Abstract
Background:
Risk-adjustment for reimbursement and quality measures omits social risk factors despite adversely affecting health outcomes. Social risk factors are not usually available in electronic health records (EHR) or administrative data. Socioeconomic status can be assessed by using United States Census data. Distressed Communities Index (DCI) is based upon zip codes and the Area Deprivation Index (ADI) provides more granular estimates at the block group level. We examined the association of neighborhood disadvantage using the ADI, DCI and patient-level insurance status on 30-day readmission risk after colorectal surgery.
Methods:
Our 677 patient cohort was derived from the 2013–2017 National Surgical Quality Improvement Program at a safety net hospital augmented with EHR data to determine insurance status and 30-day readmissions. Patients’ home addresses were linked to the ADI and DCI.
Results:
Our cohort consisted of 53.9% males and 63.8% Hispanics with a 22.9% 30-day readmission rate from the date of discharge; >50% lived in highly deprived neighborhoods. Controlling for medical comorbidities and complications, ADI was associated with increased risk of 30-day from the date of discharge readmissions among patients living in medium (OR = 2.15, p= .02) or high (OR = 1.88, p= .03) deprived areas compared to less deprived neighborhoods; but not insurance status or DCI.
Conclusions:
The ADI identified patients living in deprived communities with increased readmission risk. Our results show that block-group level ADI can potentially be used in risk-adjustment, to identify high-risk patients and to design better care pathways that improve health outcomes.
Keywords: colectomy, social risk factors, outcomes, Distressed Communities Index, National Surgical Quality Improvement Program
Introduction
Colorectal operations are among the most common surgeries performed in the US and are associated with one of the highest rates of complications and hospital readmissions of general surgery procedures [1, 2]. The Hospital Readmissions Reduction Program (HRRP) mandates financial penalties for higher than expected 30-day readmission rates. The HRRP’s unintended consequences of disproportionately penalizing safety net hospitals (SNH) constrains resources at hospitals caring for the most vulnerable populations [3–7]. A study across four states showed a 30-day readmission rate for colectomy patients of 10.9% that increased to 12.1% in the subgroup of hospitals with a high safety net burden [8]. The Centers for Medicare & Medicaid Services (CMS) recently established a new peer group-based payment adjustment system for assessing readmission penalties comparing similar hospitals stratified by patient socioeconomic status (SES) measured by the proportion of patients with dual enrollment in Medicare/Medicaid. The greatest penalty reductions occurred in hospitals serving the lowest SES patients, lessening, but not eliminating, the previously unbalanced penalty burden for SNH [9].
Patient-level SES variables that could improve risk-adjustment are limited in administrative data and electronic health records (EHR). However, and despite known limitations, dual enrollment in Medicare/Medicaid, insurances status [2], race and ethnicity are commonly used measures to identify health disparities and low SES populations [10]. Another commonly used patient characteristic is their zip code linked to US Census data to estimate individual-level SES, usually showing no or mixed effects on outcomes [11–15] due to the lack of granularity. US Census Zip Code Tabulation Areas are generalized representations of zip codes with an average population of 9,414. The National Academy of Medicine released five reports outlining the need to develop better risk adjustment methods using more granular social risk factor data [16]. A more granular proxy measure is the “block group” representing approximately 1,431 residents [17].
The Area Deprivation Index (ADI) and Distressed Communities Index (DCI) were developed to help identify populations living in deprived/disadvantaged neighborhoods. The ADI [18] uses 17 American Community Survey [19] variables from the US Census to stratify geographic areas based on socioeconomic disadvantage at the more granular block group level. Patients living in more compared to less disadvantaged neighborhoods had higher 30-day all cause readmissions [18, 20, 21]. The DCI combines five measures from the American Community Survey and two measures from the US Census Bureau Business Patterns dataset to estimate the community socioeconomic distress by zip codes [22]. Several recent studies merged DCI with the American College of Surgeons National Quality Improvement Program (NSQIP) [23–26] data improving prediction of postoperative complications and risk-adjustment [27–31]. Using ADI data provides more granularity but requires patient addresses to be geocoded, while DCI can be easily linked to patients using their zip codes but provides data on a less granular level.
Comparing studies on the effect of social risk factors on readmission risk is further complicated by the variability in defining 30-day readmissions. Many studies using NSQIP data define readmissions as 30 days from the date of surgery while the CMS defines readmissions as 30 days from the date of discharge from the index procedure. The goals of our study are to 1) examine ADI, DCI, and insurance status as proxy SES measures to predict 30-day readmissions after colorectal surgery and 2) use both definitions of 30-day readmissions in models using ADI, DCI and insurance status. We hypothesize that patients living in distressed/deprived neighborhoods have an increased risk of 30-day readmissions and that using the more granular ADI will provide increased sensitivity in predicting readmission risk than the DCI or insurance status.
Materials and Methods
Patient Population and Variables
All patients undergoing colorectal procedures present in the 2013–2017 NSQIP of University Hospital [32], a safety net county hospital, were evaluated for study inclusion (Figure 1). NSQIP registry was used for cohort identification as well providing standardized definitions of preoperative risk factors and complications [24–26]. Colorectal procedures were identified using the NSQIP Principle Current Procedural Terminology (CPT) code for the index surgery and categorized as laparoscopic, open, perineal or transsacral approaches, combined open and perineal approaches were classified as open (Table 1). All CPT codes used for billing during the index hospitalization were assessed, patients with any CPT code specifying operative placement of a stoma or use was associated with the presence of a stoma (e.g. endoscopy performed through a stoma) were classified as having stoma (Table 1) regardless of the Principle CPT code. CPT codes with optional stoma placement required chart review for assignment. Patient preoperative variables from NSQIP were used to estimate frailty using the Risk Analysis Index-A (RAI-A) [33, 34]. Case priority was determined from NSQIP variables with urgent cases being defined as “no” responses to elective and emergency variables. Clavien-Dindo level IV complications [35] were derived from the NSQIP variables of unplanned intubation, pulmonary embolism, on ventilator >48 hours, progressive renal insufficiency, acute renal failure, stroke, cardiac arrest, myocardial infarction, septic shock, and unplanned reoperation. We used the NSQIP variable for hospital readmissions 30 days from the date of surgery.
FIGURE 1.
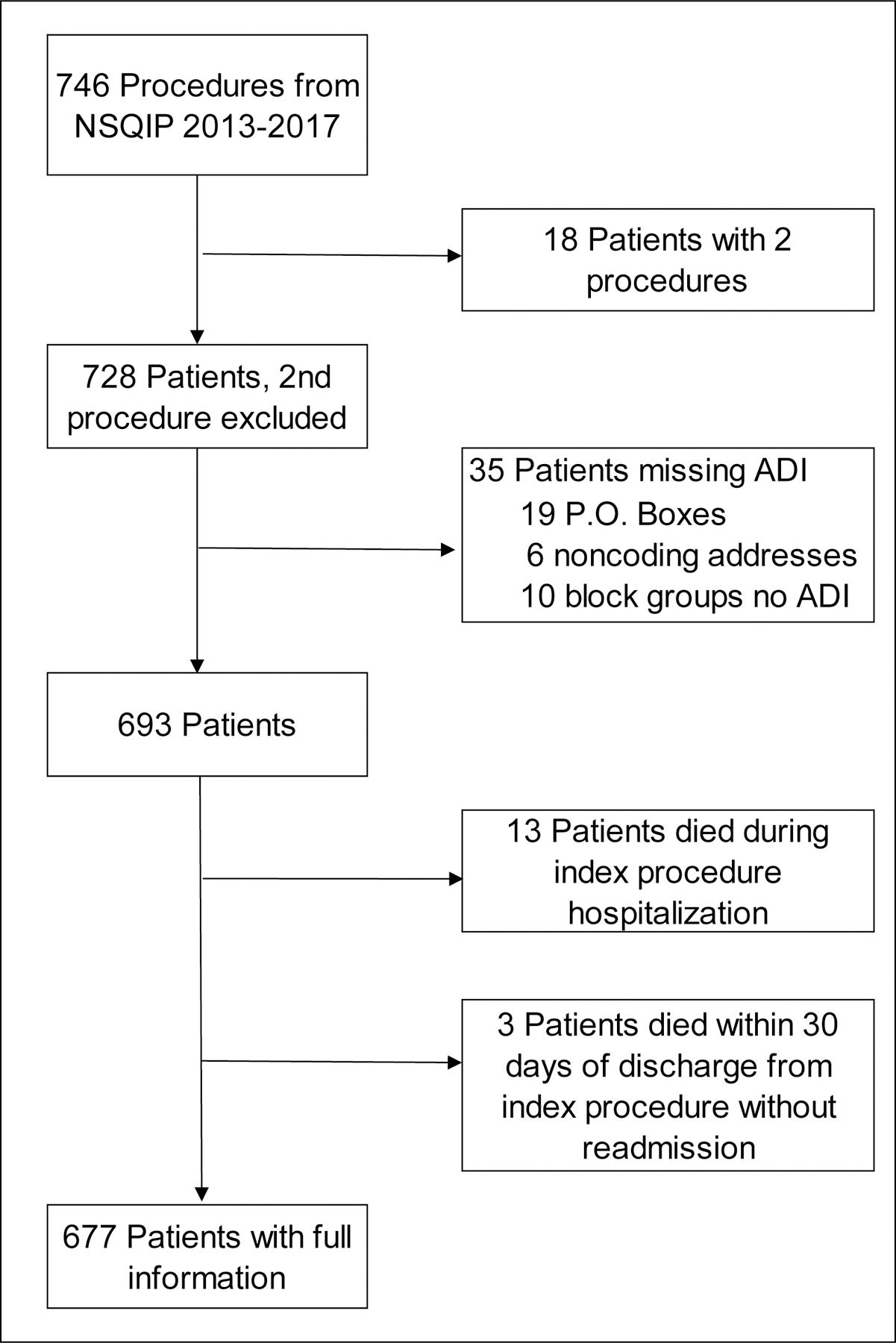
Patient cohort for 30-day hospital readmission rates for colorectal procedures. The diagram describes the numbers of unique patients and exclusion criteria for colorectal procedures in the National Surgical Quality Improvement Program (NSQIP) of an academic, safety net hospital from 2013–2017. Our cohort of 677 patients orginated from 746 procedures; only the first procedure was included for patients with more than one procedure. Patient exclusion criteria include missing an Area Deprivation Index (ADI) or mortality during or within 30 days of discharge from the index procedure without a readmission to the hospital. Three patients expiring within 30 days of discharge that were readmitted prior to death were included in the analyses.
