Abstract
Functional and nutraceutical foods provide an alternative way to improve immune function to aid in the management of various diseases. Traditionally, many medicinal products have been derived from natural compounds with healing properties. With the development of research into nutraceuticals, it is becoming apparent that many of the beneficial properties of these compounds are at least partly due to the presence of polyphenols. There is evidence that dietary polyphenols can influence dendritic cells, have an immunomodulatory effect on macrophages, increase proliferation of B cells, T cells and suppress Type 1 T helper (Th1), Th2, Th17 and Th9 cells. Polyphenols reduce inflammation by suppressing the pro-inflammatory cytokines in inflammatory bowel disease by inducing Treg cells in the intestine, inhibition of tumor necrosis factor-alpha (TNF-α) and induction of apoptosis, decreasing DNA damage. Polyphenols have a potential role in prevention/treatment of auto-immune diseases like type 1 diabetes, rheumatoid arthritis and multiple sclerosis by regulating signaling pathways, suppressing inflammation and limiting demyelination. In addition, polyphenols cause immunomodulatory effects against allergic reaction and autoimmune disease by inhibition of autoimmune T cell proliferation and downregulation of pro-inflammatory cytokines (interleukin-6 (IL-6), IL-1, interferon-γ (IFN-γ)). Herein, we summarize the immunomodulatory effects of polyphenols and the underlying mechanisms involved in the stimulation of immune responses.
Keywords: polyphenols, immunomodulation, pro-inflammatory cytokines, anti-inflammatory cytokines
1. Introduction
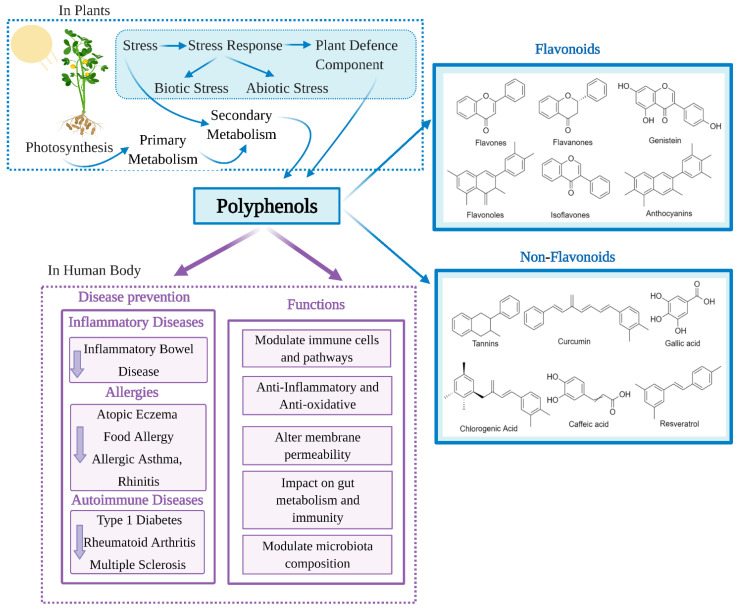
With advancing knowledge of the importance of adequate nutrition, and increased public health awareness about diet, there is growing attention on the health benefits of natural products including those that are rich in polyphenols. Polyphenols are the most extensive group of non-energetic secondary metabolites and are produced by plants in response to stress [1] (Figure 1). Polyphenols have been called ‘lifespan essentials’ due to their significant impact on health [2]. There are as many as 8000 different polyphenols which are divided into different classes based on their chemical structure. Despite the different classifications, all polyphenols have the key structural features of an aromatic ring and at least one hydroxyl group [3,4]. Dietary polyphenols are abundant in plant-based foods such as fruits, vegetables, dry legumes, cereals, olives, cocoa, tea, coffee and wine [5]. Some common dietary polyphenols include the lignins present in nuts and whole-grain cereals; pro-anthocyanidins in grapes, pine bark and cocoa; anthocyanins/anthocyanidins in brightly colored fruits and vegetables like berries; isoflavones in soybeans; catechins in green tea, grapes and wine; tannins in tea and nuts; quercetin in grapes and onion; resveratrol in wines and naringenin/hesperidin in citrus fruits [6].
Figure 1.
Classification and health benefits of polyphenols.
Research into the beneficial health effects of polyphenols has increased considerably over the last two decades [7]. Polyphenols have shown anti-inflammatory, antimicrobial, antioxidant, anticarcinogenic, antiadipogenic, antidiabetic and neuroprotective effects [8,9,10,11,12]. Polyphenols may also counteract cytotoxicity and apoptosis due to their immunomodulatory properties [13] and regulate innate and adaptive immunity. Polyphenols have also been shown to reduce oxidative stress and inflammation [14], modulate immune cells, regulate gut microbiota composition and immunity (Figure 1). Through this regulation of the immune system, polyphenols could beneficially impact a number of chronic diseases [15]. Herein, we discuss the immunomodulatory effect of polyphenols and the resulting effects on different chronic diseases, including inflammatory bowel disease, atopic eczema or dermatitis, allergic asthma, rhinitis, type 1 diabetes, multiple sclerosis and rheumatoid arthritis.
2. Methods
Extraction of current and relevant data was performed using the electronic databases, Science Direct, PubMed, Springer and Google Scholar. Searches were conducted in two sections. The first part aimed to identify evidence on the effect of polyphenols on the immune system and immune cells. Search terms used were ‘Polyphenols’ OR ‘Phytochemicals’ OR ‘Phenolic’ AND ‘Immunity’ OR ‘Immune system’ OR ‘Immune function’ AND ‘Immune cells’ OR ‘Dendritic cells’ OR ‘Macrophages’ OR ‘Monocytes’ OR ‘Neutrophils’ OR ‘Natural Killer cells’ OR ‘B cells’ OR ‘T cells’ OR ‘T helper cells. The second part aimed to identify evidence on the impact of polyphenols in chronic inflammatory and auto-immune diseases. Additional search terms included ‘Inflammatory diseases’ OR ‘Inflammatory Bowel Disease’ AND ‘Allergy’ OR ‘Atopic Eczema’ OR ‘Dermatitis’ OR ‘Food Allergy’ OR ‘Rhinitis’ OR ‘Asthma’ AND ‘Autoimmune Disease’ OR ‘Type-1 diabetes’ OR ‘Rheumatoid arthritis’ OR ‘Multiple sclerosis’. Articles published in English were included. The titles and abstracts were scanned to exclude any irrelevant studies. A total of 167 papers that focused only on the immunomodulatory effect of polyphenols on health were screened and articles containing relevant data were reviewed.
3. Immune Modulation of Polyphenols to Immune Cells
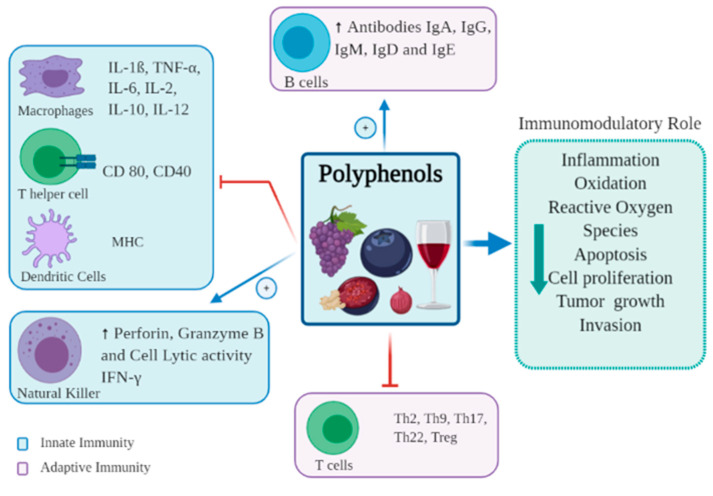
The immune system as a whole consists of innate and adaptive immunity, each with different roles and functions [16]. The innate immune system is the first line of defense, and protects against foreign antigens through the skin, pulmonary system, and gut epithelial cells, forming a barrier between the organism and its environment [17]. The innate system is broadly divided into cellular and non-cellular systems. The cellular system consists of several cell subsets, including dendritic cells (DCs), monocytes, macrophages, granulocytes and natural killer (NK) cells. The non-cellular system is very diverse, ranging from simple mucus barriers to complex protein pathways, such as the complement cascade, however all function to prevent pathogen entry, and facilitate pathogen destruction by phagocytosis [18]. The adaptive immune system comprises T and B cells. B cells secrete antibodies, whilst T cells are involved in the production of cytokines, direct cytotoxic destruction of infected or malignant tissue, and activation of other immune cells [16]. Polyphenols modulate immune responses in both the innate and adaptive systems, having both stimulatory and inhibitory effects in different areas [19] (Figure 2).
Figure 2.
Immunomodulatory effects of polyphenols on immune cells.
3.1. Effects of Polyphenols on Dendritic Cells
DCs are the most potent antigen-presenting cells which act to prime the adaptive immune system to recognize foreign antigens, and so are vital in the initiation and regulation of the adaptive immune response [20]. It has been shown that polyphenols can influence the differentiation of DCs [21]. In fact, resveratrol has been identified as affecting human DC differentiation from monocytes, with a strong potential for regulatory action [22]. Likewise, epigallocatechin gallate (EGCG) induces apoptosis and affects the phenotype of developing DCs. Molecules that are essential for antigen presentation by DCs such as CD83, CD80, CD11c, and major histocompatibility complex (MHC) class II, are downregulated by EGCG, suggesting an immunosuppressive action [23]. Other polyphenols, including EGCG, curcumin, quercetin, apigenin, silibinin, and blackberry polyphenols cause inhibition of murine bone marrow-derived DC maturation and expression of MHC molecules, reducing antigen uptake and decreasing secretion of the pro-inflammatory cytokines interleukin-1 (IL-1), IL-2, IL-6, IL-12 [24,25,26,27]. A study in an animal model showed that administration of fisetin (50 mg/kg) decreased DC migration and DC allo-stimulatory capacity [28]. Similarly, in vitro resveratrol has an inhibitory effect on DC maturation [29].
3.2. Effects of Polyphenols on Monocytes and Macrophages
Macrophages are phagocytes that ingest pathogens and dead cells, which differentiate from the transitory monocyte. Like DCs, macrophages can also function as antigen-presenting cells (albeit with less potent activity) being able to activate naïve T cells into effector T cells in the presence of an antigen [19]. Macrophages play an important role in inflammation, host defense, and tissue repair [30,31]. Importantly, macrophages also play a pathogenic role in various chronic diseases including asthma, inflammatory bowel disease, atherosclerosis and rheumatoid arthritis [31,32,33]. Macrophages are classically considered in two categories, known as polarization: the classical inflammatory M1 and immunosuppressive/anabolic M2 phenotypes. Initiation of M1 differentiation is by interferon-γ (IFN-γ) stimulation and the activation of toll-like receptors (TLRs) by bacterial lipopolysaccharides (LPS); while M2 polarization is triggered by IL-4 [34]. It has been shown that polyphenolic cocoa extract suppressed M1 mediated inflammation and drove M2 polarization of activated macrophages [35]. Polyphenol-rich green tea has anti-tumor effects secondary to the activation of macrophages and NK cells [36]. Inonotus sanghuang, a plant known for its medicinal value, rich in rutin, quercetin, quercitrin, isorhamnetin and chlorogenic acid, has been shown to reduce inflammation by modulating the interaction between macrophages and adipocytes. It was suggested that in this way it may improve insulin resistance and metabolic syndrome [37]. Moreover, Overman et al. reported that grape powder extract decreased LPS-stimulated inflammation in macrophages and reduce insulin resistance [38].
