Abstract
Objectives:
To determine if coronary computed tomography angiography (CCTA)-based radiomic analysis of pericoronary adipose tissue (PCAT) can distinguish patients with acute myocardial infarction (MI) from patients with stable or no coronary artery disease (CAD).
Background:
Imaging of PCAT with CCTA enables detection of coronary inflammation. Radiomics involves extracting quantitative features from medical images to create big data and identify novel imaging biomarkers.
Methods:
In a prospective case-control study, 60 patients with acute MI underwent CCTA within 48 hours of admission, prior to invasive angiography. These subjects were matched to patients with stable CAD (n=60) and controls with no CAD (n=60) by age, gender, risk factors, medications, and CT tube voltage. PCAT was segmented around the proximal right coronary artery (RCA) in all patients, and around culprit and non-culprit lesions in MI patients. PCAT segmentations were analyzed using Radiomics Image Analysis software.
Results:
Of 1,103 calculated radiomic parameters, 20.3% differed significantly between MI patients and controls, and 16.5% differed between MI and stable CAD patients (critical p<0.0006); whereas none differed between stable CAD patients and controls. On cluster analysis, the most significant radiomic parameters were texture- or geometry-based. At 6 months post-MI, there was no significant change in the PCAT radiomic profile around the proximal RCA or non-culprit lesions. Using machine learning (XGBoost), a model integrating clinical features (risk factors, serum lipids, high-sensitivity C-reactive protein), PCAT attenuation, and radiomic parameters provided superior discrimination of acute MI (area under the receiver operator characteristic curve [AUC] 0.87) compared to a model with clinical features and PCAT attenuation (AUC 0.77, p=0.001) or clinical features alone (AUC 0.76, p<0.001).
Conclusions:
Patients with acute MI have a distinct PCAT radiomic phenotype compared to patients with stable or no CAD. Using machine learning, a radiomics-based model outperforms a PCAT attenuation-based model in accurately identifying patients with MI.
Keywords: coronary computed tomography angiography, pericoronary adipose tissue, myocardial infarction, radiomics, machine learning
INTRODUCTION
Vascular inflammation plays a key role in atherogenesis and atherosclerotic plaque rupture (1). Patients with acute myocardial infarction (MI) have histological evidence of local inflammation both within culprit lesions (2) and throughout the entire coronary vascular bed (3). This has been supported by imaging studies using invasive intracoronary techniques (4,5) and positron emission tomography (6). Coronary computed tomography angiography (CCTA) is a widely used non-invasive modality for the diagnosis of coronary artery disease (CAD). Recent evidence demonstrates that vascular inflammation inhibits lipid accumulation in pericoronary adipose tissue (PCAT) and that this can be detected on routine CCTA as an increase in CT attenuation of PCAT surrounding the proximal right coronary artery (RCA) (7). This surrogate measure of coronary inflammation predicts plaque progression (8) and cardiac mortality (9) in patients undergoing CCTA for suspected CAD. However, measurement of PCAT CT attenuation is based only on voxel intensity values and does not account for the complex spatial relationship between voxels. Radiomics is the process of extracting thousands of quantitative features from medical images, which can then be analyzed using computational techniques such as machine learning (ML) to identify novel imaging patterns of significant clinical value. In this prospective case-control study, we sought to perform CCTA-based radiomic characterization of PCAT in patients with acute MI and compare their radiomic phenotypes to those of matched subjects with stable CAD and controls with no CAD. We then aimed to examine the natural history of PCAT radiomic features and their response to conventional treatment at 6 months following MI. Finally, we hypothesized that using ML, a radiomics-based model would outperform a PCAT-attenuation based model in identifying patients with MI.
METHODS
Study population
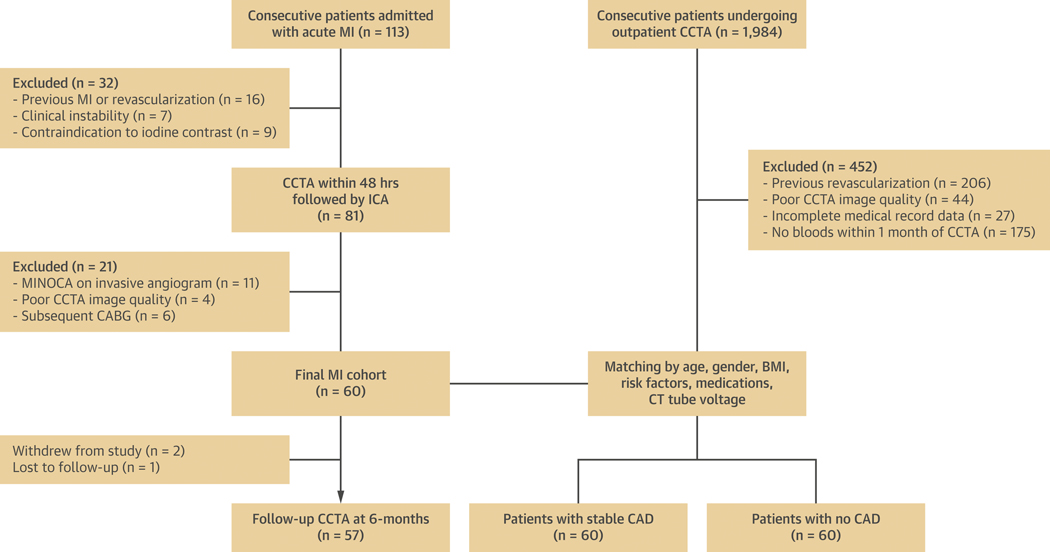
We prospectively recruited 60 consecutive patients admitted with acute MI from June 2018-January 2019 at MonashHeart (Monash Medical Centre, Melbourne, Australia) to undergo CCTA within 48 hours of admission and prior to invasive coronary angiography. We included patients who presented with post-thrombolysis ST-segment elevation MI (STEMI) or non-ST-segment elevation MI (NSTEMI) (10) and had a culprit lesion identified on invasive angiography (Supplemental Appendix). Exclusion criteria were previous MI or revascularization, clinical instability, severe renal impairment (eGFR <30 ml/m/1.73m2), or allergy to iodinated contrast. MI patients were matched to outpatients who underwent CCTA for suspected CAD during the same time period, stratified by 2 groups: i) 60 patients with stable CAD (defined by the presence of stable exertional symptoms and/or inducible myocardial ischemia on stress testing) and detection of stenosis severity between 25–99% on CCTA; and ii) 60 patients with no visually detectable CAD on CCTA who were used as controls. Matching of the 3 groups was performed for age decile, gender, body mass index (BMI) category, cardiovascular risk factors (diabetes, hypertension, dyslipidemia, smoking), baseline medications, and CT tube voltage (Supplemental Appendix). All patients in the MI cohort were discharged on optimal medical therapy and returned at 6 months for follow-up CCTA. The study was approved by the local Human Research Ethics Committee (Monash Health HREC 15244A) and all MI patients provided written informed consent. The study was registered on the Australian New Zealand Clinical Trials Registry (URL: http://www.anzctr.org.au. Unique identifier: ACTRN12618001058268). Figure 1 describes the patient selection and study design.