Table 1.
CPT Codes used to Identify Procedure Type and Presence of a Stoma
| Principle CPT Codes used to Identify Cohort |
| Laparoscopic Procedures |
| 44204, 44205, 44206, 44207, 44208, 44210 44211, 44212, 45395, 45397, 45402 |
| Open Abdominal Procedures* |
| 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 45110, 45111, 45112, 45113, 45114, 45119, 45120, 45121, 45126, 45135, 45550 |
| Perineal Procedures |
| 45123, 45130 |
| Transsacral Procedures |
| 45116, 45160 |
| CPT Codes used to Identify Stoma Absence or Presence |
| Colorectal Procedures with a Primary Anastomosis |
| 44140, 44145, 44147, 44160, 44204, 44205, 44207, 45112, 45114, 45116, 45120, 45121, 45130, 45135, 45402, 45550 |
| Colorectal Procedures not Requiring an Anastomosis or Stoma |
| 45123, 45160 |
| Colorectal Procedures Possibly having a Stoma+ |
| 44130, 44150, 44157, 44158, 44210, 44389, 45113, 45119, 45126, 45397 |
| Colorectal Procedures with a Stoma |
| 44141, 44143, 44144, 44146, 44151, 44155, 44156, 44206, 44208, 44211, 44212, 45110, 45111, 45395 |
| Other CPT Codes used to Identify a Stoma# |
| 44125, 44187, 44188, 44310, 44312, 44314, 44316, 44320, 44322, 44340, 44345, 44346, 44380, 44382, 44381, 44384, 44385, 44386, 44388, 44389, 44390, 44394, 44391, 44392, 44401, 44402, 44403, 44404, 44405, 44406, 44407, 44408, 44605, 45136, 45563, 45805, 45825 |
CPT, Current Procedural Terminology
includes combined abdominal and perineal procedures
chart review used to determine presence of a stoma
indicated presence of a stoma if CPT code used during index hospitalization
NSQIP patients were linked to EHR and administrative data in our clinical data warehouse to supplement any missing race/ethnicity variables, determine 30-day readmissions from the date of hospital discharge for the index procedure, calculate a Charlson Comorbidity Index [36], determine insurance status and obtain patients’ home addresses. ArcGIS 10.7 Desktop version was used to geocode patients’ home addresses to the block group level to assign the 2015 ADI grouped into low (ADI=1–3), medium (ADI=4–6) and high deprivation (ADI=7–10) neighborhoods as previously described [37]. The 2018 DCI at zip code level was used to assign patients into Prosperous (0–19), Comfortable (20–39), Mid-tier (40–59), At risk (60–79) and Distressed (80–100) categories. We grouped DCI scores into 2-category with scores of ≤ 75 and > 75, as previously described [27–29], and 3 category with scores of 0–39, 40–59, and 60–100. Patients were categorized based upon insurance status as 1) private insurance, Medicare/Government/Disability, or self-pay that paid their hospital bill and 2) Medicaid, dual enrollment in Medicare/Medicaid, Charity Care, self-pay with <1% of collected charges, or CareLink [38], a Bexar county indigent care program administered through University Health System. We were able to calculate the Charlson Comorbidity Index [36] for 552 patients.
Statistical Analysis
All analyses were conducted using R Studio 3.5.1. Descriptive and correlational data were estimated using frequencies (percent) and means (standard deviation). The Charlson Comorbidity Index was excluded from analyses secondary to collinearity with the RAI-A and the ability to calculate a score for only 81.5% of patients. We conducted Chi-square tests to assess associations between our two 30-day readmission outcome variables and each predictor variable. Bivariate logistic regression models between each predictor variable and 30-day readmissions were used to select variables for inclusion in the logistic regression models (p ≤ 0.10). Four separate multivariate logistic regression models were conducted to compare association of ADI, 2-category DCI, 3-category DCI and insurance status with 30-day readmission rates from date of surgery and date of discharge from the index procedure.
Results
Characteristics of Colorectal Surgery Patients
Our sample consisted of 728 patients undergoing 746 colorectal procedures at University Hospital present in the 2013–2017 NSQIP; 18 patients had two procedures. Patients were excluded for 1) addresses that could not be geocoded (n=25), 2) ADI was not assigned to their block group (n=10), 3) expiring during the index procedure hospitalization (no chance of readmission, n=13), or 4) expiring within 30 days of the discharge date without being readmitted (n=3), leaving 677 patients in our cohort (Figure 1). The 13 patients who expired during the index procedure hospitalization predominately underwent emergency or urgent procedures, 61.5%, 30.8% respectively and 84.6% experienced at least one Clavien-Dindo Level IV complication (Table 2).
Table 2.
Characteristics of Patients expiring during the index procedure hospitalization
| N (%) or Mean (SD) | |
|---|---|
| Demographics | 13 (100%) |
| Age (years) | 62.9 (12.6) |
| Age range | 42.2 −>90 |
| Sex (female) | 4 (30.8%) |
| Race | |
| White | 13 (100%) |
| Ethnicity (Hispanic) | 10 (76.9%) |
| Case Priority | |
| Elective | 1 (7.7%) |
| Urgent | 4 (30.8%) |
| Emergency | 8 (61.5%) |
| Stoma | |
| Yes | 7 (53.8%) |
| Clavien-Dindo IV Complications | |
| Yes | 11 (84.6%) |
| ADI | |
| Low (1–3) | 3 (23.1%) |
| Medium (4–6) | 2 (15.4%) |
| High (7–10) | 8 (61.5%) |
| DCI (2-Category) | |
| Low Distress (DCI ≤75) | 7 (53.8%) |
| High Distress (DCI >75) | 6 (46.2%) |
| DCI (3-Category) | |
| Pros/Comf (0–39) | 2 (15.4%) |
| Mid-Tier (40–59) | 1 (7.7%) |
| Risk/Dis (60–100) | 10 (76.9%) |
| Insurance Status | |
| Carelink | 2 (15.4%) |
| Medicaid | 4 (30.7%) |
| Medicare/Government/Disability | 3 (23.1%) |
| Dual enrollment Medicare/Medicaid | 2 (15.4%) |
| Private | 1 (7.7%) |
| Self-pay | 1 (7.7%) |
N, number of patients; SD, standard deviation; ADI, Area Deprivation Index; DCI, Distressed Communities Index.
