Abstract
Aim
Coronavirus disease (COVID-19) ranges from mild clinical phenotypes to life-threatening conditions like severe acute respiratory syndrome (SARS). It has been suggested that early liver injury in these patients could be a risk factor for poor outcome. We aimed to identify early biochemical predictive factors related to severe disease development with intensive care requirements in patients with COVID-19.
Methods
Data from COVID-19 patients were collected at admission time to our hospital. Differential biochemical factors were identified between seriously ill patients requiring intensive care unit (ICU) admission (ICU patients) versus stable patients without the need for ICU admission (non-ICU patients). Multiple linear regression was applied, then a predictive model of severity called Age-AST-D dimer (AAD) was constructed (n = 166) and validated (n = 170).
Results
Derivation cohort: from 166 patients included, there were 27 (16.3%) ICU patients that showed higher levels of liver injury markers (P < 0.01) compared with non-ICU patients: alanine aminotrasnferase (ALT) 225.4 ± 341.2 vs. 41.3 ± 41.1, aspartate aminotransferase (AST) 325.3 ± 382.4 vs. 52.8 ± 47.1, lactic dehydrogenase (LDH) 764.6 ± 401.9 vs. 461.0 ± 185.6, D-dimer (DD) 7765 ± 9109 vs. 1871 ± 4146, and age 58.6 ± 12.7 vs. 49.1 ± 12.8. With these finding, a model called Age-AST-DD (AAD), with a cut-point of <2.75 (sensitivity = 0.797 and specificity = 0.391, c − statistic = 0.74; 95%IC: 0.62-0.86, P < 0.001), to predict the risk of need admission to ICU (OR = 5.8; 95% CI: 2.2-15.4, P = 0.001), was constructed. Validation cohort: in 170 different patients, the AAD model < 2.75 (c − statistic = 0.80 (95% CI: 0.70-0.91, P < 0.001) adequately predicted the risk (OR = 8.8, 95% CI: 3.4-22.6, P < 0.001) to be admitted in the ICU (27 patients, 15.95%).
Conclusions
The elevation of AST (a possible marker of early liver injury) along with DD and age efficiently predict early (at admission time) probability of ICU admission during the clinical course of COVID-19. The AAD model can improve the comprehensive management of COVID-19 patients, and it could be useful as a triage tool to early classify patients with a high risk of developing a severe clinical course of the disease.
1. Introduction
The entire healthcare system's collapse is a serious public concern worldwide due to the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. In the United States (US), the coronavirus disease (COVID-19) has given way to a nationwide public health catastrophe. For the first time in US history, a disaster declaration has been put in place for all 50 states and most US territories [1]. Until 26 September 2020, there were 32,626,165 confirmed SARS-CoV-2 infection cases worldwide and 990,134 deaths, according to the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) [2]. Mexico is one of the countries with a higher frequency of deaths due to the COVID-19 pandemic, with more than 70,000 deaths, a tally surpassed only by the US, Brazil, and India [3]. In this catastrophic scenario, results essential to understand the main factors related to a worse prognosis in the Mexican population.
COVID-19 ranges from mild clinical phenotypes to life-threatening conditions like severe acute respiratory syndrome (SARS). Among COVID-19 patients, around 80% are present with a mild illness whose symptoms usually disappear within two weeks. However, around 20% of the patients may develop severe symptoms requiring hospitalization. The mortality rate for this group of patients is around 13.4%. Therefore, patient risk assessment, preferably in a quantitative, nonsubjective way, is essential for adequate patient management and medical resource allocation. The prognostic value of different variables is not yet fully understood [4].
Patients with COVID-19 often develop respiratory failure 8–14 days after symptom onset, with “silent hypoxemia” and a high respiratory rate [5, 6]. Other authors have described examples of patients going from being physiologically normal to decompensating just a few hours later [7]. Therefore, further to oxygen saturation, recognizing poor prognosis factors that appear earlier during the disease is the key to prioritizing medical care for these high-risk patients, thus achieving effective triage in saturated healthcare systems. Our study is aimed at identifying the early biochemical factors determined at admission time, which were independent of pulmonary parameters, related to the disease course's progression, and the development of severe illness conditioning need to admission to the intensive care unit (ICU).
