Abstract
Few studies have investigated the short-term effects of a very low carbohydrate ketogenic diet (KD) on body composition and substrate utilization in trained individuals. This study investigated effects on substrate utilization during incremental exercise, and changes in body composition, in response to seven days ad libitum consumption of a KD by athletes from endurance sports. Nine young trained males (age, 21.8 ± 1.9 y; height, 1.83 ± 0.11 m; body mass, 78.4 ± 13.8 kg; body fat, 14.9 ± 3.9%; VO2peak, 54.3 ± 5.9 mL kg-1 min-1) were assessed before (day 0; PRE) and after (day 7; POST) seven days of consuming an ad libitum KD. Following an overnight fast, body composition was measured by dual x-ray absorptiometry, and substrate utilization was measured during an incremental (3 min stages, 35 W increments) exercise test on a cycle ergometer. After KD, Wmax (PRE, 295 ± 30 W; POST, 292 ± 38 W) and VO2peak (PRE, 4.18 ± 0.33 L min-1; POST, 4.10 ± 0.43 L min-1) were unchanged, whereas body mass [-2.4 (-3.2, -1.6) kg; P < 0.001, d = 0.21], fat mass [-0.78 (-1.10, -0.46) kg; P < 0.001, d = 0.22] and fat-free mass (FFM) [-1.82 (-3.12, -0.51) kg; P = 0.013, d = 0.22] all decreased. The respiratory exchange ratio was lower, and rates of fat oxidation were higher, at POST across a range of exercise intensities. Maximal fat oxidation rate was ~1.8-fold higher after KD (PRE, 0.54 ± 0.13 g min-1; POST, 0.95 ± 0.24 g min-1; P < 0.001, d = 2.2). Short-term KD results in loss of both fat mass and FFM, increased rates of fat oxidation and a concomitant reduction in CHO utilization even at moderate-to-high intensities of exercise.
Keywords: Cycling, Crossover point, High fat diet, Lactate threshold, Low carbohydrate, Maximal fat oxidation
INTRODUCTION
Sports nutrition guidelines have generally recommended high carbohydrate (CHO) diets for performance in athletic populations [1]. However, there is much interest in modulation of the CHO and fat content of the diet, and specifically how such approaches alter body composition, adaptive responses to exercise training, and athletic performance [2].
Very low CHO ketogenic diets (KD) (< 50 g d-1 CHO, > 80% fat, and moderate protein) have emerged as an intensely-studied [3], albeit contentious [4], nutrition strategy particularly among endurance [5–12], and ultra-endurance athletes [13]. Low CHO, high fat (LCHF) diets and KDs are similar in that both restrict CHO intake, and encourage increased intake of dietary fat, but they are somewhat distinct in their metabolic consequences [3, 14]. Both LCHF and KDs are associated with an elevation in circulating free-fatty acids, but the lower glucose availability arising from a greater degree of CHO restriction imposed with a KD is associated with an elevation in the ketone bodies acetone, acetoacetate (AcAc) and β-hydroxybutyrate ( βHB) [15].
Whether nutritional ketosis as a consequence of a KD confers a performance benefit over LCHF remains to be established [16]. Proponents of the benefits of a KD for performance suggest that a period of “ketoadaptation” is necessary when adapting from a CHO-based diet to a KD, as the short term effect of a KD is often characterized by reports of low-energy, fatigue, and decreased cognitive and physical performance [3, 5, 8, 17]. Operational definitions of keto-
ABBREVIATIONS
BMI, body mass index; CHO, carbohydrate; FATmax, exercise intensity that elicits the maximal rate of fat oxidation; FFM, fat-free mass; KD, ketogenic diet; LCHF, low carbohydrate, high fat; PDH, pyruvate dehydrogenase; PRE, testing day prior to intervention; POST, testing day upon completion of intervention; RER, respiratory exchange ratio.adaptation in an exercise context are lacking however, as are objective data demonstrating that such a phenomenon exists.
Cross-sectional data from endurance [7], and ultra-endurance athletes [13], habituated to a KD for 8–36 months demonstrate marked changes in the metabolic response to exercise compared to moderate and high CHO diets. Most notable were higher rates of fat oxidation during submaximal intensity exercise including 2 h cycling at ~72%VO2peak (~1.21 g min-1) [7], and 3 h cycling at ~70%VO-2max (~1.54 g min-1) [13]. However, similar rates of fat oxidation (~1.57 g min-1) were observed in elite race walkers during exercise at ~80%VO2peak after as few as 21 days of consuming a KD [6]. More recently, 4 days of consumption of a KD produced an ~4.5-fold increase in the rate of fat oxidation (~0.14 g min-1 to ~0.63 g min-1) during a 5 km time trial (~82–84%VO2max) [12], while 5–6 days of consumption of a KD resulted in fat oxidation rates of ~1.43 g min-1 at ~ 73%VO2max during a graded exercise test [18].
Body mass, and body composition are important determinants of optimal athletic performance, with endurance athletes benefiting from a lower energy cost of movement and increased power-to-weight ratio associated with reduced body mass and fatness [1]. Various KD interventions have consistently been associated with reduced body mass [6, 8, 10, 19–21], and estimations of body fat mass [9, 10, 19, 22], within trained populations following 3 to 12 week interventions.
To date, the majority of research on KD in athletic populations have focused on ≥ 3 week interventions, but the extent to which changes in substrate utilization and body composition can occur in short-term ( ≤ 7 days) interventions in free-living conditions is largely unexplored. Therefore, the aim of the present study was to investigate effects on substrate utilization during incremental exercise, and changes in body composition, in response to short-term (seven days) ad libitum consumption of a KD by athletes from an endurance training background.
