Abstract
Objectives:
Nasal steroids, oral anti-leukotrienes and supplemental oxygen are effective in the treatment of mild obstructive sleep apnea (OSA) in otherwise healthy children. However, their efficacy is unknown in children with Down syndrome (DS). Here we examine the effect of single medication therapy versus observation versus oxygen on polysomnographic outcomes in these children.
Methods:
We reviewed children (<18 years) diagnosed with DS and mild OSA (obstructive apnea-hypopnea index [oAHI] ≥1 to <5 events/hour) treated non-surgically (with supplemental oxygen, one medication, or observation) between 2012 and 2017. Demographic data, comorbid diagnoses, and pre- and posttreatment polysomnograms were analyzed. We assessed pre- and posttreatment oAHI, oxyhemoglobin saturation nadir, percent total sleep time (%TST) in rapid eye movement (REM), and end-tidal carbon dioxide (ETCO2) >50 mmHg.
Results:
Twenty-four children met inclusion criteria; 10 treated with medication, one with oxygen, and 13 with observation (baseline oAHI was 3.5, 3.3, and 2.9 events/hour, respectively). There was no significant change in oAHI, oxyhemoglobin saturation nadir, ETCO2, or percent TST in REM after treatment for any treatment group (P = .21–.94). There was no association between reported symptoms and AHI severity or change in AHI. OSA resolved in one patient treated with observation and two treated with medication, but worsened in two each in the medication and observation groups. Resolution of OSA occurred in 20% treated with medication, 7.7% with observation, and 0% with oxygen (P = .82).
Conclusion:
In our cohort, resolution of mild OSA was low. This suggests that consideration should be given to multimodality treatments in children with DS and mild OSA. Prospective studies will help establish effectiveness in this cohort.
Keywords: Obstructive sleep apnea, mild, observation, infant, pediatric, children, medication
Editor’s Note:
This Manuscript was accepted for publication on September 6, 2019.
INTRODUCTION
Down syndrome (DS), defined as having an additional copy of chromosome 21, is the most common genetic disorder, occurring in one in 691 births.1 Individuals with DS are at an increased risk of a myriad of medical conditions, including obstructive sleep apnea (OSA). Higher than the 1% to 6% prevalence in the general population of children, the prevalence of OSA is DS children is estimated to be between 30% and 50%.2,3 This prevalence grows with age, as >90% of DS adults are estimated to have OSA.4 Additionally, individuals with DS tend to have a more severe OSA phenotype, including significant hypoxemia and hypoventilation, when compared to those without DS.4 Several anatomic factors present in DS children increase the likelihood of upper airway obstruction including a higher prevalence of maxillary and mandibular hypoplasia, relative macroglossia, lingual tonsillar hypertrophy, pharyngeal hypotonia, laryngomalacia, subglottic and tracheal stenosis, and obesity than in the general population.5–13 Additionally, comorbid medical conditions with increased prevalence in DS individuals like gastroesophageal reflux (GERD) and hypothyroidism can increase the likelihood of OSA development through airway narrowing secondary to inflammation and mucopolysaccharide deposition, respectively.14–17
Many of the manifestations of OSA in DS children, including snoring and poor sleep quality, overlap with OSA among children in the general population. However, symptoms more likely to be prominent in DS OSA include failure to thrive, hyperactivity, behavioral disruptions, and poor school performance.18
OSA in children has been associated with numerous sequelae including cardiovascular disease, metabolic syndrome, and neurocognitive dysfunction.19 Additionally, OSA can exacerbate preexisting medical conditions common in DS children such as congenital heart disease and neurocognitive dysfunction. Some studies have found that DS children with OSA performed worse on cognitive testing than DS children without OSA.20
While adenotonsillectomy (AT) is the mainstay of OSA treatment in children, normalization of PSG abnormalities following AT in children with DS is low. The aim of this study is to review different treatment modalities in children with DS and mild OSA and their respective effects on polysomnographic parameters.
METHODS
We performed a retrospective chart review of all children (<18 years of age) with DS and mild OSA who were treated non-surgically from 2012 to 2017 at Cincinnati Children’s Hospital Medical Center. We defined mild OSA as an obstructive apnea-hypopnea index (oAHI) ≥1 and <5 events/hour diagnosed by overnight polysomnogram (PSG). Patients were included if they were treated with a single modality (one medication or supplemental oxygen) or observation within 1 year of baseline PSG and had a follow-up PSG. Responders were defined as children whose oAHI was <1 event per hour on the follow-up PSG. Patients without a follow-up PSG or with PSGs less than 3 months apart were excluded. Patients were also excluded if they had a history of prior surgical intervention for OSA (ie, adenotonsillectomy), neuromuscular disease, current tracheostomy, or chronic interstitial lung disease.
Demographic data and additional comorbid diagnoses were collected. Body mass index (BMI) percentile was determined based on Centers for Disease Control and Prevention calculation for age and gender for children >2 years of age. PSG outcomes were recorded for baseline and follow-up apnea-hypopnea index (AHI), oAHI, oxyhemoglobin saturation nadir, percentage of total sleep time spent (%TST) with end-tidal carbon dioxide (ETCO2) >50 mmHg, sleep efficiency (SE), arousal index, and percentage of TST spent in REM sleep (%REM). Baseline and follow-up visits were reviewed to document patient or parent sleep-related complaints of snoring, gasping, breathing pauses, heavy breathing, noisy breathing, nocturnal enuresis, daytime behavior problems, sleepiness, or morning headaches. This study was approved by the institutional review board at the Cincinnati Children’s Hospital Medical Center.