2. Materials and Methods
2.1. Study Design and Data Collection
This was an observational cohort study. First, we prospectively identified 166 patients with COVID-19 due to SARS-CoV-2 infection admitted to our hospital from March to May 2020. Demographic, clinical, and biochemical data at admission time were obtained from the medical records of these patients. Independent variables of interest were sex, age, glucose, urea, creatinine, lactic dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (AP), gamma-glutamyl transferase (GGT), ferritin, D-dimer (DD), total platelet count, mean platelet volume (VPM), hemoglobin (Hb), red cell distribution width (RDW), leukocytes, neutrophils, lymphocytes, brain natriuretic peptide (BNP), albumin, total proteins, direct bilirubin, indirect bilirubin, total cholesterol, triglycerides, sodium, potassium, chlorine, magnesium, phosphorus, calcium, fibrinogen, international normalized ratio (INR), C-reactive protein (CRP), creatine phosphokinase (CPK), creatine phosphokinase-myocardial band (CPK-MB), troponin I, and myoglobin. Our primary outcome was to identify disease biomarkers in patients with severe disease needing ICU admission (ICU patients) and compare them with those who remained stable and needed only standard care support through supplementary oxygen by mask (non-ICU patients). There was not a search for specific predictors already reported in the literature because this sampling was time-depending. The intensive care medical staff evaluated all cases that need transfer to the ICU; SARS development was the most important reason to transfer patients to ICU. All patients transferred to ICU were intubated and supported with mechanical ventilation. The decision to transfer a patient to ICU and to initiate mechanical ventilation was always taken by the medical staff of the ICU. Patients were treated according to a previously established algorithm based on international standard care dictated by the Infectious Diseases Society of America (IDSA) [8].
2.2. Predictive Model Construction
Differential factors were identified between ICU patients versus non-ICU patients; these variables were then used to create a model to early predict (at admission time) whose patients were at risk to need transfer to the ICU at any time during the follow-up. The derivation and validation of the prediction model were designed according to the TRIPOD guidelines [9]. The Institutional Review Board approved the protocol.
2.3. Inclusion Criteria
Patients admitted to the hospitalization area because of confirmed COVID-19 due to SARS-CoV-2 infection by nasopharyngeal and oropharyngeal swab positive tests using real-time reverse transcription-polymerase chain reaction (RRT-PCR) taken at admission time.
2.4. Exclusion Criteria
Patients with incomplete information on their medical records. This was a per-protocol analysis, so the intention-to-treat analysis was not done.
2.5. Derivation Cohort
We included consecutive patients admitted from March to May 2020.
2.6. Validation Cohort
We included consecutive patients admitted from June to August 2020.
2.7. Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD). Categorical variables were expressed as frequencies and percents. Characteristics from ICU patients were compared with non-ICU patients. Differences between categorical variables were analyzed using the χ2 test or Fisher Exact test, whereas continuous variables were analyzed using two tails Student's t-test. A P ≤ 0.01 was considered significant.
To normalize the distribution of significant variables, we transformed it into their natural logarithm. The variables were ordered based on univariate significance by fitting a logistic regression model and added into the multivariate model using a forward selection procedure. Model selection was based on minimizing the Akaike information criterion and maximizing area underneath the receiver operator curve (AUROC) or concordance c-statistic, with priority given to the lowest Akaike information criterion. The final model named Age-AST-DD (AAD) was applied to both derivation and validation cohort, and AUROC analysis was performed to predict developing severe disease needing to be transferred to ICU. The model's diagnostic performance in derivation and validation cohorts was evaluated using sensitivity, 1-specificity, positive predictive value, negative predictive value, and diagnostic accuracy. All analyses were performed using IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY.
