Abstract
The emergence of methicillin-resistant Staphylococcus pseudintermedius (MRSP) antimicrobial resistance and epidemic genetic lineages is posing a challenge in veterinary medicine due to the limited therapeutical options. MRSP has been identified as an important canine pyoderma pathogen. Thus, we aimed to characterize the antimicrobial resistance and clonal lineages of MRSP isolated from canine cutaneous pyoderma. Thirty-one MRSP isolates recovered from pyoderma were further characterized. The antimicrobial susceptibility testing of the isolates was performed by the Kirby-Bauer disc diffusion method against 14 antimicrobial agents. The presence of antimicrobial and virulence genes was carried out by PCR. Multilocus sequence typing was performed in all isolates. All strains had a multidrug-resistant profile showing resistance mainly to penicillin, macrolides and lincosamides, aminoglycosides, tetracycline and trimethoprim-sulfamethoxazole, which was encoded by the blaZ, ermB, msr(A/B), aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, tetM, tetK and dfrG genes. All isolates harbored the lukS-I/lukF-I virulence factors. Isolates were ascribed to nine previously described sequence types (STs): ST123, ST339, ST727, ST71, ST537, ST45, ST1029, ST118 and ST1468; and to five STs first described in this study: ST2024, ST2025, ST2026, ST2027 and ST2028. In this study, most isolates belonged to ST123 (n = 16), which belongs to CC71 and is the most common clone in Europe. All isolates were multidrug-resistant, which may impose a serious threat to animal health.
Keywords: Staphylococcus pseudintermedius (S. pseudintermedius), MRSP, ST123, CC71, multidrug resistance
1. Introduction
The prevalence of antimicrobial resistant bacteria has been increasing over the years, and it is highly associated with the overuse and misuse of antibiotics in human and veterinary medicine, agriculture and industry [1]. Furthermore, multidrug-resistant bacteria have been increasingly reported as a cause of infections, which makes this issue a major concern in clinical practice worldwide [2]. The indiscriminative use of different antibiotics over the years has led to the emergence of multi-resistant staphylococci strains due to mutations in genes that encode target proteins, and also through the acquisition and accumulation of genes that confer resistance to antibiotics [3]. In general, staphylococci often carry resistance to antibiotics, with resistance to β-lactams such as penicillins, cephalosporins, and carbapenems, being of particular importance [4]. Staphylococci present different mechanisms of resistance to β-lactams, such as the presence of modified penicillin-binding proteins (PBP), the production of β-lactamase enzymes and the tolerance phenomena [5]. Methicillin resistant staphylococci have been recognized as a public health problem and are considered one of the antibiotic-resistant priority pathogens [6]. Staphylococcus pseudintermedius is a coagulase-positive species of staphylococci that belongs to the Staphylococcus intermedius group. S. pseudintermedius, similarly to S. aureus in humans, colonizes the skin and mucous membranes of some animal species, in particular dogs [7]. Although approximately 90% of healthy dogs are colonized by S. pseudintermedius, it is also one of the most common microorganisms causing infection in these animals, especially when the immune system of the host is compromised or if there is a breach in the skin [8,9,10]. While this pathogen is the primary cause of skin and soft tissue infections, it has also been associated with other infections such as external ear otitis, abscess formation, urinary tract infections, mastitis and endocarditis [11]. Indeed, it has been shown that up to 60% of canine cutaneous pyoderma cases are caused by S. pseudintermedius [12]. Since 2006, an increasing number of methicillin-resistant S. pseudintermedius (MRSP) has been reported in dogs [9]. Studies have reported methicillin resistance rates from 10 to 20% in diseased dogs. However, the rate of methicillin resistance was reported to increase up to 60% in strains isolated from canine pyoderma [13]. The methicillin resistance in S. pseudintermedius is conferred by the mecA gene, which encodes the penicillin-binding protein 2a (PBP2a) [14]. S. pseudintermedius shares some features with S. aureus, including some virulence factors. S. pseudintermedius produces a bicomponent leukotoxin, similar to Panton-Valentine leukocidin (PVL) from S. aureus, which is also encoded by two genes, lukS-I and lukF-I, that induce cell lysis [15]. Recent studies have shown that MRSP isolated from dogs are multidrug resistant, posing a challenge in veterinary antimicrobial therapy [16]. Several studies have identified geographical patterns of the clonal spread of S. pseudintermedius. Multilocus sequence typing has identified several dominant MRSP clones around the world, including ST71 in Europe, ST68 in the United States and ST45/ST112 in Asia [17]. Just like in the rest of Europe, in Portugal, until 2016, the predominant clone of MRSP was also ST71 [18,19]. However, the molecular epidemiology of MRSP is changing in European countries, and more recent studies are required. Thus, in this study, we aimed to characterize the antimicrobial resistance in MRSP isolated from canine pyoderma, as well as the clonal lineages.