Our cohort of 677 patients consisted of 46.1% females, 89.7% Whites, 63.8% Hispanics, with a mean age of 55.1 years old at the time of the index procedure (Table 3). Distribution of patients using the Charlson Comorbidity Index (n=552) was 22.5%, 14.3%, 12.7% and 50.5% for scores of 0, 1, 2 and ≥3, respectively. Insurance status, ADI and DCI were used to estimate patient SES and neighborhood deprivation [8, 18, 20, 22]. More than 50% of the patients were classified as low SES or living in a highly deprived neighborhood using insurance status, ADI and 3-category DCI. Using the 2-category DCI, 38.6% of patients were classified as living in highly distressed neighborhoods (Table 4). Patients were classified as robust (61.3%), normal (27.0%) and frail (11.7%) with 31.2% and 9.6% of patients having an urgent or emergency procedure, respectively. Finally, 42.7% of patients had a stoma, and 16.4% of patients had at least one life threatening Clavien-Dindo Level IV postoperative complication (Table 4). Unplanned reoperations and prolonged postoperative ventilation were the most common Clavien-Dindo Level IV complications (Table 5). Eight patients had pulmonary embolism, 7 were on appropriate chemoprophylaxis and the remaining patient was treated with a sequential compression device. Open surgical compared to laparoscopic procedures had higher readmission rates and increased length of stay averaging 13.1 ± 12.8 and 6.9 ± 6.6 days, respectively (Table 6).
Table 3.
Patient Characteristics
| N (%) or Mean (SD) | |
|---|---|
| Demographics | 677 (100%) |
| Age (years) | 55.1 (12.9) |
| Age range | 19.1 −>90 |
| Sex (female) | 312 (46.1%) |
| Race | |
| White | 607 (89.7%) |
| African-American | 51 (7.5%) |
| Asian | 10 (1.5%) |
| Multi-racial | 9 (1.3%) |
| Ethnicity (Hispanic) | 432 (63.8%) |
| BMI | 29.4 (7.1) |
| BMI Range | 13.1 – 54.1 |
| NSQIP Comorbidities | |
| Chronic Steroid Use | 55 (8.1%) |
| Congestive Heart Failure | 5 (0.7%) |
| COPD | 17 (2.5%) |
| Current Smoker | 176 (25.9%) |
| Diabetes-Insulin | 56 (8.3%) |
| Diabetes-Oral Agents | 108 (15.9%) |
| Dialysis | 17 (2.5%) |
| Disseminated Cancer | 60 (8.9%) |
| Dyspnea | 34 (5.0%) |
| Hypertension | 305 (45%) |
| Weight loss | 54 (7.9%) |
| ASA | |
| ASA 1 | 2 (0.3%) |
| ASA 2 | 107 (15.8%) |
| ASA 3 | 483 (71.3%) |
| ASA 4 | 79 (11.7%) |
| ASA 5 | 6 (0.9%) |
| Length of Stay | 10.8 (11.3) |
| Length of Stay range | 1.0 – 106.0 |
| Insurance Status | |
| Carelink | 176 (26.0%) |
| Charity Care | 5 (0.7%) |
| Medicaid | 123 (18.2%) |
| Medicare/Government/Disability | 127 (18.8%) |
| Dual enrollment Medicare/Medicaid | 49 (7.2%) |
| Private | 141 (20.8%) |
| *Self-pay | 56 (8.3%) |
N, number of patients; SD, standard deviation; ASA, American Society of Anesthesiology; BMI, body mass index; COPD, chronic obstructive pulmonary disease; NSQIP, National Quality Surgical Improvement Program; Weight Loss, weight loss >10% in previous 6 months.
Hospital received <1% of charges from 52 patients with the self-pay insurance status
Table 4.
Patient Variables for 30-day Readmissions from Date of Surgery and Date of Discharge
| All patients N (%) |
Patients with a 30-day readmission |
||||
|---|---|---|---|---|---|
| Date of surgery N (%) |
P value | Date of discharge N (%) |
P value | ||
| 677 (100%) | 130 (19.2%) | 155 (22.9%) | |||
| Proxy SES | |||||
| ADI | 0.04 | 0.07 | |||
| Low (1–3) | 125 (18.5%) | 14 (11.2%) | 19 (15.2%) | ||
| Medium (4–6) | 189 (27.9%) | 40 (21.2%) | 46 (24.3%) | ||
| High (7–10) | 363 (53.6%) | 76 (20.9%) | 90 (24.8%) | ||
| DCI (2-Category) | 0.09 | 0.1 | |||
| Low Distress (DCI ≤75) | 416 (61.4%) | 71 (17.1%) | 86 (20.7%) | ||
| High Distress (DCI >75) | 261 (38.6%) | 59 (22.6%) | 69 (26.4%) | ||
| DCI (3-Category) | 0.07 | 0.13 | |||
| Pros/Comf (0–39) | 190 (28.1%) | 26 (13.7%) | 34 (17.9%) | ||
| Mid-Tier (40–59) | 73 (10.8%) | 17 (23.3%) | 20 (27.4%) | ||
| Risk/Dis (60–100) | 414 (61.1%) | 87 (21.0%) | 101 (24.4%) | ||
| Insurance Status | 0.08 | 0.1 | |||
| Medicare/Private | 272 (40.2%) | 43 (15.8%) | 53 (19.5%) | ||
| Medicaid/Dual/Carelink/Self-pay | 405 (59.8%) | 87 (21.5%) | 102 (25.2%) | ||
| Preoperative Variables | |||||
| Age | 0.36 | 0.2 | |||
| 18–44 | 133 (19.6%) | 25 (18.8%) | 33 (24.8%) | ||
| 45–64 | 400 (59.1%) | 83 (20.8%) | 97 (24.3%) | ||
| 65+ | 144 (21.3%) | 22 (15.3%) | 25 (17.4%) | ||
| Sex | 0.91 | 0.86 | |||
| Male | 365 (53.9%) | 69 (18.9%) | 85 (23.3%) | ||
| Female | 312 (46.1%) | 61 (19.6%) | 70 (22.4% | ||
| Hispanic Ethnicity | 0.36 | 0.62 | |||
| Yes | 432 (63.8%) | 88 (20.4%) | 102 (23.6%) | ||
| No | 245 (36.2%) | 42 (17.1%) | 53 (21.6%) | ||
| RAI-A (frailty) | 0.97 | 0.07 | |||
| Robust (1–20) | 415 (61.3%) | 79 (19.0%) | 88 (21.2%) | ||
| Normal (21–29) | 183 (27.0%) | 35 (19.1%) | 41 (22.4%) | ||
| Frail (30–50) | 79 (11.7%) | 16 (20.3%) | 26 (32.9%) | ||
| Operative Variables | |||||
| Case Priority | 0.78 | 0.24 | |||
| Elective | 397 (58.6%) | 73 (18.4%) | 82 (20.7%) | ||
| Urgent | 215 (31.2%) | 43 (20.0%) | 55 (25.6%) | ||
| Emergency | 65 (9.6%) | 14 (21.5%) | 18 (27.7%) | ||
| Stoma | 0.003 | <0.001 | |||
| Yes | 289 (42.7 %) | 71 (24.6%)* | 99 (34.3%)+ | ||
| Postoperative Variables | |||||
| Clavien-Dindo IV Complications | <0.001 | <0.001 | |||
| Yes | 111 (16.4%) | 41 (36.9%) | 50 (45.0%) | ||
| No | 566 (83.6%) | 89 (15.7%) | 105 (18.6%) | ||
N, number of patients; % percent of patients readmitted within each variable category; SES, Socioeconomic Status; ADI, Area Deprivation Index; DCI, Distressed Communities Index; RAI-A, Risk Analysis Index; P values for testing association between readmission with each factor based on chi-square test
48 (67.6%) and
66 (66.7%) small bowel stomas.
Table 5.
Distribution of Clavien-Dindo Level IV Complications by 30-Day Readmissions
| All patients N (%) |
Patients with a 30-day readmission |
||
|---|---|---|---|
| Date of surgery N (%) |
Date of Discharge N (%) |
||
| 677 (100%) | 130 (19.2%) | 155 (22.9%) | |
| Number of Complications/Patient | |||
| 1 | 59 (53.2%) | 25 (61.0%) | 30 (60.0%) |
| 2 | 27 (24.3%) | 9 (21.9%) | 9 (18.0%) |
| 3 | 15 (13.5%) | 4 (9.8%) | 5 (10.0%) |
| 4 | 5 (4.5%) | 0 (0.0%) | 2 (4.0%) |
| 5 | 2 (1.8%) | 0 (0.0%) | 1 (2.0%) |
| 6 | 3 (2.7%) | 3 (7.3%) | 3 (6.0%) |
| Total Patients | 111 (100%) | 41 (100%) | 50 (100%) |
| Distribution of Complications | |||
| Unplanned Intubation | 15 (7.3%) | 6 (8.2%) | 6 (6.4%) |
| Pulmonary Embolism | 8 (3.9%) | 4 (5.5%) | 4 (4.3%) |
| On Ventilator >48 hrs. | 49 (23.8%) | 9 (12.3%) | 12 (12.8%) |
| Progressive Renal Insufficiency | 11 (5.3%) | 8 (11.0%) | 7 (7.4%) |
| Acute Renal Failure | 4 (1.9%) | 2 (2.7%) | 3 (3.2%) |
| Stroke/Cerebral Vascular Accident | 2 (1.0%) | 1 (1.4%) | 2 (2.1%) |
| Cardiac Arrest Requiring CPR | 4 (1.9%) | 2 (2.7%) | 2 (2.1%) |
| Myocardial Infarction | 9 (4.4%) | 2 (2.7%) | 3 (3.2%) |
| Septic Shock | 28 (13.6%) | 5 (6.8%) | 10 (10.6%) |
| Unplanned Reoperation 1 | 60 (29.1%) | 26 (35.6%) | 33 (35.1%) |
| Unplanned Reoperation 2 | 11 (5.3%) | 5 (6.8%) | 8 (8.5%) |
| More than 2 Unplanned Reoperations | 5 (2.4%) | 3 (4.1%) | 4 (4.3%) |
| Total Number of Complications | 206 (100%) | 73 (100%) | 94 (100%) |
N, number of patients; 111 patients experienced 206 Clavien-Dindo Level IV complications, % percents within each column based upon numbers of patients or complications, respectively.