2.8. Sample Size
In a post hoc analysis (StatMate 2 for Windows), we found a power higher than 95% in the effect sizes of main variables (Age, AST, and DD), so we conclude that the sample size used to construct and then to validate de model was enough to get statistical validity.
3. Results
The enrolment of patients is summarized on the flowchart (see Figure 1).
Figure 1.
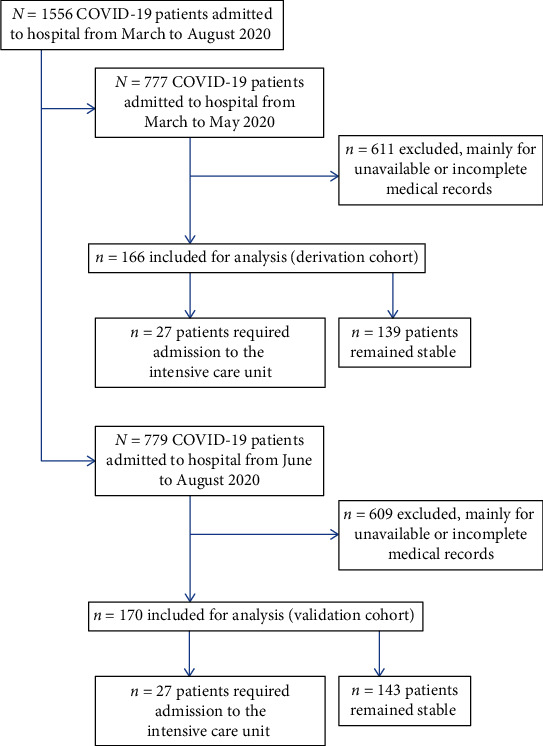
Enrolment of patients.
3.1. Derivation Cohort
One hundred and sixty-six patients were included; from those, 114 (68.7%) were men. The mean age was 50.6 ± 13.3 years old. A total of 27 (16.3%) were ICU patients. In the comparative analysis between those ICU patients versus non-ICU patients, we found significant raises of ALT (225.4 ± 341.2 vs. 41.3 ± 41.1; P = 0.003), AST (325.3 ± 382.4 vs. 52.8 ± 47.1; P = 0.001), LDH (764.6 ± 401.9 vs. 461.0 ± 185.6; P = 0.001), DD (7765 ± 9109 vs. 1871 ± 4146; P = 0.003), and older age (58.6 ± 12.7 vs. 49.1 ± 12.8; P = 0.001). See Table 1.
Table 1.
Comparison of admission characteristics between patients who developed SARS and required admission to ICU versus those with COVID-19 pneumonia without severity criteria.
| Variable | Patients with SARS requiring ICU admission (n = 27) | Patients with COVID-19 pneumonia without severity criteria for ICU admission (n = 139) | P (∗ < 0.01) |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Male/female gender, n (%) | 20/7 (74.1/25.9) | 94/45 (67.6/32.4) | 0.51 |
| Age, years old | 58.6 ± 12.7 | 49.1 ± 12.8 | 0.001∗ |
| Tobacco consumption, n (%) | 7 (25.9) | 26 (18.7) | 0.43 |
| Alcohol intake, n (%) | 3 (11.1) | 13 (9.3) | 0.73 |
| Diabetes, n (%) | 8 (29.6) | 48 (34.5) | 0.82 |
| Hypertension, n (%) | 5 (18.5) | 45 (32.4) | 0.25 |
| Weight | |||
| Normal, n (%) | 11 (40.7) | 45 (32.4) | 0.53 |
| Obesity, n (%) | 16 (59.3) | 94 (67.6) | |
| COPD, n (%) | 6 (22.2) | 8 (5.7) | 0.01 |
| Cardiovascular disease, n (%) | 3 (11.1) | 10 (7.2) | 0.45 |
| Chronic liver disease, n (%) | 4 (14.8) | 13 (9.3) | 0.30 |
| Chronic rheumatic disease, n (%) | 2 (7.4) | 5 (3.6) | 0.32 |
| Dyslipidemia, n (%) | 9 (33.3) | 17 (12.2) | 0.02 |
| Chronic kidney disease, n (%) | 3 (11.1) | 6 (22.2) | 0.12 |
| Cancer, n (%) | 1 (3.7) | 14 (10.1) | 0.46 |
| AIDS, n (%) | 1 (3.7) | 1 (0.7) | 0.30 |
| Use of immunosuppressive medication different than steroids, n (%) | 2 (7.4) | 6 (4.3) | 0.62 |
