Abstract
Background
Gallstone disease (cholelithiasis) is common. In most people it is asymptomatic and does not require treatment, but in about 20% it can become symptomatic, causing pain and other complications requiring medical attention and/or surgery. A proportion of symptomatic people with uncomplicated gallstone disease do not experience further episodes of pain and, therefore, could be treated conservatively. Moreover, surgery carries risks of perioperative and postoperative complications.
Methods and analysis
C-Gall is a pragmatic, multicentre, randomised controlled trial and economic evaluation to assess whether cholecystectomy is cost-effective compared with observation/ conservative management (here after referred to as medical management) at 18 months post-randomisation (with internal pilot).
Primary outcome measure
Patient-reported quality of life (QoL) (36-Item Short Form Survey (SF-36) bodily pain domain) up to 18 months after randomisation.
The primary economic outcome is incremental cost per quality-adjusted life year gained at 18 months.
Secondary outcome measures
Secondary outcome measures include condition-specific QoL, SF-36 domains, complications, further treatment, persistent symptoms, healthcare resource use, and costs assessed at 18 and 24 months after randomisation. The bodily pain domain of the SF-36 will also be assessed at 24 months after randomisation.
A sample size of 430 participants was calculated. Computer-generated 1:1 randomisation was used.
The C-Gall Study is currently in follow-up in 20 UK research centres. The first patient was randomised on 1 August 2016, with follow-up to be completed by 30 November 2021.
Statistical analysis
Statistical analysis of the primary outcome will be intention-to-treat and a per-protocol analysis. The primary outcome, area under the curve (AUC) for the SF-36 bodily pain up to 18 months, will be generated using the Trapezium rule and analysed using linear regression with adjustment for the minimisation variables (recruitment site, sex and age). For the secondary outcome, SF-36 bodily pain, AUC up to 24 months will be analysed in a similar way. Other secondary outcomes will be analysed using generalised linear models with adjustment for minimisation and baseline variables, as appropriate. Statistical significance will be at the two-sided 5% level with corresponding CIs.
Ethics and dissemination
The North of Scotland Research Ethics Committee approved this study (16/NS/0053). The dissemination plans include Health Technology Assessment monograph, international scientific meetings and publications in high-impact, open-access journals.
Trial registration number
ISRCTN55215960; pre-results.
Keywords: surgery, protocols & guidelines, gastroenterology, adult gastroenterology
Strengths and limitations of this study.
First multicentre randomised controlled trial comparing surgical treatment with medical management in symptomatic uncomplicated gallstone disease.
C-Gall group included patients and researchers with a wide range of experience in gallstone disease, ensuring that the study is robust for all relevant stakeholders.
Adherence to accepted international methodological standards, ensuring that high-quality recommendations are produced.
Lifelong follow-up of the recruited patients is not currently proposed.
Introduction
Gallstone disease (cholelithiasis) is one of the most common gastrointestinal disorders in industrialised societies. The prevalence of gallstones in adult populations is approximately 10%–15%.1–4 Gallstones are more common in women and people over the age of 40 years.5 In most people, gallstones are asymptomatic. About 20% of people with gallstones experience pain and complications.6
Clinical surveys conducted in Europe, North and South America, and Asia indicate that prevalence rates for gallstone disease range from 5.9% to 25%7–10 and tend to increase with age. A clinical ultrasound survey conducted in the UK reported prevalence rates of 12% among men and 22% among women over 60 years of age.9 A multicentre population-based study conducted in Italy has reported an annual incidence of gallstone disease of 0.66% in men and 0.81% in women.11
The natural course of gallstone disease is benign, with relatively low progression from asymptomatic disease to symptomatic disease.6 Natural history studies have shown low mortality from gallstone disease with typically less than 1% of people dying from gallbladder-related causes.6 12 13 In a population-based study published in 2010, the overall frequency of symptom development in asymptomatic people was around 20% over a long follow-up period (mean 8.7 years).6
In people with symptomatic uncomplicated gallstone disease, the annual rates of developing complications have been reported to be as low as 1%–3%.14–16 The Italian Group for the Epidemiology and Prevention of Cholelithiasis Study reported an annual incidence of complications of 0.7% for symptomatic people.13
In the UK and North America, the number of surgical procedures for gallstone disease increased steadily between the 1950s and 1990s, reflecting both the rise in prevalence of identified gallstone disease and the use of cholecystectomy as the treatment of choice. Rates of surgical procedures stabilised in both countries towards the end of the 20th century.
Impact of health problem
From a patient perspective, the defining symptom of gallstone disease is pain.17 18 Commonly, general abdominal symptoms intensify over a period of time and become regular pain attacks (biliary colic) and may require medical attention. Best medical therapy includes the prescription of analgesics and, when necessary, anti-inflammatory drugs or antibiotics.
The most common complications associated with gallstones are severe acute cholecystitis, which may lead to complications such as empyema, obstructive jaundice due to the obstruction of the common bile duct, acute cholangitis and acute pancreatitis.
