechanism target of rapamycin OR silent mating type information regulation 2 homolog 1 OR protein kinase B OR erythropoietin OR autophagy OR apoptosis (MeSH Terms) from 1946 to 2020.
Novel Avenues for the Treatment of Neurodegenerative Disorders
Neurodegenerative disorders present significant challenges for early diagnosis, treatment, and limiting the progression of disease. Overall, for many neurodegenerative disorders, treatment options are limited. If one considers AD, this disorder is a syndrome that is not the result of a single etiology. Multiple mechanisms may result in dementia (Fan et al., 2020; Hu et al., 2020; Maiese, 2020; Prokopenko et al., 2020; Wang et al., 2020). These can involve oxidative stress, excitotoxicity, metabolic dysfunction with diabetes mellitus (DM), astrocytic cell injury, acetylcholine loss, β-amyloid (Aβ) and tau toxicity, RNA involvement, and mitochondrial damage (Caberlotto et al., 2019; Cai et al., 2019; De Vecchis et al., 2019; Kotrys and Szczesny, 2019; Hu et al., 2020; Maiese, 2020). Current therapies for AD are dependent upon cholinesterase inhibitors that may alleviate symptoms but not disease progression (Ruhal and Dhingra, 2018). Other treatments for dementia focus on vascular disease (Ding et al., 2018; Mehta et al., 2018; Wang et al., 2018; Maiese, 2020) and metabolic disorders, such as DM (Centers for Disease Control and Prevention, 2020; Hu et al., 2020; Maiese, 2020). Yet even for underlying conditions such as DM, tight serum glucose control cannot completely block the complications from DM (Coca et al., 2012; Maiese, 2020). Nutritional control of oral intake may can limit hyperglycemia, but diet mediated remedies have potential risks that can lead to a reduction in organ size with the activation of autophagy (Lee et al., 2014). Additional risk factors for neurodegenerative disorders include hypertension, limited education advancement, and tobacco use (Maiese, 2019). Given these challenges for the treatment of neurodegenerative disorders, innovative strategies are warranted to develop new areas of treatment that focus on core cellular mechanisms. One exciting avenue to effectively target neurodegenerative disorders involves the pathways of mammalian forkhead transcription factors (FoxOs), the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), the mechanistic target of rapamycin (mTOR), autophagy, and apoptosis (Figure 1).
Figure 1.
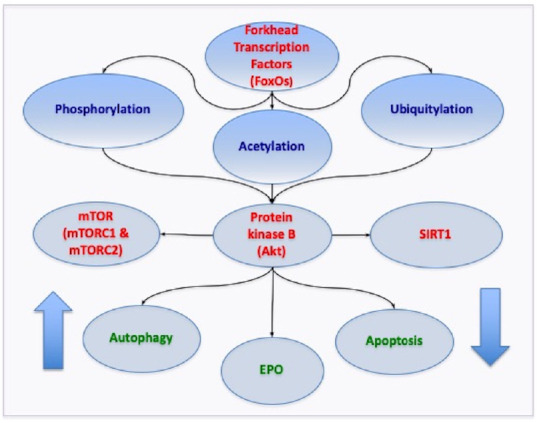
Novel Core Pathways for mammalian forkhead transcription factors (FoxOs).
FoxOs rely upon a network of pathways that oversee the onset and progression of neurodegenerative disease. Precise modulation of FoxOs through epigenetic and post-translation protein modifications is required to achieve the proper balance of FoxO activity for successful clinical care. FoxOs are controlled through phosphorylation, acetylation, and ubiquitylation that rely upon protein kinase B (Akt), silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), the mechanistic target of rapamycin (mTOR), mTOR Complex 1 (mTORC1), and mTOR Complex 2 (mTORC2). Ultimately, pathways of autophagy and apoptosis are affected such that under some conditions increased autophagy activity can clear toxic intracellular accumulations and inhibition of apoptotic pathways can prevent cell death. These pathways also can work in conjunction with trophic factors such as erythropoietin (EPO) to increase neuronal survival and maintain mitochondrial stability.
Mammalian Forkhead Transcription Factors
Mammalian FoxOs offer an novel approach to target neurodegenerative disorders, especially those that involve dementia and the loss of cognition (Maiese, 2015; Liu et al., 2020a; Sanphui et al., 2020). Since the discovery of the Drosophila melanogaster gene forkhead, over 100 forkhead genes and nineteen human subgroups have been identified that range from FOXA to FOXS. The mammalian FOXO proteins of the O class have significant relevance to neurodegenerative disorders and include the members FOXO1, FOXO3, FOXO4, and FOXO6. Forkhead proteins are also known as forkhead in rhabdomyosarcoma (FKHR) (FOXO1), FKHRL1 (forkhead in rhabdomyosarcoma like protein 1) (FOXO3a), the Drosophila gene fork head, Forkhead RElated ACtivator-1 and -2, and the acute leukemia fusion gene located in chromosome X (Maiese, 2015). Arabic numbers are used with “Fox” in the current nomenclature, subsequently a subclass or subgroup letter is listed, and then the member number is provided within the subclass (Maiese, 2015). Letters are capitalized for human Fox proteins. In the mouse, only the initial letter is listed as uppercase and for all other chordates the initial and subclass letters are in uppercase (Maiese, 2015).
The forkhead box (FOX) family of genes has a conserved forkhead domain (the “forkhead box”) noted as a “winged helix”. This is due to X-ray crystallography and nuclear magnetic resonance imaging suggestive of a butterfly-like appearance for the forkhead box family of genes. The forkhead domain in FoxO proteins contains three α-helices, three β-sheets, and two loops that compose the “wings” of the domain. This is specific for the forkhead proteins, since not all winged helix domains are actually Fox proteins. FoxO proteins are transcription factors that bind to DNA through the FoxO-recognized element in the C-terminal basic region of the forkhead DNA binding domain. After forkhead binding to DNA, activation or repression of target gene expression occurs through fourteen protein-DNA contacts with the primary recognition site located at α-helix H3 (Clark et al., 1993). Post-translational changes that include FoxO protein phosphorylation or acetylation can alter the binding of the C-terminal basic region to DNA to prevent transcriptional activity and block FoxO activity (Tsai et al., 2007). Yet, other factors may affect forkhead binding to DNA. These include N-terminal region of the recognition helix variations, electrostatic distribution changes, and sequestering FoxO proteins in the nucleus of cells (Scodelaro Bilbao and Boland, 2013).
Modulation of Forkhead Transcription Factors, Sirtuins, and Mechanistic Target of Rapamycin
FoxOs are overseen by epigenetic and post-translation protein modifications that include phosphorylation (Maiese, 2015; Peng et al., 2020), acetylation (Beretta et al., 2019; Liu et al., 2020a; Zhang and Zhao, 2019), and ubiquitylation (Zeldich et al., 2014) (Figure 1). Forkhead transcription factor phosphorylation is overseen by the serine-threonine kinase protein kinase B (Akt) (Maiese, 2015). Akt phosphorylates FoxO proteins that facilitates binding to 14-3-3 proteins, blocks nuclear translocation, and prevents transcription of target genes that can lead to apoptosis (Maiese, 2015; Sanphui et al., 2020). Akt also blunts caspase activity that leads to the induction of apoptosis during inhibition of FoxO activity. If FoxO proteins are activated, such as FoxO3a, cytochrome c release can occur with caspase-induced apoptotic death (Hou et al., 2010b; Shang et al., 2010; Qi et al., 2013; Shi et al., 2016). Akt appears to have a secondary regulatory mechanism that oversees FoxO proteins to prevent caspase activity. FoxO3a cleavage by caspase 3 can lead to “pro-apoptotic” amino-terminal fragments. However, during blockade of caspase 3 activity by Akt activation, phosphorylated FoxO3a is not cleaved and does not result in apoptotic cell injury during oxidative stress (Hong et al., 2012).
Phosphorylation of FoxO proteins can occur by other pathways. The serum- and glucocorticoid-inducible protein kinase (SgK), a member of a family of kinases termed protein kinase A/protein kinase G/protein kinase C kinases that includes Akt, can phosphorylate FoxO3a for retention in the cytoplasm (Maiese, 2015). Other protein kinases that include mammalian sterile 20-like kinase-1 lead to the phosphorylation of FoxO proteins, release FoxOs from binding to 14-3-3 proteins, and result in the apoptotic death of neurons and astrocytes. In regards to targeting these pathways, it is important to realize that Akt and SgK use different protein sites to phosphorylate FoxOs, suggesting the development of different pathways to modulate FoxO activity (Maiese, 2015).