2. Materials and Methods
2.1. Samples and Bacterial Isolates
During the period of one year (from 2019 to 2020), 31 S. pseudintermedius were isolated from dogs with cutaneous pyoderma (Figure 1). All dogs considered in this study had previously undergone antibiotic therapy to treat the infection, which was not effective. Isolates were recovered from dogs of different ages (ranging from 2 to 18 years old) and breeds (Table 1). Sterile saline-moistened swabs were used to sample the affected skin. Swabs were inoculated onto 5% Sheep Blood agar and MacConkey agar and incubated aerobically at 37 °C for 24 h. Colonies with morphology characteristic of S. pseudintermedius were recovered. The species identification and the presence of the mecA gene were confirmed by biochemical tests (Gram staining, catalase and coagulase tests) and by Polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR-RFLP) assay [20].
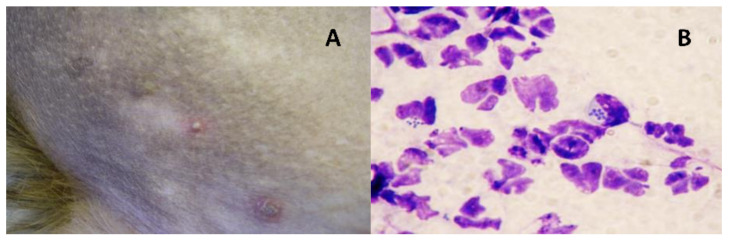
Figure 1.
(A) Example of a pustule and (B) the cytological analysis performed to confirm canine pyoderma.
Table 1.
Age, gender and breed of dogs included in study.
| Isolate | Age (Years) | Gender | Breed |
|---|---|---|---|
| VS2777 | 5 | F | Bull Terrier |
| VS2778 | 6 | M | Golden Retriever |
| VS2779 | 8 | F | German Shepherd |
| VS2780 | 2 | F | Yorkshire Terrier |
| VS2781 | 5 | M | Labrador Retriever |
| VS2782 | 6 | M | Boxer |
| VS2783 | 10 | M | Mixed breed |
| VS2784 | 4 | M | Estrela Mountain |
| VS2785 | 9 | F | Mixed breed |
| VS2786 | 7 | M | Boxer |
| VS2787 | 6 | M | Golden Retriever |
| VS2788 | 6 | M | Cocker Spaniel |
| VS2789 | 10 | M | Rafeiro do Alentejo |
| VS2790 | 6 | M | Yorkshire Terrier |
| VS2791 | 2 | M | Mixed breed |
| VS2792 | 9 | F | French Bulldog |
| VS2793 | 12 | F | Mixed breed |
| VS2794 | 5 | F | Bull Terrier |
| VS2795 | 7 | F | Cocker Spaniel |
| VS2796 | 3 | F | German Shepherd |
| VS2797 | 9 | M | Akita |
| VS2798 | 4 | F | French Bulldog |
| VS2799 | 6 | M | Spanish Water Dog |
| VS2800 | 8 | M | Labrador Retriever |
| VS2801 | 4 | F | Mixed breed |
| VS2802 | 5 | F | Belgian Shepherd |
| VS2803 | 5 | M | Beagle |
| VS2804 | 4 | F | Mixed breed |
| VS2805 | 4 | F | French Bulldog |
| VS2806 | 12 | F | Mixed breed |
| VS2807 | 18 | M | Poodle |
2.2. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing was performed by the Kirby-Bauer disk diffusion method against 14 antimicrobial agents: penicillin (1 U), cefoxitin (30 μg), ciprofloxacin (5 μg), linezolid (10 µg), gentamicin (10 μg), kanamycin (30 μg), tobramycin (10 μg), erythromycin (15 μg), clindamycin (2 μg), tetracycline (30 μg), chloramphenicol (30 μg), fusidic acid (10 μg), trimethoprim-sulfamethoxazole (1.25/23.75 μg) and mupirocin (200 μg). The interpretation was performed according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST, 2018), except for kanamycin, which followed the Clinical and Laboratory Standards Institute guidelines (CLSI, 2017). S. aureus strain ATCC 25923 was used as quality control.
2.3. Antibiotic Resistance Genes and Virulence Factors
DNA was extracted from fresh cultures as previously described [21]. Briefly, isolates were seeded onto Brain Heart Infusion agar plates and incubated at 37 °C for 18–24 h. A few colonies of each isolate were suspended in 45 µL of MiliQ-water and 5 µL of lysostaphin (1 mg/mL), and the samples were incubated at 10 min at 37 °C. Then, 45 μL of MilliQ water, 150 µL of Tris-HCl (0.1 M) and 5 μL of proteinase K (2 mg/mL) were added. The samples were then incubated at 67 °C for 10 min and then the samples were boiled for 5 min.
According to the phenotypic resistance of each isolate, the presence of 23 antibiotic resistance genes was studied by PCR using specific primers and conditions as previously described [22]: blaZ, ermA, ermB, ermC, ermT, msr(A/B), mphC, linB, vgaB, vgaC, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, tetM, tetL, tetK, tetO, dfrA, dfrD, dfrG, dfrK, fusB and fusC.
Virulence factors were determined by PCR with specific primers and conditions as previously described [22,23,24,25,26]: hla, hlb, hld, tst, eta, etb, lukS/F-PV and lukF/lukS-I.
Positive and negative controls used in all experiments belong to the strain collection of the University of Trás-os-Montes and Alto Douro.