Table 6.
Characteristics of Patients and Outcomes by Open and Laparoscopic Surgical Approach
| All patients N (%) |
Type of Surgery |
|||
|---|---|---|---|---|
| Open Surgery N (%) |
Laparoscopic N (%) |
P value | ||
| 674 (100%)+ | 418 (62.0%) | 256 (38.0%) | ||
| 30-Day Readmissions | ||||
| Date of Surgery | 0.05 | |||
| Yes | 129 (19.1%) | 90 (69.8%) | 39 (30.2%) | |
| No | 545 (80.9%) | 328 (60.2%) | 217 (39.8%) | |
| Date of Discharge | <0.001 | |||
| Yes | 154 (22.8%) | 115 (74.7%) | 39 (25.3%) | |
| No | 520 (77.2%) | 303 (58.3%) | 217 (41.7%) | |
| Proxy SES | ||||
| ADI | 0.79 | |||
| Low (1–3) | 125 (18.5%) | 76 (60.8%) | 49 (39.2%) | |
| Medium (4–6) | 189 (28.1%) | 121 (64.0%) | 68 (36.0%) | |
| High (7–10) | 360 (53.4%) | 221 (61.4%) | 139 (38.6%) | |
| DCI (2-Category) | 0.65 | |||
| Low Distress (DCI ≤75) | 414 (61.4%) | 260 (62.8%) | 154 (37.2%) | |
| High Distress (DCI >75) | 260 (38.6%) | 158 (60.8%) | 102 (39.2%) | |
| DCI (3-Category) | 0.92 | |||
| Pros/Comf (0–39) | 190 (28.2%) | 120 (63.2%) | 70 (36.8%) | |
| Mid-Tier (40–59) | 72 (10.7%) | 45 (62.5%) | 27 (37.5%) | |
| Risk/Dis (60–100) | 412 (61.1%) | 253 (61.4%) | 159 (38.6%) | |
| Insurance Status | 0.02 | |||
| Medicare/Private | 271 (40.2%) | 153 (56.5%) | 118 (43.5%) | |
| Medicaid/Dual/Carelink/Self-pay* | 403 (59.8%) | 265 (65.8%) | 138 (34.2%) | |
| Operative Variables | ||||
| Case Priority | <0.001 | |||
| Elective | 395 (58.6%) | 197 (49.9%) | 198 (50.1%) | |
| Urgent | 214 (31.8%) | 160 (74.8%) | 54 (25.2%) | |
| Emergency | 65 (9.6%) | 61 (93.8%) | 4 (6.2%) | |
| Length of Stay | <0.001 | |||
| 1–3 Days | 32 (4.7%) | 6 (18.7%) | 26 (81.3%) | |
| 4–7 Days | 306 (45.4%) | 146 (47.7%) | 160 (52.3%) | |
| 8–14 Days | 203 (30.1%) | 154 (75.9%) | 49 (24.1%) | |
| 15–30 Days | 98 (14.5%) | 80 (81.6%) | 18 (18.4%) | |
| 31+ Days | 35 (5.2%) | 32 (91.4%) | 3 (8.6%) | |
| Postoperative Variables | ||||
| Clavien-Dindo IV Complications | <0.001 | |||
| Yes | 110 (16.3%) | 85 (77.3%) | 25 (22.7%) | |
| No | 564 (83.7%) | 333 (59.0%) | 231 (41.0%) | |
N, number of patients; % percent of patients within each variable category; SES, Socioeconomic Status; ADI, Area Deprivation Index; DCI, Distressed Communities Index; P values for testing association between surgery type with each factor based on chi-square test.
Three patients with perineal only surgical approach were excluded from this analysis
Hospital received <1% of charges from 52 patients with the self-pay insurance status
Unadjusted effects of proxy socioeconomic, preoperative, operative and postoperative factors on 30-day readmissions
Surgical 30-day readmissions were defined as 1) from the date of surgery (19.2%) using the NSQIP variable and 2) from the date of hospital discharge of the index procedure (22.9%) using the CMS definition. Only ADI was significantly associated (p = 0.04) with 30-day readmissions from the date of surgery and marginally significant from date of discharge while DCI and insurance status had a marginally significant effect on 30-day readmissions. Patients readmitted within 30 days from the date of discharge were more likely to be frail, 21.2%, 22.4% and 32.9% for robust, normal and frail, respectively. Stomas were present in 42.7% of patients and were associated with increased readmissions. Of the 155 patients readmitted 30 days after the date of discharge, 99 had stomas with small bowel stomas (66) predominating. Seventeen patients with small bowel stomas were readmitted due to high ostomy output and dehydration; four of these patients required takedown of their loop ileostomies. Patients with a Clavien-Dindo Level IV life-threatening complication were more likely to be readmitted 30 days after date of surgery (36.9%) and date of discharge (45.0%) (Table 4).
Unadjusted, bivariate logistic regression models (Table 7) demonstrated that ADI was significantly associated with increased risk of 30-day readmissions. Patients from medium and high deprived areas were more likely of being readmitted within 30 days from surgery (OR = 2.13, p = 0.02, OR = 2.10, p = 0.02, respectively), or within 30 days from discharge (OR = 1.79, p = 0.05, OR = 1.84, p = 0.03 respectively) compared to patients from the least deprived (affluent) areas. The 3-category DCI (Table 7) was only significantly associated with increased 30-day readmission risk from the date of surgery for the At risk/Distressed group (OR = 1.68, p = 0.03); otherwise, 2-category DCI and insurance status were not significantly associated with readmission risk.
Table 7.
Bivariate Logistic Regression Models for 30-day Readmissions
| Date of surgery |
Date of discharge |
|||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| ADI (Ref = Low) | ||||
| Medium (4–6) | 2.13 (1.13 – 4.23) | 0.02 | 1.79 (1.01 – 3.3) | 0.05 |
| High (7–10) | 2.10 (1.17 – 4.01) | 0.02 | 1.84 (1.08 – 3.24) | 0.03 |
| DCI (Ref = Pros/Comf 0–39) | ||||
| Mid-Tier (40–59) | 1.91 (0.96 – 3.77) | 0.06 | 1.73 (0.91 – 3.25) | 0.09 |
| Risk/Dis (60–100) | 1.68 (1.06 – 2.75) | 0.03 | 1.48 (0.97 – 2.31) | 0.08 |
| DCI (Ref = Low Distress (DCI ≤75) | ||||
| High Distress (DCI >75) | 1.42 (0.96 – 2.09) | 0.07 | 1.37 (0.96 – 1.98) | 0.08 |
| Insurance Status (Ref = Medicare/Private) | ||||
| Medicaid/Dual/Carelink/Self-pay | 1.46 (0.98 – 2.19) | 0.07 | 1.39 (0.96 – 2.03) | 0.08 |
| Age (Ref = 18–44) | ||||
| 45–64 | 1.13 (0.69 – 1.89) | 0.63 | 0.97 (0.62 – 1.54) | 0.89 |
| 65+ | 0.78 (0.41 – 1.46) | 0.44 | 0.64 (0.35 – 1.14) | 0.13 |
| Sex (Ref = Female) | ||||
| Male | 0.96 (0.65 – 1.41) | 0.83 | 1.05 (0.73 – 1.51) | 0.79 |
| Hispanic Ethnicity (Ref = No) | ||||
| Yes | 1.24 (0.83 – 1.87) | 0.31 | 1.12 (0.77 – 1.64) | 0.56 |
| RAI-A (Ref = Robust) | ||||
| Normal (21–29) | 1.01 (0.64 – 1.56) | 0.98 | 1.07 (0.70 – 1.62) | 0.74 |
| Frail (30–50) | 1.08 (0.58 – 1.93) | 0.80 | 1.82 (1.07 – 3.06) | 0.02 |
| Case Priority (Ref = Elective) | ||||
| Urgent | 1.11 (0.72 – 1.68) | 0.63 | 1.32 (0.89 – 1.95) | 0.16 |
| Emergency | 1.22 (0.62 – 2.27) | 0.55 | 1.47 (0.79 – 2.63) | 0.2 |
| Stoma (Ref = No) | ||||
| Yes | 1.82 (1.24 – 2.68) | 0.002 | 3.09 (2.13 – 4.51) | <0.001 |
| Clavien-Dindo IV Complications (Ref = No) | ||||
| Yes | 3.14 (1.99 – 4.89) | <0.001 | 3.60 (2.33 – 5.53) | <0.001 |
CI, confidence interval; OR, odds ratio; Ref, reference; ADI, Area Deprivation Index; DCI, Distressed Communities Index; RAI-A, Risk Analysis Index
Frailty, measured by the RAI-A, was associated with an increased risk of 30-day readmissions from the date of discharge (OR = 1.82, p = 0.02). Age, sex, Hispanic ethnicity and case priority were not significant factors in our cohort (Table 7) and were not included in subsequent models. In addition, age and sex are variables included in the RAI-A. Patients with a stoma were more likely to be readmitted 30 days from surgery (OR = 1.82, p = 0.002) and after the date of discharge (OR = 3.09, p = < 0.001). Finally, the presence of at least one Clavien-Dindo Level IV complication was strongly associated with 30-day readmissions from date of surgery and date of discharge (OR = 3.14, p < 0.001, OR = 3.60, p = < 0.001, respectively).