A recent large prospective study conducted in the UK (8909 participants) has shown that 10.8% of people experienced complications 30 days after surgery.19 Even though removal of the gallbladder is considered the standard treatment for symptomatic gallstones, it does not guarantee eradication of symptoms.20 A proportion of people (up to 40% of people) may continue to experience pain and abdominal symptoms after surgery.21 In particular, persistent pain, similar to that experienced preoperatively, has been reported in about 20% of people after cholecystectomy,22 23 and de novo pain has been reported in up to 14% of people.24
The term ‘post-cholecystectomy syndrome’ is an umbrella term which has been widely used to describe the range of symptoms which occur after cholecystectomy.25 The term ‘persistent post-cholecystectomy symptoms’ has been suggested as a more accurate description of these symptoms.26 Symptoms include biliary and non-biliary abdominal pain, gastrointestinal disorders, dyspepsia, heartburn, nausea, vomiting, jaundice and cholangitis. Persistent diarrhoea or constipation is often reported after cholecystectomy and flatulence may arise de novo after surgery.24 In some people, functional gastrointestinal disorders and not gallstone disease may be the cause of persistent post-surgery symptoms.27 Nevertheless, there is not a consistent pathophysiological explanation for persistent post-cholecystectomy symptoms and, in about 5% of people, the reason for persistent abdominal pain remains unknown.28
Rationale for the trial
Current clinical guidelines recommend laparoscopic cholecystectomy for patients with biliary pain or acute cholecystitis and radiological evidence of gallstones.29 Cholecystectomy is, therefore, the default option for people with symptomatic gallstone disease and one of the most common and costly surgical procedures performed in the National Health Service (NHS) in the UK. Some 74 373 cholecystectomies were performed in England in 2019 (61 584 following elective admissions).30 These figures indicate that although some patients are operated in the acute hospital setting, many patients with uncomplicated symptomatic gallstone disease are put on a waiting list and operated on electively.
However, medical management may be a valid therapeutic option in people presenting with uncomplicated disease depending on their age, clinical presentation and evolution of symptoms over time. Moreover, as these symptoms are usually not urgent, it may be reasonable to take into consideration a non-surgical option first, which could save a considerable amount of NHS resources.
Recent studies stated that half of the people treated medically were symptom free; therefore, up to 30 000 cholecystectomies per year could potentially be avoided with a likely saving for the NHS of £68 million/year. These resources could be freed (disinvestment) and allocated to fund alternative healthcare within the NHS.
Early natural history studies and more recent observational and population-based studies have suggested that a proportion of people with symptomatic gallstone disease no longer experience biliary pain after the onset of symptoms.6 12 13 21 31 Larsen and Qvist21 found that 45% of symptomatic people on watchful waiting were totally relieved from symptoms during a 1-year observation period. Similarly, Festi et al observed that 58% of people with initially mild symptoms and 52% of those with more severe symptoms did not experience further episodes of pain during a follow-up period of 10 years and the severity of the disease did not increase over time.6
A recent National Institute for Health Research (NIHR) Health Technology Assessment (HTA) found that on average cholecystectomy is more costly but more effective than medical management for the treatment of symptomatic gallstones or cholecystitis.32 Nevertheless, half of the people treated medically were symptom free and did not require surgery in the long term, indicating that there is probably a proportion of patients with uncomplicated symptoms who could benefit from medical management. The specific results were that participants randomised to observation were significantly more likely to experience gallstone-related complications (risk ratio (RR) 6.69; 95% CI 1.57 to 28.51; p=0.01), in particular acute cholecystitis (RR 9.55; 95% CI 1.25 to 73.27; p=0.03); but less likely to undergo surgery (RR 0.50; 95% CI 0.34 to 0.73; p=0.0004) and experience surgery-related complications (RR 0.36; 95% CI 0.16 to 0.81; p=0.01) than those randomised to surgery. Fifty-five per cent of people randomised to observation did not require an operation during the 14-year follow-up period, and 12% of people randomised to cholecystectomy did not undergo the scheduled operation. These results were subject to major uncertainties in the reported economic model. Even when cholecystectomy occurred, medical management had between 40% and 60% chance of being cost-effective for alternative values of willingness to pay for an additional quality-adjusted life year (QALY).
Furthermore, results were strongly influenced by the proportion of individuals originally followed with medical management that needed surgery. In their base case, the authors assumed that 44% of the cohort would need surgery within 5 years. If this proportion was reduced to 25%, medical management became, on average, cost-effective. It is worth pointing out that the NIHR HTA was based on evidence from two randomised controlled trials (201 participants in total)33 34 conducted in Norway by the same research team. Due to the limited evidence available and the current lack of UK NHS data, the authors highlighted the need for a large, well-designed trial assessing the effects and safety of medical management compared with cholecystectomy.
Methods/design
Aims and objectives
The primary aim of the study is to assess the clinical and cost-effectiveness of observation/conservative management (here after referred to as medical management) compared with cholecystectomy for preventing recurrent symptoms and complications in adults presenting with uncomplicated symptomatic gallstones in a secondary care setting.
The primary patient objective is to compare medical management with cholecystectomy in terms of participants’ quality of life using the 36-Item Short Form Survey (SF-36) bodily pain domain at up to 18 months after randomisation.
The primary economic objective is to assess the cost-effectiveness of medical management versus cholecystectomy in terms of the incremental cost per QALY.
The secondary objectives are to compare medical management with surgical treatment (cholecystectomy) in terms of condition-specific quality of life, SF-36 domains, complications, the need for further treatment, persistent symptoms, healthcare resource use and costs. All secondary outcomes are assessed at 18 and 24 months after randomisation.
The bodily pain domain of the SF-36 health survey will also be assessed at 24 months after randomisation as a secondary objective.
Blinding of outcomes is not possible.