| Chronic use of steroids | |||
| No, n (%) | 27 (100) | 133 (95.7) | 0.55 |
| Low dose, n (%) | 0 (0) | 4 (2.9) | |
| High dose, n (%) | 0 (0) | 2 (1.4) | |
| Liver function tests | |||
| Albumin, g/dL | 3.27 ± 0.52 | 3.48 ± 0.50 | 0.09 |
| Alanine aminotransferase, UI/L | 225.4 ± 341.2 | 41.3 ± 41.1 | 0.003∗ |
| Aspartate aminotransferase, UI/L | 325.3 ± 382.4 | 52.8 ± 47.1 | 0.001∗ |
| Alkaline phosphatase, UI/L | 109.1 ± 74.8 | 96.8 ± 54.4 | 0.39 |
| Gamma Glutamyl Transferase, UI/L | 205.6 ± 360.4 | 125.4 ± 163.3 | 0.35 |
| Direct bilirubin, mg/dL | 0.8 ± 1.7 | 0.3 ± 0.3 | 0.23 |
| Indirect bilirubin, mg/dL | 0.8 ± 1.1 | 0.5 ± 0.3 | 0.31 |
| Biochemical serum analysis | |||
| Glucose, mg/dL | 168.2 ± 95.0 | 149.8 ± 97.8 | 0.54 |
| Urea, mg/dL | 54.7 ± 37.0 | 42.1 ± 37.7 | 0.14 |
| Creatinine, mg/dL | 1.1 ± 0.7 | 0.9 ± 0.7 | 0.29 |
| Cholesterol, mg/dL | 102.9 ± 33.8 | 123.0 ± 27.0 | 0.03 |
| Triglycerides, mg/dL | 142.4 ± 45.8 | 145.7 ± 49.4 | 0.83 |
| Total proteins, g/dL | 6.5 ± 0.7 | 6.3 ± 1.0 | 0.60 |
| Lactic dehydrogenase, UI/L | 764.6 ± 401.9 | 461.0 ± 185.6 | 0.001∗ |
| Serum electrolytes | |||
| Sodium, mmol/L | 128.8 ± ±26.8 | 135.8 ± 3.5 | 0.38 |
| Potassium, mmol/L | 4.2 ± 0.4 | 4.0 ± 0.5 | 0.19 |
| Chlorine, mmol/L | 102.2 ± 5.04 | 100.6 ± 4.35 | 0.25 |
| Calcium, mg/dL | 7.8 ± 0.47 | 8.0 ± 0.44 | 0.77 |
| Phosphorus, mg/dL | 3.2 ± 1.0 | 3.1 ± 0.8 | 0.75 |
| Magnesium, mg/dL | 2.3 ± 0.3 | 2.2 ± 0.4 | 0.27 |
| Hematic cytometry | |||
| Leukocytes, cells/mm3 | 10.3 ± 5.1 | 8.7 ± 4.5 | 0.23 |
| Neutrophils, cells/mm3 | 8.9 ± 4.6 | 7.1 ± 4.2 | 0.09 |
| Lymphocytes, cells/mm3 | 1.0 ± 0.4 | 1.0 ± 0.6 | 0.99 |
| Hemoglobin, g/dL | 14.7 ± 1.7 | 14.5 ± 2.3 | 0.82 |
| Red cells wide distribution | 14.8 ± 1.4 | 14.2 ± 1.4 | 0.15 |
| Platelets, cells/mL | 219.7 ± 73.1 | 226.4 ± 86.2 | 0.77 |
| Mean platelet volume, fL | 8.9 ± 0.9 | 8.4 ± 0.9 | 0.11 |
| Coagulation tests and inflammatory profile | |||
| International normalized ratio | 1.1 ± 0.2 | 1.0 ± 0.3 | 0.63 |
| Fibrinogen, mg/dL | 640.7 ± 207.5 | 608.6 ± 168.9 | 0.54 |
| D-dimer, ng/mL | 7765 ± 9109 | 1871 ± 4146 | 0.003∗ |
| Reactive C protein, mg/L | 210.3 ± 157.4 | 142.7 ± 121.2 | 0.17 |
| Ferritin, ng/mL | 782 ± 518 | 786 ± 1011 | 0.98 |
| Muscle enzymes | |||
| Creatine phosphokinase, UI/L | 169 ± 188 | 300 ± 462 | 0.36 |