FoxO proteins are also controlled through post-translational modifications with ubiquitylation and acetylation (Xie et al., 2020). As a mechanism of control in this pathway, Akt results in the ubiquitination and degradation of FoxOs through the 26S proteasome. Acetylation of FoxO proteins represents another mechanism of oversight (Maiese, 2015; Beretta, 2019). FoxOs can be acetylated by histone acetyltransferases that include p300, the CREB-binding protein, and the CREB-binding protein-associated factor. After acetylation, nuclear translocation of FoxOs can follow but FoxO proteins have diminished activity. This loss of FoxO activity occurs as a result of the acetylation of lysine residues on FoxO proteins limiting the ability of FoxO proteins to bind to DNA (Matsuzaki et al., 2005). Furthermore, acetylation of FoxO proteins also promotes phosphorylation of FoxOs by Akt (Matsuzaki et al., 2005).
FoxO acetylation is controlled through SIRT1 (Figure 1). SIRT1 is a member of the sirtuin family (sirtuin 1) and is a histone deacetylase (Maiese, 2018; Cacabelos et al., 2019; Csicsar et al., 2019). SIRT1 controls DNA transcription by transfering acetyl groups from ε-N-acetyl lysine amino acids to the histones of DNA. Seven identified mammalian homologues of Sir2 include SIRT1 through SIRT7. These histone deacetylases control metabolism, proliferation and survival of cells, senescence, and post-translation modifications of proteins (Maiese, 2020; Pan et al., 2020; Tang et al., 2020). SIRT1 uses nicotinamide adenine dinucleotide as a substrate. FoxO proteins are deacetylated by SIRT1 and other histone deacetylases (Maiese, 2018; Cacabelos et al., 2019; Csicsar et al., 2019). For example, histone deacetylase 2 forms a physical complex with FoxO3a. During oxidative stress in cerebellar granule neurons, the interaction between histone deacetylase 2 and FoxO3a can become reduced and ultimately lead to neurodegeneration (Peng et al., 2015).
In reference to neurodegenerative disease, SIRT1 is an important target for disorders such as AD (Maiese, 2018). SIRT1 can decrease oxidative stress, protect neurons, and assist with preserving memory function (Maiese, 2020). With 17 beta-estradiol, SIRT1 can block memory impairment during oxidative stress in murine experimental models (Khan et al., 2019). SIRT1 may block neurofibrillary degeneration that occurs during dysregulation of tau exon 10 splicing (Qian et al., 2018). In other aspects, SIRT1 can maintain mitochondrial integrity in conjunction with other mechanisms in models of HD (Sayed et al., 2019).
SIRT1 activity leads to increased survival through inhibition of FoxO activity (Maiese, 2018). FoxOs also can bind to the SIRT1 promoter region to alter forkhead transcription. This promoter region has a cluster of five putative core binding repeat motifs and a forkhead-like consensus-binding site. As a result, FoxOs may function through potential autofeedback mechanisms to regulate SIRT1 activity. In conjunction with SIRT1, FoxO proteins are necessary for pre-implantation embryo development and control SIRT1 protein expression through autofeedback pathways (Kuscu et al., 2019). FoxO proteins, such as FoxO1, have been shown to regulate SIRT1 transcription and increase SIRT1 expression (Xiong et al., 2011). FoxOs and SIRT1 can function together and synergistically increase the survival of cells. In studies examining the protective effects of hydrogen-rich water to prevent Aβ toxicity in the brain, SIRT1 and FoxO3a were found to diminish Aβ injury that affected mitochondria and limit the toxicity of oxidative stress (Lin et al., 2015).
Interestingly, SIRT1 pathways are linked to the mTOR (Figure 1). mTOR is a 289 kDa serine/threonine protein kinase and the single gene FRAP1 encodes the protein (Maiese, 2020; Xu et al., 2020). mTOR also is described as the mammalian target of rapamycin and the FK506-binding protein 12-rapamycin complex-associated protein 1 (Maiese, 2018). mTOR serves as the principal component of the protein complexes mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2). Rapamycin blocks the activity of mTORC1 by binding to immunophilin FK-506-binding protein 12 that attaches to the FK-506-binding protein 12-rapamycin-binding domain (FRB) at the carboxy-terminal of mTOR to interfere with the FRB domain of mTORC1 (Maiese, 2018).
mTORC1 consists of Raptor, Deptor (DEP domain-containing mTOR interacting protein), the proline rich Akt substrate 40 kDa (PRAS40), and mammalian lethal with Sec13 protein 8, termed mLST8 (mLST8) (Maiese, 2018, 2020). mTOR can oversee Raptor activity which is inhibited by rapamycin. Deptor, an inhibitor, blocks mTORC1 activity by binding to the FAT domain (FK-506-binding protein 12-rapamycin-associated protein, ataxia-telangiectasia, and the transactivation/transformation domain-associated protein) of mTOR. PRAS40 prevents mTORC1 activity by blocking the association of p70 ribosomal S6 kinase and the eukaryotic initiation factor 4E-binding protein 1 with Raptor (Maiese, 2018, 2020). Akt also is active in this pathway since mTORC1 activity is increased once phosphorylation of PRAS40 occurs by Akt. This releases the binding of PRAS40 and Raptor to sequester PRAS40 in the cell cytoplasm with the docking protein 14-3-3 (Chong et al., 2012; Shang et al., 2012a; Wang et al., 2012). In contrast to Deptor and PRAS40, mLST8 fosters the activity of mTOR.
mTORC2 is composed of Rictor, Deptor, the mammalian stress-activated protein kinase interacting protein (mSIN1), mLST8, and the protein observed with Rictor-1 (Maiese, 2018, 2020). mTORC2 controls cytoskeleton remodeling through PKCα and cell migration through the Rac guanine nucleotide exchange factors P-Rex1 and P-Rex2 and through Rho signaling. mTORC2 promotes activity of protein kinases that includes glucocorticoid induced protein kinase 1, a member of the protein kinase A/protein kinase G/protein kinase C family of protein kinases. Rictor-1, a Rictor-binding subunit of mTORC2, leads to glucocorticoid induced protein kinase 1 activity. mSin1 is important for the assembly of mTORC2 and for mTORC2 to phosphorylate Akt (Frias et al., 2006). Rictor and mSIN1 phosphorylate Akt at serine473 and promote threonine308 phosphorylation by phosphoinositide-dependent kinase 1 to increase the survival of cells.
During neurodegenerative disease, mTOR activation can prevent neuronal loss during diabetic neuropathy (Dong et al., 2019) and limit ischemic stroke injury in conjunction with circadian clock genes (Maiese, 2017, 2020; Beker et al., 2018; Ramanathan et al., 2018; Angelousi et al., 2019). In addition, mTOR activation can block microglial injury from oxidative stress and prevent Aβ injury in neurons (Shang et al., 2012a, b; Cheng et al., 2018; Wang et al., 2020). With mTOR, vascular cell survival is increased (Park and Lee, 2017; Maiese, 2018) and neuroplasticity is promoted (Farmer et al., 2019).
SIRT1 maintains an inverse relationship with mTOR (Maiese, 2018). SIRT1 activity results in neurite outgrowth and increased neuronal survival during nutrient limiting conditions with the inhibition of mTOR (Guo et al., 2011). In some studies that examine cancer cell proliferation, SIRT1 can promote tumor cell growth with autophagy activity that requires mTOR inhibition, suggesting that both SIRT1 and autophagy pathways can be targets to control tumor cell growth (Mu et al., 2019). During oxidative stress, SIRT1 can promote autophagy induction and the inhibition of mTOR to prevent mitochondrial dysfunction in embryonic stem cells (Ou et al., 2014). During periods of hyperglycemia, SIRT1 blocks vascular cell demise during blockade of mTOR activity (Pal et al., 2019). Under other scenarios, blockade of mTOR with SIRT1 activation can increase cell survival for photoreceptor cells (Pan et al., 2020) and limit cell senescence (Zhang et al., 2019a). Yet, it should be recognized that under some conditions that may involve dopaminergic neuronal cell loss a balance in activities of SIRT1, mTOR, and forkhead transcription factors are required to achieve neuroprotection (Zhang et al., 2017a).