Figure 1: Patient selection and study design.
Flow chart showing inclusion and exclusion criteria for the study population. ICA = invasive coronary angiography; MINOCA = myocardial infarction with non-obstructive coronaries.
Ascertainment of risk factors
Pre-existing cardiovascular risk factors and medications were documented on admission for the MI cohort and ascertained by review of electronic medical records for the outpatient cohorts. Risk factors are defined in the Supplemental Appendix.
CCTA acquisition
All scans were performed on a 320-detector-row CT scanner (Aquilion ONE ViSION, Canon Medical Systems, Otawara, Japan) as previously described (11). Our protocol is detailed in the Supplemental Appendix. Scan settings at follow-up were identical to those at baseline.
Coronary plaque analysis
CCTA images were analyzed using axial and multiplanar reconstruction views. We excluded scans deemed uninterpretable due to artefact or heavy calcification (Figure 1). All coronary segments ≥2 mm were analyzed according to an 18-segment model (12) using semi-automated software (Autoplaque v2.5, Cedars-Sinai Medical Center, Los Angeles, CA, USA). For the MI and stable CAD cohorts, plaque quantification was performed at the Cedars-Sinai Medical Center core laboratory by an expert reader blinded to clinical data (A.L.) (Supplemental Appendix).
PCAT segmentation
Quantification of PCAT around the proximal RCA is a standardized and highly reproducible method which has been used in prior studies as an imaging biomarker of global coronary inflammation (7–9,13). Accordingly, we focused on the proximal RCA (10–50 mm from RCA ostium) for our per-patient level PCAT radiomic analysis. Following plaque quantification in the proximal RCA by the blinded expert reader, segmentation of the surrounding PCAT volume was fully automated (Autoplaque v2.5). PCAT was defined as all voxels with CT attenuation between −190 and −30 Hounsfield units (HU) located within a radial distance from the outer coronary wall equal to the diameter of the vessel (7,8). As the average luminal diameter of the proximal RCA in this study was 3.4±0.3 mm, we included PCAT within a 3 mm outer radial distance from the vessel wall (7) (Central Illustration). For serial PCAT analysis, we excluded patients who had stenting of culprit lesions in the proximal RCA (n=5; 8%), as stents on follow-up CCTA may have affected radiomic features of the adjacent PCAT due to partial volume averaging.
Central Illustration: Radiomic phenotyping of pericoronary adipose tissue in acute MI.
(A) Manhattan plots of p-values for pairwise comparisons of all radiomic parameters among the 3 cohorts. Negative logarithm of p-values are plotted on the y axis for each of the 1,103 radiomic parameters (color-coded by category) lined up on the x axis. The red horizontal line indicates the Bonferroni-corrected p-value of 0.0006, and parameters above the line were considered statistically significant. (B) Automated volumetric segmentation of PCAT around the proximal RCA (10–50 mm from the ostium) on CCTA. (C) Receiver operator characteristic curves of the 3 machine learning (ML) models for identifying patients with MI.
Given that previous studies have shown PCAT surrounding coronary lesions to be a potential sensor of plaque vulnerability (14,15), we also performed PCAT radiomic characterization at a per-lesion level in MI patients. Culprit lesions and the highest-grade quantitative percent stenosis non-culprit lesions were chosen for baseline PCAT radiomic phenotyping. PCAT around non-culprit lesions was also analyzed at follow-up; culprit lesions were not assessed due to stents. For patients who had stenting of non-culprit lesions prior to 6-month follow-up (n=9; 15%), the next highest-grade stenosis lesion was chosen for serial PCAT analysis. All PCAT segmentations were exported as DICOM data, along with automated measurements of PCAT attenuation, defined as the average attenuation of all voxels within the segmented volume (7). The average computing time for each automated PCAT segmentation was <30 seconds.
Epicardial adipose tissue volume measurement
Epicardial adipose tissue (EAT) volume was quantified from non-contrast CT images by an expert reader (J.Y.) using semi-automated software (QFAT v2.0, Cedars-Sinai Medical Center), as previously described (16).
Laboratory analyses
Fasting blood samples were drawn from all MI patients within 48 hours of admission and at 6-month follow-up. Laboratory results for the outpatient cohorts were obtained from a hospital database within 1 month prior to their CCTA. All samples were analyzed by a single laboratory (Monash Pathology, Victoria, Australia) using standard techniques (Supplemental Appendix).
Radiomic analysis
Detailed methodology for our radiomic analysis has been previously published (17). In brief, exported PCAT segmentations were loaded onto the Radiomics Image Analysis open-source software in the R environment (18). The images were discretized to 8, 16 and 32 equally sized bins with identical HU ranges. A total of 1,103 radiomic parameters were calculated for each PCAT segmentation. Specifically, there were 44 first-order parameters describing the HU distribution of the tissue, 342 gray-level co-occurrence matrix (GLCM) parameters describing the probability of given voxel pairs occurring next to each other, 33 gray-level run-length matrix (GLRM) parameters enumerating the probability of identical voxel values being continuously next to each other, and 684 geometrical parameters describing the spatial complexity of lesions (19). The average computation time for radiomic feature extraction using a standard workstation was approximately 30 minutes per proximal RCA segment and 15 minutes per coronary lesion.
Machine learning with extreme gradient boosting (XGBoost)
XGBoost (20) is a state-of-the-art ensemble boosting ML algorithm which has recently demonstrated high performance in cardiovascular risk stratification using cardiac CT (21,22). This method iteratively trains a set of weak learners (one-level decision trees) using a given set of patient data or features, to build a combined strong classifier to identify an outcome. For each patient, the XGBoost algorithm computes an individualized ML score according to the weighting of each variable. We developed a comprehensive ML model to identify patients with MI by first building a model with clinical features: age, gender, risk factors (diabetes, hypertension, smoking), serum lipid levels, and hs-CRP (model 1). To this model, we added novel CCTA-based imaging biomarkers: i) PCAT attenuation (model 2); or ii) PCAT attenuation plus PCAT radiomic phenotype (model 3) derived from the top 20 radiomic features based on ML information gain.