| Myoglobin, ng/mL | 151 ± 151 | 110 ± 192 | 0.47 |
| Cardiac enzymes and peptides | |||
| Troponin I, ng/L | 49.4 ± 136.7 | 26.1 ± 96.3 | 0.45 |
| CPK-MB, ng/dL | 34 ± 42 | 25 ± 17 | 0.29 |
| Brain natriuretic peptide, pg/mL | 56.9 ± 80.5 | 136.1 ± 342.2 | 0.49 |
AIDS: acquired immunodeficiency syndrome; COPD: chronic obstructive pulmonary disease; ICU: intensive care unit; SARS: severe acute respiratory distress syndrome.
The results of the linear regression are shown in Table 2, where model 3 was the one that best explained the need for ICU admission, with these variables was constructed the model called AAD, where [AAD = 3.896 + ln(age)x − 0.218 + ln(AST)x − 0.185 + ln(DD)x0.070], where a value ≤ 2.75 had sensitivity = 0.797 and 1 − specificity = 0.391, c − statistic = 0.74 (95% CI: 0.62-0.86; P < 0.0001), to predict the risk of developing severe disease and need to ICU admission (OR = 5.8, 95% CI: 2.2-15.4; P = 0.001). See Figure 2. The shrinkage factor for derivation sampling was 0.89.
Table 2.
Multivariate linear regression models predictive of severe disease in patients with COVID-19 and requirement for ICU admission.
| Model | Nonstandardized coefficients | Standardized coefficients | P | 95% confidence interval for B | Colinearity statistics | ||||
|---|---|---|---|---|---|---|---|---|---|
| B | Error deviation | Beta | Inferior limit | Superior limit | Tolerance | VIF | |||
| 1 | C | 2.721 | 0.131 | <0.001 | 2.462 | 2.980 | |||
| AST | -0.229 | 0.033 | -0.512 | <0.001 | -0.293 | -0.164 | 1.000 | 1.000 | |
| 2 | C | 3.161 | 0.198 | <0.001 | 2.770 | 3.551 | |||
| AST | -0.194 | 0.034 | -0.435 | <0.001 | -0.261 | -0.127 | 0.878 | 1.139 | |
| DD | -0.081 | 0.028 | -0.221 | 0.01 | -0.135 | -0.026 | 0.878 | 1.139 | |
| 3 | C | 3.896 | 0.414 | <0 .001 | 3.077 | 4.714 | |||
| AST | -0.185 | 0.034 | -0.413 | <0.001 | -0.252 | -0.118 | 0.860 | 1.163 | |
| DD | -0.070 | 0.028 | -0.190 | 0.01 | -0.125 | -0.014 | 0.844 | 1.185 | |
| Age | -0.218 | 0.108 | -0.148 | 1.05 | -0.433 | -0.004 | 0.915 | 1.093 | |
AST: aspartate aminotransferase; C: constant; DD: D-dimer; VIF: variance inflation factors. Resume of the model: (1) R = 0.512, r2 = 0.262, r2 adjusted = 0.256, standard error = 0.331. (2) R = 0.552, r2 = 0.305, r2 adjusted = 0.294, standard error = 0.322. (3) R = 0.570, r2 = 0.325, r2 adjusted = 0.310, standard error = 0.318. Durbin − Watson = 1.53.