Adjusted effects of proxy socioeconomic, preoperative, operative and postoperative factors on 30-day readmissions from date of surgery and date of discharge
ADI in logistic regression models (Table 8) indicated that the risk of being readmitted within 30 days from surgery increased among patients living in medium (OR = 2.49, p = 0.008) or high (OR = 2.18, p = 0.01) deprived areas, compared to patients from the least deprived (affluent) neighborhoods. Similar effects were observed for the risk of being readmitted within 30 days from discharge among patients living in medium (OR = 2.15, p = 0.02) or high (OR = 1.88, p = 0.03) deprived areas compared to patients from the least deprived neighborhoods.
Table 8.
Multivariate Logistic Regression Models for 30-day Readmissions by ADI, 2-Category DCI, 3-Category DCI, and Insurance Status
| Date of surgery |
Date of discharge |
|||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| ADI | ||||
| ADI (Ref = Low) | ||||
| Medium (4–6) | 2.49 (1.29 – 5.06) | 0.008 | 2.15 (1.17 – 4.09) | 0.02 |
| High (7–10) | 2.18 (1.20 – 4.23) | 0.01 | 1.88 (1.08 – 3.40) | 0.03 |
| RAI-A (Ref = Robust) | ||||
| Normal (21–29) | 0.97 (0.61 – 1.53) | 0.90 | 1.01 (0.64 – 1.56) | 0.98 |
| Frail (30–50) | 0.86 (0.45 – 1.60) | 0.65 | 1.29 (0.73 – 2.24) | 0.38 |
| Stoma (Ref = No) | ||||
| Yes | 1.43 (0.93 – 2.18) | 0.09 | 2.39 (1.60 – 3.58) | <0.001 |
| Clavien-Dindo IV Complications (Ref = No) | ||||
| Yes | 2.94 (1.81 – 4.76) | <0.001 | 2.81 (1.77 – 4.45) | <0.001 |
| 2-Category DCI | ||||
| DCI (Ref = Low Distress (DCI ≤75) | ||||
| High Distress (DCI >75) | 1.34 (0.90 – 1.99) | 0.15 | 1.28 (0.87 – 1.87) | 0.21 |
| RAI-A (Ref = Robust) | ||||
| Normal (21–29) | 0.91 (0.57 – 1.42) | 0.67 | 0.95 (0.60 – 1.46) | 0.81 |
| Frail (30–50) | 0.85 (0.44 – 1.57) | 0.62 | 1.28 (0.72 – 2.22) | 0.39 |
| Stoma (Ref = No) | ||||
| Yes | 1.43 (0.94 – 2.18) | 0.09 | 2.39 (1.60 – 3.58) | <0.001 |
| Clavien-Dindo IV Complications (Ref = No) | ||||
| Yes | 2.72 (1.68 – 4.36) | <0.001 | 2.61 (1.65 – 4.11) | <0.001 |
| 3-Category DCI | ||||
| DCI (Ref = Pros/Comf) | ||||
| Mid-Tier (40–59) | 1.72 (0.84 – 3.44) | 0.13 | 1.60 (0.81 – 3.09) | 0.17 |
| Risk/Dis (60–100) | 1.62 (1.01 – 2.67) | 0.05 | 1.38 (0.89 – 2.19) | 0.16 |
| RAI-A (Ref = Robust) | ||||
| Normal (21–29) | 0.90 (0.57 – 1.42) | 0.67 | 0.95 (0.60 – 1.46) | 0.81 |
| Frail (30–50) | 0.85 (0.44 – 1.57) | 0.62 | 1.29 (0.73 – 2.24) | 0.38 |
| Stoma (Ref = No) | ||||
| Yes | 1.43 (0.94 – 2.18) | 0.09 | 2.39 (1.60 – 3.58) | <0.001 |
| Clavien-Dindo IV Complications (Ref = No) | ||||
| Yes | 2.71 (1.68 – 4.36) | <0.001 | 2.60 (1.65 – 4.10) | <0.001 |
| Insurance Status | ||||
| Insurance Status (Ref = Medicare/Private) | ||||
| Medicaid/Dual/Carelink/Self-pay | 1.44 (0.95 – 2.20) | 0.09 | 1.37 (0.92 – 2.05) | 0.12 |
| RAI-A (Ref = Robust) | ||||
| Normal (21–29) | 0.98 (0.61 – 1.55) | 0.94 | 1.02 (0.65 – 1.59) | 0.94 |
| Frail (30–50) | 0.93 (0.48 – 1.71) | 0.81 | 1.37 (0.77 – 2.39) | 0.28 |
| Stoma (Ref = No) | ||||
| Yes | 1.40 (0.91 – 2.13) | 0.12 | 2.34 (1.57 – 3.50) | <0.001 |
| Clavien-Dindo IV Complications (Ref = No) | ||||
| Yes | 2.79 (1.73 – 4.48) | <0.001 | 2.67 (1.69 – 4.21) | <0.001 |
ADI, Area Deprivation Index; CI, confidence interval; OR, odds ratio; Ref, reference; DCI, Distressed Communities Index
In contrast, DCI was only significantly associated with increased readmission risk at 30 days from surgery for the At risk/Distressed group (OR = 1.62, p = 0.05) but not at 30 days from discharge or using the 2-category variable (Table 8) and neither was insurance status (Table 8).
Significant covariates in all 4 models (Table 8) included increased 30-day readmission risk from the date of discharge among patients with a stoma (p ≤ 0.001) and the presence of a Clavien-Dindo Level IV complication increased risk of being readmitted for both 30 days from date of surgery and discharge (p ≤ 0.001) compared to patients without Clavien-Dindo Level IV complications. Results were similar when the 3 patients that died within 30 days of the date of discharge without being readmitted were included in the analysis. Patients identified as living in the most deprived neighborhoods by the ADI were more likely to be frail and experience a Clavien-Dindo Level IV complication (Table 9).
Table 9.