Repeated cross-validation and parameter tuning
To avoid reporting biased results and limit overfitting, we validated all 3 of our ML models using 10 times repeated 10-fold cross-validation, which separates training, tuning, and testing data (23) (Supplemental Appendix).
Statistical analysis
Continuous variables are presented as median (interquartile range) and categorical variables as frequencies (percentages). For continuous variables, baseline comparisons between the 3 groups were performed using the Friedman-test, and a Wilcoxon signed-rank test with Bonferroni correction was used for post-hoc pairwise comparisons. In MI patients, comparisons of continuous variables between baseline and follow-up were performed using a Wilcoxon signed-rank test. Categorical variables were compared with a Chi-square or Fisher’s exact test. For conventional parameters, a 2-sided p-value <0.05 indicated statistical significance.
For radiomic analysis, the number of unique radiomic parameters accounting for 99.5% of the variance in the data was calculated using principal component analysis, resulting in 29 unique parameters (24). Therefore, applying Bonferroni correction, p-values <0.0017 (0.05/29) were considered statistically significant for comparisons between all 3 groups; while p-values <0.0006 (0.05/29/3) were considered significant for post-hoc comparisons between 2 specific groups or between baseline and follow-up. Radiomic parameters were compared between groups using the permutation test of symmetry in the coin R package (25).
Linear regression analysis was conducted between the radiomic features that were significant among the 3 groups, and the R2 value between each radiomic feature was used as a distance measure for hierarchical clustering. The elbow method based on the total within-cluster sum of squares was used to calculate the optimal number of different clusters, which was 8. From each cluster, we identified the radiomic parameter with the smallest p-value and used this is as a representative biomarker for that cluster. We performed multivariable linear regression to evaluate whether clinical presentation with MI was independently associated with each of the 8 radiomic biomarkers, adjusted for age, gender, number of risk factors, EAT volume, and coronary plaque burden.
The performance of the ML models in identifying patients with MI was assessed using receiver operator characteristic (ROC) analysis, and the area under the curve (AUC) values were compared with the DeLong test (26). Statistical analyses were performed in the R environment (R version 3.5 and R Studio version 1.2.1335) and using Stata 14.0 (StataCorp, College Station, TX, USA).
RESULTS
Table 1 summarizes baseline characteristics of the study population stratified by clinical presentation. The 3 groups were well matched for age, gender, BMI, risk factors, medications, and CT tube voltage.
Table 1.
Baseline characteristics of the study population
| Characteristics | Acute MI (n = 60) |
Stable CAD (n = 60) |
No CAD (n = 60) |
p Value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, yrs | 58.4 (51.6 to 73.7) | 60.0 (52.0 to 68.5) | 59.5 (52.0 to 69.0) | 0.67 |
| Body mass index, kg/m2 | 27.7 (25.0 to 30.2) | 27.9 (25.0 to 32.3) | 27.2 (25.5 to 32.6) | 0.91 |
| Male gender | 52 (87) | 52 (87) | 52 (87) | 1.00 |
| Hypertension | 44 (73) | 41 (68) | 42 (70) | 0.71 |
| Diabetes | 15 (25) | 13 (22) | 12 (20) | 0.65 |
| Dyslipidemia | 32 (55) | 35 (58) | 31 (52) | 0.46 |
| Smoking | 24 (40) | 20 (33) | 19 (32) | 0.31 |
| Baseline medications | ||||
| Antiplatelet | 11 (18) | 10 (17) | 8 (13) | 0.27 |
| Statin | 15 (25) | 17 (28) | 13 (22) | 0.35 |
| Beta-blocker | 10 (17) | 12 (20) | 10 (17) | 0.54 |
| ACE-I or ARB | 14 (23) | 14 (23) | 12 (20) | 0.46 |
| Lipids, mmol/L | ||||
| Total cholesterol | 4.9 (4.1 to 5.8) | 5.1 (4.4 to 6.0) | 5.2 (4.3 to 6.2) | 0.16 |
| LDL cholesterol | 3.1 (2.3 to 3.7) | 3.1 (2.2 to 4.1) | 3.3 (2.5 to 4.1) | 0.12 |
| HDL cholesterol | 1.1 (1.0 to 1.3) | 1.3 (1.0 to 1.6) | 1.2 (1.0 to 1.5) | 0.13 |
| Triglycerides | 1.6 (1.2 to 2.5) | 1.5 (1.2 to 2.2) | 1.4 (1.1 to 2.0) | 0.10 |
| Inflammatory markers | ||||
| hs-CRP, mg/L | 9.7 (6.2 to 13.3) | 1.8 (0 to 3.6) | 1.4 (0 to 2.8) | <0.001 |
| White cell count, ×109/L | 9.0 (7.8 to 10.5) | 6.3 (5.2 to 7.4) | 6.0 (4.8 to 7.2) | 0.003 |
| Referring symptoms for CCTA | ||||
| Chest pain | 43 (72) | 39 (65) | 0.48 | |
| Dyspnea | 11 (18) | 10 (17) | 0.62 | |
| Asymptomatic | 6 (10) | 11 (18) | 0.35 | |
| Abnormal stress test | 15 (25) | 9 (15) | 0.13 | |
| CCTA acquisition parameters | ||||
| Heart rate, bpm | 55.0 (50.0 to 58.0) | 55.0 (51.0 to 58.0) | 56.5 (51.3 to 59.8) | 0.19 |
| Tube voltage | 1.00 | |||
| 100 kV | 22 (37) | 22 (37) | 22 (37) | |
| 120 kV | 38 (63) | 38 (63) | 38 (63) | |
| Tube current, mA | 710.0 (582.5 to 750.0) | 700.0 (570.0 to 750.0) | 710.0 (550.0 to 750.0) | 0.95 |
| Contrast dose, mL | 75.0 (74.3 to 75.0) | 75.0 (70.0 to 75.0) | 75.0 (72.0 to 75.0) | 0.26 |
| Radiation dose, DLP | 283.5 (154.3 to 370.6) | 268.4 (220.0 to 343.5) | 270.1 (201.7 to 360.1) | 0.22 |
| Segment involvement score | 5.5 (4.0 to 7.0) | 5.0 (4.0 to 7.0) | 0.88 | |
| Quantitative plaque measures | ||||
| Total coronary plaque burden, % | 36.1 (31.3 to 42.6) | 23.5 (18.2 to 30.1) | 0.001 | |
| Proximal RCA plaque burden, % | 19.5 (10.1 to 30.8) | 14.6 (7.1 to 23.7) | 0.04 | |
| EAT volume, cm3 | 96.6 (74.3 to 119.8) | 88.9 (72.1 to 106.5) | 79.6 (60.2 to 102.4) | <0.001 |
| PCAT attenuation (proximal RCA), HU | −83.1 (−86.6 to −79.8) | −90.4 (−95.2 to −86.6) | −93.7 (−98.2 to −87.9) | <0.001 |
Values are expressed as n (%) or median (interquartile range, 25th–75th).
ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; CAD = coronary artery disease; CCTA = coronary computed tomography angiography; EAT = epicardial adipose tissue; hs-CRP = high-sensitivity C-reactive protein; LDL = low-density lipoprotein; MI = myocardial infarction; PCAT = pericoronary adipose tissue; RCA = right coronary artery.
Clinical presentation
Of patients with MI, 55 (91.7%) presented with NSTEMI and 5 (8.3%) with post-thrombolysis STEMI. Characteristics of MI patients are detailed in the Supplemental Appendix. The median time from hospital admission to CCTA was 28.6 (19.5 to 40.2) hours. All patients underwent stenting of the culprit lesion during the index admission.
Follow-up post-MI
Of patients with MI, 57 (95.0%) returned for follow-up at a median interval of 188 (182 to 203) days; 2 patients withdrew from the study and 1 was lost to follow-up. No patients experienced MI, unstable angina, or unplanned revascularization in the intervening period. All patients were on dual antiplatelets, and 53 (93.0%) were on a moderate- or high-intensity statin at follow-up.
Radiomic parameters at baseline
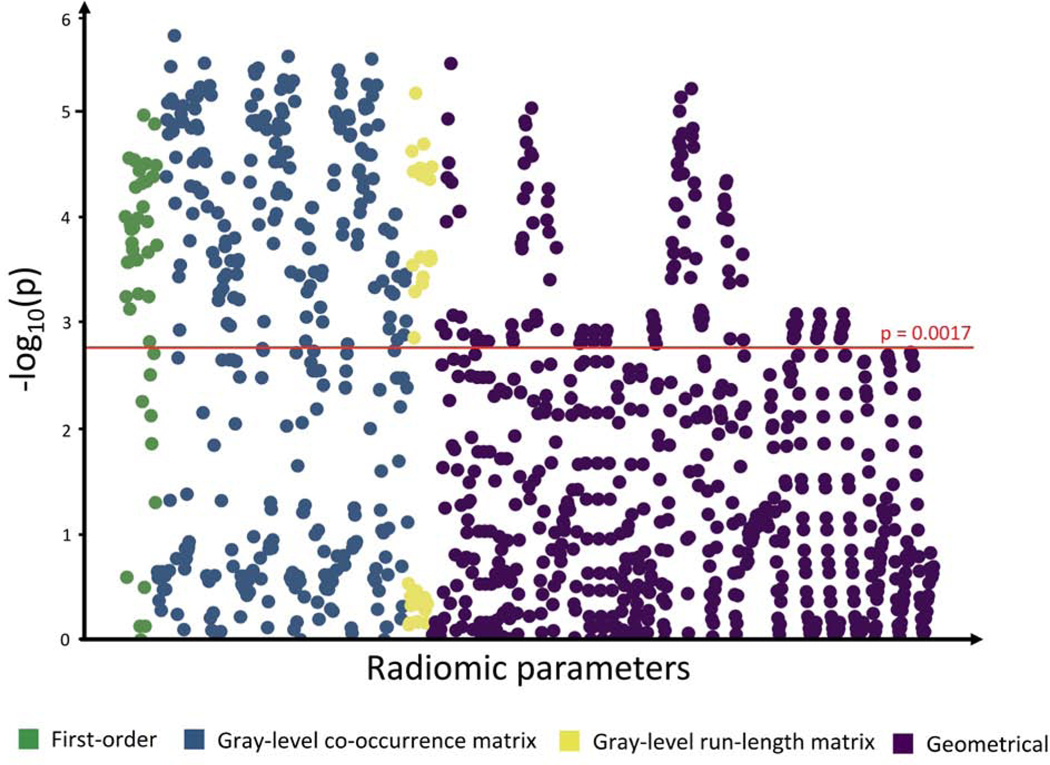
Of all 1,103 calculated radiomic parameters, 32.8% (362/1,103) showed a significant difference (p<0.0017) between the 3 groups. Specifically, 75.0% (33/44) of first-order parameters, 53.8% (184/342) of GLCM parameters, 54.6% (127/684) of GLRLM parameters, and 18.6% (127/684) of geometrical parameters were significant (Figure 2).
Figure 2: Manhattan plot of p-values for differences in all radiomic parameters between the 3 cohorts.
Negative logarithm of p-values for comparisons between all 3 cohorts (acute MI, stable CAD, controls) are plotted on the y axis for each of the 1,103 radiomic parameters lined up on the x axis. The red horizontal line indicates the Bonferroni-corrected p-value of 0.0017, and 362 parameters above the line were considered statistically significant.
In pairwise comparisons, 20.3% (224/1,103) of all radiomic features were significantly different between MI patients and controls, and 16.5% (182/1,103) of radiomic features differed between patients with MI and those with stable CAD (p<0.0006); while there were no significant differences in radiomic features between stable CAD patients and controls (Central Illustration). In patients with MI, we found no significant differences in radiomic parameters between culprit and non-culprit lesions (Supplemental Figure 1).