Figure 2.
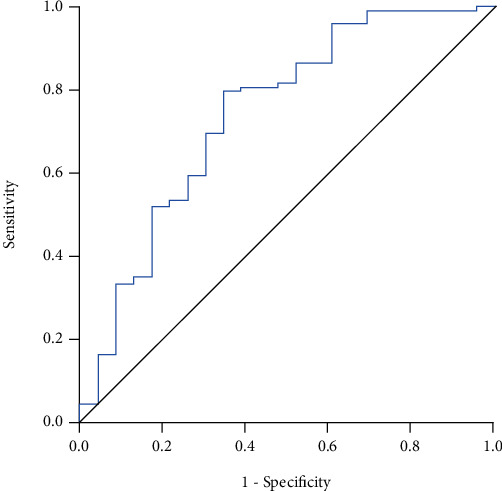
Derivation cohort (n = 166): AAD model to predict ICU admission. c − statistic = 0.74 (95% CI: 0.62-0.86; P < 0.0001).
3.2. Validation Cohort
One hundred and seventy patients were included; from those, 116 (68.2%) were men. The mean age was 50.9 ± 12.8 years old. A total of 27 (15.9%) were ICU patients. The AAD value ≤ 2.75 in this cohort had sensitivity = 0.77 and 1 − specificity = 0.26, c − statistic = 0.80 (95% CI: 0.70-0.91; P < 0.0001), to predict the risk of requiring ICU admission (OR = 8.8, 95% CI: 3.4-22.6; P < 0.0001). See Figure 3. The shrinkage factor for validation sampling was 0.88.
Figure 3.
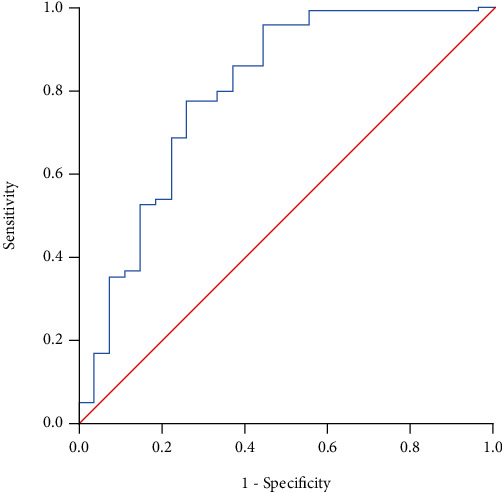
Validation cohort (n = 170): AAD model to predict ICU admission. c − statistic = 0.80 (95% CI: 0.70-0.91; P < 0.0001).
4. Discussion
In this study, we develop a regression model using early biomarkers to predict the severity of COVID-19, assessing the need for admission to ICU. Cytokine storm, SARS, and systemic inflammation-related pathology characterize severe COVID-19 [10]. Liver injury is common and is associated with disease severity in patients infected by the other two significant coronavirus—SARS-CoV and the Middle East respiratory syndrome coronavirus [11–14]. Between 14.8% and 53% of COVID-19 patients had hepatocellular liver injury demonstrated by higher ALT or AST and slightly high bilirubin levels [15]. Moreover, liver injury frequency is higher in severe COVID-19 [16–19] and increases the mortality as high as 58 to 78% [20, 21].
