Brain angiotensin II (ANG II) as a pleiotropic player: Mental disorders have been commonly associated with an imbalance in many neurotransmitter systems, such as dopamine, glutamate, and gamma-aminobutyric acid. Considering the complexity of brain functioning, all components of the neurovascular unit should be considered in studies for a better comprehension of the physiopathology and possible therapeutics. ANG II is present in the brain and binds to AT1 receptors (AT1-R), located in the neurovascular unit and has a close relationship with the mentioned neurotransmitter systems. In pathological conditions, AT1-R expressed in astrocytes, microglia, and brain endothelial cells are key mediators in the development of an oxidative/inflammatory microenvironment, as well as in glial activation. Therefore, pharmacological intervention targeting AT1-R provides a holistic and moderated approach to modulate neurotransmission systems in addition to the glial and vascular responses (Figure 1). This interaction is underscored by several studies that related brain ANG II to neurological disorders, such as Parkinson´s disease (PD) and attention deficit hyperactivity disorder (ADHD).
Figure 1.
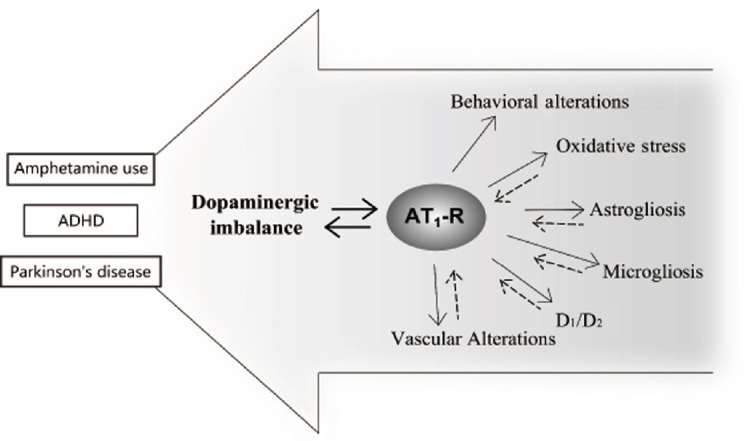
Schematic representation showing the interplay between dopaminergic altered neurotransmission and AT1 receptor (AT1-R).
Alterations in neurovascular unit components as consequence/source of dopamine imbalance should be considered. ADHD: Attention deficit hyperactivity disorder.
PD and brain ANG II: PD is the second most common neurodegenerative disorder, and the available therapies are only symptomatic. Current research are focused on elucidating the cause of dopaminergic cell death and developing treatment strategies that interfere with progression of the underlying neuropathology. The available evidence suggests that processes, such as oxidative stress, inflammation, mitochondrial dysfunction and excitotoxicity, are key players in the pathogenesis and progression of the disorder. Several findings have showed that microglial activation and/or nicotinamide adenine dinucleotide phosphate-oxidase derived superoxide participate in neurotoxin induced dopaminergic degeneration in different animal models of PD (Mertens et al., 2010). Moreover, it has been found that AT1-R overexpression and reduced insulin-like growth factor-1 availability are involved in neuroinflammatory processes, oxidative stress, microvascular rarefaction and dopaminergic neuronal degeneration. Particularly, AT1-R promote the initiation and progression of local neuroinflammation and oxidative stress under dopamine-imbalance conditions, as described in animal models of senescence and PD. Moreover, the interaction between brain ANG II and the central dopaminergic system is evident by the modulation of the dopamine release in striatum by AT1-R activation, since the acute administration of ANG II increases striatal dopamine release (Labandeira-Garcia et al., 2013; Perez-Lloret et al., 2017). It is important to highlight that AT1-R are present in the substantia nigra pars compacta and striatum of different mammals, including rats and humans (Perez-Lloret et al., 2017). Moreover, the density of AT1-R is higher in human striatum and substantia nigra compared to rats and other mammals. Interestingly, the density of AT1-R in the striatum is reduced in dopaminergic neurons of PD patients (Perez-Lloret et al., 2017).
Attention deficit hyperactive disorder and brain ANG II: The spontaneous hypertensive rats (SHR), a validated animal model of ADHD, exhibit AT1-R overexpression in brain microvessels associated with vascular oxidative stress and inflammation (Zhou et al., 2005). In this scenario, AT1-R mediate nitric oxide production (Yamakawa et al., 2003), vascular increased inflammatory cells permeability, and recruitment and activation (Ando et al., 2004). Moreover, it has been described that SHR have a high density of striatal D1-type dopamine receptors and atypical D2-type dopamine receptor activity. This suggests an imbalanced activation between the direct and indirect dopaminergic pathways in striatum of SHR rats because of the differential distribution of D1 and D2 receptors (Natsheh and Shiflett, 2018). A normal corticostriatal function should represent a balanced activation/inactivation in the direct and indirect pathways. Striatal dopamine depletion, as observed in PD, results in hypo-activation/hypo-inhibition of the direct/indirect pathways, respectively, that account for motor dysfunction as well as cognitive deficits (Natsheh and Shiflett, 2018). Some authors postulate motor hyperactivity characteristic of ADHD as a reverse Parkinsonism because of overstimulation of dopaminergic activity in the direct pathway or excessive dopaminergic inhibition of the indirect pathway. Nevertheless, others propose that specific dopaminergic receptors modulation of the direct and indirect pathways might explain motivational impairments and motor symptoms in ADHD (Natsheh and Shiflett, 2018).