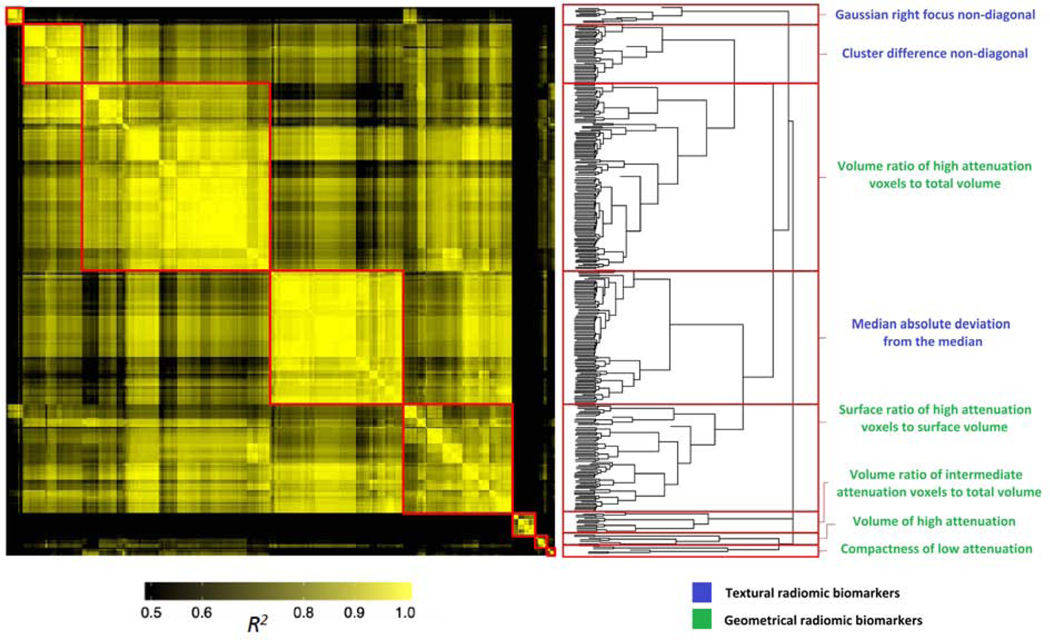
Cluster analysis of significant radiomic parameters
Results of the linear regression analysis performed between the 362 significant radiomic parameters are summarized in a heatmap (Figure 3). Hierarchical clustering revealed 8 different clusters in which the parameters were highly correlated with each other (represented by the yellow areas in the heatmap) but had minimal correlation with other parameters (black areas in in the heatmap). Within these clusters, the radiomic biomarkers with the smallest p-values were either texture-based GLCM statistics or geometry-based (Figure 3).
Figure 3: Heatmap and clustering dendrogram of significant radiomic parameters.
Heatmap of R2 values on linear regression analysis between the 362 radiomic features that were significant among the 3 cohorts. R2 values were plotted against each other and ordered to show the statistically different information clusters present in the dataset (red boxes). The color-coding indicates the value of explained variance: R2 values <0.5 are black, whereas greater values are shown in yellow with increasing intensity. The resulting hierarchical clustering dendrogram, partitioned into the 8 clusters (red boxes), is shown on the right. The radiomic biomarker from each cluster with the smallest p-value is displayed.
Association of MI with radiomic biomarkers
In multivariable linear regression models adjusted for age, gender, number of risk factors, EAT volume, and total plaque burden in the coronary tree, clinical presentation with MI was independently and positively associated with each of the 8 cluster-derived radiomic biomarkers (all p<0.001). Similar results were observed when proximal RCA plaque burden was used as the quantitative plaque measure in the models (all p<0.001).
Radiomic parameters at follow-up
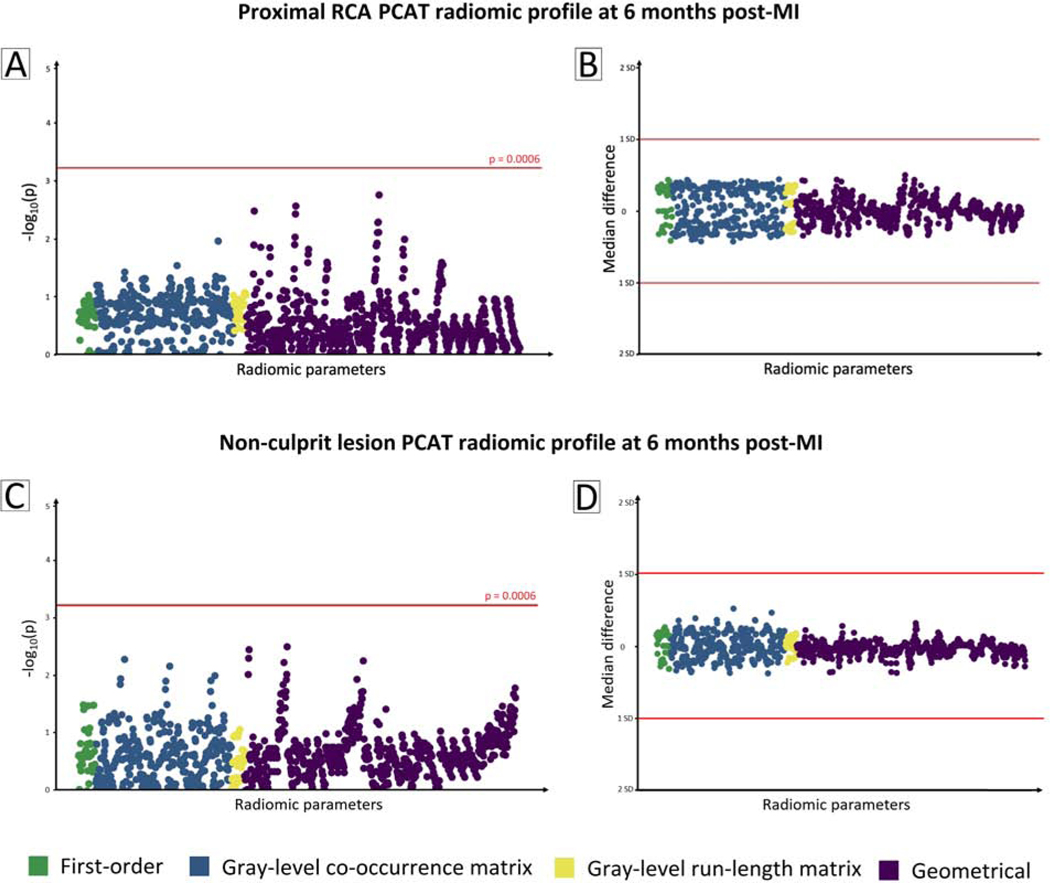
In MI patients, there was no significant change in the 1,103 radiomic parameters of PCAT surrounding the proximal RCA at 6-month follow-up (Figure 4A). The median difference between baseline and follow-up values of all radiomic parameters was within 1 standard deviation of the median baseline value (Figure 4B). Serial radiomic phenotyping of PCAT around non-culprit lesions revealed similar results (Figure 4, C and D). In subgroup analyses of patients with LDL-C <1.8 mmol (n=41) or ≥1.8 mmol/L (n=16) at 6-month follow-up, no significant temporal change was observed in radiomic parameters around the proximal RCA or non-culprit lesions.