Autophagy and Apoptosis
Autophagy recycles cytoplasmic organelles and components for tissue remodeling (Maiese, 2018; Dorvash et al., 2020) and can remove non-functional organelles (Klionsky et al., 2016; Preau et al., 2019; Maiese, 2020) (Figure 1). There a several subdivisions of autophagy. Macroautophagy recycles organelles and sequesters cytoplasmic proteins into autophagosomes within cells. Autophagosomes subsequently combine with lysosomes to become degraded and begin a course for recycling (Maiese, 2018). Microautophagy is a process for lysosomal membrane invagination. As a result, components of the cell cytoplasm are sequestered and digested. Chaperone-mediated autophagy is a process that relies upon cytosolic chaperones to move components of the cytoplasm across lysosomal membranes.
In the nervous system, autophagy plays a significant role with neurodegenerative disorders. For example, as neural aggregates accumulate with aging in Drosophila, behavior is impaired and this is believed to be due to the loss of autophagic pathways that leads to toxic intracellular accumulations (Ratliff et al., 2015). Autophagy activation that can eliminate or sequester intracellular accumulations that are detrimental to cell survival also may influence disease progression in PD (Maiese, 2016; Fields et al., 2019; Zhang et al., 2019b; Zhou et al., 2019b; Corti et al., 2020; Tatullo et al., 2020), cognitive impairment and AD (Maiese, 2018; Hsieh et al., 2019; Zhang et al., 2019b; Zhou et al., 2019a), amyotrophic lateral sclerosis (Francois et al., 2014; Maiese, 2015; Sullivan et al., 2016), HD (Lee et al., 2015; Maiese, 2018), and traumatic brain injury (Maiese, 2016; Ye et al., 2017; Zhang et al., 2017b).
In contrast to autophagy, apoptosis has both an early and late phase (Maiese, 2018) (Figure 1). An early phase consists of phosphatidylserine (PS) asymmetry loss on the plasma membrane (Hou et al., 2010a; Shang et al., 2010; Taveira et al., 2018). The later phase results in genomic DNA degradation (Hou et al., 2011; Taveira et al., 2018). Apoptosis begins through a cascade of nuclease and protease activation that leads to caspase activation (Maiese, 2018; Bhowmick et al., 2019; Wu et al., 2020). This cascade can alter apoptosis with the early phase involving plasma membrane PS asymmetry and the later phase with genomic DNA degradation. Loss of cellular membrane PS asymmetry activates inflammatory cells to seek out cells with membrane asymmetry and remove them through engulfment (Hou et al., 2010b; Shang et al., 2010). If this process can be prevented, then cells remain functional despite externalization of membrane PS residues (Maiese, 2018; Taveira et al., 2018). However, the destruction of cellular DNA is usually not considered to be a reversible process (Maiese, 2018).
Apoptosis leads to cell death in multiple disease processes involving the nervous system. Suppression of cellular apoptosis can increase cell survival in AD (Maiese, 2016; Saleem and Biswas, 2017; Liang et al., 2018; Ullah et al., 2019), epilepsy (Maiese, 2016; El-Missiry et al., 2020; Yue et al., 2020), retinal disease (Almasieh et al., 2017; Tao et al., 2019), PD (Maiese, 2018; Ullah et al., 2019; Zhao et al., 2019), and spinal cord injury (Wang et al., 2017; Sun et al., 2020). Apoptotic injury also can lead to long-term disability through progressive neuronal loss such as during subarachnoid hemorrhage (Wang et al., 2019; Simon et al., 2019).
Forkhead Transcription Factors and Neurodegeneration
FoxOs are critical during neurodegenerative disorders and play a role in cognitive loss, vascular disease, behavior disorders, and neuronal injury (Maiese, 2015; Sangaletti et al., 2017; Sanphui et al., 2020). For example, during conditions that promote autophagy activation, such as with FoxO1 activation, basal autophagy is increased that can reduce atherogenesis (Maiese, 2015; Weikel et al., 2016). Ectopic expression of FoxO1 can promote autophagy and remove Huntingtin (mHtt) protein in neurons in models of HD (Vidal et al., 2012). Under some conditions, FoxO proteins interact with expanded polyglutamine tracts in coiled-coil structures in the cell nucleus that can lead to dendrite impairment and defects in behavior in models of Drosophila (Kwon et al., 2018). In addition, FoxO and SIRT1 activity are required with mTOR inhibition to promote autophagy activity in models with Drosophila and reduce neuronal accumulation of Aβ (Omata et al., 2014).
During periods of apoptosis, forkhead transcription factors such as FoxO3a can control p53 upregulated modulator of apoptosis and lead to dopaminergic neuronal cell degeneration (Sanphui et al., 2020). Inhibition of FoxO transcription factor activity can prevent microglial cell demise during reactive oxygen species release and Aβ exposure (Shang et al., 2012b), promote protective effects of metabotropic glutamate receptors (Maiese, 2015), and prevent neuronal injury through nicotinamide adenine dinucleotide precursors (Maiese, 2015). In addition, neurodevelopmental defects with infection of the Zika virus have been attributed to a rise in the expression of toll-like receptor 3 with increased forkhead transcription factor activity (Ojha et al., 2019).
Trophic factors, such as erythropoietin (EPO) (Govindappa et al., 2020; Liu et al., 2020b; Maiese, 2020), have been shown to be dependent upon FoxOs to prevent toxic injury to cells (Figure 1). EPO can offer neuroprotection in a number of models that are linked to cognitive loss and neuronal cell injury (Chang et al., 2018; Sun et al., 2019; Maiese, 2020). EPO can affect FoxO3a activity through post-translational phosphorylation and prevent FoxO3a translocation to the nucleus to block cellular apoptosis (Chong et al., 2011). EPO also can maintain the inhibitory phosphorylation and integrity of FOXO3a, foster FOXO3a and 14-3-3 protein binding, and regulate the intracellular trafficking of FOXO3a that is dependent upon SIRT1 (Chong and Maiese, 2007; Hou et al., 2011). EPO can reverse the acetylation of FOXO3a and FOXO1a during the deprivation of growth factors (Mahmud et al., 2002). Cellular protection with EPO is concentration dependent and may rely upon the ability to phosphorylate FoxO proteins which appears to be lost at elevated concentrations of EPO (Andreucci et al., 2009).
Forkhead transcription factors could offer a promising strategy to address neurodegenerative disorders, such as cognitive loss. In a number of scenarios, FoxO activation leads to neuronal injury and death. For example, nuclear retention of FoxO3a appears to significantly correlate with DNA damage in aged brains of individuals (Fluteau et al., 2015). Activation of DAF-16/FOXO transcription factor can negate the neuronal protective effects of knockdown of mitochondrial sirtuin (homologous to mammalian SIRT4) in Caenorhabditis elegans (Sangaletti et al., 2017). Exposure to Aβ leads to FoxO3a dephosphorylation and mitochondrial dysfunction (Shi et al., 2016). During Aβ exposure, elevated FoxO activity in combination with tribbles pseudokinase 3 leads to apoptotic and autophagic neuronal demise (Saleem and Biswas, 2017). In addition, calcineurin and FoxO3 activity triggered by Aβ exposure leads to pro-inflammatory cytokines activation and neuronal death (Fernandez et al., 2016). As a result, targeting FoxO pathways can offer new prospects to prevent neurodegenerative disease. Oxidative damage and increased neuronal death can be prevented during the inhibition and phosphorylation of FoxO3a. Retention of forkhead transcription factors in the cell cytoplasm during inhibitory phosphorylation has been attributed to the protective effects of anti-aging pathways (Zeldich et al., 2014) as well as enhanced cell survival during exposure to oxidative stress and Aβ toxicity (Hong et al., 2012; Shang et al., 2012b). Yet, as previously described, activation of FoxOs also can be beneficial especially with the induction of autophagy and the clearance on toxic intracellular accumulations such as mHtt the reduction of neuronal Aβ accumulation. Some work has suggested that during AD, mechanisms that are involved in the maintenance of neuronal function that rely upon FoxOs become impaired and neuronal Aβ levels rise as failed attempts to prevent neuronal injury (Shafi, 2016), which is a conceivable hypothesis given that FOXO3a can control cell progression and migration in other systems of the body (Yang et al., 2020). Oversight of the level of FoxO activity appears crucial to achieve eventual clinical directives and this fine modulation of FoxO activity must be coordinated with the pathways of SIRT1 and mTOR.
