Abstract
This study investigated the effects of acute antioxidant supplementation on endothelial function, exercise blood flow and oxidative stress biomarkers in 9 young African American compared to 10 Caucasian males (25.7 ± 1.2 years). We hypothesized that African American males would have lower exercise blood flow and endothelial responsiveness compared to Caucasian males, and these responses would be improved following antioxidant supplementation. Ultrasonography was used to measure blood flow during handgrip exercise. Endothelial function was assessed using flow-mediated dilation, and lipid peroxidation was assessed by measuring levels of malondialdehyde-thiobarbituric acid reactive substances. African American males exhibited lower endothelial function than Caucasians at baseline (8.3 ± 1.7 vs. 12.2 ± 1.7%) and the difference was ameliorated with antioxidant supplementation (10.7 ± 1.9 % vs. 10.8 ± 1.8 %), but the interaction was not significant (p = 0.10). There were no significant changes in malondialdehyde-thiobarbituric acid reactive substances following antioxidant supplementation. There was a significant increase in brachial blood flow and forearm vascular conductance with exercise but no differences with antioxidant supplementation. There were no group differences in exercise responses and no differences with antioxidant supplementation, suggesting a lack of influence of oxidative stress during exercise in this cohort.
Keywords: endothelial function, exercise blood flow, oxidative stress, antioxidant, racial differences, vascular
Introduction
The elevated risk of cardiovascular disease (CVD), especially hypertension, in African Americans (AA) is only partially explained by an increased prevalence of CVD risk factors such as hypertension, obesity, and type 2 diabetes mellitus [17, 26], suggesting potential racial differences in arterial structure and function. Young, healthy AAs often display impaired vascular function [1, 4, 5, 40], a risk factor for future cardiovascular events [25, 35, 42]. A healthy endothelium maintains vascular tone and homeostasis through factors such as nitric oxide (NO), and endothelial dysfunction, characterized by decreased NO bioavailability, is a precursor for CVD [2]. Even after adjustment of CVD risk factors, AAs exhibit impaired macrovascular (FMD %) [32] and microvascular endothelial function [34]. In addition, AAs demonstrate decreased NO-mediated vasodilation of forearm resistance vessels to mental stress [5] and to endothelial dependent and independent pharmacological stimuli [4, 40] as well as reduced responsiveness of conductance vessels to endogenous NO compared with Caucasians (CA) [3]. Even young AAs exhibit vascular dysfunction manifesting as significantly lower peak and total reactive hyperemic forearm blood flow, greater central pressures, carotid beta stiffness, and higher carotid intima media thickness [24], and lower arterial compliance [51] compared to CAs. In addition, young AAs have shown reduced endothelial function [3, 40]. These differences in vascular function between races, even in young, healthy individuals could potentially be due to the elevated oxidative stress seen in AAs. For example, AAs have a disproportionately high risk of developing oxidative stress related conditions such as hypertension and metabolic diseases [19, 33] and there is evidence of elevated oxidative stress in AAs both in vivo and in vitro [14, 16, 29]. Elevations in oxidative stress also lead to the development of endothelial dysfunction and contribute to the subsequent development of CVD [25, 41] through the inactivation of NO by superoxide and other free radicals [13, 43].
Blood flow, arterial dilation, or vasodilatory responses with exercise can provide insight into cardiovascular function and endothelial dysfunction not seen at rest. In addition, there are known racial differences following exercise. AAs demonstrate a lack of post exercise hypotension [36] and no change or increases in arterial stiffness following exercise while CAs exhibit decreased stiffness with exercise [23, 50]. However, there is limited information about blood flow responses during exercise. NO plays an important role in exercise vasodilation [49] and elevated free radicals and oxidative stress can also lead to decrements in exercise-induced vasodilation [11]. Because reduced vasodilation can lead to a decrease in perfusion during exercise [30], it is clinically relevant to determine potential mechanisms for improved vasodilation with exercise. It is also unknown how antioxidant supplementation might differentially affect racial differences in exercise or the potential for improved exercise vasodilation and blood flow.
Acute supra-physiological dosages of antioxidants have been shown to restore endothelial function in individuals with elevated oxidative stress, such as older adults [12, 28, 43] and patients with coronary artery disease and hypertension [18] by scavenging free radicals. In addition, there is a marked improvement in brachial artery vasodilation during exercise in individuals with endothelial dysfunction following antioxidant supplementation [11], suggesting a role for NO in mediating this vasodilatory response. In contrast, in young, healthy individuals with a lack of oxidative stress, AOX supplementation does not improve vascular function and may have negative effects [11, 12, 37]. However, these studies were not performed in a racially diverse cohort. It is unknown if oxidative stress might contribute to the vascular dysfunction seen in AAs and if acute antioxidant supplementation can diminish differences in vascular function between CAs and AAs. It is also unknown how acute antioxidant supplementation with known efficacy might differentially affect blood flow in young AA and CA men at rest and during exercise [37, 48]. Therefore, the purpose of this study was to examine vascular responses of CA and AA males by measuring exercise blood flow and endothelial function and the effect of antioxidant supplementation (AOX). We hypothesized that AA males would exhibit elevated oxidative stress and would therefore have lower exercise blood flow and endothelial responsiveness compared to CA males, a cohort with a lack of oxidative stress, and these responses would be improved in AA males only following AOX supplementation.