Monocytes and macrophages play a fundamental role in the progression of atherosclerosis [35]. Increased oxidative stress causes low-density lipoprotein oxidation (oxLDL), with the resulting lipoproteins engulfed by macrophages resulting in the formation of foam cells. This process triggers an inflammatory response in the neighboring endothelial cells which secrete pro-inflammatory cytokines and chemokines [39,40,41]. When monocytes migrate towards the intima, they transform into macrophages on stimulation by macrophage colony-stimulating factor, increasing the expression of scavenger receptors outside the cell [39,40]. Polyphenols are known to regulate this interplay between immune and vascular endothelial function. Evidence has shown that polyphenols reduce atherosclerotic progression by increasing high-density lipoprotein (HDL) levels and decrease LDL accumulation in macrophages, reducing foam cell formation [3,42].
3.3. Effects of Polyphenols on Natural Killer Cells
NK cells are a subset of lymphocytes, but are part of the innate immune response, with the function of eliminating infected or malignant cells [19]. NK cells have a strong cytolytic function and a considerable role in immune regulation [43]. NK cells are activated by CD4+ T cell secretion of IL-2 and IFN-γ [44]. Once activated NK cells secrete perforin and granzyme B, which induce apoptosis and necrosis in target cells. Polyphenols have immunomodulatory effects on NK cells, increasing their number and activity. Green tea catechin metabolites increase NK cell cytotoxicity [45] and quercetin enhances NK cell lytic activity [46] in animal models. In a clinical trial, healthy participant prescribed a diet low in polyphenols and supplemented with juices rich in polyphenols increased lymphocyte proliferative responsiveness, IL-2 secretion and lytic activity by NK cells [47]. Berries rich in flavonoids and pro-anthocyanidins have a cancer-preventive effect but are also involved in the modulation of NK cells [48]. A study in marathon runners noted that daily consumption of 250 g of blueberries for six weeks resulted in doubled NK cell counts [49]. Evidence showed that purple sweet potato leaves that are rich in flavonoids enhanced the lytic activity of NK cells in 16 healthy participants [50].
3.4. Effects of Polyphenols on T and B Cells
T and B cells are the principal components of the adaptive immune system. B cells secrete antibodies known as immunoglobulins (Igs), which bind to antigens and underpin hypersensitivity reactions and antimicrobial immune responses. When B cells are activated to a specific antigen, they differentiate into plasma cells and produce Immunoglobulin (Ig)A, IgG, IgM, IgD and IgE [19]. Polyphenols have been suggested to modulate the function of B cells; however, this has been poorly described, and further research is required. In an in vitro study it was noted that green tea polyphenols and EGCG decreased the production of IgE in a dose and time-dependent manner [51], and another study showed that polyphenols inhibit the proliferation of CD19+ cells and reduce IgG production [52]. It was noted that administration of green tea extract to mice for 6 weeks reduced the production antigen-specific IgE by enhancing CD4+ CD25+ regulatory T cells (Treg) in the spleen, resulting in reduction of allergic response [53].
T cells are divided into three major types: cytotoxic T cells, T helper (Th) cells and the Treg cells depending upon expression of the CD4 or CD8 molecules. CD4+ T cells are helper T cells that assist and control immune cell activity and activation. CD8+ cytotoxic T cells act to directly lyse and destroy malignant, senescent or infected cells [19]. Polyphenols have been associated with the modulation of enzymatic signaling, via the inhibition of the serine-threonine and tyrosine-protein kinase pathways. These enzymes are primarily linked with B cell activation and T cell proliferation as well as the production of cytokines by activated monocytes [3]. Treg play a crucial role in immunity tolerance and control of auto-immunity [54]. A study on mice showed that regular treatment of EGCG for a week increased the frequency of Treg cells in the spleen, pancreatic lymph nodes and mesenteric lymph nodes. The Treg cells obtained from the treated group could suppress cytotoxic T cell function, reducing proliferation and IFN-γ production [54]. In addition, EGC-M5 (a major metabolite of EGCG) at a dosage of 10 mg/kg of body weight were provided to rats for 14 days and caused upregulation of CD4+ T cell activity and cytotoxic activity of NK cells [45].
3.5. Effects of Polyphenols on T Cell Differentiation
CD4+ T helper cells differentiate into T helper (Th)1, Th2, Th9, Th17, Th22, depending upon the cytokine environment [55]. Th1 cells are involved in cell-mediated immunity and are produced in the presence of IL-12, whilst Th2 cells are critical for humoral immunity and differentiate in the presence of IL-4 and IL-13 [56]. Th17 cells secrete IL-17, IL-22, and chemokine ligands 20 (CCL20) [57] and have been shown to have a role in the progression and pathogenesis of chronic inflammatory diseases like rheumatoid arthritis, multiple sclerosis, psoriasis, atopic dermatitis, and asthma [56,58]. Th22 cells produce IL-22, a cytokine responsible for maintaining the epithelial barrier and skin integrity [57]. In mice, polyphenols like apigenin and chrysin suppresses serum IgE induced by ovalbumin immunization by downregulating Th2 responses [59]. Similarly, tea polyphenols, such as EGCG, reduce Th1 differentiation and numbers of Th17 and Th9 cells [60], as well as resveratrol by decreasing Th17 cell numbers in an inflammatory arthritis model in rodents [61]. Grape seed pro-anthocyanidin extract also showed anti-arthritic properties and upregulated the number of Tregs and maintained the balance between Th17/Treg, attenuating inflammation [62].
3.6. Effects of Polyphenols in Inflammation
The inflammatory response of the innate immune system is a vital part of the defense against microbial infection. However despite its vital role in promoting the immune response, its timely resolution is equally important [63]. Chronic inflammation is a key cause of a number of life-threatening diseases [64]. A study on pomegranate peel polyphenols (PPPs) and its specific components such as punicalagin (PC) and ellagic acid (EA) showed a reduction in pro-inflammatory cytokines TNF-α, IL-1β and IL-6 and downregulation other inflammatory mediators including nitric oxide (NO) and prostaglandin E2 (PGE2) by decreasing inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression [65]. Similarly, PPPs, PC, and EA have shown inhibitory effects on LPS-induced production of intracellular reactive oxygen species (ROS) and suppression of TLR4 at both the mRNA and protein levels, all of which have major mechanistic roles in inflammation [66]. In addition, grape seed extract, has been shown to decrease pro-inflammatory cytokine, ROS and superoxide production whilst elevating antioxidant enzyme gene expression and secretion of anti-inflammatory mediators [67,68]. Green tea polyphenols also reduce the production of inflammatory cytokines (TNF-α, IL-6, IL-1β), and inhibit the TLR4 signaling pathway [69]. The immunomodulatory effects of polyphenols are summarized in Table 1.
Table 1.
The immunomodulatory effects of polyphenols.
| Polyphenols | Signaling Pathways | Immunomodulatory Responses |
|---|---|---|
| Curcumin [70,71] | Suppress NF-κB | ↓ Bcl-2 in PHA-activated Tcells |
| Suppress maturation of DCs | ||
| Inhibit IL-12, IL-8 | ||
| ↑ IL-4 | ||
| Resveratrol, Quercetin, Silibinin [72] |
Altering PI3K/Akt | ↓IL-6 and IL-1 |
| Genistein [71,73] | Activate AMPK | ↓ IL-1β, IL-6, IL-8 |
| Inhibit ROS/Akt/NF-κB | ↓COX-2 | |
| EGCG [74] | Suppress NF-κB and MAPK | Inhibit Th1 and Th17 differentiation |
| ↓ Transcription factors (STAT1 and T-bet for Th1, and STAT3 and RORγt for Th17) | ||
| ↑ T-reg in lymphoid tissues and central nervous system | ||
| Proanthocyanidins Procyanidins [75,76] |
Suppress NF-κB and MAPK | ↓TNF-α, IL-1β |
| Inhibit iNOS and COX-2 | ||
| Caffeic acid [77,78,79] | Suppress p38 MAPK, JNK1/2 and NF-κB | ↓ IL-1β, IL-6, TNF-α |
| ↓ Monocyte chemoattractant protein (MCP)-1 | ||
| Inhibit xanthine oxidase and COX |
PHA: Phytohemagglutinin, DC: Dendritic cell, IL: Interleukin, COX: Cyclooxygenase, Th: T helper, STAT: Signal transducer and activator of transcription, NF-κB: Nuclear factor kappa-B, ROS: Reactive oxygen species, TNF-α: Tumor necrosis factor-alpha, MCP: Monocyte chemoattractant protein, iNOS: Inducible nitric oxide synthase, PI3K/Akt: Phosphatidylinositol 3-kinase/protein kinase B, AMPK: Adenosine monophosphate-activated protein kinase, MAPK: Mitogen-Activated Protein Kinase.
4. Immune Modulation of Polyphenols to Prevent and Control Chronic Diseases
Dietary polyphenols have preventive and therapeutic potential for a number of chronic diseases whose development involves dysregulation of the immune function.