Figure 4: Serial radiomic phenotyping of PCAT in MI patients.
(A) Manhattan plot of p-values for differences in radiomic features of PCAT surrounding the proximal RCA. Negative logarithm of p-values for comparisons between baseline and 6-month follow-up are plotted on the y axis for each of the 1,103 radiomic parameters on the x axis. The red line indicates statistical significance. (B) Median differences between baseline and follow-up values of PCAT radiomic parameters around the proximal RCA are plotted on the y axis. The red lines represent 1 standard deviation from the median baseline value. Panels (C) and (D) show the corresponding plots for radiomic features of PCAT surrounding non-culprit lesions.
PCAT attenuation
Comparisons of PCAT attenuation between the 3 groups at baseline, and between baseline and follow-up for MI patients, are detailed in the Supplemental Appendix. In patients with MI, PCAT attenuation was increased around culprit lesions compared with non-culprit lesions (−80.0 [−84.3 to −75.9] HU vs. −84.6 [−89.7 to −79.5] HU, p=0.01).
Laboratory results
Baseline levels of hs-CRP, WCC, and LDL-C are shown in Table 1. There was a significant reduction in all 3 serum biomarkers at 6 months post-MI (Supplemental Appendix).
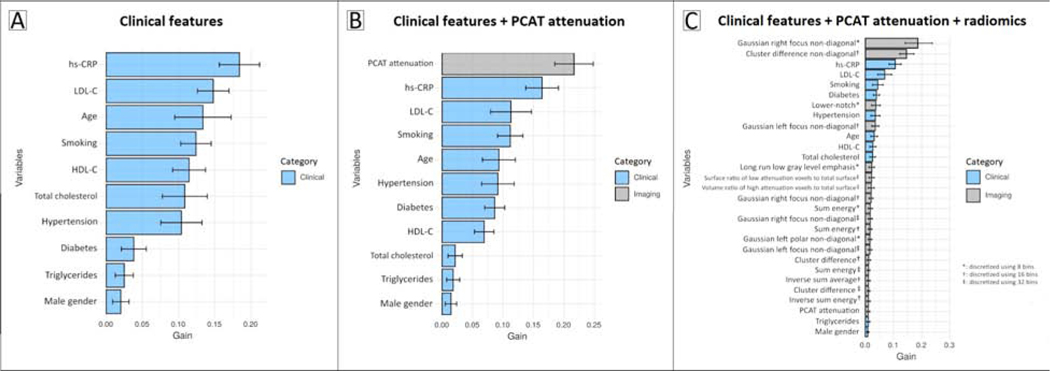
Identification of patients with MI using ML models
Ranking of variable importance for classification of MI in the 3 ML models is shown in Figure 5, A to C. On ROC analysis, the radiomics-based model (AUC 0.87; 95% confidence interval [CI]: 0.82–0.93) provided superior discrimination of MI compared to the PCAT attenuation-based model (AUC 0.77 [95% CI: 0.70–0.84], p=0.001) or clinical model (AUC 0.76 [95% CI: 0.69–0.84], p<0.001) (Central Illustration). Addition of PCAT attenuation to clinical features did not improve model discrimination of MI (p=0.59).
Figure 5: Variable importance for machine learning classification of MI.
Ranking of variable importance in ML models incorporating: (A) clinical features alone; (B) clinical features and PCAT attenuation; and (C) clinical features, PCAT attenuation, and PCAT radiomic parameters. The solid bars and error bars represent the mean gain and standard deviation, respectively, for each variable.
DISCUSSION
Our primary findings are: 1) patients with acute MI have a distinct PCAT radiomic phenotype compared to patients with stable or no CAD; 2) the most significant radiomic parameters distinguishing patients with and without MI are texture- and geometry-based, providing information not captured by PCAT attenuation; 3) there is no significant change in the per-patient or per-lesion PCAT radiomic profile at 6 months post-MI; 4) using ML, a radiomics-based model outperforms a PCAT attenuation-based model in identifying patients with acute MI.
Radiomics greatly increases the amount of quantitative information accessible from CT images. Thousands of imaging features indiscernible to the human eye are extracted from a given region of interest to create big data, from which imaging patterns associated with clinical characteristics or outcomes are derived (27). Radiomic analysis of coronary plaque has been shown to outperform conventional parameters for CCTA-based identification of plaque vulnerability (17,28). Beyond plaque, PCAT attenuation on CCTA represents a surrogate measure of coronary inflammation (7). However, this metric simply enumerates the average voxel intensity values, without considering the spatial relationship between voxels. Radiomics utilizes texture analysis, which models the spatial distribution of voxel gray-level intensities and applies higher-order statistics to provide a measure of heterogeneity (27). Further, geometrical radiomic parameters quantify the size and shape of 3D volumes within an imaging dataset.
In our study, PCAT attenuation around the proximal RCA was higher in patients with acute MI compared to patients with stable CAD and controls. However, on PCAT radiomic analysis, hierarchical clustering showed textural and geometrical features, not intensity-based metrics, to be most significant in distinguishing patients with versus without MI. Furthermore, in our radiomics-based ML model, the 2 imaging biomarkers with highest variable importance were Gaussian right focus non-diagonal and cluster difference non-diagonal – textural parameters which describe the number of times 2 voxels of a given intensity occur next to each other. These findings suggest that radiomic heterogeneity of PCAT on CCTA in acute MI may reflect pathophysiological changes in the adipose tissue that are not captured by PCAT attenuation. Indeed, a recent report showed texture-based radiomic features of PCAT surrounding the RCA and left anterior descending (LAD) artery to have higher accuracy than average attenuation in detecting adipose tissue fibrosis and vascularity on histology (29). Our study is the first to perform PCAT radiomic characterization at the per-patient and per-lesion level in acute MI; to systematically compare radiomic phenotypes among patients with MI, stable CAD, and no CAD; and to assess the temporal changes of radiomic parameters in MI patients.