Distribution of Patient Variables by ADI
| All patients N (%) |
ADI |
||||
|---|---|---|---|---|---|
| Low (1–3) |
Medium (4–6) |
High (7–10) |
P value | ||
| 677 (100%) | 125 (18.5%) | 189 (27.9%) | 363 (53.6%) | ||
| Insurance Status | <0.001 | ||||
| Medicare/Private | 272 (40.2%) | 75 (27.6%) | 87 (32.0%) | 110 (40.4%) | |
| Medicaid/Dual/Carelink/Self-pay | 405 (59.8%) | 50 (12.3%) | 102 (25.2%) | 253 (62.5%) | |
| Age | 0.27 | ||||
| 18–44 | 133 (19.6%) | 20 (15.0%) | 42 (31.6%) | 71 (53.4%) | |
| 45–64 | 400 (59.1%) | 71 (17.8%) | 106 (26.5%) | 223 (55.8%) | |
| 65+ | 144 (21.3%) | 34 (23.6) | 41 (28.5%) | 69 (47.9%) | |
| Sex | 0.46 | ||||
| Male | 365 (53.9%) | 73 (20.0%) | 97 (26.6%) | 195 (53.4%) | |
| Female | 312 (46.1%) | 52 (16.7%) | 92 (29.5%) | 168 (53.8%) | |
| Hispanic Ethnicity | <0.001 | ||||
| Yes | 432 (63.8%) | 49 (11.3%) | 105 (24.3%) | 278 (64.4%) | |
| No | 245 (36.2%) | 76 (31.0%) | 84 (34.3%) | 85 (34.7%) | |
| RAI-A | 0.02 | ||||
| Robust (1–20) | 415 (61.3%) | 69 (16.6%) | 128 (30.8%) | 218 (52.5%) | |
| Normal (21–29) | 183 (27.0%) | 46 (25.1%) | 42 (23.0%) | 95 (51.9%) | |
| Frail (30–50) | 79 (11.7%) | 10 (12.6%) | 19 (24.0%) | 50 (63.3%) | |
| Case Priority | 0.12 | ||||
| Elective | 397 (58.6%) | 71 (17.9%) | 121 (30.5%) | 205 (51.6%) | |
| Urgent | 215 (31.2%) | 36 (16.7%) | 53 (24.7%) | 126 (58.6%) | |
| Emergency | 65 (9.6%) | 18 (27.7%) | 15 (23.1%) | 32 (49.2%) | |
| Stoma | 0.45 | ||||
| Yes | 289 (42.7%) | 51 (17.6%) | 75 (26.0%) | 163 (56.4%) | |
| No | 388 (57.3%) | 74 (19.1%) | 114 (29.4%) | 200 (51.5%) | |
| Clavien-Dindo IV Complications | 0.04 | ||||
| Yes | 111 (16.4%) | 24 (21.6%) | 20 (18.0%) | 67 (60.4%) | |
| No | 566 (83.6%) | 101 (17.8%) | 169 (29.9%) | 296 (52.3%) | |
N, number of patients; % percent of patients within each variable category; ADI, Area Deprivation Index; RAI-A, Risk Analysis Index
Discussion
Our study demonstrates an association of proxy social risk factors with increased risk of 30-day readmissions using the ADI, a block group level measure of neighborhood deprivation. We examined three measures used to identify low SES patients, ADI, DCI and insurance status. Only ADI was associated with higher 30-day readmission rates from both the dates of surgery and discharge in low SES patients (Table 8). Our study is the first to apply the ADI to outcomes in patients undergoing colorectal procedures. We identified only one other study using ADI in surgical outcomes. ADI was used to identify low SES patients after curative resection in pancreatic adenocarcinoma [37]. The study retrospectively analyzed 289 patients from 2008–2015 with 24.2% of patients living in the most deprived neighborhoods (ADI 7–10) found no association with neighborhood deprivation and Clavien-Dindo Level III & IV complications, initiating or completing adjuvant chemotherapy, and survival. The authors concluded that treatment in a high-volume cancer center with standardized clinical pathways may partially address SES disparities. Additional factors potentially explaining their negative results include the median overall patient survival of 27.6 months; the clinical course of pancreatic adenocarcinoma may be the dominant factor in outcomes in contrast to most colorectal surgeries.
Previous studies with surgery patients demonstrated that the DCI was independently predictive of major complications with an odds ratio of 1.1 per quartile increase in DCI after risk-adjustment for clinical and demographic factors [29]; these outcomes were similar when the DCI was added to a regional quality improvement group using NSQIP data [28]. We used two classifications of DCI scores with differing definitions of the highest distress neighborhoods; 2-category with >75, as previously described [27–29], and 3-category combining the At risk and Distressed categories (≥60). Only the 3-category DCI was significantly associated with increased 30-day from date of surgery readmission risk, but not from date of discharge (Table 8). The greater sensitivity of the ADI may be due to the increased granularity provided by the smaller block group geography compared to DCI at the zip code level. The 2-category DCI classified only 38.6% versus > 50% of our patients as living in the highest distressed neighborhoods compared to the other measures, and therefore, may have failed to include many low SES patients treated at our SNH. In addition, prior studies using DCI for surgical outcomes [27–29] had sample sizes ranging from 2,578 – 44,451 patients, much larger than the 677 patients in the current study and larger than the sample size at most institutions. The DCI may be useful at predicting population-level risk, but not sensitive enough to identify risk at the institutional or patient levels. Predictive models from large regional or national data may have limited accuracy at a single institution due to data aggregation from many populations, institutions and regions. Multiple studies show that national data used to develop predictive models for the NSQIP risk calculator may not accurately predict risk at an institutional level [39–43]. Thus, local context in regards to patient and provider characteristics is important in risk modeling and methods to predict risk in smaller sample sizes present at an institutional level are necessary.
Insurance status, another common method to estimate patient-level SES [10], was not predictive of hospital readmissions (Table 8). Interestingly, the distribution of insurance status across the ADI categories showed that 40% of patients with Medicare or private insurance lived in the most deprived neighborhoods (Table 9) suggesting that these patients were possibly elderly and poor, or were members of the working poor. Conversely, 12% of the patients with an insurance status indicative of low SES (Medicaid, Self-pay, etc) had home addresses in the most prosperous neighborhoods (Table 9). These inconsistencies highlight some of the challenges with estimating patient-level SES and additional factors, such as living alone [44] or lack of social support that are important in hospital readmissions.
Multiple factors may contribute to observed disparities of patients residing in highly deprived areas including access to medical care [45]. Our patients had access to inpatient and outpatient enterostomal therapists, however, we cannot rule out that patients lacking insurance may have utilized these resources less than insured patients contributing to increased readmissions. While uninsured and Medicaid patients have an increased risk of postoperative hospital readmissions, [46, 47], these studies used large state or national administrative datasets and may not provide useful information at a single health system level, as demonstrated by the results from the current study. Further, it is possible that inadequate public transportation, travel times and travel distance to the nearest clinic, were significant obstacles to attending clinic appointments and obtaining care among patients from more deprived areas [48, 49]. Finally, our results show that urgent and/or emergency surgeries were more common in patients from more deprived areas, possibly contributing to the increased risk of 30-day readmission among low SES patients [50]. All of these factors may affect recovery after surgery and significantly increase readmission risks.
Consistent with previous publications [4, 20, 27–29], patients in our study who were frail, suffered Clavien-Dindo Level IV complications (Table 9) and had Charlson Comorbidity Index scores of ≥ 3 were more likely to live in the most deprived neighborhoods. Our cohort containing 63.8% Hispanic and 89.7% White patients is consistent with the demographics of San Antonio and South Texas having a predominately White and majority Hispanic population [51]. Similar to a prior study [8], Hispanic ethnicity was not associated with increased risk of hospital readmissions. There are multiple possibilities [52] to explain these findings. The mean age of San Antonio residents is 33.7 years old, younger than the national average of 38.2 years old and 13.7% of San Antonio residents were born in a country other than the United States. Interestingly, the distribution of the non-Hispanic population was roughly equivalent across the three ADI categories while 64.4% and 11.3% of Hispanic patients lived in the most and least deprived neighborhoods, respectively (Table 9). Thus, the increased poverty rate among Hispanic patients may be countered by younger age, family social structure and the heterogeneity of outcomes among multiple generations of the Hispanic population [51].
A challenge in comparing studies on readmission risk is accounting for patients that died during the index hospitalization and the variability in defining 30-day readmissions [53]. We excluded patients expiring during the index procedure hospitalization as these patients have no risk of hospital readmission [53]. Our study had 6 patients that expired within 30 days from the date of discharge; 3 patients died without readmission and 3 patients expired during the readmission. Including/excluding the 3 patients that died without readmission yielded similar results. Many studies using NSQIP data define readmissions as 30 days from the date of surgery while the CMS defines readmissions as 30 days from the date of discharge from the index procedure. We used both definitions to facilitate comparison with prior studies. Readmission rates are lower using 30 days from the date of surgery as the length of the hospital stay affects the time period of readmission risk. Interestingly, similar to prior reports [54, 55], the presence of a stoma was associated with increased readmission risk at 30 days from the date of discharge, but not from the date of surgery (Table 8) suggesting that variables associated with increased readmission risk may vary with the definition of readmissions.