4.1. Polyphenols and Inflammatory Bowel Disease
The intestinal epithelium is generally in a state of low-grade inflammation, due to microbial, chemical and mechanical stimuli that maintain a moderate inflammatory response [80]. Generally, it is controllable, but if inflammation exceeds the normal limit due to disease, it can disrupt epithelial tissues and impede intestinal dysfunction. These uncontrollable inflammatory conditions are known collectively as inflammatory bowel disease (IBD), comprised of two specific pathologies; Crohn’s disease and ulcerative colitis [81]. Globally, the annual incidence of IBD is approximately 396 cases per 100,000 persons per year [82]. There is evidence to suggest polyphenol supplementation could play a role in managing IBD. The proposed mechanism by which this occurs is through polyphenol modulation of pattern recognition receptors (PRRs), such as the TLRs and nucleotide-binding oligomerization domain proteins, which are highly expressed in intestinal epithelial and immune cells. PRRs activate immune responses against pathogens through recognition of related molecular structures [83], and polyphenols are known to be able to modulate the expression of PRRs and their associated inflammatory response in the intestine [80]. Activation of PRRs induces inflammation by increasing cytokine secretion and cyclooxygenase-2 expression. Polyphenols like curcumin and isothiocyanate inhibit TLR4 dimerization, an essential step for TLR4 activation [84,85]. Resveratrol also interferes with TLR4 signal transduction by inhibiting TANK binding kinase 1 which regulates the downstream pathways that result in cytokine production [86]. In addition, resveratrol acts as anti-inflammatory agent in intestinal mucosa [87]. Polyphenols are also known to modulate key inflammatory genes, such as cyclooxygenase-2 and the inflammatory cytokines, further implying potential for an anti-inflammatory effect in IBD [88,89]. It has also been shown that flavonoids are able to regulate the activity of Treg cells in the intestine, downregulating the expression of inflammatory cytokines, and consequently suppressing inflammation [90,91]. Polyphenols are also able to influence the gut microbiota as a probiotic. Green tea polyphenols promote the growth of beneficial microbiota like Bifidobacterium and Lactobacillus and suppress pathogenic bacteria, such as, E Coli and Salmonella [12,92]. This supports the maintenance of intestinal homeostasis and reduces inflammation [93]. Grape seed and green tea polyphenols also have potential to prevent or delay the progression of IBD [68,94,95]. Pomegranate polyphenols also provide protective effects against IBD by modulating the intestinal inflammatory response reducing expression of various pro-inflammatory cytokines, such as iNOS, COX-2, PGE2, as well as regulating the composition of the luminal microbiota [96]. A recent study reported that dietary polyphenols from mango (gallotannins and gallic acid) improved the symptoms of IBD. This study included 10 subjects who received 200–400 g/day of mango pulp for 8 weeks. A significant reduction was observed in a factors related to neutrophil-induced inflammation like IL-8, growth-regulated oncogene and granulocyte macrophage colony-stimulating factor by 16.2%, 25.0% and 28.6%, respectively [97]. Another study showed that Bronze tomatoes, which are rich in anthocyanins, flavonols, and stilbenoids, had a significant impact in alleviating the symptoms of IBD in mice [98]. Taken together, this suggests that polyphenols can help in the prevention and treatment of IBD by reducing pro-inflammatory cytokines, regulating the activity of Treg cells and promoting the growth of beneficial microbiota in the intestine.
4.2. Polyphenols and Allergies
The prevalence of allergic disorders has been increasing dramatically with competing environmental, genetic, diet and hygiene factors likely to underlie their advance [99,100]. Allergic reactions result from a hyper-reaction of the immune response against allergens such as those in the environment (dust, grass pollen) or food (milk, fish, eggs, nuts and wheat) [101]. Due to their growing incidence, there is significant attention on interventions to assist in their management, and polyphenols have been proposed as viable solutions [101] (Table 2). Certain polyphenols influence allergic responses at two critical stages: (1) allergic sensitization and (2) re-exposure to the allergen. During the sensitization phase, polyphenols such as caffeic and ferulic acid bind with allergenic proteins, forming insoluble complexes and rendering them non-reactive [101]. Additionally, flavonoids directly affect antigen presentation by DCs by either inhibiting cell surface expression of MHC-II and co-stimulatory molecules (CD80, CD86), leading to ineffective antigen presentation, or by inhibiting cytokine production [102,103]. Polyphenols like catechins and their derivatives inhibit Th2 cytokine production [104,105] as well as T cell activation and proliferation [106,107]. Recruitment of B cells to sites of allergic inflammation and their production of IgE have also been shown to be inhibited by polyphenols [59,108,109]. Of interest, the interaction between polyphenols and proteins results in the modulation of allergic sensitization and their direct effect on mast cells hence inhibiting the release of allergic mediators and eventually decreasing the symptoms of allergy [101]. In addition, polyphenols such as caffeic, ferulic, and chlorogenic acids can bind irreversibly with the peanut allergens, Ara h1 and Ara h2, reducing their allergenicity [110]. In mice, administration of polyphenol-enriched areca nut extracts suppressed the level of ovalbumin (OVA)-specific IgE, the expression of IL-4, downregulating Th2 driven immunity and enhancing the activity of myeloid-derived suppressor cells, attenuating allergic responses [111]. In another study, 30 female mice treated with cranberry and blueberry polyphenol complexed peanut protein for 6 weeks, had reduced expression of CD63 and decreased plasma IgE levels [112]. Evidence has also shown that polyphenol-rich cranberry extracts interact with wheat gliadins forming insoluble complexes in a mouse model, which decreased wheat gliadin immunogenicity and allergenicity [113]. Furthermore, polyphenolic ellagic acid effectively binds with allergenic proteins within the food matrix [114,115]. Punicalagin (a polyphenol derived from pomegranate), rutin and phloridzin increased the growth of beneficial bacteria species such as Bifidobacterium and Lactobacillus which are known to have beneficial impacts in food allergies [116,117]. Oral administration of a polyphenol-rich grape skin extract that had been fermented with Lactobacillus Plantarum had an inhibitory effect on allergic responses when compared to a non-fermented extract [118].
Table 2.
Effect of dietary polyphenols on allergic reaction.
| Dietary Polyphenols | Treatment and Duration | Results |
|---|---|---|
| Atopic Eczema or Dermatitis | ||
| Quercetin (pure isolated polyphenols) | 15 human subjects with contact dermatitis. Quercetin applied topically for five days | No change as compared to the control [119] |
| Cocoa flavanols (catechin, epicatechin, procyanidins) at a lower dose of 27 mg or a higher dose of 329 mg | Ten healthy women consumed a low and high dose. | The higher dose of cocoa drink reduced water loss and improved the blood circulation in the skin [120] |
| Water extract of whey powder dodder rich in quercetin | Randomized control trial (RCT) study recruited 52 subjects atopic dermatitis recruited for 30 days | Quercetin reduces allergy and inhibits the secretion of the mast cell. Elevate skin moisture and elasticity [121] |
| Apple condensed tannins (ACT) at a dose of 10 mg/kg | Apple polyphenols were investigated in subjects with atopic eczema for 8 weeks. | Reduced inflammation and itching in disease subjects compared with the control group. ACT has an anti-allergic effect [122] |
| Food Allergy | ||
| Polyphenol enriched extracts or purified epicatechin (1, 0.3 and 0.01%) | Female BALB/c mice treated with polyphenols for 8 days | Epicatechin exhibited a significant anti-allergic effect [123] |
| Polyphenol-enriched apple extract (>40%) | BALB/c mice treated with an apple extract for 7 weeks | Reduce allergenicity by protein–polyphenol interaction, decrease intestinal mast cell protease and pro-inflammatory genes, diminished cytokine secretion. [105] |
| Cocoa diet with 0.2% polyphenols | Rats received either a cocoa diet or a standard diet for 4 weeks | Cocoa diet decreased total serum immunoglobulin (Ig)E, Tumor necrosis factor (TNF)-α and interleukin (IL)-10 secretion. No effect on IL-4 synthesis [124] |
| Asthma and Rhinitis | ||
| Drinks containing apple polyphenols at low and high dose (50 mg and 100 mg) | 33 subjects having moderate or severe persistent allergic rhinitis treated with apple polyphenols for 4 weeks | Improve sneezing attacks nasal discharger and swelling of the nasal turbinate in the low-dose group and high dose group [125] |
| 100 mg pycnogenol mixture of water-soluble bioflavonoids | 76 patients with asthma | Decrease by 15.2% of the specific IgE, whereas IgG1 and IgG4 remained unchanged. Reduced the need for medication [126] |
| 500 mg/day Apple condensed tannins (ACT) and polyphenols | A double-blind comparative study on 36 subjects with rhinitis for 12 weeks | Significant improvement in sneezing scores and nasal discharge inhibited in perennial rhinitis due in the group taking polyphenols treatment [127] |
4.3. Polyphenols in Atopic Eczema or Dermatitis
Atopic eczema and dermatitis are allergic skin disorders that primarily occur during early infanthood (3–4 months of age) and continue to develop until 2 years of age [128]. They cause dryness of skin, itching, inflammation and erythema (redness). Polyphenols have anti-inflammatory properties that can alleviate this allergic inflammation. Green tea extracts (catechins, epicatechin, epigallocatechin gallate and their derivatives) protect against cutaneous inflammation [129]. Similarly, EGCG suppresses the secretion of the pro-inflammatory cytokine IL-2 in vitro, an important mediator in allergic dermatological conditions [130]. Polyphenols have also been shown to improve the characteristic itching and pruritis associated with these conditions. Oat-derived polyphenol avenanthramide was shown to reduce the characteristics of pruritis and itching associated with dermatological conditions [131]. The effect of polyphenols on keratinocytes and immune cells was also analyzed in vitro and shown to reduce nuclear factor κB (NF-κB) activation, TNF-α and IL-8 [132]. Likewise, quercetin and luteolin also inhibit skin itching and flush reaction [133]. By suppressing pro-inflammatory cytokines, polyphenols can reduce the symptoms and occurrence of allergic skin disorders.
4.4. Polyphenols in Allergic Asthma and Rhinitis
Asthma, an allergic inflammatory lung disease characterized by increased leukocyte infiltration into the airways, most notably granulocytes, resulting in decreased respiratory function. Often the inflammation can cause bronchoconstriction, airway hyper-responsiveness (AHR) and increased mucus production [134]. In the airways, exposure to an allergen such as pollen produces a Th2-dominated response by recruiting and activating inflammatory cells and upregulating IL-4, IL-13, and IL-5 [135]. In an animal model of asthma, it was shown that resveratrol had a suppressive effect on asthmatic parameters as it inhibited the production of Th-2 cytokines like IL-4 and IL-5 in the plasma and bronchoalveolar lavage fluid, and caused suppression of airway hyperresponsiveness, eosinophilia, and hypersecretion of mucus [136]. Moreover, quercetin is known to ameliorate the pathogenic process of asthma by decreasing IL-4 and IFN-γ synthesis and by regulating Th1/Th2 balance [137]. Recent evidence showed that the administration of polyphenol-rich ethanolic extract of Boehmeria nivea (caffeic acid, catechin, epicatechin, β-sissterol, rutin, luteolin-7-glucoside, naringin, hesperidin, chlorogenic acid, and tangeretin) reduces allergic response in mice by suppressing mast cell mediated inflammation, decreasing TNF-α, IL-1β, IL-6, Th2, extracellular-signal-regulated kinase (ERK) and Mitogen-Activated Protein Kinase (MAPK) expression [138]. In murine asthma models, it was found that sesamin (rich in flavonoids) reduced allergic inflammation induce by asthma and airway hyperresponsiveness (AHR), making them an effective adjunct for the treatment of asthma [139].
5. Immune Modulation of Polyphenols in Autoimmunity
The immune system protects against foreign substances, but it is also responsible for self-tolerance, by which host tissues are protected from immunological action. Dysfunction can lead to loss of this immune tolerance and disturbed homeostasis, resulting in autoimmune disease [140]. The prevalence of autoimmune diseases is about 5%, and approximately 80 types of autoimmune diseases have been described [141]. Several factors lead to the development of autoimmune diseases, including genetic, epigenetic, environmental, nutritional and microbiotic diseases. Polyphenols have been shown to have a beneficial role in some common autoimmune diseases.