MATERIALS AND METHODS
Participants
Nine young males [age, 21.8 ± 1.9 y; height, 1.83 ± 0.11 m; body mass, 78.4 ± 13.8 kg; body mass index (BMI), 23.3 ± 2.1 kg m-2; body fat, 14.9 ± 3.9%; fat-free mass (FFM), 66.80 ± 10.62 kg; VO2peak, 54.3 ± 5.9 mL kg-1 min-1] participated in the study. Volunteers were sought from university sports clubs who were male, aged 18 to 35 y, free from lower limb injury for the previous six months, self-reported as currently training ≥ 4 times per week in endurance-related activities, and having had a training history of at least two years at this level of activity. The final cohort consisted of n = 9 trained, sub-elite endurance athletes, namely four long distance runners, four collegiate rowers, and one cyclist.
Experimental design
Each participant performed an incremental exercise test to exhaustion on a cycle ergometer in order to measure rates of substrate utilization over a wide range of exercise intensities [23, 24]. This test was performed on two occasions, once before (PRE) and once after (POST) prescription of seven days of ad libitum consumption of a KD. This study was approved by the University College Dublin Research Ethics Committee (permit: UH-13-06-Fusco-Egan), and was performed in accordance with the Declaration of Helsinki. Each participant provided written informed consent to participate after explanation of the experimental procedures.
Pre-test preparation
All participants had previous experience of laboratory testing of lac-tate threshold and aerobic fitness as part of their participation in their respective sport. All tests were performed between 0730 and 1000 (ambient temperature ~19°C), and participants performed their POST test at the same time of day ± 0.5 h as their PRE test. Pre-test preparation was the same for each visit. For 24 h prior to each visit, participants were asked to abstain from alcohol consumption, and refrain from strenuous exercise. All testing took place after an overnight (~8 to 10 h) fast, and participants were asked to consume 500 mL of plain water upon waking, which was 2 h prior to arriving at the laboratory.
Prior to the PRE visit, each participant kept a three day portion size estimate food diary. Analysis of these food diaries (Nutritics Dietary Analysis Software; Nutritics, Dublin, Ireland) revealed that during this time participants consumed on average per kg of body mass 41.3 ± 8.5 kcal kg-1 comprised of 4.51 ± 1.16 g kg-1 CHO, 2.26 ± 0.42 g kg-1 protein, and 1.53 ± 0.69 g kg-1 fat, and equivalent to 44 ± 8%, 23 ± 3%, and 32 ± 11% of energy from CHO, protein and fat respectively.
Testing procedures
Upon arrival and after voiding of the bladder, body mass (to nearest 0.1 kg) and height (to nearest 0.01 m) were measured using a digital scales (SECA, Germany) and wall-mounted stadiometer (Holtain, UK), respectively, after which body composition was assessed by dual-energy X-ray absorptiometry (DXA; Lunar iDXA, GE Healthcare, UK).
The incremental exercise tests were based on the method for determining the intensity (FATmax) eliciting the maximal rate of fat oxidation [23]. Briefly, on an electronically-braked cycle ergometer (Lode Excalibur Sport, Netherlands), participants began exercise with a warm-up of 5 min at 50 W, before commencing the first stage at 95 W for 3 min, after which for each stage the power output was increased by 35 W every 3 min thereafter until volitional exhaustion. Participants were instructed to maintain a pedaling cadence between 70 and 80 rpm, which was kept consistent within and between tests. During the last 20 s of each stage, blood lactate was measured using a finger-prick sample analyzed by a handheld lactate analyzer (LactatePro2, Japan), and heart rate (HR; Vantage, Polar Electro, Finland) and rating of perceived exertion (RPE) were recorded at the same time. Expired air was collected continuously throughout each exercise test, and breath-by-breath measurements were performed using Quark CPET gas analysis system (Cosmed, Italy). Oxygen up-take, as a 30 s average, was considered to have peaked, if two of the following criteria were achieved: (i) levelling off of the VO2 despite increasing power output (increase of less than 2 ml kg−1 min−1), (ii) HR within 5% of the age-predicted HRmax (208 − 0.7 × age in years), and (iii) respiratory exchange ratio (RER) > 1.10. Maximal power output (Wmax) was calculated from the power output of the last completed stage plus the fraction of time spent in the final non-completed stage multiplied by the power output increment (i.e. 35 W).
Average values (L min-1) for VO2 and VCO2, and RER, were calculated during the last 2 min of each 3 min stage in the incremental tests as described by the established determination of FATmax protocol [23]. Whole-body rates (g min-1) of CHO and fat oxidation, and energy expenditure during each stage were calculated using intensity-dependent equations that assume negligible protein contribution to energy expenditure [25]. The percentage contribution of CHO and fat to total energy expenditure during exercise was also calculated [26]. The %VO2peak corresponding to lactate threshold at the fixed blood lactate concentration of 2 mM was calculated using a polynomial equation based on the curvilinear relationship between blood lactate concentration and %VO2peak.
Short-term KD intervention
Upon completion of testing at PRE, participants commenced the consumption of a KD that prescribed a limit to CHO intake to a maximum of 40 g d-1, and of total energy to derive ~75–80% from fat, ~15–20% from protein, and ~5–10% from CHO, and incorporated best practice guidelines for KDs and physical performance [27, 28]. In order to transition immediately into KD after PRE, in the days prior to PRE, participants were provided with an information booklet with guidelines for consuming a KD, a traffic light system list of foods that were advised to be eaten or avoided, an indicative meal plan and associated recipes. However, the exact foods, meals and timing to be eaten remained the choice of each participant and therefore, the diet was considered ad libitum and without caloric restrictions.
To monitor compliance to the dietary prescription, each participant was provided with reagent strips for urinanalysis of ketone body concentrations (Ketostix; Bayer, Germany). Participants were asked to measure their urine ketone body concentration each evening after dinner [29, 30], and document the evidence of their current status by providing a picture of reagent strip alongside the colorimetric scale by smartphone messaging to the study investigators each day. This messaging exchange also facilitated discussions between investigators and participants around compliance with the diet. After 72 h of KD, all participants were within the 1.5 to 4.0 mM range indicated by the colorimetric scale, and remained so until POST.