The null hypothesis being tested is that there is no difference between medical management and cholecystectomy. The alternative hypothesis is that cholecystectomy is superior.
Trial design
A pragmatic, phase 4, multicentre parallel group patient randomised superiority trial (with an internal pilot phase) to test if the strategy of standard cholecystectomy is more (cost-)effective than medical management (with internal pilot). Other than the collection of outcome data, participant care will follow standard care pathways in the participating NHS secondary care sites. A within-trial economic evaluation will be conducted. Linear regression models will be used for this. Extrapolation beyond the trial follow-up period will be considered if a definite answer on cost-effectiveness cannot be reached from the within-trial analysis.
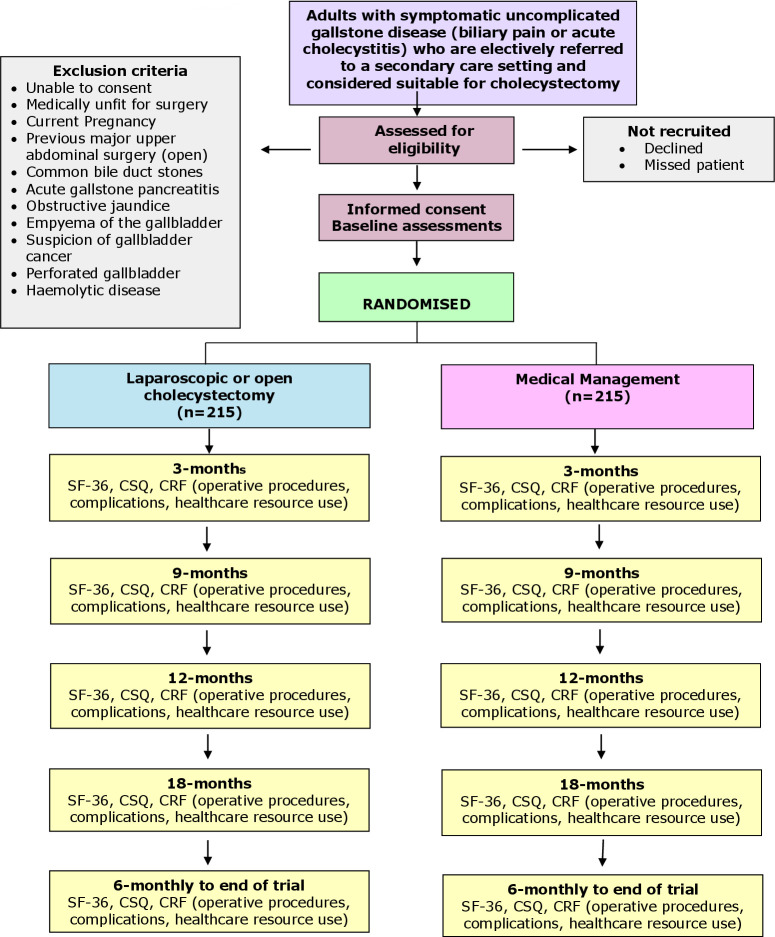
The patient-reported outcomes (SF-36; Condition Specific Questionnaire/CSQ) will be assessed by participant-completed questionnaires at baseline, 3, 9, 12 and 18 months and 6 monthly thereafter, post-randomisation, until the end of the trial. A case report form (CRF) will be completed at the time of surgery, providing details of the operative procedures, complications and resource use in the hospital. Costs of the initial intervention procedures will be estimated from resource use data recorded on the CRFs coupled with routine unit cost data. Costs associated with subsequent contacts with primary and secondary care (eg, due to symptomatic gallstones or surgical complications) will be estimated from patient questionnaires at 3, 9, 12 and 18 months and 6 monthly thereafter, post-randomisation, until the end of the trial and checked at the source. QALYs will be estimated from patients’ responses to the SF-36. The trial flow chart is shown in figure 1.
Figure 1.
Trial flow chart. CRF, case report form; CSQ, Condition Specific Questionnaire; SF-36, 36-Item Short Form Survey.
Embedded qualitative research will identify any challenges during the internal pilot related to design or conduct that can then be addressed and modified during progression to the full trial. Full details are given in trial protocol.
Interventions to be evaluated
Cholecystectomy. Laparoscopic cholecystectomy is the current standard surgical procedure for the management of symptomatic gallstone disease. The gall bladder is removed with the stones within it using keyhole techniques (laparoscopy). The procedure is undertaken under a general anaesthetic. It usually involves three to four small incisions in the abdomen, which allow the surgeon to dissect the gallbladder from its attachments and safely divide the key anatomical structures (the cystic duct and artery) that link it to the biliary tree. The gallbladder is separated from under the surface of the liver. Usually, the gallbladder (containing the stones) is removed within a retrieval bag via one of the small incisions. The operation takes between 45 and 120 min, many patients are admitted for one night, although day case laparoscopic cholecystectomy is safely undertaken in otherwise fit patients with appropriate social support.
Medical management. This includes the prescription of analgesics and antispasmodics to relieve the biliary pain. Typical therapy includes paracetamol, non-steroidal anti-inflammatory drugs (eg, ibuprofen), narcotic analgesics (eg, opiates), antispasmodics (eg, Buscopan), together with generic lifestyle advice.35 36 In the longer term, medical management also may involve these strategies for symptom management, if required, as well as advice to eat a healthy diet with regular meals (http://www.nhs.uk/Conditions/Gallstones/Pages/Treatment.aspx). For the purpose of this trial, a standard protocol for medical management was agreed with the Patient and Public Involvement group and used in all participating centres. Safety advice for patients in the medical management group was aligned with the current advice given via the NHS choices website (www.nhs.uk).