Polysomnography
All overnight PSGs were performed in an accredited sleep center within Cincinnati Children’s Hospital Medical Center. Standard PSG parameters were recorded simultaneously, including: body position, bilateral electrooculogram (ROC/A1, LOC/A2), six-channel electroencephalogram (EEG; F3A2, F4A1, C3A2, C4A1, O1A2, O2A1), chin electromyogram, anterior tibialis electromyogram, tracheal microphone, electrocardiogram, pulse oximetry, thoracic and abdominal inductance plethysmography, nasal pressure transduction, and end-tidal CO2 (ETCO2) (BCI Capnoguard, OH). If ETCO2 did not plateau and/or if malfunction of the monitoring cannulas occurred, the ETCO2 was deemed unreliable and it was not included in scoring (capillary blood gases were obtained in these instances). Scoring of the PSG was performed with standard criteria as defined by the American Academy of Sleep Medicine.21 Sleep stage and respiratory scoring were performed by a certified sleep technician and interpreted by a board-certified sleep physician. An obstructive apnea was defined as a cessation or decrease in in the oronasal thermal airflow tracing by >90% of the pre-event baseline tracing with retained effort throughout the episode. An obstructive hypopnea was defined as a decrease in the peak excursion of the nasal pressure transducer by ≥30% when compared with the preceding baseline tracing and was associated with an oxygen desaturation ≥3%, an arousal, or an awakening. All obstructive events were ≥2 breaths’ duration. The number of apneas and hypopneas (including central and mixed) per hour were calculated and reported as the AHI. The oAHI was defined as the number of obstructive or mixed apneas and hypopneas per hour. Mild OSA was defined as an oAHI ≥1 and < 5 events/hour. Oxygen nadir was determined by the lowest oxygen saturation data point during an obstructive event.
Statistical Analysis
Descriptive statistics were used to examine the demographic data. Data distributions were reported as medians with 95% confidence intervals (CI) for continuous variables and frequencies with percentages for categorical variables. The changes in PSG respiratory and EEG parameters between baseline and follow-up were analyzed using the Wilcoxon signed rank test. Changes in categorical variables were tested using the McNemar test.
RESULTS
Forty-eight patients with mild OSA and Down syndrome were identified during the study. Seventeen patients were excluded due to lack of follow-up PSG. Seven patients were excluded because they were treated with both a leukotriene-inhibitor and a nasal steroid spray. Twenty-four children met inclusion criteria: 10 treated with medication, one with oxygen, and 13 with observation. Fourteen of 24 (58.3%) were female. Sixteen (66.7%) were white, four (16.7%) were black, and four (16.7%) were identified as “other.” Median age of the observation group was 4.4 years, oxygen group was 0.6 years, medication group was 8.6 years (P = .015). There were no other significant differences in demographics or comorbidities between the three treatment groups (P = .1–0.79). Demographic data can be found in Table I.
TABLE I.
Baseline Patient Characteristics for Children with Down Syndrome and Mild Obstructive Sleep Apnea.
| Characteristic | Observation (n = 13) | Oxygen (n = 1) | Medication (n = 10) | P |
|---|---|---|---|---|
| Age, years, median (95% CI) | 4.4 (0.2–10.9) | 0.6 (–) | 8.6 (1.9–14.3) | .015 |
| Sex, female, n (%) | 8 (61.5) | 1 (100.0) | 5 (50.0) | .60 |
| Race, n (%) | .73 | |||
| White | 9 (69.2) | 1 (100.0) | 6 (60.0) | |
| Black | 2 (15.4) | – | 2 (20.0) | |
| Other | 2 (15.4) | – | 2 (20.0) | |
| Body mass index percentile,(95% CI) | 86.0 (61.0–99.9) | – | 94.0 (65.1–9.9) | .31 |
| Comorbidities, n, (%)* | ||||
| Hypertension | 1 (7.7) | 1 (100.0) | 2 (20.0) | .06 |
| Dyslipidemia | 0 (0.0) | 0 (0.0) | 3 (30.0) | .10 |
| Mood disorders | 1 (7.7) | 0 (0.0) | 0 (0.0) | .66 |
| ADHD | 2 (15.4) | 0 (0.0) | 0 (0.0) | .41 |
| Allergies | 3 (23.1) | 0 (0.0) | 3 (30.0) | .79 |
| Asthma | 2 (15.4) | 0 (0.0) | 3 (30.0) | .62 |
| Cardiac disease | 10 (76.9) | 1 (100.0) | 6 (60.0) | .56 |
| Laryngomalacia | 4 (30.8) | 0 (0.0) | 2 (20.0) | .72 |
ADHD = attention deficit hyperactivity disorder; CI = confidence interval.
Observation
Thirteen patients were managed with observation. Median time between PSGs was 15.6 months. Their median age was 4.4 years (95% CI, 0.2–10.9). Eight of the 13 (61.5%) were female; nine (69.2%) were white, two (15.4%) were black, and two (15.4%) were identified as “other.” The median BMI percentile was 86.0 (95% CI, 61.0–99.9). Comorbidities included: cardiac disease (76.9%), laryngomalacia (30.8%), allergies (23.1%), asthma (15.4%), ADHD (15.4%), pulmonary hypertension (12.5%), and hypertension (7.7%).
Sleep-related complaints were recorded upon initial consultation and follow-up in 69.2% (9/13) children. Seven of these nine children had an increase in AHI while two had a decrease in AHI. Snoring was the most common complaint. For those with an increase in AHI, snoring was reported by 71.4% at baseline and decreased to 57.1% at follow-up. For those with a decrease in AHI, snoring was reported by 50.0% at baseline and did not change at follow-up. Two patients had an improvement in symptoms that corresponded to an improvement in the AHI. Table II illustrates each patient’s symptoms at the baseline and follow-up visits. Figure 1 demonstrates the percentage of patients with symptoms at baseline and follow-up grouped by change in AHI.
