To the Editor:
Sympathetic overactivity, assessed via muscle sympathetic nerve activity (MSNA), is a hallmark characteristic of heart failure (HF) with reduced ejection fraction,1,2 a maladaptation that contributes to excess vasoconstriction, inappropriate increases in blood pressure (BP), and exercise intolerance.1,3 Conversely, despite initial evidence for increased risk of cardiac events in patients with HF with preserved ejection fraction (HFpEF) who exhibit cardiac sympathetic overactivity,4 few studies have directly assessed MSNA in this patient group,2,5 and none have evaluated both MSNA and BP responses to sympathoexcitatory maneuvers. Importantly, alterations (both blunted and exaggerated) in cardiovascular reactivity have been associated with poor health outcomes in several populations.6–8 Herein, we report for the first time the direct assessment of MSNA in response to isometric handgrip exercise (HG), a sympathoexcitatory maneuver, in a patient with HFpEF and a healthy control of similar age.
A 65-year-old woman (body mass index of 31.1 kg/m2, resting BP 116/71 mm Hg) was diagnosed with HFpEF (New York Heart Association functional class II, grade 1 diastolic dysfunction with elevated right ventricular systolic pressure) in 2016, with a left ventricular ejection fraction of 70%. Medical history includes pulmonary hypertension, depression/anxiety, rheumatic heart disease, and acquired hypothyroidism. The patient was on optimal pharmacotherapy, including spironolactone (25 mg), lorazepam (0.5 mg), levothyroxine (75 mcg), and aspirin (81 mg). Laboratory results revealed hemoglobin 16.0 g/dL, creatinine 1.03 mg/dL, sodium 139 mmol/L, and N-terminal pro-brain natriuretic peptide 81 pg/mL. A 70-year-old woman (body mass index of 22.2 kg/m2, resting BP 127/74 mm Hg) with no history of cardiovascular or metabolic disease was a healthy, older control. Both participants were postmenopausal and not on hormone replacement therapy. HG was performed for 2 minutes at 30% and 40% of maximal voluntary contraction, and the last minute of each exercise bout was used for analysis.
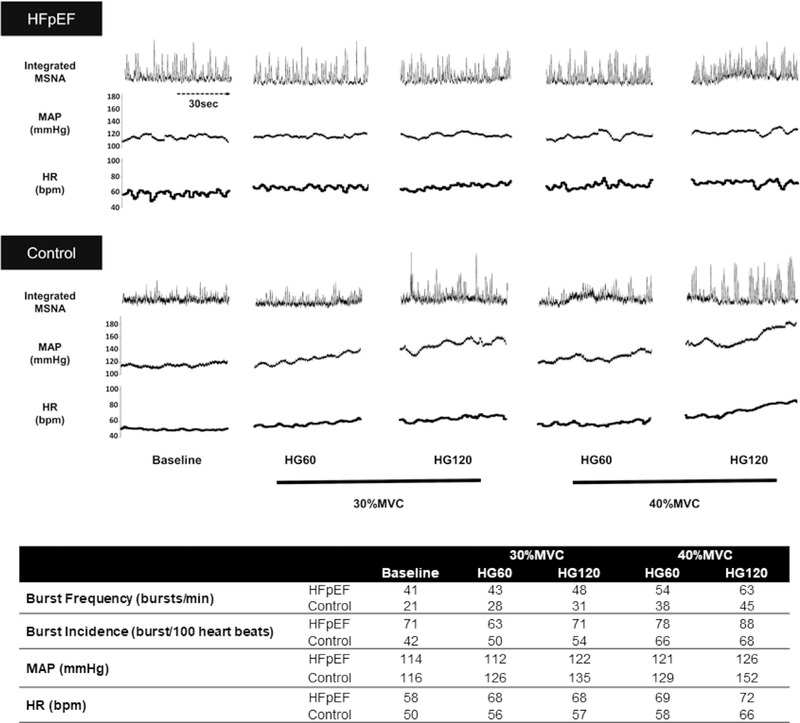
In Figure 1, the patient with HFpEF exhibited markedly increased MSNA (burst frequency and incidence), compared with the control at baseline and during both exercise intensities. MSNA increased during HG in both participants in an intensity-dependent manner, but the changes in MSNA from baseline seemed to be smaller in the patient with HFpEF than in the control, possibly owing to an overall greater sympathoexcitation at baseline in the patient with HFpEF. Mean arterial pressure was similar between participants at baseline, but increased more in the control patients than in the patient with HFpEF during HG at both intensities. The baseline HR was slightly higher in the patient with HFpEF, but HR during HG increased similarly in both participants.
Fig. 1.