Amphetamine exposure can be considered as a useful animal model of dopamine- imbalance: In rodents, amphetamine is commonly used as a pharmacological tool to promote dopamine-imbalance modifying future responses to environmental stimuli. Accordingly, our findings support the AT1-R involvement in the development of amphetamine-induced neuroadaptations at neurochemical, structural, and behavioral levels (Basmadjian et al., 2019). We observed a long-lasting overexpression of functional AT1-R in dopamine-innervated areas after amphetamine exposure, along with an altered AT1-R functionality regarding the classical ANG II-elicited actions (Basmadjian et al., 2019). Moreover, our recent findings recreate a neuroinflammatory environment involving glial and vascular alterations by using repeated amphetamine administration (Marchese et al., 2020). It is extensively known that astrocytes, microglia, and endothelial cells sense the disturbed neuronal activity after neurotoxic agents or disease. Furthermore, amphetamine promotes astrocyte reactivity, stimulating cytokine production, and enhancing dopamine neurotransmission in brain areas receiving dopaminergic inputs. At a vascular level, non-toxic doses of amphetamine increased pro-inflammatory markers, oxidative stress, and heat shock proteins expression in meningeal vasculature in rodents (Thomas et al., 2009). It has also been reported that chronic methylphenidate -at an equivalent clinical dosage used to treat attention deficit in humans- promotes vascular, glial, and neuronal changes in rats (Coelho-Santos et al., 2019). We recently found that amphetamine affects the microvascular network rearrangement in prefrontal cortex (PFC) similar to vascular alterations described in chronic inflammatory disease and schizophrenia (Marchese et al., 2020). Remarkably, we showed that AT1-R are involved in the development of the amphetamine-induced increased glial markers expression and vascular rearrangement in PFC. The role of AT1-R in these alterations may be attributed to their multiple actions, including dopamine neurotransmission modulation, dopaminergic neuronal degeneration by oxidative stress, astrocyte and microglial reactivity, and angiogenesis (Perez-Lloret et al., 2017; Basmadjian et al., 2019; Marchese et al., 2020). Particularly in neuroinflammatory responses involving dopamine-imbalance, the brain renin-angiotensin system is considered a key mediator in triggering glial cell activation with the subsequent progression of inflammation (Marchese et al., 2020). Moreover, we found long-term amphetamine effects over brain microvessels, observed as sensitized oxidative and cellular stress elicited by an amphetamine challenge. From our knowledge, this was the first report showing that amphetamine exposure induces sensitized oxidative/inflammatory responses in brain microvessels (Marchese et al., 2020). There is an established link between elevated reactive oxygen species levels and further stimulating heat shock protein 70 synthesis as a protective response to apoptotic signaling. Concerning brain ANG II involvement, we found that pretreatment with an AT1-R blocker avoids the sensitized response to the amphetamine challenge in brain microvessels. We also found that amphetamine exposure induced an increase in AT1-R expression in brain microvessels that might trigger the sensitized response. In this sense, AT1-R up-regulation in SHR brain microvessels has been related to increased levels of heat shock family components proinflammatory mediators and endothelial imbalance of nitric oxide and reactive oxygen species levels (Ando et al., 2004; Zhou et al., 2005).
Amphetamine-induced vascular rearrangement in PFC, observed as increased vascular density and vessel tortuosity and decreased branching points, was prevented by AT1-R antagonism (Marchese et al., 2020). These results are in agreement with the ANG II key role in several types of angiogenesis through local AT1-R activation and subsequent vascular endothelial growth factor synthesis and release. The interplay between dopamine and AT1-R is clear because dopamine shows anti-angiogenic effects by decreasing AT1-R expression and activity after ischemic injury. Since blood flow regulation in PFC is highly dependent on the direct influence of dopamine over the local microvessels, it is not surprising that this brain area is functionally responsive and plays a major role in brain vascular disorders (Marchese et al., 2020).