Limitations of the study include data derived from a single institution with sample size that may bias subgroup analyses. Multiple factors can influence surgical readmissions [56–59]. The 89.7% of White patients in the cohort limits analyses based upon race. Using CPT codes to assess the presence of a stoma can misclassify patients if the patient has a pre-existing stoma at the time of the index hospitalizations or if CPT codes were not appropriately used. ADI and DCI are estimates of patient-level SES and misclassifications can occur for multiple reasons. Our 30-day readmissions may have not captured patients readmitted to outside health care systems and factors that may have influenced readmissions, such as access to home health care visits, were not available. In addition, observation stays and emergency department visits were not included yet are important aspects of outcomes and patient-centered care.
Conclusions
The ADI identified patients living in deprived communities with increased readmission risk with increased sensitivity compared to the DCI and insurance status. Our results show that ADI can potentially be used 1) for risk adjustment to improve predictive models at an institutional level, 2) to identify high-risk patients and 3) to design better care pathways that improve health outcomes.
Source of Funding:
This work was supported by National Institutes of Health grants U01TR002393, UL1TR002645 and T32HL07446.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Meeting Presentation: Presented at the Academic Surgical Congress, Orlando, FL, February 2020
Conflicts of Interest: The authors have no additional conflicts of interest to disclose for this work. The views expressed are those of the authors and do not represent the views of the National Institutes of Health. The National Institutes of Health did not play any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
References
- 1.Kassin MT, Owen RM, Perez SD, Leeds I, Cox JC, Schnier K, Sadiraj V, and Sweeney JF, Risk factors for 30-day hospital readmission among general surgery patients. J Am Coll Surg, 2012. 215(3): p. 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sastow DL, White RS, Mauer E, Chen Y, Gaber-Baylis LK, and Turnbull ZA, The Disparity of Care and Outcomes for Medicaid Patients Undergoing Colectomy. J Surg Res, 2019. 235: p. 190–201. [DOI] [PubMed] [Google Scholar]
- 3.Favini N, Hockenberry JM, Gilman M, Jain S, Ong MK, Adams EK, and Becker ER, Comparative Trends in Payment Adjustments Between Safety-Net and Other Hospitals Since the Introduction of the Hospital Readmission Reduction Program and Value-Based Purchasing. JAMA, 2017. 317(15): p. 1578–1580. [DOI] [PubMed] [Google Scholar]
- 4.Shih T, Ryan AM, Gonzalez AA, and Dimick JB, Medicare’s Hospital Readmissions Reduction Program in Surgery May Disproportionately Affect Minority-serving Hospitals. Ann Surg, 2015. 261(6): p. 1027–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salerno AM, Horwitz LI, Kwon JY, Herrin J, Grady JN, Lin Z, Ross JS, and Bernheim SM, Trends in readmission rates for safety net hospitals and non-safety net hospitals in the era of the US Hospital Readmission Reduction Program: a retrospective time series analysis using Medicare administrative claims data from 2008 to 2015. BMJ Open, 2017. 7(7): p. e016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson MP, Waters TM, Kaplan CM, Cao Y, and Bazzoli GJ, Most Hospitals Received Annual Penalties For Excess Readmissions, But Some Fared Better Than Others. Health Aff (Millwood), 2017. 36(5): p. 893–901. [DOI] [PubMed] [Google Scholar]
- 7.Glance LG, Kellermann AL, Osler TM, Li Y, Li W, and Dick AW, Impact of Risk Adjustment for Socioeconomic Status on Risk-adjusted Surgical Readmission Rates. Ann Surg, 2016. 263(4): p. 698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park HS, White RS, Ma X, Lui B, and Pryor KO, Social determinants of health and their impact on postcolectomy surgery readmissions: a multistate analysis, 2009–2014. J Comp Eff Res, 2019. 8(16): p. 1365–1379. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy CP, Vaduganathan M, Patel KV, Lalani HS, Ayers C, Bhatt DL, Januzzi JL Jr., de Lemos JA, Yancy C, Fonarow GC, and Pandey A, Association of the New Peer Group-Stratified Method With the Reclassification of Penalty Status in the Hospital Readmission Reduction Program. JAMA Netw Open, 2019. 2(4): p. e192987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shavers VL, Measurement of socioeconomic status in health disparities research. J Natl Med Assoc, 2007. 99(9): p. 1013–1023. [PMC free article] [PubMed] [Google Scholar]
- 11.Bernheim SM, Parzynski CS, Horwitz L, Lin Z, Araas MJ, Ross JS, Drye EE, Suter LG, Normand SL, and Krumholz HM, Accounting For Patients’ Socioeconomic Status Does Not Change Hospital Readmission Rates. Health Aff (Millwood), 2016. 35(8): p. 1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martsolf GR, Barrett ML, Weiss AJ, Kandrack R, Washington R, Steiner CA, Mehrotra A, SooHoo NF, and Coffey R, Impact of Race/Ethnicity and Socioeconomic Status on Risk-Adjusted Hospital Readmission Rates Following Hip and Knee Arthroplasty. J Bone Joint Surg Am, 2016. 98(16): p. 1385–1391. [DOI] [PubMed] [Google Scholar]
- 13.Keyhani S, Myers LJ, Cheng E, Hebert P, Williams LS, and Bravata DM, Effect of clinical and social risk factors on hospital profiling for stroke readmission: a cohort study. Ann Intern Med, 2014. 161(11): p. 775–784. [DOI] [PubMed] [Google Scholar]
- 14.Blum AB, Egorova NN, Sosunov EA, Gelijns AC, DuPree E, Moskowitz AJ, Federman AD, Ascheim DD, and Keyhani S, Impact of socioeconomic status measures on hospital profiling in New York City. Circ Cardiovasc Qual Outcomes, 2014. 7(3): p. 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eapen ZJ, McCoy LA, Fonarow GC, Yancy CW, Miranda ML, Peterson ED, Califf RM, and Hernandez AF, Utility of socioeconomic status in predicting 30-day outcomes after heart failure hospitalization. Circ Heart Fail, 2015. 8(3): p. 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NASEM, Accounting for Social Risk Factors in Medicare Payment, available at https://www.nap.edu/download/23635, accessed 1/28/2020., Steinwachs D, Straton K, Kwan LY Editor. 2017. [PubMed]
- 17.Samson LW, Finegold K, Ahmed A, Jensen M, Filice CE, and Joynt KE, Examining Measures of Income and Poverty in Medicare Administrative Data. Med Care, 2017. 55(12): p. e158–e163. [DOI] [PubMed] [Google Scholar]
- 18.Kind AJH and Buckingham WR, Making Neighborhood-Disadvantage Metrics Accessible - The Neighborhood Atlas. N Engl J Med, 2018. 378(26): p. 2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Census Bureau, US Census Bureau: American Community Survey design and methodology,available at https://www2.census.gov/programs-surveys/acs/methodology/design_and_methodology/acs_design_methodology_report_2014.pdf 2014, Version 2, accessed 5/20/2017. 2014.
- 20.Hu J, Kind AJH, and Nerenz D, Area Deprivation Index Predicts Readmission Risk at an Urban Teaching Hospital. Am J Med Qual, 2018. 33(5): p. 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, Greenberg C, and Smith M, Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med, 2014. 161(11): p. 765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Economic Innovations Group, From great recession to great reshuffling: Charting a decade of change across American communities, findings from the 2018 Distressed Communities Index, available at https://eig.org/wp-content/uploads/2018/10/2018-DCI.pdf, accessed 10/15/2019. 2018.
- 23.American College of Surgeons, American College of Surgeons National Quality Improvement Program (ACS NSQIP), available at https://www.facs.org/quality-programs/acs-nsqip, accessed 7/5/2017. 2017.