2.4. Molecular Characterization
Multilocus-sequence-typing (MLST) was performed in all isolates as previously described [27,28]. Sequence types (STs) were assigned based on the S. pseudintermedius MLST database (http://pubmlst.org/spseudintermedius accessed on 14 September 2020.).
3. Results and Discussion
S. pseudintermedius is frequent in dogs and other companion animals and is one of the most frequent causes of cutaneous pyoderma in dogs. Furthermore, the prevalence of MRSP in canine pyoderma is moderate to high according to some studies [29,30]. In this study, samples were collected from 31 dogs with cutaneous pyoderma. All animals were client-owned dogs visiting a Veterinary Hospital in Lisbon, Portugal. Demographic data comprised 16 females and 15 males with ages ranging from 2 to 18 years old. Seven dogs were mixed breed, while the remaining ones belonged to several different breeds (Table 1). However, there seems to be no relation between gender, breed or age and antimicrobial resistance patterns or clonal lineages. In this study, only samples from dogs that had already undergone antibiotic therapy for the treatment of pyoderma but that the treatment was not effective were considered. As expected, all isolates were multidrug-resistant since they showed resistance to at least three antibiotic classes. All isolates harbored the mecA gene and were classified as MRSP. The antimicrobial resistance characteristics of the isolates are shown in Table 2. Studies have shown that S. pseudintermedius isolates are commonly resistant to penicillins, tetracyclines and macrolides, which are the most frequently used antibiotics in dogs [31]. All MRSP were phenotypically resistant to penicillin and all (except one) harbored the blaZ gene. Penicillin resistance in staphylococci is very common and is usually conferred by the staphylococcal beta-lactamase encoded by the blaZ gene [32]. Resistance to macrolides and lincosamides was detected in all isolates and was conferred by the genes ermB (n = 30), msr(A/B) (n = 1) or both (n= 7). Previous studies conducted in Europe, North America and Asia revealed that ermB is the predominant erm gene in canine S. pseudintermedius [7,29,33]. Resistance to aminoglycosides was observed in all isolates. Yet, unlike what happens in methicillin-resistant S. aureus (MRSA) isolates, as most have resistance to gentamicin, in MRSP, resistance to kanamycin seems to be more prevalent than to gentamycin. In our study, all MRSP were phenotypically resistant to kanamycin and all harbored the aph(3′)-IIIa gene. However, resistance to gentamicin and tobramycin was observed in 25 and 24 isolates, respectively, of which 20 harbored the bi-funtional enzyme aac(6′)-Ie-aph(2′′)-Ia and 11 had the ant(4′)-Ia gene. Other studies had similar results, with resistance to kanamycin conferred by the aph(3′)-IIIa gene as the most prevalent resistance to aminoglycosides in MRSP [34,35]. Resistance to tetracycline in S. pseudintermedius and MRSP is common, and is often mediated by tetM followed by tetK genes [12,36]. In agreement with this, 25 of our isolates showed resistance to tetracycline and harbored either the tetM (n = 16) or tetK (n = 9) genes. tetM codes for ribosome protective proteins, whereas tetK codes for efflux pumps [37]. Trimethoprim-sulfamethoxazole resistance was found in 29 isolates. All isolates carried only the dfrG gene, which codes for a trimethoprim-resistant dihydrofolate reductase, and this is in accordance with the results of other studies that report the dfrG gene as the most common in S. pseudintermedius isolates [7,38]. A high prevalence of dfrG has been found in both MRSP and methicillin-susceptible S. pseudintermedius (MSSP) isolates [36,39]. Previous studies have shown that the vast majority of MRSP isolates often carry a Tn5405-like transposable element that carries several antimicrobial resistance genes including ermB, aph(3′)-IIIa and dfrG, which were the most frequently detected genes in this study [39,40]. Phenotypic resistance to ciprofloxacin (n = 24), chloramphenicol (n = 6) and fusidic acid (n = 1) was also detected in our study. Resistance to linezolid and mupirocin was not detected. Regarding the virulence genes, lukF-I/lukS-I was detected in all isolates; however, none of the other virulence genes tested were detected. These results are in accordance with other studies that obtained a high prevalence of lukF-I and lukS-I genes in S. pseudintermedius canine isolates [19,41,42]. The MRSP isolates were ascribed to nine previously described STs: ST123 (n = 16), ST339 (n = 2), ST727 (n = 2), ST71, ST537, ST45, ST1029, ST118 and ST1468; and to five STs first described in this study: ST2024, ST2025, ST2026, ST2027 and ST2028 (Figure S1). The high genetic diversity is in accordance with other studies conducted in S. pseudintermedius [17,43]. ST71 (clonal complex 71) is a widespread clone that has been described as the epidemic European clone. A systematic review on the epidemiology of MRSP conducted in 2016 confirmed that Clonal Complex (CC) 71 was the most reported CC among