TABLE II.
Baseline and Follow-Up Obstructive Apnea-Hypopnea Index and Subjective Sleep-Related Complaints Grouped by Symptom Change for Children with Down Syndrome and Mild Obstructive Sleep Apnea.
| Nocturnal symptoms | Daytime symptoms | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change in symptoms | Therapy | Appointment type | oAHI | Snoring | Gasping | Breath pauses | Heavy breathing | Noisy breathing | Nocturnal enuresis | Behavior problems | Sleepiness | Morning headache |
| Improved | Observation | Initial | 3.4 | Yes | Yes | Yes | ||||||
| Follow-up | 3.9 | |||||||||||
| Observation | Initial | 2.2 | Yes | Yes | ||||||||
| Follow-up | 3.7 | Yes | ||||||||||
| Observation | Initial | 1.1 | Yes | Yes | ||||||||
| Follow-up | 1.4 | Yes | ||||||||||
| Observation | Initial | 3.7 | Yes | Yes | Yes | |||||||
| Follow-up | 4.5 | Yes | Yes | |||||||||
| Medication | Initial | 1.6 | Yes | |||||||||
| Follow-up | 1.4 | |||||||||||
| Medication | Initial | 3.3 | Yes | |||||||||
| Follow-up | 3.5 | |||||||||||
| Medication | Initial | 3.8 | Yes | |||||||||
| Follow-up | 1.4 | |||||||||||
| No Change | Observation | Initial | 2.4 | Yes | Yes | |||||||
| Follow-up | 3.6 | Yes | Yes | |||||||||
| Observation | Initial | 1.5 | ||||||||||
| Follow-up | 5.2 | |||||||||||
| Medication | Initial | 1.3 | Yes | |||||||||
| Follow-up | 1.7 | Yes | ||||||||||
| Medication | Initial | 4.4 | Yes | |||||||||
| Follow-up | 11.3 | Yes | ||||||||||
| Medication | Initial | 3.6 | ||||||||||
| Follow-up | 13.8 | |||||||||||
| Medication | Initial | 1.3 | ||||||||||
| Follow-up | 12.2 | |||||||||||
| Worsened | Observation | Initial | 4.9 | |||||||||
| Follow-up | 2.9 | Yes | ||||||||||
| Observation | Initial | 1.8 | Yes | Yes | ||||||||
| Follow-up | 1.3 | Yes | Yes | Yes | ||||||||
| Oxygen | Initial | 3.3 | ||||||||||
| Follow-up | 1.1 | Yes | Yes | |||||||||
| Medication | Initial | 4.7 | Yes | Yes | Yes | Yes | Yes | |||||
| Follow-up | 3.6 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||
oAHI = obstructive apnea-hypopnea index.
Children with an increase in the apnea-hypopnea index are highlighted in grey.
Fig. 1.
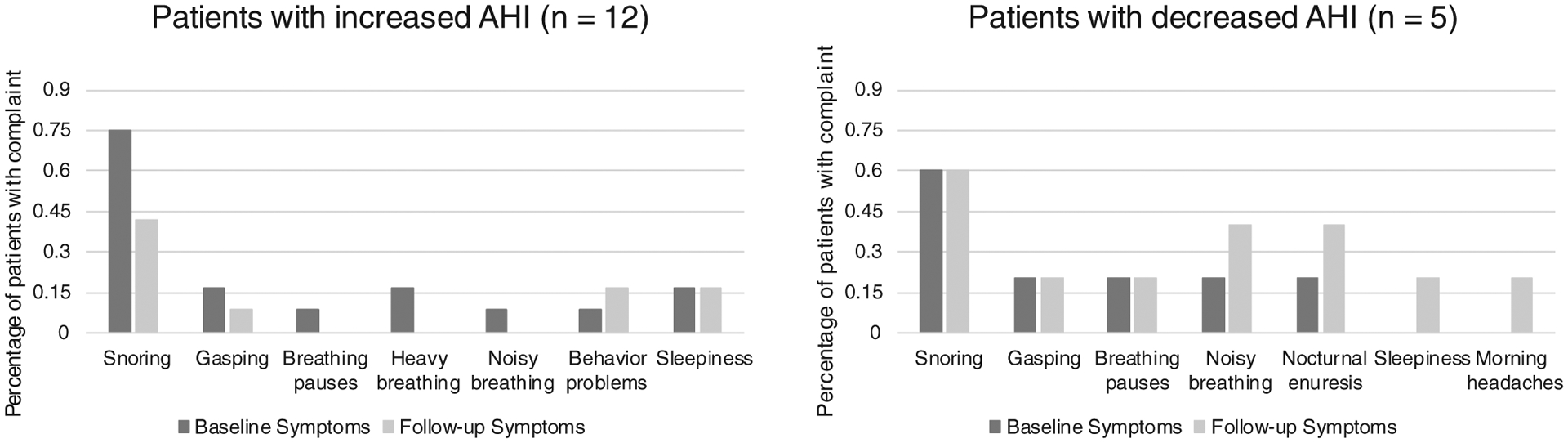
Percentage of patients with sleep-related complaints at baseline and follow-up appointments grouped by increase or decrease in AHI.