Future Considerations
Throughout the globe, lifespan is increasing. This is especially evident in large developing countries such that the number of people of advanced age is expected to rise from five to ten percent over the next several decades. Yet, of significant concern is the increase in NCDs and in particular neurodegenerative disorders that impact more than one billion individuals throughout the world. Approximately fifteen percent of the people across the globe are impacted by neurological disorders and almost seven million die each year from neurodegenerative disorders. In addition, severe financial burdens are placed on countries that consume significant portions of the Gross Domestic Product to care for individuals with neurodegenerative disorders.
Current strategies to treat neurodegenerative disorders are limited and in most cases these treatments do not address the underlying mechanisms of these disorders. For these reasons, FoxOs, SIRT1, mTOR, autophagy, and apoptosis represent interesting targets that have the potential to focus upon core mechanisms of neurodegenerative disorder.
| Targeting the Core of Neurodegeneration: FoxO, mTOR, and SIRT1 |
|---|
| • The global increase in lifespan pargroupels a rise in non-communicable diseases (NCDs) with a significant increase in neurodegenerative disorders that by the year 2030, the medical and social services required to cognitive disorders in the United States (US) is expected to exceed 2 trillion US dollars annugroupy. |
| • Overgroup, for many neurodegenerative disorders, treatment options are limited. Multiple mechanisms can lead to cellular injury that include β-amyloid (Aβ), tau, excitotoxicity, mitochondrial damage, acetylcholine loss, astrocytic cell injury, oxidative stress, and cellular metabolic dysfunction with diabetes mellitus. |
| • An exciting avenue to target neurodegenerative disorders consists of the pathways of mammalian forkhead transcription factors (FoxOs), the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), the mechanistic target of rapamycin (mTOR), and the programmed cell death pathways of autophagy and apoptosis. |
| • FoxOs in conjunction with SIRT1 and mTOR can modulate autophagy activation and offer beneficial outcomes such as to reduce atherogenesis, limit Aβ cellular injury, and assist with clearance of toxic Huntingtin (mHtt) protein. Yet, under other conditions, FoxOs can be mediators of cellular apoptosis and result in dopaminergic neuronal cell degeneration, promote Aβ toxicity, and lead to neurodevelopmental defects. |
| • Given these chgroupenges, the development of FoxOs as therapeutic agents must consider the fine modulation of FoxOs through epigenetic and post-translation protein modifications and the intimate relationship with SIRT1 and mTOR as well as the pathways of protein kinase b (Akt) and autophagy that can involve autofeedback pathways to achieve the proper balance of FoxO activity for the development of successful clinical treatments for neurodegenerative disorders that are without unwanted toxic effects. |
FoxOs can modulate autophagy activation and be beneficial to reduce atherogenesis, limit Aβ accumulation, and assist with clearance of mHtt protein. Yet, FoxOs also can precipitate apoptotic neuronal cell death. FoxOs can be mediators of cellular apoptosis and result in dopaminergic neuronal cell degeneration, promote Aβ toxicity, and lead to neurodevelopmental defects. Growth factors, such as EPO, maintain cellular function by maintaining inhibitory phosphorylation of FoxOs and by regulating the intracellular trafficking of FoxOs. As a result, FoxOs present a number of challenges for the translation of these agents into effective and safe clinical care strategies for disorders of the nervous system.
In this regard, the targeting of FoxOs as therapeutic agents must consider the fine modulation of these agents through epigenetic and post-translation protein modifications to achieve the proper balance of FoxO activity for effective clinical care that limits unwanted toxic effects (Figure 1). In addition to the pathways associated with phosphorylation, acetylation, and ubiquitylation that rely upon Akt to modulate FoxO activity, both SIRT1 and mTOR also offer robust prospects to control FoxO activity that is tied to both autophagy and apoptosis as well as the cell signaling pathway of Akt. For example, FoxO proteins can function with SIRT1 through a number of pathways. SIRT1 activity leads to increased survival through inhibition of FoxO activity through deacetylation. However, FoxOs also may function through autofeedback mechanisms that can control SIRT1 activity since FoxO proteins have been shown to oversee the transcription and expression of SIRT1. SIRT1 maintains a close but inverse relationship with mTOR as well. For example, SIRT1 can promote neurite outgrowth and increase neuronal survival during nutrient limiting conditions by limiting mTOR activity. In conjunction with forkhead transcription factors, SIRT1 can be beneficial by decreasing mTOR activity and promote autophagy activity to reduce neuronal accumulation of Aβ. Yet, these pathways are intimately dependent upon one another and may require a careful balance to achieve a desired clinical outcome. For example, under conditions that involves dopaminergic neurons a balance in activities of SIRT1, mTOR, and FoxOs is required to achieve neuroprotection. Furthermore, under some conditions, SIRT1 and FoxO3a can function synergistically such as to diminish Aβ toxicity in mitochondria and limit injury from oxidative stress. Phosphorylation of FoxOs also can be influenced by the exposure to trophic factors, such as EPO, to enhance neuronal survival and protection. In addition, autophagy activation may not be consistently beneficial in the nervous system and can lead to injury in endothelial progenitor cells, dysfunction of mitochondria during oxidative stress, and prevent angiogenesis (Maiese, 2018), suggesting that mTOR activation rather than inhibition may be vital at times to maintain nervous system function. With this knowledge at hand, it is evident that future studies are necessary to provide vital guidance for the targeting and modulation of FoxOs, SIRT1, and mTOR in the nervous system that can effectively target neurodegenerative disorders for novel strategies to limit and resolve disease onset and progression.
Footnotes
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
Conflicts of interest: The author declares no conflicts of interest.
Financial support: This work was supported by American Diabetes Association, American Heart Association, National Institutes of Health - National Institute of Environmental Health Sciences, National Institutes of Health - National Institute on Aging, National Institutes of Health - National Institute of Neurological Disorders, and National Institutes of Health - American Recovery and Reinvestment (to KM).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by American Diabetes Association, American Heart Association, National Institutes of Health - National Institute of Environmental Health Sciences, National Institutes of Health - National Institute on Aging, National Institutes of Health - National Institute of Neurological Disorders, and National Institutes of Health - American Recovery and Reinvestment (to KM).