Statistical analysis
Data were evaluated using Prism v8.4 (GraphPad Software, Inc., San Diego, CA, USA), and are presented as mean ± SD, or mean difference (lower, upper 95% confidence limits of the mean) as appropriate. Two-way (intensity x condition) repeated measures analysis of variance (ANOVA) were used to determine differences between PRE and POST for variables with serial measurements e.g. VO2, VCO2, %Wmax, HR, RER and blood lactate. When a main effect of condition
n, or an interaction effect between condition and intensity, was indicated, post-hoc testing was performed using Bonferroni’s multiple comparisons test to compare PRE to POST at respective time points for which multiplicity-adjusted P values are reported. A paired t-test was used to compare differences between PRE and POST for variables as single measurements. The data were tested for normality using the Shapiro-Wilk test prior to proceeding with the parametric tests described. The significance level was set at P ≤ 0.05 for all statistical tests. Stan-dardised differences in the mean were used to assess magnitudes of effects within and between groups, with effect sizes calculated as Cohen’s d and interpreted as trivial for < 0.2, small for ≥ 0.2 to < 0.5, moderate for ≥ 0.5 to < 0.8, and large for ≥ 0.8.
RESULTS
Body mass [-2.4 (-3.2, -1.6) kg; P < 0.001, d = 0.21], fat mass [-0.78 (-1.10, -0.46) kg; P < 0.001, d = 0.22] and FFM [-1.82 (-3.12, -0.51) kg; P = 0.013, d = 0.22] were all decreased by KD (Table 1). These changes equated to decreases of 3.0%, 8.3% and 2.5%, for body mass, fat mass and FFM, respectively, with the magnitude of effect size for each variable interpreted as small. VO2peak and Wmax, whether expressed in absolute terms or relative to body mass, were not different from PRE to POST (Table 1).
TABLE 1.
Participant characteristics and changes in response to seven days of a ketogenic diet.
| PREmean ± SD | POSTmean ± SD | Changemean (95% CI) | %changemean (95%CI) | P-value | |
|---|---|---|---|---|---|
| Body mass (kg) | 78.4 ± 13.8 | 76.0 ± 13.1 | -2.4 (-3.2, -1.6) | -3.0 (-3.9, -2.1) | < 0.001 |
| Body fat (%) | 14.9 ± 3.9 | 14.3 ± 4.2 | -0.6 (-1.3, 0.0) | -5.1 (-10.2, 0.0) | 0.055 |
| Fat mass (kg) | 11.34 ± 3.97 | 10.56 ± 4.18 | -0.78 (-1.10, -0.46) | -8.3 (-12.7, -4.0) | < 0.001 |
| Fat-free mass (kg) | 66.80 ± 10.62 | 64.98 ± 9.33 | -1.82 (-3.12, -0.51) | -2.5 (-4.2, -0.9) | 0.013 |
| Wmax(W) | 295 ± 30 | 292 ± 38 | -2 (-18, 13) | -1.0 (-6.2, 4.2) | 0.723 |
| Wmax(W kg-1) | 3.81 ± 0.41 | 3.87 ± 0.30 | -0.06 (-0.16, 0.28) | 2.1 (-3.5, 7.7) | 0.541 |
| VO2peak(L min-1) | 4.18 ± 0.33 | 4.10 ± 0.43 | -0.09 (-0.39, 0.21) | -1.9 (-8.8, 5.0) | 0.518 |
| VO2peak(mL kg-1 min-1) | 54.4 ± 5.9 | 54.7 ± 6.3 | 0.4 (-3.3, 4.2) | 1.2 (-6.3, 8.7) | 0.802 |
| 2 mM LT (%VO2peak) | 70.1 ± 6.4 | 81.0 ± 7.6 | 10.3 (4.1, 16.6) | 15.2 (5.6, 24.8) | 0.005 |
Note: Date are n = 9. SD, standard deviation; 95%CI, 95% confidence interval; Wmax, maximal power output; VO2peak, peak oxygen uptake; LT, lactate threshold.
Across the incremental power output with each stage, %Wmax, %VO2peak and HR were similar at PRE and POST as indicated by the absence of main effects for condition, or interaction effects for intensity x condition (Table 2). Despite similar oxygen consumption at each stage at PRE and POST (Table 2), the RER was lower (P < 0.001 for each stage) at POST compared to PRE (Figure 1A). For rates of CHO oxidation, main effects for intensity (P < 0.001) and condition (P < 0.001), and an interaction effect (P < 0.001) were observed (Figure 1B), whereas for rates of fat oxidation, main effects for intensity (P < 0.001) and condition (P < 0.001) were observed, but there was no interaction effect (P = 0.075) (Figure 1C). Therefore, rates of fat oxidation were higher at POST across a range of exercise intensities, with the maximal fat oxidation rate being 1.8-fold higher after KD (PRE, 0.54 ± 0.13 g min-1; POST, 0.95 ± 0.24 g min-1; P < 0.001, d = 2.2) (Figure 2).
TABLE 2.