Methods
Participants
19 young (25.7 ± 1.2 years, 9 AAs and 10 CAs), sedentary and recreationally active healthy male volunteers participated in this study. Physical activity was reported using the Paffenbarger Questionnaire. Participants were recruited amongst the local university population and the greater Chicago area though flyers and word of mouth. Participants completed a health history questionnaire and were excluded if they had known cardiovascular, metabolic, inflammatory, renal or respiratory diseases. Participants were also excluded if they were smokers or taking any medications. None of the participants were regularly consuming antioxidant supplements. Prior to any data collection, all participants provided written consent and the study was approved by the University of Illinois at Chicago Institutional Review Board. The study followed the procedures for protection of human participants as provided in the 2013 Declaration of Helsinki. The study protocol complies with the ethical standards of the International Journal of Sports Medicine [22].
Study design
Subjects reported to the lab for 2 visits, separated by at least 48 h. Previous pilot research utilizing the same antioxidant supplement found separation of the 2 conditions (placebo and antioxidant) by 24 h to be sufficient [48]. Participants received an AOX or placebo supplementation in a double-blind, randomized, cross-over design. Supplements were consumed in 2 dosages separated by 30 min: dosages were consumed 90 and 60 min prior to the FMD protocol. The first dose contained 300 mg of alpha-lipoic acid, 500 mg vitamin C and 200 International Units (I. U.) vitamin E. The second dose contained 300 mg alpha-lipoic acid, 500 mg vitamin C and 400 I. U. of vitamin E. The dosage and timing of this supplement is the result of previous research in young subjects demonstrating free radical concentration reduction measured by electron paramagnetic resonance spectroscopy without greatly exceeding the over-thecounter dosage of each antioxidant [37]. In addition, this supplement has been shown to reduce levels of oxidative stress and improve endothelial function in older CA adults [47]. Placebo supplementation was consumed in a similar fashion and capsules were of the same color, taste and appearance. Both were encapsulated by the University of Illinois at Chicago Investigational Drug Service.
Visits
Subjects were instructed to abstain from alcohol for 48 h and caffeine and exercise for 24 h, and were fasted for 12 h prior to each visit. Measurements of height were taken using a stadiometer to the nearest 0.1 cm and body weight was obtained using a beam balance platform scale to the nearest 0.1 kg. Body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters sguared. Subjects underwent a blood draw following the completed protocol at the end of both visits.
Subjects then assumed a supine position and rested quietly for 10 min in a temperature controlled room before systolic and diastolic blood pressure (SBP, DBP) measurements were obtained using an automated oscillometric cuff (HEM-907 XL; Omron, Japan). Brachial BP measurements were repeated, and if values were within 5 mm Hg of each other, the average of the 2 values was used for analysis. If measurements were not within 5 mmHg, readingswere taken until 2 values within 5 mmHg were obtained. Participants remained in a supine position for the remainder of the measurements.
Flow-mediated vasodilation
Flow-mediated vasodilatation (FMD) was assessed using ultrasonography (SSD-5500; Aloka) utilizing a standardized protocol [44]. A rapid-release cuff (Hokanson DE, Bellevue, WA, USA) was placed below the elbow joint on the widest part of the forearm. The subjects rested in the supine position with their right arm stabilized using an immobilizer cushion. The brachial artery was imaged in longitudinal sections, 5–10 cm proximal to placement of a blood-pressure cuff, using a high-frequency(5–13 MHz) linear-array probe. In B-mode, split mode was used to simultaneously measure the arterial diameter (displayed on the left side of the screen), and Doppler velocity (displayed on the right side of the screen). The flow signals were corrected at an insonation angle of 55 °. The sample volume was placed in the middle of the artery, with a large sampling area. Gated images were recorded at 5 frames s-1 using Vascular Tools (Medical Imaging Applications, Coralville, IA, USA). Baseline measures of resting brachial flow velocity and diameter were taken for 60 s. The blood pressure cuff was then inflated and the ischemic stimulus was maintained for 5 min. Image capture was restarted 30 s prior to cuff deflation and continued until 180 s post-deflation. A single technician collected and analyzed all FMD data and was blinded to all patient demographics during analysis (age, sex, race). Brachial analysis was completed offline using automated edge-detection software (Brachial Analyzer, Medical Imaging Applications, Coralville, IA, USA) and followed by visual inspection. The coefficient of variation for day-to-day variability was 10 %. FMD (%) was measured using the equation:
Dynamic handgrip exercise
Brachial blood flow was measured during a dynamic hand grip exercise. In a supine position, the subjects performed 3 maximal contractions, sgueezing a dynamometer (TSD121C hand dynamometer, Biopac Systems, Inc., Goleta, CA). The best attempt of the 3 contractions was used as the subject’s maximal voluntary contraction (MVC). Following a 5-min rest period, subjects performed rhythmic handgrip exercises at 10 and 20 % of MVC. Following auditory prompts from a metronome, the subjects contracted their dominant hand for one second and relaxed their hand for 2 s for a total of 5 min at each exercise intensity. A 10-min rest occurred between the 10% MVC and 20 % MVC exercise bouts. Brachial artery diameter and flow velocity were measured using ultrasonography (SSD-5500; Aloka) with a high-frequency (5–13 MHz) linear-array probe. Images were recorded using Vascular Tools (Medical Imaging Applications, Coralville, IA, USA), during diastole [7]. Resting brachial diameter and flow velocity were recorded for 60 s prior to the start of exercise. Exercise diameter and flow velocities were recorded and averaged during the final 60 s of each exercise bout (10 and 20 % MVC). Average brachial diameter (in cm) and average brachial flow velocity (in cm/s) were used to calculate forearm blood flow (FBF) using the equation:
Blood pressure values were measured at rest and during each exercise bout using a manual blood pressure cuff and sphygmomanometer. The mean arterial blood pressure (in mmHg) during the final minute of exercise and average forearm blood flow (mL/min) over the final 60 s was used to calculate forearm vascular conductance (FVC) using the following equation:
This protocol has been previously shown to be effective for evaluating the FBF response to exercise [9, 39].