Interval between PSGs was a median of 15.6 months. Median oAHI was 2.9 events/hour at baseline and 3.6 events/hour on follow-up PSG (P = .60). Changes in AHI (P = .60), oxygen saturation nadir (P = .80), sleep efficiency (P = .32), arousal index (P = .46), %REM sleep (P = .72), and %TST with ETCO2 > 50 mmHg (P = .9) were not significantly different within the group or between treatment groups (Table III). One (7.7%) patient had resolution of OSA, 10 (76.9%) continued to exhibit mild OSA, and two (15.4%) had worsening to moderate OSA on follow-up PSG.
TABLE III.
P-values for Change in Polysomnographic Values with Treatment.
| Observation (n = 13) | Oxygen (n = 1) | Medication (n = 10) | |
|---|---|---|---|
| Pre- vs. post oAHI | .60 | – | .21 |
| Pre- vs. post-AHI | .60 | – | .24 |
| Pre- vs. post-CAI | .81 | – | .48 |
| Pre- vs. post-Oxyhemoglobin nadir | .80 | – | .99 |
| Pre- vs. post-Sleep Efficiency | .32 | – | .15 |
| Pre- vs. post-Arousal Index | .46 | – | .56 |
| Pre- vs. post-ETCO2 > 50 mmHg | .90 | – | .84 |
| Pre- vs. post-Percentage of REM sleep | .72 | – | .94 |
AHI = apnea-hypopnea index; CAI = central apnea index; ETCO2 = end tidal carbon dioxide; REM = rapid eye movement; oAHI = obstructive apnea-hypopnea index; OSA = obstructive sleep apnea.
Medication
Ten patients (50.0% female and 60.0% white) were treated with a single medication: eight (80%) with montelukast and two (20%) with fluticasone. Medication was started at the follow up appointment after baseline PSG and continued until follow up after interval PSG. Median time between PSGs was 15.4 months. The median age of these patients was 8.6 years (95% CI, 1.9–14.3) and the median BMI percentile was 94.0 (95% CI, 65.1–99.9). Comorbidities included: cardiac disease (60.0%), allergies (30.0%), asthma (30.0%), dyslipidemia (30.0%), hypertension (20.0%), and laryngomalacia (20.0%).
Sleep-related complaints were recorded upon initial consultation and follow-up in 70.0% (7/10) children treated with medication; snoring was the most common complaint. Five of these seven children had an increase in AHI and two children experienced a decrease in AHI. For those with an increase in AHI, snoring was reported by 60.0% at baseline and decreased to 40.0% at follow-up. For those with a decrease in AHI, snoring was reported by 100% at baseline and decreased to 50.0% at follow-up. See Table II for individual patient symptoms. Figure 1 illustrates baseline and follow-up symptoms grouped by change in the AHI.
Changes in AHI (P = .24), oAHI (P = .21), oxygen saturation nadir (P = .99), sleep efficiency (P = .15), arousal index (P = .56), %REM sleep (P = .94), and %TST ETCO2 > 50 mmHg (P = .84) were not significantly different. One (10.0%) patient had resolution of OSA, six (60.0%) continued to exhibit mild OSA, and three (30.0%) had severe OSA (oAHI >10) on follow-up PSG.
Oxygen
One patient was treated with supplemental oxygen. She was a 7.2-month-old white female with comorbid diagnoses of hypertension and cardiac disease. Upon initial evaluation she did not exhibit any sleep-related complaints. She was treated with 1/8 liter per minute oxygen for mixed sleep apnea with an oAHI of 3.3 events/hour. After 12 months, her follow-up PSG showed persistent mild OSA with a posttreatment oAHI 1.1 events/h and her parents complained of new snoring and noisy breathing. Table IV illustrates pre- and posttreatment PSG parameters for the entire cohort. Figure 2 demonstrates the baseline and follow-up oAHI for each treatment group.
TABLE IV.
Polysomnographic Parameters at Baseline and After Treatment for Children with Down Syndrome and Mild Obstructive Sleep Apnea by Treatment Group.
| Characteristic | Observation (n = 13) | Oxygen (n = 1) | Medication (n = 10) | P |
|---|---|---|---|---|
| Baseline parameters | ||||
| oAHI, events/hour (95% CI) | 2.9 (1.1–4.9) | 3.3 (–) | 3.5 (1.3–4.7) | .96 |
| AHI, events/hour (95% CI) | 3.3 (1.8–7.2) | 8.2 (–) | 3.5 (1.7–4.7) | .24 |
| CAI, events/hour (95% CI) | 0.5 (0–2.3) | 4.9 (–) | 0.25 (0–2) | .20 |
| Oxyhemoglobin nadir, % (95% CI) | 90 (82–96) | 73 (–) | 89 (80–94) | .22 |
| Sleep efficiency, % total sleep time (95% CI) | 83 (62–91) | 87 | 87 (68–95) | .67 |
| Arousal Index, event/hour (95% CI) | 14.2 (9.1–32.7) | 19.6 | 10.4 (6.2–18.5) | .18 |
| End-tidal CO2 > 50 mmHg, min (95% CI) | 0.1 (0–62) | 0 (–) | 0.1 (0–69) | .63 |
| Percentage of REM sleep, % (95% CI) | 22 (10–40) | 28 (–) | 22 (16–28) | .55 |
| Posttreatment polysomnogram parameters | ||||
| Posttreatment oAHI, events/hour, (95% CI) | 3.6 (0.7–6.8) | 1.1 (–) | 3.6 (1.5–13.8) | .45 |
| Posttreatment AHI, events/hour (95% CI) | 4.1 (0.7–7.8) | 4.7 (–) | 3.7 (0.8–13.8) | .82 |
| Posttreatment CAI, events/hour (95% CI) | 0.4 (0–3.9) | 3.6 (–) | 0.25 (0–0.6) | .18 |
| Posttreatment oxygen nadir, % (95% CI) | 91 (73–95) | 85 (–) | 90 (73–92) | .25 |
| Posttreatment sleep efficiency, % total sleep time (95% CI) | 86 (71–96) | 85 (–) | 86 (77–98) | .77 |
| Posttreatment Arousal Index, event/hour (95% CI) | 12.3 (6.8–24.1) | 12.1 (–) | 13.3 (6.4–22.5) | .97 |
| Posttreatment CO2 > 50 mmHg min (95% CI) | 0 (0–79) | 1.1 (–) | 2.8 (0–79) | .47 |
| Posttreatment percentage of REM sleep, % (95% CI) | 24 (13–37) | 25 (–) | 24 (3–38) | .69 |
| Posttreatment OSA severity (n, %) | .82 | |||
| None | 1 (7.7) | 0 (0) | 1 (10.0) | |
| Mild | 10 (76.9) | 1 (100.0) | 6 (60.0) | |
| Moderate/severe | 2 (15.4) | 0 (0) | 3 (30.0) |
AHI = apnea-hypopnea index; CAI = central apnea index; CO2 = carbon dioxide; REM = rapid eye movement; oAHI = obstructive apnea-hypopnea index; OSA = obstructive sleep apnea.