Trial population
Adults with symptomatic uncomplicated gallstone disease (biliary pain) who are electively referred to a secondary care setting and considered suitable for cholecystectomy.
A sample size of 430 participants was calculated.
Setting
Adult patients with diagnosed symptomatic uncomplicated gallstone disease electively referred to a secondary care setting by a general practitioner (GP), accident and emergency department or elsewhere, not requiring emergency surgical or endoscopic intervention will be approached by the research teams.
Planned inclusion and exclusion criteria
Inclusion criteria
All adult patients with confirmed symptomatic gallstone disease electively referred to a secondary care setting for consultation.
Clinical diagnosis of gallstone disease will be confirmed by imaging. Transabdominal ultrasonography is the standard imaging technique for the diagnosis of gallbladder stones, but diagnosis by any imaging technique is acceptable.
Exclusion criteria
Unable to consent.
Medically unfit for surgery.
Current pregnancy.
Previous open major upper abdominal surgery.
Gallstones in common bile duct or evidence of previous choledocholithiasis (gallstones in common bile duct) on latest imaging or evidence of previous choledocholithiasis.
A history of acute pancreatitis.
Evidence of empyema of the gallbladder with sepsis.
Suspicion of gallbladder cancer.
Perforated gallbladder (refers to recent or old perforation detected on imaging).
Haemolytic disease.
Gallstone ileus.
Recruitment and trial procedures
Identifying participants
GPs within the study area have an important role in raising awareness among those potential recruits to the study that they are referring or admitting to hospital. We will provide information about the study to all referring GPs within the study areas. In Scotland, we will contact and attend the relevant health boards’ GP subcommittees. Subsequently, we will work with the boards’ primary care directorate to cascade information to individual GP practices and registered locums. In England, we will contact the relevant Clinical Research Network primary care leads and seek permission to contact referring GPs within their grouping.
Additionally, in the regions where the study is taking place, we will liaise with the relevant clinical commissioning groups as a further means of cascading information to relevant GPs. We will provide GPs with standardised information about the study and make the protocol available to them. We will encourage GPs to make patients aware of the study and why it is being conducted when they refer or admit potential recruits to the study. Participants will be identified by the local research team at participating centres. Local procedures at the participating hospitals are different, and the timing and mode of approach to patients and the consent process may vary to accommodate both the specific circumstances at each site and the needs of the patients.
Following identification of potential participants, an invitation letter and patient information leaflet (PIL) detailing the trial will be sent out, inviting them to attend an outpatient clinic where the trial and their treatment will be discussed. Potential participants, not identified prior to a clinic visit or at sites that are unable to send the PIL in advance, will be given the PIL at the outpatient clinic. The PIL will also highlight that the clinical consultation may be audio-recorded if participants consent. At the clinic consultation, the research team will outline the trial and ask the patient if they are willing to discuss participation and have their conversation audio-recorded. For those patients who are happy with this proposal, the process will follow as described. A member of the local research team will complete a trial screening form using information from the prospective participant and from the clinical record to document fulfilment of the entry criteria. Eligibility criteria will be cross-checked with the clinical record. If the patient is eligible and in provisional agreement, a local research team member will meet with the patient immediately in the clinic. Eligible participants who express interest in participating will have the study explained to them by local research staff and asked if they have any questions or concerns about participating in the trial. If they agree to take part, they will give written consent to be randomised. Standard local arrangements concerning pre-assessment, admission, consent for surgery, conduct of surgery and aftercare will continue unimpaired.
The PIL and consent form refer to the possibility of long-term follow-up to determine the incidence of future operations. The PIL and consent form also refer to the possibility of participants being contacted in the future to participate in other relevant research. Eligible and randomised participants, as well as those who are not willing to consider randomisation, may be contacted to participate in a semistructured audio-recorded interview.
The PIL provides clear details of the anticipated risks and benefits of trial participation. Risks associated with both treatment arms are explicitly mentioned. The risk and benefits of the study will also be discussed by the local research nurses and the patient’s consultant as part of the process of obtaining informed consent.
Informed consent
Informed consent to participate in the trial will be sought and obtained according to Good Clinical Practice (GCP) guidelines. Informed signed consent forms will be obtained from the participants in all centres by an appropriately trained individual. Participants will be given sufficient time to accept or decline involvement and will be free to come out of the study at any time. Patients may decide to participate during an initial consultation, during a subsequent visit to the hospital or alternatively at home. If the patient agrees to be contacted at home, he/she may receive a telephone call from the local research nurse to discuss any queries. Patients who decide to participate following telephone counselling can either send their completed documents (consent form and baseline questionnaire) through the post to the local team at their treating hospital or bring it with them if they are returning to the hospital for another consultation.
An embedded evaluation using qualitative research methods will underpin recruitment development and inform how best to interpret the results of the trial. The qualitative component is entirely optional, but consent will be sought to audio-record the initial consultation when the trial is discussed (to explore opportunities to improve the informed consent discussion) and for interviews with both those who consent and refuse randomisation (to identify opportunities to improve trial processes).37
Participants who cannot give informed consent (eg, due to their mental state) are not eligible for either the randomised trial or the qualitative work.