MRSP in Europe, and that it was only detected in methicillin-resistant strains [44]. More recent studies continue to report CC71 as the predominant clone in MRSP in Italy, Spain and Germany [33,38,45,46]. However, in contrast with these recent studies underlying a higher detection of MRSP belonging to ST71, in our study, only one ST71 was found, with ST123 being the most prevalent clone. Nevertheless, ST123 also belongs to CC71, and it differs from ST71 by a one-point mutation on the sar locus. ST123 has been previously described in a study by Duim et al. conducted in MRSP isolated from dogs in the Netherlands in 2016 [47]. Bergot et al. (2018) reported that, although ST71 still remains the most prevalent clone, between 2012–2013 and 2015–2016 the prevalence of ST71 rapidly decreased from 65.3% to 55.2% [17]. In Portugal, in studies performed until 2016, ST71 was also the most prevalent clone [19]. However, the clonal lineages of MRSP isolated from dogs have not been reported since 2016 in this country, and a shift might have occurred. Other plausible explanations may be that the ST123 clone has a higher evolutionary rate or some unknown factor has favored its recent prevalence in canine pyoderma in Portugal. Furthermore, the high occurrence of this clone, closely related to ST71, may be in agreement to Ishihara et al., who stated that the ST71 lineage may not be as clonal as previously believed [48]. ST71 seems to be linked to MRSP, while ST123 has been detected among both MRSP and MSSP [44]. In our study, the ST71 isolate had an extensively drug-resistant profile, showing resistance to penicillin, kanamycin, erythromycin, clindamycin, tetracycline and trimethoprim-sulfamethoxazole. Some studies reported that ST71 typically has tetracycline and chloramphenicol susceptibility [44]. However, the frequency of tetracycline resistance seems to vary geographically in ST71 isolates, and other studies have also found a multidrug-resistant profile, including resistance to tetracycline, in ST71 strains [12,38,46,49]. In our study, among the CC71 isolates, there seems to be a connection between the ST and the resistance profile since, unlike the ST71 isolate, all ST123 strains showed resistance to gentamicin, tobramycin and ciprofloxacin. Two isolates belonged to ST727, which was first described in Sweden and associated with a MRSP isolate according to the S. pseudintermedius PubMLST database (http://pubmlst.org/spseudintermedius accessed on 25 January 2021). Two strains belonged to ST339, which is a singleton and has been reported in MRSP from canine pyoderma [14,47]. All of the other STs found in this study belonged to one isolate each. ST1468 was only described in MSSP recovered from a dog nasal swab in a recent study, and ST537 was reported in MRSP from a diseased dog in Australia [7,12]. ST45 is the most prevalent clone in Asia and it is also widely disseminated in Australia. However, it is also relatively common in Europe, often being the second most prevalent clone in dog infections [43,47]. In our study, the ST45 isolate was one of those that showed resistance to more antibiotics, harboring seven antibiotic resistance genes. One isolate belonged to ST1029, which, according to the S. pseudintermedius PubMLST database, was first described in MRSP causing canine pyoderma in 2016 in France. Finally, one isolate was ascribed to ST118, which is included in CC258. ST118 has been reported in multiple European countries [44,47].
Table 2.
Genetic characteristics of methicillin-resistant Staphylococcus pseudintermedius (MRSP) isolated from canine pyoderma in Portugal.
| Isolate | Antimicrobial resistance | Virulence | ST (CC) | ||
|---|---|---|---|---|---|
| Phenotype | Genotype | ||||
| VS2777 | PEN-KAN-ERY-CD | blaZ, ermB, aph(3′)-IIIa | lukF-I/lukS-I | ST2024 | |
| VS2778 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aph(3′)-IIIa, tetK, dfrG | lukF-I/lukS-I | ST2025 | |
| VS2779 | PEN-KAN-ERY-CD-TET-CHL | blaZ, ermB, aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST2026 | |
| VS2780 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aph(3′)-IIIa, aac(6′)-Ie-aph(2′′)-Ia, ant(4′)-Ia, tetM, dfrG | lukF-I/lukS-I | ST2027 | |
| VS2781 | PEN-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, tetM, dfrG | lukF-I/lukS-I | ST2028 | |
| VS2782 | PEN-KAN-ERY-CD-TET-SXT | blaZ, ermB, aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST71 (71) | |
| VS2783 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-CHL-SXT | blaZ, msr(A/B), aph(3′)-IIIa, aac(6′)-Ie-aph(2′′)-Ia, ant(4′)-Ia, tetM, dfrG | lukF-I/lukS-I | ST1468 | |
| VS2784 | PEN-CIP-CN-TOB-KAN-ERY-CD-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2785 | PEN-CIP-KAN-ERY-CD-TET-CHL-SXT | blaZ, ermB, tetM, dfrG | lukF-I/lukS-I | ST727 | |
| VS2786 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST339 | |
| VS2787 | PEN-CIP-KAN-ERY-CD-SXT | blaZ, ermB, aph(3′)-IIIa, dfrG | lukF-I/lukS-I | ST537 | |
| VS2788 | PEN-CIP-KAN-ERY-CD-SXT | blaZ, ermB, aph(3′)-IIIa, dfrG | lukF-I/lukS-I | ST339 | |