Fig. 2.
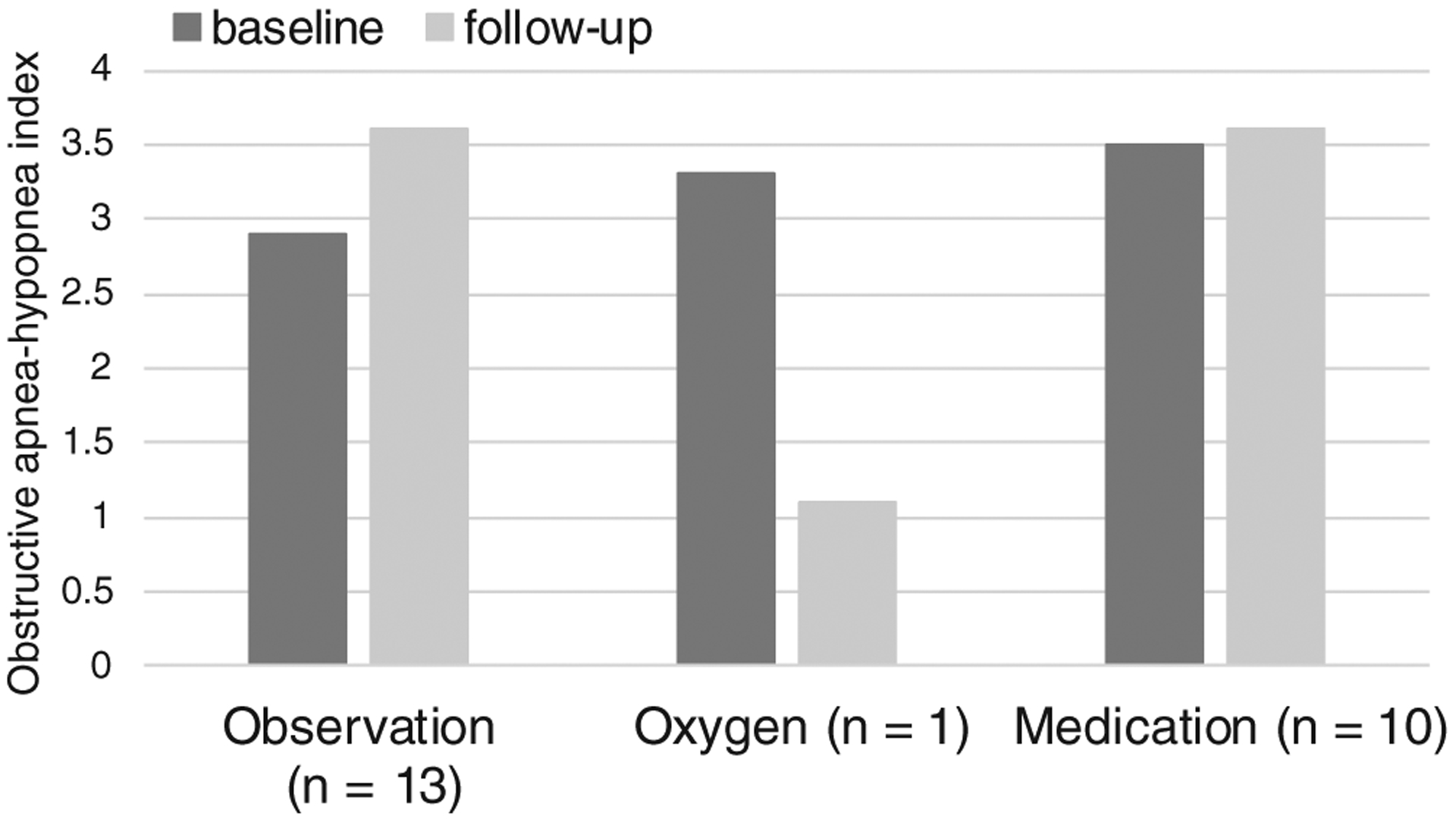
Graph of baseline and follow-up obstructive apnea-hypopnea index for children with Down syndrome and mild obstructive sleep apnea treated with medication, supplemental oxygen, or observation.