Thiobarbituric Acid Reactive Substances (TBARS):
Lipid peroxidation was assessed through the measurement of MDA-TBARS in the plasma utilizing a TBARS Assay Kit (Item 10009055, Cayman Chemical, Ann Arbor, MI). TBARS are formed as a byproduct of the oxidative degradation of lipids by reactive oxygen species (lipid peroxidation), a commonly used marker of oxidative stress [38]. TBARS have been shown to change acutely following ingestion of the antioxidant utilized in the current study [47]. The assay involves the reaction of malondialdehyde (MDA), a product of lipid peroxidation, with thiobarbituric acid (TBA) under high temperature and acidic conditions to form a MDA-TBA complex that can be measured colorimetrically. Plasma samples were mixed with sodium dodecyl sulfate solution and TBA reagent (530 mg thiobarbituric acid solubilized in a mixed solution containing 50 ml of sodium hydroxide and 50 ml acetic acid). Samples were measured in duplicate, and standards were measured in triplicate. Absorbance was read at 540 nm using a Softmax Pro 5.0 Microplate Reader (Molecular Devices, Sunnyvale, CA). Any CVs above 10 % were re-run. Inter-assay and intra-assay CVs were 3.4 and 2.3 %, respectively.
Statistical analysis
Power analyses:
Power calculations were based upon previous research utilizing the same antioxidant supplement [11, 37, 47], suggesting that an n of 9 in each group would yield a power of 0.9. This was based on the expectation of a 3 % decrease in the CA group and a 4 % increase in the AA group. AOX effectiveness appears to be related to the baseline levels of oxidative stress [47] and because this is the first study to utilize antioxidant supplementation in a racially diverse cohort, the understanding that low levels of free radicals play a beneficial role in vasodilation [31] led to the expectation of a decrease in the CA group and an increase in a population with elevated oxidative stress like AAs [14]. Previous research utilizing the same antioxidant supplement [47] demonstrated that in young, healthy populations with low oxidative stress levels, antioxidant supplementation can have a negative effect on FMD, whereas in populations with elevated oxidative stress, such as elderly individuals, antioxidant supplementation can lead to improvements in FMD.
All data are presented as the mean ± standard error. Group differences between CAs and AAs in anthropometrics were measured using t-tests and all dependent variables (FMD %, TBARS) were tested using a repeated measures analysis of variance (ANOVA). To determine exercise blood flow and FVC responses, a 3-way ANOVA was performed (supplement by race by exercise intensity). An ANCOVA was performed to determine the effects of shear rate on FMD %, controlling for peak shear rate and AUC. Any significant findings were followed up with independent and dependent T-tests. Statistical significance was set at an alpha < 0.05. All analyses were conducted using IBM Statistical Package for Social Sciences software, version 22.0 (SPSS, Armonk, New York).
Results
Subject characteristics are reported in Table 1. There were no significant differences between groups.
Table 1.
Subject characteristics.
| AA (n = 9) | CA (n = 10) | Total (n = 19) | |
|---|---|---|---|
| Age (yr) | 25.6 ± 1.9 | 25.8 ± 1.5 | 25.7 ± 1.2 |
| Height (cm) | 177.3 ± 1.9 | 180.7 ± 2.3 | 179.1 ± 1.5 |
| Weight (kg) | 88.0 ± 5.8 | 84.2 ± 4.4 | 86.0 ± 3.5 |
| BMI (kg/m2) | 25.4 ± 1.7 | 23.3 ± 1.3 | 24.3 ± 1.0 |
| Brachial SBP (mmHg) | 131 ± 4 | 124 ± 3 | 127 ± 3 |
| Brachial DBP (mmHg) | 73 ± 3 | 67 ± 3 | 70 ± 2 |
| Brachial MAP (mmHg) | 92 ± 3 | 86 ± 3 | 89 ± 2 |
All data reported as mean ± SEM. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure
Although there was a baseline difference (placebo condition) in FMD (p < 0.043), with AAs exhibiting lower values, this difference was ameliorated by AOX; however, the interaction effect from the repeated measures ANOVA was not significant (p = 0.10, Fig. 1). There were also no group or condition differences in brachial artery baseline diameter (p = 0.30), peak brachial artery diameter (p = 0.66), and no differences when controlling for baseline brachial artery diameter. There were no differences when controlling for shear rate (peak shear rate p = 0.75, shear rate AUC p = 0.14, Table 2) and there was no effect of shear rate on FMD % when controlling for both peak shear rate and AUC.