Randomisation and allocation
Eligible and consenting participants will be randomised to one of the two intervention groups using the 24-hour telephone Interactive Voice Response randomisation application or via the web-based application, both hosted by the Centre for Healthcare Randomised Trials. The randomisation algorithm will use recruitment site, sex (male/female) and age (<35, 35–64, ≥65 years) as minimisation covariates to allocate treatment to intervention and control groups in a 1:1 ratio. A random element will be incorporated into the randomisation algorithm. The principal investigator (PI) at each site, or individual with delegated authority, will access the telephone or web-based system. Patient screening identification information, including patient initials, sex, date of birth and recruiting site (the stratifying variables), will be entered into the voice-activated or web-based system, which will return the allocation status. After obtaining patient consent, randomisation will happen in the clinic, and participants will be informed of their allocated treatment group following randomisation. If the participants are not present in the clinic, they will be contacted by the research teams to inform them of the allocated treatment group after randomisation.
Follow-up procedures
The patient reported outcomes (SF-36; CSQ) will be assessed by participant-completed questionnaires at baseline, 3, 9, 12 and 18 months and 6 monthly thereafter, post-randomisation, until the end of the trial. A CRF at the time of any gallstone surgery will be collected providing details of the operative procedures, complications and resource use in the hospital. Costs of the initial intervention procedures will be estimated from resource use data recorded on the CRFs coupled with routine unit cost data. Costs associated with subsequent contacts with primary and secondary care (due to symptomatic gallstones) will be estimated from patient questionnaires at 3, 9, 12 and 18 months and 6 monthly thereafter, post-randomisation, until the end of the trial and checked at the source. QALYs will be estimated from patients’ responses to the SF-36.
Change of status/withdrawal procedures
Participants will remain in the trial unless they choose to withdraw consent or if they are unable to continue for a clinical reason. All changes in status with the exception of complete withdrawal of consent will mean the participant is still followed up for all trial outcomes, wherever possible. All data collected up to the point of complete withdrawal will be retained and used in the analysis.
Subsequent arrangements
Informing key people
Following formal trial entry:
The study office will:
Inform the participant’s GP (by letter) enclosing information about C-Gall and the study office contact details.
The local research nurse/recruitment officer and/or PI will:
File the hospital copy of the consent form in the hospital notes along with information about C-Gall.
Inform the ward and theatre staff as appropriate of the participant’s entry to the trial and details of the intervention allocation (theatre only).
Use the C-Gall internet database to enter data regarding the participant, including data required to complete randomisation; and intraoperative and postoperative information abstracted from local medical records.
Maintain and archive study documentation at the site. A copy of the signed consent form is returned to the study office in Aberdeen after database entry.
Provide any relevant, follow-up clinical data.
Monitoring the participants
Participants will be contacted by phone, post or email as appropriate. Participants will be asked to contact the study office when they are given an appointment for their surgery. In case of non-return of questionnaires or non-attendance at outpatient appointments, attempts will be made by staff at the study office to trace the participant directly using these means or indirectly by contacting the GP.
Notification by GPs
GPs are asked to contact the study office if the participant moves, becomes too ill to continue or dies, or any other notifiable or adverse event occurs. Alternatively, the staff at the study office may contact the GP.
Offices for National Statistics (NHS digital data in England, Information Statistics Division data in Scotland)
Consent will be sought from all participants to trace their medical records and addresses from local records and centrally held computerised databases. This should facilitate long-term follow-up.
Patient and public involvement
Patients and the public have been key to the development of the study, and this is facilitated through a named contact within the research team. Two patient partners (one as a member of the Project Management Group has been involved since the design stage, one as an independent member of the Trial Steering Committee with an oversight role) have been involved throughout key decision points of the trial conduct with opportunities to comment on and help inform the improvement of aspects of conduct and recruitment. Additional patient perspectives are obtained through a dedicated patient group of six people set up through the trial who contribute their perspectives on disease and the two interventions being studied.
Sample size
The primary outcome is the area under the curve (AUC) measure from SF-36 bodily pain up to 18 months. To detect a 0.33 SD difference, with 90% power and alpha at 5%, 194 participants per group (388 in total) are required. To allow for 10% of participants for whom outcome data will be completely missing, and there the AUC cannot be calculated, it is proposed to randomise 430 participants in total.
Internal pilot
The internal pilot was primarily designed to verify that recruitment was possible. There were three areas of uncertainty that we proposed to verify during the internal pilot study. These areas were (1) the generalisability of the randomised participants, (2) the willingness to randomise and (3) ability to scale up the number of centres. To address these areas, we initially set up four selected pilot centres in the first 12 months of the study. Within these four centres, detailed clinical screening logs were implemented. The screening logs recorded the number of screened participants, the number ineligible and number eligible. The four centres also took part in embedded qualitative research to help the trial team understand barriers and facilitators to recruitment during this phase. From months 12 to 24, the internal pilot continued with the scaling up of the trial to the rest of the trial centres.
Stop/go criteria
During the internal pilot phase, we proposed two decision points—one at month 12 and another at month 18. By month 12, 14 centre months of recruitment should have occurred, and 20 participants randomised across the four centres. By end of month 18, 94 centre months of recruitment should have occurred, and 111 participants randomised across 20 centres. After the internal pilot, we also proposed an early check of the AUC assumption, average recruitment rate and rate of crossovers during follow-up. The proposed stop/go criteria were:
At 12 months:
Recruited projected participants (currently 20).