- 24.Shiloach M, Frencher SK Jr., Steeger JE, Rowell KS, Bartzokis K, Tomeh MG, Richards KE, Ko CY, and Hall BL, Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg, 2010. 210(1): p. 6–16. [DOI] [PubMed] [Google Scholar]
- 25.Kao LS, Dimick JB, Porter GA, and Evidence-Based Reviews in Surgery G, How do administrative data compare with a clinical registry for identifying 30-day postoperative complications? J Am Coll Surg, 2014. 219(6): p. 1187–1191. [DOI] [PubMed] [Google Scholar]
- 26.Lawson EH, Louie R, Zingmond DS, Brook RH, Hall BL, Han L, Rapp M, and Ko CY, A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg, 2012. 256(6): p. 973–981. [DOI] [PubMed] [Google Scholar]
- 27.Hawkins RB, Charles EJ, Mehaffey JH, Williams CA, Robinson WP, Upchurch GR, Kern JA, Tracci MC, and Virginias Vascular G, Socioeconomic Distressed Communities Index associated with worse limb-related outcomes after infrainguinal bypass. J Vasc Surg, 2019. 70(3): p. 786–794 e782. [DOI] [PubMed] [Google Scholar]
- 28.Mehaffey JH, Hawkins RB, Charles EJ, Turrentine FE, Kaplan B, Fogel S, Harris C, Reines D, Posadas J, Ailawadi G, Hanks JB, Hallowell PT, and Jones RS, Community level socioeconomic status association with surgical outcomes and resource utilisation in a regional cohort: a prospective registry analysis. BMJ Qual Saf, 2019. [DOI] [PubMed] [Google Scholar]
- 29.Mehaffey JH, Hawkins RB, Charles EJ, Turrentine FE, Hallowell PT, Friel C, Jones RS, and Tracci MC, Socioeconomic “Distressed Communities Index” Improves Surgical Risk-adjustment. Ann Surg, 2019. [DOI] [PubMed] [Google Scholar]
- 30.Mehaffey JH, Hawkins RB, Charles EJ, Sahli ZT, Schirmer BD, and Hallowell PT, Socioeconomically Distressed Communities Associated With Long-term Mortality After Bariatric Surgery. J Surg Res, 2019. 243: p. 8–13. [DOI] [PubMed] [Google Scholar]
- 31.Mehaffey JH, Hawkins RB, Charles EJ, Thibault D, Williams ML, Brennan M, Thourani VH, Badhwar V, and Ailawadi G, Distressed communities are associated with worse outcomes after coronary artery bypass surgery. J Thorac Cardiovasc Surg, 2019. [DOI] [PubMed] [Google Scholar]
- 32.University Health System, University Health System, Bexar County, San Antonio Texas, available at https://www.universityhealthsystem.com/, accessed 04/05/2020. 2020.
- 33.Arya S, Varley P, Youk A, Borrebach JA, Perez S, Massarweh NN, Johanning JM, and Hall DE, Recalibration and External Validation of the Risk Analysis Index: A Surgical Frailty Assessment Tool. Ann Surg, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall DE, Arya S, Schmid KK, Blaser C, Carlson MA, Bailey TL, Purviance G, Bockman T, Lynch TG, and Johanning J, Development and Initial Validation of the Risk Analysis Index for Measuring Frailty in Surgical Populations. JAMA Surg, 2017. 152(2): p. 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dindo D, Demartines N, and Clavien PA, Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg, 2004. 240(2): p. 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charlson ME, Pompei P, Ales KL, and MacKenzie CR, A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis, 1987. 40(5): p. 373–383. [DOI] [PubMed] [Google Scholar]
- 37.Powers BD, Fulp W, Dhahri A, DePeralta DK, Ogami T, Rothermel L, Permuth JB, Vadaparampil ST, Kim JK, Pimiento J, Hodul PJ, Malafa MP, Anaya DA, and Fleming JB, The Impact of Socioeconomic Deprivation on Clinical Outcomes for Pancreatic Adenocarcinoma at a High-volume Cancer Center: A Retrospective Cohort Analysis. Ann Surg, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.University Health System, CareLink, available at https://www.universityhealthsystem.com/patients/support/carelink, accessed 04/05/2020. 2020.
- 39.Cologne KG, Keller DS, Liwanag L, Devaraj B, and Senagore AJ, Use of the American College of Surgeons NSQIP Surgical Risk Calculator for Laparoscopic Colectomy: how good is it and how can we improve it? J Am Coll Surg, 2015. 220(3): p. 281–286. [DOI] [PubMed] [Google Scholar]
- 40.Adegboyega TO, Borgert AJ, Lambert PJ, and Jarman BT, Applying the National Surgical Quality Improvement Program risk calculator to patients undergoing colorectal surgery: theory vs reality. Am J Surg, 2017. 213(1): p. 30–35. [DOI] [PubMed] [Google Scholar]
- 41.Samson P, Robinson CG, Bradley J, Lee A, Broderick S, Kreisel D, Krupnick AS, Patterson GA, Puri V, Meyers BF, and Crabtree T, The National Surgical Quality Improvement Program risk calculator does not adequately stratify risk for patients with clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg, 2016. 151(3): p. 697–705 e691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivard C, Nahum R, Slagle E, Duininck M, Isaksson Vogel R, and Teoh D, Evaluation of the performance of the ACS NSQIP surgical risk calculator in gynecologic oncology patients undergoing laparotomy. Gynecol Oncol, 2016. 141(2): p. 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasad KG, Nelson BG, Deig CR, Schneider AL, and Moore MG, ACS NSQIP Risk Calculator: An Accurate Predictor of Complications in Major Head and Neck Surgery? Otolaryngol Head Neck Surg, 2016. 155(5): p. 740–742. [DOI] [PubMed] [Google Scholar]
- 44.Arbaje AI, Wolff JL, Yu Q, Powe NR, Anderson GF, and Boult C, Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community-dwelling Medicare beneficiaries. Gerontologist, 2008. 48(4): p. 495–504. [DOI] [PubMed] [Google Scholar]
- 45.Dupre ME, Xu H, Granger BB, Lynch SM, Nelson A, Churchill E, Willis JM, Curtis LH, and Peterson ED, Access to routine care and risks for 30-day readmission in patients with cardiovascular disease. Am Heart J, 2018. 196: p. 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Telem DA, Yang J, Altieri M, Patterson W, Peoples B, Chen H, Talamini M, and Pryor AD, Rates and Risk Factors for Unplanned Emergency Department Utilization and Hospital Readmission Following Bariatric Surgery. Ann Surg, 2016. 263(5): p. 956–960. [DOI] [PubMed] [Google Scholar]
- 47.Strom JB, Kramer DB, Wang Y, Shen C, Wasfy JH, Landon BE, Wilker EH, and Yeh RW, Short-term rehospitalization across the spectrum of age and insurance types in the United States. PLoS One, 2017. 12(7): p. e0180767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turrentine FE, Buckley PJ, Sohn MW, and Williams MD, Travel Time Influences Readmission Risk: Geospatial Mapping of Surgical Readmissions. Am Surg, 2017. 83(6): p. 573–582. [DOI] [PubMed] [Google Scholar]
- 49.Kelley KA, Young JI, Bassale S, Herzig DO, Martindale RG, Sheppard BC, Lu KC, and Tsikitis VL, Travel distance influences readmissions in colorectal cancer patients-what the primary operative team needs to know. J Surg Res, 2018. 227: p. 220–227. [DOI] [PubMed] [Google Scholar]
- 50.Havens JM, Olufajo OA, Cooper ZR, Haider AH, Shah AA, and Salim A, Defining Rates and Risk Factors for Readmissions Following Emergency General Surgery. JAMA Surg, 2016. 151(4): p. 330–336. [DOI] [PubMed] [Google Scholar]
- 51.DataUSA, San Antonio, TX, available at https://datausa.io/profile/geo/san-antonio-tx/, accessed 2/2/2020.
- 52.Vega WA, Rodriguez MA, and Gruskin E, Health disparities in the Latino population. Epidemiol Rev, 2009. 31: p. 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zogg CK, Pawlik TM, and Haider AH, Three Common Methodological Issues in Studies of Surgical Readmission Rates: The Trouble With Readmissions. JAMA Surg, 2018. 153(12): p. 1074–1076. [DOI] [PubMed] [Google Scholar]
- 54.Fish DR, Mancuso CA, Garcia-Aguilar JE, Lee SW, Nash GM, Sonoda T, Charlson ME, and Temple LK, Readmission After Ileostomy Creation: Retrospective Review of a Common and Significant Event. Ann Surg, 2017. 265(2): p. 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bliss LA, Maguire LH, Chau Z, Yang CJ, Nagle DA, Chan AT, and Tseng JF, Readmission After Resections of the Colon and Rectum: Predictors of a Costly and Common Outcome. Dis Colon Rectum, 2015. 58(12): p. 1164–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wahl TS and Hawn MT, How to Predict 30-Day Readmission. Adv Surg, 2018. 52(1): p. 101–111. [DOI] [PubMed] [Google Scholar]
- 57.Wahl TS and Hawn MT, How Do We Prevent Readmissions After Major Surgery? Adv Surg, 2017. 51(1): p. 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hollis RH, Graham LA, Richman JS, Morris MS, Mull HJ, Wahl TS, Burns E, Copeland LA, Telford GL, Rosen AK, Itani KF, Whittle J, Wagner TH, and Hawn MT, Hospital Readmissions after Surgery: How Important Are Hospital and Specialty Factors? J Am Coll Surg, 2017. 224(4): p. 515–523. [DOI] [PubMed] [Google Scholar]
- 59.Graham LA, Wagner TH, Richman JS, Morris MS, Copeland LA, Harris AH, Itani KM, and Hawn MT, Exploring Trajectories of Health Care Utilization Before and After Surgery. J Am Coll Surg, 2019. 228(1): p. 116–128. [DOI] [PubMed] [Google Scholar]