5.1. Polyphenols and Type-1 Diabetes
Type-1 diabetes is a multifactorial disease linked to a combination of genetic and environmental factors. It is characterized by the autoimmune destruction of pancreatic β cells, resulting in severe insulin deficiency and resultant hyperglycemia [142]. Polyphenols help in the regulation of pancreatic β-cells, type-1 diabetes and complications associated with type-1 diabetes [143]. Polyphenols involved in activation of the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway, thus helping to reduce the progression of type-1 diabetes [140]. It was shown that pomegranate peel extract inhibited immune cell infiltration into pancreatic islets and interferes with IL-17 and IFN-γ synthesis in gut-associated lymphoid tissue in type-1 diabetes [144]. A study in mice with type-1 diabetes quercetin treatment modulated Th1/Th2 balance and had glucose-lowering potential [145]. In addition, butein (a plant polyphenol) was able to prevent cytokine-induced β-cell damage by inhibiting NO production, iNOS expression, NF-κB translocation and glucose-stimulated insulin secretion which, prevented the progression of type-1 diabetes in rats [146]. Similarly, Broussonetia kazinoki polyphenols have been shown to have therapeutic potential in the prevention of cytokine-induced β-cell damage and reduce/delay the extent of pancreatic β-cell damage in type-1 diabetes [147]. Consequently, polyphenols may play a role in modulating key signaling pathways, T helper cell response and reducing cytokine induced β-cell damage, which may aid in the management of type-1 diabetes.
5.2. Polyphenols and Rheumatoid Arthritis
Rheumatoid arthritis, is a systemic autoimmune disease, characterized by erosive and symmetric synovitis, particularly in the peripheral joints. Degradation of cartilage and bone erosion leads to the eventual destruction of a joint [148]. In developed countries, rheumatoid arthritis affects about 0.5–1% of the population and women are at three times greater risk [149]. While the reason for the development of this disease is still unknown, genetics is thought to play an important role. Polyphenols may also have an impact in the management of rheumatoid arthritis. For instance, curcumin, a potent anti-inflammatory agent, decreases IL-1β, induces IL-6 and vascular endothelial growth factor by rheumatoid arthritis-fibroblast-like synoviocytes [150]. In addition, curcumin also induces apoptosis of rheumatoid arthritis-fibroblast-like synoviocytes, which are typically resistant to apoptotic signaling, by upregulating pro-apoptotic proteins, such as Bax, and downregulating the anti-apoptotic protein Bcl-2 [151]. In addition, resveratrol has both protective and therapeutic effects in inflammatory arthritis, by inhibiting the function of Th-17, B-cells and MAPK signaling pathways [61,152]. A clinical trial reported that a 1 g capsule of resveratrol for 3 months decreased the swelling and tenderness of joints by regulating pro-inflammatory cytokines [153]. Moreover, EGCG suppresses osteoclast differentiation and decreases clinical symptoms in an animal model of rheumatoid arthritis [154]. Grape polyphenols have shown immuno-regulatory effects, establishing a balance between Th17 and Treg cells and inhibiting TNFα in rheumatoid arthritis, hence mitigating inflammation, oxidative stress and rheumatoid arthritis associated symptoms [155,156,157]. Administration of extra virgin olive oil polyphenol extract (100 and 200 mg/kg body weight) to arthritic mice decreased the pro-inflammatory cytokines, PGE2, COX-2, and microsomal prostaglandin E synthase-1 as well as NF-κB translocation, resulting in decreased progression of the joint disease [158]. Evidence showed that quercetin supplements (500 mg/day) for 8 weeks resulted in a significant reduction of morning stiffness, morning pain, and after-activity pain [159]. Therefore, polyphenols may improve the quality of life of patients with rheumatoid arthritis.
5.3. Polyphenols and Multiple Sclerosis
Multiple sclerosis, is a chronic neurological autoimmune disorder characterized by the breakdown of the myelin sheath, alongside dysfunction of the blood–brain barrier, perivascular inflammation, as well as damaged axons and oligodendrocytes-all of which lead to progressive nervous system damage and clinical disability [140]. Polyphenols may play a role in the prevention and treatment of multiple sclerosis [160]. Quercetin exerts an immunomodulatory effect aiding in the treatment of multiple sclerosis by inhibiting proliferation of autoimmune T-cells, and the expression of TNF-α by mononuclear cells in vitro [161,162]. It has also been shown to reduce peripheral blood mononuclear cell proliferation [163]. The polyphenolic flavones apigenin and luteolin have a robust inhibitory potential on T-cell proliferation while also reducing IFN-γ production [162]. Strikingly, flavonoids have been shown to limit demyelination in multiple sclerosis and so may confer neuroprotective benefits [164]. In a mouse model, resveratrol prevented neuronal loss, and delayed the onset of autoimmune encephalomyelitis suggesting that resveratrol could play an immunomodulatory role in managing multiple sclerosis [165]. Similarly, administration of resveratrol (250 mg/kg/day) for 3 weeks showed therapeutic potential as an adjunct in the treatment of multiple sclerosis by improving motor coordination and balance, mitochondrial function, reducing oxidative stress, and inhibiting NF-κB signaling [166]. In a study of 66 patients with multiple sclerosis who were treated with grape seed capsules for one month it was found that the capsules positively impacted physical and mental functioning, improving quality of life [167]. Given this, polyphenols may have therapeutic potential as an adjunct treatment in multiple sclerosis patients.
6. Conclusions
Polyphenols are promising candidates for novel adjunct therapeutic approaches. They can modulate multiple immune system processes and reduce the burden of various diseases such as IBD, allergies and autoimmune disorders. In addition to their demonstrated antioxidant qualities, polyphenols have broad health-promoting effects, due to their ability to modulate inflammation and immune responses. They improve the interplay between immune cells and decrease expression of pro-inflammatory cytokines. Further research is required to clinically validate the therapeutic potential of polyphenols on immunomodulation as well as to explore their interaction with gut microbiota.
Acknowledgments
H.S., L.S., H.I.A, A.S.A.D. and C.P. would like to acknowledge the Department of Nutrition and Health, United Arab Emirates University for their ongoing support. L.C. thanks the College of Heatlh Sciences and RIMHS University of Sharjah for their support and M.B. acknowledges the St. Ciril and Methodius Faculty of Medicine University of Cardiology clinic for their research support. J.F. would like to acknowledge the Australian Government for the support for an RTP training scholarship and the University of Melbourne Stipend. J.F. and V.A. would like to thank the Immunology and Translational Research Group within the Institute for Health and Sport, Victoria University Australia, for their support.
Author Contributions
Conceptualization, L.S. and C.P.; formal analysis, H.S.; investigation, H.S.; writing—original draft preparation, H.S. and J.F.; writing—review and editing, J.F., V.A., H.I.A., A.S.A.D., L.C.I., M.B. and L.S.; visualization, H.S.; supervision, L.S. and C.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Swallah M.S., Sun H., Affoh R., Fu H., Yu H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int. J. Food Sci. 2020;2020:9081686. doi: 10.1155/2020/9081686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandrasekara A., Shahidi F. Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J. Agric. Food Chem. 2010;58:6706–6714. doi: 10.1021/jf100868b. [DOI] [PubMed] [Google Scholar]
- 3.Santhakumar A.B., Battino M., Alvarez-Suarez J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018;113:49–65. doi: 10.1016/j.fct.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Yamagata K., Tagami M., Yamori Y. Dietary polyphenols regulate endothelial function and prevent cardiovascular disease. Nutrition. 2015;31:28–37. doi: 10.1016/j.nut.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Cardona F., Andrés-Lacueva C., Tulipani S., Tinahones F.J., Queipo-Ortuño M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013;24:1415–1422. doi: 10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Martin K.R., Appel C.L. Polyphenols as dietary supplements: A double-edged sword. Nutr. Diet. Suppl. 2009;2:1–12. [Google Scholar]
- 7.Quiñones M., Miguel M., Aleixandre A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 2013;68:125–131. doi: 10.1016/j.phrs.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Cassidy A., O’Reilly É.J., Kay C., Sampson L., Franz M., Forman J.P., Curhan G., Rimm E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011;93:338–347. doi: 10.3945/ajcn.110.006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiva-Blanch G., Urpi-Sarda M., Ros E., Valderas-Martinez P., Casas R., Arranz S., Guillén M., Lamuela-Raventós R.M., Llorach R., Andres-Lacueva C. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013;32:200–206. doi: 10.1016/j.clnu.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Hanhineva K., Törrönen R., Bondia-Pons I., Pekkinen J., Kolehmainen M., Mykkänen H., Poutanen K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010;11:1365–1402. doi: 10.3390/ijms11041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooper L., Kay C., Abdelhamid A., Kroon P.A., Cohn J.S., Rimm E.B., Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012;95:740–751. doi: 10.3945/ajcn.111.023457. [DOI] [PubMed] [Google Scholar]
- 12.Ahtesh F.B., Stojanovska L., Feehan J., de Courten M.P., Flavel M., Kitchen B., Apostolopoulos V. Polyphenol Rich Sugar Cane Extract Inhibits Bacterial Growth. Prilozi. 2020;41:49–57. doi: 10.2478/prilozi-2020-0045. [DOI] [PubMed] [Google Scholar]