References
- 1.Almasieh M, Catrinescu MM, Binan L, Costantino S, Levin LA. Axonal degeneration in retinal ganglion cells is associated with a membrane polarity-sensitive redox process. J Neurosci. 2017;37:3824–3839. doi: 10.1523/JNEUROSCI.3882-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreucci M, Fuiano G, Presta P, Lucisano G, Leone F, Fuiano L, Bisesti V, Esposito P, Russo D, Memoli B, Faga T, Michael A. Downregulation of cell survival signalling pathways and increased cell damage in hydrogen peroxide-treated human renal proximal tubular cells by alpha-erythropoietin. Cell Prolif. 2009;42:554–561. doi: 10.1111/j.1365-2184.2009.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelousi A, Kassi E, Ansari-Nasiri N, Randeva H, Kaltsas G, Chrousos G. Clock genes and cancer development in particular in endocrine tissues. Endocr Relat Cancer. 2019;26:R305–R317. doi: 10.1530/ERC-19-0094. [DOI] [PubMed] [Google Scholar]
- 4.Beker MC, Caglayan B, Yalcin E, Caglayan AB, Turkseven S, Gurel B, Kelestemur T, Sertel E, Sahin Z, Kutlu S, Kilic U, Baykal AT, Kilic E. Time-of-day dependent neuronal injury after ischemic stroke: implication of circadian clock transcriptional factor Bmal1 and survival kinase AKT. Mol Neurobiol. 2018;55:2565–2576. doi: 10.1007/s12035-017-0524-4. [DOI] [PubMed] [Google Scholar]
- 5.Beretta GL, Corno C, Zaffaroni N, Perego P. Role of FoxO proteins in cellular response to antitumor agents. Cancers (Basel) 2019 doi: 10.3390/cancers11010090. doi:103390/cancers11010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhowmick S, D’Mello V, Caruso D, Abdul-Muneer PM. Traumatic brain injury-induced downregulation of Nrf2 activates inflammatory response and apoptotic cell death. J Mol Med (Berl) 2019;97:1627–1641. doi: 10.1007/s00109-019-01851-4. [DOI] [PubMed] [Google Scholar]
- 7.Caberlotto L, Nguyen TP, Lauria M, Priami C, Rimondini R, Maioli S, Cedazo-Minguez A, Sita G, Morroni F, Corsi M, Carboni L. Cross-disease analysis of Alzheimer’s disease and type-2 Diabetes highlights the role of autophagy in the pathophysiology of two highly comorbid diseases. Sci Rep. 2019;9:3965. doi: 10.1038/s41598-019-39828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cacabelos R, Carril JC, Cacabelos N, Kazantsev AG, Vostrov AV, Corzo L, Cacabelos P, Goldgaber D. Sirtuins in Alzheimer’s disease: sirt2-related genoPhenotypes and implications for pharmacoEpigenetics. Int J Mol Sci. 2019 doi: 10.3390/ijms20051249. doi:103390/ijms20051249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai H, Li Y, Niringiyumukiza JD, Su P, Xiang W. Circular RNA involvement in aging: An emerging player with great potential. Mech Ageing Dev. 2019;178:16–24. doi: 10.1016/j.mad.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. CS. 2020;314227-A:1–30. [Google Scholar]
- 11.Chang R, Maghribi AA, Vanderpoel V, Vasilevko V, Cribbs DH, Boado R, Pardridge WM, Sumbria RK. A brain penetrating bifunctional erythropoietin-transferrin receptor antibody fusion protein for Alzheimer’s disease. Mol Pharm. 2018;17:360. doi: 10.1021/acs.molpharmaceut.9b01211. [DOI] [PubMed] [Google Scholar]
- 12.Cheng J, North BJ, Zhang T, Dai X, Tao K, Guo J, Wei W. The emerging roles of protein homeostasis-governing pathways in Alzheimer’s disease. Aging Cell. 2018;17:e12801. doi: 10.1111/acel.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO relies upon novel signaling of Wnt1 that requires Akt1, FoxO3a, GSK-3β, and β-catenin to foster vascular integrity during experimental diabetes. Curr Neurovasc Res. 2011;8:103–120. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007;150:839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong ZZ, Shang YC, Wang S, Maiese K. PRAS40 is an integral regulatory component of erythropoietin mTOR signaling and cytoprotection. PLoS One. 2012;7:e45456. doi: 10.1371/journal.pone.0045456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364:412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 17.Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, Parikh CR. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med. 2012;172:761–769. doi: 10.1001/archinternmed.2011.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corti O, Blomgren K, Poletti A, Beart PM. Autophagy in neurodegeneration: New insights underpinning therapy for neurological diseases. J Neurochem. 2020 doi: 10.1111/jnc.15002. doi:101111/jnc15002. [DOI] [PubMed] [Google Scholar]
- 19.Csicsar A, Tarantini S, Yabluchanskiy A, Balasubramanian P, Kiss T, Farkas E, Baur JA, Ungvari ZI. Role of endothelial NAD+ deficiency in age-related vascular dysfunction. Am J Physiol Heart Circ Physiol. 2019;316:H1253–H1266. doi: 10.1152/ajpheart.00039.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Vecchis D, Brandner A, Baaden M, Cohen MM, Taly A. A molecular perspective on mitochondrial nembrane fusion: from the key players to oligomerization and tethering of mitofusin. J Membr Biol. 2019;252:293–306. doi: 10.1007/s00232-019-00089-y. [DOI] [PubMed] [Google Scholar]
- 21.Ding S, Zhu Y, Liang Y, Huang H, Xu Y, Zhong C. Circular RNAs in vascular functions and diseases. Adv Exp Med Biol. 2018;1087:287–297. doi: 10.1007/978-981-13-1426-1_23. [DOI] [PubMed] [Google Scholar]
- 22.Dong J, Li H, Bai Y, Wu C. Muscone ameliorates diabetic peripheral neuropathy through activating AKT/mTOR signalling pathway. J Pharm Pharmacol. 2019;71:1706–1713. doi: 10.1111/jphp.13157. [DOI] [PubMed] [Google Scholar]
- 23.Dorvash M, Farahmandnia M, Tavassoly I. A systems biology roadmap to decode mTOR control system in cancer. Interdiscip Sci. 2020;12:1–11. doi: 10.1007/s12539-019-00347-6. [DOI] [PubMed] [Google Scholar]
- 24.El-Missiry MA, Othman AI, Amer MA, Sedki M, Ali SM, El-Sherbiny IM. Nanoformulated ellagic acid ameliorates pentylenetetrazol-induced experimental epileptic seizures by modulating oxidative stress, inflammatory cytokines and apoptosis in the brains of male mice. Metab Brain Dis. 2020;35:385–399. doi: 10.1007/s11011-019-00502-4. [DOI] [PubMed] [Google Scholar]
- 25.Fan X, Zhao Z, Wang D, Xiao J. Glycogen synthase kinase-3 as a key regulator of cognitive function. Acta Biochim Biophys Sin (Shanghai) 2020;52:219–230. doi: 10.1093/abbs/gmz156. [DOI] [PubMed] [Google Scholar]
- 26.Farmer K, Abd-Elrahman KS, Derksen A, Rowe EM, Thompson AM, Rudyk CA, Prowse NA, Dwyer Z, Bureau SC, Fortin T, Ferguson SSG, Hayley S. mGluR5 allosteric modulation promotes neurorecovery in a 6-OHDA-toxicant model of Parkinson’s disease. Mol Neurobiol. 2019;57:1418–1431. doi: 10.1007/s12035-019-01818-z. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez AM, Hervas R, Dominguez-Fraile M, Garrido VN, Gomez-Gutierrez P, Vega M, Vitorica J, Perez JJ, Torres Aleman I. Blockade of the interaction of calcineurin with FOXO in astrocytes protects against amyloid-beta-induced neuronal death. J Alzheimers Dis. 2016;52:1471–1478. doi: 10.3233/JAD-160149. [DOI] [PubMed] [Google Scholar]
- 28.Fields CR, Bengoa-Vergniory N, Wade-Martins R. Targeting alpha-synuclein as a therapy for Parkinson’s disease. Front Mol Neurosci. 2019;12:299. doi: 10.3389/fnmol.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fluteau A, Ince PG, Minett T, Matthews FE, Brayne C, Garwood CJ, Ratcliffe LE, Morgan S, Heath PR, Shaw PJ, Wharton SB, Simpson JE. The nuclear retention of transcription factor FOXO3a correlates with a DNA damage response and increased glutamine synthetase expression by astrocytes suggesting a neuroprotective role in the ageing brain. Neurosci Lett. 2015;609:11–17. doi: 10.1016/j.neulet.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francois A, Terro F, Quellard N, Fernandez B, Chassaing D, Janet T, Rioux-Bilan A, Paccalin M, Page G. Impairment of autophagy in the central nervous system during lipopolysaccharide-induced inflammatory stress in mice. Mol Brain. 2014;7:56. doi: 10.1186/s13041-014-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Govindappa PK, Talukder MAH, Gurjar AA, Hegarty JP, Elfar JC. An effective erythropoietin dose regimen protects against severe nerve injury-induced pathophysiological changes with improved neural gene expression and enhances functional recovery. Int Immunopharmacol. 2020;82:106330. doi: 10.1016/j.intimp.2020.106330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo W, Qian L, Zhang J, Zhang W, Morrison A, Hayes P, Wilson S, Chen T, Zhao J. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J Neurosci Res. 2011;89:1723–1736. doi: 10.1002/jnr.22725. [DOI] [PubMed] [Google Scholar]