We showed that MI was associated with each of our 8 unique, cluster-derived radiomic biomarkers, independently of risk factors and coronary plaque burden. This suggests that the PCAT radiomic phenotype in MI may potentially be driven by plaque rupture and the associated local inflammatory response. Histopathologic and invasive imaging studies have demonstrated that patients with MI have diffuse coronary inflammation independently of the location of the culprit lesion (3–5). Our results suggest that CCTA-based radiomic characterization of PCAT around the proximal RCA may potentially capture this acute, global coronary inflammation. We found no significant difference between the PCAT radiomic profiles of culprit and non-culprit lesions, lending further support to the pan-coronary inflammatory hypothesis.
There were no significant changes in PCAT radiomic features surrounding the proximal RCA and non-culprit lesions at 6-months post-MI; similar results were observed for PCAT attenuation. Meanwhile, there was a reduction in circulating levels of inflammatory markers, consistent with resolution of the systemic inflammation associated with acute MI. These findings suggest that the imaging phenotype of PCAT at these 2 sites in the coronary tree is not modified by optimal medical therapy within 6 months following an event. This may be due to irreversible morphological changes in PCAT, including extracellular fibrosis and angiogenesis (30), that occur in response to coronary inflammation soon after MI. Alternatively, conventional medications such as statins and antiplatelets may not exert a significant, detectable effect on the local inflammatory status of PCAT. Certainly, no studies have specifically examined the influence of medical therapy for CAD on CCTA-derived measures of PCAT. Elnabawi et al. (13) showed a reduction in PCAT attenuation around the proximal RCA in response to a year of systemic biologic therapy for psoriasis. Recently, Oikonomou et al. (29) observed no changes in the radiomic profile of PCAT around the RCA and LAD on standard treatment at 6 months post-MI. However, this was a small cohort (n = 16), and radiomic features of PCAT surrounding plaques were not examined. Our study extends these findings to a larger population at both a per-patient and per-lesion level, and includes measures of systemic inflammation.
There has been much interest into detection of the ‘vulnerable patient’ at high risk for MI or cardiac death in the near term (31,32). Traditional clinical risk factors are well-studied and represent fixed, chronic conditions better suited to predicting long-term risk (32). Hence, novel biomarkers that reflect acute processes influencing coronary plaque rupture are needed. Local vascular inflammation increases plaque instability and risk of atherothrombosis (2). Moreover, systemic levels of CRP are increased in MI and may reflect the presence of ruptured plaque (33). Application of advanced CCTA-based quantitative techniques to PCAT in acute MI may enhance our understanding of the inflammatory activity in the pericoronary milieu at a non-invasive imaging level. By integrating these imaging biomarkers with clinical risk factors and circulating hs-CRP levels, we developed comprehensive, individualized ML models for identifying patients with acute MI. In these models, radiomic parameters added incremental discriminatory value over and above PCAT attenuation and clinical features. By contrast, PCAT attenuation did not improve discrimination of MI beyond clinical features. On conventional statistical analysis, PCAT attenuation was higher around culprit lesions compared to non-culprit lesions; however, no significant differences were found in radiomic parameters. These findings suggest that the radiomic phenotype of PCAT may be more useful for per-patient comparisons in this setting, while PCAT attenuation may have better utility as a lesion-specific imaging biomarker.
Limitations
The present analysis has several limitations. All patients underwent CCTA at a single center using the same CT scanner and protocol. Hence, the generalizability of our findings to other populations may be limited as image acquisition and reconstruction settings can affect the reproducibility of radiomic features (34). Standardized CCTA acquisition protocols and data analysis techniques are required to provide a robust framework for radiomic analysis. Current radiomic techniques are also computationally expensive and time-consuming (~30 min per case on a standard workstation in this study); hence limiting their applicability to clinical practice. Advancements in hardware (such as Graphics Processing Units) and artificial intelligence algorithms will facilitate increasing automation of radiomic feature extraction and analysis. Culprit lesions on invasive angiography were determined by visual assessment, and intravascular imaging was used in only a small proportion of patients. We do not have histological correlation for our radiomic phenotypes; yet recent evidence has shown textural radiomic features to associate with adverse PCAT remodelling on cardiac surgical biopsies (29). Finally, our radiomics-based ML model requires external validation in an independent cohort.
CONCLUSION
Patients with acute MI have a distinct PCAT radiomic phenotype on CCTA compared to patients with stable or no CAD. Characterization of textural and geometrical radiomic features provides magnitudes more information than is captured by PCAT attenuation. Using ML, a radiomics-based model outperforms a PCAT attenuation-based model in accurately identifying patients with MI. Further studies in cardiac CT radiomics may lead to identification of new imaging biomarkers which can aid in the detection of the ‘vulnerable patient’.
Supplementary Material
PERSPECTIVES.
Competency in medical knowledge
Imaging of pericoronary adipose tissue (PCAT) with routine coronary computed tomography angiography (CCTA) enables detection of coronary inflammation. CCTA-based radiomic analysis of PCAT can distinguish patients with acute MI from patients with stable or no CAD. PCAT radiomic features do not change significantly in response to conventional treatment within 6 months following MI. Using machine learning, a radiomics-based model outperforms a PCAT attenuation-based model in accurately identifying patients with acute MI.
Translational outlook
The present study results suggest that acute MI is associated with significant changes in textural and geometrical radiomic features of PCAT on CCTA. Quantification of these features provides magnitudes more information than is captured by measuring the average CT attenuation of PCAT. Further studies in cardiac CT radiomics, along with advancements in computational techniques, will enable identification of novel imaging biomarkers which can aid in the detection of the ‘vulnerable patient’.
Acknowledgments
Funding: This study was supported by grants from the National Health and Medical Research Council, Australia, and the National Heart, Lung, and Blood Institute, USA [1R01HL133616]. These funding bodies had a role in the collection, analysis, and interpretation of the data.