- 13.Scalbert A., Manach C., Morand C., Rémésy C., Jiménez L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 14.Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C.B., Rahu N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolowczuk I., Verwaerde C., Viltart O., Delanoye A., Delacre M., Pot B., Grangette C. Feeding our immune system: Impact on metabolism. Clin. Dev. Immunol. 2008;2008:639803. doi: 10.1155/2008/639803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medzhitov R., Janeway C. Innate immunity. N. Engl. J. Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 17.Beutler B. Innate immunity: An overview. Mol. Immunol. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Clark R., Kupper T. Old meets new: The interaction between innate and adaptive immunity. J. Investig. Dermatol. 2005;125:629–637. doi: 10.1111/j.0022-202X.2005.23856.x. [DOI] [PubMed] [Google Scholar]
- 19.Hachimura S., Totsuka M., Hosono A. Immunomodulation by food: Impact on gut immunity and immune cell function. Biosci. Biotechnol. Biochem. 2018;82:584–599. doi: 10.1080/09168451.2018.1433017. [DOI] [PubMed] [Google Scholar]
- 20.Buckwalter M.R., Albert M.L. Orchestration of the immune response by dendritic cells. Curr. Biol. 2009;19:R355–R361. doi: 10.1016/j.cub.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 21.del Cornò M., Scazzocchio B., Masella R., Gessani S. Regulation of dendritic cell function by dietary polyphenols. Crit. Rev. Food Sci. Nutr. 2016;56:737–747. doi: 10.1080/10408398.2012.713046. [DOI] [PubMed] [Google Scholar]
- 22.Švajger U., Obermajer N., Jeras M. Dendritic cells treated with resveratrol during differentiation from monocytes gain substantial tolerogenic properties upon activation. Immunology. 2010;129:525–535. doi: 10.1111/j.1365-2567.2009.03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoneyama S., Kawai K., Tsuno N.H., Okaji Y., Asakage M., Tsuchiya T., Yamada J., Sunami E., Osada T., Kitayama J. Epigallocatechin gallate affects human dendritic cell differentiation and maturation. J. Allergy Clin. Immunol. 2008;121:209–214. doi: 10.1016/j.jaci.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Lee J.S., Kim S.G., Kim H.K., Lee T.H., Jeong Y.I., Lee C.M., Yoon M.S., Na Y.J., Suh D.S., Park N.C. Silibinin polarizes Th1/Th2 immune responses through the inhibition of immunostimulatory function of dendritic cells. J. Cell. Physiol. 2007;210:385–397. doi: 10.1002/jcp.20852. [DOI] [PubMed] [Google Scholar]
- 25.Huang R.-Y., Yu Y.-L., Cheng W.-C., OuYang C.-N., Fu E., Chu C.-L. Immunosuppressive effect of quercetin on dendritic cell activation and function. J. Immunol. 2010;184:6815–6821. doi: 10.4049/jimmunol.0903991. [DOI] [PubMed] [Google Scholar]
- 26.Yoon M.-S., Lee J.S., Choi B.-M., Jeong Y.-I., Lee C.-M., Park J.-H., Moon Y., Sung S.-C., Lee S.K., Chang Y.H. Apigenin inhibits immunostimulatory function of dendritic cells: Implication of immunotherapeutic adjuvant. Mol. Pharmacol. 2006;70:1033–1044. doi: 10.1124/mol.106.024547. [DOI] [PubMed] [Google Scholar]
- 27.Gupta S.C., Tyagi A.K., Deshmukh-Taskar P., Hinojosa M., Prasad S., Aggarwal B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys. 2014;559:91–99. doi: 10.1016/j.abb.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Liu S.-H., Lin C.-H., Hung S.-K., Chou J.-H., Chi C.-W., Fu S.-L. Fisetin inhibits lipopolysaccharide-induced macrophage activation and dendritic cell maturation. J. Agric. Food Chem. 2010;58:10831–10839. doi: 10.1021/jf1017093. [DOI] [PubMed] [Google Scholar]
- 29.Buttari B., Profumo E., Facchiano F., Ozturk E.I., Segoni L., Saso L., Riganò R. Resveratrol prevents dendritic cell maturation in response to advanced glycation end products. Oxidative Med. Cell. Longev. 2013;2013:574029. doi: 10.1155/2013/574029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bottazzi B., Doni A., Garlanda C., Mantovani A. An integrated view of humoral innate immunity: Pentraxins as a paradigm. Annu. Rev. Immunol. 2009;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 31.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansson G.K., Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 2011;12:204. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 33.Kamada N., Hisamatsu T., Okamoto S., Chinen H., Kobayashi T., Sato T., Sakuraba A., Kitazume M.T., Sugita A., Koganei K. Unique CD14+ intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-γ axis. J. Clin. Investig. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sica A., Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dugo L., Belluomo M.G., Fanali C., Russo M., Cacciola F., Maccarrone M., Sardanelli A.M. Effect of cocoa polyphenolic extract on macrophage polarization from proinflammatory M1 to anti-inflammatory M2 state. Oxidative Med. Cell. Longev. 2017;2017:6293740. doi: 10.1155/2017/6293740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park H.-R., Hwang D., Suh H.-J., Yu K.-W., Kim T.Y., Shin K.-S. Antitumor and antimetastatic activities of rhamnogalacturonan-II-type polysaccharide isolated from mature leaves of green tea via activation of macrophages and natural killer cells. Int. J. Biol. Macromol. 2017;99:179–186. doi: 10.1016/j.ijbiomac.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 37.Zhang M., Xie Y., Su X., Liu K., Zhang Y., Pang W., Wang J. Inonotus sanghuang polyphenols attenuate inflammatory response via modulating the crosstalk between macrophages and adipocytes. Front. Immunol. 2019;10:286. doi: 10.3389/fimmu.2019.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overman A., Bumrungpert A., Kennedy A., Martinez K., Chuang C.C., West T., Dawson B., Jia W., McIntosh M. Polyphenol-rich grape powder extract (GPE) attenuates inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. Int. J. Obes. 2010;34:800–808. doi: 10.1038/ijo.2009.296. [DOI] [PubMed] [Google Scholar]
- 39.McLaren J.E., Michael D.R., Ashlin T.G., Ramji D.P. Cytokines, macrophage lipid metabolism and foam cells: Implications for cardiovascular disease therapy. Prog. Lipid Res. 2011;50:331–347. doi: 10.1016/j.plipres.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Moss J.W.E., Ramji D.P. Nutraceutical therapies for atherosclerosis. Nat. Rev. Cardiol. 2016;13:513. doi: 10.1038/nrcardio.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramji D.P., Davies T.S. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015;26:673–685. doi: 10.1016/j.cytogfr.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sevov M., Elfineh L., Cavelier L.B. Resveratrol regulates the expression of LXR-α in human macrophages. Biochem. Biophys. Res. Commun. 2006;348:1047–1054. doi: 10.1016/j.bbrc.2006.07.155. [DOI] [PubMed] [Google Scholar]
- 43.Ruggeri L., Capanni M., Urbani E., Perruccio K., Shlomchik W.D., Tosti A., Posati S., Rogaia D., Frassoni F., Aversa F. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 44.Hu J.-Y., Zhang J., Cui J.-L., Liang X.-Y., Lu R., Du G.-F., Xu X.-Y., Zhou G. Increasing CCL5/CCR5 on CD4+ T cells in peripheral blood of oral lichen planus. Cytokine. 2013;62:141–145. doi: 10.1016/j.cyto.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Kim Y.H., Won Y.-S., Yang X., Kumazoe M., Yamashita S., Hara A., Takagaki A., Goto K., Nanjo F., Tachibana H. Green tea catechin metabolites exert immunoregulatory effects on CD4+ T cell and natural killer cell activities. J. Agric. Food Chem. 2016;64:3591–3597. doi: 10.1021/acs.jafc.6b01115. [DOI] [PubMed] [Google Scholar]
- 46.Exon J.H., Magnuson B.A., South E.H., Hendrix K. Dietary quercetin, immune functions and colonic carcinogenesis in rats. Immunopharmacol. Immunotoxicol. 1998;20:173–190. doi: 10.3109/08923979809034816. [DOI] [PubMed] [Google Scholar]
- 47.Bub A., Watzl B., Blockhaus M., Briviba K., Liegibel U., Müller H., Pool-Zobel B.L., Rechkemmer G. Fruit juice consumption modulates antioxidative status, immune status and DNA damage. J. Nutr. Biochem. 2003;14:90–98. doi: 10.1016/s0955-2863(02)00255-3. [DOI] [PubMed] [Google Scholar]
- 48.McAnulty L.S., Nieman D.C., Dumke C.L., Shooter L.A., Henson D.A., Utter A.C., Milne G., McAnulty S.R. Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running. Appl. Physiol. Nutr. Metab. 2011;36:976–984. doi: 10.1139/h11-120. [DOI] [PubMed] [Google Scholar]
- 49.McAnulty L.S., Collier S.R., Landram M.J., Whittaker D.S., Isaacs S.E., Klemka J.M., Cheek S.L., Arms J.C., McAnulty S.R. Six weeks daily ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutr. Res. 2014;34:577–584. doi: 10.1016/j.nutres.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Chen C.-M., Li S.-C., Lin Y.-L., Hsu C.-Y., Shieh M.-J., Liu J.-F. Consumption of purple sweet potato leaves modulates human immune response: T-lymphocyte functions, lytic activity of natural killer cell and antibody production. World J. Gastroenterol. WJG. 2005;11:5777. doi: 10.3748/wjg.v11.i37.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hassanain E., Silverberg J.I., Norowitz K.B., Chice S., Bluth M.H., Brody N., Joks R., Durkin H.G., Smith-Norowitz T.A. Green tea (Camelia sinensis) suppresses B cell production of IgE without inducing apoptosis. Ann. Clin. Lab. Sci. 2010;40:135–143. [PubMed] [Google Scholar]
- 52.Sanbongi C., Suzuki N., Sakane T. Polyphenols in chocolate, which have antioxidant activity, modulate immune functions in humansin vitro. Cell. Immunol. 1997;177:129–136. doi: 10.1006/cimm.1997.1109. [DOI] [PubMed] [Google Scholar]
- 53.Kuo C.-L., Chen T.-S., Liou S.-Y., Hsieh C.-C. Immunomodulatory effects of EGCG fraction of green tea extract in innate and adaptive immunity via T regulatory cells in murine model. Immunopharmacol. Immunotoxicol. 2014;36:364–370. doi: 10.3109/08923973.2014.953637. [DOI] [PubMed] [Google Scholar]