Physiological responses, as a function of power output, during incremental exercise on a cycle ergometer before (PRE) and after (POST) seven days of a ketogenic diet.
| 50 | 95 | 130 | 165 | 200 | 235 | 270 | Intensity P-value | Condition P-value | Interaction P-value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | 17.1 | 32.5 | 44.5 | 56.5 | 68.5 | 80.5 | 92.5 | ||||
| %Wmax | POST | 17.1 ± 1.8 |
32.5 ± 3.3 |
44.5 ± 4.6 |
56.5 ± 5.8 |
68.5 ± 7.0 |
80.5 ± 8.2 |
92.5 ± 9.5 |
|||
| PRE | 17.4 ± 2.3 |
33.0 ± 4.3 |
45.2 ± 5.9 |
57.4 ± 7.6 |
69.6 ± 9.2 |
81.7 ± 10.8 |
91.5 ± 8.9 |
< 0.001 | 0.336 | 0.939 | |
| %VO2peak | POST | 31.4 ± 3.8 |
42.7 ± 3.6 |
52.6 ± 4.1 |
62.8 ± 5.3 |
73.5 ± 6.0 |
82.4 ± 7.8 |
91.2 ± 6.2 |
|||
| PRE | 31.7 ± 3.4 |
45.0 ± 4.3 |
55.3 ± 5.0 |
65.8 ± 5.8 |
76.2 ± 7.2 |
85.7 ± 7.1 |
93.4 ± 5.9 |
< 0.001 | 0.011 | 0.982 | |
| VO2(L min-1) | POST | 1.30 ± 0.14 |
1.77 ± 0.13 |
2.18 ± 0.15 |
2.60 ± 0.15 |
3.04 ± 0.19 |
3.41 ± 0.28 |
3.78 ± 0.30 |
|||
| PRE | 1.30 ± 0.17 |
1.84 ± 0.17 |
2.25 ± 0.14 |
2.68 ± 0.15 |
3.10 ± 0.18 |
3.49 ± 0.24 |
3.81 ± 0.28 |
< 0.001 | 0.059 | 0.985 | |
| VCO2(L min-1) | POST | 1.05 ± 0.13 |
1.46 ± 0.10 |
1.88 ± 0.11 |
2.34 ± 0.15 |
2.85 ± 0.20 |
3.39 ± 0.41 |
4.05 ± 0.42 |
|||
| PRE | 0.92 ± 0.12 |
1.35 ± 0.11 |
1.74 ± 0.09 |
2.16 ± 0.14* |
2.63 ± 0.24** |
3.19 ± 0.34* |
3.74 ± 0.42** |
< 0.001 | < 0.001 | 0.315 | |
| Blood lactate(mM) | POST | 1.2 ± 0.3 |
1.4 ± 0.4 |
1.4 ± 0.4 |
1.9 ± 0.7 |
2.8 ± 1.6 |
4.8 ± 3.0 |
7.1 ± 4.8 |
|||
| PRE | 1.3 ± 0.4 |
1.3 ± 0.4 |
1.3 ± 0.5 |
1.5 ± 0.5 |
1.9 ± 1.0 |
3.4 ± 2.1* |
6.5 ± 4.2 |
< 0.001 | 0.009 | 0.259 | |
| 92 ± 17 |
110 ± 12 |
124 ± 12 |
141 ± 12 |
155 ± 12 |
168 ± 13 |
176 ± 12 |
|||||
| HR(bpm) | POST | 83 ± 21 |
111 ± 13 |
126 ± 11 |
142 ± 13 |
158 ± 13 |
170 ± 14 |
179 ± 11 |
< 0.001 | 0.761 | 0.688 |
Note: Data presented as mean ± SD (n = 9). *P < 0.05, **P < 0.01, and ***P < 0.001 are for PRE vs. POST at same power output (W). %Wmax, percentage of maximal power output; %VO2peak, percentage peak oxygen uptake; VO2, oxygen uptake; VCO2, carbon dioxide production; HR, heart rate.
FIG. 1.
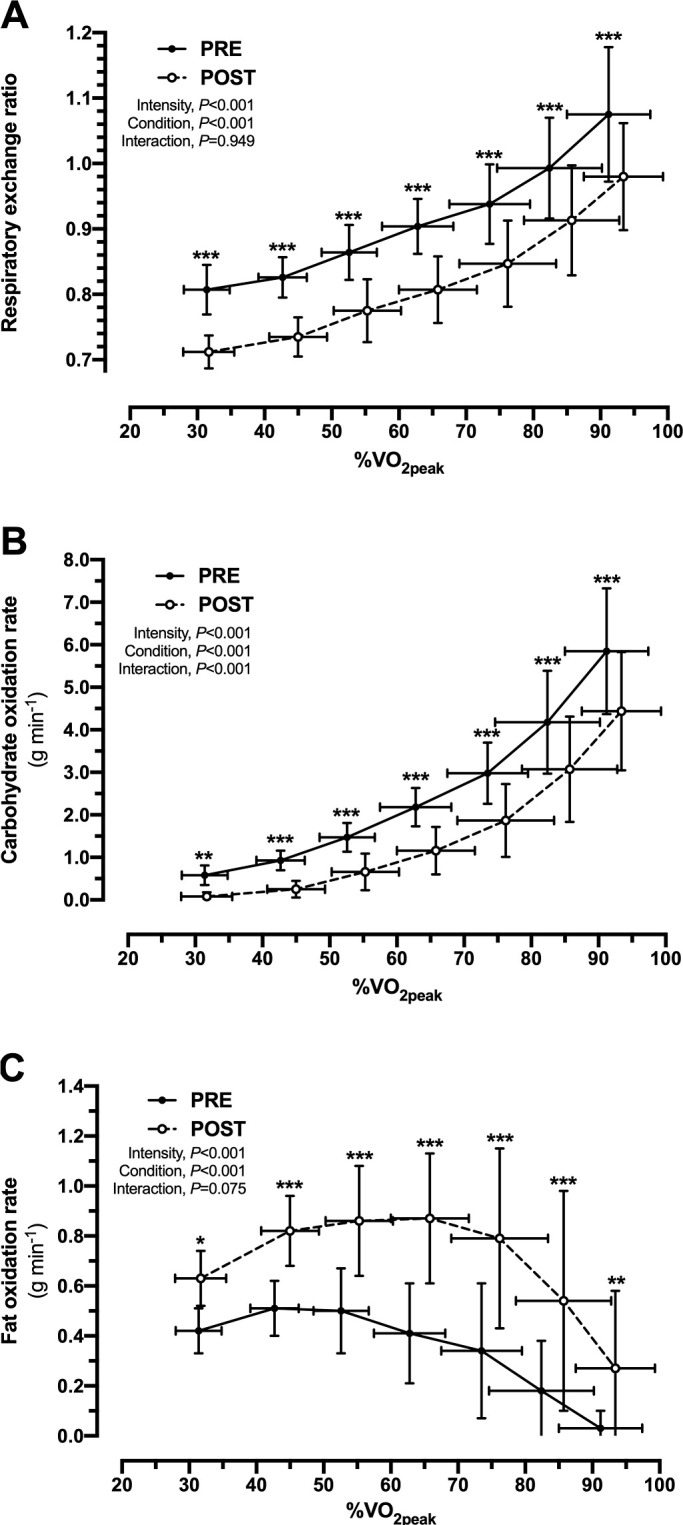
Substrate utilization during exercise across a range of intensities before (PRE) and in response to seven days of consuming a ketogenic diet (POST). A, Respiratory exchange ratio (RER); B, rates of carbohydrate oxidation; C, rates of fat oxidation. *P < 0.05, **P < 0.01, and ***P < 0.001 are for PRE vs. POST at same stage of the incremental exercise test.