Our study found that early liver injury, assessed by elevated aminotransferases, particularly AST, is a factor related to the worst progression in COVID-19 patients who require entering to ICU. Huang et al. [19] showed that AST elevation was observed in 8 (62%) of 13 patients in the ICU compared with 7 (25%) of 28 patients who did not require ICU admission. Wang et al. [22] also found that patients admitted to ICU had significantly higher ALT (35 vs. 23, P = 0.007) and AST (52 vs. 29, P < 0.001) levels. Our study results confirm the finding that liver injury is more prevalent in severe cases of COVID-19.
According to several studies, high values of CRP, ferritin, DD, procalcitonin, LDH, prothrombin time, activated partial thromboplastin time, amyloid serum protein A, CPK, GGT, urea, and creatinine are risk factors for severe disease, thromboembolic complications, myocardial damage, and worse prognosis [23–26]. In addition to aminotransferases, in our study, many of these factors were higher in ICU patients than in non-ICU patients, but the most important associated with severe disease were LDH and DD. The most severely ill patients usually present with coagulopathy, and disseminated intravascular coagulation- (DIC-) like massive intravascular clot formation is frequently seen in this group of patients [27, 28]. Therefore, as we found in our AAD predictive model, coagulation tests, specifically DD [29], may be considered useful to discriminate severe cases of COVID-19. Changes in hemostatic biomarkers represented by an increase in DD and fibrin/fibrinogen degradation products indicate the essence of coagulopathy is massive fibrin formation [28].
Liver injury in patients with COVID-19 might be due to viral infection in liver cells or due to other causes such as drug-induced liver injury (DILI) and systemic inflammation induced by cytokine storm or pneumonia-associated hypoxia [30]. A significant limitation of our study is that we were not able to correlate the biochemical findings at admission with liver biopsy in these patients; therefore, we are unable to determine if the serum alterations observed in liver function tests, particularly aminotransferases, are due to direct viral infection of the liver parenchyma. Another significant limitation is that the received therapy in these patients was heterogeneous regarding the date of starting, type of medication, and the dose of the medications, then we do not collect data from the received therapy of these patients; therefore, we cannot perform a subanalysis to try to identify potential DILI contributing to liver injury.
5. Conclusions
The elevation of AST (a possible marker of early liver injury) along with D-dimer and age efficiently early predict (at admission time) the probability of needing ICU admission during the clinical course of COVID-19. Our findings support using the AAD model to accurately determine those patients who would need to be transferred to ICU because of a severe clinical course of their disease. The AAD model can improve the comprehensive management of COVID-19 patients, and it could be useful as a triage tool to early classify patients with a high risk of developing a severe clinical course of the disease.
Acknowledgments
Luis Servín-Abad, MD, Gastroenterologist at Saint Cloud Hospital, Centracare, St. Cloud, Minnesota, 56303, United States of America contributed to edit the English language version of this manuscript.
Data Availability
The datasets generated and analyzed in this study are not publicly available because of respect to and protect patient privacy but are available from the corresponding authors on reasonable request.
Disclosure
Preliminary results of this work were presented as an abstract as cartel at the Annual Meeting of the Mexican Association of Hepatology (AMH)–XV Congreso Nacional de Hepatología, Online modality held on July 23-25, 2020.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Authors' Contributions
Fátima Higuera-de-la-Tijera designed the overall concept of the study, performed the statistical analysis, and wrote the final manuscript; Alfredo Servín-Caamaño designed the overall concept of the study and supervised the writing of the manuscript; Daniel Reyes-Herrera, Argelia Flores-López, Enrique J.A. Robiou-Vivero, Felipe Martínez-Rivera, Victor Galindo-Hernández, Victor H. Rosales-Salyano, Catalina Casillas-Suárez, Oscar Chapa-Azuela, Alfonso Chávez-Morales, Billy Jiménez-Bobadilla, María L. Hernández-Medel, Benjamín Orozco-Zúñiga, and Jed R. Zacarías-Ezzat collected the data of all patients; Santiago Camacho provided support to perform the statistical analysis, tables, and figures; José L. Pérez-Hernández helped to design the mathematical model and contributed to edit the final manuscript.