| VS2789 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, msr(A/B), aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2790 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-CHL-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2791 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-CHL-SXT | blaZ, ermB, msr(A/B), aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST45 (45) | |
| VS2792 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2793 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2794 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetK, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2795 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2796 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-CHL-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2797 | PEN-CN-TOB-KAN-ERY-CD-TET-SXT-RD | blaZ, ermB, aph(3′)-IIIa, ant(4′)-Ia, tetK, dfrG | lukF-I/lukS-I | ST1029 | |
| VS2798 | PEN-CIP-CN-TOB-KAN-ERY-CD-FD-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2799 | PEN-CN-KAN-ERY-CD-TET-SXT | blaZ, ermB, msr(A/B), aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetK, dfrG | lukF-I/lukS-I | ST727 | |
| VS2800 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, msr(A/B), aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, tetK, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2801 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, msr(A/B), aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, tetK, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2802 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2803 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetK, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2804 | PEN-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, msr(A/B), aph(3′)-IIIa, ant(4′)-Ia, tetK, dfrG | lukF-I/lukS-I | ST118 (258) | |
| VS2805 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | ermB, msr(A/B), aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetK, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2806 | PEN-CIP-CN-TOB-KAN-ERY-CD-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2807 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
4. Conclusions
In this study, multidrug-resistant MRSP were detected in all isolates and they seem to be a common cause of cutaneous pyoderma, leading to an incidence of subsequent infections. MRSP presented frequent resistance to aminoglycosides, macrolides, tetracycline and trimethoprim-sulfamethoxazole. Given that ST123 was the most prevalent clone detected in this study and has not been previously reported in Portugal, it is likely that it may represent a locally evolved clone. Nevertheless, CC71 clones remain the most common in Europe. Due to the multidrug-resistant profile of MRSP isolated from cutaneous infections, veterinary antibiotic therapy is becoming less effective, imposing a serious threat to animal health.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/9/3/482/s1, Figure S1: Clonal lineages of MRSP isolated from pyoderma. goeBURST analysis in which the branches are connected with a single locus variant level to show the relation of STs. Red circles indicate STs detected in this study.
Author Contributions
Conceptualization, V.S., G.I. and P.P.; formal analysis, R.C. and C.A.-C.; methodology, V.S., V.M. and M.C.; validation, R.C., C.A.-C. and P.P.; investigation, V.S. resources, A.O.; data curation, V.S.; writing—original draft preparation, V.S.; visualization D.C. and I.C.; supervision, J.L.C., G.I. and P.P.; funding acquisition, R.C. and C.A.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministerio de Ciencia, Innovación y Universidades (Spain, Project RTI2018-098267-R-C33) and the Junta de Castilla y León (Consejería de Educación, Spain, Project LE018P20). This work was funded by the R&D Project CAREBIO2: Comparative assessment of antimicrobial resistance in environmental biofilms through proteomics—towards innovative theranostic biomarkers, with reference NORTE-01-0145-FEDER-030101 and PTDC/SAU-INF/30101/2017, financed by the European Regional Development Fund (ERDF) through the Northern Regional Operational Program (NORTE 2020) and the Foundation for Science and Technology (FCT). This work was supported by the Associate Laboratory for Green Chemistry-LAQV, which is financed by national funds from FCT/MCTES (UIDB/50006/2020 and UIDP/50006/2020). Vanessa Silva is grateful to FCT (Fundação para a Ciência e a Tecnologia) for financial support through the PhD grant SFRH/BD/137947/2018.
Institutional Review Board Statement
The study was conducted according to the Helsinki Declaration (ICH-GCP principles), compliance with Schedule Y/ICMR Guidelines, the Oviedo Convention, and approved by the Ethics Committee of University of Trás-os-Montes e Alto Douro (EC-UTAD, 8 November 2019)
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Syed A. Antibiotic Use and Resistance. Int. J. Curr. Res. Med. Sci. 2019;5:17–23. [Google Scholar]
- 2.Christaki E., Marcou M., Tofarides A. Antimicrobial resistance in bacteria: Mechanisms, evolution, and persistence. J. Mol. Evol. 2020;88:26–40. doi: 10.1007/s00239-019-09914-3. [DOI] [PubMed] [Google Scholar]