Recruited at least 20% of eligible patients.
At 18 months:
Recruited the appropriate number of centres to achieve recruitment target (currently 20).
The average recruitment rate per site per month is at least one.
At 24 months:
The AUC estimate is no more than 10% larger than current estimate of 0.33.
The crossover rate is greater than 50%.
Statistical analysis
The primary outcome, AUC for the SF-36 bodily pain up to 18 months, will be generated for each participant using the Trapezium rule. The primary outcome (difference in mean AUC) measure will be analysed using linear regression with adjustment for the minimisation variables (site of recruitment, sex and age). Sensitivity analyses of all treatment effect estimates for participants who have missed a scheduled questionnaire will be estimated using a multiple imputation approach to make use of partial outcome data. We will also assess the robustness of the treatment effect estimates to these approaches. Missing items on the health-related outcome measures will be treated as per the instructions for that particular measure. For the secondary outcome, SF-36 bodily pain AUC up to 24 months will be analysed in a similar way to the primary outcome. All other secondary outcomes will be analysed using generalised linear models with adjustment for minimisation and baseline variables, as appropriate. Statistical significance will be at the two-sided 5% level with corresponding CIs derived. Subgroup analyses will explore the possible modification of treatment effect by clinically important factors: sex and age. This will be done by including treatment-by-factor interactions in the model, and they will be classified as exploratory analyses with 99% CIs calculated. All analyses will initially be performed on an intention-to-treat basis, although we will consider additional analysis groups such as per-protocol (definitions of the per-protocol group will be detailed in the Statistical Analysis Plan). The main statistical analyses will be based on all participants as randomised, irrespective of subsequent compliance with the treatment allocation. From the internal pilot phase, we will report estimates of recruitment rates and potential participant availability, together with appropriate CIs. There are no planned interim outcome analyses; all analyses will occur following completion of trial follow-up. Interim analyses will be performed if requested by the Data Monitoring and Ethics Committee.
Economic evaluation
A within-trial cost-utility analysis will be conducted. The need to extrapolate beyond the study follow-up period will be considered if a definite answer on cost-effectiveness cannot be obtained from within the trial analysis.
Resource use and costs will be estimated for each participant. Hospital inpatient and outpatient resource use data (eg, hospital admissions by type of service, outpatient visits and so on) will be retrieved from participants’ hospital case notes. In addition, primary care resource use (eg, GP visits), time off work, out-of-pocket purchases of medications and quality of life data (eg, SF-36) will be obtained from patient questionnaires at 3, 9, 12, 18 and 24 months. The analysis will be conducted from the UK NHS and personal social services’ perspective, and resource use will be valued using standard UK sources.38–40 Based on participant responses to the SF-36, utility scores will be obtained and used to calculate QALYs for each trial participant.41–43
Cost-effectiveness will be measured in terms of costs of the care pathways and QALYs at 24 months post-randomisation for the within the trial analysis. Mean NHS costs, patient costs and QALYs will be compared between randomised groups at 24 months. Incremental costs, incremental QALYs and incremental cost-effectiveness ratios will be reported. Appropriate linear regression models with adjustment for minimisation variables and baseline variables (eg, baseline utility scores) will be used. Uncertainty will be characterised and presented graphically using cost-effectiveness acceptability curves.33 34 Guidelines for economic evaluation advocate for a long enough time horizon to consider all cost and consequences relevant to the analysis.44 If extrapolating the results beyond the clinical trial follow-up, a simple state transition model (eg, Markov model) will be developed.
Ethics approval
The North of Scotland Research Ethics Committee (REC) has reviewed and approved this study. The study will be conducted according to the principles of GCP provided by the research governance guidelines. We believe this study does not pose any specific risks to individual participants beyond standard surgical procedures, nor does it raise any extraordinary ethical issues. Annual progress reports and a final report at the conclusion of the trial will be submitted to the North of Scotland REC within the timelines defined in the regulations.
Finance and insurance
The trial is funded by a grant awarded by the UK NIHR HTA Programme. The University of Aberdeen provides the necessary trial insurance.
Discussion
In the light of available clinical guidelines, laparoscopic cholecystectomy remains the default option for all people with symptomatic uncomplicated gallstone disease. At present, healthcare professionals have little information available to guide them in identifying people who might benefit from a conservative approach. Current national data from the UK have shown that more than 70 000 surgical procedures to remove gallbladder are performed nationally every year. It is uncertain whether these procedures are warranted, and high-quality evidence to inform this debate is needed. The C-Gall trial is designed to contribute to this discussion.
Recruitment has completed, and the trial is in its follow-up phase. It is expected that results will be available in 2022.
Dissemination
The dissemination plans include (1) publication in the NIHR Journals Library, (2) presentation at international scientific meetings and (3) publications in high-impact, open-access, peer-reviewed journals.
Trial status
The C-Gall Study has completed recruitment in 20 UK research centres and is currently in follow-up in these 20 UK research centres. The first patient was randomised on 1 August 2016, with follow-up to be completed by 30 November 2021.
Protocol V.11.0, 06 July 2020.
Supplementary Material
Acknowledgments
Previous CHaRT director John Norrie for his work with the concept of this study.