In our studies using a repeated amphetamine administration protocol, we described an attentional deficit-like behavior in rats, concomitant with morphological and structural changes in the non-neuronal cell types in PFC (Marchese et al., 2020). Catecholamine neurotransmission in PFC is implicated in the attention, coordination, and integration of cues required for spatial recognition of novel environments; thus, behavioral tests evaluating exploration are indicative of PFC functional integrity, together with the hippocampus and other limbic structures (Marchese et al., 2020). Cortical hypoperfusion, glial reactivity, inflammation, and oxidative stress have been related to neuropsychiatric impairments in psychostimulant users, such as attention or decision making deficits. This last goes along with the improvement in the attention deficit obtained in our experiments when animals received a low dose of amphetamine that promotes catecholamine release (Marchese et al., 2020); coincidently with what is observed in amphetamine users that show improved attention performance after re-exposure to low doses of the psychostimulant.
Collectively, our findings mirror a complex dopamine imbalance-derived environment where the neurovascular unit alterations and the functional outcomes indicate a crucial role for ANG II acting through its AT1-R.
Additional file: Open peer review report 1 (75.9KB, pdf) .
Footnotes
P-Reviewer: Kalani K; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Komal Kalani, Florida International University, USA.
References
- 1.Ando H, Zhou J, Macova M, Imboden H, Saavedra JM. Angiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke. 2004;35:1726–1731. doi: 10.1161/01.STR.0000129788.26346.18. [DOI] [PubMed] [Google Scholar]
- 2.Basmadjian OM, Armonelli S, Occhieppo VB, Jaime A, Baiardi G, Bregonzio C. How deep amphetamine impacts our brain and why to focus on angiotensin II. In Horizons in Neuroscience Research. NOVA. 2019;38:147–176. [Google Scholar]
- 3.Coelho-Santos V, Cardoso FL, Magalhaes A, Ferreira-Teixeira M, Leitao RA, Gomes C, Rito M, Barbosa M, Fontes-Ribeiro CA, Silva AP. Effect of chronic methylphenidate treatment on hippocampal neurovascular unit and memory performance in late adolescent rats. Eur Neuropsychopharmacol. 2019;29:195–210. doi: 10.1016/j.euroneuro.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Labandeira-Garcia JL, Rodriguez-Pallares J, Dominguez-Meijide A, Valenzuela R, Villar-Cheda B, Rodriguez-Perez AI. Dopamine-angiotensin interactions in the basal ganglia and their relevance for Parkinson’s disease. Mov Disord. 2013;28:1337–1342. doi: 10.1002/mds.25614. [DOI] [PubMed] [Google Scholar]
- 5.Marchese NA, Occhieppo VB, Basmadjian OM, Casarsa BS, Baiardi G, Bregonzio C. Angiotensin II modulates amphetamine-induced glial and brain vascular responses, and attention deficit via angiotensin type 1 receptor: Evidence from brain regional sensitivity to amphetamine. Eur J Neurosci. 2020;51:1026–1041. doi: 10.1111/ejn.14605. [DOI] [PubMed] [Google Scholar]
- 6.Mertens B, Vanderheyden P, Michotte Y, Sarre S. The role of the central renin-angiotensin system in Parkinson’s disease. J Renin Angiotensin Aldosterone Syst. 2010;11:49–56. doi: 10.1177/1470320309347789. [DOI] [PubMed] [Google Scholar]
- 7.Natsheh JY, Shiflett MW. Dopaminergic Modulation of goal-directed behavior in a rodent model of attention-deficit/hyperactivity disorder. Front Integr Neurosci. 2018;12:45. doi: 10.3389/fnint.2018.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Lloret S, Otero-Losada M, Toblli JE, Capani F. Renin-angiotensin system as a potential target for new therapeutic approaches in Parkinson’s disease. Expert Opin Investig Drugs. 2017;26:1163–1173. doi: 10.1080/13543784.2017.1371133. [DOI] [PubMed] [Google Scholar]
- 9.Thomas M, George NI, Patterson TA, Bowyer JF. Amphetamine and environmentally induced hyperthermia differentially alter the expression of genes regulating vascular tone and angiogenesis in the meninges and associated vasculature. Synapse. 2009;63:881–894. doi: 10.1002/syn.20661. [DOI] [PubMed] [Google Scholar]
- 10.Yamakawa H, Jezova M, Ando H, Saavedra JM. Normalization of endothelial and inducible nitric oxide synthase expression in brain microvessels of spontaneously hypertensive rats by angiotensin II AT1 receptor inhibition. J Cereb Blood Flow Metab. 2003;23:371–380. doi: 10.1097/01.WCB.0000047369.05600.03. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Ando H, Macova M, Dou J, Saavedra JM. Angiotensin II AT1 receptor blockade abolishes brain microvascular inflammation and heat shock protein responses in hypertensive rats. J Cereb Blood Flow Metab. 2005;25:878–886. doi: 10.1038/sj.jcbfm.9600082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