- 54.Wong C.P., Nguyen L.P., Noh S.K., Bray T.M., Bruno R.S., Ho E. Induction of regulatory T cells by green tea polyphenol EGCG. Immunol. Lett. 2011;139:7–13. doi: 10.1016/j.imlet.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorenec L., Lepej S.Z., Grgic I., Planinic A., Bes J.I., Vince A., Begovac J. The comparison of Th1, Th2, Th9, Th17 and Th22 cytokine profiles in acute and chronic HIV-1 infection. Microb. Pathog. 2016;97:125–130. doi: 10.1016/j.micpath.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Louten J., Boniface K., de Waal Malefyt R. Development and function of TH17 cells in health and disease. J. Allergy Clin. Immunol. 2009;123:1004–1011. doi: 10.1016/j.jaci.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Cavani A., Pennino D., Eyerich K. New Trends in Allergy and Atopic Eczema. Volume 96. Karger Publishers; Basel, Switzerland: 2012. Th17 and Th22 in Skin Allergy; pp. 39–44. [DOI] [PubMed] [Google Scholar]
- 58.Zheng Y., Danilenko D.M., Valdez P., Kasman I., Eastham-Anderson J., Wu J., Ouyang W. Interleukin-22, a TH 17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 59.Yano S., Umeda D., Yamashita T., Ninomiya Y., Sumida M., Fujimura Y., Yamada K., Tachibana H. Dietary flavones suppresses IgE and Th2 cytokines in OVA-immunized BALB/c mice. Eur. J. Nutr. 2007;46:257–263. doi: 10.1007/s00394-007-0658-7. [DOI] [PubMed] [Google Scholar]
- 60.Wang J., Pae M., Meydani S.N., Wu D. Green tea epigallocatechin-3-gallate modulates differentiation of naïve CD4+ T cells into specific lineage effector cells. J. Mol. Med. 2013;91:485–495. doi: 10.1007/s00109-012-0964-2. [DOI] [PubMed] [Google Scholar]
- 61.Xuzhu G., Komai-Koma M., Leung B.P., Howe H.S., McSharry C., McInnes I.B., Xu D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann. Rheum. Dis. 2012;71:129–135. doi: 10.1136/ard.2011.149831. [DOI] [PubMed] [Google Scholar]
- 62.Ahmad S.F., Zoheir K.M.A., Abdel-Hamied H.E., Ashour A.E., Bakheet S.A., Attia S.M., Abd-Allah A.R.A. Grape seed proanthocyanidin extract has potent anti-arthritic effects on collagen-induced arthritis by modifying the T cell balance. Int. Immunopharmacol. 2013;17:79–87. doi: 10.1016/j.intimp.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. [Google Scholar]
- 64.Dandona P., Aljada A., Chaudhuri A., Mohanty P., Garg R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 65.Du L., Li J., Zhang X., Wang L., Zhang W. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264. 7 macrophages via the suppression of MAPKs activation. J. Funct. Foods. 2018;43:62–69. doi: 10.29219/fnr.v63.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du L., Li J., Zhang X., Wang L., Zhang W., Yang M., Hou C. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264. 7 macrophages via the suppression of TLR4/NF-κB pathway activation. Food Nutr. Res. 2019;63 doi: 10.29219/fnr.v63.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nallathambi R., Poulev A., Zuk J.B., Raskin I. Proanthocyanidin-Rich Grape Seed Extract Reduces Inflammation and Oxidative Stress and Restores Tight Junction Barrier Function in Caco-2 Colon Cells. Nutrients. 2020;12:1623. doi: 10.3390/nu12061623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y., Wang Y., Shen W., Wang Y., Cao Y., Nuerbulati N., Chen W., Lu G., Xiao W., Qi R. Grape Seed Polyphenols Ameliorated Dextran Sulfate Sodium-Induced Colitis via Suppression of Inflammation and Apoptosis. Pharmacology. 2020;105:9–18. doi: 10.1159/000501897. [DOI] [PubMed] [Google Scholar]
- 69.Li Y., Rahman S.U., Huang Y., Zhang Y., Ming P., Zhu L., Chu X., Li J., Feng S., Wang X. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. J. Nutr. Biochem. 2020;78:108324. doi: 10.1016/j.jnutbio.2019.108324. [DOI] [PubMed] [Google Scholar]
- 70.Abdollahi E., Momtazi A.A., Johnston T.P., Sahebkar A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: A nature-made jack-of-all-trades? J. Cell. Physiol. 2018;233:830–848. doi: 10.1002/jcp.25778. [DOI] [PubMed] [Google Scholar]
- 71.Zheng M., Zhang Q., Joe Y., Lee B.H., Kwon K.B., Ryter S.W., Chung H.T. Curcumin induces apoptotic cell death of activated human CD4+ T cells via increasing endoplasmic reticulum stress and mitochondrial dysfunction. Int. Immunopharmacol. 2013;15:517–523. doi: 10.1016/j.intimp.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 72.Busch F., Mobasheri A., Shayan P., Lueders C., Stahlmann R., Shakibaei M. Resveratrol modulates interleukin-1β-induced phosphatidylinositol 3-kinase and nuclear factor κB signaling pathways in human tenocytes. J. Biol. Chem. 2012;287:38050–38063. doi: 10.1074/jbc.M112.377028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Venkatachalam K., Mummidi S., Cortez D.M., Prabhu S.D., Valente A.J., Chandrasekar B. Resveratrol inhibits high glucose-induced PI3K/Akt/ERK-dependent interleukin-17 expression in primary mouse cardiac fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2078–H2087. doi: 10.1152/ajpheart.01363.2007. [DOI] [PubMed] [Google Scholar]
- 74.Wu D., Wang J., Pae M., Meydani S.N. Green tea EGCG, T cells, and T cell-mediated autoimmune diseases. Mol. Asp. Med. 2012;33:107–118. doi: 10.1016/j.mam.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Bak M.-J., Truong V.L., Kang H.-S., Jun M., Jeong W.-S. Anti-inflammatory effect of procyanidins from wild grape (Vitis amurensis) seeds in LPS-induced RAW 264.7 cells. Oxidative Med. Cell. Longev. 2013;2013:409321. doi: 10.1155/2013/409321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chu H., Tang Q., Huang H., Hao W., Wei X. Grape-seed proanthocyanidins inhibit the lipopolysaccharide-induced inflammatory mediator expression in RAW264. 7 macrophages by suppressing MAPK and NF-κb signal pathways. Environ. Toxicol. Pharmacol. 2016;41:159–166. doi: 10.1016/j.etap.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 77.Armutcu F., Akyol S., Ustunsoy S., Turan F.F. Therapeutic potential of caffeic acid phenethyl ester and its anti-inflammatory and immunomodulatory effects. Exp. Ther. Med. 2015;9:1582–1588. doi: 10.3892/etm.2015.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Juman S., Yasui N., Ikeda K., Ueda A., Sakanaka M., Negishi H., Miki T. Caffeic acid phenethyl ester suppresses the production of pro-inflammatory cytokines in hypertrophic adipocytes through lipopolysaccharide-stimulated macrophages. Biol. Pharm. Bull. 2012;35:1941–1946. doi: 10.1248/bpb.b12-00317. [DOI] [PubMed] [Google Scholar]
- 79.Zhang M., Zhou J., Wang L., Li B., Guo J., Guan X., Han Q., Zhang H. Caffeic acid reduces cutaneous tumor necrosis factor alpha (TNF-α), IL-6 and IL-1β levels and ameliorates skin edema in acute and chronic model of cutaneous inflammation in mice. Biol. Pharm. Bull. 2014;37:347–354. doi: 10.1248/bpb.b13-00459. [DOI] [PubMed] [Google Scholar]
- 80.Shimizu M. Multifunctions of dietary polyphenols in the regulation of intestinal inflammation. J. Food Drug Anal. 2017;25:93–99. doi: 10.1016/j.jfda.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh D., Srivastava S., Pradhan M., Kanwar J.R., Singh M.R. Inflammatory bowel disease: Pathogenesis, causative factors, issues, drug treatment strategies, and delivery approaches. Crit. Rev. Ther. Drug Carr. Syst. 2015;32:181–214. doi: 10.1615/critrevtherdrugcarriersyst.2015011095. [DOI] [PubMed] [Google Scholar]
- 82.Center for Disease Control and Prevention Inflammatory Bowel Disease. [(accessed on 10 December 2020)];2012 Available online: https://www.cdc.gov/ibd/data-statistics.htm.
- 83.Fukata M., Arditi M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013;6:451–463. doi: 10.1038/mi.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang S., Zhao L., Kim K., Lee D.S., Hwang D.H. Inhibition of Nod2 signaling and target gene expression by curcumin. Mol. Pharmacol. 2008;74:274–281. doi: 10.1124/mol.108.046169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shibata T., Nakashima F., Honda K., Lu Y.-J., Kondo T., Ushida Y., Aizawa K., Suganuma H., Oe S., Tanaka H. Toll-like receptors as a target of food-derived anti-inflammatory compounds. J. Biol. Chem. 2014;289:32757–32772. doi: 10.1074/jbc.M114.585901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Youn H.S., Lee J.Y., Fitzgerald K.A., Young H.A., Akira S., Hwang D.H. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: Molecular targets are TBK1 and RIP1 in TRIF complex. J. Immunol. 2005;175:3339–3346. doi: 10.4049/jimmunol.175.5.3339. [DOI] [PubMed] [Google Scholar]
- 87.Nunes S., Danesi F., Del Rio D., Silva P. Resveratrol and inflammatory bowel disease: The evidence so far. Nutr. Res. Rev. 2018;31:85–97. doi: 10.1017/S095442241700021X. [DOI] [PubMed] [Google Scholar]
- 88.Kostyuk V.A., Potapovich A.I., Suhan T.O., de Luca C., Korkina L.G. Antioxidant and signal modulation properties of plant polyphenols in controlling vascular inflammation. Eur. J. Pharmacol. 2011;658:248–256. doi: 10.1016/j.ejphar.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 89.Mackenzie G.G., Delfino J.M., Keen C.L., Fraga C.G., Oteiza P.I. Dimeric procyanidins are inhibitors of NF-κB–DNA binding. Biochem. Pharmacol. 2009;78:1252–1262. doi: 10.1016/j.bcp.2009.06.111. [DOI] [PubMed] [Google Scholar]
- 90.Wang H.-K., Yeh C.-H., Iwamoto T., Satsu H., Shimizu M., Totsuka M. Dietary flavonoid naringenin induces regulatory T cells via an aryl hydrocarbon receptor mediated pathway. J. Agric. Food Chem. 2012;60:2171–2178. doi: 10.1021/jf204625y. [DOI] [PubMed] [Google Scholar]
- 91.Josefowicz S.Z., Lu L.-F., Rudensky A.Y. Regulatory T cells: Mechanisms of differentiation and function. Annu. Rev. Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee H.C., Jenner A.M., Low C.S., Lee Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006;157:876–884. doi: 10.1016/j.resmic.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 93.Magrone T., Jirillo E. The interplay between the gut immune system and microbiota in health and disease: Nutraceutical intervention for restoring intestinal homeostasis. Curr. Pharm. Des. 2013;19:1329–1342. doi: 10.2174/138161213804805793. [DOI] [PubMed] [Google Scholar]