- 3.Foster T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017;41:430–449. doi: 10.1093/femsre/fux007. [DOI] [PubMed] [Google Scholar]
- 4.Osman K., Alvarez-Ordóñez A., Ruiz L., Badr J., ElHofy F., Al-Maary K.S., Moussa I.M.I., Hessain A.M., Orabi A., Saad A., et al. Antimicrobial resistance and virulence characterization of Staphylococcus aureus and coagulase-negative staphylococci from imported beef meat. Ann. Clin. Microbiol. Antimicrob. 2017;16:35. doi: 10.1186/s12941-017-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fishovitz J., Hermoso J.A., Chang M., Mobashery S. Penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. IUBMB Life. 2014;66:572–577. doi: 10.1002/iub.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . World Health Organization Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. WHO; Geneva, Switzerland: 2017. [Google Scholar]
- 7.Smith J.T., Amador S., McGonagle C.J., Needle D., Gibson R., Andam C.P. Population genomics of Staphylococcus pseudintermedius in companion animals in the United States. Commun. Biol. 2020;3:282. doi: 10.1038/s42003-020-1009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balachandran M., Bemis D.A., Kania S.A. Expression and function of protein A in Staphylococcus pseudintermedius. Virulence. 2018;9:390–401. doi: 10.1080/21505594.2017.1403710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krapf M., Müller E., Reissig A., Slickers P., Braun S.D., Müller E., Ehricht R., Monecke S. Molecular characterisation of methicillin-resistant Staphylococcus pseudintermedius from dogs and the description of their SCCmec elements. Vet. Microbiol. 2019;233:196–203. doi: 10.1016/j.vetmic.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Somayaji R., Priyantha M.A.R., Rubin J.E., Church D. Human infections due to Staphylococcus pseudintermedius, an emerging zoonosis of canine origin: Report of 24 cases. Diagn. Microbiol. Infect. Dis. 2016;85:471–476. doi: 10.1016/j.diagmicrobio.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Abouelkhair M.A., Bemis D.A., Giannone R.J., Frank L.A., Kania S.A. Characterization of a leukocidin identified in Staphylococcus pseudintermedius. PLoS ONE. 2018;13:e0204450. doi: 10.1371/journal.pone.0204450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Worthing K.A., Abraham S., Coombs G.W., Pang S., Saputra S., Jordan D., Trott D.J., Norris J.M. Clonal diversity and geographic distribution of methicillin-resistant Staphylococcus pseudintermedius from Australian animals: Discovery of novel sequence types. Vet. Microbiol. 2018;213:58–65. doi: 10.1016/j.vetmic.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami T., Shibata S., Murayama N., Nagata M., Nishifuji K., Iwasaki T., Fukata T. Antimicrobial susceptibility and methicillin resistance in Staphylococcus pseudintermedius and Staphylococcus schleiferi subsp. coagulans isolated from dogs with pyoderma in Japan. J. Vet. Med. Sci. 2010;72:1615–1619. doi: 10.1292/jvms.10-0172. [DOI] [PubMed] [Google Scholar]
- 14.Gagetti P., Wattam A.R., Giacoboni G., De Paulis A., Bertona E., Corso A., Rosato A.E. Identification and molecular epidemiology of methicillin resistant Staphylococcus pseudintermedius strains isolated from canine clinical samples in Argentina. BMC Vet. Res. 2019;15:264. doi: 10.1186/s12917-019-1990-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maali Y., Badiou C., Martins-Simões P., Hodille E., Bes M., Vandenesch F., Lina G., Diot A., Laurent F., Trouillet-Assant S. Understanding the Virulence of Staphylococcus pseudintermedius: A Major Role of Pore-Forming Toxins. Front. Cell. Infect. Microbiol. 2018;8:221. doi: 10.3389/fcimb.2018.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabatabaei S., Najafifar A., Askari Badouei M., Zahraei Salehi T., Ashrafi Tamai I., Khaksar E., Abbassi M.S., Ghazisaeedi F. Genetic characterisation of methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in pets and veterinary personnel in Iran: New insights into emerging methicillin-resistant S. pseudintermedius (MRSP) J. Glob. Antimicrob. Resist. 2019;16:6–10. doi: 10.1016/j.jgar.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Bergot M., Martins-Simoes P., Kilian H., Châtre P., Worthing K.A., Norris J.M., Madec J.-Y., Laurent F., Haenni M. Evolution of the Population Structure of Staphylococcus pseudintermedius in France. Front. Microbiol. 2018;9:3055. doi: 10.3389/fmicb.2018.03055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couto N., Belas A., Couto I., Perreten V., Pomba C. Genetic Relatedness, Antimicrobial and Biocide Susceptibility Comparative Analysis of Methicillin-Resistant and -Susceptible Staphylococcus pseudintermedius from Portugal. Microb. Drug Resist. 2013;20:364–371. doi: 10.1089/mdr.2013.0043. [DOI] [PubMed] [Google Scholar]