DISCUSSION
In this study, we report low rates of resolution of mild OSA in a cohort of 24 children with DS treated non-surgically with observation, medication, or supplemental oxygen. Across the entire cohort, only two (8.3%) patients displayed resolution of OSA on follow-up PSG, and five (20.8%) patients had moderate or severe disease on follow-up. Patient symptoms did not correspond to the degree of AHI, and improvement or worsening of symptoms did not parallel the improvement or worsening of AHI. This is consistent with previous literature that has demonstrated either weak correlation or no correlation between symptoms and sleep apnea severity.22,23 However, baseline and follow-up symptoms were only available for 72% of the cohort.
Numerous anatomic and neurologic features of children with DS, including oropharyngeal crowding, midface hypoplasia, lymphoid hyperplasia, and generalized hypotonia, contribute to their substantially increased risk of developing OSA compared to healthy children.10,24 A recent meta-analysis of 18 studies and 1,200 children with DS calculated an OSA (defined by AHI > 1/h) prevalence of 69% (95% CI, 59–77%).25 OSA severity in children with DS tends to fall within the severe range (AHI ≥10/h), with rates as high as 45% to 68% reported among affected individuals.24–26
While adenotonsillectomy can yield improvements in AHI and quality of life measures for children with DS and moderate-to-severe OSA, surgical cure rates are lower than in non-DS children.26–28 Furthermore, a retrospective review of tonsillectomy in 323 children with DS and OSA showed no association between preoperative OSA severity and polysomnographic outcomes including likelihood of disease resolution, underlining the challenge of identifying patients most likely to benefit from surgery.26 While not curative, continuous positive airway pressure (CPAP) in patients that can tolerate it has immediate benefits for relief of airway obstruction and has been shown to be an effective treatment for children who are either not candidates for adenotonsillectomy or with refractory OSA following surgery.29 One prospective randomized study demonstrated equivalency of CPAP and adenotonsillectomy on PSG, Epworth Sleepiness Scale-Children, and OSA-18 outcomes at one year follow-up for 80 children with DS or mucopolysaccharidoses and OSA.28 However, the application of CPAP to children with OSA is limited due to behavioral and neurocognitive factors, and one retrospective study measured a CPAP adherence rate of 50% in 25 children with DS.30
The natural history of mild OSA in children has not been clearly elucidated in the general pediatric population nor specifically for children with DS.31 For children with DS and mild OSA, the benefits of available treatment modalities are unclear and have never been directly investigated. In a retrospective study by Trosman et al. analyzing adenotonsillectomy versus watchful waiting for 62 pediatric patients with mild OSA, a subgroup analyses for 15 syndromic patients (including seven with DS) revealed no significant differences in rates of resolution of OSA on follow up PSG (33% vs. 22%, respectively, P = .54) or change in mean AHI (3.08 to 2.03 and 3.31 to 2.84 events/hour respectively, P = .36) between the two treatment groups.32 In our cohort of patients treated with observation, only one patient (7.7%) demonstrated resolution of OSA on follow up PSG, and two patients (15.4%) were found to have OSA worsened to moderate severity. Our low spontaneous resolution rate is consistent with Trosman’s study and indicate that the disease in this population may be unlikely to resolve without active intervention.
Anti-inflammatory medications including corticosteroids and montelukast have been shown to effectively treat OSA in otherwise healthy children as both a primary treatment and for residual disease after adenotonsillectomy through several prospective studies. The reduction in AHI ranged from 1 to 4.9 events/hour depending on the study, and the therapies have been shown to be effective in reducing both apneas and hypopneas.33–37 Another large retrospective review demonstrated a benefit of combined intranasal corticosteroids and oral montelukast in the treatment of mild OSA among otherwise healthy children, with 62% of patients displaying resolution of OSA on follow up PSG after 12 weeks of treatment.38 However, no studies to date have evaluated the effect of medication on OSA in the DS population. One retrospective chart review investigated the frequency of OSA in symptomatic children with craniofacial malformations and the polysomnographic effect of treatment. However, only two patients identified in the cohort were treated with medication, and the subgroup results were not reported.39 Only one patient (10%) in our cohort of patients treated with steroids or montelukast exhibited resolution of OSA on follow up PSG. This significant difference from findings reported previously in the literature for non-DS children aligns with our current understanding of airway obstruction in DS as multifactorial.
Supplemental oxygen helps to reduce nighttime desaturations in children with OSA but does not treat the source of these events. Oxygen therapy in children at risk or with known pulmonary hypertension may be a reasonable option but yields variable reduction of the AHI.29,40,41 The one patient in our cohort treated with oxygen had comorbid hypertension and cardiac disease, and she displayed persistent mild OSA on follow-up PSG.
There is currently no standard protocol for the treatment of children with mild OSA; thus the decision to treat these patients with medication, surgery, or oxygen versus observation is dependent on the practitioner. At our institution, our treatment protocol is more likely to recommend observation for children with no daytime symptoms. However, assessment of the subjective complaints in this cohort does not suggest that there was a significant difference in daytime or nighttime symptoms between groups as children in the observation group were just as symptomatic as those in the other groups. This may suggest that provider or family preference plays a large role in treatment selection for children with mild OSA.