ABBREVIATIONS
- BMI
body mass index
- CAD
coronary artery disease
- CCTA
coronary computed tomography angiography
- LAD
left anterior descending
- MI
myocardial infarction
- ML
machine learning
- NSTEMI
non-ST-segment elevation myocardial infarction
- PCAT
pericoronary adipose tissue
- RCA
right coronary artery
- STEMI
ST-segment elevation myocardial infarction
Footnotes
Relationship with industry: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Libby P, Loscalzo J, Ridker PM et al. Inflammation, Immunity, and Infection in Atherothrombosis: JACC Review Topic of the Week. J Am Coll Cardiol 2018;72:2071–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852–66. [DOI] [PubMed] [Google Scholar]
- 3.Mauriello A, Sangiorgi G, Fratoni S et al. Diffuse and active inflammation occurs in both vulnerable and stable plaques of the entire coronary tree: a histopathologic study of patients dying of acute myocardial infarction. J Am Coll Cardiol 2005;45:1585–93. [DOI] [PubMed] [Google Scholar]
- 4.Rioufol G, Finet G, Ginon I et al. Multiple atherosclerotic plaque rupture in acute coronary syndrome: a three-vessel intravascular ultrasound study. Circulation 2002;106:804–8. [DOI] [PubMed] [Google Scholar]
- 5.Sugiyama T, Yamamoto E, Bryniarski K et al. Nonculprit Plaque Characteristics in Patients With Acute Coronary Syndrome Caused by Plaque Erosion vs Plaque Rupture: A 3-Vessel Optical Coherence Tomography Study. JAMA Cardiol 2018;3:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joshi NV, Vesey AT, Williams MC et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014;383:705–13. [DOI] [PubMed] [Google Scholar]
- 7.Antonopoulos AS, Sanna F, Sabharwal N et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med 2017;9. [DOI] [PubMed] [Google Scholar]
- 8.Goeller M, Tamarappoo BK, Kwan AC et al. Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oikonomou EK, Marwan M, Desai MY et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 2018;392:929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thygesen K, Alpert JS, Jaffe AS et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol 2018;72:2231. [DOI] [PubMed] [Google Scholar]
- 11.Wong DT, Soh SY, Ko BS et al. Superior CT coronary angiography image quality at lower radiation exposure with second generation 320-detector row CT in patients with elevated heart rate: a comparison with first generation 320-detector row CT. Cardiovasc Diagn Ther 2014;4:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leipsic J, Abbara S, Achenbach S et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014;8:342–58. [DOI] [PubMed] [Google Scholar]
- 13.Elnabawi YA, Oikonomou EK, Dey AK et al. Association of Biologic Therapy With Coronary Inflammation in Patients With Psoriasis as Assessed by Perivascular Fat Attenuation IndexAssociation of Biologic Therapy With Coronary Inflammation in PsoriasisAssociation of Biologic Therapy With Coronary Inflammation in Psoriasis. JAMA Cardiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goeller M, Achenbach S, Cadet S et al. Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared With Stable Coronary Artery Disease. JAMA Cardiol 2018;3:858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwiecinski J, Dey D, Cadet S et al. Peri-Coronary Adipose Tissue Density Is Associated With (18)F-Sodium Fluoride Coronary Uptake in Stable Patients With High-Risk Plaques. J Am Coll Cardiol Img 2019;12:2000–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dey D, Suzuki Y, Suzuki S et al. Automated quantitation of pericardiac fat from noncontrast CT. Invest Radiol 2008;43:145–53. [DOI] [PubMed] [Google Scholar]
- 17.Kolossváry M, Karády J, Szilveszter B et al. Radiomic Features Are Superior to Conventional Quantitative Computed Tomographic Metrics to Identify Coronary Plaques With Napkin-Ring Sign. Circ Cardiovasc Imaging 2017;10:e006843–e006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolossvary M. RIA: Radiomics Image Analysis Toolbox for Grayscale Images. 2017. [Google Scholar]
- 19.Kolossváry M, Karády J, Kikuchi Y et al. Radiomics versus Visual and Histogram-based Assessment to Identify Atheromatous Lesions at Coronary CT Angiography: An ex Vivo Study. Radiology 2019;293:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen T, Guestrin C. XGBoost: A Scalable Tree Boosting System. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. San Francisco, California, USA: Association for Computing Machinery, 2016:785–794. [Google Scholar]
- 21.Commandeur F, Slomka PJ, Goeller M et al. Machine learning to predict the long-term risk of myocardial infarction and cardiac death based on clinical risk, coronary calcium, and epicardial adipose tissue: a prospective study. Cardiovasc Res 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Rosendael AR, Maliakal G, Kolli KK et al. Maximization of the usage of coronary CTA derived plaque information using a machine learning based algorithm to improve risk stratification; insights from the CONFIRM registry. J Cardiovasc Comput Tomogr 2018;12:204–209. [DOI] [PubMed] [Google Scholar]
- 23.Kim J-H. Estimating classification error rate: Repeated cross-validation, repeated hold-out and bootstrap. Comput Stat Data Anal 2009;53:3735–3745. [Google Scholar]
- 24.Johnson RC, Nelson GW, Troyer JL et al. Accounting for multiple comparisons in a genome-wide association study (GWAS). BMC Genomics 2010;11:724–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hothorn T, Hornik K, van de Wiel MA, Zeileis A. Implementing a Class of Permutation Tests: The coin Package. 2008 2008;28:23. [Google Scholar]
- 26.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 27.Kolossvary M, Kellermayer M, Merkely B, Maurovich-Horvat P. Cardiac Computed Tomography Radiomics: A Comprehensive Review on Radiomic Techniques. J Thorac Imaging 2018;33:26–34. [DOI] [PubMed] [Google Scholar]
- 28.Kolossváry M, Park J, Bang J-I et al. Identification of invasive and radionuclide imaging markers of coronary plaque vulnerability using radiomic analysis of coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oikonomou EK, Williams MC, Kotanidis CP et al. A novel machine learning-derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CT angiography. Eur Heart J 2019;40:3529–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest 2017;127:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arbab-Zadeh A, Fuster V. From Detecting the Vulnerable Plaque to Managing the Vulnerable Patient. J Am Coll Cardiol 2019;74:1582. [DOI] [PubMed] [Google Scholar]
- 32.Eagle KA, Ginsburg GS, Musunuru K et al. Identifying patients at high risk of a cardiovascular event in the near future: current status and future directions: report of a national heart, lung, and blood institute working group. Circulation 2010;121:1447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sano T, Tanaka A, Namba M et al. C-reactive protein and lesion morphology in patients with acute myocardial infarction. Circulation 2003;108:282–5. [DOI] [PubMed] [Google Scholar]
- 34.Kolossvary M, Szilveszter B, Karady J, Drobni ZD, Merkely B, Maurovich-Horvat P. Effect of image reconstruction algorithms on volumetric and radiomic parameters of coronary plaques. J Cardiovasc Comput Tomogr 2019;13:325–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.