Fig. 1.
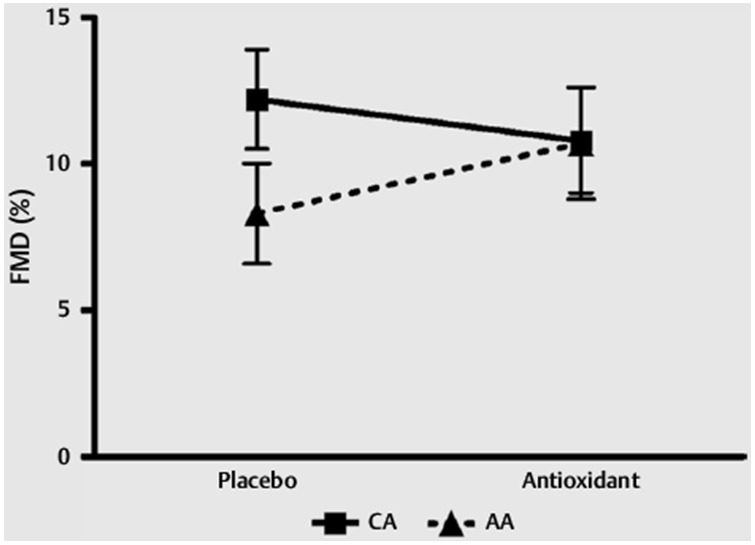
Flow Mediated Dilation. Flow mediated dilation response in CA and AA following placebo and antioxidant supplementation. Data are presented as mean ± SEM.
Table 2.
FMD values: brachial artery, flow velocity and shear rate.
| AA (n = 9) | CA (n = 10) | |||
|---|---|---|---|---|
| Condition | Placebo | AOX | Placebo | AOX |
| Brachial baseline diameter (mm) | 4.24 ± 0.22 | 4.20 ± 0.24 | 3.89 ± 0.15 | 3.96 ± 0.16 |
| Peak brachial diameter (mm) | 4.58 ± 0.20 | 4.62 ± 0.21 | 4.40 ± 0.18 | 4.40 ± 0.20 |
| FMD flow velocity at peak diameter (cm/s) | 42.19 ± 10.15 | 36.37 ± 9.87 | 39.07 ± 7.18 | 37.54 ± 6.98 |
| FMD shear rate at peak diameter (s−1) | 896.5 ± 169.7 | 582.3 ± 157.9 | 740.7 ± 151.8 | 710.2 ± 141.2 |
| Maximal Flow Velocity (cm/s) | 89.26 ± 6.95 | 77.26 ± 6.49 | 100.02 ± 6.22 | 99.51 ± 5.80 |
| Maximal Shear Rate (s−1) | 1 751 ± 191 | 1 533 ± 236 | 2 095 ± 171 | 1 989 ± 211 |
| Shear Rate AUC (AU) | 12 655 ± 2063 | 9 746 ± 2 163 | 13 543 ± 1 598 | 16 277 ± 1 676 |
All data reported as mean ± SEM. FMD, flow mediated dilation; AUC, area under the curve; AU, arbitrary units
Fig. 2 shows responses to handgrip exercise. Fig. 2a shows brachial blood flow responses at rest and with handgrip exercise. There was a significant effect of exercise (p = 0.0001) with both AOX and placebo, but there were no significant group differences or interaction effects. Fig. 2b shows FVC responses at rest and with handgrip exercises, and there was a significant effect of exercise (p = 0.0001)with both AOX and placebo. There were no significant differences between AAs and CAs in resting or exercise brachial artery diameter with either AOX or placebo (Table 3) and no significant group differences in MVC (AA = 40.7 ± 4.03 kg vs. CA = 42.4 ± 3.10 kg).
Fig. 2.