FIG. 2.
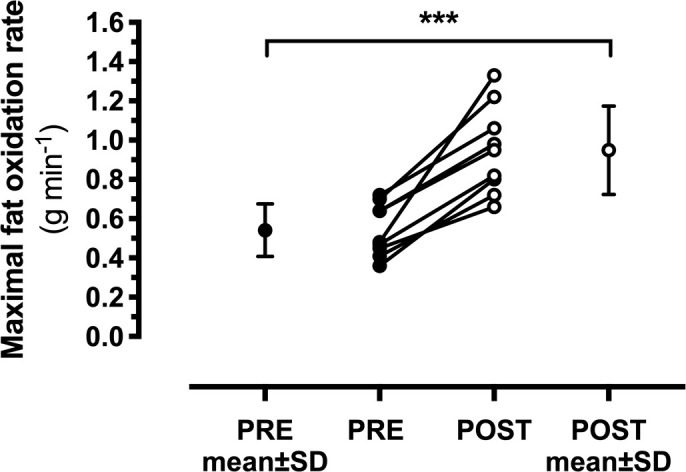
Maximal rates of fat oxidation before (PRE) and in response to seven days of consuming a ketogenic diet (POST). Data are shown as individual data points and grouped mean ± SD for the respective PRE and POST time points. ***P < 0.001 PRE vs. POST.
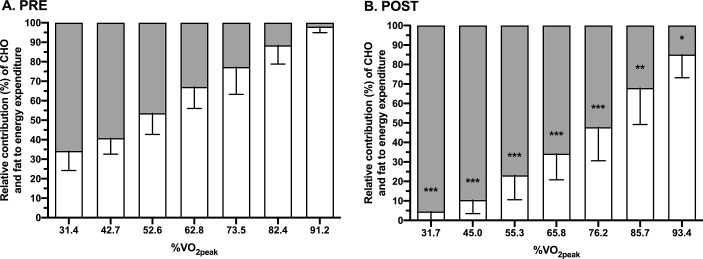
The relative contribution of fat to energy expenditure was higher at each stage at POST compared to PRE (Figure 3B). Consequently, the crossover point for substrate utilization, i.e. the exercise intensity after which CHO oxidation predominates for energy provision [31], occurred at a higher relative exercise intensity after KD (PRE, 48.4 ± 12.4%VO2peak; POST, 75.0 ± 9.7%VO2peak; P < 0.001, d = 2.4). For blood lactate concentrations, main effects for intensity (P < 0.001) and condition (P = 0.009) were observed, but there was no interaction effect (P = 0.259) (Table 2). The exercise intensity at which the FBLC 2 mM lactate threshold occurred increased from 70.1 ± 6.4%VO2peak at PRE to 81.0 ± 7.6%VO2peak at POST (P = 0.005, d = 1.5) (Table 1).
FIG. 3.
Relative contribution (%) of carbohydrate (CHO) and fat to energy expenditure during exercise across a range of intensities before (PRE; A) and in response to seven days of consuming a ketogenic diet (POST; B). *P < 0.05, **P < 0.01, and ***P < 0.001 are for PRE vs. POST at same stage of the incremental exercise test.
DISCUSSION
The main findings of the present study were that seven days of consumption of a KD by male endurance athletes resulted in rapid alterations in body composition, an almost two-fold increase in the maximal rate of fat oxidation, a rightward shift of the exercise intensities at which the 2 mM lactate threshold and the crossover point of substrate utilization occurred.
Seven days of a KD with ad libitum caloric intake resulted in decreases in body mass [-2.4 (-3.2, -1.6) kg], fat mass [-0.78 (-1.10, -0.46) kg and FFM [-1.82 (-3.12, -0.51) kg]. Of the eighteen studies that have assessed the impact of 3 to 12 weeks of a KD on changes in body mass in normal weight individuals, fifteen studies noted decreases in body mass (~2 kg on average), while twelve of the fourteen studies that assessed body composition noted decreases in fat mass [32]. To our knowledge, changes in body composition have not been reported previously in studies of short-term ( ≤ 7 days) KD or LCHF, but CHO restriction is commonly used in the later stages of the weight-making process in weight category sports [33]. Dual X-ray absorptiometry is a measurement that is dependent on body water, and therefore has been shown to be unreliable unless a participant’s hydration status, and dietary intake, especially in the form of CHO, is standardised given the coupling of ~3 g of water to each 1 g of CHO stored as glycogen [34–36]. For example, one week of CHO restoration (263 ± 42 g d-1) following ten weeks of KD within resistance trained males increased body mass (+3.4 kg) and DXA-based measurement of lean body mass by ~4.5 kg [22]. Similarly, three days of rest and CHO loading (8.1 g kg-1) increased body mass (+0.6 kg) and FFM (+0.9 kg) in non-obese men [37]. Due the present KD intervention being ad libitum caloric intake, and the limitations of DXA in measuring FFM under conditions where CHO intake is modulated, it is unknown if these participants were in a caloric deficit, and therefore, if loss of FFM observed was in the form of tissue mass or water, or a combination of both. Notwithstanding potential limitations of our body composition assessment, the reduction in body mass is noteworthy to endurance athletes, as carrying mass distally increases the aerobic demand of exercise [1]. Similarly, a short-term dietary approach that reliably produces acute changes in body mass may be attractive to athletes making weight in weight category sports [33], assuming there is no detrimental effect on performance.