Footnotes
Twitter: @GilliesKatie, @CAPCAberdeen
Contributors: IA, CR and MB conceived the idea. IA and CR led the overall design of the study. KG and RN led the qualitative analysis aspects. GM led the Clinical Trial Unit aspects, including adherence to relevant governance and ethics. RH designed and wrote the economic analysis plan. AA, JB and PM contributed to the clinical aspects of the protocol. JH wrote the Statistical Analysis Plan. BC contributed to the lay summary as the patient representative. KM contributed to the trial conduct aspects. KI and AM contributed to trial oversight and the protocol amendments. All contributors were involved in the study design and agreed the protocol.
Funding: The trial is funded by the NIHR Health Technology Assessment (HTA) Programme (funder number: 14/192/71).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.American College of Physicians . Guidelines for the treatment of gallstones. Ann Intern Med 1993;119:620–2. 10.7326/0003-4819-119-7_Part_1-199310010-00011 [DOI] [PubMed] [Google Scholar]
- 2.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet 2006;368:230–9. 10.1016/S0140-6736(06)69044-2 [DOI] [PubMed] [Google Scholar]
- 3.Shaffer EA. Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol 2006;20:981–96. 10.1016/j.bpg.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 4.Stinton LM, Myers RP, Shaffer EA. Epidemiology of gallstones. Gastroenterol Clin North Am 2010;39:157–69. 10.1016/j.gtc.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 5.Bateson MC. Gallstones and cholecystectomy in modern Britain. Postgrad Med J 2000;76:700–3. 10.1136/pmj.76.901.700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Festi D, Reggiani MLB, Attili AF, et al. Natural history of gallstone disease: expectant management or active treatment? results from a population-based cohort study. J Gastroenterol Hepatol 2010;25:719–24. 10.1111/j.1440-1746.2009.06146.x [DOI] [PubMed] [Google Scholar]
- 7.Barbara L, Sama C, Morselli Labate AM, et al. A population study on the prevalence of gallstone disease: the Sirmione study. Hepatology 1987;7:913–7. 10.1002/hep.1840070520 [DOI] [PubMed] [Google Scholar]
- 8.Everhart JE, Khare M, Hill M, et al. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology 1999;117:632–9. 10.1016/S0016-5085(99)70456-7 [DOI] [PubMed] [Google Scholar]
- 9.Heaton KW, Braddon FE, Mountford RA, et al. Symptomatic and silent gall stones in the community. Gut 1991;32:316–20. 10.1136/gut.32.3.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams JG, Roberts SE, Ali MF, et al. Gastroenterology services in the UK. The burden of disease, and the organisation and delivery of services for gastrointestinal and liver disorders: a review of the evidence. Gut 2007;56:1–113. 10.1136/gut.2006.117598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Festi D, Dormi A, Capodicasa S, et al. Incidence of gallstone disease in Italy: results from a multicenter, population-based Italian study (the MICOL project). World J Gastroenterol 2008;14:5282–9. 10.3748/wjg.14.5282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McSherry CK, Ferstenberg H, Calhoun WF, et al. The natural history of diagnosed gallstone disease in symptomatic and asymptomatic patients. Ann Surg 1985;202:69–63. 10.1097/00000658-198507000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attili AF, De Santis A, Capri R, et al. The natural history of gallstones: the GREPCO experience. The GREPCO group. Hepatology 1995;21:656–60. 10.1002/hep.1840210309 [DOI] [PubMed] [Google Scholar]
- 14.Beckingham IJ. ABC of diseases of liver, pancreas, and biliary system. gallstone disease. BMJ 2001;322:91–4. 10.1136/bmj.322.7278.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman GD, Raviola CA, Fireman B. Prognosis of gallstones with mild or no symptoms: 25 years of follow-up in a health maintenance organisation. J Clin Epidemiol 1986;1989:42. [DOI] [PubMed] [Google Scholar]
- 16.Thistle JL, Cleary PA, Lachin JM, et al. The natural history of cholelithiasis: the National cooperative gallstone study. Ann Intern Med 1984;101:171–5. 10.7326/0003-4819-101-2-171 [DOI] [PubMed] [Google Scholar]
- 17.Berhane T, Vetrhus M, Hausken T, et al. Pain attacks in non-complicated and complicated gallstone disease have a characteristic pattern and are accompanied by dyspepsia in most patients: the results of a prospective study. Scand J Gastroenterol 2006;41:93–101. 10.1080/00365520510023990 [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M, Dumot JA, Søreide O, et al. Diagnosis and management of gallbladder calculus disease. Scand J Gastroenterol 2012;47:1257–65. 10.3109/00365521.2012.704934 [DOI] [PubMed] [Google Scholar]
- 19.CholeS Study Group, West Midlands Research Collaborative . Population-Based cohort study of outcomes following cholecystectomy for benign gallbladder diseases. Br J Surg 2016;103:1704–15. 10.1002/bjs.10287 [DOI] [PubMed] [Google Scholar]
- 20.Vetrhus M, Søreide O, Eide GE, et al. Quality of life and pain in patients with acute cholecystitis. Results of a randomized clinical trial. Scand J Surg 2005;94:34–9. 10.1177/145749690509400109 [DOI] [PubMed] [Google Scholar]
- 21.Larsen TK, Qvist N. The influence of gallbladder function on the symptomatology in gallstone patients, and the outcome after cholecystectomy or expectancy. Dig Dis Sci 2007;52:760–3. 10.1007/s10620-006-9498-1 [DOI] [PubMed] [Google Scholar]