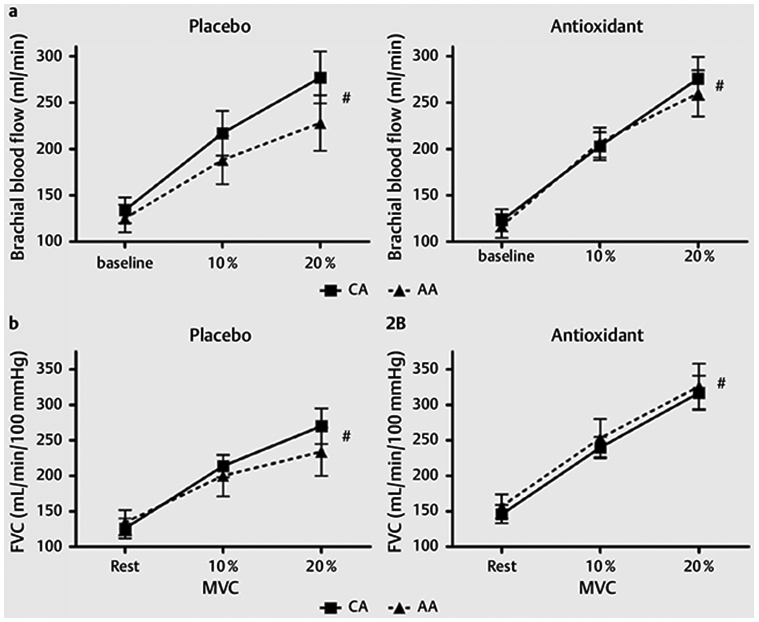
a (left) Brachial blood flow responses following placebo supplementation at rest and at 10 %MVC and 20 %MVC handgrip exercise. * p<0.05 significant difference between races. #p < 0.05 significant exercise response. a (right) Brachial blood flow responses following antioxidant supplementation at rest and at 10 %MVC and 20 %MVC handgrip exercise. * p < 0.05 significant difference between races. #p < 0.05 significant exercise response. b (left) Forearm vascular conductance (FVC) response following placebo supplementation at rest and at 10 %MVCand 20 %MVC handgrip exercise. * p < 0.05 significant difference between races. # p < 0.05 significant exercise response. b (right) Forearm vascular conductance (FVC) response following antioxidant supplementation at rest and at 10 %MVC and 20 %MVC handgrip exercise. * p < 0.05 significant difference between races. # p < 0.05 significant exercise response.
Table 3.
Brachial artery diameters.
| African Americans | |||
|---|---|---|---|
| Rest | 10 %MVC | 20 %MVC | |
| Placebo (mm) | 4.250 ± 0.197 | 4.328 ± 0.193 | 4.311 ± 0.205 |
| AOX (mm) | 4.201 ± 0.208 | 4.243 ± 0.195 | 4.367 ± 0.195 |
| Caucasians | |||
| Rest | 10 %MVC | 20 %MVC | |
| Placebo (mm) | 3.895 ± 0.187 | 3.957 ± 0.183 | 3.906 ± 0.194 |
| AOX (mm) | 3.967 ± 0.197 | 4.079 ± 0.185 | 4.121 ± 0.185 |
All data are reported as mean ± SEM. AOX, antioxidant condition; MVC10 %, 10 % of maximal voluntary contraction; MVC20 %, 20 % of maximal voluntary contraction
Fig. 3 shows MDA-TBARS response to supplementation. There were no differences between races and no effect with AOX.
Fig. 3.
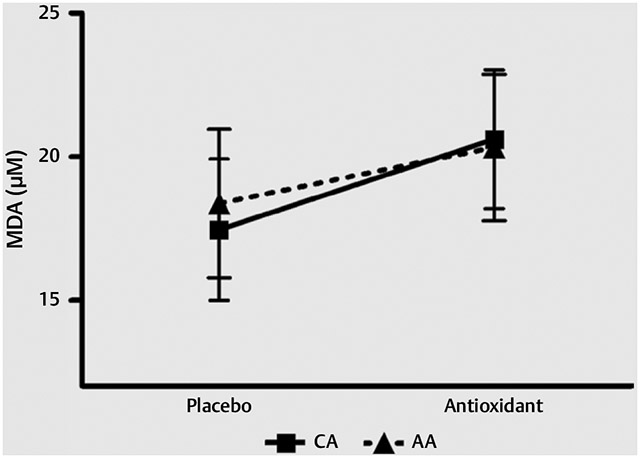
TBARS. MDA-TBARS values measured in plasma following placebo and AOX supplementation. Data are reported as mean ± SEM.
Conclusions
The novel findings of the present study are as follows: 1) AAs exhibited lower endothelial function (FMD %) at baseline (placebo condition), which was ameliorated following AOX, but there were no statistically significant changes following AOX supplementation; 2) there were no racial differences or supplemental differences in brachial blood flow or forearm vascular conductance with exercise; and 3) there were no racial differences in MDA-TBARS and no change with AOX supplementation.
There has been previous data suggesting elevated oxidative stress [16] and decreased NO bioavailability [1] in AAs, which manifests as endothelial dysfunction, potentially leading to reduced exercise responsiveness (brachial artery vasodilation or blood flow). However, in this study we demonstrated in a young, healthy male cohort, although there were racial differences in endothelial function in the placebo condition, the changes with antioxidant supplementation were not significant. The lack of racial differences in response to AOX could be due an inadequate sample size (9 AAs vs. 10 CAs). Due to the lack of previous studies of AAs in this area, power analyses were based upon hypothesized responses (i. e., a high oxidative stress group vs. a low oxidative stress group). In addition, it is possible that we were unable to reach power due to the measurement differences between studies.