This study is limited by its retrospective design, which may bias both available clinical and demographic data. Our patient selection process was also limited in that those patients that responded clinically to treatment may have not undergone posttreatment PSG, thereby biasing our results to those that remained symptomatic or concerning. The retrospective design of this study also did not allow for complete data collection of subjective complaints, as only 72% of patients in the cohort had complete baseline and follow-up symptoms documented. Additionally, only 59% of patients with baseline PSG findings underwent a follow-up PSG within 1 year. Some of this was due to lack of follow-up by families, while for others with symptoms resolution, a second PSG was not performed. Therefore, it is likely that the PSG-based resolution rate reported here is artificially low. Another limitation was the statistically significant difference in age between treatment groups; children who were observed were significantly younger than those that were treated with medication, and the patient treated with oxygen was an infant. With a small sample size, it is difficult to determine the reason for this difference. In addition, the medication group was significantly older than the oxygen and observation groups. Studies investigating the neurobehavior effects of mild OSA have been mainly carried out in children of school age and older,20,42 so perhaps the decision to treat with medications rather than observe was based upon evidence-based concern for worsening functioning. It is also likely that the symptoms and signs that are commonly elicited during our sleep history (enuresis, behavior changes, snoring, apneic episodes, morning headaches) are more apparent in older children while in younger children these may be considered less concerning or are less common.43–46 In addition, older children may present due to a teacher’s concern for behavior or learning difficulties which is an additional daytime factor which may drive the decision to treat with medication. Finally, only one patient in our cohort was treated with oxygen, so we were unable to draw any conclusions about oxygen treatment in this cohort.
Despite these limitations, our study is the first to assess response to nonsurgical treatment among children with DS and mild OSA and therefore contributes uniquely to the limited body of literature.
CONCLUSION
In our cohort, resolution of mild OSA was low for all treatment groups. These findings are consistent with the current understanding that OSA in children with DS is likely the result of several overlapping abnormalities contributing to obstructive pathology and suggest that a multimodal approach may be most appropriate in this population. Prospective studies will be helpful in the future to establish a better understanding of treatment outcomes in children with DS and mild OSA.
Footnotes
The authors have no funding or conflicts of interest to declare.
Presented at The Triological Society Combined Sections Meeting, Coronado, CA, U.S.A., January 24, 2019.
BIBLIOGRAPHY
- 1.Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol 2010;88:1008–1016. [DOI] [PubMed] [Google Scholar]
- 2.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008;5:242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus CL, Keens TG, Bautista DB, von Pechmann WS, Ward SL. Obstructive sleep apnea in children with Down syndrome. Pediatrics 1991;88:132–139. [PubMed] [Google Scholar]
- 4.Trois MS, Capone GT, Lutz JA, et al. Obstructive sleep apnea in adults with Down syndrome. J Clin Sleep Med 2009;5:317–323. [PMC free article] [PubMed] [Google Scholar]
- 5.Uong EC, McDonough JM, Tayag-Kier CE, et al. Magnetic resonance imaging of the upper airway in children with Down syndrome. Am J Respir Crit Care Med 2001;163:731–736. [DOI] [PubMed] [Google Scholar]
- 6.Guimaraes CV, Donnelly LF, Shott SR, Amin RS, Kalra M. Relative rather than absolute macroglossia in patients with Down syndrome: implications for treatment of obstructive sleep apnea. Pediatr Radiol 2008;38:1062–1067. [DOI] [PubMed] [Google Scholar]
- 7.Fricke BL, Donnelly LF, Shott SR, et al. Comparison of lingual tonsil size as depicted on MR imaging between children with obstructive sleep apnea despite previous tonsillectomy and adenoidectomy and normal controls. Pediatr Radiol 2006;36:518–523. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly LF, Shott SR, LaRose CR, Chini BA, Amin RS. Causes of persistent obstructive sleep apnea despite previous tonsillectomy and adenoidectomy in children with down syndrome as depicted on static and dynamic cine MRI. AJR Am J Roentgenol 2004;183:175–181. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand P, Navarro H, Caussade S, Holmgren N, Sanchez I. Airway anomalies in children with Down syndrome: endoscopic findings. Pediatr Pulmonol 2003;36:137–141. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs IN, Gray RF, Todd NW. Upper airway obstruction in children with Down syndrome. Arch Otolaryngol Head Neck Surg 1996;122:945–950. [DOI] [PubMed] [Google Scholar]
- 11.Bravo MN, Kaul A, Rutter MJ, Elluru RG. Down syndrome and complete tracheal rings. J Pediatr 2006;148:392–395. [DOI] [PubMed] [Google Scholar]
- 12.Shires CB, Anold SL, Schoumacher RA, Dehoff GW, Donepudi SK, Stocks RM. Body mass index as an indicator of obstructive sleep apnea in pediatric Down syndrome. Int J Pediatr Otorhinolaryngol 2010;74: 768–772. [DOI] [PubMed] [Google Scholar]
- 13.van Gameren-Oosterom HB, van Dommelen P, Schonbeck Y, Oudesluys-Murphy AM, van Wouwe JP, Buitendijk SE. Prevalence of overweight in Dutch children with Down syndrome. Pediatrics 2012;130:e1520–1526. [DOI] [PubMed] [Google Scholar]