- 22.Jørgensen T, Teglbjerg JS, Wille-Jørgensen P, et al. Persisting pain after cholecystectomy. A prospective investigation. Scand J Gastroenterol 1991;26:124–8. 10.3109/00365529108996493 [DOI] [PubMed] [Google Scholar]
- 23.Ahmed R, Freeman JV, Ross B, et al. Long term response to gallstone treatment--problems and surprises. Eur J Surg 2000;166:447–54. 10.1080/110241500750008754 [DOI] [PubMed] [Google Scholar]
- 24.Lamberts MP, Lugtenberg M, Rovers MM, et al. Persistent and de novo symptoms after cholecystectomy: a systematic review of cholecystectomy effectiveness. Surg Endosc 2013;27:709–18. 10.1007/s00464-012-2516-9 [DOI] [PubMed] [Google Scholar]
- 25.Girometti R, Brondani G, Cereser L, et al. Post-cholecystectomy syndrome: spectrum of biliary findings at magnetic resonance cholangiopancreatography. Br J Radiol 2010;83:351–61. 10.1259/bjr/99865290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luman W, Adams WH, Nixon SN, et al. Incidence of persistent symptoms after laparoscopic cholecystectomy: a prospective study. Gut 1996;39:863–6. 10.1136/gut.39.6.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt M, Søndenaa K, Dumot JA, et al. Post-cholecystectomy symptoms were caused by persistence of a functional gastrointestinal disorder. World J Gastroenterol 2012;18:1365–72. 10.3748/wjg.v18.i12.1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bisgaard T, Rosenberg J, Kehlet H. From acute to chronic pain after laparoscopic cholecystectomy: a prospective follow-up analysis. Scand J Gastroenterol 2005;40:1358–64. 10.1080/00365520510023675 [DOI] [PubMed] [Google Scholar]
- 29.van Dijk AH, de Reuver PR, Besselink MG, et al. Assessment of available evidence in the management of gallbladder and bile duct stones: a systematic review of international guidelines. HPB 2017;19:297–309. 10.1016/j.hpb.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 30.Surgical Workload Outcome Audit Database (SWORD) . An Internet-based platform which collects up to date NHS Hospital episodes statistics data.
- 31.Schmidt M, Hausken T, Glambek I, et al. A 24-year controlled follow-up of patients with silent gallstones showed no long-term risk of symptoms or adverse events leading to cholecystectomy. Scand J Gastroenterol 2011;46:949–54. 10.3109/00365521.2011.571710 [DOI] [PubMed] [Google Scholar]
- 32.Brazzelli M, Cruickshank M, Kilonzo M, et al. Clinical effectiveness and cost-effectiveness of cholecystectomy compared with observation/conservative management for preventing recurrent symptoms and complications in adults presenting with uncomplicated symptomatic gallstones or cholecystitis: a systematic review and economic evaluation. Health Technol Assess 2014;18:1–102. 10.3310/hta18550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt M, Søndenaa K, Vetrhus M, et al. Long-term follow-up of a randomized controlled trial of observation versus surgery for acute cholecystitis: non-operative management is an option in some patients. Scand J Gastroenterol 2011;46:1257–62. 10.3109/00365521.2011.598548 [DOI] [PubMed] [Google Scholar]
- 34.Schmidt M, Søndenaa K, Vetrhus M, et al. A randomized controlled study of uncomplicated gallstone disease with a 14-year follow-up showed that operation was the preferred treatment. Dig Surg 2011;28:270–6. 10.1159/000329464 [DOI] [PubMed] [Google Scholar]
- 35.Portincasa P, Di Ciaula A, Wang HH, et al. Medicinal treatments of cholesterol gallstones: old, current and new perspectives. Curr Med Chem 2009;16:1531–42. 10.2174/092986709787909631 [DOI] [PubMed] [Google Scholar]
- 36.McGillicuddy EA, Schuster KM, Barre K, et al. Non-operative management of acute cholecystitis in the elderly. Br J Surg 2012;99:1254–61. 10.1002/bjs.8836 [DOI] [PubMed] [Google Scholar]
- 37.Donovan JL, Rooshenas L, Jepson M, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the quintet recruitment intervention (QRI). Trials 2016;17:283. 10.1186/s13063-016-1391-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.UK department of health . NHS reference costs 2013-14, 2015. Available: http://www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 [Accessed Dec 2017].
- 39.Curtis L. Unit costs of health and social care 2012. Personla social services research unit 2014, 2014. Available: http://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2014/ [Accessed Dec 2017].
- 40.British National Formulary . Medicines complete 2015, 2015. Available: http://www.medicinescomplete.com/about/publications.htm [Accessed Dec 2017].
- 41.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271–92. 10.1016/S0167-6296(01)00130-8 [DOI] [PubMed] [Google Scholar]
- 42.Brazier JE, Rowen D, Hanmer J. Revised SF-6D scoring programme: a summary of improvements. PRO Newsletter 2008:14–15. [Google Scholar]
- 43.Kharroubi SA, Brazier JE, Roberts J, et al. Modelling SF-6D health state preference data using a nonparametric Bayesian method. J Health Econ 2007;26:597–612. 10.1016/j.jhealeco.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 44.National Institute for Health & Care Excellence . Guide to the methods of technology appraisal. National Institute for health and care excellence 2013, 2013. Available: https://www.nice.org.uk/process/pmg9/chapter/foreward [Accessed Dec 2017]. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.