Three well-controlled investigations containing elite racewalkers [6, 18], and runners [11], achieved fat oxidation rates of ~1.43 g min-1 at ~73%VO2max (RER, 0.75)[18], 1.57 ± 0.32 g min-1 at ~80%VO2peak (RER, 0.75), and 1.20 ± 0.18 g min-1 at ~ 77%VO2max (RER, 0.79), respectively, following 5–6 days [18], 21 days [6], and 31 days [11] of adherence to a KD. These figures are noteworthy, as they equal or surpass figures achieved in cross-sectional investigations containing ultra-endurance athletes [13], and cyclists [7], habituated to a KD for > 8 months, who achieved fat oxidation rates of 1.54 ± 0.18 g min-1 at ~70%VO2max [13], and 1.21 ± 0.15 g min-1 (RER, 0.78) at ~72%VO2max [7]. Here we demonstrate that seven days of a KD produced an almost two-fold increase in fat oxidation (to 0.95 ± 0.24 g min-1 at an RER of 0.77), with these maximal rates of fat oxidation occurring at 62.0 ± 14.7%VO-2max. These results are similar to those reported at ~70%VO2peak (increase from 0.67 ± 0.16 g min-1 to 0.91 ± 0.20 g min-1) following just 5 days of adherence to a LCHF diet [38]. Notably, no further increases in the rates of fat oxidation were noted following 10 and 15 days of adherence to LCHF [38]. Differing rates of fat oxidation between the various studies are likely a consequence of training status of participants, and different exercise protocols implemented e.g. steady-state versus incremental exercise. The present study (54.7 ± 1.3 mL kg-1 min-1) and a study in cyclists (59.4 ± 5.2 mL kg-1 min-1) [11] contained trained individuals, while previous studies contained well-trained (64.7 ± 3.7 mL kg-1min-1 [13]; 61 ± 5 mL kg-1 min-1 [7]; 66.3 ± 4.8 mL kg-1 min-1 [6]; 67.7 ± 6.1 mL kg-1 min-1 [18]) individuals. Moreover, maximal rates of fat oxidation were reportedly ~34% lower in “low performance” (59.4 ± 5.9 mL kg-1 min-1) participants compared to “moderate performance” (64.3 ± 7.1 mL kg-1 min-1) participants during the incremental FATmax protocol [39]. This difference in rates of fat oxidation based on the stratification by aerobic fitness is similar to differences observed in the present study compared to investigations involving well-trained individuals following 5–6 day (~34% lower) [18], 21 day (~40% lower) [6], and > 8 month (~38% lower) [13], periods of KD consumption. Overall, we contend that the maximal rate of fat oxidation is influenced to a greater extent by training status rather than the duration of an adherence and/or adaptation to a KD.
The crossover point describes the intensity at which CHO oxidation predominates energy provision during exercise [31]. A rightward shift in the crossover point was observed after seven days of a KD as indicated by the intensity at which this occurred being 48.4 ± 3.7% VO2peak at PRE, and 72.1 ± 4.1%VO2peak at POST. This was accompanied by a rightward shift in the blood lactate response curve and the 2 mM lactate threshold occurring at a higher intensity of exercise at POST (81.0 ± 7.6%VO2peak) compared to PRE (70.1 ± 6.4% VO2peak). The mechanistic basis of these marked metabolic effects are unclear e.g. as a consequence of reduced dietary CHO consumed, or reduced flux through pyruvate dehydrogenase (PDH), as low CHO diets (< 10% total energy) are associated with metabolic acidosis due to elevation in free-fatty acids and βHB [40]. Acute LCHF feeding, even with CHO restoration, is associated with a reduction in glycogenolysis, and attenuation in maximal enzymatic activity of the active form of PDH during moderate (~70%VO2peak) and near-maximal (150% of peak power output) intensity exercise in well-trained cyclists [41]. However, no effects on blood lactate concentrations during exercise were observed following 21 [6], and 31 [11], days of adherence to a KD, and in general the effect of KD on the blood lactate response to exercise has been equivocal [32].
Whether the metabolic changes observed after short-term KD would translate into a performance benefit is doubtful in well-trained athletes, at least at high intensities of exercise. For instance, with increasing intensity of exercise in the present study, the relative contribution of carbohydrate to energy expenditure was reduced between PRE and POST, which may be problematic given the dependency of high intensity performance on carbohydrate as a substrate [42]. While a study in recreationally-trained athletes noted no decrement in 5 km time-trial running performance with short-term (4 days) consumption of a KD [12], additional studies containing athletes of higher calibre have noted potentially detrimental effects for athletic performance [6, 11, 18]. For example, adherence to a KD for 21 days resulted in reduced exercise economy at ~91%VO2peak, and negated the benefits of training adaptations for completion of a 10 km racewalk [6]. Moreover, a recent study of 5–6 days of a KD was associated with an ~5–8% increase in oxygen cost during a 25 km race walk (~75%VO2max) [18]. Lastly, a so-called “throttling” of RER and an adaptation-intensity interaction during the final stage of graded exercise test has been described [11]. Despite increased rates of fat oxidation and reduced RER throughout incremental exercise after KD, the present study noted no decrement to efficiency as oxygen consumption and HR remained similar between PRE and POST up to ~93%VO2peak.