- 14.Macchini F, Leva E, Torricelli M, Valade A. Treating acid reflux disease in patients with Down syndrome: pharmacological and physiological approaches. Clin Exp Gastroenterol 2011;4:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May JG, Shah P, Lemonnier L, Bhatti G, Koscica J, Coticchia JM. Systematic review of endoscopic airway findings in children with gastroesophageal reflux disease. Ann Otol Rhinol Laryngol 2011;120:116–122. [DOI] [PubMed] [Google Scholar]
- 16.Attal P, Chanson P. Endocrine aspects of obstructive sleep apnea. J Clin Endocrinol Metab 2010;95:483–495. [DOI] [PubMed] [Google Scholar]
- 17.Lin CC, Tsan KW, Chen PJ. The relationship between sleep apnea syndrome and hypothyroidism. Chest 1992;102:1663–1667. [DOI] [PubMed] [Google Scholar]
- 18.Charleton PMD J;Marder E Medical management of children with Down syndrome. Paediatr Child Health 2010;20:331–337. [Google Scholar]
- 19.Tan HL, Gozal D, Kheirandish-Gozal L. Obstructive sleep apnea in children: a critical update. Nat Sci Sleep 2013;5:109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breslin J, Spano G, Bootzin R, Anand P, Nadel L, Edgin J. Obstructive sleep apnea syndrome and cognition in Down syndrome. Dev Med Child Neurol 2014;56:657–664. [DOI] [PubMed] [Google Scholar]
- 21.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8:597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shott SR, Amin R, Chini B, Heubi C, Hotze S, Akers R. Obstructive sleep apnea: should all children with Down syndrome be tested? Arch Otolaryngol Head Neck Surg 2006;132:432–436. [DOI] [PubMed] [Google Scholar]
- 23.Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Prevalence of obstructive sleep apnea in children with down syndrome. Sleep 2016;39:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Sleep problems and obstructive sleep apnea in children with down syndrome, an overwiew. Int J Pediatr Otorhinolaryngol 2016;82:12–15. [DOI] [PubMed] [Google Scholar]
- 25.Lee CF, Lee CH, Hsueh WY, Lin MT, Kang KT. Prevalence of obstructive sleep apnea in children with down syndrome: a meta-analysis. J Clin Sleep Med 2018;14:867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingram DG, Ruiz AG, Gao D, Friedman NR. Success of tonsillectomy for obstructive sleep apnea in children with down syndrome. J Clin Sleep Med 2017;13:975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shete MM, Stocks RM, Sebelik ME, Schoumacher RA. Effects of adenotonsillectomy on polysomnography patterns in Down syndrome children with obstructive sleep apnea: a comparative study with children without Down syndrome. Int J Pediatr Otorhinolaryngol 2010;74:241–244. [DOI] [PubMed] [Google Scholar]
- 28.Sudarsan SS, Paramasivan VK, Arumugam SV, Murali S, Kameswaran M. Comparison of treatment modalities in syndromic children with obstructive sleep apnea—a randomized cohort study. Int J Pediatr Otorhinolaryngol 2014;78:1526–1533. [DOI] [PubMed] [Google Scholar]
- 29.Marcus CL, Ward SL, Mallory GB, et al. Use of nasal continuous positive airway pressure as treatment of childhood obstructive sleep apnea. J Pediatr 1995;127:88–94. [DOI] [PubMed] [Google Scholar]
- 30.Chawla JF C, Heussler H CPAP to treat obstructive sleep apnoea (OSA) in children with Down syndrome (DS). Eur Respir J 2016;48:PA3066. [Google Scholar]
- 31.Baldassari CM, Choi S. Mild obstructive sleep apnea in children: what is the best management option? Laryngoscope 2018;128:2671–2672. [DOI] [PubMed] [Google Scholar]
- 32.Trosman SJ, Eleff DJ, Krishna J, Anne S. Polysomnography results in pediatric patients with mild obstructive sleep apnea: Adenotonsillectomy vs. watchful waiting. Int J Pediatr Otorhinolaryngol 2016;83:25–30. [DOI] [PubMed] [Google Scholar]
- 33.Brouillette RT, Manoukian JJ, Ducharme FM, et al. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr 2001;138: 838–844. [DOI] [PubMed] [Google Scholar]
- 34.Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics 2008;122: e149–155. [DOI] [PubMed] [Google Scholar]
- 35.Goldbart AD, Goldman JL, Veling MC, Gozal D. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med 2005;172:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldbart AD, Greenberg-Dotan S, Tal A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics 2012;130:e575–580. [DOI] [PubMed] [Google Scholar]
- 37.Wang B, Liang J. The Effect of montelukast on mild persistent osa after adenotonsillectomy in children: a preliminary study. Otolaryngol Head Neck Surg 2017;156:952–954. [DOI] [PubMed] [Google Scholar]
- 38.Kheirandish-Gozal L, Bhattacharjee R, Bandla HPR, Gozal D. Antiinflammatory therapy outcomes for mild OSA in children. Chest 2014;146:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moraleda-Cibrian M, Edwards SP, Kasten SJ, Buchman SR, Berger M, O’Brien LM. Obstructive sleep apnea pretreatment and posttreatment in symptomatic children with congenital craniofacial malformations. J Clin Sleep Med 2015;11:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aljadeff G, Gozal D, Bailey-Wahl SL, Burrell B, Keens TG, Ward SL. Effects of overnight supplemental oxygen in obstructive sleep apnea in children. Am J Respir Crit Care Med 1996;153:51–55. [DOI] [PubMed] [Google Scholar]
- 41.Das P, Kashyap R, Kotagal S. Impact of supplemental oxygen on obstructive sleep apnea of infants. Children (Basel) 2018;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc 2004;10:772–785. [DOI] [PubMed] [Google Scholar]
- 43.Chawla JWK. Evaluation of infant obstructive sleep apnea. Minerva Pneumocologica 2016;55:64–70. [Google Scholar]
- 44.Gaultier C, Praud JP, Canet E, Delaperche MF, D’Allest AM. Paradoxical inward rib cage motion during rapid eye movement sleep in infants and young children. J Dev Physiol 1987;9:391–397. [PubMed] [Google Scholar]
- 45.Kahn A, Groswasser J, Sottiaux M, et al. Clinical symptoms associated with brief obstructive sleep apnea in normal infants. Sleep 1993;16:409–413. [DOI] [PubMed] [Google Scholar]
- 46.Ramgopal S, Kothare SV, Rana M, Singh K, Khatwa U. Obstructive sleep apnea in infancy: a 7-year experience at a pediatric sleep center. Pediatr Pulmonol 2014;49:554–560. [DOI] [PubMed] [Google Scholar]