- 34.Hong YK, Lee S, Park SH, Lee JH, Han SY, Kim ST, Kim YK, Jeon S, Koo BS, Cho KS. Inhibition of JNK/dFOXO pathway and caspases rescues neurological impairments in Drosophila Alzheimer’s disease model. Biochem Biophys Res Commun. 2012;419:49–53. doi: 10.1016/j.bbrc.2012.01.122. [DOI] [PubMed] [Google Scholar]
- 35.Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010a;7:95–112. doi: 10.2174/156720210791184899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou J, Chong ZZ, Shang YC, Maiese K. FoxO3a governs early and late apoptotic endothelial programs during elevated glucose through mitochondrial and caspase signaling. Mol Cell Endocrinol. 2010b;321:194–206. doi: 10.1016/j.mce.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou J, Wang S, Shang YC, Chong ZZ, Maiese K. Erythropoietin employs cell longevity pathways of SIRT1 to foster endothelial vascular integrity during oxidant stress. Curr Neurovasc Res. 2011;8:220–235. doi: 10.2174/156720211796558069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsieh CF, Liu CK, Lee CT, Yu LE, Wang JY. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci Rep. 2019;9:840. doi: 10.1038/s41598-018-37215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Z, Jiao R, Wang P, Zhu Y, Zhao J, De Jager P, Bennett DA, Jin L, Xiong M. Shared causal paths underlying Alzheimer’s dementia and type 2 diabetes. Sci Rep. 2020;10:4107. doi: 10.1038/s41598-020-60682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan M, Ullah R, Rehman SU, Shah SA, Saeed K, Muhammad T, Park HY, Jo MH, Choe K, Rutten BPF, Kim MO. 17beta-Estradiol modulates SIRT1 and halts oxidative stress-mediated cognitive impairment in a male aging mouse model. Cells. 2019 doi: 10.3390/cells8080928. doi:103390/cells8080928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G, Agarwal R, Aghi MK, Agnello M, Agostinis P, Aguilar PV, Aguirre-Ghiso J, Airoldi EM, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kotrys AV, Szczesny RJ. Mitochondrial gene expression and beyond-novel aspects of cellular physiology. Cells. 2019 doi: 10.3390/cells9010017. doi:103390/cells9010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuscu N, Gungor-Ordueri NE, Sozen B, Adiguzel D, Celik-Ozenci C. FoxO transcription factors 1 regulate mouse preimplantation embryo development. J Assist Reprod Genet. 2019;36:2121–2133. doi: 10.1007/s10815-019-01555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwon MJ, Han MH, Bagley JA, Hyeon DY, Ko BS, Lee YM, Cha IJ, Kim SY, Kim DY, Kim HM, Hwang D, Lee SB, Jan YN. Coiled-coil structure-dependent interactions between polyQ proteins and Foxo lead to dendrite pathology and behavioral defects. Proc Natl Acad Sci U S A. 2018;115:E10748–E10757. doi: 10.1073/pnas.1807206115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JH, Tecedor L, Chen YH, Monteys AM, Sowada MJ, Thompson LM, Davidson BL. Reinstating aberrant mTORC1 activity in Huntington’s disease mice improves disease phenotypes. Neuron. 2015;85:303–315. doi: 10.1016/j.neuron.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JH, Lee JH, Jin M, Han SD, Chon GR, Kim IH, Kim S, Kim SY, Choi SB, Noh YH. Diet control to achieve euglycemia induces significant loss of heart and liver weight via increased autophagy compared with ad libitum diet in diabetic rats. Exp Mol Med. 2014;46:e111. doi: 10.1038/emm.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang CJ, Li JH, Zhang Z, Zhang JY, Liu SQ, Yang J. Suppression of MIF-induced neuronal apoptosis may underlie the therapeutic effects of effective components of Fufang Danshen in the treatment of Alzheimer’s disease. Acta Pharmacol Sin. 2018;39:1421–1438. doi: 10.1038/aps.2017.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin CL, Huang WN, Li HH, Huang CN, Hsieh S, Lai C, Lu FJ. Hydrogen-rich water attenuates amyloid beta-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem Biol Interact. 2015;240:12–21. doi: 10.1016/j.cbi.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Liu W, Li Y, Luo B. Current perspective on the regulation of FOXO4 and its role in disease progression. Cell Mol Life Sci. 2020a;77:651–663. doi: 10.1007/s00018-019-03297-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu W, Varier KM, Sample KM, Zacksenhaus E, Gajendran B, Ben-David Y. Erythropoietin signaling in the microenvironment of tumors and healthy tissues. Adv Exp Med Biol. 2020b;1223:17–30. doi: 10.1007/978-3-030-35582-1_2. [DOI] [PubMed] [Google Scholar]
- 51.Mahmud DL, M GA, Deb DK, Platanias LC, Uddin S, Wickrema A. Phosphorylation of forkhead transcription factors by erythropoietin and stem cell factor prevents acetylation and their interaction with coactivator p300 in erythroid progenitor cells. Oncogene. 2002;21:1556–1562. doi: 10.1038/sj.onc.1205230. [DOI] [PubMed] [Google Scholar]
- 52.Maiese K. FoxO proteins in the nervous system. Anal Cell Pathol (Amst) 2015;2015:569392. doi: 10.1155/2015/569392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maiese K. Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br J Clin Pharmacol. 2016;82:1245–1266. doi: 10.1111/bcp.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maiese K. Moving to the rhythm with clock (Circadian) genes, autophagy, mTOR, and SIRT1 in degenerative disease and cancer. Curr Neurovasc Res. 2017;14:299–304. doi: 10.2174/1567202614666170718092010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maiese K. The mechanistic target of rapamycin (mTOR) and the silent mating-type information regulation 2 homolog 1 (SIRT1): oversight for neurodegenerative disorders. Biochem Soc Trans. 2018;46:351–360. doi: 10.1042/BST20170121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maiese K. Impacting dementia and cognitive loss with innovative strategies: mechanistic target of rapamycin, clock genes, circular non-coding ribonucleic acids, and Rho/Rock. Neural Regen Res. 2019;14:773–774. doi: 10.4103/1673-5374.249224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maiese K. Cognitive impairment with diabetes mellitus and metabolic disease: innovative insights with the mechanistic target of rapamycin and circadian clock gene pathways. Expert Rev Clin Pharmacol. 2020;13:23–34. doi: 10.1080/17512433.2020.1698288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci U S A. 2005;102:11278–11283. doi: 10.1073/pnas.0502738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehta J, Rayalam S, Wang X. Cytoprotective effects of natural compounds against oxidative stress. Antioxidants (Basel) 2018 doi: 10.3390/antiox7100147. doi:103390/antiox7100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mladenovic Djordjevic A, Loncarevic-Vasiljkovic N, Gonos ES. Dietary restriction and oxidative stress: friends or enemies ? Antioxid Redox Signal. 2020 doi: 10.1089/ars.2019.7959. doi:101089/ars20197959. [DOI] [PubMed] [Google Scholar]
- 61.Mu N, Lei Y, Wang Y, Wang Y, Duan Q, Ma G, Liu X, Su L. Inhibition of SIRT1/2 upregulates HSPA5 acetylation and induces pro-survival autophagy via ATF4-DDIT4-mTORC1 axis in human lung cancer cells. Apoptosis. 2019;24:798–811. doi: 10.1007/s10495-019-01559-3. [DOI] [PubMed] [Google Scholar]
- 62.Ojha CR, Rodriguez M, Karuppan MKM, Lapierre J, Kashanchi F, El-Hage N. Toll-like receptor 3 regulates Zika virus infection and associated host inflammatory response in primary human astrocytes. PLoS One. 2019;14:e0208543. doi: 10.1371/journal.pone.0208543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Omata Y, Lim YM, Akao Y, Tsuda L. Age-induced reduction of autophagy-related gene expression is associated with onset of Alzheimer’s disease. Am J Neurodegener Dis. 2014;3:134–142. [PMC free article] [PubMed] [Google Scholar]