The present study is not without limitations. While we included an objective urinary measure to monitor adherence to the KD and provided recipes, meal plans and daily support for the participants, we did not perform a dietary analysis for the seven day intervention period. Therefore, whether a short-term calorie deficit contributed to the urinary ketone body concentrations or the changes in body mass and composition is unknown. Using a single arm, pre-post design means that the absence of a control group can be considered a limitation. Moreover, we did not monitor training load for the seven day intervention period. However due to the short duration of the study, the low P values, and small-to-large magnitude of effects for reported outcomes, it is unlikely that effects observed are invalidated by a lack of control group, or that a training effect explains any of the PRE to POST differences.
CONCLUSIONS
The present study demonstrates that a short-term (seven days) ad libitum KD consumed by trained men produces ~3% loss of body mass, and marked changes in substrate utilization across a range of exercise intensities. Although such changes in substrate utilization may be considered to be ergogenic in some performance contexts e.g. ultra-endurance exercise, and generally reflect the outcomes of longer term adherence to a KD, it remains to be confirmed whether there are performance contexts in which a short-term KD could confer a performance benefit.
Acknowledgements
The authors would like to acknowledge the technical support of Mr. Romain Denis throughout the study, and Dr. Lorna Doyle (Waterford Institute of Technology, Ireland) for helpful comments on an early draft of the manuscript.
Conflict of interest statement
All authors report no conflicts of interest associated with this manuscript.
Footnotes
CITATION: McSwiney FT, Ben Fusco B, McCabe L et al. Changes in body composition and substrate utilization after a short-term ketogenic diet in endurance-trained males. Biol Sport. 2021;38(1):145–152.
Author contributions
FTMS and BE conceived the study; FTMS, BF, LMC, AL, PC, JW and MH collected the data; FTMS, MH and BE analyzed the data and interpreted the results; FTMS and BE wrote the first draft of the manuscript; All authors reviewed and contributed to the final manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
References
- 1.Thomas DT, Erdman KA, Burke LM. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J Acad Nutr Diet. 2016;116(3):501–28. [DOI] [PubMed] [Google Scholar]
- 2.Burke LM, Hawley JA. Swifter, higher, stronger: What’s on the menu? Science. 2018;16(362):781–7. [DOI] [PubMed] [Google Scholar]
- 3.Volek JS, Noakes T, Phinney SD. Rethinking fat as a fuel for endurance exercise. Eur J Sport Sci. 2015; 15(1):13–20. [DOI] [PubMed] [Google Scholar]
- 4.Burke LM. Ketogenic Low CHO, High Fat diet: the future of elite endurance sport? J Physiol. 2020; doi: 10.1113/JP278928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phinney SD, Bistrian BR, Evans WE, Gervino E, Blackburn GL. The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism. 1983;32(8):769–76. [DOI] [PubMed] [Google Scholar]
- 6.Burke LM, Ross ML, Garvican-Lewis LA, Welvaert M, Heikura IA, Forbes SG, Mirtschin JG, Cato LE, Stobel N, Sharma AP, Hawley JA. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J Physiol. 2017;595(9):2785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webster CC, Noakes TD, Chacko SK, Swart J, Kohn TA, Smith JAH. Gluconeogenesis during endurance exercise in cyclists habituated to a long-term low carbohydrate high-fat diet. J Physiol. 2016; 594(15):4389–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zinn C, Wood M, Williden M, Chatterton S, Maunder E. Ketogenic diet benefits body composition and well-being but not performance in a pilot case study of New Zealand endurance athletes. J Int Soc Sports Nutr. 2017;14(22):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heatherly AJ, Killen LG, Smith AF, Waldman HS, Hollingsworth A, Seltmann CL, O’Neal EK. Effects of ad libitum Low Carbohydrate High-Fat Dieting in Middle-Age Male Runners. Med Sci Sport Exerc. 2017; 50(3):570–9. [DOI] [PubMed] [Google Scholar]
- 10.McSwiney FT, Wardrop B, Hyde PN, Lafountain RA, Volek JS, Doyle L. Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism. 2018;81:25–34. [DOI] [PubMed] [Google Scholar]
- 11.Shaw DM, Merien F, Braakhuis A, Maunder E, Dulson DK. Effect of a Ketogenic Diet on Submaximal Exercise Capacity and Efficiency in Runners. Med Sci Sport Exerc. 2019;51(10):2135–46. [DOI] [PubMed] [Google Scholar]
- 12.Prins PJ, Noakes TD, Welton GL, Haley SJ, Esbenshade NJ, Atwell AD, Scott KE, Abraham J, Raabe AS, Buxton JD, Ault DL. High rates of fat oxidation induced by a low-carbohydrate, high-fat diet, do not impair 5–km running performance in competitive recreational athletes. J Sport Sci Med. 2019; 18(4):738–50. [PMC free article] [PubMed] [Google Scholar]
- 13.Volek JS, Freidenreich DJ, Saenz C, Kunces LJ, Creighton BC, Bartley JM, Davitt PM, Munoz CX, Anderson JM, Maresh CM, Lee EC, Schuenke MD, Aerni G, Kraemer WJ, Phinney SD. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism. 2016;65(3):100–10. [DOI] [PubMed] [Google Scholar]
- 14.Burke LM. Re-Examining High-Fat Diets for Sports Performance: Did We Call the ’Nail in the Coffin’Too Soon? Sport Med. 2015;45(1):33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poff AM, Koutnik AP, Egan B. Nutritional Ketosis with Ketogenic Diets or Exogenous Ketones: Features, Convergence, and Divergence. Curr Sport Med Rep. 2020;19(8):251–9. [DOI] [PubMed] [Google Scholar]