References
- 1.The Lancet. COVID-19 in the USA: a question of time. Lancet. 2020;395(10232):p. 1229. doi: 10.1016/S0140-6736(20)30863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins Resource Center. COVID-19 map. Johns Hopkins Coronavirus Resource Center; 2020. [Google Scholar]
- 3.Agren D. Understanding Mexican health worker COVID-19 deaths. Lancet. 2020;396(10254):p. 807. doi: 10.1016/S0140-6736(20)31955-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu G., Yang P., Xie Y., et al. Development of a clinical decision support system for severity risk prediction and triage of COVID-19 patients at hospital admission: an international multicentre study. European Respiratory Journal. 2020;56(2) doi: 10.1183/13993003.01104-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y., Xiao S.-Y. Hepatic involvement in COVID-19 patients: pathology, pathogenesis, and clinical implications. Journal of Medical Virology. 2020;92(9):1491–1494. doi: 10.1002/jmv.25973. [DOI] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ottestad W., Seim M., Mæhlen J. O. Covid-19 med stille hypoksemi. Tidsskr den Nor Laegeforening. 2020;140 doi: 10.4045/tidsskr.20.0299. [DOI] [PubMed] [Google Scholar]
- 8.Infectious Diseases Society of America. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ [DOI] [PMC free article] [PubMed]
- 9.Collins G. S., Reitsma J. B., Altman D. G., Moons K. G. M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Annals of Internal Medicine. 2015;162(1):55–63. doi: 10.7326/M14-0697. [DOI] [PubMed] [Google Scholar]
- 10.Pence B. D. Severe COVID-19 and aging: are monocytes the key? Gero Science. 2020;42(4):1051–1061. doi: 10.1007/s11357-020-00213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J., Fan J.-G. Characteristics and mechanism of liver injury in 2019 coronavirus disease. Journal of Clinical and Translational Hepatology. 2020;8(1):1–5. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan Y.-J., Fielding B. C., Goh P.-Y., et al. Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway. Journal of Virology. 2004;78(24):14043–14047. doi: 10.1128/JVI.78.24.14043-14047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng D. L., Al Hosani F., Keating M. K., et al. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of middle east respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. The American Journal of Pathology. 2016;186(3):652–658. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsaad K. O., Hajeer A. H., Al Balwi M., et al. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection - clinicopathological and ultrastructural study. Histopathology. 2018;72(3):516–524. doi: 10.1111/his.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu L., Liu J., Lu M., Yang D., Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver International. 2020;40(5):998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai Q., Huang D., Ou P., et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75(7):1742–1752. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 17.Fan Z., Chen L., Li J., et al. Clinical features of COVID-19-related liver functional abnormality. Clinical Gastroenterology and Hepatology. 2020;18(7):1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. The New England Journal of Medicine. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B., Zhou X., Qiu Y., et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15(7):p. e0235458. doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ying Huang C., Yang R., Xu Y., Gong P. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. 2020. [DOI]
- 22.Wang D., Hu R., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vabret N., Britton G. J., Gruber C., et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52(6):910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azkur A. K., Akdis M., Azkur D., et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kermali M., Khalsa R. K., Pillai K., Ismail Z., Harky A. The role of biomarkers in diagnosis of COVID-19 - a systematic review. Life Sciences. 2020;254:p. 117788. doi: 10.1016/j.lfs.2020.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. The Journal of Clinical Investigation. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iba T., Levy J. H., Levi M., Thachil J. Coagulopathy in COVID-19. Journal of Thrombosis and Haemostasis. 2020;18(9):2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Connors J. M., Levy J. H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miesbach W., Makris M. COVID-19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clinical and Applied Thrombosis/Hemostasis. 2020;26:p. 107602962093814. doi: 10.1177/1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. The Lancet Gastroenterology & Hepatology. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed in this study are not publicly available because of respect to and protect patient privacy but are available from the corresponding authors on reasonable request.