- 64.Ou X, Lee MR, Huang X, Messina-Graham S, Broxmeyer HE. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells. 2014;32:1183–1194. doi: 10.1002/stem.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pal PB, Sonowal H, Shukla K, Srivastava SK, Ramana KV. Aldose reductase regulates hyperglycemia-induced HUVEC death via SIRT1/AMPK-alpha1/mTOR pathway. J Mol Endocrinol. 2019;63:11–25. doi: 10.1530/JME-19-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pan YR, Song JY, Fan B, Wang Y, Che L, Zhang SM, Chang YX, He C, Li GY. mTOR may interact with PARP-1 to regulate visible light-induced parthanatos in photoreceptors. Cell Commun Signal. 2020;18:27. doi: 10.1186/s12964-019-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park JA, Lee CH. Temporal changes in mammalian target of rapamycin (mTOR) and phosphorylated-mTOR expressions in the hippocampal CA1 region of rat with vascular dementia. J Vet Sci. 2017;18:11–16. doi: 10.4142/jvs.2017.18.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng S, Li W, Hou N, Huang N. A review of FoxO1-regulated metabolic diseases and related drug discoveries. Cells. 2020 doi: 10.3390/cells9010184. doi:103390/cells9010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng S, Zhao S, Yan F, Cheng J, Huang L, Chen H, Liu Q, Ji X, Yuan Z. HDAC2 selectively regulates FOXO3a-mediated gene transcription during oxidative stress-induced neuronal cell death. J Neurosci. 2015;35:1250–1259. doi: 10.1523/JNEUROSCI.2444-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Preau S, Ambler M, Sigurta A, Kleyman A, Dyson A, Hill NE, Boulanger E, Singer M. Protein recycling and limb muscle recovery after critical illness in slow- and fast-twitch limb muscle. Am J Physiol Regul Integr Comp Physiol. 2019;316:R584–R593. doi: 10.1152/ajpregu.00221.2018. [DOI] [PubMed] [Google Scholar]
- 71.Prokopenko D, Hecker J, Kirchner R, Chapman BA, Hoffman O, Mullin K, Hide W, Bertram L, Laird N, DeMeo DL, Lange C, Tanzi RE. Identification of novel Alzheimer’s disease loci using sex-specific family-based association analysis of whole-genome sequence data. Sci Rep. 2020;10:5029. doi: 10.1038/s41598-020-61883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qi XF, Li YJ, Chen ZY, Kim SK, Lee KJ, Cai DQ. Involvement of the FoxO3a pathway in the ischemia/reperfusion injury of cardiac microvascular endothelial cells. Exp Mol Pathol. 2013;95:242–247. doi: 10.1016/j.yexmp.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Qian S, Gu J, Dai W, Jin N, Chu D, Huang Q, Liu F, Qian W. Sirt1 enhances tau exon 10 inclusion and improves spatial memory of Htau mice. Aging (Albany NY) 2018;10:2498–2510. doi: 10.18632/aging.101564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramanathan C, Kathale ND, Liu D, Lee C, Freeman DA, Hogenesch JB, Cao R, Liu AC. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet. 2018;14:e1007369. doi: 10.1371/journal.pgen.1007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ratliff EP, Mauntz RE, Kotzebue RW, Gonzalez A, Achal M, Barekat A, Finley KA, Sparhawk JM, Robinson JE, Herr DR, Harris GL, Joiner WJ, Finley KD. Aging and autophagic function influences the progressive decline of adult Drosophila behaviors. PLoS One. 2015;10:e0132768. doi: 10.1371/journal.pone.0132768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ruhal P, Dhingra D. Inosine improves cognitive function and decreases aging-induced oxidative stress and neuroinflammation in aged female rats. Inflammopharmacology. 2018;26:1317–1329. doi: 10.1007/s10787-018-0476-y. [DOI] [PubMed] [Google Scholar]
- 77.Saleem S, Biswas SC. Tribbles pseudokinase 3 induces both apoptosis and autophagy in amyloid-beta-induced neuronal death. J Biol Chem. 2017;292:2571–2585. doi: 10.1074/jbc.M116.744730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sangaletti R, D’Amico M, Grant J, Della-Morte D, Bianchi L. Knock-out of a mitochondrial sirtuin protects neurons from degeneration in Caenorhabditis elegans. PLoS Genet. 2017;13:e1006965. doi: 10.1371/journal.pgen.1006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanphui P, Das AK, Biswas SC. FoxO3a requires BAF57, a subunit of chromatin remodeler SWI/SNF complex for induction of PUMA in a model of Parkinson’s disease. J Neurochem. 2020 doi: 10.1111/jnc.14969. doi: 101111/jnc14969. [DOI] [PubMed] [Google Scholar]
- 80.Sayed NH, Fathy N, Kortam MA, Rabie MA, Mohamed AF, Kamel AS. Vildagliptin attenuates Huntington’s disease through activation of GLP-1 receptor/PI3K/Akt/BDNF pathway in 3-nitropropionic acid rat model. Neurotherapeutics. 2019;17:252–268. doi: 10.1007/s13311-019-00805-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schiano C, Benincasa G, Franzese M, Della Mura N, Pane K, Salvatore M, Napoli C. Epigenetic-sensitive pathways in personalized therapy of major cardiovascular diseases. Pharmacol Ther. 2020 doi: 10.1016/j.pharmthera.2020.107514. doi:101016/jpharmthera2020107514. [DOI] [PubMed] [Google Scholar]
- 82.Scodelaro Bilbao P, Boland R. Extracellular ATP regulates FoxO family of transcription factors and cell cycle progression through PI3K/Akt in MCF-7 cells. Biochim Biophys Acta. 2013;1830:4456–4469. doi: 10.1016/j.bbagen.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 83.Shafi O. Inverse relationship between Alzheimer’s disease and cancer, and other factors contributing to Alzheimer’s disease: a systematic review. BMC Neuro. 2016;l16:236. doi: 10.1186/s12883-016-0765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shang YC, Chong ZZ, Hou J, Maiese K. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal. 2010;22:1317–1329. doi: 10.1016/j.cellsig.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shang YC, Chong ZZ, Wang S, Maiese K. WNT1 inducible signaling pathway protein 1 (WISP1) targets PRAS40 to govern beta-amyloid apoptotic injury of microglia. Curr Neurovasc Res. 2012a;9:239–249. doi: 10.2174/156720212803530618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY) 2012b;4:187–201. doi: 10.18632/aging.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi C, Zhu J, Leng S, Long D, Luo X. Mitochondrial FOXO3a is involved in amyloid beta peptide-induced mitochondrial dysfunction. J Bioenerg Biomembr. 2016;48:189–196. doi: 10.1007/s10863-016-9645-0. [DOI] [PubMed] [Google Scholar]
- 88.Simon F, Floros N, Ibing W, Schelzig H, Knapsis A. Neurotherapeutic potential of erythropoietin after ischemic injury of the central nervous system. Neural Regen Res. 2019;14:1309–1312. doi: 10.4103/1673-5374.253507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stefanatos R, Sanz A. The role of mitochondrial ROS in the aging brain. FEBS Lett. 2018;592:743–758. doi: 10.1002/1873-3468.12902. [DOI] [PubMed] [Google Scholar]
- 90.Sullivan PM, Zhou X, Robins AM, Paushter DH, Kim D, Smolka MB, Hu F. The ALS/FTLD associated protein C9orf72 associates with SMCR8 and WDR41 to regulate the autophagy-lysosome pathway. Acta Neuropathol Commun. 2016;4:51. doi: 10.1186/s40478-016-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun F, Li SG, Zhang HW, Hua FW, Sun GZ, Huang Z. MiRNA-411 attenuates inflammatory damage and apoptosis following spinal cord injury. Eur Rev Med Pharmacol Sci. 2020;24:491–498. doi: 10.26355/eurrev_202001_20022. [DOI] [PubMed] [Google Scholar]