- 16.McSwiney FT, Doyle L, Plews DJ, Zinn C. Impact Of Ketogenic Diet On Athletes: Current Insights. Open Access J Sport Med. 2019;10:171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volek JS, Phinney SD. The Art and Science of Low Carbohydrate Performance. Miami, FL: Beyond Obesity; 2012. [Google Scholar]
- 18.Burke LM, Whitfield J, Heikura IA, Ross ML, Tee N, Forbes SF, Hall R, McKay AK, Wallett AM, Avish P. Adaptation to Low Carbohydrate High Fat diet is rapid but impairs endurance exercise metabolism and performance despite enhanced glycogen availability. J Physiol. 2020. doi 10.1113/JP280221 (online ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paoli A, Grimaldi K, D’Agostino D, Cenci L, Moro T, Bianci A, Palma A. Ketogenic diet does not affect strength performance in elite artistic gymnasts. J Int Soc Sports Nutr. 2012;9(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhyu H, Cho S. The effect of weight loss by ketogenic diet on the body composition, performance-related physical fitness factors and cytokines of Taekwondo athletes. J Exerc Rehabil. 2014;10(5):326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green D, Varley B, Hartwig T, Chapman P, Rigley M. A low-carbohydrate ketogenic diet reduces body weight without compromising performance in powerlifting and olympic weightlifting athletes. J Strength Cond Res. 2018; 32(12):1–10. [DOI] [PubMed] [Google Scholar]
- 22.Wilson JM, Lowery RP, Roberts MD, Sharp MH, Joy JM, Shields KA, Partl J, Volek JS, D’Agostino D. The Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Males. J Strength Cond Res. 2017; doi: 10.1519/JSC.0000000000001935. [DOI] [PubMed] [Google Scholar]
- 23.Achten J, Gleeson M, Jeukendrup AE. Determination of the exercise intensity that elicits maximal fat oxidation. Med Sci Sport Exerc. 2002; 34(1):92–7. [DOI] [PubMed] [Google Scholar]
- 24.Achten J, Venables MC, Jeukendrup AE. Fat oxidation rates are higher during running compared with cycling over a wide range of intensities. Metabolism. 2003;52(6):747–52. [DOI] [PubMed] [Google Scholar]
- 25.Jeukendrup AE, Wallis GA. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sport Med Suppl. 2005; 26(1):S28–37. [DOI] [PubMed] [Google Scholar]
- 26.Kuo CC, Fattor JA, Henderson GC, Brooks GA. Lipid oxidation in fit young adults during postexercise recovery. J Appl Physiol. 2005;99(1):349–56. [DOI] [PubMed] [Google Scholar]
- 27.Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004; 1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paoli A, Bianco A, Grimaldi K. The ketogenic diet and sport: a possible marriage? Exerc Sport Sci Rev. 2015; 43(3):153–62. [DOI] [PubMed] [Google Scholar]
- 29.Musa-Veloso K, Likhodii SS, Cunnane SC. Breath acetone is a reliable indicator of ketosis in adults consuming ketogenic meals. Am J Clin Nutr. 2002; 76(1):65–70. [DOI] [PubMed] [Google Scholar]
- 30.Urbain P, Bertz H. Monitoring for compliance with a ketogenic diet: what is the best time of day to test for urinary ketosis? Nutr Metab. 2016;13(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J Appl Physiol. 1994;76(6):2253–61. [DOI] [PubMed] [Google Scholar]
- 32.Kang J, Ratamess NA, Faigenbaum AD, Bush JA. Ergogenic Properties of Ketogenic Diets in Normal-Weight Individuals: A Systematic Review. J Am Coll Nutr. 2020; doi: 10.1080/07315724.2020.1725686 (online ahead of print). [DOI] [PubMed] [Google Scholar]
- 33.Reale R, Slater G, Burke LM. Acute-Weight-Loss Strategies for Combat Sports and Applications to Olympic Success. Int J Sport Physiol Perform. 2017; 12(2):142–51. [DOI] [PubMed] [Google Scholar]
- 34.Wagner DR, Heyward VH. Techniques of body composition assessment: A review of laboratory and field methods. Res Q Exerc Sport. 1999;70(2):135–49. [DOI] [PubMed] [Google Scholar]
- 35.Ackland TR, Lohman TG, SundgotBorgen J, Maughan RJ, Meyer NL, Stewart AD, Müller W. Current status of body composition assessment in sport: review and position statement on behalf of the ad hoc research working group on body composition health and performance, under the auspices of the I.O.C. Medical Commission. Sport Med. 2012;42(3):227–49. [DOI] [PubMed] [Google Scholar]
- 36.Kerr A, Slater GJ, Byrne N. Impact of food and fluid intake on technical and biological measurement error in body composition assessment methods in athletes. Br J Nutr. 2017; 117(4):591–601. [DOI] [PubMed] [Google Scholar]
- 37.Rouillier M-A, David-Riel S, Brazeau A-S, St-Pierre DH, Karelis AD. Effect of an Acute High Carbohydrate Diet on Body Composition Using DXA in Young Men. Ann Nutr Metab. 2015;66(4):233–6. [DOI] [PubMed] [Google Scholar]
- 38.Goedecke J, Christie C, Wilson G, Dennis S, Noakes TD, Hopkins WG, Lambert EV. Metabolic adaptations to a high-fat diet in endurance cyclists. Metabolism. 1999;48(12):1509–17. [DOI] [PubMed] [Google Scholar]
- 39.Lima-Silva AE, Bertuzzi RC, Pires FO, Gagliardi JF, Barros RV, Hammond J, Kiss MA. Relationship between training status and maximal fat oxidation rate. J Sport Sci Med. 2010;9(1):31–5. [PMC free article] [PubMed] [Google Scholar]
- 40.Maughan RJ, Greenhaff P. Diet composition and the performance of high-intensity exercise. J Sport. 1997; 15(3):265–75. [DOI] [PubMed] [Google Scholar]
- 41.Stellingwerff T, Spriet LL, Watt MJ, Kimber NE, Hargreaves M, Hawley JA, Burke LM. Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Am J Physiol Endocrinol Metab. 2006;290(2):380–8. [DOI] [PubMed] [Google Scholar]
- 42.Hawley JA, Leckey JJ. Carbohydrate dependence during prolonged, intense endurance exercise. Sport Med. 2015; 45(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]