- 19.Couto N., Belas A., Oliveira M., Almeida P., Clemente C., Pomba C. Comparative RNA-seq-based transcriptome analysis of the virulence characteristics of methicillin-resistant and-susceptible Staphylococcus pseudintermedius strains isolated from small animals. Antimicrob. Agents Chemother. 2016;60:962–967. doi: 10.1128/AAC.01907-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bannoehr J., Franco A., Iurescia M., Battisti A., Fitzgerald J.R. Molecular diagnostic identification of Staphylococcus pseudintermedius. J. Clin. Microbiol. 2009;47:469–471. doi: 10.1128/JCM.01915-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva V., Almeida F., Carvalho J.A., Castro A.P., Ferreira E., Manageiro V., Tejedor-Junco M.T., Caniça M., Igrejas G., Poeta P. Emergence of community-acquired methicillin-resistant Staphylococcus aureus EMRSA-15 clone as the predominant cause of diabetic foot ulcer infections in Portugal. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:179–186. doi: 10.1007/s10096-019-03709-6. [DOI] [PubMed] [Google Scholar]
- 22.Silva V., Almeida F., Silva A., Correia S., Carvalho J.A., Castro A.P., Ferreira E., Manageiro V., Caniça M., Igrejas G., et al. First report of linezolid-resistant cfr-positive methicillin-resistant Staphylococcus aureus in humans in Portugal. J. Glob. Antimicrob. Resist. 2019;17:323–325. doi: 10.1016/j.jgar.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Yu F., Liu Y., Lv J., Qi X., Lu C., Ding Y., Li D., Liu H., Wang L. Antimicrobial susceptibility, virulence determinant carriage and molecular characteristics of Staphylococcus aureus isolates associated with skin and soft tissue infections. Braz. J. Infect. Dis. 2015;19:614–622. doi: 10.1016/j.bjid.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarraud S., Mougel C., Thioulouse J., Lina G., Meugnier H., Forey F., Etienne J., Vandenesch F., Nesme X. Relationships between Staphylococcus aureus Genetic Background, Virulence Factors, agr Groups (Alleles), and Human Disease. Infect. Immun. 2002;70:631–641. doi: 10.1128/IAI.70.2.631-641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lina G., Piemont Y., Godail-Gamot F., Bes M., Peter M.-O., Gauduchon V., Vandenesch F., Etienne J. Involvement of Panton-Valentine Leukocidin-Producing Staphylococcus aureus in Primary Skin Infections and Pneumonia. Clin. Infect. Dis. 1999;29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 26.Futagawa-Saito K., Sugiyama T., Karube S., Sakurai N., Ba-Thein W., Fukuyasu T. Prevalence and characterization of leukotoxin-producing Staphylococcus intermedius in isolates from dogs and pigeons. J. Clin. Microbiol. 2004;42:5324–5326. doi: 10.1128/JCM.42.11.5324-5326.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solyman S.M., Black C.C., Duim B., Perreten V., Van Duijkeren E., Wagenaar J.A., Eberlein L.C., Sadeghi L.N., Videla R., Bemis D.A. Multilocus sequence typing for characterization of Staphylococcus pseudintermedius. J. Clin. Microbiol. 2013;51:306–310. doi: 10.1128/JCM.02421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bannoehr J., Ben Zakour N.L., Waller A.S., Guardabassi L., Thoday K.L., Broek A.H.M.V.D., Fitzgerald J.R. Population genetic structure of the Staphylococcus intermedius group: Insights into agr diversification and the emergence of methicillin-resistant strains. J. Bacteriol. 2007;189:8685–8692. doi: 10.1128/JB.01150-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakaminami H., Okamura Y., Tanaka S., Wajima T., Murayama N., Noguchi N. Prevalence of antimicrobial-resistant staphylococci in nares and affected sites of pet dogs with superficial pyoderma. J. Vet. Med. Sci. 2020 doi: 10.1292/jvms.20-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.González-Domínguez M.S., Carvajal H.D., Calle-Echeverri D.A., Chinchilla-Cárdenas D. Molecular Detection and Characterization of the mecA and nuc Genes From Staphylococcus Species (S. aureus, S. pseudintermedius, and S. schleiferi) Isolated From Dogs Suffering Superficial Pyoderma and Their Antimicrobial Resistance Profiles. Front. Vet. Sci. 2020;7:376. doi: 10.3389/fvets.2020.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruzauskas M., Couto M., Pavilonis A., Klimiene I., Siugzdiniene R., Virgailis M., Vaskeviciute L., Anskiene L., Pomba C. Characterization of Staphylococcus pseudintermedius isolated from diseased dogs in Lithuania. Pol. J. Vet. Sci. 2016;19:7–14. doi: 10.1515/pjvs-2016-0002. [DOI] [PubMed] [Google Scholar]
- 32.Priyantha R., Gaunt M.C., Rubin J.E. Antimicrobial susceptibility of Staphylococcus pseudintermedius colonizing healthy dogs in Saskatoon, Canada. Can. Vet. J. 2016;57:65. [PMC free article] [PubMed] [Google Scholar]
- 33.Menandro M.L., Dotto G., Mondin A., Martini M., Ceglie L., Pasotto D. Prevalence and characterization of methicillin-resistant Staphylococcus pseudintermedius from symptomatic companion animals in Northern Italy: Clonal diversity and novel sequence types. Comp. Immunol. Microbiol. Infect. Dis. 2019;66:101331. doi: 10.1016/j.cimid.2019.101331. [DOI] [PubMed] [Google Scholar]