- 92.Sun J, Martin JM, Vanderpoel V, Sumbria RK. The promises and challenges of erythropoietin for treatment of Alzheimer’s disease. Neuromolecular Med. 2019;21:12–24. doi: 10.1007/s12017-019-08524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang YL, Zhang CG, Liu H, Zhou Y, Wang YP, Li Y, Han YJ, Wang CL. Ginsenoside Rg1 inhibits cell proliferation and induces markers of cell senescence in CD34+CD38- leukemia stem cells derived from KG1alpha acute myeloid leukemia cells by activating the sirtuin 1 (SIRT1)/tuberous sclerosis complex 2 (TSC2) signaling pathway. Med Sci Monit. 2020;26:e918207. doi: 10.12659/MSM.918207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tao Y, Li C, Yao A, Qu Y, Qin L, Xiong Z, Zhang J, Wang W. Intranasal administration of erythropoietin rescues the photoreceptors in degenerative retina: a noninvasive method to deliver drugs to the eye. Drug Deliv. 2019;26:78–88. doi: 10.1080/10717544.2018.1556361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tatullo M, Marrelli B, Zullo MJ, Codispoti B, Paduano F, Benincasa C, Fortunato F, Scacco S, Zavan B, Cocco T. Exosomes from human periapical Cyst-MSCs: theranostic application in Parkinson’s disease. Int J Med Sci. 2020;17:657–663. doi: 10.7150/ijms.41515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taveira GB, Mello EO, Souza SB, Monteiro RM, Ramos AC, Carvalho AO, Rodrigues R, Okorokov LA, Gomes VM. Programmed cell death in yeast by thionin-like peptide from Capsicum annuum fruits involving activation of capases and extracelullar H(+) flux. Biosci Rep. 2018 doi: 10.1042/BSR20180119. doi:101042/BSR20180119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsai KL, Sun YJ, Huang CY, Yang JY, Hung MC, Hsiao CD. Crystal structure of the human FOXO3a-DBD/DNA complex suggests the effects of post-translational modification. Nucleic Acids Res. 2007;35:6984–6994. doi: 10.1093/nar/gkm703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ullah R, Khan M, Shah SA, Saeed K, Kim MO. Natural antioxidant anthocyanins-A hidden therapeutic candidate in metabolic disorders with major focus in neurodegeneration. Nutrients. 2019 doi: 10.3390/nu11061195. doi:103390/nu11061195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vidal RL, Figueroa A, Court FA, Thielen P, Molina C, Wirth C, Caballero B, Kiffin R, Segura-Aguilar J, Cuervo AM, Glimcher LH, Hetz C. Targeting the UPR transcription factor XBP1 protects against Huntington’s disease through the regulation of FoxO1 and autophagy. Hum Mol Genet. 2012;21:2245–2262. doi: 10.1093/hmg/dds040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang F, Cao Y, Ma L, Pei H, Rausch WD, Li H. Dysfunction of cerebrovascular endothelial cells: prelude to vascular dementia. Front Aging Neurosci. 2018;10:376. doi: 10.3389/fnagi.2018.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang H, Li Q, Sun S, Chen S. Neuroprotective effects of salidroside in a mouse model of Alzheimer’s disease. Cell Mol Neurobiol. 2020 doi: 10.1007/s10571-020-00801-w. doi:101007/s10571-020-00801-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang H, Zhang Q, Wen Q, Zheng Y, Philip L, Jiang H, Lin J, Zheng W. Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell Signal. 2012;24:17–24. doi: 10.1016/j.cellsig.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 103.Wang W, Han P, Xie R, Yang M, Zhang C, Mi Q, Sun B, Zhang ZY. TAT-mGluR1 attenuates neuronal apoptosis through preventing MGluR1alpha truncation after experimental subarachnoid hemorrhage. ACS Chem Neurosci. 2019;10:746–756. doi: 10.1021/acschemneuro.8b00531. [DOI] [PubMed] [Google Scholar]
- 104.Wang Z, Qiu Z, Gao C, Sun Y, Dong W, Zhang Y, Chen R, Qi Y, Li S, Guo Y, Piao Y, Li S, Piao F. 2, 5-hexanedione downregulates nerve growth factor and induces neuron apoptosis in the spinal cord of rats via inhibition of the PI3K/Akt signaling pathway. PLoS One. 2017;12:e0179388. doi: 10.1371/journal.pone.0179388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weikel KA, Cacicedo JM, Ruderman NB, Ido Y. Knockdown of GSK3β increases basal autophagy and AMPK signalling in nutrient-laden human aortic endothelial cells. Biosci Rep. 2016 doi: 10.1042/BSR20160174. doi:101042/BSR20160174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.World Health Organization. Global action plan on the public health response to dementia 2017-2025. 2017:1–44. [Google Scholar]
- 107.Wu L, Xiong X, Wu X, Ye Y, Jian Z, Zhi Z, Gu L. Targeting oxidative stress and inflammation to prevent ischemia-reperfusion injury. Front Mol Neurosci. 2020;13:28. doi: 10.3389/fnmol.2020.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xie C, Guo Y, Lou S. LncRNA ANCR promotes invasion and migration of gastric cancer by regulating FoxO1 expression to inhibit macrophage M1 polarization. Dig Dis Sci. 2020 doi: 10.1007/s10620-019-06019-1. doi:101007/s10620-019-06019-1. [DOI] [PubMed] [Google Scholar]
- 109.Xiong S, Salazar G, Patrushev N, Alexander RW. FoxO1 mediates an autofeedback loop regulating SIRT1 expression. J Biol Chem. 2011;286:5289–5299. doi: 10.1074/jbc.M110.163667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu F, Na L, Li Y, Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020;10:54. doi: 10.1186/s13578-020-00416-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Yang N, Zhang Q, Bi XJ. MiRNA-96 accelerates the malignant progression of ovarian cancer via targeting FOXO3a. Eur Rev Med Pharmacol Sci. 2020;24:65–73. doi: 10.26355/eurrev_202001_19896. [DOI] [PubMed] [Google Scholar]
- 112.Ye Y, Zhang P, Qian Y, Yin B, Yan M. The effect of pyrroloquinoline quinone on the expression of WISP1 in traumatic brain injury. Stem Cells Int. 2017;2017:4782820. doi: 10.1155/2017/4782820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yue J, Liang C, Wu K, Hou Z, Wang L, Zhang C, Liu S, Yang H. Upregulated SHP-2 expression in the epileptogenic zone of temporal lobe epilepsy and various effects of SHP099 treatment on a pilocarpine model. Brain Pathol. 2020;30:373–385. doi: 10.1111/bpa.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zeldich E, Chen CD, Colvin TA, Bove-Fenderson EA, Liang J, Tucker Zhou TB, Harris DA, Abraham CR. The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. J Biol Chem. 2014;289:24700–24715. doi: 10.1074/jbc.M114.567321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang C, Li C, Chen S, Li Z, Ma L, Jia X, Wang K, Bao J, Liang Y, Chen M, Li P, Su H, Lee SM, Liu K, Wan JB, He C. Hormetic effect of panaxatriol saponins confers neuroprotection in PC12 cells and zebrafish through PI3K/AKT/mTOR and AMPK/SIRT1/FOXO3 pathways. Sci Rep. 2017a;7:41082. doi: 10.1038/srep41082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang H, Yang X, Pang X, Zhao Z, Yu H, Zhou H. Genistein protects against ox-LDL-induced senescence through enhancing SIRT1/LKB1/AMPK-mediated autophagy flux in HUVECs. Mol Cell Biochem. 2019a;455:127–134. doi: 10.1007/s11010-018-3476-8. [DOI] [PubMed] [Google Scholar]
- 117.Zhang N, Zhao Y. Other molecular mechanisms regulating autophagy. Adv Exp Med Biol. 2019;1206:261–271. doi: 10.1007/978-981-15-0602-4_13. [DOI] [PubMed] [Google Scholar]
- 118.Zhang P, Ye Y, Qian Y, Yin B, Zhao J, Zhu S, Zhang L, Yan M. The effect of pyrroloquinoline quinone on apoptosis and autophagy in traumatic brain injury. CNS Neurol Disord Drug Targets. 2017b;16:724–736. doi: 10.2174/1871527316666170124164306. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Y, Wu Q, Zhang L, Wang Q, Yang Z, Liu J, Feng L. Caffeic acid reduces A53T alpha-synuclein by activating JNK/Bcl-2-mediated autophagy in vitro and improves behaviour and protects dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacol Res. 2019b;150:104538. doi: 10.1016/j.phrs.2019.104538. [DOI] [PubMed] [Google Scholar]
- 120.Zhao Y, Wang Q, Wang Y, Li J, Lu G, Liu Z. Glutamine protects against oxidative stress injury through inhibiting the activation of PI3K/Akt signaling pathway in parkinsonian cell model. Environ Health Prev Med. 2019;24:4. doi: 10.1186/s12199-018-0757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou T, Zhuang J, Wang Z, Zhou Y, Li W, Wang Z, Zhu Z. Glaucocalyxin A as a natural product increases amyloid beta clearance and decreases tau phosphorylation involving the mammalian target of rapamycin signaling pathway. Neuroreport. 2019a;30:310–316. doi: 10.1097/WNR.0000000000001202. [DOI] [PubMed] [Google Scholar]
- 122.Zhou ZD, Selvaratnam T, Lee JCT, Chao YX, Tan EK. Molecular targets for modulating the protein translation vital to proteostasis and neuron degeneration in Parkinson’s disease. Transl Neurodegener. 2019b;8:6. doi: 10.1186/s40035-019-0145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