(Top) Original recordings of muscle sympathetic nerve activity (MSNA), mean arterial pressure (MAP), and heart rate (HR) at baseline and at 60 (HG60) and 120 seconds (HG120) of 30% and 40% of maximal voluntary contraction (MVC) isometric handgrip exercise (HG). (Bottom) Autonomic and cardiovascular responses at baseline and during HG exercise, expressed as 1-minute averages.
We observed an approximately 2-fold greater resting MSNA in the patient with HFpEF than in the control, which is similar to a previous report in patients with HF with reduced ejection fraction (approximately 53 bursts/min, approximately 79 bursts/100 heartbeats).1 To our knowledge, this report is the first of marked sympathetic overactivity observed at rest persisting during HG in a patient with HFpEF compared with a control. Interestingly, the attenuated increase in the mean arterial pressure in response to HG in the patient with HFpEF suggests a decreased “exercise pressor” response in HFpEF that may be attributed to alterations in alpha-adrenergic receptor sensitivity and/or sympathetic neurovascular transduction, although the possibility that the expected increase in the BP was mitigated by antihypertensive medication cannot be ruled out.
Given the strong relationship between sympathetic overactivity and exercise intolerance in HF,1,3 our observation suggests that sympathetic overactivity may be an underlying mechanism of the HFpEF-related exertional symptoms. Sympathetic overexpression may contribute to excess vasoconstriction in the peripheral circulation during exercise and limit the requisite increase in skeletal muscle blood flow to meet the metabolic demand of the exercising muscle. This notion is consistent with our recent findings of dysregulated exercising blood flow in patients with HFpEF, as evidenced by a 15%–20% decreased in leg blood flow during small muscle mass exercise (knee extensor) compared with age-matched, healthy controls.9 Sustained sympathetic vasoconstriction during exercise may have possibly contributed to the impaired skeletal muscle vasodilation, although further studies are required to confirm this postulate.
This case, identifying a striking elevation in both resting and exercising MSNA in HFpEF, indicates that autonomic dysfunction may be present in this patient group and reinforces the importance of considering noncardiac complications of HFpEF pathophysiology. However, it is important to recognize that the HFpEF clinical syndrome represents a “constellation of comorbidities”10 with significant phenotypic heterogeneity, and thus additional studies are needed to confirm the presence, and explore the consequences, of sympathetic overactivity in this patient group. That said, it is tempting to speculate that a disease-related change in autonomic function may represent a new therapeutic target in the ongoing quest to successfully treat these challenging patients.
References
- 1.Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol 2001;280: H969–76. [DOI] [PubMed] [Google Scholar]
- 2.Seravalle G, Quarti-Trevano F, Dell’Oro R, et al. Sympathetic and baroreflex alterations in congestive heart failure with preserved, midrange and reduced ejection fraction. J Hypertens 2019;37:443–8. [DOI] [PubMed] [Google Scholar]
- 3.Notarius CF, Millar PJ, Murai H, et al. Divergent muscle sympathetic responses to dynamic leg exercise in heart failure and age-matched healthy subjects. J Physiol 2015;593: 715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katoh S, Shishido T, Kutsuzawa D, et al. Iodine-123-metaiodobenzylguanidine imaging can predict future cardiac events in heart failure patients with preserved ejection fraction. Ann Nuclear Med 2010;24:679–86. [DOI] [PubMed] [Google Scholar]
- 5.Tokuhisa H, Murai H, Okabe Y, et al. Differential effects of lipophilic and hydrophilic statins on muscle sympathetic nerve activity in heart failure with preserved left ventricular ejection fraction. Auton Neurosci 2018;213:8–14. [DOI] [PubMed] [Google Scholar]
- 6.Kupper N, Denollet J, Widdershoven J, et al. Cardiovascular reactivity to mental stress and mortality in patients with heart failure. JACC Heart Fail 2015;3:373–82. [DOI] [PubMed] [Google Scholar]
- 7.Simoes GM, Campagnaro BP, Tonini CL, et al. Hemodynamic reactivity to laboratory stressors in healthy subjects: influence of gender and family history of cardiovascular diseases. Int J Med Sci 2013;10:848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souza GG, Mendonca-de-Souza AC, Duarte AF, et al. Blunted cardiac reactivity to psychological stress associated with higher trait anxiety: a study in peacekeepers. BMC Neurosci 2015;16:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JF, Barrett-O’Keefe Z, Nelson AD, et al. Impaired skeletal muscle vasodilation during exercise in heart failure with preserved ejection fraction. Int J Cardiol 2016;211:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohammed SF, Borlaug BA, Roger VL, et al. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ Heart Fail 2012;5:710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]