- 34.Riley M.C., Perreten V., Bemis D.A., Kania S.A. Complete genome sequences of three important methicillin-resistant clinical isolates of Staphylococcus pseudintermedius. Genome Announc. 2016;4:e01194-16. doi: 10.1128/genomeA.01194-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onuma K., Tanabe T., Sato H. Antimicrobial resistance of Staphylococcus pseudintermedius isolates from healthy dogs and dogs affected with pyoderma in Japan. Vet. Dermatol. 2012;23:17-e5. doi: 10.1111/j.1365-3164.2011.00995.x. [DOI] [PubMed] [Google Scholar]
- 36.Wegener A., Broens E.M., Zomer A., Spaninks M., Wagenaar J.A., Duim B. Comparative genomics of phenotypic antimicrobial resistances in methicillin-resistant Staphylococcus pseudintermedius of canine origin. Vet. Microbiol. 2018;225:125–131. doi: 10.1016/j.vetmic.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Petersen A., Stegger M., Heltberg O., Christensen J., Zeuthen A., Knudsen L.K., Urth T., Sorum M., Schouls L., Larsen J., et al. Epidemiology of methicillin-resistant Staphylococcus aureus carrying the novel mecC gene in Denmark corroborates a zoonotic reservoir with transmission to humans. Clin. Microbiol. Infect. 2013;19:E16–E22. doi: 10.1111/1469-0691.12036. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz-Ripa L., Simón C., Ceballos S., Ortega C., Zarazaga M., Torres C., Gómez-Sanz E.S. pseudintermedius and S. aureus lineages with transmission ability circulate as causative agents of infections in pets for years. BMC Vet. Res. 2021;17:42. doi: 10.1186/s12917-020-02726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gómez-Sanz E., Torres C., Lozano C., Zarazaga M. High diversity of Staphylococcus aureus and Staphylococcus pseudintermedius lineages and toxigenic traits in healthy pet-owning household members. Underestimating normal household contact? Comp. Immunol. Microbiol. Infect. Dis. 2013;36:83–94. doi: 10.1016/j.cimid.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy A.J., Harrison E.M., Stanczak-Mrozek K., Leggett B., Waller A., Holmes M.A., Lloyd D.H., Lindsay J.A., Loeffler A. Genomic insights into the rapid emergence and evolution of MDR in Staphylococcus pseudintermedius. J. Antimicrob. Chemother. 2015;70:997–1007. doi: 10.1093/jac/dku496. [DOI] [PubMed] [Google Scholar]
- 41.Pitchenin L.C., Brandão L.N.S., Rosa J.M.A., Kagueyama F.C., da Silva Alves A., Rocha Í.S.M., Nakazato L., Dutra V. Occurrence of toxin genes in Staphylococcus pseudintermedius from diseased dogs and other domestic and wild species. J. Infect. Dev. Ctries. 2018;11:957–961. doi: 10.3855/jidc.8261. [DOI] [PubMed] [Google Scholar]
- 42.Melter O., Svec P., Tkadlec J., Doskar J., Kinská H., Pantucek R. Characterisation of methicillin-susceptible Staphylococcus pseudintermedius isolates from canine infections and determination of virulence factors using multiplex PCR. Vet. Med. (Praha) 2017;62:81–89. doi: 10.17221/105/2016-VETMED. [DOI] [Google Scholar]
- 43.Damborg P., Moodley A., Aalbæk B., Ventrella G., Dos Santos T.P., Guardabassi L. High genotypic diversity among methicillin-resistant Staphylococcus pseudintermedius isolated from canine infections in Denmark. BMC Vet. Res. 2016;12:131. doi: 10.1186/s12917-016-0756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pires dos Santos T., Damborg P., Moodley A., Guardabassi L. Systematic Review on Global Epidemiology of Methicillin-Resistant Staphylococcus pseudintermedius: Inference of Population Structure from Multilocus Sequence Typing Data. Front. Microbiol. 2016;7:1599. doi: 10.3389/fmicb.2016.01599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meroni G., Soares Filipe J.F., Drago L., Martino P.A. Investigation on antibiotic-resistance, biofilm formation and virulence factors in multi drug resistant and non multi drug resistant Staphylococcus pseudintermedius. Microorganisms. 2019;7:702. doi: 10.3390/microorganisms7120702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soimala T., Lübke-Becker A., Hanke D., Eichhorn I., Feßler A.T., Schwarz S., Eule J.C. Molecular and phenotypic characterization of methicillin-resistant Staphylococcus pseudintermedius from ocular surfaces of dogs and cats suffering from ophthalmological diseases. Vet. Microbiol. 2020;244:108687. doi: 10.1016/j.vetmic.2020.108687. [DOI] [PubMed] [Google Scholar]
- 47.Duim B., Verstappen K.M., Broens E.M., Laarhoven L.M., van Duijkeren E., Hordijk J., de Heus P., Spaninks M., Timmerman A.J., Wagenaar J.A. Changes in the Population of Methicillin-Resistant Staphylococcus pseudintermedius and Dissemination of Antimicrobial-Resistant Phenotypes in the Netherlands. J. Clin. Microbiol. 2016;54:283–288. doi: 10.1128/JCM.01288-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishihara K., Koizumi A., Saito M., Muramatsu Y., Tamura Y. Detection of methicillin-resistant Staphylococcus pseudintermedius ST169 and novel ST354 SCCmec II–III isolates related to the worldwide ST71 clone. Epidemiol. Infect. 2016;144:434–442. doi: 10.1017/S0950268815001545. [DOI] [PubMed] [Google Scholar]
- 49.Videla R., Solyman S.M., Brahmbhatt A., Sadeghi L., Bemis D.A., Kania S.A. Clonal Complexes and Antimicrobial Susceptibility Profiles of Staphylococcus pseudintermedius Isolates from Dogs in the United States. Microb. Drug Resist. 2017;24:83–88. doi: 10.1089/mdr.2016.0250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.