Although not statistically significant when comparing race and supplement effects in FMD %, a t-test on the placebo condition alone demonstrated that CA males exhibited a higher FMD % compared to the AA cohort. This difference was eliminated with AOX supplementation, with the CA group experiencing a slight decrease in FMD % whereas the AA group demonstrated a slight increase. However, the lack of significant interaction encourages future research with a larger number of subject in order to determine potential racial differences. The lack of an effect with AOX supplementation may be due to the population recruited being young (mean age of 26), of normal weight (BMI = 24.3) and free of disease and medication usage. It has been shown that in individuals free of elevated oxidative stress levels, antioxidant supplementation has either a negative or no effect on endothelial function [21]. The MDA-TBARS analysis confirmed no racial differences between AAs and CAs, indicating a lack of elevated oxidative stress in AA individuals, which was not concordant with our hypothesis. This lack of elevated oxidative stress is also possibly why antioxidant supplementation did not alter MDA-TBARS. In addition, a previous study showing a decrease in MDA-TBARS following antioxidant supplementation [47] was performed in an elderly cohort, a group that demonstrated measured elevated oxidative stress and therefore was significantly affected by antioxidant supplementation, leading to reductions in oxidative stress and MDA-TBARS. This antioxidant supplement, although previously established to decrease oxidative stress in individuals exhibiting elevated oxidative stress, did not have an effect on MDA-TBARS possibly due to a lack of elevated lipid peroxidation in the young, AA male cohort in this study, in contrast to our hypothesis.
During exercise, arterioles in the skeletal muscle dilate to allow for increased muscle blood flow and subsequent elevations in oxygen delivery to the working muscle to meet metabolic demand [20]. This dilation can occur as a result of vasoactive substances such as prostaglandins, adenosine and NO [6, 46] and mechanical signaling factors in exercising muscle [45] and/or shear stress. Abnormal responses in blood flow, dilation or vasodilatory factors, resulting in altered cardiovascular responses to exercise can provide insight regarding cardiovascular risk and group differences that are not always evident at rest. The role of NO in exercise-induced conduit artery vasodilation [49] could be diminished in the presence of an oxidative stress environment. The importance of NO in exercise vasodilation can be seen in aging individuals who suffer from reduced perfusion during exercise [11].
There were no observed differences between AAs and CAs in brachial blood flow or FVC at rest. With exercise, there were no differences in brachial blood flow or FVC, and there were also no differences following AOX. Similar to the FMD % results, CAs displayed slightly higher brachial artery blood flow compared to AAs, and although this gap was eliminated following AOX, this did not reach statistical significance and warrants further research with a higher subject number to determine potential racial differences and AOX effects. Conflicting with the results of Richardson et al. (2007), who demonstrated attenuated brachial artery vasodilation with exercise following AOX [37], brachial diameter with exercise was not significantly different between AOX and placebo conditions in our study.
The overall lack of racial differences in exercise blood flow and oxidative stress is somewhat inconsistent with previous work [1, 16] and may be due to several factors. In studies observing elevated oxidative stress in AAs and CAs and differential vascular responses, the subject population varies widely. Mean ages were older [4, 5], there was a mixture of males and females [4, 5, 16] some with both pre- and post-menopausal women [5] and smokers [4, 5]. Because these factors could affect oxidative stress and NO bioavailability, our study was designed to eliminate variability by including only young, healthy, nonsmoking males.
Measurements and exercise stimuli vary between studies and may also be a reason behind differing results. Basset et al. (1992) found racial differences in forearm vascular resistance, and although Bassett utilized similar measures and had a similar subject mean age, the sample size was double the current study’s (21 blacks and 20 whites) [1]. Additionally, although measures were similar, the equipment and exercise stimulus were not identical. Venous occlusion plethysmography was used to determine forearm blood flow, and the exercise stimulus was 10 min of ischemic handgrip exercise. In addition, the vascular bed studied was the forearm resistance vessels whereas the current study measured a conduit artery. However, it should be noted that despite different vascular beds, conduit artery blood flow is reflective of forearm resistance vessel function [27].
There are a few limitations in the current study. First, the AA cohort did have slightly higher BMI and blood pressure values, as seen in Table 1. Although this was not significantly different between groups, it should be noted that these values are less than ideal and could indicate this group was not as healthy as the CA cohort.
Physical activity was not directly assessed and therefore it is possible that there were varying levels of fitness within groups, which could have an effect on FMD. However, only sedentary and recreationally active individuals were recruited, so there was little variation between individuals in terms of physical activity levels.
We also performed blood draws following the completion of the entire protocol, which might have affected the MDA-TBARS measurement. Because the blood draw was performed following the acute handgrip exercise and exercise can lead to acute increases in TBARS [10], it is possible that the lack of increase in TBARS could be due to the antioxidant supplement. However, conflicting studies demonstrated no change in TBARS following acute exercise [8, 14] in age groups and intensities similar to that of our current study. In comparison to a previous study observing oxidative stress in a similar cohort [15], our MDA-TBARS values are higher (an average of 17 μM compared to 4 μM). However, our values are well within the standard curve and our low CVs suggest these differences could be attributed to equipment, reagents, or protocols, in additional to the overall difficulty of precisely quantifying oxidative stress measurements. Therefore we refrained from comparing our MDA-TBARS values to any previous studies.
In addition, we did not see significant changes in MDA-TBARS between conditions and did not perform additional blood measures, therefore we were unable to directly document reduced free radical concentration or a reduction in oxidative stress levels with the antioxidant supplement. However, we chose to utilize this supplement because of the well-documented research regarding the efficacy of this antioxidant and its ability to reduce free radicals and decrease oxidative stress [11, 37, 47]. In addition, the dosage and timing of the supplement remained identical to these studies; however, the lack of direct blood measures of oxidative stress in this current study is a limitation.