- 94.Rahman S.U., Li Y., Huang Y., Zhu L., Feng S., Wu J., Wang X. Treatment of inflammatory bowel disease via green tea polyphenols: Possible application and protective approaches. Inflammopharmacology. 2018;26:319–330. doi: 10.1007/s10787-018-0462-4. [DOI] [PubMed] [Google Scholar]
- 95.Barbalho S.M., Bosso H., Salzedas-Pescinini L.M., de Alvares Goulart R. Green tea: A possibility in the therapeutic approach of inflammatory bowel diseases?: Green tea and inflammatory bowel diseases. Complementary Ther. Med. 2019;43:148–153. doi: 10.1016/j.ctim.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 96.Hollebeeck S., Larondelle Y., Schneider Y.-J., During A. The use of pomegranate (Punica granatum l.) phenolic compounds as potential natural prevention against IBDs. Inflamm. Bowel Dis. Adv. Pathog. Manag. Intech-Publ. Belg. 2012:275–300. doi: 10.5772/27532. [DOI] [Google Scholar]
- 97.Kim H., Venancio V.P., Fang C., Dupont A.W., Talcott S.T., Mertens-Talcott S.U. Mango (Mangifera indica L.) polyphenols reduce IL-8, GRO, and GM-SCF plasma levels and increase Lactobacillus species in a pilot study in patients with inflammatory bowel disease. Nutr. Res. 2020;75:85–94. doi: 10.1016/j.nutres.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 98.Scarano A., Butelli E., De Santis S., Cavalcanti E., Hill L., De Angelis M., Giovinazzo G., Chieppa M., Martin C., Santino A. Combined dietary anthocyanins, flavonols, and stilbenoids alleviate inflammatory bowel disease symptoms in mice. Front. Nutr. 2018;4:75. doi: 10.3389/fnut.2017.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Devereux G. The increase in the prevalence of asthma and allergy: Food for thought. Nat. Rev. Immunol. 2006;6:869–874. doi: 10.1038/nri1958. [DOI] [PubMed] [Google Scholar]
- 100.Tai A., Volkmer R., Burton A. Prevalence of asthma symptoms and atopic disorders in preschool children and the trend over a decade. J. Asthma. 2009;46:343–346. doi: 10.1080/02770900802660998. [DOI] [PubMed] [Google Scholar]
- 101.Singh A., Holvoet S., Mercenier A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clin. Exp. Allergy. 2011;41:1346–1359. doi: 10.1111/j.1365-2222.2011.03773.x. [DOI] [PubMed] [Google Scholar]
- 102.Gong J., Chen S.-S. Polyphenolic antioxidants inhibit peptide presentation by antigen-presenting cells. Int. Immunopharmacol. 2003;3:1841–1852. doi: 10.1016/j.intimp.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 103.Kim J.-Y., Kina T., Iwanaga Y., Noguchi H., Matsumura K., Hyon S.-H. Tea polyphenol inhibits allostimulation in mixed lymphocyte culture. Cell Transplant. 2007;16:75–83. [PubMed] [Google Scholar]
- 104.Iwamura C., Shinoda K., Yoshimura M., Watanabe Y., Obata A., Nakayama T. Naringenin chalcone suppresses allergic asthma by inhibiting the type-2 function of CD4 T cells. Allergol. Int. 2010;59:67–73. doi: 10.2332/allergolint.09-OA-0118. [DOI] [PubMed] [Google Scholar]
- 105.Zuercher A.W., Holvoet S., Weiss M., Mercenier A. Polyphenol-enriched apple extract attenuates food allergy in mice. Clin. Exp. Allergy. 2010;40:942–950. doi: 10.1111/j.1365-2222.2010.03460.x. [DOI] [PubMed] [Google Scholar]
- 106.Aires V., Adote S., Hirchami A., Moutairou K., Boustani E.-S.E., Khan N.A. Modulation of intracellular calcium concentrations and T cell activation by prickly pear polyphenols. Mol. Cell. Biochem. 2004;260:103–110. doi: 10.1023/b:mcbi.0000026061.57326.28. [DOI] [PubMed] [Google Scholar]
- 107.Schoene N.W., Kelly M.A., Polansky M.M., Anderson R.A. A polyphenol mixture from cinnamon targets p38 MAP kinase-regulated signaling pathways to produce G2/M arrest. J. Nutr. Biochem. 2009;20:614–620. doi: 10.1016/j.jnutbio.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 108.Kawai K., Tsuno N.H., Kitayama J., Sunami E., Takahashi K., Nagawa H. Catechin inhibits adhesion and migration of peripheral blood B cells by blocking CD11b. Immunopharmacol. Immunotoxicol. 2011;33:391–397. doi: 10.3109/08923973.2010.522195. [DOI] [PubMed] [Google Scholar]
- 109.Yano S., Umeda D., Maeda N., Fujimura Y., Yamada K., Tachibana H. Dietary apigenin suppresses IgE and inflammatory cytokines production in C57BL/6N mice. J. Agric. Food Chem. 2006;54:5203–5207. doi: 10.1021/jf0607361. [DOI] [PubMed] [Google Scholar]
- 110.Chung S.-Y., Champagne E.T. Reducing the allergenic capacity of peanut extracts and liquid peanut butter by phenolic compounds. Food Chem. 2009;115:1345–1349. [Google Scholar]
- 111.Wang C.-C., Lin Y.-R., Liao M.-H., Jan T.-R. Oral supplementation with areca-derived polyphenols attenuates food allergic responses in ovalbumin-sensitized mice. Bmc Complementary Altern. Med. 2013;13:154. doi: 10.1186/1472-6882-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bansode R.R., Randolph P.D., Plundrich N.J., Lila M.A., Williams L.L. Peanut protein-polyphenol aggregate complexation suppresses allergic sensitization to peanut by reducing peanut-specific IgE in C3H/HeJ mice. Food Chem. 2019;299:125025. doi: 10.1016/j.foodchem.2019.125025. [DOI] [PubMed] [Google Scholar]
- 113.Peérot M., Lupi R., Guyot S., Delayre-Orthez C., Gadonna-Widehem P., Theébaudin J.-Y., Bodinier M., Larreé C. Polyphenol interactions mitigate the immunogenicity and allergenicity of gliadins. J. Agric. Food Chem. 2017;65:6442–6451. doi: 10.1021/acs.jafc.6b05371. [DOI] [PubMed] [Google Scholar]
- 114.Anderson K.C., Teuber S.S. Ellagic acid and polyphenolics present in walnut kernels inhibit in vitro human peripheral blood mononuclear cell proliferation and alter cytokine production. Ann. N. Y. Acad. Sci. 2010;1190:86–96. doi: 10.1111/j.1749-6632.2009.05259.x. [DOI] [PubMed] [Google Scholar]
- 115.Labuckas D.O., Maestri D.M., Perello M., Martínez M.L., Lamarque A.L. Phenolics from walnut (Juglans regia L.) kernels: Antioxidant activity and interactions with proteins. Food Chem. 2008;107:607–612. [Google Scholar]
- 116.Graff J.C., Jutila M.A. Differential regulation of CD11b on γδ T cells and monocytes in response to unripe apple polyphenols. J. Leukoc. Biol. 2007;82:603–607. doi: 10.1189/jlb.0207125. [DOI] [PubMed] [Google Scholar]
- 117.Parkar S.G., Stevenson D.E., Skinner M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008;124:295–298. doi: 10.1016/j.ijfoodmicro.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 118.Tominaga T., Kawaguchi K., Kanesaka M., Kawauchi H., Jirillo E., Kumazawa Y. Suppression of type-I allergic responses by oral administration of grape marc fermented with Lactobacillus plantarum. Immunopharmacol. Immunotoxicol. 2010;32:593–599. doi: 10.3109/08923971003604786. [DOI] [PubMed] [Google Scholar]
- 119.Katsarou A., Davoy E., Xenos K., Armenaka M., Theoharides T.C. Effect of an antioxidant (quercetin) on sodium-lauryl-sulfate-induced skin irritation. Contact Dermat. 2000;42:85–89. doi: 10.1034/j.1600-0536.2000.042002085.x. [DOI] [PubMed] [Google Scholar]
- 120.Neukam K., Stahl W., Tronnier H., Sies H., Heinrich U. Consumption of flavanol-rich cocoa acutely increases microcirculation in human skin. Eur. J. Nutr. 2007;46:53–56. doi: 10.1007/s00394-006-0627-6. [DOI] [PubMed] [Google Scholar]
- 121.Mehrbani M., Choopani R., Fekri A., Mehrabani M., Mosaddegh M., Mehrabani M. The efficacy of whey associated with dodder seed extract on moderate-to-severe atopic dermatitis in adults: A randomized, double-blind, placebo-controlled clinical trial. J. Ethnopharmacol. 2015;172:325–332. doi: 10.1016/j.jep.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 122.Kojima T., Akiyama H., Sasai M., Taniuchi S., Goda Y., Toyoda M., Kobayashi Y. Anti-allergic effect of apple polyphenol on patients with atopic dermatitis: A pilot study. Allergol. Int. 2000;49:69–73. [Google Scholar]
- 123.Singh A., Demont A., Actis-Goretta L., Holvoet S., Lévêques A., Lepage M., Nutten S., Mercenier A. Identification of epicatechin as one of the key bioactive constituents of polyphenol-enriched extracts that demonstrate an anti-allergic effect in a murine model of food allergy. Br. J. Nutr. 2014;112:358–368. doi: 10.1017/S0007114514000877. [DOI] [PubMed] [Google Scholar]
- 124.Abril-Gil M., Massot-Cladera M., Pérez-Cano F.J., Castellote C., Franch À., Castell M. A diet enriched with cocoa prevents IgE synthesis in a rat allergy model. Pharmacol. Res. 2012;65:603–608. doi: 10.1016/j.phrs.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 125.Enomoto T., Nagasako-Akazome Y., Kanda T., Ikeda M., Dake Y. Clinical effects of apple polyphenols on persistent allergic rhinitis: A randomized double-blind placebo-controlled parallel arm study. J. Investig. Allergol. Clin. Immunol. 2006;16:283. [PubMed] [Google Scholar]
- 126.Belcaro G., Luzzi R., Cesinaro P.D.R., Cesarone M.R., Dugall M., Feragalli B., Errichi B.M., Ippolito E., Grossi M.G., Hosoi M. Pycnogenol® improvements in asthma management. Panminerva Med. 2011;53:57–64. [PubMed] [Google Scholar]
- 127.Kishi K., Saito M., Saito T., Kumemura M., Okamatsu H., Okita M., Takazawa K. Clinical efficacy of apple polyphenol for treating cedar pollinosis. Biosci. Biotechnol. Biochem. 2005;69:829–832. doi: 10.1271/bbb.69.829. [DOI] [PubMed] [Google Scholar]
- 128.Spergel J.M. Epidemiology of atopic dermatitis and atopic march in children. Immunol. Allergy Clin. 2010;30:269–280. doi: 10.1016/j.iac.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 129.Katiyar S.K., Elmets C.A., Agarwal R., Mukhtar H. Protection against ultraviolet-B radiation-induced local and systemic suppression of contact hypersensitivity and edema responses in C3H/HeN mice by green tea polyphenols. Photochem. Photobiol. 1995;62:855–861. doi: 10.1111/j.1751-1097.1995.tb09147.x. [DOI] [PubMed] [Google Scholar]
- 130.Ichikawa D., Matsui A., Imai M., Sonoda Y., Kasahara T. Effect of various catechins on the IL-12p40 production by murine peritoneal macrophages and a macrophage cell line, J774. 1. Biol. Pharm. Bull. 2004;27:1353–1358. doi: 10.1248/bpb.27.1353. [DOI] [PubMed] [Google Scholar]
- 131.Meydani M. Potential health benefits of avenanthramides of oats. Nutr. Rev. 2009;67:731–735. doi: 10.1111/j.1753-4887.2009.00256.x. [DOI] [PubMed] [Google Scholar]