In conclusion, the results of this study demonstrate that these young, apparently healthy AA males had lower FMD at baseline, but the AOX did not significantly change FMD in either group. Further-more, FBF and FVC in response to exercise was similar between AAs and CAs, and AOX had no effect on the responses in either group. However, a lack of statistical power may have impacted our findings and further study utilizing a larger sample size is warranted.
Acknowledgements
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000050. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- [1].Bassett DR Jr., Duey WJ, Walker AJ, Howley ET, Bond V. Racial differences in maximal vasodilatory capacity of forearm resistance vessels in normotensive young adults. Am J Hypertens 1992; 5: 781–786 [DOI] [PubMed] [Google Scholar]
- [2].Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 2000; 87: 840–844 [DOI] [PubMed] [Google Scholar]
- [3].Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. J Am Coll Cardiol 2002; 40: 754–760 [DOI] [PubMed] [Google Scholar]
- [4].Cardillo C, Kilcoyne CM, Cannon RO 3rd, Panza JA. Attenuation of cyclic nucleotide-mediated smooth muscle relaxation in blacks as a cause of racial differences in vasodilator function. Circulation 1999; 99: 90–95 [DOI] [PubMed] [Google Scholar]
- [5].Cardillo C, Kilcoyne CM, Cannon RO 3rd, Panza JA. Racial differences in nitric oxide-mediated vasodilator response to mental stress in the forearm circulation. Hypertension 1998; 31: 1235–1239 [DOI] [PubMed] [Google Scholar]
- [6].Casey DP, Mohamed EA, Joyner MJ. Role of nitric oxide and adenosine in the onset of vasodilation during dynamic forearm exercise. Eur J Appl Physiol 2013; 113: 295–303 [DOI] [PubMed] [Google Scholar]
- [7].Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002; 39: 257–265 [DOI] [PubMed] [Google Scholar]
- [8].Diaz KM, Feairheller DL, Sturgeon KM, Williamson ST, Brown MD. Oxidative Stress Response to Short Duration Bout of Submaximal Aerobic Exercise in Healthy Young Adults. Int J Exerc Sci 2011; 4: 247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic alpha-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 2005; 567: 311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Djordjevic DZ, Cubrilo DG, Barudzic NS, Vuletic MS, Zivkovic VI, Nesic M, Radovanovic D, Djuric DM, Jakovljevic V. Comparison of blood pro/antioxidant levels before and after acute exercise in athletes and non-athletes. Gen Physiol Biophys 2012; 31: 211–219 [DOI] [PubMed] [Google Scholar]
- [11].Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol 2010; 298: H671–H678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 2004; 556: 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol 2004; 24: 1367–1373 [DOI] [PubMed] [Google Scholar]
- [14].Feairheller DL, Diaz KM, Sturgeon KM, Williamson ST, Brown MD. Racial Differences in the Time-Course Oxidative Stress Responses to Acute Exercise. J Exerc Physiol Online 2011; 14: 49–59 [PMC free article] [PubMed] [Google Scholar]
- [15].Feairheller DL, Park JY, Rizzo V, Kim B, Brown MD. Racial differences in the responses to shear stress in human umbilical vein endothelial cells. Vasc Health Risk Manag 2011; 7: 425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Feairheller DL, Park JY, Sturgeon KM, Williamson ST, Diaz KM, Veerabhadrappa P, Brown MD. Racial differences in oxidative stress and inflammation: in vitro and in vivo. Clin Transl Sci 2011; 4: 32–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics – 2014 update: a report from the American Heart Association. Circulation 2014; 129: e28–e292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gokce N, Keaney JF Jr., Frei B, Holbrook M, Olesiak M, Zachariah BJ, Leeuwenburgh C, Heinecke JW, Vita JA. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 1999; 99: 3234–3240 [DOI] [PubMed] [Google Scholar]
- [19].Gower BA, Ard JD, Hunter GR, Fernandez J, Ovalle F. Elements of the metabolic syndrome: association with insulin sensitivity and effects of ethnicity. Metab Syndr Relat Disord 2007; 5: 77–86 [DOI] [PubMed] [Google Scholar]
- [20].Granger HJ, Goodman AH, Granger DN. Role of resistance and exchange vessels in local microvascular control of skeletal muscle oxygenation in the dog. Circ Res 1976; 38: 379–385 [DOI] [PubMed] [Google Scholar]
- [21].Harris RA, Nishiyama SK, Wray DW, Tedjasaputra V, Bailey DM, Richardson RS. The effect of oral antioxidants on brachial artery flow-mediated dilation following 5 and 10 min of ischemia. Eur J Appl Physiol 2009; 107: 445–453 [DOI] [PubMed] [Google Scholar]
- [22].Harriss DJ, Atkinson G. Ethical Standards in Sport and Exercise Science Research: 2016 Update. Int J Sports Med 2015; 36: 1121–1124 [DOI] [PubMed] [Google Scholar]
- [23].Heffernan KS, Jae SY, Fernhall B. Racial differences in arterial stiffness after exercise in young men. Am J Hypertens 2007; 20: 840–845 [DOI] [PubMed] [Google Scholar]
- [24].Heffernan KS, Jae SY, Wilund KR, Woods JA, Fernhall B. Racial differences in central blood pressure and vascular function in young men. Am J Physiol 2008; 295: H2380–H2387 [DOI] [PubMed] [Google Scholar]
- [25].Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001; 104: 2673–2678 [DOI] [PubMed] [Google Scholar]
- [26].Hozawa A, Folsom AR, Sharrett AR, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects – Atherosclerosis Risk in Communities Study. Arch Intern Med 2007; 167: 573–579 [DOI] [PubMed] [Google Scholar]
- [27].Irace C, Ceravolo R, Notarangelo L, Crescenzo A, Ventura G, Tamburrini O, Perticone F, Gnasso A. Comparison of endothelial function evaluated by strain gauge plethysmography and brachial artery ultrasound. Atherosclerosis 2001; 158: 53–59 [DOI] [PubMed] [Google Scholar]
- [28].Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol 2007; 103: 1715–1721 [DOI] [PubMed] [Google Scholar]
- [29].Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation 2004; 109: 2511–2517 [DOI] [PubMed] [Google Scholar]
- [30].Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol 2003; 285: H1023–H1031 [DOI] [PubMed] [Google Scholar]
- [31].Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 2003;93: 573–580 [DOI] [PubMed] [Google Scholar]
- [32].Loehr LR, Espeland MA, Sutton-Tyrrell K, Burke GL, Crouse JR 3rd, Herrington DM. Racial differences in endothelial function in postmenopausal women. Am Heart J 2004; 148: 606–611 [DOI] [PubMed] [Google Scholar]
- [33].Minor DS, Wofford MR, Jones DW. Racial and ethnic differences in hypertension. Curr Atheroscler Rep 2008; 10: 121–127 [DOI] [PubMed] [Google Scholar]
- [34].Morris AA, Patel RS, Binongo JN, Poole J, Al Mheid I, Ahmed Y, Stoyanova N, Vaccarino V, Din-Dzietham R, Gibbons GH, Quyyumi A. Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J Am Heart Assoc 2013; 2: e002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 2001; 104: 191–196 [DOI] [PubMed] [Google Scholar]
- [36].Pescatello LS, Bairos L, Vanheest JL, Maresh CM, Rodriguez NR, Moyna NM, DiPasguale C, Collins V, Meckes CL, Krueger L, Thompson PD. Postexercise hypotension differs between white and black women. Am Heart J 2003; 145: 364–370 [DOI] [PubMed] [Google Scholar]
- [37].Richardson RS, Donato AJ, Uberoi A, Wray DW, Lawrenson L, Nishiyama S, Bailey DM. Exercise-induced brachial artery vasodilation: role of free radicals. Am J Physiol 2007; 292: H1516–H1522 [DOI] [PubMed] [Google Scholar]
- [38].Schisterman EF, Faraggi D, Browne R, Freudenheim J, Dorn J, Muti P, Armstrong D, Reiser B, Trevisan M. TBARS and cardiovascular disease in a population-based sample. J Cardiovasc Risk 2001; 8: 219–225 [DOI] [PubMed] [Google Scholar]
- [39].Schrage WG, Wilkins BW, Dean VL, Scott JP, Henry NK, Wylam ME, Joyner MJ. Exercise hyperemia and vasoconstrictor responses in humans with cystic fibrosis. J Appl Physiol 2005; 99: 1866–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Stein CM, Lang CC, Nelson R, Brown M, Wood AJ. Vasodilation in black Americans: attenuated nitric oxide-mediated responses. Clin Pharmacol Ther 1997; 62: 436–443 [DOI] [PubMed] [Google Scholar]
- [41].Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev 2004; 84: 1381–1478 [DOI] [PubMed] [Google Scholar]
- [42].Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr., Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000; 101: 948–954 [DOI] [PubMed] [Google Scholar]
- [43].Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 2001; 38: 274–279 [DOI] [PubMed] [Google Scholar]
- [44].Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol 2011; 300: H2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 2002; 541: 623–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wilson JR, Kapoor SC. Contribution of prostaglandins to exercise-induced vasodilation in humans. Am J Physiol 1993; 265: H171–H175 [DOI] [PubMed] [Google Scholar]
- [47].Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MA, Ives SJ, Barrett-O’Keefe Z, Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 2012; 59: 818–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin Sci (Lond) 2009; 116: 433–441 [DOI] [PubMed] [Google Scholar]
- [49].Wray DW, Witman MA, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Progressive handgrip exercise: evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. Am J Physiol 2011; 300: H1101–H1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yan H, Ranadive SM, Heffernan KS, Lane AD, Kappus RM, Cook MD, Wu PT, Sun P, Harvey IS, Woods JA, Wilund KR, Fernhall B. Hemodynamic and arterial stiffness differences between African-Americans and Caucasians after maximal exercise. Am J Physiol 2014; 306: H60–H68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zion AS, Bond V, Adams RG, Williams D, Fullilove RE, Sloan RP, Bartels MN, Downey JA, De Meersman RE. Low arterial compliance in young African-American males. Am J Physiol 2003; 285: H457–H462 [DOI] [PubMed] [Google Scholar]