- 132.Guo W., Wise M.L., Collins F.W., Meydani M. Avenanthramides, polyphenols from oats, inhibit IL-1β-induced NF-κB activation in endothelial cells. Free Radic. Biol. Med. 2008;44:415–429. doi: 10.1016/j.freeradbiomed.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 133.Papaliodis D., Boucher W., Kempuraj D., Theoharides T.C. The flavonoid luteolin inhibits niacin-induced flush. Br. J. Pharmacol. 2008;153:1382–1387. doi: 10.1038/sj.bjp.0707668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Djukanovic R., Roche W., Wilson J., Beasley C., Twentyman O., Howarth P., Holgate S. Mucosal Inflammation In Asthma1. 2. Am. Rev. Respir. Dis. 1990;142:434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- 135.Bisset L.R., Schmid-Grendelmeier P. Chemokines and their receptors in the pathogenesis of allergic asthma: Progress and perspective. Curr. Opin. Pulm. Med. 2005;11:35–42. doi: 10.1097/01.mcp.0000144502.50149.e0. [DOI] [PubMed] [Google Scholar]
- 136.Lee M., Kim S., Kwon O.-K., Oh S.-R., Lee H.-K., Ahn K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int. Immunopharmacol. 2009;9:418–424. doi: 10.1016/j.intimp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 137.Park H.-j., Lee C.-M., Jung I.D., Lee J.S., Jeong Y.-i., Chang J.H., Chun S.-H., Kim M.-J., Choi I.-W., Ahn S.-C. Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int. Immunopharmacol. 2009;9:261–267. doi: 10.1016/j.intimp.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 138.Lim J.-Y., Lee J.-H., Lee B.-R., Kim M., Lee Y.-M., Kim D.-K., Choi J.K. Extract of Boehmeria nivea Suppresses Mast Cell-Mediated Allergic Inflammation by Inhibiting Mitogen-Activated Protein Kinase and Nuclear Factor-κB. Molecules. 2020;25:4178. doi: 10.3390/molecules25184178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lin C.-H., Shen M.-L., Zhou N., Lee C.-C., Kao S.-T., Wu D.C. Protective Effects of the Polyphenol Sesamin on Allergen-Induced TH 2 Responses and Airway Inflammation in Mice. PLoS ONE. 2014;9:e96091. doi: 10.1371/journal.pone.0096091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Khan H., Sureda A., Belwal T., Çetinkaya S., Süntar İ., Tejada S., Devkota H.P., Ullah H., Aschner M. Polyphenols in the treatment of autoimmune diseases. Autoimmun. Rev. 2019;18:647–657. doi: 10.1016/j.autrev.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sudres M., Verdier J., Truffault F., Le Panse R., Berrih-Aknin S. Pathophysiological mechanisms of autoimmunity. Ann. N. Y. Acad. Sci. 2018;1413:59–68. doi: 10.1111/nyas.13560. [DOI] [PubMed] [Google Scholar]
- 142.Thomas N.J., Jones S.E., Weedon M.N., Shields B.M., Oram R.A., Hattersley A.T. Frequency and phenotype of type 1 diabetes in the first six decades of life: A cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018;6:122–129. doi: 10.1016/S2213-8587(17)30362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Apaya M.K., Kuo T.-F., Yang M.-T., Yang G., Hsiao C.-L., Chang S.-B., Lin Y., Yang W.-C. Phytochemicals as modulators of β-cells and immunity for the therapy of type 1 diabetes: Recent discoveries in pharmacological mechanisms and clinical potential. Pharmacol. Res. 2020;156:104754. doi: 10.1016/j.phrs.2020.104754. [DOI] [PubMed] [Google Scholar]
- 144.Stojanović I., Šavikin K., Đedović N., Živković J., Saksida T., Momčilović M., Koprivica I., Vujičić M., Stanisavljević S., Miljković Đ. Pomegranate peel extract ameliorates autoimmunity in animal models of multiple sclerosis and type 1 diabetes. J. Funct. Foods. 2017;35:522–530. [Google Scholar]
- 145.Ravikumar N., Kavitha C.N. Immunomodulatory effect of Quercetin on dysregulated Th1/Th2 cytokine balance in mice with both type 1 diabetes and allergic asthma. J. Appl. Pharm. Sci. 2020;10:80–87. [Google Scholar]
- 146.Jeong G.-S., Lee D.-S., Song M.-Y., Park B.-H., Kang D.-G., Lee H.-S., Kwon K.-B., Kim Y.-C. Butein from Rhus verniciflua protects pancreatic β cells against cytokine-induced toxicity mediated by inhibition of nitric oxide formation. Biol. Pharm. Bull. 2011;34:97–102. doi: 10.1248/bpb.34.97. [DOI] [PubMed] [Google Scholar]
- 147.Bae U.-J., Jang H.-Y., Lim J.M., Hua L., Ryu J.-H., Park B.-H. Polyphenols isolated from Broussonetia kazinoki prevent cytokine-induced β-cell damage and the development of type 1 diabetes. Exp. Mol. Med. 2015;47:e160. doi: 10.1038/emm.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Glossop J.R., Dawes P.T., Mattey D.L. Association between cigarette smoking and release of tumour necrosis factor α and its soluble receptors by peripheral blood mononuclear cells in patients with rheumatoid arthritis. Rheumatology. 2006;45:1223–1229. doi: 10.1093/rheumatology/kel094. [DOI] [PubMed] [Google Scholar]
- 149.Mateen S., Moin S., Zafar A., Khan A.Q. Redox signaling in rheumatoid arthritis and the preventive role of polyphenols. Clin. Chim. Acta. 2016;463:4–10. doi: 10.1016/j.cca.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 150.Kloesch B., Becker T., Dietersdorfer E., Kiener H., Steiner G. Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like synoviocytes. Int. Immunopharmacol. 2013;15:400–405. doi: 10.1016/j.intimp.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 151.Mateen S., Moin S., Khan A.Q., Zafar A., Fatima N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS ONE. 2016;11:e0152925. doi: 10.1371/journal.pone.0152925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang G., Chang C.-C., Yang Y., Yuan L., Xu L., Ho C.-T., Li S. Resveratrol alleviates rheumatoid arthritis via reducing ROS and inflammation, inhibiting MAPK signaling pathways, and suppressing angiogenesis. J. Agric. Food Chem. 2018;66:12953–12960. doi: 10.1021/acs.jafc.8b05047. [DOI] [PubMed] [Google Scholar]
- 153.Khojah H.M., Ahmed S., Abdel-Rahman M.S., Elhakeim E.H. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin. Rheumatol. 2018;37:2035–2042. doi: 10.1007/s10067-018-4080-8. [DOI] [PubMed] [Google Scholar]
- 154.Morinobu A., Biao W., Tanaka S., Horiuchi M., Jun L., Tsuji G., Sakai Y., Kurosaka M., Kumagai S. (−)-Epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2008;58:2012–2018. doi: 10.1002/art.23594. [DOI] [PubMed] [Google Scholar]
- 155.Park M.-K., Park J.-S., Cho M.-L., Oh H.-J., Heo Y.-J., Woo Y.-J., Heo Y.-M., Park M.-J., Park H.-S., Park S.-H. Grape seed proanthocyanidin extract (GSPE) differentially regulates Foxp3+ regulatory and IL-17+ pathogenic T cell in autoimmune arthritis. Immunol. Lett. 2011;135:50–58. doi: 10.1016/j.imlet.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 156.Gonçalves G.A., Soares A.A., Correa R.C.G., Barros L., Haminiuk C.W.I., Peralta R.M., Ferreira I.C.F.R., Bracht A. Merlot grape pomace hydroalcoholic extract improves the oxidative and inflammatory states of rats with adjuvant-induced arthritis. J. Funct. Foods. 2017;33:408–418. [Google Scholar]
- 157.Stamer D.K., Nizami S.A., Lee F.Y., Soung D.Y. Whole grape alleviates inflammatory arthritis through inhibition of tumor necrosis factor. J. Funct. Foods. 2017;35:458–465. [Google Scholar]
- 158.Rosillo M.Á., Alcaraz M.J., Sánchez-Hidalgo M., Fernández-Bolaños J.G., Alarcón-de-la-Lastra C., Ferrándiz M.L. Anti-inflammatory and joint protective effects of extra-virgin olive-oil polyphenol extract in experimental arthritis. J. Nutr. Biochem. 2014;25:1275–1281. doi: 10.1016/j.jnutbio.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 159.Javadi F., Ahmadzadeh A., Eghtesadi S., Aryaeian N., Zabihiyeganeh M., Rahimi Foroushani A., Jazayeri S. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: A double-blind, randomized controlled trial. J. Am. Coll. Nutr. 2017;36:9–15. doi: 10.1080/07315724.2016.1140093. [DOI] [PubMed] [Google Scholar]
- 160.Riccio P. The molecular basis of nutritional intervention in multiple sclerosis: A narrative review. Complementary Ther. Med. 2011;19:228–237. doi: 10.1016/j.ctim.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 161.Nair M.P., Mahajan S., Reynolds J.L., Aalinkeel R., Nair H., Schwartz S.A., Kandaswami C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-κβ system. Clin. Vaccine Immunol. 2006;13:319–328. doi: 10.1128/CVI.13.3.319-328.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Verbeek R., Plomp A.C., van Tol E.A.F., van Noort J.M. The flavones luteolin and apigenin inhibit in vitro antigen-specific proliferation and interferon-gamma production by murine and human autoimmune T cells. Biochem. Pharmacol. 2004;68:621–629. doi: 10.1016/j.bcp.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 163.Sternberg Z., Chadha K., Lieberman A., Hojnacki D., Drake A., Zamboni P., Rocco P., Grazioli E., Weinstock-Guttman B., Munschauer F. Quercetin and interferon-β modulate immune response (s) in peripheral blood mononuclear cells isolated from multiple sclerosis patients. J. Neuroimmunol. 2008;205:142–147. doi: 10.1016/j.jneuroim.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 164.Hendriks J.J.A., de Vries H.E., van der Pol S.M.A., van den Berg T.K., van Tol E.A.F., Dijkstra C.D. Flavonoids inhibit myelin phagocytosis by macrophages; a structure–activity relationship study. Biochem. Pharmacol. 2003;65:877–885. doi: 10.1016/s0006-2952(02)01609-x. [DOI] [PubMed] [Google Scholar]
- 165.Fonseca-Kelly Z., Nassrallah M., Uribe J., Khan R.S., Dine K., Dutt M., Shindler K.S. Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Front. Neurol. 2012;3:84. doi: 10.3389/fneur.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ghaiad H.R., Nooh M.M., El-Sawalhi M.M., Shaheen A.A. Resveratrol promotes remyelination in cuprizone model of multiple sclerosis: Biochemical and histological study. Mol. Neurobiol. 2017;54:3219–3229. doi: 10.1007/s12035-016-9891-5. [DOI] [PubMed] [Google Scholar]
- 167.Siahpoosh A., Majdinasab N., Derakhshannezhad N., Khalili H.R., Malayeri A. Effect of grape seed on quality of life in multiple sclerosis patients. [(accessed on 10 December 2020)];J. Contemp. Med Sci. 2018 4 Available online: http://www.jocms.org/index.php/jcms/article/view/453/243. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.